Abstract
The role of the innate immune response in colorectal cancer is understudied. We examined the survival of colorectal cancer patients in relation to eosinophils, innate immune cells, infiltrating the tumor. Tissue microarrays were constructed from paraffin-embedded tumor tissues collected between 1986–2002 from 441 post-menopausal women diagnosed with colorectal cancer in the Iowa Women’s Health Study. Tissue microarrays were stained with an eosinophil peroxidase antibody. Eosinophils in epithelial and stromal tissues within the tumor (called epithelial and stromal eosinophils, hereafter) were counted and scored into 3 and 4 categories, respectively. In addition, the degree of eosinophil degranulation (across epithelial and stromal tissues combined) was quantified and similarly categorized. We used Cox regression to estimate the hazard ratios and 95% confidence interval for all-cause and colorectal cancer death during five-year follow-up after diagnosis and during follow-up through 2011 (“total follow-up”). The hazard ratios associated with eosinophil scores were adjusted for age of diagnosis, SEER stage, tumor grade, body mass, and smoking history. High tumor stromal eosinophil score was inversely correlated with age and stage, and was associated with a decreased risk for all-cause and colorectal cancer death: hazard ratios (95% confidence intervals) were 0.61 (0.36–1.02; P-trend =0.02) and 0.48 (0.24–0.93; P-trend =0.01), respectively, during the five-year follow-up for the highest versus lowest category. The inverse associations also existed for total follow-up for all-cause and colorectal cancer death for the highest versus lowest stromal eosinophil score: hazard ratios (95% confidence intervals) were 0.72 (0.48–1.08; P-trend =0.04) and 0.61 (0.34–1.12; P-trend =0.04), respectively. Further adjustment for treatment, comorbidities, additional lifestyle factors, tumor location or molecular markers did not markedly change the associations, while adjustment for cytotoxic T-cells slightly attenuated all associations. The infiltration of tumors with eosinophils, especially in stromal tissue, may be an important prognostic factor in colorectal cancer.
INTRODUCTION
Colorectal cancer is a leading cause of cancer death worldwide. Currently, stage is a reference standard for colorectal cancer prognosis, but methods to increase predictive value for survival of colorectal cancer patients are needed.(1) Given that colorectal tumors may be recognized by the immune system and that colorectal cancer development and progression may be inhibited by immune response, tumor-infiltrating immune cells hold promise as novel prognostic biomarkers.(1–5) Most previous studies have focused on the adaptive immune response, in particular the infiltration of T-cells in colorectal tumors,(3, 5) although tissue-infiltrating innate immune cells may be essential for colorectal tumor control.(2, 4, 6) This prompted us to examine the role of innate immune cells – namely, eosinophils that are present in gastrointestinal epithelium of both healthy people and those diagnosed with colorectal cancer.
Eosinophils are multifunctional white blood cells that develop in bone marrow from myeloid progenitors. Once activated, eosinophils migrate into the blood stream and subsequently into tissues of the gastrointestinal tract and uterus (reviewed in (7–10)). Blood eosinophil counts are typically elevated in parasitic infection, allergy, and malignant disorders.(8, 10)
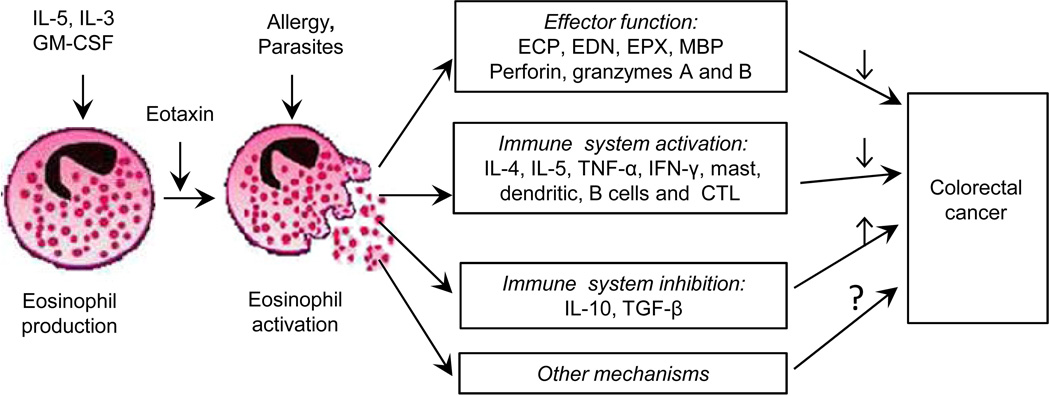
A distinct feature of eosinophils is that they contain large granules, which store a variety of preformed cytokines/chemokines and four cationic proteins: eosinophil cationic protein, eosinophil peroxidase, eosinophil-derived neurotoxin, and major basic protein, all known to be cytotoxic (Fig. 1). Under activation, the granules can rapidly release their cytotoxic contents, which in turn may induce tissue remodeling and direct killing of tumor cells.(10–12) Eosinophils may also affect carcinogenesis via modulating immune response.(6, 8, 10) A recent murine study reported that eosinophils improve vascularization and enhance the infiltration of cytotoxic T-cells, resulting in tumor rejection, which supports the immunomodulatory function of eosinophils.(13)
Figure 1.
Eosinophil cells and their link with colorectal cancer (adapted from (20)).
The role of tumor-infiltrating eosinophils in cancer progression and survival has been examined in the studies of different cancers and differed by cancer type (reviewed in (14, 15)). Eosinophil accumulation was associated with poorer prognosis in cervical cancer(16) and Hodgkin’s lymphoma,(17) and better prognosis of head and neck, bladder, gastric cancer, and esophageal carcinoma,(14, 15, 18, 19) while the eosinophil role in oral cancer was inconsistent across studies.(20) Colorectal cancer provides the most consistent evidence for a beneficial role of eosinophils in cancer prognosis, despite the small size of most conducted studies and difference in their designs and statistical methods (Supplementary Table 1).(21–27) Among seven studies that examined eosinophils in colorectal cancer patients, six studies suggested that eosinophils protect against colorectal cancer progression,(21–27) but they did not calculate the risk of cancer death or recurrence in spite of their importance to the colorectal cancer patients’ prognosis. A recent well-conducted study of colorectal cancer patients by Harbaum et al [2015] found an improved progression-free and cancer-specific survival associated with peritumoral eosinophils, i.e., those located in the stroma at the invasive tumor margin.(28) A critical question is whether or not eosinophils are a novel independent prognostic factor that should be routinely measured in colorectal cancer patients.
Therefore, before eosinophils are considered for therapeutic purposes, it is necessary to address the following issues essential to understanding the eosinophil-related survival of colorectal cancer patients that were not examined in previous studies: 1) the influence of eosinophil degranulation on colorectal cancer progression, since the eosinophil-specific proteins with cytotoxic properties are produced during degranulation; 2) potentially different roles of eosinophil infiltration into the epithelium and stroma within the colorectal tumor; and 3) the effect of lifestyle factors (smoking, obesity, alcohol, physical activity), molecular characteristics, and immune cytotoxic T-cells (also called CD8+) on the association between tumor-infiltrating eosinophils and survival.
We hypothesized that eosinophil accumulation in stroma and epithelium along with eosinophil degranulation are associated with better survival of colorectal cancer patients. We investigated this hypothesis in paraffin-embedded tissues from post-menopausal women diagnosed with colorectal cancer in the Iowa Women’s Health Study. Our study is novel, because we immunostained tumor tissues with a specific, previously validated antibody(29–31) that is able to discriminate between eosinophil peroxidase stored in intact eosinophils and proteins secreted from degranulated eosinophils, while the previous studies used conventional haematoxylin and eosin or Giemsa staining.
