Abstract
Heme oxygenase-1 has been identified to protect allograft from ischemia/reperfusion and immunologic rejection. Activity of heme oxygenase-1 is regulated by a guanine-thymine dinucleotide length polymorphism in the heme oxygenase-1 gene promoter. In this study, we aimed to explore the impact of the heme oxygenase-1 gene promoter polymorphism of donors and recipients on the orthotopic liver graft function after transplantation. Sixty recipients and their accompanying donors of orthotopic liver allografts were included retrospectively in this study. Heme oxygenase-1 gene promoter polymorphism was assessed using genomic DNA isolated from cryopreserved splenocytes or peripheral blood mononuclear cells and analyzed by genetic analyzer. Small allele of the donor heme oxygenase-1 gene polymorphism significantly prolonged the graft survival (p = 0.017). Recipients of allografts from a class of small-allele carrier had significantly lower serum total bilirubin compared with recipients of a nonclass small-allele donor liver (p < 0.01). Additionally, in recipients of small-carrier allografts, cold ischemia time (<10 h or ≥10 h) did not affect the total bilirubin significantly. Our study suggested a protective function of donor-derived heme oxygenase-1 gene promoter polymorphism on orthotopic liver allograft function after transplantation.
Keywords: Heme oxygenase-1, Polymorphism, Liver transplantation, Graft survival, Graft function
Introduction
Heme oxygenase-1 (HO-1) catalyzes the rate-limiting steps in heme degradation to carbon monoxide, iron, and bilirubin. HO-1 has the ability of protecting against oxidative stress in vitro and in vivo [1, 2]. In organ transplantation, the experimental study provided evidences that HO-1 effectively protected liver, heart, and islet allografts or xenografts against ischemia/reperfusion injury and improved the grafts’ survival [3–6].
A guanine-thymine (GT)n dinucleotide repeat polymorphism that modulates the level of HO-1 inducibility was identified in the promoter region on chromosome 22q13.1 of human HO-1 gene facing oxidative stress [7, 8]. Short (GT) repeats were found to be associated with significant upregulation of HO-1 in response to inflammatory stimuli [7, 8]. The biological activity of HO-1 is associated with the number of (GT)n repeats [8]. It was demonstrated that a cutoff of 25 GT had been associated with a differential expression of postangioplasty inflammatory response in patients with peripheral artery disease [9]. However, up to date, the clinical significance of HO-1 gene polymorphism remains unknown in liver transplantation. Therefore, in the present study, we hypothesized that the HO-1 promoter gene polymorphism would influence orthotopic liver allograft function.
Methodology
Patients
Between June 2005 and December 2010, a consecutive 60 cases of non-tumoral hepatitis B-related liver cirrhosis who underwent orthotopic liver transplantation performed by our surgical team were retrospectively enrolled. Cryopreserved splenocytes or peripheral blood mononuclear cells from the 60 recipients and their accompanying donors were available for DNA isolation. Complete database was available for all the recipients and donors. Graft survival was defined as the period from the time of surgery to the time of graft failure. This study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee of Shanghai Jiao Tong University, and informed consent was obtained from each patient included in the study.
Genotyping of the Polymorphism in the HO-1 Promoter
Genomic DNA was isolated from peripheral blood mononuclear cells or spleen cells using the MagNA Pure isolation kit I (Roche Diagnostics GmbH, Mannheim, Germany). The 5′ flanking region containing (GT)n repeats of the HO-1 gene on chromosome 22q13.1 was amplified by PCR followed by fragment analysis of the amplified PCR product. For PCR, a 5′ FAM labeled forward primer (5′-FAM-AGA GCC TGC AGC TTC TCA GA-3′) and a reverse primer (5′-ACA AAG TCT GGC CAT AGG AC-3′) were used to amplify a 91- to 151-bp fragment containing the GT-repeat. Fragment length depends on the number of GT repeats. PCR was performed in a GeneAmp® PCR System 9700 (Applied Biosystems). After 10 min, at 94 °C, the DNA samples were subjected to 40 cycles of denaturation at 94 °C for 20 s, annealing at 60 °C for 10 s and extension at 72 °C for 20 s. The last cycle was extended with 7 min at 72 °C. Amplified DNA was denatured and genotyped using the fragment analysis method by an ABI PRISMTM 3100 Genetic Analyzer with GeneScan TM analysis software 3.7 (Applied Biosystems).
