Abstract
For more than 60 years drug delivery systems have produced numerous controlled release formulations helping patients improve compliance and maximize the drug efficacy. Development of new controlled drug delivery systems was very productive during the period 1950-1980. The productivity, as measured by the number of clinically used formulations, dropped significantly during 1980-2010. This reduced productivity needs to be understood so that the future development of drug delivery systems can be accelerated and prolific again. This requires critical evaluation of the current drug delivery field, so that the factors inhibiting rapid progress can be identified and resolved. The current drug delivery field is faced with an invisible gorilla syndrome, i.e., seeing a gorilla when it is not present and missing a gorilla when it actually exists. Overcoming this syndrome requires a new way of thinking, questioning the status quo. Advances in drug delivery technologies occur by an evolutionary process, and thus, the more trials and errors lead to faster advances. The drug delivery area needs to nurture the environment where vastly different ideas can be tested, and all data, positive or negative, need to be exchanged freely as they have equal importance.
Keywords: drug delivery, future, nanoparticle, invisible gorilla
1. Drug delivery systems that changed the world
Many things in the human history have revolutionized the world. Several examples in science and technology include the germ theory by Louis Pasteur, anesthesia by Crawford Long, X-ray by Wilhelm R?ntgen, aspirin by Felix Hoffman, water chlorination by John Leal, insulin by Frederick Banting and Charles Best, DNA structure by James Watson and Francis Crick, and recombinant DNA technology by Paul Berg, Stanley Cohen and Herbert Boyer. The drugs of historical significance include penicillin by Alexander Fleming, chlorpromazineblocking dopamine receptors for treating schizophrenia[1], zidovudine (also known as azidothymidine) inhibiting reverse transcriptase for treating acquired immunodeficiency syndrome[2], and omeprazole blocking the gastric hydrogen potassium ATPase (proton pump)[3]. The world may be quite a different place without these discoveries.
In drug delivery, the first formulation that changed the world is the Spansule® technology introduced in 1952 by Smith Kline & French[4]. The Spansule technology was used to develop 12-hour drug release formulations for the first time.Each Spansule capsule contains hundreds of micropellets which are coated with a water-soluble wax, poly(ethylene oxide) or PEO, at different thicknesses to provide slow release of a drug for 12 hours. This formulation was revolutionary, as patients could then take drugs only twice a day, dramatically improving patients’ convenience and compliance, as compared with taking drugs 3 or 4 times a day. Commercial success of this new controlled-release formulation prompted the drug delivery field to develop other controlled release technologies. These include dissolution-controlled, diffusion-controlled, osmosis-controlled, and ion-exchange-controlled formulations. The term “controlled release” evolved to include sustained release, timed release, extended release, and other names, but currently they are used interchangeably. Controlled release drug delivery systems (DDSs) have been improving patient care by providing a sustained level of effective drug concentration as compared with the conventional “immediate release” formulations. Controlled release formulations have also been beneficial to the pharmaceutical industry by making drugs more effective with fewer side effects.
The progress made during the 30 years following the introduction of the Spansule technology, i.e., during 1950-1980 or the first generation (1G), was remarkable in the quality of technology developed and the quantity of the clinical formulations introduced. The drug delivery technologies developed in the subsequent 30 years, i.e., 1980-2010 or the second generation (2G), were also significant, butsuccess in introducing clinical formulations was considerably reduced. Currently, we are in the third generation (3G) of drug delivery technology starting from around 2010. Drug delivery scientists will continue to change the world through their research and technologies leading to innovative formulations benefiting patients.
2. Candid discussion on the progress of drug delivery technology
To have a clear view on the progress of DDS, a historical perspective is necessary[5, 6]. The simplest version of the DDS history is that oral and transdermal DDSs (developed during the 1G period) have been very productive in producing clinicalformulations, while advanced DDSs, mostly studied during the 2G period, were not translated into clinical applications as much. The main difficulty of the 2G technologies was, in part, due to dealing with biological barriers that cannot be easily overcome by altering the physicochemical properties of DDSs. Understanding the reasons behind it will help us find the solutions for further, and hopefully rapid, progress in the future. To achieve the goal of introducing clinically effective DDSs, the scientists in this area need to find out the reasons for the difficulties, and thus, the solutions to overcome them if possible. Understanding the reasons, however, requires more than search of scientific reasons. It also requires reasons based on human behavior.