MATERIALS AND METHODS
Approvals for the current study were obtained from the Institutional Review Boards for Human Research at University of Minnesota, Mayo Clinic Rochester, and the University of Iowa.
The Iowa Women’s Health Study design
Details of the Iowa Women’s Health Study have been previously published.(32, 33) In 1986, a questionnaire was mailed to 98,030 women ages 55 to 69 years; 41,836 completed the questionnaire and constituted the cohort that was followed up to 2011. The follow-up of this cohort is nearly complete: the annual migration rate from Iowa is <1%.(32) Five follow-up surveys were sent in 1987, 1989, 1992, 1997, and 2004 to update vital status, residence, and exposure information (response rates were 91, 90, 83, 79, and 69%, respectively).
Incident colorectal cancer cases were ascertained through annual linkage to the State Health Registry of Iowa, part of the Surveillance, Epidemiology, and End Results (SEER). Colorectal cancer subsites were categorized as proximal colon (the cecum, ascending colon, hepatic flexure, transverse colon, and splenic flexure; International Classification of Diseases for Oncology (ICD-O-3) codes 18.0, 18.2–18.5) and distal colon or rectal cancers (descending colon, sigmoid colon, sigmoid colon, rectosigmoid junction and rectum; ICD-O-3 codes 18.6, 18.7, 19.9, 20.9). The registry also provided information on the extent of cancer at diagnosis, grade, and first course of treatment (surgery, radiation, chemotherapy).
Participants' deaths in Iowa were ascertained through the State Health Registry of Iowa through 2011. Deaths among non-respondents and emigrants from Iowa were found through the National Death Index resulting in 99% ascertainment of deaths in the Iowa Women’s Health Study.(32) Colorectal cancer deaths were assessed using codes for underlying cause of death from colorectal cancer (ICD9: 153.0–154.1, 159.0; ICD10: C18–C20, C26.0).
Tissue Selection and Processing
Archived, paraffin-embedded tissue specimens were requested from incident colorectal cancer cases diagnosed through December 31, 2002. In total, tissue specimens were retrieved from 732/1255 (58%) cases, which is similar to colorectal cancer tissue retrieval rates recently reported from the Health Professionals Follow-up Study (51%)(34) and the Nurses’ Health Study (58%).(35) Paraffin blocks were serially sectioned onto 5 or 10 µm slides. The last slide was stained with haematoxylin and eosin, so that areas of neoplastic tissue (defined as >50% dysplastic cells) could be identified. From these marked slides, three tumor cores (unstained) were taken from each pathology tissue block and placed into a tissue microarray block along with liver controls. The tissue microarrays was produced by the Mayo Clinic Pathology Research Core lab (Rochester) using the Beecher ATA-27 automated array. From the tissue microarray, 5 µm slides were cut for hematoxylin and eosin and immunohistochemistry staining of eosinophils.
Characterization of eosinophils and cytotoxic T-cells
Immunohistochemistry for eosinophil peroxidase and CD8 was performed by the Pathology Research Core at the Mayo Clinic (Rochester) using the Leica Bond III Stainer. Briefly, slides were dewaxed and retrieved for 20 minutes using the following reagents: Bond Dewax (Leica, Buffalo, IL) and Epitope Retrieval 2 (EDTA) for eosinophil peroxidase or Epitope Retrieval 1 (citrate) for CD8. The tissue slides were retrieved for 10 minutes (CD8) or 20 minutes (eosinophil peroxidase). The primary monoclonal eosinophil peroxidase antibody (clone 144B, homebrew from Dr. James Lee, Mayo Clinic, Arizona) was applied at 1:750 dilution in Background Reducing Diluent (Leica). The CD8 antibody (Clone 144B; Dako) was diluted in Bond Diluent (Leica) and used at 1:200. Both antibodies were incubated for 15 minutes.
An experienced gastrointestinal pathologist (T.S.) reviewed each tissue core. Eosinophils and cytotoxic T-cells were semi-quantified in two tumor areas – epithelium and stroma. Using a scoring algorithm developed by Protheroe et al,(30) three sets of eosinophil scores were created: mean and maximum epithelial eosinophil score, stromal eosinophil score and degranulation score. The last score was based on eosinophil peroxidase secretion in the tumor epithelium and stroma areas combined. Mean and maximum scores were associated in a similar fashion with clinicopathological characteristics and survival; therefore, only mean score was used in all the analyses. The scores for eosinophils in stroma and epithelium and complete degranulation ranged from 1 to 4: 1 – non-detected; 2 – mild (1–5 eosinophils. per 0.28 mm2); 3 – moderate (6–10 eosinophils per 0.28 mm2); 4 – strong infiltration (≥10 eosinophils per 0.28 mm2), or complete degranulation (Fig. 2). The same pathologist created the following categories for cytotoxic T-cells in epithelium and stroma: 1 – non-detected; 2 – mild (1–10 cells per 0.28 mm2); 3 – moderate (11–29 cells per 0.28 mm2); 4 – strong infiltration (≥30 cells per 0.28 mm2). The different cut-points for cytotoxic T-cells versus eosinophils reflect the fact that there were more cytotoxic T-cells than eosinophils in most cores. Necrotic areas were not counted. Another characteristic of host response –Crohn's-like lymphoid aggregates around colorectal tumor cells – have been quantified in a recent study by Graham et al.(36)
Figure 2.
Classification of eosinophil peroxidase protein expression in tumor epithelium and stroma of tissue microarray cores in two colorectal cancer patients. The eoinsophil scores were quantified as follows: 1 – non-detected; mild (2 – 1–5 eosinophils per 0.28 mm2); 3 – moderate (6–10 eosinophils per 0.28 mm2); 4 – strong infiltration (≥10 eosinophils per 0.28 mm2).
The data on molecular pathways for these colorectal tumors, including molecular subtypes, have been previously described.(37) Tumors were characterized as microsatellite stable (MSS), MSI high or MSI low; CpG island methylator phenotype (CIMP) high or CIMP low; and positive or negative for BRAF and/or KRAS mutations. Based on these mutations, integrated pathways were assigned: traditional (MSS, CIMP negative, BRAF mutation negative, and KRAS mutation negative), alternate (MSS, CIMP low, BRAF mutation negative, and KRAS mutation positive), serrated (any MSI, CIMP high, BRAF mutation positive, and KRAS mutation negative), or unassigned.
Statistical methods
Subject-specific summary epithelial eosinophil, stromal eosinophil and degranulation scores were calculated by averaging the scores from the multiple tumor cores per subject. For all analyses, these summary scores were then categorized, so that each category included more than 15 colorectal cancer deaths. As a result, 3 categories were created for the epithelial eosinophil (1, >1–<2, and ≥2) and degranulation scores (1, >1–1.5, and >1.5), and 4 categories for the stromal eosinophil score (1, >1–2, >2–3, and >3). A chi-squared test was used to test for differences in eosinophil scores across demographic, lifestyle and clinicopathologic characteristics of women diagnosed with colorectal cancer. To compare five-year all-cause and colorectal cancer survival across eosinophil categories, we used Kaplan–Meier plots and log-rank tests. We utilized Cox proportional hazards regression to estimate hazard ratios for all-cause and colorectal cancer death and 95% confidence intervals during the five-year follow-up after diagnosis. In the analyses of all-cause and colorectal cancer death, participants who died or survived five years were censored. Additionally, in the analysis of colorectal cancer death, participants who died from other causes were censored. We tested the proportional hazards assumption quantitatively by adding an interaction term between follow-up time and each eosinophil score and qualitatively, and the assumption not violated for any eosinophil score. The tests for trend were created for each score by numbering the categories from lowest to highest and fitting a linear term in the Cox regression model.
