Abstract
AIM: To calculate cost effectiveness of the treatment of critically ill patients in a medical intensive care unit (ICU) of a middle income country with limited access to ICU resources.
METHODS: A prospective cohort study and economic evaluation of consecutive patients treated in a recently established medical ICU in Sarajevo, Bosnia and Herzegovina. A cost utility analysis of the intensive care of critically ill patients compared to the hospital ward treatment from the perspective of the health care system was subsequently performed. Incremental cost effectiveness was calculated using estimates of ICU vs non-ICU treatment effectiveness based on a formal systematic review of published studies. Decision analytic modeling was used to compare treatment alternatives. Sensitivity analyses of the key model parameters were performed.
RESULTS: Out of 148 patients, seventy patients (47.2%) survived to one year after critical illness with a median quality of life index 0.64 [interquartile range(IQR) 0.49-0.76]. Median number of life years gained per patient was 30 (IQR 16-40) or 18 quality adjusted life years (QALYs) (IQR 7-28). The cost of treatment of critically ill patients varied between 1820 dollar and 20109 dollar per hospital survivor and between 100 dollar and 2514 dollar per QALY saved. Mean factors that influenced costs were: Age, diagnostic category, ICU and hospital length of stay and number and type of diagnostic and therapeutic interventions. The incremental cost effectiveness ratio for ICU treatment was estimated at 3254 dollar per QALY corresponding to 35% of per capita GDP or a Very Cost Effective category according to World Health Organization criteria.
CONCLUSION: The ICU treatment of critically ill medical patients in a resource poor country is cost effective and compares favorably with other medical interventions. Public health authorities in low and middle income countries should encourage development of critical care services.
Keywords: Cost benefit analysis, Intensive care, Quality of life, Intensive care unit, Mortality, Decision analysis, Economics
Core tip: The first of a kind prospective cost effectiveness study in the intensive care unit in low resource settings. The study provides important evidence that critical care is cost effective medical intervention that favorably compares with most standard medical treatments but is unfortunately grossly underdeveloped in low resource settings.
INTRODUCTION
Cost-effectiveness analysis (CEA) and related techniques are methods for evaluating the economic efficiency of health-related programs and interventions[1,2].
CEA is designed to incorporate the medical and economic aspects of health care programs or interventions, to examine these interventions from perspectives of different actors and to allow objective comparisons with alternative interventions or programs[3]. In an environment with limited resources, it is crucial to ensure fairness in the allocation of resources on the one hand and efficiency on the other[4]. Health care providers, public health officials, and other decision makers require accurate information about economic efficiency, or “cost-effectiveness”, of different options in order to maximize the impact of health care spending[5]. Ideally this will lead to the most effective allocation of resources. For a post-war developing country such as Bosnia and Herzegovina optimal allocation of resources is crucial.
Treatment of critically ill patients includes expensive equipment, highly skilled personnel, and often costly procedures to save lives. Studies that deal with the outcome of treatment in an intensive care unit (ICU), have reported mortality of critically ill patients between 8% and 33%, with a further 11% to 64% mortality rate on general hospital wards after ICU treatment[6,7].
Intensive care is an expensive specialty due to its need for highly trained personnel and modern technology[8] and is a low priority for public health authorities in resource limited countries[9-11]. Objective analysis of costs and outcomes is needed to determine whether an admission to an ICU is a reasonable use of limited resources in a population of critically ill patients[12].
The high mortality of ICU patients, high cost, and a certain possibility of survival of patients without intensive care raise a question of whether the treatment in an ICU provides good value for invested resources, especially in a resource limited country such as Bosnia and Herzegovina?
A search of the literature on the analysis of costs and effectiveness in the field of intensive care medicine found no studies published from the Balkans, South-Eastern Europe or other resource limited settings. The objective of this study was to calculate the incremental cost per quality-adjusted life year (QALY) obtained by the treatment of medical critically ill patients in the ICU compared to the treatment without intensive care at the Sarajevo University Clinical Center. We sought to understand whether the recently established medical ICU provides a good value for the invested resources by the health care system of a developing country.
Our original hypothesis was that critical care is cost-effective even in a resources poor setting.
MATERIALS AND METHODS
Design and setting
We performed a prospective cohort study and an economic evaluation of consecutive medical critically ill patients treated in a recently established five bed medical ICU in a tertiary care medical institution with around 1800 beds in Sarajevo, the capital city of Bosnia and Herzegovina. The ICU has approximately 140-180 admissions annually.
Study population
Consecutive medical critically ill patients treated between June 1 2011 and June 29 2012 was included. Patients that stayed in the ICU less than 24 h and hospital readmissions were excluded from the analysis. Critical care survivors were interviewed at one year follow up after hospital release.
Patient characteristics, acute illness severity scores[13-15], diagnostic and therapeutic interventions along with costs were prospectively recorded. Data were recorded in a predefined paper form and afterwards entered in a specially constructed database.
Control group of patients
Ethical and administrative constraints precluded the use of historical or concurrent control group of the critically ill patients without access to the ICU in our institution. To overcome these constraints and to increase the generalizability of our findings, we calculated the incremental cost effectiveness using estimates of ICU vs non-ICU treatment effectiveness based on a formal systematic review of published studies.
Outcome definitions
Primary outcome measure was one year survival. Secondary outcome measures were: 28 d ventilator free days, 28 d ICU free days, 30 d and 60 d mortality, the health related quality of life (HRQOL) and incremental cost effectiveness ratio (ICER). Ventilator-free days were defined as the number of days between successful weaning from mechanical ventilation and 28 d after study enrollment[16]. ICU-free days were defined as the number of days between successful transfer to a normal ward and 28 d after study enrollment[16].
ICER is the ratio of the difference in costs and difference in effects between two competing choices[17].
Economic evaluation
Recommendations of the Panel of Cost-effectiveness in Health and Medicine adjusted for critical care were followed for economic evaluation[18].
With primary data obtained, a full economic evaluation in the form of cost utility analysis of intensive care of critically ill patients compared to hospital ward treatment from the perspective of the health care system was performed. The analytical horizon was the life time of patients. Decision analytic modeling was used to construct a decision tree (Figure 1), which served to calculate the ICER.
Figure 1.
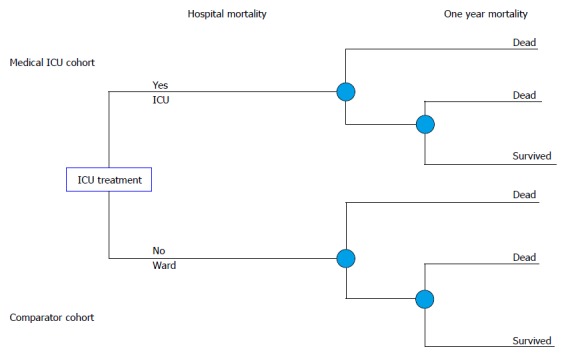
The decision tree in the decision analysis. Two branches of the decision tree represent two cohorts of critically ill patients. First one is represented with the patients that were treated in the medical ICU. The other branch represents comparator model of the same patients and shows the treatment outcomes if patients were treated on the hospital ward instead of the ICU. data on treatment outcomes for the comparator were extracted from the systemetic review of the literature. ICU: Intensive care unit.
Model: The decision tree
Two branches of the decision tree present two cohorts of critically ill patients. First one is represented with the medical ICU patients (Figure 1). The other branch represents the comparator model of the patients with the same characteristics and shows treatment outcomes if patients were treated on the hospital ward instead of the ICU.
The data on the treatment outcomes of the comparator cohort were extracted from a systematic review of the literature.
Systematic review
A systematic review of the available literature was performed in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines[19].
A comprehensive search strategy was developed in order to identify the studies that dealt with outcomes of the treatment of adult critically ill patients in and out of an ICU. The following electronic databases were searched: PubMed, EBSCO and Web of science. The search targeted original articles, published in English on adult patient population (≥ 19 years) in the date range from the year 1990 to May 31, 2012.