Statistics
Continuous data were expressed as the mean ± standard deviation. The chi-square test was used to compare proportions, and the Student’s t test was used for comparison of continuous data. Cumulative survival was analyzed by Kaplan-Meier method. All statistics were performed with SPSS 13 (Chicago, IL, USA). A p value <0.05 was considered statistically significant.
Results
HO-1 Genotype Distribution in the Recipients and Donors
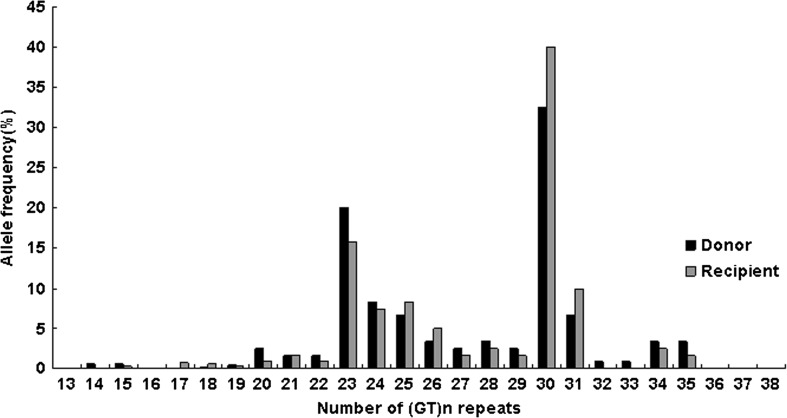
The allele distribution in the donors and recipients was given in Fig. 1. The allele distribution between recipients and donors was comparable, and 18 different repeat alleles for the recipients and 20 alleles for the donors were found, ranging from 14 to 35 repeats (median 25 repeats). The distribution of the (GT)n repeat alleles was comparable between recipients and donors (p = 0.250). The distribution of the (GT)n repeat alleles was bimodal, with one peak at allele 23 and the other at allele 30, similar as reported in other literatures [7, 10]. Based on this observation, a previous description [11], and because the number of repeats was inversely correlated to HO-1 production and activity, we divided the alleles into two subclasses, one with ≤25 repeats (the short (S) class) and one with >25 repeats (the long (L) class). The HO-1 genotype was composed of a combination of S- and/or L-alleles with genotypes SS, SL, and LL. The frequencies of the genotype groups were shown in Table 1. Genotype distribution for the recipients and donors was found to be in Hardy-Weinberg equilibrium. A comparison of recipients of class S-allele liver and nonclass S-allele liver was shown in Table 2. No significant imbalances were found between the genotypes in age, gender, cold or warm ischemia time, or occurrence of acute rejection.
Fig. 1.
Allele frequencies of the heme oxygenase-1 guanine-thymine repeat polymorphism in liver allograft donors and recipients. (GT)n guanine-thymine
Table 1.
Distribution of heme oxygenase-1 genotypes in the recipients and donors
| Recipient | Donor | |
|---|---|---|
| SS | 14 | 12 |
| SL | 19 | 20 |
| LL | 27 | 28 |
| Total | 60 | 60 |
SS short-short, SL short-long, LL long-long
Table 2.