Here it is important to clarify why drug delivery scientists do their research. Many do their research, because they want to do basic research which may not result in near-term clinical applications. The ultimate goal of DDS research, however, is to contribute to development of clinically useful formulations that can prevent and/or treat diseases in human patients. The lack of clinical translations of the 2G advanced DDS is due to inadequate understanding of the complex behavior of the body with too optimistic assumptions that are not supported by facts. If drug delivery scientists continue the current way of doing research by patting each other on the back without critical assessment of others’ work, the real progress of understanding the obstacles and developing formulationsthat can be used clinically will be unnecessarily slow. It is time for the drug delivery science community to review the field critically. This article discusses the reasons for slow progress that the field has been experiencing, and what each drug delivery scientist can do about it. The views described in this article are personal views which may be very different from many in the drug delivery field. However, exchanging different views and ideas is the first stepnecessary to overcome the current stalemate in drug delivery technologies.
2.1. Current complacency in drug delivery technology
The current technology that dominates the drug delivery field is the nanotechnology-based targeted drug delivery, and it serves as a good example illustrating the difficulties facing the field. The “nanoparticle” technology attracted a lot of attention since the late 1990s for improved, although modest, drug delivery to target tumors as compared with the control.The nanoparticle technology was hailed as a new, disruptive technology. In drug delivery, the term “nanoparticle” was first used in 1976 [7] and many scientists used nanoparticles to find novel ways of drug delivery [8], but it caught on since the National Nanotechnology Initiative by the US government in 2000[9]. The current small animal experiments using nanoparticles and their data interpretation rely on conventional wisdom that nanoparticle formulations are effective in targeted drug delivery to the target tumor site due to the enhanced permeability and retention (EPR) effect. This assumption was accepted without any critical evaluation, especially in human patients. The hard data cumulated over the last few decadesare not conclusive even in mice[10-13]. The assumption based on intuition should be accompanied by hard data supporting it [14].The data from small animal models have been misinterpreted or over-interpreted.
If the results inmouse studies are not reproduced in humans, the small animal model needs to be changed to something else that represents the human condition better. Otherwise, the research done using the mouse models will become irrelevant to our ultimate goal of developing clinical formulations. In a larger scale, the drug delivery field as a whole may become irrelevant in pharmaceutical industry without drug delivery systems for clinical applications. Here, drug delivery scientists need to have a growth mindset that welcomes challenges and tries to stretch existing abilities through failures, instead of a fixed mindset striving for success and avoiding failure at all costs[15], and developthe willingness to listen to different points of views. The drug delivery science community need frank discussion on the lack of translation from mouse to human. There may be nanoparticle systems that may appear to be working in xenograft mouse models, but there is really no point in dwelling on such systems when the data are not translated to clinical applications. Drug delivery scientists need to listen to others who do not share the same views. Such open mindedness is essential for accurate assessment of the current status, and thus, hopefully, for breaking the current complacency and finding solutions to the problems at hand.
2.2. Diversification of drug delivery technologies
Analysis of the drug delivery technologies developed for the last 60 years indicates that drug delivery research follows a trend which prevails in a given time period. Since the dawn of the nanotechnology era in 2000, the majority of the drug delivery researchers have focused on nanoparticle-based targeted drug delivery to tumors. The nanoparticle-based research on tumor-targeted delivery has been sweeping the world for much more than a decade. The prevailing idea has been that a number of shortcomings of conventional small molecule drugs can be overcome by multiplexed nanoparticle formulations through tailoring the chemistry and identity of variable nanoparticle constituents [16]. Numerous articles have been published on the topic over the years. Those research articles have certainly enhanced our understanding on nanoparticle formulations. But there is a lingering question: Why are all those seemingly very promising nanoparticle systems failing in clinical trials? Many clinical trials of tumor-targeted drug delivery using nanoparticleshave failed[11, 13, 17]. According to Gene Therapy Clinical Trials Worldwide provided by the Journal of Gene Medicine [18], there have been more 2,210 gene therapy clinical studies as of September 2015. Out of these, only two are in Phase IV. If so many clinical studies have failed, the approach used is usually reviewed and adjusted to find a better approach. Instead, the nanoparticle field has been churning outover-engineered nanoparticleswith minimal improvement in treatment. The overshooting technology adds more complexity with minimal return [19, 20]. The nanoparticle formulations have become more sophisticated but the goal of treating tumors is still beyond our grasp. The field needs a new radical innovationin place of incremental improvements by the current nanoparticle technology.
A large number of drug delivery scientists are involved in nanoparticle research, which is often described as nanomedicine. What has made so many scientists in the world jump on the nanoparticle band wagon?It is simply due to the trend of the time. Research funds are readily available, and thus, there is no reason not to do nanoparticle research. One may ask why we even need to consider such a question. Many researchers work on the same topic, and thus, significant advances occur at least as measured by the number of publications. On the surface, there seems to be no problem. In its core, however, this breeds long-term problems. If our collective efforts are focused on the technology that has shown a lot of potential in small animal studies, but has no clear sign of efficacy in clinical applications, we are simply diverting important resources to something less useful. We need to have a system which encourages those researchers who have different views to do research differently. Otherwise, the same thing will repeat itself. Only after trying a wide variety of different ideas, will we be able to find a technology that works in humans. We need to promote a culture and environment that allows scientists with different views and ideas can test theirs without any difficulty.