The following terms were used: Critical OR acute AND ill AND rationing OR selection OR withholding treatment OR refusal to treat OR triage OR utilization AND mortality OR outcome OR statistics OR numerical data OR economics. In addition we searched through related articles on PubMed and also found additional articles from the reference lists of retrieved articles.
Comparative, validation, evaluation, observational studies, clinical trials and controlled trials were included in the study screening process. Review articles, letters to the editor, commentaries, expert opinions and studies for which the full text was unavailable were excluded.
The search process is presented on Figure 2 (PRISMA flow diagram). The first search returned 501 studies or 487 after the duplicates were removed. Studies that did not fulfill the inclusion criteria or the ones that met the exclusion criteria (n = 369) were excluded. A total of 118 articles were screened based on the title and abstract and among them full texts of 18 studies were identified and reviewed. Secondarily, the studies that showed highly increased mortality among ICU treated critically ill were excluded from the selection, since it was very unlikely that such outcome would be possible in the case of the Clinical Center University of Sarajevo. A total of 14 studies were included[12,20-32] (Figure 3 and Table 1). The following data were recorded: The year of publication, country of origin, patient population, reasons for ICU refusal, and mortality of critically ill patients treated within the ICU and outside of an ICU. Data were extracted in a previously defined form.
Figure 2.
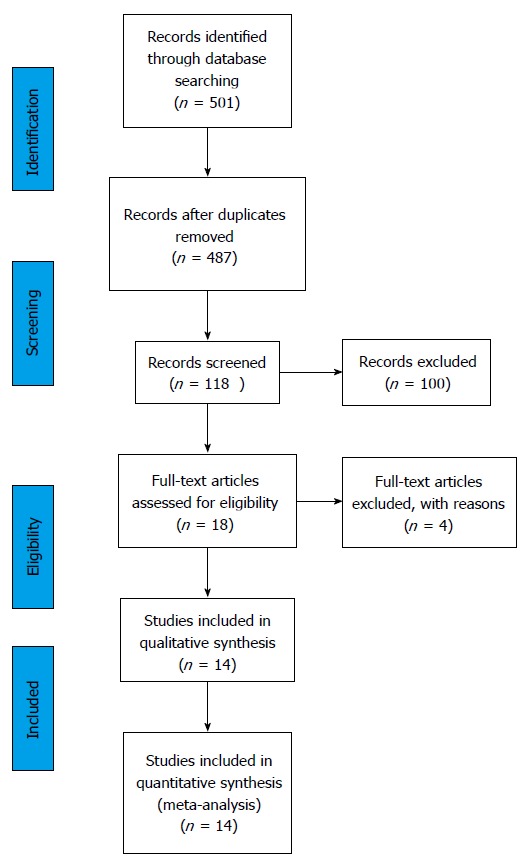
The Preferred Reporting Items for Systematic Reviews and Meta-analyses flow diagram. The information flow in the different phases of the systematic review is presented. The first search through the data bases returned 501 studies, or 487 after the duplicates were removed. A total of 118 studies were screened based on the abstract and title and 18 full text articles were reviewed. Among them 14 studies were included in the meta-analysis.
Figure 3.
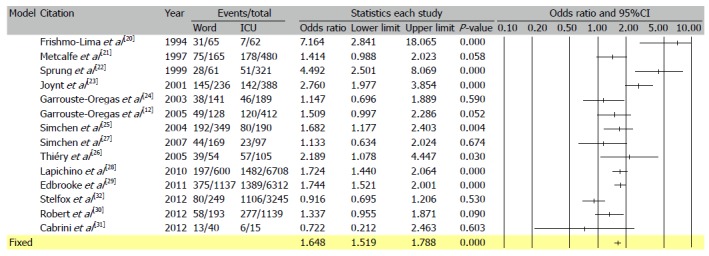
Meta-analysis of unadjusted mortality rates from the 14 studies reporting mortality rates in patients admitted to and refused intensive care unit. Based on the reported international data odds ratio for mortality of critically ill patients when treated outside of intensive care unit on standard care wards was calculated, OR = 1.64, 95%CI: 1.51-1.78.
Table 1.
Characteristics of the studies included in the systematic review
| Ref. | Patients population and country | Year of publication | Refusal reasons | No. of deaths with ICU support out of total patients treated in ICU (% mortality) | No. of deaths without ICU support out of total patients not treated in ICU (% mortality) | Difference in mortality rates (%) | OR (95%CI) |
| Frisho-Lima et al[20] | General, Israel | 1994 | Moribund, no beds | 7/62 (11.3) | 31/65 (47.7) | 36.4 | 7.16 (2.84-18.07) |
| Metcalfe et al[21] | 6 general, United Kingdom | 1997 | Lack of beds or staff or other | 178/480 (37.1) | 75/165 (45.5) | 8.4 | 1.41 (0.99-2.02) |
| Sprung et al[22] | General, Israel | 1999 | Too good or too poor prognosis, no beds, more data required, another crit. care area more appropriate | 51/321 (15.9) | 28/61 (45.9) | 30 | 4.49 (2.50-8.07) |
| Joynt et al[23] | General, Hong Kong | 2001 | Triage, futility, inappropriate referral. | 142/388 (36.6) | 145/236 (61.4) | 24.8 | 2.76 (1.98-3.85) |
| Garrouste-Oregas et al[24] | Medical and surgical, France | 2003 | too well/too sick | 46/189 (24.3) | 38/141 (27.0) | 2.6 | 1.15 (0.70-1.89) |
| Simchen et al[25] | 5 ICUs. Medical and surgical, Israel | 2004 | All hospital patients screened | 80/190 (42.1) | 192/349 (55.0) | 12.9 | 1.68 (1.18-2.40) |
| Garrouste-Oregas et al[12] | 4 medical and 7 general, France | 2005 | Too well/too sick, patients refusal, ICU occupied | 120/412 (29.1) | 49/128 (38.3) | 9.2 | 1.51 (1.00-2.29) |
| Thiéry G et al[26] | Medical ICU, cancer patients 30 d mortality, France | 2005 | Too sick | 57/105 (54.3) | 39/54 (74.0) | 19.7 | 2.19 (1.08-4.45) |
| Simchen et al[27] | 5 acute care hospitals, medical and surgical patients Israel | 2007 | Died > 24 h deteriorated on ward | 23/97 (23.7) | 44/169 (26.0) | 2.3 | 1.13 (0.63-2.02) |
| Died < 24 h | 4/97 (4.1) | 55/414 (13.3) | 9.2 | 3.56 (1.26-10.08) | |||
| Iapichino et al[28] | 11 university hospitals from 7 countries: Denmark, France, Israel, Italy, The Netherlands, Spain, United Kingdom | 2010 | 28 d mortality | 1482/6708 (22.1) | 197/600 (32.8) | 10.7 | 1.72 (1.44-2.06) |
| Edbrooke et al[29] | 11 hospitals in 7 EU countries | 2011 | 28 d mortality | 1389/6312 (22.0) | 375/1137 (33.0) | 11.0 | 1.74 (1.52-2.00) |
| Robert et al[30] | 10 medical ICUs, France | 2012 | Too good or too sick 28 d | 277/1,139 (24.3) | 58/193 (30.1) | 5.7 | 1.34 (0.96-1.87) |
| Cabrini et al[31] | Early ICU transfer, medical patients | 2012 | Too well/too sick or lack of ICU beds | 6/15 (40.0) | 13/40 (32.5) | -7.5 | 0.72 (0.21-2.46) |
| Italy | |||||||
| Stelfox et al[32] | Canada, medical ICU | 2012 | Too well/too sick or lack of ICU beds | 1106/3245 (34.1) | 80/249 (32.1) | -2.0 | 0.92 (0.69-1.21) |
| Total | 4968/19760 (25.1) | 1419/4001 (35.5) | 10.3 | 1.64 (1.52-1.76) |
ICU: Intensive care unit.
Data from the above mentioned studies were used for the calculation of an Odds Ratio for mortality of critically ill patients when treated on the general hospital wards instead of the ICU. Similar calculations were previously done by Ridley et al[8], further details in the Electronic data supplement.