Demographic characteristics of recipients of class S-allele liver allografts versus recipients of non-class S-allele allografts
| Characteristics | Class S liver recipients (n = 32) | Non-class S liver recipients (n = 28) | p value |
|---|---|---|---|
| Age (years) | 49.2 ± 8.1 | 50.4 ± 7.1 | NS |
| Gender (male/female) | 22/10 | 18/10 | NS |
| Cold ischemia time (min) | 630 ± 315 | 798 ± 243 | NS |
| Warm ischemia time (min) | 29.1 ± 8.9 | 31.3 ± 6.3 | NS |
| Acute rejection (N/Y) | 25/7 | 21/7 | NS |
S short, NS not significant, N/Y No/Yes
HO-1 Genotype and Liver Allograft Outcome
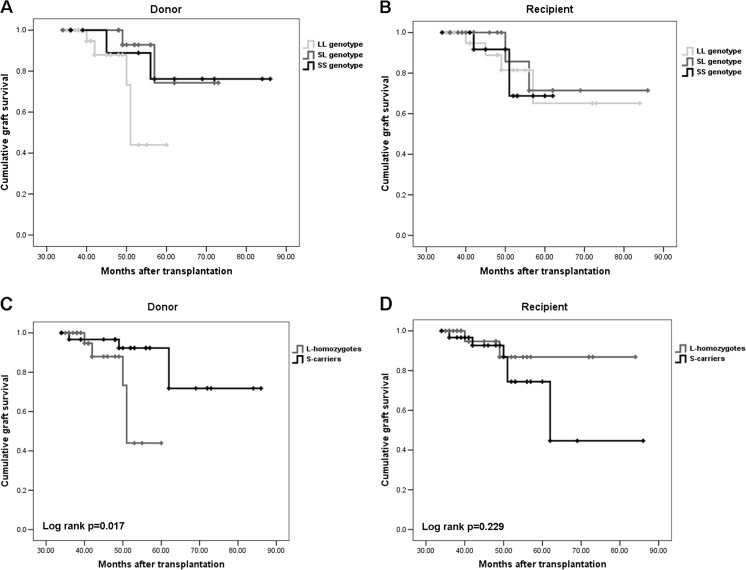
Graft survival of donor livers with the SL genotype were comparable to that of the SS homozygotes (log rank, p = 0.81) and significantly higher than the donor livers with LL-genotype (log rank, p = 0.015) (Fig. 2a). However, graft survival did not differ significantly according to the recipients’ HO-1 genotypes (log rank p = 0.93) (Fig. 2b). The above results indicated that the S-allele was dominant over the L-allele. For this reason and for statistical power, we performed the analysis based on S-carriers (SS and SL) and L-homozygotes. As a result, graft survival was found to be associated with donor and not recipient HO-1 genotype (log rank p = 0.017 vs 0.229) (Fig. 2c, d).
Fig. 2.
a, b Kaplan-Meier curves for graft survival in relation to donor and recipient heme oxygenase-1 guanine-thymine dinucleotide repeat gene polymorphism. The heme oxygenase-1 genotype is composed of a combination of short ≤25 alleles, and/or long (>25 repeats) alleles with the genotypes short-short, short-long, and long-long. According to donors’ heme oxygenase-1 genotypes, graft survival rates of donor livers with the short-long genotype are comparable to that of the short-short homozygotes (log rank p = 0.81), and significantly higher than the donor livers with long-long genotype (log rank p = 