Abstract
Barrett's esophagus (BE) is a common condition that develops as a consequence of gastroesophageal reflux disease. The significance of Barrett's metaplasia is that predisposes to cancer development. This article provides a current evidence-based review for the management of BE and related early neoplasia. Controversial issues that impact the management of patients with BE, including definition, screening, clinical aspects, diagnosis, surveillance, and management of dysplasia and early cancer have been assessed.
Keywords: Barrett’s esophagus, Barrett metaplasia, Esophageal adenocarcinoma, Gastroesophageal reflux disease, Radiofrequency ablation
Core tip: Barrett’s esophagus (BE) is a common condition that predisposes to cancer development. This article provides a current evidence-based review for controversial issues that impact the management of patients with BE, including clinical aspects, diagnosis, surveillance, and management of dysplasia and early cancer.
INTRODUCTION
Since Barrett’s esophagus (BE) was first described in 1950[1], the definition of this condition has been modified on several occasions. Presently, it is defined as the condition in which any extent of metaplastic columnar epithelium that predisposes to cancer development replaces the stratified squamous epithelium that normally lines the distal esophagus[2]. Since only intestinal metaplasia (with globet cells) clearly predisposes to malignancy, its presence is required for the diagnosis. Nevertheless, some scientific societies consider that the presence of cardiac mucosa (with mucus-secreting columnar cells without goblet cells) are also diagnostic of BE[3]. However, because the risk for malignancy of cardia-type epithelium remains unclear, it is not generally recommended to use the term “Barrett’s esophagus” in that context[2,4].
EPIDEMIOLOGY AND CLINICAL ISSUES
BE is a common condition, with an estimated prevalence in the general adult population between 2% and 7%[5,6], and an incidence rate varying between 23.1 and 32.7 per 100000 person-year[5,7,8]. It is observed in 4% of patients undergoing an upper gastrointestinal endoscopy, and in 9% of men over 50 years[9].
Risk factors for BE include the presence of severe and longstanding gastroesophageal reflux, for which the biliary-pancreatic content seems to have a significant role[10], as well as advanced age, male sex, white race, obesity, and tobacco use. Conversely, factors that might protect against BE include the use of NSAIDs, Helicobacter pylori, and high consumption of fruits and vegetables[11].
Although BE is an asymptomatic disease, it is the most important known risk factor for the development of esophageal adenocarcinoma (EA), a tumor that has increased its incidence six-fold over the last four decades in Western countries, becoming the fastest growing cause of cancer mortality[12]. For BE patients, with a probability of 0.5% per year[13], the risk of developing EA is between 40 and 50 higher than for the general population[14-16].
The malignant degeneration cascade is thought to occur from nondysplastic intestinal metaplasia, to low-grade (LGD) and then high-grade dysplasia (HGD), and eventually EA[2,17]. The rate of progression from LGD to either HGD or EA ranges from 0.5% to 13.4% per patient per year[18]. The annual risk of progression from HGD to EA is 10% (ranges between 6% and 19%)[19,20].
SCREENIG FOR BE
Although this practice is not supported by high-quality evidence, screening for BE can be suggested in patients with chronic gastroesophageal reflux disease (GERD) symptoms who have at least one additional risk factor for EA. The risk factors include: 50 years or older, male sex, white race, hiatal hernia, elevated body-mass index, intrabdominal body-fat distribution, or tobacco use[21-24].
ENDOSCOPIC DIAGNOSIS
The gastroesophageal junction (GEJ) is not anatomically well defined, but it is accepted as the proximal limit of the gastric folds under partial insufflation. The squamocolumnar junction is bounded by the pale pink squamous mucosa of the esophagus, which contrasts with the red columnar gastric mucosa. The diagnosis of BE requires that the columnar epithelium extends above the GEJ, and the presence of columnar metaplasia confirmed in the esophageal biopsy[21].
BE has been divided in short (< 3 cm) or long-segment (≥ 3 cm), depending on the length of the metaplastic epithelium[25], but it is not clear that this classification can be clinical helpful, since there is no definitive evidence that the extent of the metaplastic segment increases the risk of cancer[26]. Prague’s classification is a more recent system for describing BE endoscopically that evaluates both the circumferential extent (C) and the maximum extent (M) of Barrett’s metaplasia[27]. However, since no endoscopic technique allows to either differentiate the intestinal metaplasia from gastric metaplasia or recognize the presence of dysplasia, a biopsy specimen is always required for diagnosis.
SURVEILLANCE IN BE
Although there is no data from randomized controlled trials, surveillance is generally recommended because it has been correlated in published studies with earlier stage diagnosis and improved survival from cancer[3]. However, surveillance strategies are limited by the low incidence of cancer in patients with BE, and by the various difficulties in the interpretation of the presence of dysplasia (because of random sample collection, possibility of false negatives in the evaluation of the biopsies, or high variability for the interpretation of dysplasia). Nevertheless, most clinical guidelines[2,3,28] recommend endoscopic surveillance in patients with BE. The goal is the early detection of LGD and of its progression to HGD or early stage cancer (lymph node involvement varies between 0% and 2%, respectively). However, no long-term trials have been performed to definitively answer the question of whether endoscopic surveillance really reduces cancer incidence or mortality.