3. The scientists’ invisible gorilla syndrome
It is common for scientists, or any individual for that matter, to believe that one’s opinions and decisions are based on experience, facts, and well-designed experimental data, while ignoring others’ conclusions if they do not match with their own thinking [21]. Most drug delivery scientists tend to accept unproven hypothesis with few questions, if it is provided by an authority figure in the field. When the majority of scientists in the field follow a certain trend, theyare prone to accept the data without any critical evaluation, if they match with the prevalent views at the time. This is known as conformity. The majority may have a certain opinion, but it may not be a correct one. Scientific facts are not determined by the majority votes.
As shown by the famous selective attention test (also known as the invisible gorilla experiment) by Simons and Chabris in 1999 [22], when we are intensely focusing on a task, we tend to miss other things happening at the same time. Our attention is limited, and not designed for multi-tasking. This seems to be happening in scientific experiments also. This is called “the invisible gorilla syndrome” in this article. The invisible gorilla syndrome can be divided into two categories: seeing a non-existing gorilla and missing an existing gorilla.
3.1. Seeingagorilla when it is nowhere in sight
Seeing a gorilla when it does not exist at all is simply due to the tendency to see the world the way we like it to be[23]. In this situation it is easy to see a meaningful pattern out of random events, especially if it helps confirm the preexisting belief. Apophenia, which is adding special meaning to random coincidental events, has no scientific basis other than blind trust. Scientists may believe that their conclusions are based on careful experiments and critical analysis of the data, but the first perception obtained in the literature or from other authorities affects later perceptions and decisions[21]. Such anchoring effects lead to conclusions that fit the currently prevailing views. Once the majority builds up a certain make-believe story, everyone follows without any critical questions. In this situation, any logic is accepted, if the conclusion fits the current knowledge basis. As noted previously by Ioannidis, many claimed findings may simply reflect accurate measures of the prevailing bias [24-27]
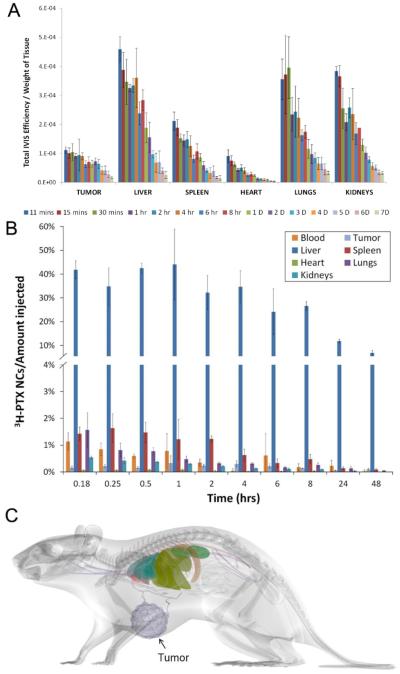
A good example of seeing a non-existent gorilla is nanoparticle-based tumor targeted drug delivery.Many experimental data exist showing the tumor targeting property of nanoparticles, but most of them are based on images of a fluorescent probe, not an actual anticancer drug. The fluorescence images usually show extended presence of a fluorescent probe at the tumor site much longer than in other organs, and this has been mistaken as the prolonged presence of an anticancer drug. To clarify that the distribution of a fluorescent probe in a mouse is not the same as the distribution of an anticancer drug, a clever experiment was done by Li and his colleagues [12]. The prepared paclitaxel nanocrystals contained both 3H-paclitaxel and FPI-749 fluorophore for quantitative measurement of paclitaxel distribution and visual observation, respectively, in different organs. Figure 1-A shows the relative distribution of FPI-749, and the amount of the fluorophore accumulated in the tumor is about 1/4 of that in the liver. Furthermore, the fluorescent signal was visible even after a week. The data in Figure 1-A, if taken at its face value, clearly indicates the tumor-targeting property of nanocrystals. If Figure 1-A was the only data generated from the study, it would have been easy to claim that nanocrystals accumulate at the tumor site in a significant quantity and last for more than a week. In fact, this type of fluorescence imaging data is very common and used widely, as it is easy to prove the superiority of nanoparticles. But it is important to note that the data in Figure 1-A is for distribution of a fluorescent probe, not paclitaxel. The distribution of paclitaxel in a mouse was quantitatively measured using 3H-paclitaxel. As shown in Figure 1-B, the quantitative measurement of paclitaxel shows that less than 0.4% of the administered paclitaxel is found at the tumor, while paclitaxel in the liver is more than 40% of the total administered dose. A comparison study with Taxol® formulation (paclitaxel in Cremophor EL and ethanol) indicates that the paclitaxel nanocrystal is not as effective as Taxol® which delivers more than 0.8%. To visualize the relative distribution of paclitaxel in different organs, the data in Figure 1-B was transformed into a digital mouse image in Figure 1-C. As shown in the figure, the tumor is as large as the liver, but only a fraction of the drug going to the liver was found around the tumor. Clearly, the fluorescence imaging data does not reflect the actual distribution of paclitaxel at the target tumor and other organs.