We created the following three models. Model 1 was adjusted for age at diagnosis. Model 2 was a multivariable-adjusted model that also included SEER stage (in situ or local, regional, or distant), tumor grade (well, moderately, poorly differentiated, lymphomas/not stated), body mass index before colorectal cancer diagnosis (continuous), and smoking history (current, former, or never). The covariates in Model 2 were chosen a priori; we included variables that were associated with all-cause or colorectal cancer survival or with eosinophil levels in previous studies in the Iowa Women’s Health Study. Further adjustment for first course treatment (yes, no or missing for each of surgery, radiation, chemotherapy), integrated pathways of colorectal carcinogenesis (traditional, serrated, alternate and other), colorectal cancer anatomic subsite (colon proximal, colon distal or rectal cancer; the latter two were studied together), alcohol use, physical activity, history of diabetes, hypertension or heart disease did not markedly change the associations, and those variables were not included in the final model. Model 3 was created by adjusting Model 2 for cytotoxic T-cells as follows: the model with epithelial eosinophil score was adjusted for epithelial cytotoxic T-cells score and the model with stromal eosinophil score, for stromal cytotoxic T-cells score.
Further, we conducted several additional analyses. We studied the association between eosinophil score and non- colorectal cancer mortality. Since AJCC-TNM stage is most commonly used in clinical practice, we also (1) examined the association between AJCC-TNM stage and eosinophil score and (2) stratified the observed associations by AJCC-TNM stage. Of note, we used the algorithm previously created for deriving AJCC-TNM stage in colorectal tumors in the Iowa Women’s Health Study. It was based on the information provided by SEER: tumor extension and size, the number of lymph nodes examined, and the number of positive lymph nodes. In addition, we analyzed all-cause and colorectal cancer survival in relation to the combined eosinophil score created as a sum of ordinal epithelial and stromal scores, which was further categorized into 4 groups (≤2.5, >2.5–3, >3–4, >4). Also, we repeated all analyses for the follow-up until 2011 (“total follow-up”). To test for a potential selection bias, we conducted a sensitivity analysis by creating an additional category for those subjects missing eosinophil data and re-ran the Cox regression models for each eosinophil score. All analyses were carried out with the SAS (release 9.3) and all statistical tests were 2-sided.
RESULTS
After excluding any cancer before colorectal cancer diagnosis (n=146) and the participants with less than one day of follow-up time (n=6), our analytical cohort included 580 women diagnosed with colorectal cancer. Among them, 441 colorectal cancer cases had high-quality, usable cores and were included in the analyses.
Colorectal cancer cases were 56–89 years at diagnosis (mean was 73 years); 32.4% had in situ or localized disease (among them, there were 3 in situ cases (0.5%)), 41.0% had regional spread, 13.2% had distant spread, and 13.4% had an unspecified stage of disease. During the five-year follow-up after diagnosis, 121 women died from colorectal cancer as an underlying cause (27%), 51 (12%) died from other causes, and 269 women were alive (61%), whereas during the total follow-up (median=8.7 years, max=25 years), 138 women died from colorectal cancer (31%), 161 (37%) died from other causes, and 142 (32%) were alive in 2011.
The three eosinophil scores were interrelated (Spearman correlation coefficients ρ=0.46–0.56); while the correlation between stromal eosinophil and cytotoxic T-cells scores was ρ=0.29, and between epithelial eosinophil and cytotoxic T-cells scores, ρ=0.23. The distribution of participants' characteristics across categories of stromal and epithelial eosinophil scores are shown in Table 1, and the data for degranulation score are presented in Supplementary Table 2. Older patients (≥72 years at diagnosis) and those with lower cytotoxic T-cells score were more likely to have lower eosinophil scores and weaker degranulation. Patients with higher stromal eosinophil score tended to have lower SEER stage and were less likely to have proximal colon cancer, while the epithelial eosinophil score was positively associated with hypertension (Table 1). An inverse association was also observed between stromal eosinophil score and AJCC-TNM stage (P for chi-square test=0.04). There was no association between Crohn's-like lymphoid aggregates and any eosinophil score.
Table 1.
Characteristics of women diagnosed with colorectal cancer across tumor stromal and epithelial eosinophil scoresa.
| Participant’s characteristics | Stromal eosinophilscore | Epithelial eosinophil score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 n=80 |
>1 – 2 n=164 |
>2 – 3 n=111 |
>3 n=86 |
P-valuef | 1 n=250 |
>1 – <2 n=114 |
≥2 n=77 |
P-valuef | |
| Age at colorectal cancer diagnosis | |||||||||
| ≤ 71 y | 21.3 | 26.8 | 36.1 | 40.7 | 25.6 | 34.2 | 42.9 | ||
| 72–77 y | 37.5 | 45.7 | 35.1 | 37.3 | 42.4 | 40.4 | 31.2 | ||
| >77 y | 41.3 | 27.4 | 28.8 | 22.1 | 0.02 | 32.0 | 25.4 | 26.0 | 0.05 |
