Abstract
Oxygen supplemented at a concentration higher than 40–50 % for at least 2 h perioperatively is expected to reduce surgical site infections (SSI). Although supplementation of 80 % of oxygen perioperatively has shown to reduce SSI in various studies, this concentration is known to be associated with airway complications. This study was taken up to assess the efficacy of 60 %, i.e. <80 and >50 %, inspired oxygen supplemented perioperatively in reducing SSI. One hundred and eighty-eight patients who underwent elective class I and II surgeries were studied. Patients were divided equally into two groups and subgroups and matched for age, sex, type of surgeries, etc. The control group received 30 % and the study group received 60 % oxygen supplementation perioperatively for 2 h. Wounds were observed for the development of SSI. 8/94 patients in the study group and 13/94 patients in the control group developed SSI (p < 0.01). The results indicate a relative risk of 1.62, risk difference of 0.0531 and attributable risk of 38.42 %. Hence, it may be concluded that perioperative oxygen supplementation at 60 % concentration reduces SSI.
Keywords: Hyperoxia, 60 % inspired oxygen, Surgical site infections, Perioperative oxygen supplementation
Introduction
Surgical site infections (SSI) are the third most frequently reported nosocomial infections and comprise one third of total infections in surgical patients [1]. There are various factors which predispose to SSI and perioperative hypoxia is one of them [2]. Oxygen is routinely supplemented at 30 % concentrations perioperatively. Supplementing higher concentrations of oxygen in the perioperative period for at least 2 h is one of the methods suggested to overcome perioperative hypoxia. This is vital for healing [3] and critical for prevention of infections by releasing reactive oxygen species [4]. Although higher concentrations of oxygen supplementation (80 %) were found to reduce SSI by half, inspiring a higher concentration of oxygen is not devoid of complications [5, 6]. It can induce lung injury and atelectasis in patients at risk [7–10]. As evidence regarding the rational use of hyperoxia in the perioperative period in reducing SSI is very less, hence this study was planned with an objective of assessing the efficacy of supplementing oxygen at 60 % concentration perioperatively in reducing SSI.
Methods
Study design: A prospective comparative study was conducted at a teaching hospital mainly catering to rural and semirural patients. Patients admitted for class I (clean) and class II (clean contaminated) elective general surgeries between October 2009 and May 2011 were included in the study. Detailed information was given to the patients and informed consent was collected from them. The study design was approved by the Institutional Ethics Committee constituted as per ICMR guidelines. With an incidence rate of 2 % at ±2 margin of error and 95 % level of confidence, the calculated sample size was 188. An equal number of patients (94 each) were included from class 1 and class 2 surgeries. Similarly, an equal number of patients from each group were further classified into subgroups as the study and control groups (47 each). Both groups were similar in their demographic profiles. All the surgeries were performed by faculty and residents of a single surgical unit in a medical college. Major surgeries were done by surgeons of asst. prof. level and above with a minimum of 3 years of post-MS experience. Majority of the medium procedures were performed by third-year residents and minor surgeries by first- and second-year residents under supervision. Duration of the surgical procedures ranged from 15 min in minor procedures to 3 h maximum in radical procedures. Major, medium and minor cases were distributed equally to both the groups. Patients with diabetes mellitus, immunosuppression, chronic renal and respiratory diseases and who received chemotherapy or steroids were excluded from the study. Patients who did not complete the follow-up or who needed implants like mesh were excluded in the study. Patients were alternatively allotted to either the study or control group.
Details of patients were recorded including history and clinical examination. Necessary pre-operative investigations were performed and operated upon. All the patients received a single dose of prophylactic antibiotic, third-generation cephalosporin (ceftriaxone), and a second dose was given whenever surgery extended beyond 2 h. Hypovolaemia correction was done wherever required and normothermia at 20–22 °C was maintained. Both the groups matched in age, sex and type of surgeries (Table 1).
Table 1.