Figure 1.
Relative distributions of a fluorescence probe (A) and paclitaxel (B) after administration of paclitaxel nanocrystals containing 3H-paclitaxel and FPI-749 fluorophore in the same crystals. (C) The data at t=0.25 h in Panel B was constructed into a digital image. Data in Panels A and B are from Reference [12], and the digital image in Panel C was prepared by Professor Tonglei Li at Purdue University.
The significance of the data in Figure 1 cannot be overemphasized. First, the experimental data based on fluorescence images do not represent the actual distribution of an anticancer drug. A fluorescence probe cannot be considered as a model drug for anticancer drugs. Second, a fluorescent probe, at best, is another chemical which is different from an anticancer drug of interest. Different chemicals have different physicochemical properties, making it difficult to predict anticancer drug distribution from the distribution of a fluorescent probe. Even the two different anticancer drugs would have two different distribution profiles in the body.Yet, numerous studies concludedtumor-targeting properties of nanoparticlesbased on fluorescence imaging studies. Third, different distributions of a fluorescent probe and paclitaxel, even when both were delivered in the same nanocrystal, indicate that the value of the so-called theranosis is dubious. If fluorescence imaging data do not accurately describe distribution of anticancer drugs, theranosis may not work as intended.
A conclusion obtained from only fluorescent imaging data is nothing more than self-fulfilling prophecy,which is seeing a gorilla when it is nowhere in sight. The real danger of seeing an absent gorilla is focusing on a certain result that a researcher is looking for, while ignoring other data which may provide important clues to the problems. Such illusion of positive results leads to conclusions which are very different from the inconvenient fact. Scientists, no matter how seasoned they may be, are equally prone to illusion of attention [23]. Drug delivery scientists, maybe all scientists for that matter, need to get out of this illusion of attention. This is especially important for young scientists whose minds are still pure.
3.2. Missing a gorilla when it is in sight
Missing an existing gorilla is known as inattentional blindness, or motivated blindness.Inattentional blindness is the failure to notice a fully-visible but unexpected object or event when attention is focused on something else[23, 28]. Furthermore, inattentional blindness ignores new findings with overwhelming evidence instead of changing the existing prevailing view. Kodak developed a digital camera but it ignored it, because the company wanted to believe continued sales of films. IBM made a similar mistake by ignoring the personal computer revolution. Madoff’s Ponzi scheme lasted so long, even though it was impossible to have 12% annual return of investment, because the regulators ignored the evidence. The 2008 financial crisis was born out of the belief that the housing prices in America always go up and never go down. Such motivated blindness resulted in missing/ignoring important information, sometimes with disastrous outcomes.
To see the gorilla when it is present requires an open mind. Scientists need to be trained to see a gorilla when it exists. This, for the drug delivery area, means that the scientists need examine the data critically with questions, instead of blindly accepting others’ data just because they fit the current thinking of the majority. The invisible gorilla syndrome is important, as it may hamper the real progress to be made in drug delivery. Finding a pattern or a meaning out of complete randomness not only leads to inaccurate conclusions, but also it causes missing real important information. The current drug delivery field is full of seeing non-existing gorillas, and this leads to an important consequential mistake of missing a real one.
4. A few changes to be made in the drug delivery field
It is easy to make predictions of the future. Simply, they most likely will be wrong[20, 29, 30]. If a prediction does not pan out, everybody forgets and nobody is liable for anything. If a prediction, out of hundreds, turns out to be reasonably close to reality, then the person with such a prediction is considered a visionary. What the field needs is not visionaries but doers. Instead of making predictions on the new technologies that may or may not be available in the future, let’s discuss the current problems in drug delivery research. Finding answers starts from clearly understanding the problems. Without clearly defining the problem, any attempts to finding solutions will be a shot in the dark. Table 1 describes some of the difficulties facing the drug delivery field [31]. Each problem in Table 1 is unique, and thus, requires different solutions. Nanoparticles, or nanotechnology in general, may not be able to provide solutions to all these problems. This is another reason why drug delivery scientists need to explore different technologies for solving various important problems.
Table 1.