| BMIb | |||||||||
| <25 kg/m2 | 27.5 | 37.2 | 36.0 | 46.5 | 36.8 | 35.1 | 40.3 | ||
| 25–30 kg/m2 | 37.5 | 39.0 | 33.3 | 25.6 | 36.8 | 31.6 | 33.8 | ||
| >30 kg/m2 | 35.0 | 23.8 | 30.6 | 27.9 | 0.13 | 26.4 | 33.3 | 26.0 | 0.65 |
| Smokingc | |||||||||
| Current | 12.7 | 14.7 | 13.6 | 15.7 | 13.8 | 13.1 | 17.3 | ||
| Former | 16.5 | 23.9 | 21.8 | 16.9 | 20.7 | 21.1 | 20.0 | ||
| Never | 70.9 | 61.4 | 64.6 | 67.5 | 0.76 | 65.5 | 65.8 | 62.7 | 0.95 |
| History of diabetesb | |||||||||
| Yes | 13.8 | 12.8 | 17.1 | 17.4 | 0.68 | 21.6 | 15.8 | 16.9 | 0.36 |
| History of hypertensionb | |||||||||
| Yes | 48.8 | 43.9 | 50.5 | 52.3 | 0.57 | 45.6 | 43.9 | 62.3 | 0.02 |
| History of heart diseaseb | |||||||||
| Yes | 25.0 | 15.9 | 24.3 | 14.0 | 0.10 | 21.6 | 15.8 | 16.9 | 0.36 |
| Stage at diagnosis | |||||||||
| In situ or local | 20.0 | 30.5 | 33.3 | 45.4 | 28.6 | 41.2 | 32.4 | ||
| Regional | 48.8 | 46.3 | 35.1 | 32.6 | 46.4 | 29.0 | 41.6 | ||
| Distant | 20.0 | 12.2 | 13.5 | 8.1 | 13.5 | 12.3 | 13.0 | ||
| Unknown | 11.3 | 11.0 | 18.0 | 14.0 | 0.03 | 11.5 | 17.5 | 13.0 | 0.12 |
| Grade | |||||||||
| Well differentiated | 3.8 | 4.9 | 6.3 | 7.0 | 5.2 | 6.1 | 5.2 | ||
| Moderately differentiated | 50.0 | 61.6 | 66.7 | 62.8 | 58.4 | 64.9 | 63.6 | ||
| Poorly differentiated | 41.3 | 29.3 | 23.4 | 24.4 | 31.2 | 24.6 | 28.6 | ||
| Lymphomas/ not stated | 5.0 | 4.3 | 3.6 | 5.8 | 0.37 | 5.2 | 4.4 | 2.6 | 0.84 |
| Colorectal cancer site | |||||||||
| Colon proximal | 63.8 | 60.4 | 52.3 | 43.0 | 56.0 | 53.5 | 58.4 | ||
| Colon distal/rectal | 33.8 | 37.8 | 47.8 | 55.8 | 42.4 | 44.8 | 41.6 | ||
| Unspecified | 2.5 | 1.8 | 0 | 1.2 | 0.04 | 1.6 | 1.7 | 0 | 0.80 |
| Integrated pathway | |||||||||
| Traditional | 33.9 | 33.8 | 37.1 | 50.8 | 35.0 | 44.4 | 39.7 | ||
| Alternate | 6.8 | 13.2 | 7.9 | 15.9 | 9.4 | 14.8 | 12.7 | ||
| Serrated | 32.2 | 25.7 | 28.1 | 17.5 | 26.1 | 22.2 | 30.2 | ||
| Unassigned | 27.1 | 27.2 | 27.0 | 15.9 | 0.18 | 29.6 | 18.5 | 17.5 | 0.20 |
| Surgeryd | |||||||||
| No | 43.8 | 32.9 | 39.6 | 29.1 | 36.8 | 36.8 | 31.2 | ||
| Yes | 56.3 | 67.1 | 60.4 | 70.9 | 0.16 | 63.2 | 63.2 | 68.8 | 0.64 |
| Chemotherapyd | |||||||||
| No | 73.7 | 78.1 | 78.4 | 81.4 | 78.8 | 79.0 | 74.0 | ||
| Yes | 26.3 | 21.9 | 21.6 | 18.6 | 0.70 | 21.2 | 21.1 | 26.0 | 0.65 |
| Radiationd | |||||||||
| No | 96.1 | 97.6 | 95.4 | 97.6 | 96.3 | 96.4 | 98.7 | ||
| Yes | 3.9 | 2.4 | 4.6 | 2.4 | 0.25 | 3.7 | 3.6 | 1.3 | 0.59 |
| Cytotoxic T cells categoriese | |||||||||
| 1 | 24.9 | 10.5 | 10.4 | ||||||
| >1 – 2 | 37.9 | 27.7 | 13.8 | 14.0 | 42.9 | 38.6 | 29.9 | ||
| >2 – 3 | 38.0 | 41.4 | 34.0 | 29.1 | 14.7 | 29.8 | 24.7 | ||
| >3 | 24.1 | 30.9 | 52.3 | 57.0 | 0.0001 | 17.6 | 21.1 | 35.1 | <0.0001 |
Epithelial and stromal eosinophil scores were assessed in epithelial and stromal areas of tumor, respectively.
Value at the colorectal cancer diagnosis.
Value at baseline (in 1986).
Initial course of therapy.
Cytotoxic T-cells (or CD8+ T cells) scores in epithelium and stroma were cross-tabulated with corresponding eosinophil scores in epithelium and stroma. The categories in stromal cytotoxic T-cells 1 and >1–2 were combined because of a very low frequency of stromal cytotoxic T-cells in category=1.
This is based on a chi-square test.
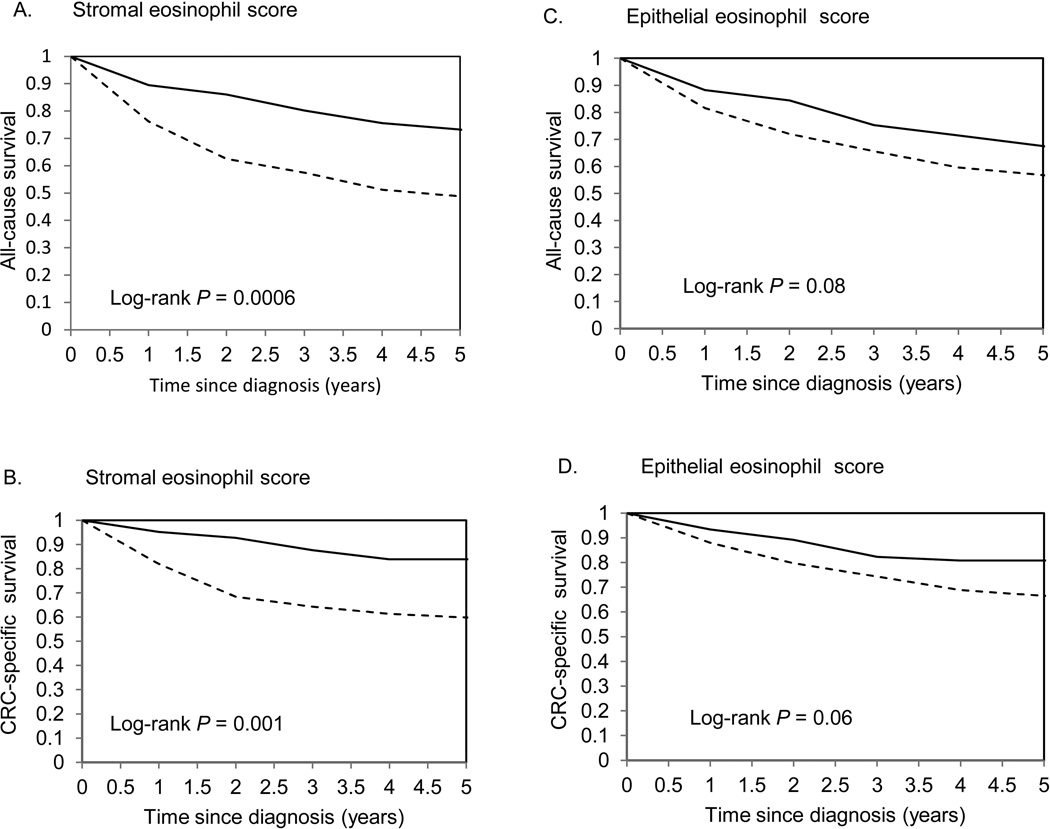
We tested the hypothesis that eosinophil score was associated with patient survival comparing the highest versus lowest eosinophil score categories (Fig. 3). In a univariable analysis, all-cause and colorectal cancer five-year survival was significantly better for colorectal cancer patients with the highest vs lowest stromal eosinophil scores (log-rank P =0.0006 and 0.001, respectively, in Fig. 3A, B). For epithelial eosinophil score, colorectal cancer patients with the highest score tended to have better all-cause and cancer-specific survival but the difference was not statistically significant (Fig. 3C, D). In a multivariable analysis, adding each of the confounders –age at diagnosis, BMI, smoking status, stage and grade at diagnosis – slightly attenuated the observed associations; the strongest attenuation was found for age and stage at diagnosis. In Model 2, the highest category of stromal eosinophils was associated with a 39% decrease in risk of all-cause five-year death (P-trend =0.02) and a 52% decrease in risk of colorectal cancer death (P-trend =0.01) compared to the lowest category (Table 2). After stratification by AJCC-TNM stage, the inverse associations were observed in Stages 2 and 3, but the association was not statistically significant for Stage 2, although the power was limited. To increase power, we combined colorectal cancer patients in Stages 2 and 3: hazard ratio was 0.30 (95% confidence interval: 0.08–1.07, P-trend =0.01) for the highest versus lowest eosinophil score. The associations of stromal eosinophil score with all-cause and colorectal cancer death remained during the total follow-up: for the highest versus lowest category, hazard ratios (95% confidence intervals) were 0.72 (0.48–1.08, P-trend =0.04) and 0.61 (0.34–0.12, P-trend =0.04), respectively (Table 2). Of note, stromal eosinophil score was not associated with non- colorectal cancer death during 5-year or total follow-up.