For discounting of costs and health effects an annual rate of 3% was used, with sensitivity analysis for 0% and 6%. Uncertainty was controlled by the use of sensitivity analysis of the key model parameters: The attributable risk reduction of death for ICU treatment, the daily ICU cost, the daily standard care general ward cost, the discount rate, and the quality of life after critical illness, the quality of life index calculation method, the adjustment for excess mortality after critical illness (Post-ICU survival rate, PICUS).
One and two-way sensitivity analysis was performed; along with best and worst case scenarios. A simplified overview of methodology used is presented in Figure 4.
Figure 4.
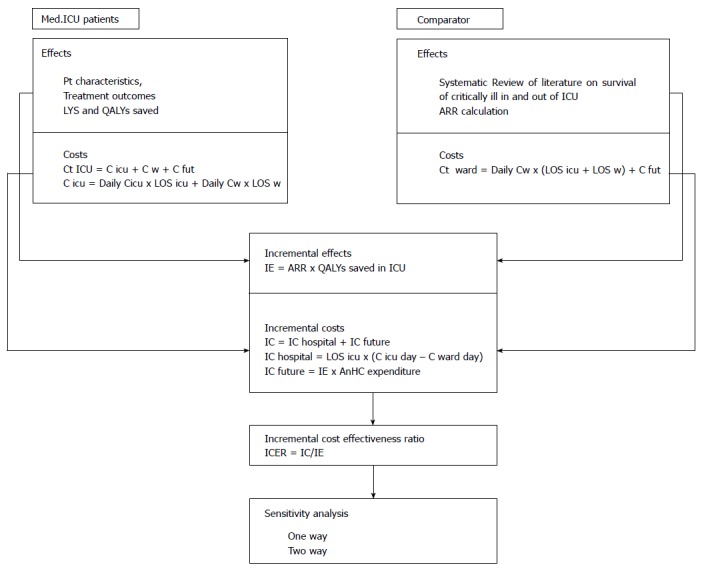
Overview of methodology used in the study. The first phase was data collection for two patient cohorts, observed medical ICU patients and comparator cohort, which was represented by the same number of critically ill patients, with the same characteristics who would be treated on general hospital ward. Costs and treatment outcomes (effects) were recorded and calculated. In the second phase incremental analysis of costs and effects was done. The third phase gave the main incremental cost effectiveness ratio and in fourth a sensitivity analysis of cost effectiveness ratio to key model parameters was performed. Costs and effects were discounted at an annual rate of 3%. LYS: Life years saved; QALY: Quality adjusted life years; ARR: Attributable risk reduction for mortality with treatment of critically ill in ICU; Ct: Total costs; C icu: Costs of ICU treatment; C w: Costs of ward treatment; C fut: Future health care costs, after hospital release; Daily C icu: Average cost per ICU day; Daily C w: Average cost per general hospital ward day in internal medical department; LOS icu/w: Length of stay in the ICU or on general ward; IE: Incremental effects; IC: Incremental costs; AnHC expenditure: Annual health care expenditure per capita for Bosnia and Herzegovina; ICER: Incremental cost-effectiveness ratio. ICU: Intensive care unit.
Costing methodology
From the perspective of health system Costs consist of costs of hospitalization and long term post-hospitalization costs.
The cost of hospital stay was calculated for the ICU patient cohort and for the comparator cohort. The ICU patient cohort hospital stay cost was calculated as the sum of the ICU cost and the post-ICU ward cost. The cost of the ICU stay was calculated as the product of the cost per ICU day and the length of ICU stay. The daily ICU cost was calculated based on fixed ICU cost and patient specific variable costs and costing methodology recommended by the Health Insurance Institute of Federation of Bosnia and Herzegovina.
The fixed cost was an estimate made by the Health insurance Institute after an analysis of costs of personnel, equipment amortization, overheads, cost of a hospital bed-maintenance, costs of routine laboratory and X-ray and microbiology. The Health Insurance institute of Federation of Bosnia and Herzegovina is the main financier of the Clinical center University of Sarajevo.
Patient variable costs were prospectively recorded and included all additional medical interventions such as radiology procedures (CT, MRI, United States), the placement of central venous or dialysis catheters, hemodialysis (HD), peritoneal dialysis, continuous veno-venous HD and other related techniques etc. The variable costs were calculated based on present market values of specific medical interventions[9].
The total ICU-stay cost of all 148 patients was used to calculate the daily unit cost for the ICU. The daily post ICU ward cost was calculated as the average daily cost for standard care on internal medical clinics in the Feredation of Bosnia and Herzegovina. This data was also published by the Federal Health Insurance Institute[9].
The comparator cohort cost was calculated as the product of the average ward daily cost and the hospital length of stay of the real patient cohort. For the purposes of the model, it was assumed that the hypothetical patient cohort would have the same length of stay as real ICU patients that were analyzed.
Incremental hospital costs were then calculated using the following formula: IC hospital = ICU cost - Ward cost = LOS icu × (C icu day - C ward day); LOS-length of stay, C-cost. Future cost for the ICU survivors were approximated using the mean annual health care expenditure per capita for the population of Bosnia and Herzegovina, which was 928 dollar for the year 2011, as reported by the WHO[33]. This approach has been previously used in economic evaluations[8,34].
Costs were reported using a World Health Organization (WHO) suggested currency, the International dollar. This is a theoretical currency, calculated from the WHO published purchasing power parities, and represents what can be bought in a country with one United States dollar. In practice, it corresponds to United States dollar[35].
Incremental future cost was calculated using the following formula: IC future = IE (number of QALYs gained by the treatment in the ICU) × mean annual health care expenditure per capita for the population of Bosnia and Herzegovina and were discounted to present value for the year 2012. The incremental cost was calculated as the sum of incremental hospital and the incremental future costs, or: IC = IC hospital + IC future. IC= [LOS icu × (C icu day - C ward day)] + [IE × mean annual health care expenditure per capita].
Prediction of life time QALYs
For one year survivors a life expectancy was calculated using life tables for the population of Bosnia and Herzegovina for the year 2011, obtained from the WHO[36]. For one year survivors the life expectancy was calculated as expected life duration from a patient’s age at admission.
For patients who did not survive one year the exact length of life after hospital release was used. Life time QALYs were calculated by multiplying the predicted life expectancy or real life-time with utility values for HRQOL.
The HRQOL was assessed using the EQ-5D questionnaire[37]. Written permission along with official translation of the questionnaire was granted by the Euro-QOl Group[37]. A HRQOL index was calculated from the resulting five digit EQ-5D profile using reference values for population of Europe and Slovenia. Resulting the HRQOL index was compared to reference values. An adjustment was made for missing data on the quality of life index for patients who did not answer the EQ5D questionnaire and for those who died before one year from the hospital release. For one year survivors who did not answer the questionnaire it was assumed that they had the mean value of the HRQOL index of the age and sex matched respondents. For one year non-survivors it was assumed they had the 75% of the mean HRQOL of respondents. This approach was previously taken in cost effectiveness analysis by Linko et al[35]. Life time QALY was calculated as the product of life years saved and HQOL utility value.
Statistical analysis
Discrete data are reported as frequencies and continuous data as means (confidence interval-CI) or medians [interquartile ranges-Interquartile range (IQR)], as appropriate. All hypotheses in the study were two-sided, with the level of significance 0.05. Discrete variables were compared using the χ2 test. Normally distributed continuous variables were compared using the Student’s t test, and non-normally distributed variables were compared using the Mann Whitney test. Multiple groups were compared using the Kruskal Wallis test. Comparison of one year survivors EQ-5D index values to the reference values of the age and sex matched population of Slovenia was done using the Wilcoxon signed matched pair test. Statistical analysis was done using SPSS 19 and MS Excel 2010 programs. No funding was received for the conduct of this study.