Barriers to overcome by the 3G DDSs. (From reference [31]).
| 1. | Delivery of poorly soluble drugs |
| Non-toxic excipients | |
| 2. | Peptide/protein/nucleic acid delivery |
| Control of the initial burst release and subsequent release rate | |
| Non-invasive delivery | |
| In vitro-in vivo correlation | |
| 3. | Targeted drug delivery |
| Tumor targeting in human | |
| Overcoming blood-brain barrier | |
| 4. | Self-regulated drug delivery |
| Functional in the body for extended period of time |
As understanding on any topic increases, hypotheses are developed and assumptions are made so that at some point well-established theories emerge. Assumptions are valid only under the conditions that they were designed to be valid. Yet, it is common to see that the unproven assumptions are taken as facts to support the conclusions derived from experiments even though the actual data are not conclusive enough to warrant such conclusions. The problem becomes even more compounded when the data of animal experiments with predictable results are extended to predict clinical outcomes. There may be many reasons for this mistake by scientists. One of them is that drug delivery scientists rely too much on small animal modelswith facile extrapolation and inappropriate titles on papers often alluding to a novel solution to a clinical problem.
4.1. Toomuch reliance on the mouse model
Of the thousands of articles in nanoparticle-based tumor targeting, most of them used xenograft mouse models or some type of mouse model. A formulation may have shown some efficacy in mice. Here it is important to see the accumulatingdata without any preconceived bias. Most of the mouse experiments show some type of efficacy of nanoparticles in treating tumors. This is where our preconceived notion of nanoparticle superiority has to be abandoned. If the mouse data in the literature are analyzed, the seemingly effective nanoparticle delivery systems do not really show the effective treatment of xenograft tumors. If the treatment really worked, the nanoparticle formulations could have been administered repeatedly for the mouse to live longer as normal mice would. The more important fact from all of those mouse experiments is that none of the results in mouse studies was reproduced in clinical trials[17].
Here, a review of experimental models used in the development of omeprazole is particularly relevant. When a proton-pump inhibitor was developed in the late 1960s, the efficacy of antisecretory compounds was screened using the rat model. Those compounds which were very effective in the rat, however, were completely ineffective in man [3]. This led to other animal models, such as anesthetized dogs, and simpler in vitroscreening models, including isolated gastric-acid-secreting mucosa of the guinea pig, isolated rabbit acid-secreting glands, and a micromethod for isolating acid-secreting glands from human gastric biopsies, all of which allowed accurate screening of a large number of candidate drugs [3]. If the rat model was continued as a screening tool, even when the rat data did not reflect the clinical efficacy, the development of omeprazole could have been delayed significantly. Currently, most peptic ulcers are known to be caused by infection with Helicobacter pylori, and thus, a proton-pump inhibitor is used in combination with antibiotics [3]. Thus, reflecting the human condition accurately may require even more sophisticated models.
The current xenograft mouse models are known to be incapable of predicting clinical efficacy of nanoparticle-based tumortargeted drug delivery. This is not surprising considering the vast differences between mouse and man. The size ratio of a tumor in a mouse is orders of magnitude larger than that of a tumor in a man. More importantly, the blood volume in a mouse is only a few milliliters, while that in a man is several liters. In addition, the use of unrealistically high dose in mice will not provide useful information for clinical applications. Nevertheless, most of the studies still rely on the mouse experiments. It is time to rethink the xenograft mouse model and find alternative experimental methods that can accurately screen the efficacy of drug delivery in man. We too often design models to test our ideas, but not to test the complexities of diseases. More thoughtful, and likely more complex, animal modeling is required.
4.2. Not asking simple questions
The EPR effect has been the dominant theoretical basis for claiming targeted drug delivery to tumors. Most articles, however, describe improved results without actually showing the quantitative data suggesting the existence of the EPR effect. Many rely on fluorescence imaging data which may not show the actual distribution of the drug used in the study. As long as the data fit the preset box of thinking, i.e., nanoparticle, PEGylation, ligand grafting, and the EPR effect, the conclusion is made fast without asking other important questions. Since those who use nanoparticles are seeking any evidence showing the efficacy of nanoparticles, they tend to see the invisible gorilla, while ignoring the overwhelming evidence that the gorilla actually does not exist.
Whenever a new article is published on nanoparticle based tumor targeting, one can ask simple questions. Does the study present quantitative data to prove that the drug actually accumulates at the target? Is the drug accumulated at the target effective? Is the shrinking in tumor size enough to predict that the formulation is effective in treating tumors? Can the nanoparticle formulation be administered repeatedly to make the mice live longer? What makes a particular nanoparticle formulation different? Can we still assume the presence of the EPR effect without any quantitative data? Even if it exists, if the mouse data cannot predict the efficacy in humans, what value does it add to our understanding? Doing the same thing over and over again in mice and expect something that works in humans may not be the right approach in finding effective ways to treating tumors in humans.