A high-resolution endoscopy is strongly recommended for the accurate evaluation of BE. A 4-quadrant biopsy sampling should be performed every 2 cm or every 1 cm (if known or suspected dysplasia). Additionally, specific biopsies of any suspicious lesions should be submitted separately.
Advanced imaging techniques (such as chromoendoscopy or electronic chromoendoscopy, narrow band imaging, confocal laser endomicroscopy or magnification) are not superior to standard white light endoscopy and, therefore, are not recommended for routine use. However, these technologies may be helpful to adequately address biopsies if dysplasia is suspected[2,29].
When no dysplasia is detected after 2 consecutive endoscopies within 6-12 mo, the usual recommendation is to repeat the test after 3-5 years. When indeterminate-grade dysplasia is detected, it is recommended to increase the antisecretory treatment to heal the esophageal inflammation and then repeat the biopsy after 6 mo. When LGD is detected, the recommendation is to perform an endoscopic control after 6-12 mo, and then an annual endoscopy until the absence of dysplasia is confirmed in two consecutive annual controls; alternatively, endoscopic eradication therapy can also be considered. When HGD is detected, endoscopic eradication therapy is strongly advised (consider surveillance every 3 mo only in selected cases). An algorithm for the screening, surveillance and management of BE is shown in Figure 1.
Figure 1.
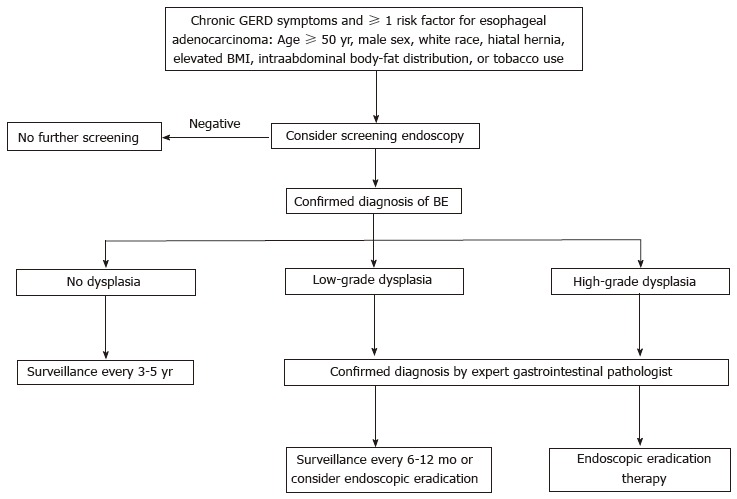
Algorithm for the screening, surveillance and management of Barrett's esophagus. GERD: Gastroesophageal reflux disease; BMI: Body mass index. BE: Barrett's esophagus.
MANAGEMENT OF DYSPLASIA AND EARLY CANCER
HGD and intramucosal EA
Traditionally, esophagectomy has been recommended for patients with BE and either HGD or early EA, but the high morbidity and mortality of this technique, alongside with the development of new endoscopic techniques, are modifying this approach. Even in high-volume centers, the mortality rate of esophagectomy for HGD or early EA ranges from 0% to 4%[30]. Actually, surgery should be reserved for those patients with infiltration of the submucosal layer, and/or low grade or lack of response to endoscopic treatment.
The goal of endoscopic eradication therapy for patients with BE is to permanently eradicate all intestinal metaplasia and achieve a complete reversion to squamous epithelium[2,31]. Several studies have shown that HGD/T1m neoplasms can be eradicated in up 80%-100% cases, as well as the BE with intestinal metaplasia can be removed of in > 75% of cases[20,32-36]. Moreover, a significantly higher rate of progression to cancer has been shown in the endoscopic surveillance group comparing with the ablative treatment group (after initial endoscopic mucosal resection (EMR) where appropriate)[20,37]. Therefore, instead of surveillance, endoscopic eradication therapy with radiofrequency ablation (RFA), photodynamic therapy (PDT), or EMR is recommended for treatment of patients with confirmed HGD or intramucosal adenocarcinoma (T1a) within BE[2]. Major complications of these techniques include strictures, hemorrhage and perforation. Minor complications include temporary chest pain, fever and odynophagia.
Survival after endoscopic resection is similar to that expected after surgical treatment, but with less morbidity[32-34]. Therefore, since HGD is not associated with metastatic nodal spread when the existence of a deeper invasion has been excluded by EMR, endoscopic treatment is preferred over surgery in most patients with BE and HGD[32,33,38-40]. But, on the other hand, endoscopic therapies are associated with a higher rate of recurrence of the HGD[32-34,38,41], although it can usually be treated endoscopically[32-34,42]. Because of that, surgical resection should be reserved until the endoscopic treatment fails[32-34].