Details of surgeries
| Sl. no. | Study group | Control group | Total | |
|---|---|---|---|---|
| Class I surgeries | ||||
| 1 | Thyroidectomies | 7 | 5 | 12 |
| 2 | Modified radical mastectomy | 2 | 1 | 3 |
| 3 | Laparoscopic cholecystectomy | 13 | 12 | 25 |
| 4 | Hernia surgeries without mesh | 3 | 1 | 4 |
| 5 | Minor procedures like fibroadenoma, lipoma, dermoid cyst, sebaceous cyst, lymph node biopsy ganglion excision, etc | 24 | 27 | 51 |
| Class II surgeries | ||||
| 6 | Appendectomies | 16 | 20 | 36 |
| 7 | Anal fissure | 13 | 13 | 26 |
| 8 | Hemorrhoidectomy | 12 | 13 | 25 |
| 9 | Hemicolectomies and anterior resections | 4 | 2 | 6 |
| Total | 94 | 94 | 188 | |
While the study group received 60 % fraction of inspired oxygen intraoperatively and for 2 h after surgery, the control group received 30 % fraction of inspired oxygen intraoperatively and for 2 h after surgery. Oxygen was delivered through the Boyle machine at the required rate of either 30 or 60 % during surgery under general anaesthesia. For individuals who underwent surgery under regional anaesthesia, oxygen was delivered through Ventimask, a HAFOE device, at a rate of 4 l/min for the control group. For individuals in the study group, oxygen was supplemented with an MC oronasal mask at a rate of 6 l/min [11]. Operative wounds were examined on the second, fifth and eighth postoperative days for signs of surgical site infection. Follow-up was done for 30 days. Patients from both the study and control groups were compared for final analyses.
Statistical analysis was carried out using diagrammatic presentation. Risk difference, relative risk and attributable risks were also calculated. Chi-square test was applied to calculate the p value, and a p value <0.01 was considered significant.
The study was conducted on a total of 188 patients aged between 1 and 90, of which 94 underwent clean general surgical procedures and 94 underwent clean contaminated general surgical procedures.
The study group included 47 patients who underwent clean surgeries and 47 clean contaminated surgical procedures, and both of them received 60 % fraction of inspired oxygen. Similarly, the control group included 47 patients who underwent clean surgeries and 47 clean contaminated surgical procedures. Both of these subgroups of patients received 30 % fraction of inspired oxygen.
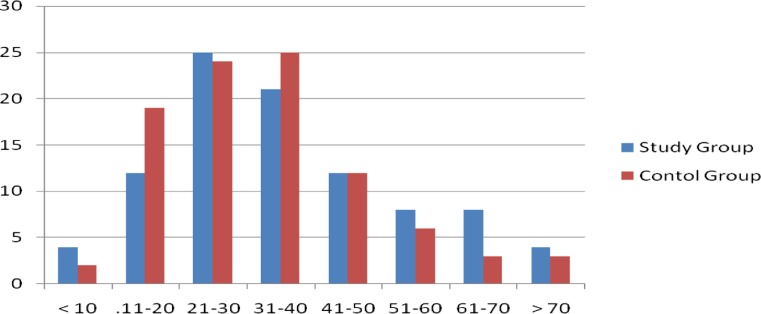
Of the 94 patients, the study group had 57 % (54) males and 43 % (40) females. In the control group of 94 patients, 42 % (42) were males and 58 % (52) were females (Fig. 1).
Fig. 1.
Age distribution (X axis: age in years; Y axis: no. of patients)
Table 2 depicts that out of 94 patients who underwent clean surgeries, three from the study group of 47 developed features of SSI. All of them had erythema. Similarly, among the control group of 47, 5 developed features of SSI of which 3 of them had erythema and two had purulent discharge as shown in Table 1. The patients with purulent discharge had grade II SSI.
Table 2.
Results in the class I group (clean surgeries, n = 94)
| O2 supplementation | Presence of surgical site infection | Absence of surgical site infection | Percentage (%) of surgical site infection |
|---|---|---|---|
| 60 % inspired oxygen (n = 47) | 3 | 44 | 6.4 |
| 30 % inspired oxygen (n = 47) | 5 | 42 | 10.6 |
Table 3 shows that out of the 94 patients who underwent class II general surgical procedures, 5 of 47 from the study group developed features of SSI. Two had only erythema, one had erythema with tenderness and two had purulent discharge. In 47 patients of the control group, 8 developed features of SSI of which 6 had erythema and 2 had purulent discharges. The grade of SSI in the study group was I in 2 and II in 3 of them. The control group had 8 with SSI, 6 had grade I and 2 had grade II.
Table 3.