RESULTS
Patient characteristics
In the period from June 2011 to June 2012 a total of 183 patients were treated in the medical ICU of the Sarajevo University Clinical Center, out of which 148 were included in the study (Figure 5). Overall patient characteristics separated by status one year after hospital release are reported in Table 2. Patients differed in various terms, with patients that survived one year longer, significantly younger (mean 49 years vs 63 years, P < 0.01), less severely ill (mean SAPSII 42 vs 59, P < 0.001), mainly admitted through the emergency department (44% vs 26%, P = 0.027), with a shorter hospital stay before the ICU [median 1 IQR (0-4) vs 1.5 IQR (0-8), P = 0.039] and shorter courses of mechanical ventilation [median 3 IQR (0-9) vs 5 IQR (3-9), P = 0.024] and vasopressor use (11% vs 37%, P = 0.01). The most frequent admission diagnoses among one year survivors were: Sepsis (20%), cardiac arrest (18.6%), intoxication (15.7%) and hypoxemic acute respiratory failure (12.9%). Non-survivors were admitted to the ICU mostly due to: Sepsis (24.4%), comma (17.9%), cardiac arrest (15.4%) and hypoxemic acute respiratory failure (12.8%). All critically ill patients had median ICU length of stay of 6 d, IQR (3-12), that did not differ between survivors and non- survivors. Median length of post ICU hospital stay was 14 d, IQR (13.75-15.75) which refers to one year survivors, because non survivors died mostly in the ICU or very shortly after ICU release. One year survivors had significantly higher median 28 d ICU free days [12.5, IQR (0-23)] compared to non-survivors [0, IQR (0-19)]. Ventilator free days at 28 d did not differ significantly between survivors and non survivors, and overall median was 23 d, IQR (18-25).
Figure 5.
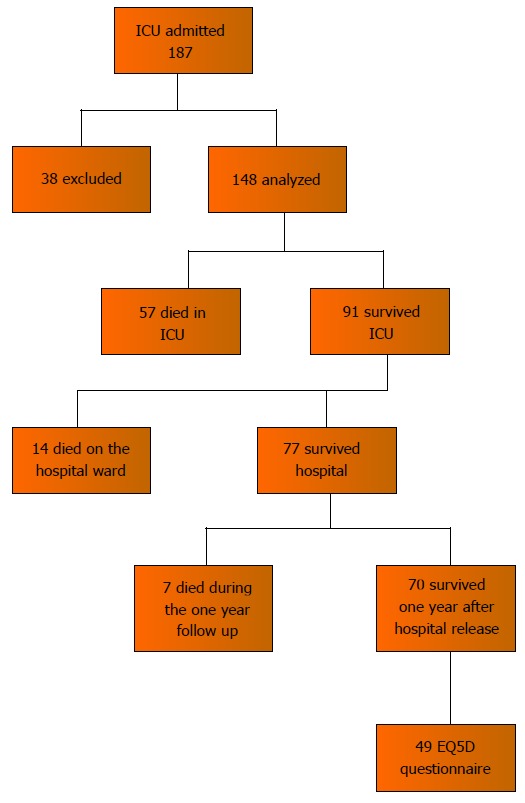
Patient flow diagram. Out of 148 patients that were included in the study, 57 (38.5%) died in the ICU, 14 (9.4%) died on the hospital wards after the ICU, 7 (4.7%) died during one year follow up, 70 (47.2%) survived one year after hospital release and 49 were interviewed for the health related quality of life assessment using the EuroQol-5D questionnaire. ICU: Intensive care unit.
Table 2.
Patient characteristics
| All n = 148 | 1 yr survivors | Dead after one year | P | ||
| Age | |||||
| Mean (SD) | 56 (17) | 49 (17) | 63 (15) | < 0.01a | |
| Male (%) | 58.6 | 57.7 | 1b | ||
| SAPSII | Mean (SD) | 51 (20) | 42 (19) | 59 (17) | < 0.001a |
| Dg.(%) | |||||
| Cardiac arrest | 16.9 | 18.6 | 15.4 | 0.17b | |
| Cardiogenic shock | 1.4 | 0 | 2.6 | ||
| Coma | 12.2 | 5.7 | 17.9 | ||
| Status epilepticus. | 4.7 | 2.9 | 6.4 | ||
| CHF | 3.4 | 4.3 | 2.6 | ||
| Hypercapnic ARF | 10.1 | 10.0 | 10.3 | ||
| Hypoxic ARF | 12.8 | 12.9 | 12.8 | ||
| Intoxication | 9.5 | 15.7 | 3.8 | ||
| Malignant neurolept.sy | 0.7 | 1.4 | 0 | ||
| Neuromuscular ARF | 2.7 | 4.3 | 1.3 | ||
| Other | 2 | 2.9 | 1.3 | ||
| Renal/metabolic | 1.4 | 1.4 | 1.3 | ||
| Sepsis (including shock) | 22.3 | 20.0 | 24.4 | ||
| Other | 16.20% | 12.90% | 1.30% | ||
| ICU LOS | |||||
| Sum | 1474 | ||||
| Median (IQR) | 6 (3-12) | 6 (4-16) | 5 (3-10) | 0.116c | |
| 28 d ICU free days | Median (IQR) | 0 (0-22) | 12.5 (0-23) | 0 (0-19) | 0.002c |
| Ward LOS after ICU | |||||
| Sum | 1694 | ||||
| Median (IQR) | 14 (0-14) | 14 (13.75-15.25) | 0 (0-1.25) | < 0.001c | |
| Mechanical ventilation | % | 80 | 74 | 87 | 0.097b |
| Ventilator-free days at 28 d | Median (IQR) | 23 (18-25) | 23.5 (18-26) | 22 (18-25) | 0.150c |
Student’s t test;
χ2 test,
Mann Whitney U test; Dg: Admission diagnosis; ICU: Intensive care unid; LOS: Length of stay; SAPSII: Simplified Acute Physiology Score II. IQR: Interquartile range; CHF: Chronic heart failure.
Patient mortality
Patient flow diagram presents the phases where mortality was recorded (Figure 5). Seventy patients (47.2%) survived one year after hospital discharge, 7 (4.7%) died during the one year follow up, 14 (9.4%) died on the hospital wards after the ICU, and 57 (38.5%) died in the ICU. Hospital mortality was 48%, 30 d mortality was 41.9%, 60 d mortality was 48.6%, and one year mortality was 52.7%.
Follow up surveys
The only statistically significant difference between respondents and non-respondents was that respondents were older (mean age 51 years) than non-respondents (mean 44 years). The respondents were mainly females (79.3%), compared to non-respondents where only 20.7% were females, which was not statistically significant.
The results of interviews are shown in Table 3. Most of the patients did not have problems or had some problems with mobility (95.9%), while severe problems with mobility had 4.1% of the respondents. Most of the patients did not have severe problems with self-care (91.8%). Severe problems with every activites had 20.4%, with pain or discomfort had 10.2% and with anxiety or depression had 6.10% of patients. The patients evaluated their HRQOL lower [0.63 IQR (0.56-0.70)] than the value of the resultant quality of life index calculated from their answers 0.69, IQR (0.49-0.84).
Table 3.
Distribution of responses to EQ-5D modalities at one year
| Problems | n = 49 | % | |
| Mobility | No | 25 | 51.0 |
| Some | 22 | 44.9 | |
| Severe | 2 | 4.10 | |
| Self-care | No | 37 | 75.5 |
| Some | 8 | 16.3 | |
| Severe | 4 | 8.2 | |
| Usual activities | |||
| No | 13 | 26.5 | |
| Some | 26 | 53.1 | |
| Severe | 10 | 20.4 | |
| Pain/discomfort | |||
| No | 30 | 61.2 | |
| Some | 14 | 28.6 | |
| Severe | 5 | 10.2 | |
| Anxiety/depression | |||
| No | 27 | 55.1 | |
| Some | 19 | 38.8 | |
| Severe | 3 | 6.10 | |
| VAS | 0.63 (0.56-0.70) |
VAS: Visual analogue scale. Data are presented as numbers (percentage). EQ-5D-3Lquestionnaire is an instrument developed by the EuroQol group with five health related quality of life dimensions and a visual analogue scale for patients to subjectively evaluate the state of their quality of life.
After adjustment for non-respondents and patients who died during the one year follow up median HRQOL was 0.64, IQR (0.49-0.76). The value of HRQOL index at one year was significantly lower than the age and sex matched reference value.
