Abstract
Osteoarthritis is a progressive joint disease that results in degradation of cartilage in load-bearing joints. Pain and inflammation in the joint are the hallmarks of this condition, which further exacerbate the cartilage destruction and health of the patient. It is hence imperative to treat the joint inflammation at the earliest. Interleukin 1 (IL-1) blockade by IL-1 receptor antagonist (IL-1Ra) has shown promise in the clinic but this therapy suffers from rapid clearance, high doses, and frequent intervention. Use of carrier particles that result in longer residence time has been proposed. Here we have synthesized a new class of nanoparticles presenting IL-1Ra on the surface and with tunable size from 300 to 700 nm. These IL-1Ra-poly(2-hydroxyethyl methacrylate)-pyridine nanoparticles are cytocompatible and stable in serum-containing solutions for several days. Our results further demonstrate that these nanoparticles are capable of blocking IL-1β signaling in an NF-κB inducible reporter cell line. These engineered nanoparticles are promising for localized intra-articular delivery in joint space to reduce inflammation in osteoarthritis and other inflammatory diseases.
Keywords: protein delivery, biomaterials, polymeric particles, immunomodulation, cytokine
INTRODUCTION
Osteoarthritis (OA) is a debilitating degenerative disease affecting the joints, mainly found in elderly individuals.1 One in four people may develop symptomatic hip osteoarthritis in his/her lifetime2 and the lifetime risk of developing OA increases with body mass index (BMI), with a risk of 2 in 3 among those who are obese.3 OA affects an estimated total of 27 million people in the United States,4 resulting in a total (direct and indirect) annual cost of $5,700 per patient.5
OA causes degradation of the cartilage, usually in load-bearing areas such as the hip and knee. Inflammation plays a key role in disease progression and results in an imbalance of chondrocytes catabolic and anabolic processes.6 Inflammatory cytokines, including IL-1, IL-6, TNF-α, and proteases such as matrix metalloproteinases (MMPs), are upregulated which decrease chondrocyte viability and degrade cartilage. Hence, the use of anti-inflammatory drugs has been proposed to prevent further degradation of the joint and provide pain relief. Particularly, IL-1 is identified as a key target as it induces production of free radicals and MMPs6,7 and is also involved in transmission of pain.8
Interleukin 1 receptor antagonist (IL-1Ra) is a naturally occurring IL-1 inhibitor that binds to the IL-1 receptor (IL-1R) without triggering an agonist response and thus functions as a receptor antagonist.9 As such, IL-1Ra has been explored as a therapy to treat OA via local delivery of bolus protein injections9–12 and gene therapy approaches.13–16 Though intraarticular injections of IL-1Ra in OA knees of human patients was found to be safe and resulted in pain relief,17 such anti-inflammatory therapies have not been effective due to rapid clearance of the protein with residence times as short as 1–2 h leading to suboptimal therapeutic effect even after local administration. Several strategies have been explored to prolong the IL-1Ra biological half-life.18 IL-1Ra-loaded PLGA microspheres increased protein residence times but the loading efficiency and bioactivity of the released protein were limited by the hydrophobic nature of this carrier.19–21 Pluronics-based gels have also been developed for IL-1Ra delivery,22,23 but IL-1Ra release from such gels is limited to several hours. PEGylation of IL-1Ra prolongs its biological half-life but at a significant cost of reduced bioactivity.24 Notably, Setton et al. developed an injectable drug depot consisting of IL-1Ra fused to elastin-like polypeptides (ELP) domains and showed that this fusion protein retained the characteristic ELP inverse phase-transitioning behavior as well as the bioactivity of the IL-1Ra domain.25
We previously reported self-assembling nanoparticles made up of a block-co-polymer (tetraethylene glycol and cyclohexyl methacrylate) presenting IL-1Ra for enhanced delivery, retention, and bioactivity in the OA joint.26 This copolymer assembled into 300 nm particles that provided longer retention times for IL-1Ra in the rat stifle joint compared to that of soluble IL-1Ra with no adverse effects on the cartilage structure. However, the residence time for these particles was still limited to 3 days. We hypothesized that larger particle sizes will increase IL-1Ra retention time in the joint. In order to generate nanoparticles with larger sizes, we recently reported another polymeric system composed of a poly-hydroxyethylmethacrylate (pHEMA) with pyridinyl groups as side chains that allowed the modulation particle size in the range from 500 to 900 nm.27 This nanoparticle system improved the in vivo retention of bovine serum albumin (BSA, model protein) delivered to the rat joint.27 In this study, we extend this nanoparticle platform to engineer IL-1Ra-presenting particles based on self-assembly using a pHEMA polymer with hydrophobic side chains of pyridine to present and deliver IL-1Ra protein. We synthesized IL-1Ra-presenting particles of various sizes by varying the mass ratio of protein:polymer. The IL-1Ra remained bioactive following nanoparticle formation, showing the potential of the system for use in treatment of osteoarthritis and other inflammatory conditions.