Figure 3.
Kaplan-Meier survival curves for the highest versus lowest category of tumor eosinophil scores. P-values for log-rank test were calculated across all categories.
Table 2.
Hazard ratios for all-cause and colorectal cancer-specific death and 95% confidence intervals in relation to stromal eosinophil scorea in the colorectal cancer patients, Iowa Women’s Health Study.
| Stromal eosinophil score | 1 | >1 – 2 | >2 – 3 | >3 | P-trend | |
|---|---|---|---|---|---|---|
| All-cause death | ||||||
| Five-year follow-up | ||||||
| No. deaths (n=172) | 41 | 74 | 34 | 23 | ||
| Person-years | 254 | 587 | 445 | 358 | ||
| HR (95% CI)b | Reference | 0.83 (0.56–1.21) | 0.51 (0.32–1.21) | 0.44 (0.26–0.73) | 0.0001 | |
| HR (95% CI)c | Reference | 0.97 (0.65–1.44) | 0.68 (0.42–1.08) | 0.61 (0.36–1.02) | 0.02 | |
| HR (95% CI)d | Reference | 0.97 (0.65–1.44) | 0.77 (0.47–1.25) | 0.69 (0.40–1.19) | 0.11 | |
| Total follow-upe | ||||||
| No. deaths (n=299) | 56 | 123 | 72 | 48 | ||
| Person-years | 523 | 1267 | 998 | 892 | ||
| HR (95% CI)b | Reference | 0.99 (0.72–1.36) | 0.74 (0.52–1.05) | 0.59 (0.40–0.87) | 0.0009 | |
| HR (95% CI)c | Reference | 1.08 (0.78–1.50) | 0.88 (0.61–1.27) | 0.72 (0.48–1.08) | 0.04 | |
| HR (95% CI)d | Reference | 1.07 (0.77–1.50) | 0.95 (0.65–1.38) | 0.79 (0.52–1.20) | 0.18 | |
| Colorectal cancer death | ||||||
| Five-year follow-up | ||||||
| No. deaths (n=121) | 30 | 53 | 25 | 13 | ||
| Person-years | 254 | 587 | 445 | 358 | ||
| HR (95% CI)b | Reference | 0.77 (0.49–1.21) | 0.49 (0.29–0.84) | 0.32 (0.17–0.62) | <.0001 | |
| HR (95% CI)c | Reference | 0.96 (0.60–1.52) | 0.70 (0.41–1.22) | 0.48 (0.24–0.93) | 0.01 | |
| HR (95% CI)d | Reference | 0.97 (0.61–1.55) | 0.84 (0.47–1.49) | 0.57 (0.29–1.15) | 0.11 | |
| Total follow-upe | ||||||
| No. deaths (n=138) | 30 | 61 | 29 | 18 | ||
| Person-years | 523 | 1267 | 998 | 892 | ||
| HR (95% CI)b | Reference | 0.88 (0.57–1.37) | 0.56 (0.34–0.94) | 0.43 (0.24–0.77) | 0.0005 | |
| HR (95% CI)c | Reference | 1.05 (0.67–1.64) | 0.76 (0.45–1.29) | 0.61 (0.34–1.12) | 0.04 | |
| HR (95% CI)d | Reference | 1.07 (0.68–1.68) | 0.92 (0.53–1.59) | 0.75 (0.40–1.41) | 0.31 | |
Stromal eosinophil score was assessed in stromal area of tumor.
Model 1: Adjusted for age.
Model 2: Model 1 + BMI, smoking status, stage and grade at diagnosis.
Model 3: Model 2 + cytotoxic T-cells (also called CD8+) score.
Total follow- up lasted from the date of diagnosis until death or the end of this study in 2011.
Similarly, the highest category of epithelial eosinophils was associated with decreased all-cause and colorectal cancer death during the 5-year and total follow-up periods, but did not reach statistical significance in multivariable models (Table 3). Considering the combined effect of epithelial and stromal eosinophils, there were inverse associations of the highest eosinophil with both all-cause and colorectal cancer five-year death (hazard ratios (95% confidence intervals) were 0.62 (0.41–0.93, P-trend =0.01) and 0.54 (0.32–0.90, P-trend =0.01), respectively, that mirrored associations for stromal eosinophil score (Supplementary Table 3).
Table 3.
Hazard ratios for all-cause and colorectal cancer death and 95% confidence intervals in relation to epithelial eosinophil scorea in the colorectal cancer patients, Iowa Women’s Health Study.
| Epithelial eosinophil score | 1 | >1 – <2 | ≥2 | P-trend | |
|---|---|---|---|---|---|
| All-cause death | |||||
| Five-year follow-up | |||||
| No. deaths (n=172) | 108 | 39 | 25 | ||
| Person-years | 884 | 450 | 309 | ||
| HR (95% CI)b | Reference | 0.74 (0.51–1.06) | 0.69 (0.44–1.06) | 0.04 | |
| HR (95% CI)c | Reference | 0.91 (0.63–1.33) | 0.74 (0.48–1.15) | 0.18 | |
| HR (95% CI)d | Reference | 0.95 (0.65–1.38) | 0.82 (0.52–1.29) | 0.40 | |
| Total follow-upe | |||||
| No. deaths (n=299) | 180 | 74 | 45 | ||
| Person-years | 1899 | 1039 | 739 | ||
| HR (95% CI)b | Reference | 0.81 (0.61–1.06) | 0.68 (0.49–0.95) | 0.01 | |
| HR (95% CI)c | Reference | 0.92 (0.69–1.22) | 0.74 (0.53–1.03) | 0.08 | |
| HR (95% CI)d | Reference | 0.92 (0.69–1.22) | 0.79 (0.56–1.12) | 0.22 | |
| Colorectal cancer death | |||||
| Five-year follow-up | |||||
| No. deaths (n=121) | 77 | 30 | 14 | ||
| Person-years | 884 | 450 | 309 | ||
| HR (95% CI)b | Reference | 0.78 (0.51–1.19) | 0.53 (0.30–0.94) | 0.02 | |
| HR (95% CI)c | Reference | 1.03 (0.67–1.58) | 0.58 (0.33–1.03) | 0.11 | |
| HR (95% CI)d | Reference | 1.08 (0.70–1.67) | 0.70 (0.39–1.26) | 0.36 | |
| Total follow-upe | |||||
| No. deaths (n=138) | 85 | 33 | 20 | ||
| Person-years | 1899 | 1039 | 739 | ||
| HR (95% CI)b | Reference | 0.76 (0.50–1.14) | 0.67 (0.41–1.09) | 0.07 | |
| HR (95% CI)c | Reference | 0.98 (0.65–1.48) | 0.74 (0.45–1.20) | 0.27 | |
| HR (95% CI)d | Reference | 1.02 (0.67–1.54) | 0.88 (0.53–1.46) | 0.69 | |
Epithelial eosinophil score was assessed in epithelial area of tumor.