Results in the class II group (clean contaminated surgeries, n = 94)
| O2 supplementation | Presence of SSI | Absence of SSI | Percentage (%) of surgical site infection |
|---|---|---|---|
| 60 % inspired oxygen (n = 47) | 5 | 42 | 10.6 |
| 30 % inspired oxygen (n = 47) | 8 | 39 | 17 |
Thus, it was seen that the 8 out of the 94 patients who received 60 % fraction of inspired oxygen perioperatively developed surgical site infections and 13 of the 94 patients who received 30 % fraction of inspired oxygen perioperatively developed surgical site infections as depicted in Tables 3 and 4.
Table 4.
Overall rate of SSI for the study and control groups
| Oxygen supplementation | SSI | No SSI | Total |
|---|---|---|---|
| Oxygen 30 % | 13(a) | 81(b) | 94(r1) |
| Oxygen 60 % | 8(c) | 86(d) | 94(r2) |
| Total | 21 | 167 | 188 |
Further evaluation of risk difference, attributable risk and relative risk reductions reveals the following [12]:
Risk difference: The risk of infection in the 30-%-inspired-oxygen group is (P1) = a/r1 = 0.1382, whereas the risk of infection in the 60-%-inspired-oxygen group is (P2) = c/r2 = 0.0851. The risk difference is (P1 − P2) 0.053112. So, for every 10,000 patients, supplementing 60 % oxygen can prevent infection in 531 patients.
Attributable risk (AR): AR = P1 − P2/P1 × 100 = 38.42. Thus, the 30-%-inspired-oxygen group suffered 38.42 % more infections compared to the 60-%-inspired-oxygen group.
Relative risk (RR): P1/P2 = 1.62 [12]. Patients in the 30-%-inspired-oxygen group have 1.62 times more risk of infection as compared to the 60-%-inspired-oxygen group.
p value ≤0.001 (significant).
Discussion
Surgical site infection (SSI) is a major complication of surgery, associated with prolonged hospitalization, increased costs and increased morbidity and mortality. In recent years, randomized trials have identified a number of preventive measures that can substantially reduce the risk of SSI. These include appropriate perioperative antibiotic prophylaxis, maintenance of perioperative normothermia and control of hyperglycaemia [13, 14]. The effect of perioperative oxygen supplementation continues to be under debate with proponents and opponents firmly divided over the issue. It has been argued by some researchers that there is benefit in reducing surgical site infection [5, 6, 15].
The wound healing process involves numerous functions, many of which depend on the presence of oxygen. Collagen production and development influence the strength of the wound which is directly correlated with the partial pressure of oxygen (PO2) of the tissue. Synthesis of collagen, cross-linking and the resulting wound strength depend on the normal function of specific enzymes. The functions of these enzymes are directly related to the amount of oxygen present, e.g. hydroxylation of proline and lysine by hydroxylase enzymes [16].
The production of epithelial tissue depends primarily on the degree of hydration and oxygen. Although a moist wound environment increases the rate of epithelialization by a factor of 2 to 3, the optimal growth of epidermal cells is found at an oxygen concentration of 10 to 50 % [16, 17]. Hyperbaric oxygen treatment increases the proliferation of the fibroblasts and the differentiation and epidermopoiesis of the keratinocytes [18]. This probably led to the idea of achieving a higher concentration of oxygen to increase epithelialization and reduce SSI. Achieving high oxygen tension at the site of surgery has been proposed as a means of reducing the risk of SSI, based on data that oxygen can enhance the oxidative processes in white cells, thus facilitating bacterial killing by oxidative phosphorylation. Thus, oxygen plays an important role in both wound healing and host resistance to microbial contamination.
There are a number of devices through which supplemental oxygen can be delivered. A nasal cannula which can provide oxygen at low flow rates, 2–6 l/min, delivers a concentration of 24–40 %. A face mask often used for controlled air entrainment known as Ventimask, at the rate of 4 l/min, can provide a concentration of 30 %, and an MC oronasal mask at the rate of 6 l/min of oxygen can provide FiO2 at 60 % concentration [19].
In our study, we used an MC or Mary Cattrell oronasal mask for oxygen supplementation at the rate of 6 l/min with a concentration of 60 % and Ventimask at the rate of 4 l/min for 30 % respectively for the study and control groups.