MATERIALS AND METHODS
Synthesis of pHEMA-pyridine
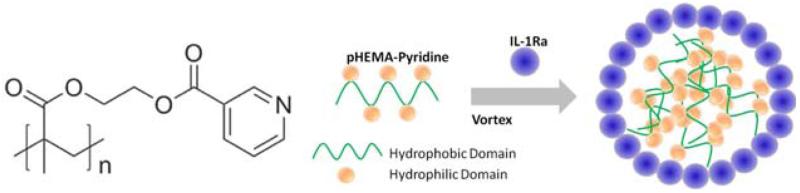
pHEMA-pyridine polymer [Fig. 1(A)] was synthesized and characterized as previously described.27 Poly(2-hydroxyethyl methacrylate) (MW 300 K, 0.13 g, 1.0 mmol −OH equiv.) and nicotinoyl chloride hydrochloride (0.21 g, 1.2 mmol) were dissolved in THF (4 mL) and pyridine (2 mL). After the mixture was stirred at room temperature for 24 h, the solvents were removed under reduced pressure, and then the crude mixture was redissolved in dichloromethane. The organic layer was washed by water, brine, and dried with anhydrous sodium sulfate. The solution was concentrated under reduced pressure until the final volume reached 1 mL, and then it was added dropwise to cold ethyl ether. Finally, the precipitate was collected and dried under vacuum.
FIGURE 1.
(A) Chemical structure of pHEMA-pyridine polymer. (B) Schematic representation of nanoparticle formation. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Nanoparticle formation
A 2 mg/mL solution of pHEMA-pyridine in dimethylformamide (DMF, Sigma Aldrich, St. Louis, MO, USA) was prepared and centrifuged at 1000g to remove any precipitates. To generate particles, 0.05 mL of 2.0 mg/mL IL-1Ra or BSA was added to 0.4 mL of phosphate-buffered saline (PBS, Life Technologies, Carlsbad, CA, USA). pHEMA-pyridine solution was slowly added to the protein solution while vortexing (VWR mini vortexer, speed 4) gently (the amount of polymer was varied to modulate protein:polymer ratio). The final volume percentage of DMF was <5%. Figure 1(B) shows the schematic representation of the particle formation process. After spinning in a rotary shaker for 30 min, the particle solutions were transferred to 10 kDa dialysis cassettes (Thermo Scientific, Waltham, MA, USA) and dialyzed overnight against distilled water with at least 3 buffer exchanges. The particles were transferred to a sterile Eppendorf tube and stored at 4°C until further use.