0.015). However, graft survival rates did not differ significantly according to the recipients’ heme oxygenase-1 genotypes (log rank p = 0.93). SS short-short, SL short-long, LL long-long. c, d Kaplan-Meier curves for graft survival in relation to donor and recipient heme oxygenase-1 guanine-thymine dinucleotide repeat gene polymorphism. The heme oxygenase-1 genotype was stratified in patients and donors as carriers vs. noncarriers of short allele. According to donors’ heme oxygenase-1 genotypes, the survival of short-allele grafts were significantly longer than grafts of long homozygotes (log rank p = 0.017). However, graft survival rates did not differ significantly according to the recipients’ heme oxygenase-1 genotypes (log rank p = 0.229). S short, L long
HO-1 Genotype and Liver Allograft Function
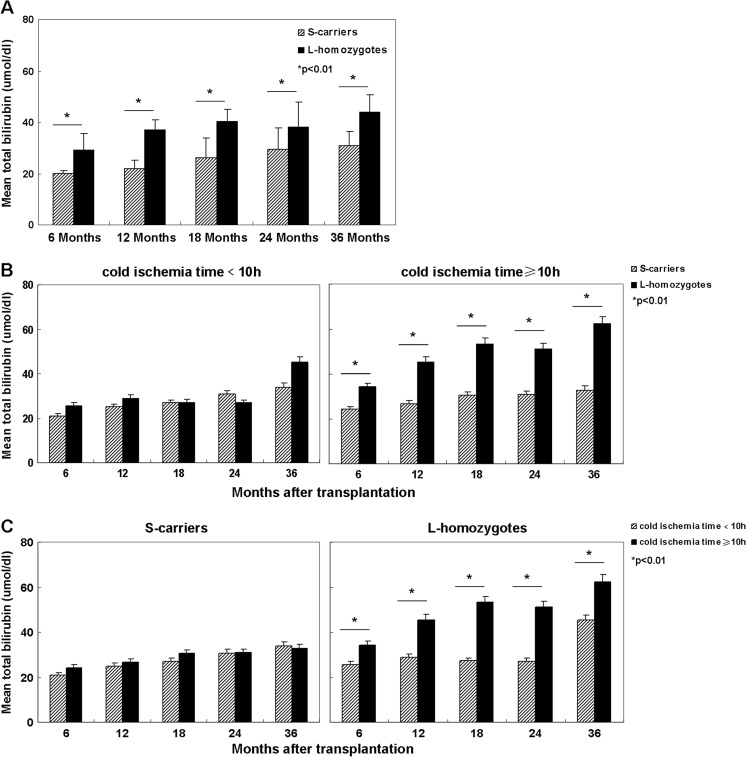
The recipients of liver allografts carrying class S-allele had significantly lower serum total bilirubin levels compared with recipients of allografts carrying no class S-allele at months of 6, 12, 18, 24, and 36 (p < 0.01) (Fig. 3a). The cold ischemia time was ≥10 h in 38.3 % (23/60) of the donors. A substantial lower serum bilirubin level in recipients of S-carrier allografts than recipients of non S-carrier grafts was found at months of 6, 12, 18, 24, and 36 postoperatively when the cold ischemia time of liver allografts was ≥10 h (p < 0.01) (Fig. 3b). However, serum bilirubin level in recipients of liver allografts from S-carriers that were exposed to <10 h of cold ischemia were not significantly lower than that of recipients of allografts from L-homozygotes (Fig. 3b). Additionally, in recipients of S-carrier allografts, cold ischemia time (<10 h or ≥10 h) did not affect the total bilirubin level significantly (Fig. 3c). But in recipients of allografts from L-homozygotes, cold ischemia time dramatically influenced the total bilirubin level (Fig. 3c).
Fig. 3.