Model 1: Adjusted for age.
Model 2: Adjusted for age of diagnosis, BMI, smoking status, stage and grade at diagnosis.
Model 3: Model 2 + cytotoxic T-cells (also called CD8+) score.
Total follow- up lasted from the date of diagnosis through death or the end of this study in 2011.
The pattern of degranulation was not related to the survival of colorectal cancer patients (Supplementary Table 3). No interactions were observed between eosinophil scores and age, BMI, smoking, stage, grade, surgery, chemotherapy, radiation, or integrated pathway of colorectal carcinogenesis (all P-values were >0.18). The results of the analysis for stromal eosinophil score stratified by tumor location are presented in Supplementary Table 4. The hazard ratios were decreased in both groups: among women with proximal cancer and among those with distal colon or rectal cancer; however, only for those with distal colon or rectal cancer the trends were statistically significant for all-cause and colorectal cancer five-year death P-trend =0.05 and P-trend =0.03, respectively).
After the adjustment for cytotoxic T-cells, the strength of associations for both eosinophil scores attenuated for all-cause and colorectal cancer death (Table 2). There was no interaction between any eosinophil score and cytotoxic T-cells score in relation to any death (all Pinteraction ≥0.45). Finally, there was no association between the category for missing eosinophils and all-cause or colorectal cancer survival, implying that the data were missing at random.
DISCUSSION
Among 441 older colorectal cancer patients in the Iowa Women’s Health Study, higher eosinophil score in the tumor stroma was associated with a statistically significant decreased risk of all-cause and colorectal cancer five-year death by 39% and 52%, respectively for the highest versus lowest category. The significant associations remained for both all-cause and colorectal cancer death during the total follow-up through 2011. Additionally, higher eosinophil score in the tumor stroma was inversely associated with SEER and AJCC-TNM stages at diagnosis, suggesting that stromal eosinophils may participate in inhibiting colorectal cancer progression.(27) Similar, but non-significant results were observed for elevated eosinophils in the tumor epithelium.
Our finding of an inverse correlation between eosinophil infiltration and stage in this large cohort is consistent with the findings from several smaller studies that assessed eosinophil accumulation across the colorectal cancer progression continuum (Supplementary Table 1).(21–23, 27) Also consistent with our results, several studies reported better survival of colorectal cancer patients with eosinophil accumulation in the tumor.(24, 25, 28) A study of 67 patients by Pretlow et al. [1983] found that eosinophil count >30 versus ≤30 eosinophils/mm2 was associated with better all-cause survival (P =0.028) including those patients without metastases (P =0.04), but that study did not control for other patients’ characteristics.(26) Likewise, another small study of 126 colorectal cancer patients in Spain observed a stronger eosinophil infiltration among those with better 5-year recurrence-free and all-cause survival independently of age, stage, grade, p53 expression, vascular invasion, and vascularization.(24)
Also in line with our results, a large Dutch study of 1416 rectal cancer patients reported a significantly better all-cause survival (P <0.007) and lower number of distant metastases (P <0.03) in those with many versus few peritumoral eosinophils, i.e. eosinophils located in the boundary zone between tumor and normal tissue.(22) However, not all studies of rectal cancer were consistent: Fisher et al. [1989] found that higher eosinophil count was associated with improved all-cause survival only before adjusting for stage.(27) Due to limited power, we were not able to separately examine the survival of rectal cancer patients; however, we found significantly decreased hazard ratios for all-cause and colorectal cancer five-year death associated with stromal eosinophil score in the combined group of patients with distal colon or rectal cancer.
One limitation of our study was the use of tissue microarrays, making it impossible to investigate peritumoral eosinophils. In addition to the Dutch study,(22) two other studies highlighted the importance of peritumoral eosinophils in the survival of colorectal cancer patients.(27) A Danish study of 584 colorectal cancer patients found a 41% decreased risk of all-cause death for the highest versus lowest category of peritumoral eosinophils in a multivariable model.(25) Similarly, a recent study by Harbaum et al [2015] reported a 25% lower hazard ratio for progression-free death and a 30% lower hazard ratio was for colorectal cancer death among those with high versus low peritumoral eosinophil count, while the association with intratumoral eosinophils was not statistically significant after adjusting for overall inflammatory cell response, stage, tumor invasion, and tumor budding.(28)
While we were not able to study peritumoral eosinophils, we did assess eosinophils in epithelial and stromal tumor areas separately, and found that only tumor stromal eosinophil score was significantly associated with both colorectal cancer and all-cause survival. To our knowledge, no other study examined eosinophil infiltration of epithelial and stromal tumor separately. Importantly, for colorectal cancer death, the associations with tumor stromal eosinophil score in our study were similar (but stronger) to the associations with peritumoral eosinophil count observed by Harbaum et al. (hazard ratio was 0.7; 95% confidence interval, 0.52–0.93; P =0.01)(28); these findings are consistent with the view that eosinophils play a beneficial role in response to colorectal cancer.
A protective role of eosinophils in carcinogenesis is supported by many animal(38–42) and in vitro studies(12, 40, 43), although the mechanisms underlying this association have not been established. One of the potential mechanisms is the cytotoxic effect of eosinophils, i.e., a direct killing via degranulation and release of their granule contents such as eosinophil specific proteins (major basic protein, eosinophil cationic protein, eosinophil peroxidase, and eosinophil-derived neurotoxin), perforins, and granzymes.(10, 11) A mouse study of B-cell lymphoma suggested that eosinophils employ perforin and granzyme-B to kill tumors,(40) and another mouse study of a melanoma resistant for cytotoxic T-cells demonstrated that the degranulation of eosinophils leads to the regression of lung and visceral metastases.(42) Consistent with animal studies, an in vitro study of a colon cancer cell line (Colo-205) showed that, upon degranulation, eosinophils adhere to cancer cells and induce apoptosis via secreting eosinophil-derived neurotoxin, eosinophil peroxidase, granzymes A, and tumor necrosis factor alpha.(12) Also, indirect support for this mechanism comes from human studies, in which degranulated eosinophils were detected in tumors after successful immunotherapy with cytokines – interleukin-2 and interleukin-4, suggesting that degranulated eosinophils participated in the tumor killing.(44, 45)
In our study, we did not observe an association of eosinophil degranulation score with the survival of colorectal cancer patients or their stage at diagnosis. The absence of association could be explained in two ways (1) eosinophil degranulation is actually not involved in killing tumor cells or (2) most of the released granules originated not from activated but from dying eosinophils that do not participate in the cytotoxic mechanisms towards tumor cells.(12, 46) Alternatively, there could be a random measurement error in quantifying degranulated eosinophils that moved the association towards null.
There is emerging evidence that eosinophils may exert their anti-tumor effect not only through their cytotoxicity, but also via immunomodulatory mechanisms: eosinophils may act through the secretion of T-cell cytokines, activation of dendritic cells or through antigen presentation to T-cells.(6, 8, 10) A recent murine study of melanoma reported that eosinophils were involved in tumor rejection via repolarizing macrophages towards the M1 phenotype, normalizing tumor vasculature and attracting cytotoxic T-cells into tumors, while depletion of eosinophils resulted in a decreased number of activated cytotoxic T-cells in the tumor and reduced mouse survival.(13) In our study, after additional adjustment for cytotoxic T-cells, the hazard ratios associated with stromal and epithelial eosinophil scores were slightly attenuated for both all-cause and colorectal cancer death, suggesting that eosinophils may act partially via cytotoxic T-cells.