Particle characterization
Particle size and polydispersity index were measured using a Brookhaven 90 Plus Particle Size analyzer (Brookhaven Instruments Corporation, Holtsville, NY, USA). Zeta potential measurements were performed using Zetasizer Nano ZS (Malvern Instruments, Westborough, MA, USA). Particle size and stability tests were performed in PBS and serum conditions (1% fetal bovine serum (FBS), Life Technologies). The 1% FBS solution was prefiltered using a 40 μm cell strainer to eliminate large size clusters of serum proteins that might interfere with the nanoparticle size readings. At each time point (1, 3, and 7 days), particle size was measured. For scanning electron microscopy (SEM, LEO 1550), nanoparticle suspension (5 μL) was dispensed on an SEM stub, air dried for 2 h, and sputter coated with 5 nm of gold to make the sample conductive. Imaging was done with a 5 KeV electron beam.
Cytotoxicity assay
RAW 264.7 macrophage cells (TIB-71, ATCC, Manassas, VA, USA) were cultured in Dulbecco’s Minimum Essential Media (DMEM, Life Technologies) supplemented with 10% fetal bovine serum (FBS) at 37°C, 5% CO2. Cells (10,000 cells in 90 μL) were seeded onto a 96-well tissue culture-treated plate and allowed to adhere overnight. Next morning, particle suspensions (10 μL) were added to the plates to a final concentration of 0.5, 0.1, and 0.01 mg/mL. in vitro cell metabolic activity assay was performed using CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA). Assays were performed by adding 20 μL of the reagent solution to culture wells and recording the absorbance plate reader at 570 nm (HTS 7000 Plus, Perkin Elmer, Waltham, MA, USA) after particle incubation for 24 h. All the experiments were done in groups of 4.
Inhibition of IL-1β induced signaling
A B-cell precursor acute lymphoblastic leukemia cell line (EU1) co-expressing Renilla (constitutive) and firefly (NF-κB reporter) luciferase28 was used to monitor IL-1β signaling. These cells express firefly luciferase in response to NF-κB activation. Luciferase reporter activity was quantified using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) according to manufacturer’s protocol. NF-κB-luc EU1 cells (100,000 cells) in RPMI-1640 with 10% FBS and 1% penicillin/streptomycin were placed in 96-well plates. IL-1Ra nanoparticles, BSA nanoparticles, or soluble IL-1Ra was added to each well (10 μg/mL IL-1Ra or equivalent amount of protein in form of particles) and incubated with cells for 1 h. IL-1β (50 ng/mL) was added to each well and incubated overnight to stimulate NF-κB activation and luciferase expression. Cells were then washed three times with PBS by centrifugation at 1000 RPM for 5 min. Cells were lysed in 20 μL/well passive lysis buffer (Promega, Madison, WI, USA) under gentle shaking at room temperature for 20 min on a rocker. Lysate (20 μL) was added to 100 μL of luciferase substrate in an opaque white 96-well plate. Renilla and firefly luciferase activity was measured using a Synergy 4 hybrid microplate reader (Biotek, Winooski, VT, USA).
RESULTS
Generation of IL-1Ra nanoparticles
To generate polymer-protein particles, the polymer was incubated with IL-1Ra or BSA in PBS. The protein–polymer complexation occurs rapidly (<5 s) and the resultant nanoparticles could be washed and collected by centrifugation. By varying the mass ratio of protein:polymer, particle sizes from 319 ± 22 nm (1:1 ratio, PDI: 0.35) to 611 ± 38 nm (3:2 ratio, PDI: 0.31) were obtained [Fig. 2(A)], which was measured by dynamic light scattering and SEM. SEM micrographs confirm particle sizes of ~600 nm for 3:2 protein: polymer ratio [Fig. 2(B,C)]. These IL-1Ra nanoparticles had a slight positive charge (+13.3 mV) as determined by zeta potential measurements [Fig. 2(D)].
FIGURE 2.