Cost analysis
The cost per ICU day was 193 dollar, and the cost per ward day was 73 dollar. The cost of ICU-patient cohort equals the sum of the ICU-stay cost (126312 dollar) and the ward after ICU-stay cost (126312 dollar) resulting in total cost of 410212 dollar, and the mean per patient cost of 2772 dollar (95%CI: 2373-3170 dollar). The minimal hospital cost per patient was 140 dollar and the maximum was 13706 dollar. The highest cost per hospital survivor and the cost per QALY were with neuromuscular acute respiratory failure and sepsis (Figure 6).
Figure 6.
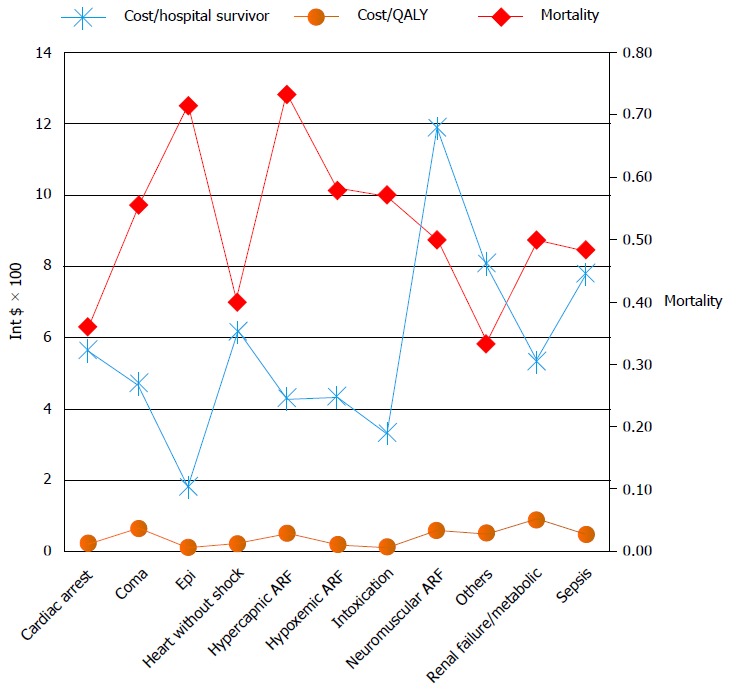
Cost effectiveness measures (costs per hospital survivor, costs per quality adjusted life year saved by the hospital treatment) and hospital mortality, presented depending on the diagnostic category. The highest mortality had patients admitted for the treatment of status epilepticus, hypercapnic acute respiratory failure, acute renal failure and sepsis. The highest costs were with the neuromuscular ARF and the sepsis diagnostic category. ARF: Acute respiratory failure; QALY: Quality adjusted life year.
The cost of treatment of critically ill patients varied between 1820 dollar and 20109 dollar per hospital survivor and between 100 dollar and 2514 dollar per QALY saved. Mean factors that influenced costs were: Age, diagnostic category, ICU and hospital length of stay and number and type of diagnostic and therapeutic interventions.
The ICER
Results of the systematic review summarizing effectiveness of ICU vs non-ICU treatment are presented in Figure 3 and Table 1. With the average ICU refusal rate, of 15% and the odds ratio for mortality of 1.64 (95%CI: 1.51-1.78) on a ward, the attributable mortality risk reduction (ARR) was calculated to be 13.4% for ICU-treated critically ill patients. Details can be found in the electronic data supplement and similar approach was previously applied by Ridley and Morris[8]. In the cohort of one year ICU survivors, a total of 2235 life years and 1455 QALYs were saved, which is 567 QALYs after discounting. The incremental effect was then equal to 75.7 QALYs after applying the attributable risk reduction from the systematic review. The incremental cost was calculated as the sum of the incremental hospital cost and the present value of the incremental future-cost and was equal to 246246 dollar. The ICER for intensive care was 3254 dollar per QALY (Table 4).
Table 4.
Costs, effects, incremental analysis of costs and effects and cost effectiveness ratio
| Hospital treatment costs | Future costs (present value) | Total | |
| Cost-ICU ($) | 410212 | 524432 | 934644 |
| Cost-ward ($) | 234240 | 454158 | 688398 |
| Incremental cost ($) | 175972 | 70274 | 246246 |
| Effects-ICU (QALYs) | 567 | ||
| Effects-ward (QALYs) | 4913 | ||
| Incremental effects (QALYs) | 757 | ||
| ICER ($/QALY) | 3254 | ||
Incremental cost represents the difference between the total ICU patient cohort cost and the comparator cohort cost and was calculated based on the decision model assumptions and attributable mortality risk reduction, using the formula presented in detail in the methods section, and after discounting future costs and health effects at an annual rate of 3% to the year 2012. ICU: Intensive care unit; ICER: Incremental cost-effectiveness ratio; QALY: Quality adjusted life year.
Sensitivity analysis
Analysis of sensitivity of the ICER values to key model parameters is presented in Figures 7 and 8.
Figure 7.
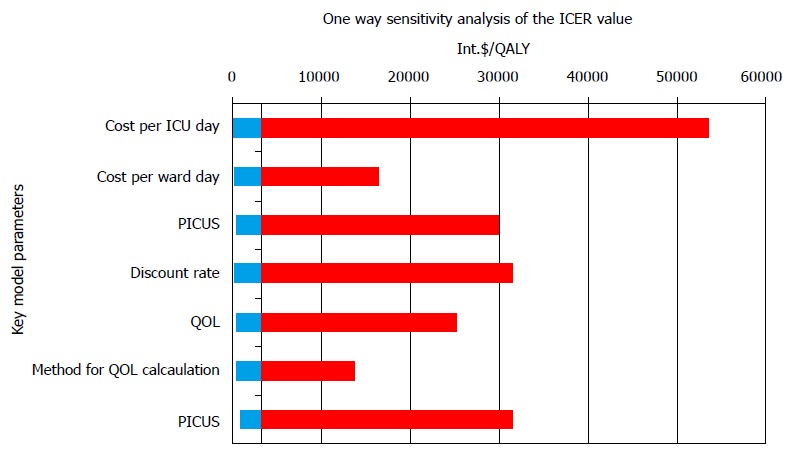
Variations of incremental cost effectiveness ratio values in the one way sensitivity analysis. The values are given in International dollar. The highest variations in ICER values and consequently the impact on ICER values had cost per ICU day, followed with post ICU survival rate and discount rate. ICU: Intensive care unit; PICUS: Post ICU survival; QOL: Quality of life; ICER: Incremental cost effectiveness ratio.
Figure 8.
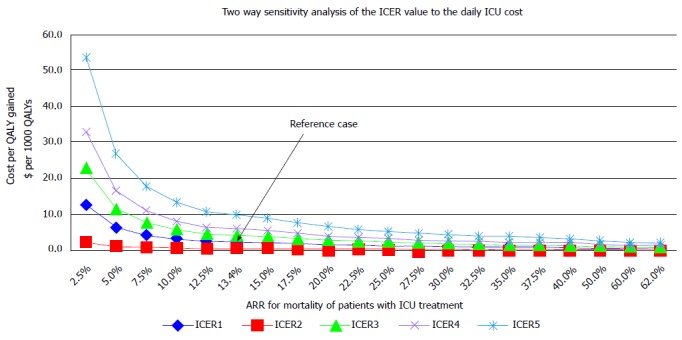
The two way sensitivity analysis. Horizontal axis represents different values of the ARR. Vertical axis-values of ICER when cost per ICU day and ARR change: ICER 1 - daily ICU cost 193 dollar, ICER 2 - daily ICU cost 96.5 dollar (50% of reference cost), ICER 3 - daily ICU cost 289.5 (150% of reference cost), ICER 4 - daily ICU cost 386 dollar (200% of reference cost), ICER 5 - daily ICU cost 579 dollar (300% of reference cost). Reference case, the ICER of 3254 dollar/ QALY when cost per ICU day is 193 dollar and ARR are value of 13.4. ICER: Incremental cost effectiveness ratio; ICU: Intensive care unit; QALY: Quality adjusted life year; ARR: Attributable mortality risk reduction.
