Abstract
AIM: To investigate the nature and severity of AE related to sacral neurostimulation (SNS).
METHODS: Based on Pubmed and Embase searches, we identified published trials and case series of SNS for fecal incontinence (FI) and extracted data on adverse events, requiring an active intervention. Those problems were operationally defined as infection, device removal explant or need for lead and/or generator replacement. In addition, we analyzed the Manufacturer and User Device Experience registry of the Federal Drug Administration for the months of August - October of 2015. Events were included if the report specifically mentioned gastrointestinal (GI), bowel and FI as indication and if the narrative did not focus on bladder symptoms. The classification, reporter, the date of the recorded complaint, time between initial implant and report, the type of AE, steps taken and outcome were extracted from the report. In cases of device removal or replacement, we looked for confirmatory comments by healthcare providers or the manufacturer.
RESULTS: Published studies reported adverse events and reoperation rates for 1954 patients, followed for 27 (1-117) mo. Reoperation rates were 18.6% (14.2-23.9) with device explants accounting for 10.0% (7.8-12.7) of secondary surgeries; rates of device replacement or explant or pocket site and electrode revisions increased with longer follow up. During the period examined, the FDA received 1684 reports of AE related to SNS with FI or GI listed as indication. A total of 652 reports met the inclusion criteria, with 52.7% specifically listing FI. Lack or loss of benefit (48.9%), pain or dysesthesia (27.8%) and complication at the generator implantation site (8.7%) were most commonly listed. Complaints led to secondary surgeries in 29.7% of the AE. Reoperations were performed to explant (38.2%) or replace (46.5%) the device or a lead, or revise the generator pocket (14.6%). Conservative management changes mostly involved changes in stimulation parameters (44.5%), which successfully addressed concerns in 35.2% of cases that included information about treatment results.
CONCLUSION: With reoperation rates around 20%, physicians need to fully disclose the high likelihood of complications and secondary interventions and exhaust non-invasive treatments, including transcutaneous stimulation paradigms.
Keywords: Sacral nerve stimulation, Implanted medical devices, Treatment complications, Defecation disorders, Device registry
Core tip: Sacral neuromodulation can improve fecal incontinence refractory to other treatments. However, adverse events are very common and often require additional operations. Many of the reported patient concerns surface early after stimulator implantation, respond to changes in stimulation parameters and may thus be considered a part of the routine maintenance of this treatment modality. Nonetheless, rates of surgical re-interventions are high and increase over time. Physicians counseling patients about this treatment for fecal incontinence should emphasize the likely need for such secondary surgeries and consider emerging non-invasive treatment options. In addition, prospective studies should compare less invasive paradigms, such as transcutaneous stimulation, with permanently implanted devices to more clearly define their differential impact.
INTRODUCTION
Fecal incontinence affects millions of Americans with an estimated prevalence between 6%-10% in adults[1]. While dietary changes, physical therapy with or without biofeedback, bulking agents or medications alleviate or control symptoms[2-5], a significant number of patients will not respond to these interventions. In March of 2011, the Federal Drug Administration (FDA) approved sacral neurostimulation (SNS) for patients with otherwise refractory fecal incontinence. Short-term studies consistently show high response rates[6,7]. However, the lasting benefit of SNS is difficult to define with an apparent increase in device explants over time[8]. Consistent with this impression, an early analysis of published data suggested efficacy in select groups, but emphasized the high likelihood of secondary surgeries[9]. A recent systematic review of data from published studies concluded that differences in endpoints and reporting complicated the overall assessment; despite remaining conceptual concerns about the validity of their estimates, the authors pooled trial data for 518 patients with a rate of complete continence of 36.5%[10].
Research frontier
With lasting improvement rates around 50% or even less, a better understanding of adverse effects is essential to appropriately weigh risks and benefits.
Innovation and breakthrough
The aim of this study was to systematically analyze published data on side effects based on a systematic literature search and define the type, relative frequency and resulting interventions of adverse events. We also performed an assessment of adverse events described in the Manufacturer and User Device Experience (MAUDE) databank of the FDA, which offers detailed descriptions of device-related problems and concerns, but has been underutilized in outcomes research related to gastrointestinal disorders.