RFA is effective transforming an esophagus with pathological cells into an esophagus with a normal mucosa, without genetic abnormalities that may become premalignant[43]. A recent systematic review[44] suggests that success rates are higher with RFA, with a sustained disappearance of the HGD in up to 90% of patients[20,23,35,43,45]. RFA ablation is a safe, long-lasting therapy (up to 5 years) that is associated with a significant reduction in the relative risk for neoplastic progression[20,35,46-48]. This technique is usually performed in various sessions to completely eradicate the metaplasia. The most common adverse event is the stenosis (up to 5% of patients)[49], but the rate of severe side effects of RFA is lower than with other ablative techniques[2]. Compared to other options, such as surgical treatment, photodynamic therapy, or follow up, RFA ablation is the most cost-effective strategy in patients with HGD[50].
In cases of HGD on a visible mucosal lesion, EMR is needed for an adequate diagnosis and depth staging[51] that can lead to a significant change in the management[52-55], since if an EA is found in the EMR sample, the risk of malignant adenopathy is related to the depth of invasion[56,57]. In this sense, the cap and snare technique with submucosal injection, and the band ligation technique without submucosal injection are considered to be equally effective[3]. Ideally, EMR should be applied in less than two thirds of the circumference of the esophagus to avoid strictures[29]. If a stenosis appears, it can be usually treated with endoscopic dilatation[58-60].
Endoscopic ablation of residual BE is currently recommended after completion of EMR of all visible HGD/T1a lesions. Several case series have reported recurrence of neoplasia if any residual BE is left untreated (11% to 30%, with a mean follow-up of 3 years)[32,58], and ablation of the residual BE is associated with a lower recurrence[20,36,40,41,61,62]. Consequently, RFA is currently the best available technique for the treatment of flat HGD and for eradicating residual BE after EMR[3,29,63].
Among alternative ablative techniques, PDT has been effectively used to ablate HGD, reducing the risk of progression to cancer compared with surveillance alone[64]. However, adverse events associated with this technology are common (development of esophageal stricture, 36% after PDT vs 6% after RFA) and may be severe[22,42]. Moreover, HGD can even persist in up to 33%-50% of the patients[65,66]. Long follow-up controlled studies comparing PDT with surgical resection and the other endoscopic therapies are needed to adequately assess this technique. Cryotherapy has not been assessed in randomized controlled trials, and it is not currently indicated as an alternative endoscopic eradication therapy. Small randomized controlled trials using argon plasma coagulation have reported anecdotal high-success rates[67].
In the case of an early EA extending into the submucosal layer, surgery should be considered as the best option[29], since in T1a context the rate of lymph node involvement is extremely low (< 3%) but the risk increases up to 20%-25% when the submucosal layer is affected. However, in selected T1b-Sm1 cases (invasion limited to the superficial layer of the submucosa), and with low-risk histopathologic features (invasion < 500 μm; G1-G2 grade, no lympho-vascular invasion), endoscopic therapy could be an option instead of esophagectomy (especially in high surgical risk patients)[68,69]. Endoscopic ultrasound evaluation of visible lymph nodes is advised in this setting.
The algorithm for the management of BE with HGD or early cancer is shown in Figure 2.
Figure 2.
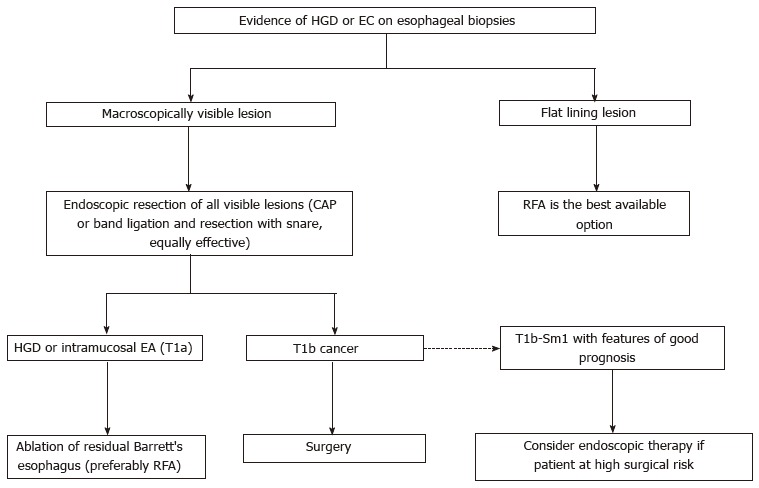
Management of high-grade dysplasia and early cancer in Barrett's esophagus. RFA: Radiofrequency ablation; BE: Barrett's esophagus; HGD: High-grade dysplasia; EC: Early cancer; EA: Esophageal adenocarcinoma.