A tornado plot in the one way sensitivity analysis was constructed to observe the impact of different parameters on ICER values, Figure 7. From the plot it can be seen that the ICU-daily cost had the highest impact on ICER values.
Values of ICER varied between 1026 dollar and 54495 dollar per QALY, with the mean of 4137 dollar (SD 4863 dollar), and the median of 2580 dollar /QALY. The minimal value of ICER was calculated for the case when the cost per ICU day was 50% lower (97 dollar), with the cost of a general ward day constant (74 dollar), the QOL index 0.64, the annual discount rate of 3%, and the ARR of 62%. The ICER would be maximum, if the cost per ICU day were 300% (580 dollar), with the constant ward cost and the ARR of 2.5%, which is only a hypothetical and not real possibility.
If the ARR varied between 13.4 and 62%, the ICER would vary from 1438 dollar to 3286 dollar per QALY (mean 2121 dollar, median 1982 dollar /QALY). As the ARR value rises its impact on the ICER significantly drops, two way sensitivity analysis (Figure 8).
DISCUSSION
The main finding of our study is a favorable ICER for the treatment of critically ill medical patients in an ICU in a resource limited setting. Severity of illness, short and long term mortality, and the quality of life after critical illness were mainly comparable to studies performed in a resource-rich setting.
Our practice before the establishment of this medical ICU showed that we could not provide effective critical care on general hospital wards due to the lack of equipment and personnel. Indeed, a decision on ICU establishment should not be made on utilitarian grounds. However, our study proves that in a resource poor setting having an ICU is also cost effective and can compete with other health care priorities for resource allocation. Therefore, investment in critical care facilities even in a resource poor setting can be justified not only by ethical and altruistic reasons but also by very good cost effectiveness.
Similar to previous studies, the health related QOL of patients one year after critical illness was significantly lower than the QOL of the age and sex matched general population[22,23,35,38-42]. Cost effectiveness of critical care in our study might as well be underestimated as we used international data on survival of critically ill patients outside of an ICU as one of the main input parameters in our decision analytic model. The ICU patient cohort had a substantially low fixed cost estimate. Such low fixed cost estimate is a consequence of the fact that, unfortunately, personnel costs in a resources limited setting are much lower than such costs in developed countries. Further, a higher incremental effectiveness of the ICU treatment of the critically ill at the Sarajevo University Clinical Center can be expected, simply because most interventions that are needed for vital function support are not available on general wards in our hospital. Data on patient survival in and out of ICU come from observational studies on treatment outcomes of ward care for the critically ill, when those patients were refused an ICU admission with careful admission triage due to the lack of beds. Ethical issues preclude conduction of randomized control trials on this subject. Therefore, the actual effectiveness of the ICU care can only be roughly estimated. Similarly, ethical and administrative reasons precluded data collection on a comparator patient cohort in our institution. However, any bias can only be in the direction of higher effectiveness of the ICU treatment because hospital floor outcomes in this limited resource setting are far worse.
Another question that could be raised is what was the opportunity cost of an investment in critical care? Opportunity costs depend on an individual situation within the health care system of a country because health care investment priorities differ among countries. Our results indorse the argument that investment in critical care facilities can also fairly compete with other health care priorities.
A number of cost utility analyses in intensive care have been published to date, but none has been done in Bosnia and Herzegovina, south-eastern Europe or any other resource limited setting. Consequently, our results cannot be adequately compared. Cost effectiveness analyses of critical care have been performed in Western Europe, and in the United States. Sznajder et al[42], reported the cost of 1150 dollar/year of life preserved, and the cost of 4100 dollar/QALY in France in1996. Graf et al[43] in Germany reported the cost of 28354 dollar/year of life preserved in the year 1998. Linko et al[35] reported on a prospective multicenter study conducted in 25 ICUs in Finland. The average life expectancy of survivors was 16.8 years, and 11.3 QALY, with the cost effectiveness ratio of 1391 dollar/QALY. Aforementioned studies did not use a standard comparator for ICU care, assuming that the alternative to the ICU treatment was a theoretical certainty of death. If we had assumed 100% mortality in our comparator group, medical ICU patients in Sarajevo would have had an even more favorable CEA profile with the hospital cost per patient of 2771 dollar, the cost per hospital survivor 5327 dollar, and the cost per QALY 295 dollar, with the average life expectancy of 32 years. Edbrooke et al[29] conducted a large study which analyzed cost effectiveness of ICU treatment on the population of over 7000 patients in 11 medical and surgical units in 7 different countries of the European Union. Results of this study indicate the cost of 103771 dollar/life saved, 7065 dollar/year of life saved, and the average expected length of life of 15 years for survivors. This study did not take future health care costs into account.
Inter-study comparability is generally difficult with respect to different methodological approaches such as the costing, perspective, analytical horizon, and the QOL estimation. Our study is different from other published cost effectiveness studies in critical care because decision analytic modeling was used as an legitimate instrument in economic evaluation studies in order to implement methodological recommendations for cost effectiveness analyses in critical care given by the American Thoracic Society[18,44].
Defining what cost-effective is requires judgment about the social willingness to pay for a QALY or life year saved. Although there is no absolute cutoff, an incremental cost-effectiveness ratio of 50000 dollar to 100000 dollar/year of life gained is generally considered a good value for money in the United States today[45]. Similarly, the National Institute for Clinical Excellence in the United Kingdom accepts technologies with cost effectiveness ratios ranging from £ 5000 to £ 15000 per QALY[46]. The WHO gives a general interpretation of cost effectiveness of health interventions in the context of a country’s regional position and economic development[47]. Cost effectiveness of other medical interventions, such as coronary bypass surgery, mitral valve replacement for rheumatic heart disease, medical management for hypertension and tertiary management for lung, liver, esophageal, and stomach cancer was classified by the World Bank as so high that was recommended that public policy should discourage their use in settings where resources are severely constrained[10]. In spite of those recommendations, these interventions are being supported by public health authorities in developing countries. According to the WHO, values of ICER less than Gross Domestic Product (GDP) per capita are highly cost-effective, between one to three times GDP per capita are effective in terms of cost, while the value of ICER over three times GDP are not cost-effective. For Bosnia and Herzegovina, with the GDP per capita of 9100 dollar, the limit of acceptable ICER is approximately 27300 dollar for the year 2011 (Figure 9). Thus critical care in Sarajevo with the ICER less than Bosnian GDP per capita is very cost effective[47]. However, these estimations may not reflect affordability and social willingness to pay.
Figure 9.
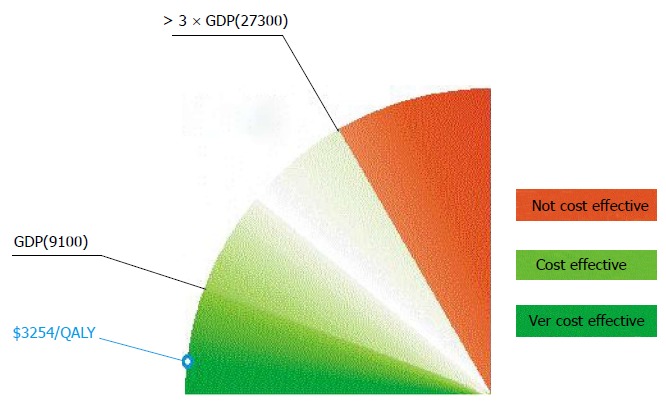
The World Health Organization interpretation of the cost effectiveness of health care interventions. The WHO interprets the cost effectiveness of a health care intervention in relation to the country’s economic development represented by the GDP per capita. If the value of ICER is less than country’s GDP per capita, then the intervention is very cost effective. If the value of ICER falls between one and three times GDP per capita, then the intervention is cost effective and if the ICER is more than three times GDP per capita, the intervention is considered not cost effective. WHO: World Health Organization; GDP: Gross domestic product; ICER: Incremental cost effectiveness ratio.