There are several contradicting reports on the beneficial role of supplemental perioperative oxygenation in reducing surgical site infections. A study on 500 patients undergoing elective colorectal surgeries of which 250 received 80 % oxygen and 250 received 30 % oxygen noticed that the study group who received 80 % oxygen had 13 surgical wound infections, as compared with 28 of the 250 patients receiving 30 % oxygen [20]. Belda FJ and others in their study showed that SSI occurred in 24.4 % of patients who were administered 30 % FiO2 and 14.9 % of patients in the 80 %-FiO2-administered group. The risk of SSI was found to be 39 % lower in the 80 %-FIO2 group [6].
Al Niaimi et al. observed that supplemental perioperative oxygenation resulted in a reduced incidence of SSI in a fixed effects model and found to be beneficial in preventing SSI in patients undergoing colorectal surgery [5].
Similarly, Motaz Qadan et al. analysed randomized controlled trials between 1998 and 2007 and observed infection rates of 12 % in the control group and 9 % in the hyperoxic group, with a relative risk reduction of 25.3 % [15].
In contradiction to the various studies discussed, Pryor and others found a higher rate of SSI in patients receiving a higher concentration of supplemental oxygen. It is interesting to observe that this study was terminated after 160 patients were enrolled early in view of increased infection rates in the hyperoxia group [21].
Majority of the studies conducted to test the efficacy of supplemental oxygen in reducing SSI have used O2 at 80 % concentration and observed benefits at variable rates. But none of these attempted to look at the pulmonary complications due to perioperative hyperoxia, like increased risk of airway inflammation and poor regulation of blood glucose levels or oxidative stress [22, 23]. The PROXI Randomized Control Trial, led by Meyhoff et al. [7], observed that there was an increase in atelectasis (7.9 versus 7.1 %) and respiratory failure (5.5 versus 4.4 %) in the group of patients who received 80 % oxygen supplementation compared to 30 %. Considering these aspects, our study was designed at fixed 60 % oxygen supplementation. This saturation was more than 50 % which is an optimum saturation [16, 17] and less than 80 % which may be considered too high and potentially dangerous.
With supplementation of 60 % oxygen perioperatively and 2 h postoperatively, the present study had infection rates of 8 and 13 respectively in the study and control groups (p < 0.001). Risk difference was 0.0531, attributable risk (AR) was 38.42 % and relative risk (RR) was 1.62. All these results suggest that there is similar reduction in the rates of SSI with 60 % oxygen supplementation in comparison with studies of Belda FJ et al. showing 39 % lowered risk [6]. Al Niami and team had a relative risk of 0.70. Motaz Qadan and group observed a relative risk reduction of 25.3 % [15]. It should be noted that all these studies have used 80 % oxygen supplementation. This study is a small attempt in rationalizing the perioperative oxygen supplementation with reasonable reduction in SSI and possible reduction in pulmonary complications of the patients receiving higher oxygen supplementation.
Conclusion
To conclude, supplementation of oxygen at 60 % concentration for 2 h perioperatively in clean and clean contaminated surgeries is effective in reducing postoperative surgical site infections.
Acknowledgments
The authors acknowledge the support of the heads of the institution and its hospital for all the support.
References
- 1.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guidelines for prevention of surgical site infection. Infect Control Hosp Epidemiol. 1999;20(4):247–78. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez PG, Frances NF, Woodley DT, Shim EK. The role of oxygen in wound healing: a review of the literature. Dermatol Surg. 2008;34:1–11. doi: 10.1097/00042728-200801000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Hopf HW, Hunt TK, West JM, Blomquist P, Goodson WH, III, Jenson A, et al. Wound tissue oxygen predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132:997–1004. doi: 10.1001/archsurg.1997.01430330063010. [DOI] [PubMed] [Google Scholar]