(A) Dynamic light scattering measurement of nanoparticle size. ANOVA p < 0.0001. #p < 0.01 vs 5:4, 3:2, and 2:1 groups, &p < 0.01 vs 3:2 and 2:1 groups. (B,C) Scanning electron microscopy images of sample with 3:2 IL-1Ra:polymer ratio. (D) Zeta potential measurement of sample with 3:2 IL-1Ra:polymer ratio. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Stability of IL-1Ra nanoparticles
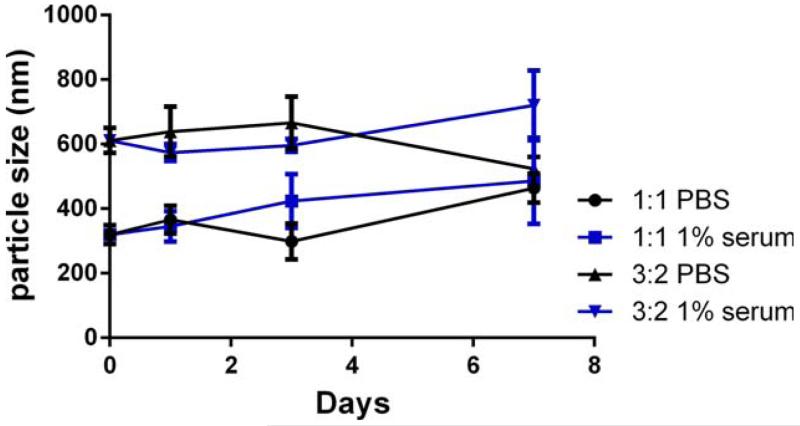
The stability of the IL-1Ra nanoparticles in PBS and serum are important characteristics for storage and application of these therapeutic particles. Therefore, the size stability of these nanoparticles was tested in PBS and 1% fetal bovine serum solution at 37°C. As determined by dynamic light scattering (Fig. 3), IL-1Ra nanoparticles generated by 1:1 and 3:2 protein:polymer ratios maintained their size for at least 7 days.
FIGURE 3.
Stability under PBS and 1% serum condition of samples 3:2 and 1:1 IL-1Ra:polymer ratio for up to 7 days. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Cytocompatibility of IL-1Ra nanoparticles
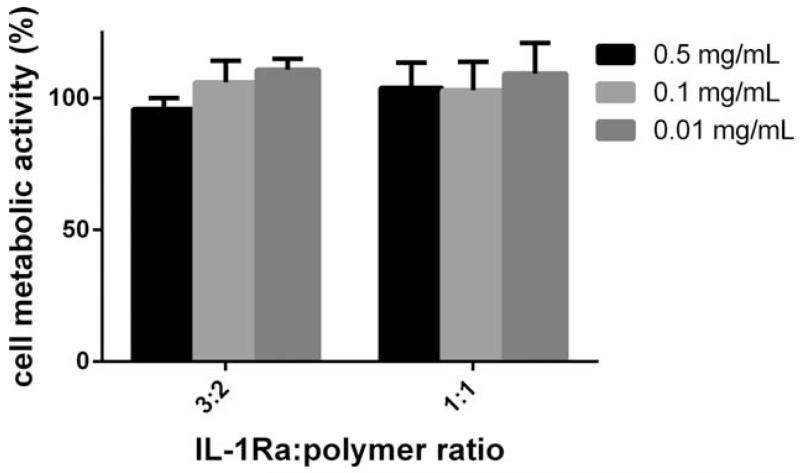
The cytocompatibility of the IL-1Ra nanoparticles was assessed by incubating particles with the RAW 264.7 macrophage cell line for 24 h and assessing metabolic activity. No change in metabolic activity was observed for increasing nanoparticle concentrations up to 0.5 mg/mL for nanoparticles synthesized with 3:2 and 1:1 IL-1Ra:polymer ratios, respectively (Fig. 4).
FIGURE 4.
Metabolic activity assay for RAW 264.7 macrophage cells incubated for 24 h with increasing dose of 3:2 and 1:1 IL-1Ra:polymer nanoparticles.