5. Scientific advances by evolutionary process
The current process of going through the nanoparticle era is simply a process of evolution toward making better drug delivery systems. The fact that nanoparticle formulations whichseemingly work in mice do not work in man is a direct result of trials. No animal models are sufficiently predictive enough to substitute for clinical trials [26]. Without such trials, we would not know and the high expectation would have remained. The process of trial and error will have to continue to find a method that is better than previous ones. The question here is whether such evolutionary processes can be made faster and shorter. Evolution provides an answer that is good enough to solve a particular problem at the time [32]. It is the same as reaching a local minimum energy state, when the minimum state of the whole system resides somewhere else. This means that any solution that can be found by trial and error is not the best solution but is good enough for solving a particular problem. Thus, even if we find a better nanoparticle system, it simply means a small improvement over the previous ones, still far from the life-saving formulations for which we are looking. This is where the idea of failing fast is important. Failing fast, or failing smart, means using the minimal resources to find whether a test method will work or not[33, 34]. Failing smart allows testing a large variety of different methods, increasing the chance of finding the method that works. In this context, failing is actually a great learning experience.
When we are faced with adaptive challenges, we need to “think in different boxes” to find creative answers[35]. This requires methodological skepticism[36], which is asking the right questions, instead of blindly accepting others’ data as proven facts. We need to think outside the box and elevate ourselves above the ground to see the big picture, i.e., the forest as a whole, instead of individual trees. The mosaic image seen outside the box is lot different from an image in each box.
6. The future drug delivery research
People rely on available information more, especially when the information matches with their expectation, instead of the all information that can be found. This is known as the availability heuristic. This explains why people remember the first news story, even though it turns out to be false later[21]. Nobody remembers the later news debunking the first one. The initial publications indicating the improved delivery to tumor by nanoparticles have dominated our thinking, and it is difficult to change that belief even if more information shows otherwise. It is a powerful human impulse to resist change[37], and we tend to mistake status quo for the natural order of things [38]. Since many scientists use nanoparticles, it is not likely for anyone to suggest alternative approaches. Raising a flag against the majority opinion is not easy[39]. Despite this general tendency, a question arises why scientists, who are trained to think logically, continue the same approach.Even though it is clear that the xenograft mouse model does not represent the human condition, especially for nanoparticle targeting to tumors, scientists continue using the same model. This is similar to holding a stock, even though the price continues to dive, until it bounces back to the purchase price. The funding agencies and investors have already spent so much time and energy on nanomedicine, and, thus, they cannot stop now untiltheirinvestment is recovered. Thus, the result is spending more time and efforts on the same thing. Another reason is that ignoring new findings is easier than changing the existing prevailing view[39]. But we have to understand that further studies in nanoparticles in the current form will have diminishing marginal utility. As we produce more me-too data, each additional informationbecomes less significant[40]. At some point, it becomes useless.
All successful people, whether they are businessmen, entrepreneurs, artists, or solders, have one thing in common: they never quit. Drug delivery scientistsshould never quit in developing effective DDSs. Some misunderstand this as never quitting in doing nanoparticle research. If the goal of doing research is just doing nanoparticle work, they of course want continue the same work. But the goal of doing research for the drug delivery scientist is to understand better the whole gamut of issues that can eventually lead to development of clinically effective delivery systems. If a certain approach of delivering a drug to a target does not work, we need to seek out other methods in an effort to achievethe goal. Continuing the work towards the goal despite repeated setbacks require tremendous will power, and we drug delivery scientists need to support each other. Instead of self-serving praises to each other, we need to be more critical of in each other’s work in a constructive way. We need to cultivate the younger generation of scientists with the ability to see things critically, instead of just accepting whatever is given to them simply because it was given by their advisors or the authority figures in the field. Our current environment is not suited to cultivate frank discussion and critical yet constructive exchange of opinions. Questioning is not disagreeing or disrespecting. It is an ultimate form of respect, but this has been lost.
For the research in the future with more productive outcomes, we need to provide a genuine experience of future possibilities when things are done right, instead of doing things driven by hype and short-termism. This requires a good framing of the issues that can enable better strategic thinking and more creative ideas.We need to find out what and where to look, and gather smart data, not just any data obtained from the self-serving experiments. Since there are so much data out there, it is virtually impossible to look for certain information without a frame. The frame will narrow the complex data. Framing, however, comes with several perils: (i) a too broad frame will obscure the data; (ii) a too narrow or familiar frame will miss important information; (iii) too many frames will only add confusion to the already difficult data; and (iv) a biased or overly prescriptive frame will look for only what one wants to see [23]. These undesirable framing results in either seeing an invisible gorilla or missing an existing gorilla. Only a good frame will allow us to see the gorilla when it is present. The scientists in the future need a good frame, and finding a good frame is also a process of trial and error. This is why we need to communicate so-called negative data, instead of data showing only the marginally improved results. Knowing what and why a particular approach failed is more important than seeing another me-too data which did not really advance the field.