LGD
Up to 25%-40% of BE patients will be diagnosed with LGD during follow-up[70]. Most guidelines recommend endoscopic surveillance (every 6-12 mo) to rule out dysplastic progression. However, there are several doubts related to the evolution of the LGD. In some cases LGD may progress to HGD or EA, but it can also remain stable or even disappear in subsequent controls. Still, a significant progression rate from LGD to HGD or EA (13.4% per person-year) has been recently reported[18], suggesting that the endoscopic treatment in this population may also be justified.
The impact of RFA on the risk of neoplastic progression in BE patients with LGD is not clear, but RFA leads to reversion to normal-appearing squamous epithelium in > 90% of LGD cases[2]. A recent randomized controlled study[71] including 136 BE patients with confirmed LGD (68 patients undergoing RFA ± EMR vs 68 patients followed endoscopically) showed that RFA was associated with a significant reduction on the risk of neoplastic progression at 3 years follow-up: 26.5% in the follow-up group vs 1.5% in the ablative treatment group (95%CI: 14.1% to 35.9%; P < 0.001). This result corresponds to an NNT of 4. Full eradication of dysplasia and intestinal metaplasia were persistently achieved in most patients of the ablative group. Therefore, the authors conclude that ablative therapy should also be considered for patients with a confirmed LGD.
BE without dysplasia
Endoscopic eradication therapy could not yet be recommended in patients with BE without dysplasia, because the low risk of progression to EA (0.1% to 0.3% per year)[14,72-74] and the side effects potentially associated with the endoscopy therapy (10%-15%).
FOLLOW-UP AFTER ERADICATION
After endoscopic or surgery eradication of HGD, endoscopic follow-up is mandatory[75,76]. An evidence-based strategy for surveillance after subtotal esophagectomy is to perform endoscopy at 2, 5, and 10 years after surgery, and every 2-year once BE has been detected[29]. The follow-up interval for the endoscopic ablative therapy is still unclear.
CHEMOPREVENTION AND SYMPTOMATIC CONTROL IN BE
GERD therapy is clearly indicated in the presence of GERD symptoms and/or reflux esophagitis. Although chemoprevention with acid-suppressing drugs can not yet be recommended, some observational studies have found an association between anti-reflux therapy and a lower rate of progression to EA, even in patients without GERD symptoms[77]. These results indirectly suggest a cancer-protective role for proton pump inhibitors (PPIs) in BE, and are strong enough to warrant conventional-dose PPI treatment for patients who have no symptoms or endoscopic signs of GERD[11]. However, acid- suppressing therapies, specifically PPIs, have not proven to reduce risk of progression to dysplasia or cancer[2,3]. PPIs are also used to prevent acid reflux and allow for reepithelialization by squamous epithelium after EMR or ablation.
The risk of EA among patients treated with antireflux surgery, and among those who received medical treatment with PPIs is similar[78]. Thus, antireflux surgery does not protect against cancer, and its indications in BE patients are the same as in GERD patients.
There is currently no definitive evidence to advise the use of aspirin or other chemopreventive agents in BE patients. The use of aspirin is only recommended in BE patients with cardiovascular risk factors (for which aspirin therapy is indicated) because the benefit-risk balance is clearly favorable only in this situation.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 27, 2015
First decision: September 8, 2015
Article in press: February 16, 2016
P- Reviewer: Nagahara H S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
References
- 1.Barrett NR. Chronic peptic ulcer of the oesophagus and “oesophagitis”. Br J Surg. 1950;38:175–182. doi: 10.1002/bjs.18003815005. [DOI] [PubMed] [Google Scholar]
- 2.Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084–1091. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, Patel P, Kaye PV, Sanders S, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 4.Riddell RH, Odze RD. Definition of Barrett’s esophagus: time for a rethink--is intestinal metaplasia dead? Am J Gastroenterol. 2009;104:2588–2594. doi: 10.1038/ajg.2009.390. [DOI] [PubMed] [Google Scholar]
- 5.Ronkainen J, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E, Vieth M, Stolte M, Talley NJ, Agréus L. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825–1831. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 6.Rex DK, Cummings OW, Shaw M, Cumings MD, Wong RK, Vasudeva RS, Dunne D, Rahmani EY, Helper DJ. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125:1670–1677. doi: 10.1053/j.gastro.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Hayeck TJ, Kong CY, Spechler SJ, Gazelle GS, Hur C. The prevalence of Barrett’s esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus. 2010;23:451–457. doi: 10.1111/j.1442-2050.2010.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Soest EM, Dieleman JP, Siersema PD, Sturkenboom MC, Kuipers EJ. Increasing incidence of Barrett’s oesophagus in the general population. Gut. 2005;54:1062–1066. doi: 10.1136/gut.2004.063685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford AC, Forman D, Reynolds PD, Cooper BT, Moayyedi P. Ethnicity, gender, and socioeconomic status as risk factors for esophagitis and Barrett’s esophagus. Am J Epidemiol. 2005;162:454–460. doi: 10.1093/aje/kwi218. [DOI] [PubMed] [Google Scholar]
- 10.Huo X, Juergens S, Zhang X, Rezaei D, Yu C, Strauch ED, Wang JY, Cheng E, Meyer F, Wang DH, et al. Deoxycholic acid causes DNA damage while inducing apoptotic resistance through NF-κB activation in benign Barrett’s epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2011;301:G278–G286. doi: 10.1152/ajpgi.00092.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med. 2014;371:836–845. doi: 10.1056/NEJMra1314704. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute. Fast stats: esophagus cancer. Available from: http://seer.cancer.gov/faststats/selections.php.