Bioactivity of IL-1Ra nanoparticles
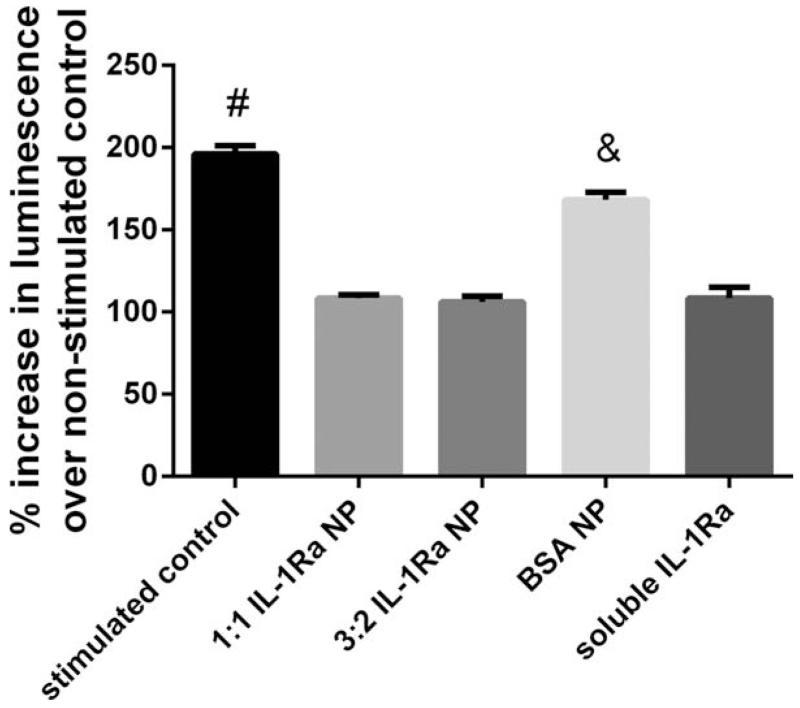
We next examined the ability of the IL-1Ra-presenting nanoparticles to inhibit IL-1β-induced signaling. To monitor IL-1β signaling, we used a reporter cell line that expresses luciferase in response to NF-κB activation. Stimulation with IL-1β resulted in significant increases in luminescence signal compared to the unstimulated control (Fig. 5). Pretreatment with IL-1Ra-nanoparticles of different sizes (3:2 and 1:1 IL-1Ra:polymer ratios) completely inhibited IL-1β-dependent increase in luminescence signal (Fig. 5). The level of inhibition was comparable to that obtained with soluble IL-1Ra. Importantly, nanoparticles formed using bovine serum albumin did not inhibit IL-1β signaling, demonstrating that the inhibitory effects of the IL-1Ra-nanoparticles were specific to the IL-1Ra protein. Taken together, these results demonstrate that the IL-1Ra protein on the nanoparticle retains significant biological activity.
FIGURE 5.
Bioactivity of IL-1Ra-nanoparticles as assessed by inhibition of IL-1β activation of NF-κB activity. ANOVA p < 0.0001. #p < 0.001 vs all other groups, &p < 0.001 vs all other groups. No significant difference was found between any of the IL-1Ra nanoparticles and soluble IL1-Ra.
DISCUSSION
We synthesized IL-1Ra-presenting particles based on the complexation and self-assembly of IL-1Ra and pHEMA-pyridine polymer. This polymer is insoluble at pH > 5, and when mixed with protein at neutral pH in an aqueous solution, protein acts as an amphiphile at the interface of water and the polymer. The pHEMA-pyridine polymer structure is critical for successful self-assembly as the hydrophobic nature of the polymer allows protein to assemble on the surface while pyridinyl units can form hydrogen bonds to provide stability and conformational preservation of protein structure. This strategy also provides control over the size of the nanoparticles by varying the ratio of protein and polymer. We were able to modulate nanoparticle size from 300 to 700 nm in diameter with acceptable polydispersity.