A few decades ago, the nanoparticle approach in tumor targeting was considered a disruptive innovation, although no tangible clinical relevance was realized. Since then, the progress of nanoparticle approach has been incremental. As time passes, once a disruptive technology becomes just another sustaining technology that focuses on overshooting technology, instead of further disruptive innovation. It is time for the drug delivery field to have a new drastic, radical innovation. This requires a growth mindset, away from the fixed mindset trapped in an old box. It is never too late to learn something new. At the same time, as the saying goes, it is difficult to teach an old dog new tricks. Neither may be right. But it is right for sure to nurture the new, budding drug delivery scientists to think differently. Both the federal funding agencies and academic institutions need to reward those who are brave enough to break the prevailing bias. There is no reason for them to follow the current dogma, as it will severely slow the progresses necessary for the future. Drug delivery scientists need to cultivate an environment where they can try new things without fear of failure and their achievements are not measured by immediate metric outcomes. We need to get our sanity back to research.
Acknowledgment
This work was supported by the Showalter Research Trust Fund and the National Institute of Health through CA129287 and GM095879.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatric Disease and Treatment. 2007;3:495–500. [PMC free article] [PubMed] [Google Scholar]
- [2].Moore RD, Hidalgo J, Sugland BW, Chaisson RE. Zidovudine and the natural history of the acquired immunodeficiency syndrome. N. Engl. J. Med. 1991;324:1412–1416. doi: 10.1056/NEJM199105163242006. [DOI] [PubMed] [Google Scholar]
- [3].Olbe L, Carlsson E, Lindberg P. A proton-pump inhibitor expedition: the case histories of omeprazole and esomeprazole. Nature Reviews: Drug Discovery. 2003;2:132–139. doi: 10.1038/nrd1010. [DOI] [PubMed] [Google Scholar]
- [4].Lee PI, Li J-X. Evolution of oral controlled release dosage forms. In: Wen H, Park K, editors. Oral Controlled Release Formulation Design and Drug Delivery. John Wiley & Sons, Inc.; Hoboken, NJ: 2010. pp. 21–31. [Google Scholar]
- [5].Park K. Facing the truth about nanotechnology in drug delivery. ACS Nano. 2013;7:7442–7447. doi: 10.1021/nn404501g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Park K. Controlled drug delivery systems: Past forward and future back. J. Control. Release. 2014;190:3–8. doi: 10.1016/j.jconrel.2014.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kreuter J, Speiser P. In vitro studies of poly(methylmethacrylate) adjuvants. J. Pharm. Sci. 1976;65:1624–1627. doi: 10.1002/jps.2600651115. [DOI] [PubMed] [Google Scholar]
- [8].Jani P, Halbert GA, Langridge J, Florence AT. Nanoparticle uptake by the rat gastrointestinal mucosa: Quantitation and particle size dependence. J. Pharm. Pharmacol. 1990;42:821–826. doi: 10.1111/j.2042-7158.1990.tb07033.x. [DOI] [PubMed] [Google Scholar]
- [9].The White House Office of the Press Secretary National Nanotechnology Initiative: Leading to the Next Industrial Revolution. http://clinton4.nara.gov/WH/New/html/20000121_4.html.
- [10].Bae YH, Park K. Targeted Drug Delivery to Tumors: Myths, Reality, and Possibility. J. Control. Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stirland DL, Nichols JW, Miura S, Bae YH. Mind the gap: A survey of how cancer drug carriers are susceptible to the gap between research and practice. J. Control. Release. 2013;172:1045–1064. doi: 10.1016/j.jconrel.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hollis CP, Weiss HL, Leggas M, Evers BM, Gemeinhart RA, Li T. Biodistribution and bioimaging studies of hybrid paclitaxel nanocrystals: lessons learned of the EPR effect and image-guided drug delivery. J. Control. Release. 2013;172:12–21. doi: 10.1016/j.jconrel.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nichols JW, Bae YH. EPR: Evidence and fallacy. Journal of Controlled Release. 2014;190:451–464. doi: 10.1016/j.jconrel.2014.03.057. [DOI] [PubMed] [Google Scholar]
- [14].Bazerman MH. The Power of Noticing: What the Best Leaders See. Simon & Schuster; New York, NY: 2014. p. 215. [Google Scholar]
- [15].Dweck CS. How We Can Learn to Fulfill Our Potential. Ballantine Books; New York, NY.: 2006. Mindset: The New Psychology of Success; p. 277. [Google Scholar]
- [16].Eifler AC, Thaxton CS. Nanoparticle therapeutics: FDA approval, clinical trials, regulatory pathways, and case study. Methods Mol. Biol. 2011;726:325–338. doi: 10.1007/978-1-61779-052-2_21. [DOI] [PubMed] [Google Scholar]
- [17].Park K. Translation from mouse to human: Time to think in new boxes. J. Control. Release. 2014;189:187. doi: 10.1016/j.jconrel.2014.07.046. [DOI] [PubMed] [Google Scholar]
- [18].Gene Therapy Clinical Trials Worldwide provided by the Journal of Gene Medicine. 2015 http://www.abedia.com/wiley/phases.php.