- 13.Spechler SJ. Barrett’s esophagus: Clinical issues. Gastrointest Endosc Clin N Am. 2011;21:1–7. doi: 10.1016/j.giec.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung KW, Talley NJ, Romero Y, Katzka DA, Schleck CD, Zinsmeister AR, Dunagan KT, Lutzke LS, Wu TT, Wang KK, et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett’s esophagus: a population-based study. Am J Gastroenterol. 2011;106:1447–1455; quiz 1456. doi: 10.1038/ajg.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 16.Solaymani-Dodaran M, Logan RF, West J, Card T, Coupland C. Risk of oesophageal cancer in Barrett’s oesophagus and gastro-oesophageal reflux. Gut. 2004;53:1070–1074. doi: 10.1136/gut.2003.028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet. 2009;373:850–861. doi: 10.1016/S0140-6736(09)60487-6. [DOI] [PubMed] [Google Scholar]
- 18.Curvers WL, ten Kate FJ, Krishnadath KK, Visser M, Elzer B, Baak LC, Bohmer C, Mallant-Hent RC, van Oijen A, Naber AH, et al. Low-grade dysplasia in Barrett’s esophagus: overdiagnosed and underestimated. Am J Gastroenterol. 2010;105:1523–1530. doi: 10.1038/ajg.2010.171. [DOI] [PubMed] [Google Scholar]
- 19.Rastogi A, Puli S, El-Serag HB, Bansal A, Wani S, Sharma P. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc. 2008;67:394–398. doi: 10.1016/j.gie.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, Galanko JA, Bronner MP, Goldblum JR, Bennett AE, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 21.Spechler SJ. Barrett esophagus and risk of esophageal cancer: a clinical review. JAMA. 2013;310:627–636. doi: 10.1001/jama.2013.226450. [DOI] [PubMed] [Google Scholar]
- 22.Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology. 2011;140:e18–52; quiz e13. doi: 10.1053/j.gastro.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans JA, Early DS, Fukami N, Ben-Menachem T, Chandrasekhara V, Chathadi KV, Decker GA, Fanelli RD, Fisher DA, Foley KQ, et al. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012;76:1087–1094. doi: 10.1016/j.gie.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Shaheen NJ, Weinberg DS, Denberg TD, Chou R, Qaseem A, Shekelle P. Upper endoscopy for gastroesophageal reflux disease: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2012;157:808–816. doi: 10.7326/0003-4819-157-11-201212040-00008. [DOI] [PubMed] [Google Scholar]
- 25.Sharma P, Morales TG, Sampliner RE. Short segment Barrett’s esophagus--the need for standardization of the definition and of endoscopic criteria. Am J Gastroenterol. 1998;93:1033–1036. doi: 10.1111/j.1572-0241.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- 26.Rudolph RE, Vaughan TL, Storer BE, Haggitt RC, Rabinovitch PS, Levine DS, Reid BJ. Effect of segment length on risk for neoplastic progression in patients with Barrett esophagus. Ann Intern Med. 2000;132:612–620. doi: 10.7326/0003-4819-132-8-200004180-00003. [DOI] [PubMed] [Google Scholar]
- 27.Sharma P, Dent J, Armstrong D, Bergman JJ, Gossner L, Hoshihara Y, Jankowski JA, Junghard O, Lundell L, Tytgat GN, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & amp; M criteria. Gastroenterology. 2006;131:1392–1399. doi: 10.1053/j.gastro.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 28.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 29.Bennett C, Vakil N, Bergman J, Harrison R, Odze R, Vieth M, Sanders S, Gay L, Pech O, Longcroft-Wheaton G, et al. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology. 2012;143:336–346. doi: 10.1053/j.gastro.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tseng EE, Wu TT, Yeo CJ, Heitmiller RF. Barrett’s esophagus with high grade dysplasia: surgical results and long-term outcome--an update. J Gastrointest Surg. 2003;7:164–170; discussion 170-171. doi: 10.1016/s1091-255x(02)00153-1. [DOI] [PubMed] [Google Scholar]
- 31.Dunbar KB. Endoscopic eradication therapy for mucosal neoplasia in Barrett’s esophagus. Curr Opin Gastroenterol. 2013;29:446–453. doi: 10.1097/MOG.0b013e3283622848. [DOI] [PubMed] [Google Scholar]
- 32.Peters FP, Kara MA, Rosmolen WD, Aalders MC, Ten Kate FJ, Bultje BC, Krishnadath KK, Fockens P, van Lanschot JJ, van Deventer SJ, et al. Endoscopic treatment of high-grade dysplasia and early stage cancer in Barrett’s esophagus. Gastrointest Endosc. 2005;61:506–514. doi: 10.1016/s0016-5107(05)00063-5. [DOI] [PubMed] [Google Scholar]