A potential concern with this complexation system is that whether serum components may interfere with the stability of the protein–polymer complex. We tested the stability of nanoparticles in the presence of serum-containing media and found no significant change in nanoparticle size for up to 7 days. This result is consistent with our previous finding with serum albumin:pHEMA-pyridine nanoparticles; these particles were stable in physiological solutions for up to 14 days.27 Additionally, IL-1Ra-nanoparticles were cytocompatible as shown via metabolic activity assay on a macrophage cell line. We tested the bioactivity of these particles to block the induction of NF-κB by IL-1β. Both sizes of nanoparticles tested were able to completely block the activation of NF-κB for 24 h. The IL-1Ra remains associated with the particle as the protein is required for the formation and stability of the particle. Our previous analysis with ELISA using protein:pHEMA-pyridine particles showed retention and no exchange of the protein used to form the particles.27 Additionally, the functional assay used in this study (Fig. 5) involved an overnight incubation in 10% serum and demonstrated high bioactivity for IL-1Ra:pHEMA-pyridine particles. Taken together, these results indicate that the protein used to generate the particles remains on the particle and is not significantly replaced by other proteins.
Use of IL-1Ra carrying nanoparticles offers several advantages over soluble protein. Soluble IL-1Ra injected in the joint clears within few hours compared to residence time of few days for larger nanoparticles.26,27 The use of nanoparticles presenting ligands allows multiple ligand–receptor interactions and hence can result in higher binding avidity to IL-1 receptor compared to soluble IL-1Ra, the so-called multi-valency effect.29 PEGylation of IL-1Ra increases its size and residence time but results in loss of bioactivity and only offers marginal improvement in residence time.24 Pluronics-based gels have increased the residence time of IL-1Ra but it is still limited to several hours.22,23 Encapsulation of IL-1Ra in PLGA particles increased its residence time but proteins can denature due to the harsh fabrication process and hydrophobic properties of the polymer.19–21 Further degradation products of PLGA particles (lactic and glycolic acid) reduces the surrounding pH and can often lead to inflammation.30 Major advantages of protein–pHEMA-pyridine system are its high residence time in the joint (several days) and availability of IL-1Ra on particle surface for interaction with its target receptor.
Future studies will focus on use of these nanoparticles to evaluate in vivo retention and efficacy over soluble protein in arthritic joints. Although we have previously tested nanoparticles formed using this polymer and bovine serum albumin in rat knee and found no inflammatory responses,27 it would be necessary to test this new IL-1Ra protein–polymer complex for any nonspecific immune response. In addition, the stability of nanoparticles under in vivo conditions will also need to be tested.
CONCLUSIONS
We synthesized a new class of self-assembly polymer that contains a pHEMA backbone with pyridinyl side chains to nanocomplex and present the therapeutic IL-1Ra protein. Size of the nanoparticles thus formed is controllable and particles are stable in presence of serum. Importantly, the protein retains its activity and prevents IL-1β signaling to cells. Such IL-1Ra presenting nanoparticles can be used for localized delivery in joint space to prevent IL-1 signaling without the need for frequent therapeutic doses. Such particles can have applications in inflammatory diseases like osteoarthritis and can help regenerate tissues.
ACKNOWLEDGMENTS
TMV was supported as a visiting scholar by CAPES, a Foundation affiliated with the Ministry of Education of Brazil. We thank Richard S Sullivan III and Dr Valeria Milam for their help with zeta potential measurements. The EU1 reporter cell line was a kind gift from Dr Melissa Kemp (Georgia Institute of Technology).