Serum total bilirubin values (umol/dl) at 6, 12, 18, 24, and 36 months in recipients of livers from carriers versus noncarriers of the class short allele. a Recipients of liver allografts carrying at least one class short allele had significantly lower serum total bilirubin levels compared with recipients of allografts carrying nonclass short-allele at months of 6, 12, 18, 24, and 36 (p < 0.01). b When the time of cold ischemia time was ≥10 h, a substantial lower serum bilirubin level in recipients of short-carrier allografts than recipients of non-short-carrier grafts at months of 6, 12, 18, 24, and 36 was observed (p < 0.01). However, serum bilirubin levels in recipients of liver allografts from short-carriers that were exposed to <10 h of ischemia were not significantly different from those of recipients of allografts from long homozygotes. c In recipients of short-carrier allografts, cold ischemia time (<10 h or ≥10 h) did not affect the total bilirubin level significantly. But in recipients of allografts from long homozygotes, cold ischemia time dramatically influenced the total bilirubin level. S short, L long
Discussion
It is well-known that HO-1 is induced by a variety of different stimuli and HO-1 expression may protect cells from stress after heat shock, inflammation, or ischemia [2]. The catalytic by-products, CO, iron, and bilirubin, have been demonstrated to mediate anti-inflammatory, antioxidative, and antiapoptotic effects [2, 12–15]. CO was identified to suppress inflammatory cytokines and influence neutrophil migration [16, 17]. Biliverdin and bilirubin have been shown to inhibit monocyte chemotaxis and complement activation [18, 19]. HO-1 inducibility is modulated by a dinucleotide length polymorphism in the promoter region of the HO-1 gene. Short repeats (less than 25 GT repeats) were reported to confer a preferential inducibility of HO-1[7, 8]. A series of transplant models demonstrated that upregulation of HO-1 is beneficial for both allograft function and survival [3–5]. In addition, HO-1 affects xenograft as we found in our previous study that HO-1 overexpression prolonged the islet xenograft function and survival [6].
In the present study, we firstly demonstrated that a donor HO-1 gene promoter polymorphism might influence long-term orthotopic liver allograft outcome. Recipients of livers from S-allele carriers showed significantly longer graft survival and lower total bilirubin levels compared with recipients of livers from non-S-allele carriers, indicating a better graft function and survival. In addition, we found that donor livers with S-allele exposed to prolonged cold ischemia times (≥10 h) had a comparable graft function as donor livers with S-allele experiencing short ischemia times (<10 h) and donor livers with S-allele displayed a better graft function than L-homozygote counterparts experiencing prolonged cold ischemia times (≥10 h), suggesting that donor derived HO-1 S-allele counteracts the late effects of ischemia/reperfusion injury. This finding has important implications. When the frequency of grafts is L-homozygote, the injury is not limited by HO-1, and therefore, the graft should be transplanted as soon as possible.
Donor-derived HO-1 S-allele was not able to prevent against the occurrence of acute rejection shown in the present study (Table 2). In spite of our findings in clinical liver transplantation, HO-1 was shown to modulate inflammatory responses in a transgenic mouse model [20]. We speculate that in the mice, the HO-1 expression levels are much higher than the intrinsic HO-1 in human stressed liver cells. This might explain why in the clinical setting HO-1 does not prevent the occurrence of rejection. Furthermore, recipients of liver transplant, in contrast to the transgenic mice, receive immunosuppressive medication which may affect the benefits of HO-1.
Taken together, the present study provided the first evidence that the HO-1 gene promoter genotype of the donor may affect long-term orthotopic liver allograft function and survival. However, the mechanisms underlying the described findings in the present study should be further clarified in the experimental studies.
Acknowledgments
Grant Information
This work was supported by grants from the National Natural Science Foundations of China (81170721), Shanghai Pujiang Program (14PJ1407300), and Medical Guiding Project of Shanghai Municipal Science and Education Commission (14411960700).
Ethics Committee Approval
This study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee of Shanghai Jiao Tong University, and informed consent was obtained from each patient included in the study.
Conflict of Interest
The authors declare that they have no competing interests.
Footnotes
Zheng-Yun Zhang and Jiao Guan contributed equally to this work.
Contributor Information
Zheng-Yun Zhang, Email: coolboyzzy@126.com.
Jiao Guan, Email: 0727guanjiao@163.com.
Hao Li, Email: lihao6656@163.com.
Zun-Qiang Zhou, Email: zunqiang1978@hotmail.com.
Guang-Wen Zhou, Phone: +86-21-64369181, Email: gwzhoushsph@126.com.