- 4.Gottrup F. Measurement and evaluation of tissue perfusion in surgery. In: Leaper DJ, Branicki FJ, editors. International surgical practice Oxford. UK: Oxford University Press; 1992. pp. 15–39. [Google Scholar]
- 5.Al-Niaimi A. Supplemental perioperative oxygen for reducing surgical site infection: a meta-analysis. J Eval Clin Pract. 2009;15(2):360–365. doi: 10.1111/j.1365-2753.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 6.Belda FJ, Aguilera L, Jose Garcia DLA, Alberti J, Vicente R, Ferrandiz L, et al. Supplemental perioperative oxygen and the risk of surgical wound infection. JAMA. 2005;294(16):2035–2048. doi: 10.1001/jama.294.16.2035. [DOI] [PubMed] [Google Scholar]
- 7.Meyhoff CS, Wetterslev J, Jorgansen LN, Henneberg SW, Hogdall C, Lundvall L, et al. Effect of high perioperative oxygen on surgical site infection and pulmonary complications after abdominal surgery. JAMA. 2009;302(14):1543–1550. doi: 10.1001/jama.2009.1452. [DOI] [PubMed] [Google Scholar]
- 8.Zwemer CF, Whitesall SE, D’Alecy LG. Cardiopulmonary-cerebral resuscitation with 100% oxygen exacerbates neurological dysfunction following nine minutes of normothermic cardiac arrest in dogs. Resuscitation. 1994;27:159–170. doi: 10.1016/0300-9572(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 9.Rubertsson S, Karlsson T, Wiklund L. Systemic oxygen uptake during experimental closed-chest cardiopulmonary resuscitation using air or pure oxygen ventilation. Acta Anaesthesiol Scand. 1998;42:32–38. doi: 10.1111/j.1399-6576.1998.tb05077.x. [DOI] [PubMed] [Google Scholar]
- 10.Gottrup F. Physiology and measurement of tissue perfusion. Ann Chir Gynecol. 1994;83:183–189. [PubMed] [Google Scholar]
- 11.Atkinson RS, Rushman GB, Davies NJH (1997) in Lee’s synopsis of anaesthesia. 11th edition.Ch no 30.Butterworth Heinmann.Publications, Oxford. pp. 822-23
- 12.Sundaram KR, Dwivedi SN, Sreenivas V. Medical statistics, principles and practice, ch no 8, epidemological methods. New Delhi: BI Publications; 2010. pp. 202–204. [Google Scholar]
- 13.Kirby JP, Mazuski JE. Prevention of surgical site infection. Surg Clin N Am. 2009;89(2):365–389. doi: 10.1016/j.suc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical wound infection and shorten hospitalization. NEJM. 1996;334:1209–1216. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 15.Qadan M, Akca O, Mahid SS, Hornung CA, Polk HC., Jr Perioperative supplemental oxygen therapy and surgical site infection. A meta analysis of randomized controlled trials. Arch Surg. 2009;144(4):359–366. doi: 10.1001/archsurg.2009.1. [DOI] [PubMed] [Google Scholar]
- 16.Jonsson K, Hunt TK, Mathes SJ. Oxygen as an isolated variable influences resistance to infection. Ann Surg. 1988;208:783–787. doi: 10.1097/00000658-198812000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horikoshi T, Balin AK, Carter DM. Effect of oxygen on the growth of human epidermal keratinocytes. J Invest Dermatol. 1986;86(4):424–427. doi: 10.1111/1523-1747.ep12285695. [DOI] [PubMed] [Google Scholar]
- 18.Dimitrijevich SD, Paranjape S, Wilson JR. Effect of hyperbaric oxygen on human skin cells in culture and in human dermal and skin equivalents. Wound Repair Regen. 1999;7:53–64. doi: 10.1046/j.1524-475X.1999.00053.x. [DOI] [PubMed] [Google Scholar]
- 19.Wiseman DM, Rovee DT, Alvarez OM. Wound healing. Biochemical and clinical aspects. Philadelphia: W.B. Saunders; 1992. pp. 562–580. [Google Scholar]
- 20.Greif R, Akca O, Horn EP, Kurz A, Daniel IS. Supplemental perioperative oxygen to reduce the incidence of surgical wound infection. N Engl J Med. 2000;342(3):161–177. doi: 10.1056/NEJM200001203420303. [DOI] [PubMed] [Google Scholar]
- 21.Pryor KO, Fahey TJ, III, Lien CA, Goldstien PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgical population. A randomized controlled trial. JAMA. 2004;291(1):79–87. doi: 10.1001/jama.291.1.79. [DOI] [PubMed] [Google Scholar]
- 22.Carpagnano GE, Khairotonov SA, Foschino-Barbaro MP, Resta O, Grammiccioni E, Barnes PJ. Supplementary oxygen in healthy subjects and those with COPD increases oxidative stress and airway inflammation. Thorax. 2004;59(12):1016–1019. doi: 10.1136/thx.2003.020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bandali KS, Belanger MP, Wittnich C. Does hyperoxia affect glucose regulation and transport in newborn? J Thorac Cardiovasc Surg. 2003;126(6):1730–35. doi: 10.1016/S0022-5223(03)01044-4. [DOI] [PubMed] [Google Scholar]