Contract grant sponsor: NIH; contract grant numbers: P20 HL113451 and R01 AR062920
REFERENCES
- 1.Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: Implications for research. Clin Orthop Relat Res. 2004:S6–S15. doi: 10.1097/01.blo.0000143938.30681.9d. [DOI] [PubMed] [Google Scholar]
- 2.Murphy LB, Helmick CG, Schwartz TA, Renner JB, Tudor G, Koch GG, Dragomir AD, Kalsbeek WD, Luta G, Jordan JM. One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthritis and Cartilage. 2010;18:1372–1379. doi: 10.1016/j.joca.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, Dragomir A, Kalsbeek WD, Luta G, Jordan JM. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maetzel A, Li LC, Pencharz J, Tomlinson G, Bombardier C, Community H. Arthritis Project Study T The economic burden associated with osteoarthritis, rheumatoid arthritis, and hypertension: A comparative study. Ann Rheum Dis. 2004;63:395–401. doi: 10.1136/ard.2003.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelletier J-P, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: Potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 7.Chevalier X, Conrozier T, Richette P. Desperately looking for the right target in osteoarthritis: The anti-IL-1 strategy. Arthritis Res Ther. 2011;13:124. doi: 10.1186/ar3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sachs D, Cunha FQ, Poole S, Ferreira SH. Tumour necrosis factor-α, interleukin-1β and interleukin-8 induce persistent mechanical nociceptor hypersensitivity. Pain. 2002;96:89–97. doi: 10.1016/s0304-3959(01)00433-x. [DOI] [PubMed] [Google Scholar]
- 9.Kraus VB, Birmingham J, Stabler TV, Feng S, Taylor DC, Moorman CT, 3rd, Garrett WE, Toth AP. Effects of intraarticular IL1-Ra for acute anterior cruciate ligament knee injury: A randomized controlled pilot trial ( NCT00332254) Osteoarthritis Cartilage. 2012;20:271–278. doi: 10.1016/j.joca.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Smeets RL, van de Loo FA, Joosten LA, Arntz OJ, Bennink MB, Loesberg WA, Dmitriev IP, Curiel DT, Martin MU, van den Berg WB. Effectiveness of the soluble form of the interleukin-1 receptor accessory protein as an inhibitor of interleukin-1 in collagen-induced arthritis. Arthritis Rheum. 2003;48:2949–2958. doi: 10.1002/art.11234. [DOI] [PubMed] [Google Scholar]
- 11.Inoue K, Masuko-Hongo K, Okamoto M, Nishioka K. Efficacy of daily compared to intermittent administration of IL-1Ra for protection against bone and cartilage destruction in collagen-challenged mice. Clin Exp Rheumatol. 2003;21:33–39. [PubMed] [Google Scholar]
- 12.Kosmidis ML, Alexopoulos H, Tzioufas AG, Dalakas MC. The effect of anakinra, an IL1 receptor antagonist, in patients with sporadic inclusion body myositis (sIBM): A small pilot study. J Neurol Sci. 2013;334:123–125. doi: 10.1016/j.jns.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Frisbie DD, Ghivizzani SC, Robbins PD, Evans CH, McIlwraith CW. Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin-1 receptor antagonist gene. Gene Ther. 2002;9:12–20. doi: 10.1038/sj.gt.3301608. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes J, Tardif G, Martel-Pelletier J, Lascau-Coman V, Dupuis M, Moldovan F, Sheppard M, Krishnan BR, Pelletier JP. In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints: Prevention of osteoarthritis progression. Am J Pathol. 1999;154:1159–1169. doi: 10.1016/S0002-9440(10)65368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang HJ, Yu CL, Kishi H, Motoki K, Mao ZB, Muraguchi A. Suppression of experimental osteoarthritis by adenovirus-mediated double gene transfer. Chin Med J (Engl) 2006;119:1365–1373. [PubMed] [Google Scholar]