The strength of our study is that we used a large prospective Iowa Women’s Health Study linked to SEER that had very accurate ascertainment of cancer and underlying cause of death, almost complete information on follow-up(32) and detailed information about potential confounders from Iowa Women’s Health Study and SEER. Another major strength of our study is that we utilized a very sensitive, specific, and validated anti-eosinophil peroxidase antibody for eosinophil immunostaining,(30, 31) while all the previous studies used standard hematoxylin and eosin or Giemsa staining. An additional strength of our study is that we were able to account for molecular pathways identified in previous studies in the Iowa Women’s Health Study.(37).
Although using tissue microarrays precluded studying spatial distribution, it allowed us to conduct immunostaining under very similar conditions for all specimens. In addition, to account for within-tumor heterogeneity, we obtained three tissue cores from each case and averaged the scores across the cores. A limitation of our study is that only a subset of the Iowa Women’s Health Study participants diagnosed with colorectal cancer had tissues retrieved (58%) and had usable cores after immunostaining (441/580*100%=76%). However, the analyses in the earlier studies in the Iowa Women’s Health Study demonstrated that participants’ demographics, exposure patterns, and tumor characteristics did not differ significantly between colorectal cancer cases with retrieved versus non-retrieved tissue specimens.(37) Further, our analysis showed that the survival of colorectal cancer patients with missing eosinophil scores was not statistically different from the survival of the whole cohort. Hence, we have no evidence that there was selection bias. In addition, we had limited statistical power to conduct subgroup analysis by stage, grade, colorectal cancer subtype or molecular pathways. Finally, our population included only post-menopausal, predominantly Caucasian women, which may limit our ability to infer beyond individuals with these characteristics. Although the fact that we see similar results compared to other studies alleviates this concern.
Our study differed from all the other studies in terms of eosinophil staining (anti-eosinophil peroxidase staining in ours versus hematoxylin and eosin or Giemsa staining in the others), type of tumor slides (tissue microarrays versus full slides, respectively), methods for eosinophil quantification, and included confounders. Despite these variations, we corroborated the previous results that the infiltration with eosinophils is associated with improved survival of colorectal cancer patients and showed this association is independent of clinicopathologic, lifestyle factors, or integrated molecular pathways. Our study suggests that eosinophil infiltration should be further investigated as an independent prognostic marker in colorectal cancer patients. However, before eosinophil infiltration is considered as prognostic marker in clinical practice, it is necessary to develop a standardized, easily reproducible approach for quantifying eosinophil infiltrate in the stroma.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health (NIH) Grant R01 CA039742, R01 CA107333 (Limburg, PI), HHSN261201000032C (contract awarded to the University of Iowa). Anna Prizment was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. Anti- eosinophil peroxidase antibody was produced in the lab of James Lee (Mayo Clinic, Scottsdale). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
P.J. Limburg has received other commercial research support from Olympus America, Fujinon, Boston Scientific, Bayer Healthcare, BENEO-Orafti, Astra-Zeneca, Thorne Research, and Ironwood Pharmaceuticals; honoraria from Imedex; and royalties from Exact Sciences.
Footnotes
Supplementary information is available at Modern Pathology’s website at the end of the article and before the references.
DISCLOSURE/CONFLICT OF INTEREST: No potential conflicts of interest were disclosed by the other authors.
References
- 1.Svrcek Chaput, N, Aupérin M, Locher A, Drusch C, Malka F, Taïeb D, et al. Tumour-infiltrating CD68+ and CD57+ cells predict patient outcome in stage II–III colorectal cancer. Br J Cancer. 2013;109:1013–1022. doi: 10.1038/bjc.2013.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shunyakov L, Ryan CK, Sahasrabudhe DM, Khorana AA. The influence of host response on colorectal cancer prognosis. Clin Colorectal Cancer. 2004;4:38–45. doi: 10.3816/ccc.2004.n.008. [DOI] [PubMed] [Google Scholar]
- 3.Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: Cohort study and literature review. J Pathol. 2010;222:350–366. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 5.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, Th2, Treg, Th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 6.Ellyard J, Simson L, Parish C. Th2-mediated anti-tumour immunity: Friend or foe? Tissue Antigens. 2007;70:1–11. doi: 10.1111/j.1399-0039.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- 7.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, et al. Eosinophils: Biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 8.Kita H. Eosinophils: Multifaceted biological properties and roles in health and disease. Immunol Rev. 2011;242:161–177. doi: 10.1111/j.1600-065X.2011.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: The LIAR hypothesis. Clin Exp Allergy. 2010;40:563–575. doi: 10.1111/j.1365-2222.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotfi R, Lee JJ, Lotze MT. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): Role in the inflammatory response within tumors. J Immunother. 2007;30:16–28. doi: 10.1097/01.cji.0000211324.53396.f6. [DOI] [PubMed] [Google Scholar]
- 11.Venge R, Byström J, Carlson M, Håkansson L, Karawacjzyk M, Peterson C, et al. Eosinophil cationic protein (ECP): Molecular and biological properties and the use of ECP as a marker of eosinophil activation in disease. Clin Exp Allergy. 1999;29:1172–1186. doi: 10.1046/j.1365-2222.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 12.Legrand F, Driss V, Delbeke M, Loiseau S, Hermann E, Dombrowicz D, et al. Human eosinophils exert TNF-α and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J Immunol. 2010;185:7443–7451. doi: 10.4049/jimmunol.1000446. [DOI] [PubMed] [Google Scholar]
- 13.Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hämmerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8 T cells. Nat Immunol. 2015;16:609–617. doi: 10.1038/ni.3159. [DOI] [PubMed] [Google Scholar]
- 14.Gatault S, Legrand F, Delbeke M, Loiseau S, Capron M. Involvement of eosinophils in the anti-tumor response. Cancer Immunol Immunother. 2012;61:1527–1534. doi: 10.1007/s00262-012-1288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis BP, Rothenberg ME. Eosinophils and cancer. Cancer Immunol Res. 2014;2:1–8. doi: 10.1158/2326-6066.CIR-13-0196. [DOI] [PubMed] [Google Scholar]
- 16.Van Driel WJ, Kievit-Tyson P, van den Broek, Lambert CJM, Zwinderman AH, Trimbos BJ, et al. Presence of an eosinophilic infiltrate in cervical squamous carcinoma results from a type 2 immune response. Gynecol Oncol. 1999;74:188–195. doi: 10.1006/gyno.1999.5431. [DOI] [PubMed] [Google Scholar]
- 17.Pinto A, Aldinucci D, Gloghini A, Zagonel V, Degan M, Perin V, et al. The role of eosinophils in the pathobiology of Hodgkin's disease. Ann Oncol. 1997;8(Suppl 2):89–96. [PubMed] [Google Scholar]