- 33.Ell C, May A, Pech O, Gossner L, Guenter E, Behrens A, Nachbar L, Huijsmans J, Vieth M, Stolte M. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer) Gastrointest Endosc. 2007;65:3–10. doi: 10.1016/j.gie.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 34.Pech O, Behrens A, May A, Nachbar L, Gossner L, Rabenstein T, Manner H, Guenter E, Huijsmans J, Vieth M, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200–1206. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 35.Pouw RE, Wirths K, Eisendrath P, Sondermeijer CM, Ten Kate FJ, Fockens P, Devière J, Neuhaus H, Bergman JJ. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett’s esophagus with early neoplasia. Clin Gastroenterol Hepatol. 2010;8:23–29. doi: 10.1016/j.cgh.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Pouw RE, Seewald S, Gondrie JJ, Deprez PH, Piessevaux H, Pohl H, Rösch T, Soehendra N, Bergman JJ. Stepwise radical endoscopic resection for eradication of Barrett’s oesophagus with early neoplasia in a cohort of 169 patients. Gut. 2010;59:1169–1177. doi: 10.1136/gut.2010.210229. [DOI] [PubMed] [Google Scholar]
- 37.Overholt BF, Lightdale CJ, Wang KK, Canto MI, Burdick S, Haggitt RC, Bronner MP, Taylor SL, Grace MG, Depot M. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488–498. doi: 10.1016/j.gie.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 38.Pohl H, Sonnenberg A, Strobel S, Eckardt A, Rösch T. Endoscopic versus surgical therapy for early cancer in Barrett’s esophagus: a decision analysis. Gastrointest Endosc. 2009;70:623–631. doi: 10.1016/j.gie.2008.11.047. [DOI] [PubMed] [Google Scholar]
- 39.Westerterp M, Koppert LB, Buskens CJ, Tilanus HW, ten Kate FJ, Bergman JJ, Siersema PD, van Dekken H, van Lanschot JJ. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch. 2005;446:497–504. doi: 10.1007/s00428-005-1243-1. [DOI] [PubMed] [Google Scholar]
- 40.Vieth M, Ell C, Gossner L, May A, Stolte M. Histological analysis of endoscopic resection specimens from 326 patients with Barrett’s esophagus and early neoplasia. Endoscopy. 2004;36:776–781. doi: 10.1055/s-2004-825802. [DOI] [PubMed] [Google Scholar]
- 41.Prasad GA, Wu TT, Wigle DA, Buttar NS, Wongkeesong LM, Dunagan KT, Lutzke LS, Borkenhagen LS, Wang KK. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology. 2009;137:815–823. doi: 10.1053/j.gastro.2009.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Badreddine RJ, Prasad GA, Wang KK, Song LM, Buttar NS, Dunagan KT, Lutzke LS, Borkenhagen LS. Prevalence and predictors of recurrent neoplasia after ablation of Barrett’s esophagus. Gastrointest Endosc. 2010;71:697–703. doi: 10.1016/j.gie.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pouw RE, Gondrie JJ, Rygiel AM, Sondermeijer CM, ten Kate FJ, Odze RD, Vieth M, Krishnadath KK, Bergman JJ. Properties of the neosquamous epithelium after radiofrequency ablation of Barrett’s esophagus containing neoplasia. Am J Gastroenterol. 2009;104:1366–1373. doi: 10.1038/ajg.2009.88. [DOI] [PubMed] [Google Scholar]
- 44.Semlitsch T, Jeitler K, Schoefl R, Horvath K, Pignitter N, Harnoncourt F, Siebenhofer A. A systematic review of the evidence for radiofrequency ablation for Barrett’s esophagus. Surg Endosc. 2010;24:2935–2943. doi: 10.1007/s00464-010-1087-x. [DOI] [PubMed] [Google Scholar]
- 45.Beaumont H, Gondrie JJ, McMahon BP, Pouw RE, Gregersen H, Bergman JJ, Boeckxstaens GE. Stepwise radiofrequency ablation of Barrett’s esophagus preserves esophageal inner diameter, compliance, and motility. Endoscopy. 2009;41:2–8. doi: 10.1055/s-0028-1103451. [DOI] [PubMed] [Google Scholar]
- 46.Phoa KN, Pouw RE, van Vilsteren FG, Sondermeijer CM, Ten Kate FJ, Visser M, Meijer SL, van Berge Henegouwen MI, Weusten BL, Schoon EJ, et al. Remission of Barrett’s esophagus with early neoplasia 5 years after radiofrequency ablation with endoscopic resection: a Netherlands cohort study. Gastroenterology. 2013;145:96–104. doi: 10.1053/j.gastro.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 47.van Vilsteren FG, Pouw RE, Seewald S, Alvarez Herrero L, Sondermeijer CM, Visser M, Ten Kate FJ, Yu Kim Teng KC, Soehendra N, Rösch T, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett’s oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut. 2011;60:765–773. doi: 10.1136/gut.2010.229310. [DOI] [PubMed] [Google Scholar]