Like any research, this study has limitations. The study measured life expectancy using life tables for the population of Bosnia and Herzegovina to predict a remaining lifespan of patients. This approach assumes that the life expectancy of survivors will be comparable to the life expectancy of an age-matched and sex-matched general population. ICU patients were followed up for one year after discharge from the hospital. This period appears to be long enough to determine possible additional mortality of patients after critical illness, because it appears that mortality in our patients stabilized five months after critical illness. This is consistent with previously published results[39,48,49]. Besides aforementioned, the post ICU survival rate was one of the key parameters for which we performed the sensitivity analysis. Secondly, the HRQOL was measured one year after hospital discharge and assumed to be constant over time. However, it is known that the HRQOL declines with age. In our patient sample, there was no statistically significant difference in the quality of life between age groups. Thirdly, patients that died within 24 h in the ICU were excluded from the analysis, which could introduce bias into the analysis. However, the overall number of those patients was small and unlikely to significantly impact on our results. Further, we had only 70% EQ-5D questionnaire respondents among one year survivors, which could also distort our results. For the rest of survivors, an approximation was made according to the methodology previously used[35]. With our questionnaire results we were able to roughly estimate the value of HRQOL one year after critical illness, and gain a sense of the order, which we found satisfactory for the rest of calculations. The fifth potential source of concern is the absence of the concurrent control group of critically ill patients treated on the ward. Since none of the studies came from the countries in development there is also the possibility of the publication bias, which might distort our results. We controlled the uncertainty around this parameter by the sensitivity analysis. The value of ICER for intensive care in Bosnia and Herzegovina would exceed 50000 dollar/QALY only in cases where the attributable risk reduction for mortality was implausibly small. Contrary to that possibility, the highest values of attributable risk reduction are more appropriate for the Sarajevo University Clinical Center and consequently even better cost effectiveness of critical care.
In conclusion, public health authorities should support the development of critical care services in low and middle income countries because the ICU treatment of critically ill medical patients is cost effective.
COMMENTS
Background
Considering the fact that critical care is an expensive specialty, question can be raised whether investment erase - critical care in a resource constrained setting is cost effective. High costs of treatment in the intensive care unit (ICU) have limited access to critical care in low and middle income countries. The authors’ objective was to assess the cost effectiveness of the treatment of critically ill patients in medical ICU compared to no ICU treatment in a low-middle income country with limited access to ICU resources.
Research frontiers
The economic evaluation studies of critical care have been extensively performed in developed countries with resource rich health care setting. A search of the literature on the analysis of costs and effectiveness in the field of intensive care medicine found no studies published from the Balkans, South-Eastern Europe or other resource limited settings.
Innovations and breakthroughs
Care for critically ill is considered expensive and is a low priority for public health decision makers in resource limited countries. The authors’ results suggest that critical care is and can be cost effective even in the settings of constrained resources.
Applications
This study can serve as an argument for public health authorities to support the development of critical care services in low and middle income countries.
Terminology
Cost effectiveness analysis is a form of the full economic evaluation which incorporates medical and economical aspects of health care programes or interventions. Cost utility analysis is a form of economic evaluation of health care programes which uses quality adjusted life years (QALYs) as a measure of health effects and shows the number of QALYs saved by the intervention at a certain cost expressed in Dollar or international Dollar.
Peer-review
The authors have performed a good study, the manuscript is interesting.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Institutional review board of Sarajevo University Clinical Center and Ehics commitee of the Sarajevo University Faculty of Medicine.
Informed consent statement: All study participants, or their legal guardian, provided written informed consent prior to study enrollment.
Conflict-of-interest statement: The authors of this study declare that they have no conflict of interest to disclose.
Data sharing statement: There is no additional data.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 9, 2015
First decision: September 14, 2015
Article in press: November 17, 2015
P- Reviewer: Ewers A, Normadeane A, Robertson-Smith J, Zhang ZH S- Editor: Qiu S L- Editor: A E- Editor: Jiao XK
References
- 1.Russel LB, Siegel JE, Daniels N, Gold MR, Luce BR, Mandelblatt JS. Cost-effectiveness analysis as a guide to resource allocation in health: roles and limitations. In: Gold MR, Siegel JE, Russel LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. Oxford University Press; 1996. pp. 3–24. [Google Scholar]
- 2.Weinstein MC, Stason WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med. 1977;296:716–721. doi: 10.1056/NEJM197703312961304. [DOI] [PubMed] [Google Scholar]
- 3.Basic types of economic evaluation. In: Drummond MF, Sculpher MJ, Torrance GW, O¡¯Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford University Press, 2005: 7-26 [Google Scholar]
- 4.Cost Effectiveness Analysis: Introduction. CDC Econ Eval Tutorials (E). [updated 2013 Nov 22] Available from: http//www.cdc.gov/owcd/eet/costeffect2/fixed/1.html.
- 5.Sukcharoen N. Basic Principles of Health Economics for Obstetricians and Gynecologists. Thai J Obstet Gynaecol. 2003;15:3–7. [Google Scholar]
- 6.Cuthbertson BH, Rattray J, Campbell MK, Gager M, Roughton S, Smith A, Hull A, Breeman S, Norrie J, Jenkinson D, et al. The PRaCTICaL study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. BMJ. 2009;339:b3723. doi: 10.1136/bmj.b3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams TA, Dobb GJ, Finn JC, Webb SA. Long-term survival from intensive care: a review. Intensive Care Med. 2005;31:1306–1315. doi: 10.1007/s00134-005-2744-8. [DOI] [PubMed] [Google Scholar]
- 8.Ridley S, Morris S. Cost effectiveness of adult intensive care in the UK. Anaesthesia. 2007;62:547–554. doi: 10.1111/j.1365-2044.2007.04997.x. [DOI] [PubMed] [Google Scholar]
- 9.Strateski plan razvoja zdravstva u FBiH. [updated 2013 Nov 25] Available from: http//www.fmoh.gov.ba/index.php/zakoni-i-strategije/strategije-i-politike/53-strateski-plan-razvoja-zdravstva-u-fbih.
- 10.Jamison DT, Mosley WH. Disease control priorities in developing countries: health policy responses to epidemiological change. Am J Public Health. 1991;81:15–22. doi: 10.2105/ajph.81.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bank W. World Development Report 1993: Investing in Health. [updated 2013 Nov 25] Available from: http//openknowledge.worldbank.org/handle/10986/5976.
- 12.Garrouste-Orgeas M, Montuclard L, Timsit JF, Reignier J, Desmettre T, Karoubi P, Moreau D, Montesino L, Duguet A, Boussat S, et al. Predictors of intensive care unit refusal in French intensive care units: a multiple-center study. Crit Care Med. 2005;33:750–755. doi: 10.1097/01.ccm.0000157752.26180.f1. [DOI] [PubMed] [Google Scholar]
- 13.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 14.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 15.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 16.Kümpers P, Hafer C, Lukasz A, Lichtinghagen R, Brand K, Fliser D, Faulhaber-Walter R, Kielstein JT. Serum neutrophil gelatinase-associated lipocalin at inception of renal replacement therapy predicts survival in critically ill patients with acute kidney injury. Crit Care. 2010;14:R9. doi: 10.1186/cc8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kümpers P Definitions. [accessed 2015 Sept 15] Available from: http//research.tufts-nemc.org/cear4/SearchingtheCEARegistry/Definitions.aspx.