- 18.Iwasaki K, Torisu M, Fujimura T. Malignant tumor and eosinophils. I. prognostic significance in gastric cancer. Cancer. 1986;58:1321–1327. doi: 10.1002/1097-0142(19860915)58:6<1321::aid-cncr2820580623>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Ishibashi S, Ohashi Y, Suzuki T, Miyazaki S, Moriya T, Satomi S, et al. Tumor-associated tissue eosinophilia in human esophageal squamous cell carcinoma. Anticancer Res. 2006;26:1419–1424. [PubMed] [Google Scholar]
- 20.Martinelli-Kly CP, Mendis BR, Lombardi T. Eosinophils and oral squamous cell carcinoma: A short review. J Oncol. 2009;2009:310132. doi: 10.1155/2009/310132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moezzi J, Gopalswamy N, Haas RJ, Markert RJ, Suryaprasad S, Bhutani MS. Stromal eosinophilia in colonic epithelial neoplasms. Am J Gastroenterol. 2000;95:520–523. doi: 10.1111/j.1572-0241.2000.01778.x. [DOI] [PubMed] [Google Scholar]
- 22.Nagtegaal I, Marijnen C, Kranenbarg EK, Mulder-Stapel A, Hermans J, van de Velde C, et al. Local and distant recurrences in rectal cancer patients are predicted by the nonspecific immune response; specific immune response has only a systemic effect-a histopathological and immunohistochemical study. BMC Cancer. 2001;1:7. doi: 10.1186/1471-2407-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiziltaş S, Ramadan SS, Topuzoğlu A, Kullu S. Does the severity of tissue eosinophilia of colonic neoplasms reflect their malignancy potential? Turk J Gastroenterol. 2008;19:239–244. [PubMed] [Google Scholar]
- 24.Fernandez-Aceero MJ, Galindo-Gallego M, Sanz J, Aljama A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer. 2000;88:1544–1548. [PubMed] [Google Scholar]
- 25.Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brunner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol. 1999;189:487–495. doi: 10.1002/(SICI)1096-9896(199912)189:4<487::AID-PATH484>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 26.Pretlow TP, Keith EF, Cryar AK, Bartolucci AA, Pitts AM, Pretlow TG, et al. Eosinophil infiltration of human colonic carcinomas as a prognostic indicator. Cancer Res. 1983;43:2997–3000. [PubMed] [Google Scholar]
- 27.Fisher ER, Paik S, Rockette H, Jones J, Caplan R, Fisher B. Prognostic significance of eosinophils and mast cells in rectal cancer: Findings from the national surgical adjuvant breast and bowel project (protocol R-01) Hum Pathol. 1989;20:159–163. doi: 10.1016/0046-8177(89)90180-9. [DOI] [PubMed] [Google Scholar]
- 28.Harbaum L, Pollheimer MJ, Kornprat P, Lindtner RA, Bokemeyer C, Langner C. Peritumoral eosinophils predict recurrence in colorectal cancer. Mod Pathol. 2015;28:403–413. doi: 10.1038/modpathol.2014.104. [DOI] [PubMed] [Google Scholar]
- 29.Willetts L, Parker K, Wesselius L, Protheroe C, Jaben E, Graziano P, et al. Immunodetection of occult eosinophils in lung tissue biopsies may help predict survival in acute lung injury. Respir Res. 2011;12:116. doi: 10.1186/1465-9921-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Protheroe C, Woodruff SA, de Petris G, et al. A novel histologic scoring system to evaluate mucosal biopsies from patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009;7:749.e11–755.e11. doi: 10.1016/j.cgh.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nunez-Nateras R, Castle EP, Protheroe CA, Stanton ML, Ocal TI, Ferrigni EN, et al. Predicting response to bacillus Calmette-Guérin (BCG) in patients with carcinoma in situ of the bladder. Urol Oncol. 2014;32:45.e23–45.e30. doi: 10.1016/j.urolonc.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S, Folsom AR, Sellers TA, Kushi LH, Potter JD. Better breast cancer survival for postmenopausal women who are less overweight and eat less fat. The Iowa Women's Health Study. Cancer. 1995;76:275–283. doi: 10.1002/1097-0142(19950715)76:2<275::aid-cncr2820760218>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Folsom AR, Kushi LH, Anderson KE, Mink PJ, Olson JE, Hong CP, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women's Health Study. Arch Intern Med. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 34.Lee JE, Baba Y, Ng K, Giovannucci E, Fuchs CS, Ogino S, et al. Statin use and colorectal cancer risk according to molecular subtypes in two large prospective cohort studies. Cancer Prev Res. 2011;4:1808–1815. doi: 10.1158/1940-6207.CAPR-11-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schernhammer ES, Giovannucci E, Baba Y, Fuchs CS, Ogino S. B vitamins, methionine and alcohol intake and risk of colon cancer in relation to BRAF mutation and CpG island methylator phenotype (CIMP) PLoS One. 2011;6:e21102. doi: 10.1371/journal.pone.0021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graham RP, Vierkant RA, Tillmans LS, Wang AH, Laird PW, Weisenberger DJ, et al. Tumor budding in colorectal carcinoma: Confirmation of prognostic significance and histologic cutoff in a population-based cohort. Am J Surg Pathol. 2015;39:1340–1346. doi: 10.1097/PAS.0000000000000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Limsui D, Vierkant RA, Tillmans LS, Wang AH, Weisenberger DJ, Laird PW, et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst. 2010;102:1012–1022. doi: 10.1093/jnci/djq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodruff SA, Masterson JC, Fillon S, Robinson ZD, Furuta GT. Role of eosinophils in inflammatory bowel and gastrointestinal diseases. J Pediatr Gastroenterol Nutr. 2011;52:650–661. doi: 10.1097/MPG.0b013e3182128512. [DOI] [PubMed] [Google Scholar]
- 39.Tepper RI, Coffman RL, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;257:548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 40.Costain DJ, Guha AK, Liwski RS, Lee TDG. Murine hypodense eosinophils induce tumour cell apoptosis by a granzyme B-dependent mechanism. Cancer Immunol Immunother. 2001;50:293–299. doi: 10.1007/PL00006690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simson L, Ellyard JI, Dent LA, Matthaei KI, Rothenberg ME, Foster PS, et al. Regulation of carcinogenesis by IL-5 and CCL11: A potential role for eosinophils in tumor immune surveillance. J Immunol. 2007;178:4222–4229. doi: 10.4049/jimmunol.178.7.4222. [DOI] [PubMed] [Google Scholar]
- 42.Mattes J, Hulett M, Xie W, Hogan S, Rothenberg ME, Foster P, et al. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: An eotaxin and STAT6-dependent process. J Exp Med. 2003;197:387–393. doi: 10.1084/jem.20021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clarke CA, Smith MA, Laniyan I, Vaughn TR, Parish-Gause D, Green W, et al. Eosinophil MBP extract modulates oncogene expression in prostate tumor cells: A preliminary study with monolayer cultures. J Cancer Ther. 2015;6:482–492. [Google Scholar]
- 44.Sosman JA, Bartemes K, Offord KP, Kita H, Fisher SG, Kefer C, et al. Evidence for eosinophil activation in cancer patients receiving recombinant interleukin-4: Effects of interleukin-4 alone and following interleukin-2 administration. Clin Cancer Res. 1995;1:805–812. [PubMed] [Google Scholar]
- 45.Huland E, Huland H. Tumor-associated eosinophilia in interleukin-2-treated patients: Evidence of toxic eosinophil degranulation on bladder cancer cells. J Cancer Res Clin Oncol. 1992;118:463–467. doi: 10.1007/BF01629431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cormier S, Taranova A, Bedient C, Nguyen T, Protheroe C, Pero R, et al. Pivotal advance: Eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol. 2006;79:1131–1139. doi: 10.1189/jlb.0106027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.