- 16.van de Loo FA, van den Berg WB. Gene therapy for rheumatoid arthritis. Lessons from animal models, including studies on interleukin-4, interleukin-10, and interleukin-1 receptor antagonist as potential disease modulators. Rheum Dis Clin North Am. 2002;28:127–149. doi: 10.1016/s0889-857x(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 17.Chevalier X, Giraudeau B, Conrozier T, Marliere J, Kiefer P, Goupille P. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: A multicenter study. J Rheumatol. 2005;32:1317–1323. [PubMed] [Google Scholar]
- 18.Akash MS, Rehman K, Chen S. IL-1Ra and its delivery strategies: Inserting the association in perspective. Pharm Res. 2013;30:2951–2966. doi: 10.1007/s11095-013-1118-0. [DOI] [PubMed] [Google Scholar]
- 19.Lavi G, Voronov E, Dinarello CA, Apte RN, Cohen S. Sustained delivery of IL-1Ra from biodegradable microspheres reduces the number of murine B16 melanoma lung metastases. J Control Release. 2007;123:123–130. doi: 10.1016/j.jconrel.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Giteau A, Venier-Julienne MC, Aubert-Pouëssel A, Benoit JP. How to achieve sustained and complete protein release from PLGA-based microparticles? Int J Pharma. 2008;350:14–26. doi: 10.1016/j.ijpharm.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Yuan W, Liu Z. Controlled-release and preserved bioactivity of proteins from (self-assembled) core-shell double-walled microspheres. Int J Nanomed. 2012;7:257–270. doi: 10.2147/IJN.S27621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Akash MS, Rehman K, Li N, Gao JQ, Sun H, Chen S. Sustained delivery of IL-1Ra from pluronic F127-based thermosensitive gel prolongs its therapeutic potentials. Pharm Res. 2012;29:3475–3485. doi: 10.1007/s11095-012-0843-0. [DOI] [PubMed] [Google Scholar]
- 23.Akash MS, Rehman K, Sun H, Chen S. Assessment of release kinetics, stability and polymer interaction of poloxamer 407-based thermosensitive gel of interleukin-1 receptor antagonist. Pharm Dev Technol. 2014;19:278–284. doi: 10.3109/10837450.2013.775158. [DOI] [PubMed] [Google Scholar]
- 24.Yu P, Zheng C, Chen J, Zhang G, Liu Y, Suo X, Zhang G, Su Z. Investigation on PEGylation strategy of recombinant human interleukin-1 receptor antagonist. Bioorg Med Chem. 2007;15:5396–5405. doi: 10.1016/j.bmc.2007.05.061. [DOI] [PubMed] [Google Scholar]
- 25.Kimmerling KA, Furman BD, Mangiapani DS, Moverman MA, Sinclair SM, Huebner JL, Chilkoti A, Kraus VB, Setton LA, Guilak F. Sustained intra-articular delivery of IL-1RA from a thermally-responsive elastin-like polypeptide as a therapy for post-traumatic arthritis. Eur Cell Mater. 2015;29:124–139. doi: 10.22203/ecm.v029a10. others. discussion 139-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitmire RE, Wilson DS, Singh A, Levenston ME, Murthy N, García AJ. Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins. Biomaterials. 2012;33:7665–7675. doi: 10.1016/j.biomaterials.2012.06.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh A, Agarwal R, Diaz-Ruiz CA, Willett NJ, Wang P, Lee LA, Wang Q, Guldberg RE, Garcia AJ. Nanoengineered particles for enhanced intra-articular retention and delivery of proteins. Adv Healthc Mater. 2014;3:1562–1567. 1525. doi: 10.1002/adhm.201400051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finn NA, Kemp ML. Pro-oxidant and antioxidant effects of N-acetylcysteine regulate doxorubicin-induced NF-kappa B activity in leukemic cells. Mol Biosyst. 2012;8:650–662. doi: 10.1039/c1mb05315a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tassa C, Duffner JL, Lewis TA, Weissleder R, Schreiber SL, Koehler AN, Shaw SY. Binding affinity and kinetic analysis of targeted small molecule-modified nanoparticles. Bioconjug Chem. 2010;21:14–19. doi: 10.1021/bc900438a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ara M, Watanabe M, Imai Y. Effect of blending calcium compounds on hydrolytic degradation of poly(DL-lactic acid-co-glycolic acid) Biomaterials. 2002;23:2479–2483. doi: 10.1016/s0142-9612(01)00382-9. [DOI] [PubMed] [Google Scholar]