References
- 1.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms and clinical application. FASEB J. 1988;2(10):2557–2568. [PubMed] [Google Scholar]
- 2.Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1029–L1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 3.Soares MP, Lin Y, Anrather J, et al. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;4(9):1073–1077. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- 4.Amersi F, Shen XD, Anselmo D, et al. Ex vivo exposure to carbon monoxide prevents hepatic ischemia/reperfusion injury through p38 MAP kinase pathway. Hepatology. 2002;35(4):815–823. doi: 10.1053/jhep.2002.32467. [DOI] [PubMed] [Google Scholar]
- 5.Katori M, Buelow R, Ke B, et al. Heme oxygenase-1 overexpression protects rat hearts from cold ischemia/reperfusion injury via an antiapoptotic pathway. Transplantation. 2002;73(2):287–292. doi: 10.1097/00007890-200201270-00023. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Zhang ZY, Zhou GW, et al. Protective effect of heme oxygenase-1 to pancreas islet xenograft. J Surg Res. 2010;164(2):336–343. doi: 10.1016/j.jss.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Yamada N, Yamaya M, Okinaga S, et al. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet. 2000;66(1):187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirai H, Kubo H, Yamaya M, et al. Microsatellite polymorphism in heme oxygenase-1 gene promoter is associated with susceptibility to oxidant-induced apoptosis in lymphoblastoid cell lines. Blood. 2003;102(5):1619–1621. doi: 10.1182/blood-2002-12-3733. [DOI] [PubMed] [Google Scholar]
- 9.Schillinger M, Exner M, Mlekusch W, et al. Heme oxygenase-1 genotype is a vascular anti-inflammatory factor following balloon angioplasty. J Endovasc Ther. 2002;9(4):385–394. doi: 10.1177/152660280200900401. [DOI] [PubMed] [Google Scholar]
- 10.Chen YH, Lin SJ, Lin MW, et al. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet. 2002;111(1):1–8. doi: 10.1007/s00439-002-0769-4. [DOI] [PubMed] [Google Scholar]
- 11.Exner M, Schillinger M, Minar E, et al. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with restenosis after percutaneous transluminal angioplasty. J Endovasc Ther. 2001;8(5):433–440. doi: 10.1177/152660280100800501. [DOI] [PubMed] [Google Scholar]
- 12.Stocker R, Yamamoto Y, Ames BN, et al. Bilirubin is an antioxidant of possible physiologic importance. Science. 1987;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 13.Willis D, Moore AR, Willoughby DA, et al. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat Med. 1996;2(1):87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- 14.Brouard S, Berberat PO, Soares MP, et al. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha mediated apoptosis. J Biol Chem. 2002;277(20):17950–17961. doi: 10.1074/jbc.M108317200. [DOI] [PubMed] [Google Scholar]
- 15.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8(3):240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 16.Otterbein LE, Bach FH, Alam J, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6(4):422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 17.VanUffelen BE, de Koster BM, Elferink JG, et al. Carbon monoxide enhances human neutrophil migration in a cyclic GMP-dependent way. Biochem Biophys Res Commun. 1996;226(1):21–26. doi: 10.1006/bbrc.1996.1305. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa K, Navab M, Lusis AJ, et al. Induction of heme oxygenase-1 inhibits monocyte transmigration induced by mildly oxidized LDL. J Clin Invest. 1997;100(5):1209–1216. doi: 10.1172/JCI119634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arriaga SM, Mottino AD, Almara AM, et al. Inhibitory effect of bilirubin on complement-mediated hemolysis. Biochim Biophys Acta. 1999;1473(2–3):329–336. doi: 10.1016/S0304-4165(99)00201-9. [DOI] [PubMed] [Google Scholar]
- 20.Araujo JA, Meng L, Tward AD, et al. Systemic rather than local heme oxygenase-1 overexpression improves cardiac allograft outcomes in a new transgenic mouse. J Immunol. 2003;171(3):1572–1580. doi: 10.4049/jimmunol.171.3.1572. [DOI] [PubMed] [Google Scholar]