- [19].Christensen CM. The Innovator's Dilemma. Harvard Business School Publishing Corp.; Boston, MA: 1997. p. 286. [Google Scholar]
- [20].Christensen CM, Anthony SD, Roth EA. Seeing What's Next: Using the Theories of Innovation to Predict Industry Change. Harvard Business School Publishing Corp.; Boston, MA: 2004. p. 315. [Google Scholar]
- [21].McRaney D. Why You Have Too Many Friends on Facebook, Why Your Memory Is Mostly Fiction, and 46 Other Ways You're Deluding Yourself. Gotham Books; New York, NY: 2011. You are Not so Smart; p. 320. [Google Scholar]
- [22].Selective attention test. 1999 https://www.youtube.com/watch?v=vJG698U2Mvo.
- [23].Chabris C, Simons D. How Our Intuitions Deceive Us. Crown Publishers; New York: 2009. The Invisible Gorilla; p. 306. [Google Scholar]
- [24].Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. J.A.M.A. 2005;294:218–228. doi: 10.1001/jama.294.2.218. [DOI] [PubMed] [Google Scholar]
- [25].Ioannidis JPA. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. (0696-0701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hartung T. Food for thought. Look back in anger – What clinical studies tell us about preclinical work. ALTEX. 2013;30:275–291. doi: 10.14573/altex.2013.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tsilidis KK, Panagiotou OA, Sena ES, Aretouli E, Evangelou E, Howells DW, Salman RA-S, Macleod MR, Ioannidis JPA. Evaluation of excess significance bias in animal studies of neurological diseases. PLOS Biology. 2013;11:e1001609. doi: 10.1371/journal.pbio.1001609. (1001601-1001610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].The invisible gorilla. 2015 http://theinvisiblegorilla.com/blog/
- [29].Gardner D. Future Babble: Why Expert Predictions Fail - and Why We Believe Them Anyway. Penguin Group; New York, NY: 2011. p. 305. [Google Scholar]
- [30].Tetlock PE, Gardner D. Superforecasting: The Art and Science of Prediction. Crown; New York, NY: 2015. p. 352. [Google Scholar]
- [31].Yun YH, Lee Byung Kook, Park K. Controlled drug delivery: Historical perspective for the next generation. J. Control. Release. doi: 10.1016/j.jconrel.2015.10.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Harford T. Adapt: Why Success Always Starts with Failure. Farrar, Straus and Giroux; New York: 2012. p. 309. [Google Scholar]
- [33].Sims P. Little Bets: How Breakthrough Ideas Emerge from Small Discoveries. Simon and Schuster; New York, NY: 2011. p. 224. [Google Scholar]
- [34].Levitt SD, Dubner SJ. Think Like A Freak. HarperCollins Publishers; New York, NY: 2014. p. 286. [Google Scholar]
- [35].Brabandere LD, Iny A. Thinking in New Boxes: A New Paradigm for Business Creativity. The Boston Consulting Group. 2013:330. [Google Scholar]
- [36].Fisk P. Creative Genius: An Innovation Guide for Business Leaders, Border Crossers and Game Changers. Capstone Publishing Ltd. (a Wiley Company); Chichester, West Sussex, United Kingdom: 2011. p. 386. [Google Scholar]
- [37].Catmull E, Wallace A. Creativity, Inc.: Overcoming the Unseen Forces That Stand in the Way of True Inspiration. Random House; Boston, MA: 2014. p. 340. [Google Scholar]
- [38].Daniels M. Keeping the Republic: Saving America by Trusting Americans. Sentinel; Boston, MA: 2011. p. 272. [Google Scholar]
- [39].Brafman O, Brafman R. Sway: The Irresistable Pull of Irrational Behavior. The Double Day Publishing Group; New York, NY: 2008. p. 206. [Google Scholar]
- [40].Mullainathan S. Scarcity: The New Science of Having Less and How It Defines Our Lives. Times Books; New York, NY: 2013. p. 288. [Google Scholar]