- 48.Fleischer DE, Overholt BF, Sharma VK, Reymunde A, Kimmey MB, Chuttani R, Chang KJ, Muthasamy R, Lightdale CJ, Santiago N, et al. Endoscopic radiofrequency ablation for Barrett’s esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy. 2010;42:781–789. doi: 10.1055/s-0030-1255779. [DOI] [PubMed] [Google Scholar]
- 49.Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett’s Esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1245–1255. doi: 10.1016/j.cgh.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inadomi JM, Somsouk M, Madanick RD, Thomas JP, Shaheen NJ. A cost-utility analysis of ablative therapy for Barrett’s esophagus. Gastroenterology. 2009;136:2101–2114.e1-6. doi: 10.1053/j.gastro.2009.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larghi A, Lightdale CJ, Memeo L, Bhagat G, Okpara N, Rotterdam H. EUS followed by EMR for staging of high-grade dysplasia and early cancer in Barrett’s esophagus. Gastrointest Endosc. 2005;62:16–23. doi: 10.1016/s0016-5107(05)00319-6. [DOI] [PubMed] [Google Scholar]
- 52.Peters FP, Brakenhoff KP, Curvers WL, Rosmolen WD, Fockens P, ten Kate FJ, Krishnadath KK, Bergman JJ. Histologic evaluation of resection specimens obtained at 293 endoscopic resections in Barrett’s esophagus. Gastrointest Endosc. 2008;67:604–609. doi: 10.1016/j.gie.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 53.Hull MJ, Mino-Kenudson M, Nishioka NS, Ban S, Sepehr A, Puricelli W, Nakatsuka L, Ota S, Shimizu M, Brugge WR, et al. Endoscopic mucosal resection: an improved diagnostic procedure for early gastroesophageal epithelial neoplasms. Am J Surg Pathol. 2006;30:114–118. doi: 10.1097/01.pas.0000180438.56528.a0. [DOI] [PubMed] [Google Scholar]
- 54.Mino-Kenudson M, Hull MJ, Brown I, Muzikansky A, Srivastava A, Glickman J, Park DY, Zuckerberg L, Misdraji J, Odze RD, et al. EMR for Barrett’s esophagus-related superficial neoplasms offers better diagnostic reproducibility than mucosal biopsy. Gastrointest Endosc. 2007;66:660–66; quiz 767, 769. doi: 10.1016/j.gie.2007.02.063. [DOI] [PubMed] [Google Scholar]
- 55.Moss A, Bourke MJ, Hourigan LF, Gupta S, Williams SJ, Tran K, Swan MP, Hopper AD, Kwan V, Bailey AA. Endoscopic resection for Barrett’s high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol. 2010;105:1276–1283. doi: 10.1038/ajg.2010.1. [DOI] [PubMed] [Google Scholar]
- 56.Stein HJ, Feith M, Bruecher BL, Naehrig J, Sarbia M, Siewert JR. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242:566–573; discussion 573-575. doi: 10.1097/01.sla.0000184211.75970.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bollschweiler E, Baldus SE, Schröder W, Prenzel K, Gutschow C, Schneider PM, Hölscher AH. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy. 2006;38:149–156. doi: 10.1055/s-2006-924993. [DOI] [PubMed] [Google Scholar]
- 58.Lopes CV, Hela M, Pesenti C, Bories E, Caillol F, Monges G, Giovannini M. Circumferential endoscopic resection of Barrett’s esophagus with high-grade dysplasia or early adenocarcinoma. Surg Endosc. 2007;21:820–824. doi: 10.1007/s00464-006-9187-3. [DOI] [PubMed] [Google Scholar]
- 59.Giovannini M, Bories E, Pesenti C, Moutardier V, Monges G, Danisi C, Lelong B, Delpero JR. Circumferential endoscopic mucosal resection in Barrett’s esophagus with high-grade intraepithelial neoplasia or mucosal cancer. Preliminary results in 21 patients. Endoscopy. 2004;36:782–787. doi: 10.1055/s-2004-825813. [DOI] [PubMed] [Google Scholar]
- 60.Seewald S, Akaraviputh T, Seitz U, Brand B, Groth S, Mendoza G, He X, Thonke F, Stolte M, Schroeder S, et al. Circumferential EMR and complete removal of Barrett’s epithelium: a new approach to management of Barrett’s esophagus containing high-grade intraepithelial neoplasia and intramucosal carcinoma. Gastrointest Endosc. 2003;57:854–859. doi: 10.1016/s0016-5107(03)70020-0. [DOI] [PubMed] [Google Scholar]
- 61.Gondrie JJ, Pouw RE, Sondermeijer CM, Peters FP, Curvers WL, Rosmolen WD, Krishnadath KK, Ten Kate F, Fockens P, Bergman JJ. Stepwise circumferential and focal ablation of Barrett’s esophagus with high-grade dysplasia: results of the first prospective series of 11 patients. Endoscopy. 2008;40:359–369. doi: 10.1055/s-2007-995567. [DOI] [PubMed] [Google Scholar]