- 18.Understanding costs and cost-effectiveness in critical care: report from the second American Thoracic Society workshop on outcomes research. Am J Respir Crit Care Med. 2002;165:540–550. doi: 10.1164/ajrccm.165.4.16541. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 20.Frisho-Lima P, Gurman G, Schapira A, Porath A. Rationing critical care -- what happens to patients who are not admitted? Theor Surg. 1994;9:208–211. [PubMed] [Google Scholar]
- 21.Metcalfe MA, Sloggett A, McPherson K. Mortality among appropriately referred patients refused admission to intensive-care units. Lancet. 1997;350:7–11. doi: 10.1016/S0140-6736(96)10018-0. [DOI] [PubMed] [Google Scholar]
- 22.Sprung CL, Geber D, Eidelman LA, Baras M, Pizov R, Nimrod A, Oppenheim A, Epstein L, Cotev S. Evaluation of triage decisions for intensive care admission. Crit Care Med. 1999;27:1073–1079. doi: 10.1097/00003246-199906000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Joynt GM, Gomersall CD, Tan P, Lee A, Cheng CA, Wong EL. Prospective evaluation of patients refused admission to an intensive care unit: triage, futility and outcome. Intensive Care Med. 2001;27:1459–1465. doi: 10.1007/s001340101041. [DOI] [PubMed] [Google Scholar]
- 24.Garrouste-Orgeas M, Montuclard L, Timsit JF, Misset B, Christias M, Carlet J. Triaging patients to the ICU: a pilot study of factors influencing admission decisions and patient outcomes. Intensive Care Med. 2003;29:774–781. doi: 10.1007/s00134-003-1709-z. [DOI] [PubMed] [Google Scholar]
- 25.Simchen E, Sprung CL, Galai N, Zitser-Gurevich Y, Bar-Lavi Y, Gurman G, Klein M, Lev A, Levi L, Zveibil F, et al. Survival of critically ill patients hospitalized in and out of intensive care units under paucity of intensive care unit beds. Crit Care Med. 2004;32:1654–1661. doi: 10.1097/01.ccm.0000133021.22188.35. [DOI] [PubMed] [Google Scholar]
- 26.Thiéry G, Azoulay E, Darmon M, Ciroldi M, De Miranda S, Lévy V, Fieux F, Moreau D, Le Gall JR, Schlemmer B. Outcome of cancer patients considered for intensive care unit admission: a hospital-wide prospective study. J Clin Oncol. 2005;23:4406–4413. doi: 10.1200/JCO.2005.01.487. [DOI] [PubMed] [Google Scholar]
- 27.Simchen E, Sprung CL, Galai N, Zitser-Gurevich Y, Bar-Lavi Y, Levi L, Zveibil F, Mandel M, Mnatzaganian G, Goldschmidt N, et al. Survival of critically ill patients hospitalized in and out of intensive care. Crit Care Med. 2007;35:449–457. doi: 10.1097/01.CCM.0000253407.89594.15. [DOI] [PubMed] [Google Scholar]
- 28.Iapichino G, Corbella D, Minelli C, Mills GH, Artigas A, Edbooke DL, Pezzi A, Kesecioglu J, Patroniti N, Baras M, et al. Reasons for refusal of admission to intensive care and impact on mortality. Intensive Care Med. 2010;36:1772–1779. doi: 10.1007/s00134-010-1933-2. [DOI] [PubMed] [Google Scholar]
- 29.Edbrooke DL, Minelli C, Mills GH, Iapichino G, Pezzi A, Corbella D, Jacobs P, Lippert A, Wiis J, Pesenti A, et al. Implications of ICU triage decisions on patient mortality: a cost-effectiveness analysis. Crit Care. 2011;15:R56. doi: 10.1186/cc10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robert R, Reignier J, Tournoux-Facon C, Boulain T, Lesieur O, Gissot V, Souday V, Hamrouni M, Chapon C, Gouello JP. Refusal of intensive care unit admission due to a full unit: impact on mortality. Am J Respir Crit Care Med. 2012;185:1081–1087. doi: 10.1164/rccm.201104-0729OC. [DOI] [PubMed] [Google Scholar]
- 31.Cabrini L GM. Observed versus predicted hospital mortality in general wards patients assisted by a medical emergency team. Signa Vitae. 2012;7:38–42. [Google Scholar]
- 32.Stelfox HT, Hemmelgarn BR, Bagshaw SM, Gao S, Doig CJ, Nijssen-Jordan C, Manns B. Intensive care unit bed availability and outcomes for hospitalized patients with sudden clinical deterioration. Arch Intern Med. 2012;172:467–474. doi: 10.1001/archinternmed.2011.2315. [DOI] [PubMed] [Google Scholar]
- 33.WHO WHO Guide to Cost-Effectiveness Analysis. [updated 2013 Oct 30] Available from: http//www.who.int/choice/book/en/
- 34.Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, Payne E, Cuthbertson BH. Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris) for the treatment of severe sepsis in adults: a systematic review and economic evaluation. Health Technol Assess. 2005;9:1–126, iii-iv. doi: 10.3310/hta9110. [DOI] [PubMed] [Google Scholar]
- 35.Linko R, Suojaranta-Ylinen R, Karlsson S, Ruokonen E, Varpula T, Pettilä V. One-year mortality, quality of life and predicted life-time cost-utility in critically ill patients with acute respiratory failure. Crit Care. 2010;14:R60. doi: 10.1186/cc8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Global Health Observatory Data Repository. Life tables Bosnia and Herzegovina. [updated 2013 Nov 25] Available from: http//apps.who.int/gho/data/view.main.60200?lang=en.
- 37.EuroQol EQ-5D-3L. [updated 2013 Nov 25] Available from: http//www.euroqol.org/eq-5d-products/eq-5d-3l.html.
- 38.Manns BJ, Lee H, Doig CJ, Johnson D, Donaldson C. An economic evaluation of activated protein C treatment for severe sepsis. N Engl J Med. 2002;347:993–1000. doi: 10.1056/NEJMsa020969. [DOI] [PubMed] [Google Scholar]
- 39.Granja C, Teixeira-Pinto A, Costa-Pereira A. Quality of life after intensive care--evaluation with EQ-5D questionnaire. Intensive Care Med. 2002;28:898–907. doi: 10.1007/s00134-002-1345-z. [DOI] [PubMed] [Google Scholar]
- 40.Hurel D, Loirat P, Saulnier F, Nicolas F, Brivet F. Quality of life 6 months after intensive care: results of a prospective multicenter study using a generic health status scale and a satisfaction scale. Intensive Care Med. 1997;23:331–337. doi: 10.1007/s001340050336. [DOI] [PubMed] [Google Scholar]
- 41.Gordon AC, Oakervee HE, Kaya B, Thomas JM, Barnett MJ, Rohatiner AZ, Lister TA, Cavenagh JD, Hinds CJ. Incidence and outcome of critical illness amongst hospitalised patients with haematological malignancy: a prospective observational study of ward and intensive care unit based care. Anaesthesia. 2005;60:340–347. doi: 10.1111/j.1365-2044.2005.04139.x. [DOI] [PubMed] [Google Scholar]
- 42.Sznajder M, Aegerter P, Launois R, Merliere Y, Guidet B. A cost-effectiveness analysis of stays in intensive care units. Intensive Care Med. 2001;27:146–153. doi: 10.1007/s001340000760. [DOI] [PubMed] [Google Scholar]
- 43.Graf J, Wagner J, Graf C, Koch KC, Janssens U. Five-year survival, quality of life, and individual costs of 303 consecutive medical intensive care patients--a cost-utility analysis. Crit Care Med. 2005;33:547–555. doi: 10.1097/01.ccm.0000155990.35290.03. [DOI] [PubMed] [Google Scholar]
- 44.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 45.Talmor D, Greenberg D, Howell MD, Lisbon A, Novack V, Shapiro N. The costs and cost-effectiveness of an integrated sepsis treatment protocol. Crit Care Med. 2008;36:1168–1174. doi: 10.1097/CCM.0b013e318168f649. [DOI] [PubMed] [Google Scholar]
- 46.Health inequalities and population health. NICE. [updated 2013 Nov 03] Available from: http//www.nice.org.uk/
- 47.WHO WHO-CHOICE. WHO. [updated 2013 Oct 14] Available from: http//www.who.int/choice/en/
- 48.Ridley S, Plenderleith L. Survival after intensive care. Comparison with a matched normal population as an indicator of effectiveness. Anaesthesia. 1994;49:933–935. doi: 10.1111/j.1365-2044.1994.tb04306.x. [DOI] [PubMed] [Google Scholar]
- 49.Delannoy B, Floccard B, Thiolliere F, Kaaki M, Badet M, Rosselli S, Ber CE, Saez A, Flandreau G, Guérin C. Six-month outcome in acute kidney injury requiring renal replacement therapy in the ICU: a multicentre prospective study. Intensive Care Med. 2009;35:1907–1915. doi: 10.1007/s00134-009-1588-z. [DOI] [PubMed] [Google Scholar]