- 62.Gondrie JJ, Pouw RE, Sondermeijer CM, Peters FP, Curvers WL, Rosmolen WD, Ten Kate F, Fockens P, Bergman JJ. Effective treatment of early Barrett’s neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy. 2008;40:370–379. doi: 10.1055/s-2007-995589. [DOI] [PubMed] [Google Scholar]
- 63.Rees JR, Lao-Sirieix P, Wong A, Fitzgerald RC. Treatment for Barrett’s oesophagus. Cochrane Database Syst Rev. 2010;(1):CD004060. doi: 10.1002/14651858.CD004060.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Fayter D, Corbett M, Heirs M, Fox D, Eastwood A. A systematic review of photodynamic therapy in the treatment of pre-cancerous skin conditions, Barrett’s oesophagus and cancers of the biliary tract, brain, head and neck, lung, oesophagus and skin. Health Technol Assess. 2010;14:1–288. doi: 10.3310/hta14370. [DOI] [PubMed] [Google Scholar]
- 65.Overholt BF, Wang KK, Burdick JS, Lightdale CJ, Kimmey M, Nava HR, Sivak MV, Nishioka N, Barr H, Marcon N, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett’s high-grade dysplasia. Gastrointest Endosc. 2007;66:460–468. doi: 10.1016/j.gie.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 66.Hage M, Siersema PD, van Dekken H, Steyerberg EW, Haringsma J, van de Vrie W, Grool TE, van Veen RL, Sterenborg HJ, Kuipers EJ. 5-aminolevulinic acid photodynamic therapy versus argon plasma coagulation for ablation of Barrett’s oesophagus: a randomised trial. Gut. 2004;53:785–790. doi: 10.1136/gut.2003.028860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li YM, Li L, Yu CH, Liu YS, Xu CF. A systematic review and meta-analysis of the treatment for Barrett’s esophagus. Dig Dis Sci. 2008;53:2837–2846. doi: 10.1007/s10620-008-0257-3. [DOI] [PubMed] [Google Scholar]
- 68.Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 69.Manner H, May A, Pech O, Gossner L, Rabenstein T, Günter E, Vieth M, Stolte M, Ell C. Early Barrett’s carcinoma with “low-risk” submucosal invasion: long-term results of endoscopic resection with a curative intent. Am J Gastroenterol. 2008;103:2589–2597. doi: 10.1111/j.1572-0241.2008.02083.x. [DOI] [PubMed] [Google Scholar]
- 70.Vieth M. Low-grade dysplasia in Barrett’s esophagus - an innocent bystander? Contra. Endoscopy. 2007;39:647–649. doi: 10.1055/s-2007-966638. [DOI] [PubMed] [Google Scholar]
- 71.Phoa KN, van Vilsteren FG, Weusten BL, Bisschops R, Schoon EJ, Ragunath K, Fullarton G, Di Pietro M, Ravi N, Visser M, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311:1209–1217. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 72.Desai TK, Krishnan K, Samala N, Singh J, Cluley J, Perla S, Howden CW. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: a meta-analysis. Gut. 2012;61:970–976. doi: 10.1136/gutjnl-2011-300730. [DOI] [PubMed] [Google Scholar]
- 73.Wani S, Falk G, Hall M, Gaddam S, Wang A, Gupta N, Singh M, Singh V, Chuang KY, Boolchand V, et al. Patients with nondysplastic Barrett’s esophagus have low risks for developing dysplasia or esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2011;9:220–227; quiz e226. doi: 10.1016/j.cgh.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 74.Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, Murray LJ. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049–1057. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hamilton SR, Yardley JH. Regnerative of cardiac type mucosa and acquisition of Barrett mucosa after esophagogastrostomy. Gastroenterology. 1977;72:669–675. [PubMed] [Google Scholar]
- 76.Dresner SM, Griffin SM, Wayman J, Bennett MK, Hayes N, Raimes SA. Human model of duodenogastro-oesophageal reflux in the development of Barrett’s metaplasia. Br J Surg. 2003;90:1120–1128. doi: 10.1002/bjs.4169. [DOI] [PubMed] [Google Scholar]
- 77.Kastelein F, Spaander MC, Steyerberg EW, Biermann K, Valkhoff VE, Kuipers EJ, Bruno MJ. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2013;11:382–388. doi: 10.1016/j.cgh.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 78.Spechler SJ. Does Barrett’s esophagus regress after surgery (or proton pump inhibitors)? Dig Dis. 2014;32:156–163. doi: 10.1159/000357184. [DOI] [PubMed] [Google Scholar]