MATERIALS AND METHODS
Literature search
We queried the PubMed and Embase databanks using sacral neurostimulation or sacral nerve stimulation and fecal incontinence as search terms. We retrieved full length articles if the title and abstract described SNS as treatment modality and if cohorts included at least 5 patients with fecal incontinence. We abstracted sample size, definition of endpoints, results defined as responders or changes in symptom scores, duration of follow up and adverse events, separately examining pocket site infections, pain or displacement at the generator pocket, pain in other areas, device erosion, device explant, lead migration or break, battery depletion and lack of effectiveness. Whenever possible, we assessed the total number of reoperation. Only data focusing on the use definite rather than temporary stimulation were included.
MAUDE databank
We used the electronic search option of the MAUDE database (http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/TextResults.cfm) for the years 2005 to 2010 as a reference time period prior to FDA approval of SNS for fecal incontinence, and extended our queries to October 2015, the most recent month with completed reporting at the time of the study. We recorded the number of reports and reviewed the detailed descriptions of adverse events for the period between August 1 and October 31, 2015, using the key word Interstim as trade name of the device. The MAUDE databank collects voluntarily reported adverse events related to the use of medical devices. Unlike users or healthcare providers, manufacturers are required to forward all information about side effects to the FDA. Prior work demonstrated that information obtained from this site enables the identification of rare, but potentially important adverse events, and provides insight into the type and relative frequency of complications related to medical devices marketed in the United States[11-13]. Reports include information provided by manufacturers, distributors, healthcare providers, and patients, list device type, date of event report and a narrative of the event as well as information about follow up interactions and potential responses of the manufacturer. Patient identifiers have been removed, and dates are limited to the reporting day and year of initial implant or other intervention, thus protecting privacy and not allowing linkage between the publically available records and other data banks. As SNS received FDA approval for fecal incontinence in 2011, we only included reports that provided information about the year of implantation and that fell between 2011 and 2015. We searched the narrative for indications and key symptoms and extracted data only if the terms fecal incontinence, bowel or gastrointestinal dysfunction were listed and if the narrative did not focus on urinary symptoms. We abstracted the date of the report, year of permanent stimulator implantation, the reporter (healthcare provider, manufacturer representative or patient/patient’s relative or partner), and the classification of the complaint as entered into the database, the nature of the problem, steps taken and outcome. Reported outcomes were summarized as resolved, persisting or unknown. We separately coded operative interventions, assessed whether they were confirmed by the company, dividing them into groups based on the nature of the steps as explant, replacement, lead or pocket site revision.
Statistical analysis
We used Comprehensive Meta-Analysis version 2.0 (Biostat, Englewood, NJ) to calculate incidence of adverse events based on the more conservative random effect method. Unless mentioned otherwise, data are given as mean with 95%CI. Categorical variables were compared with the χ2 test.
RESULTS
Published data
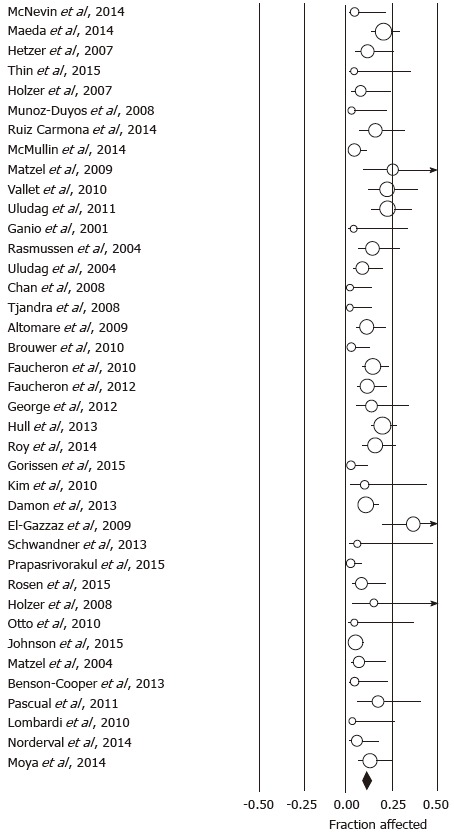
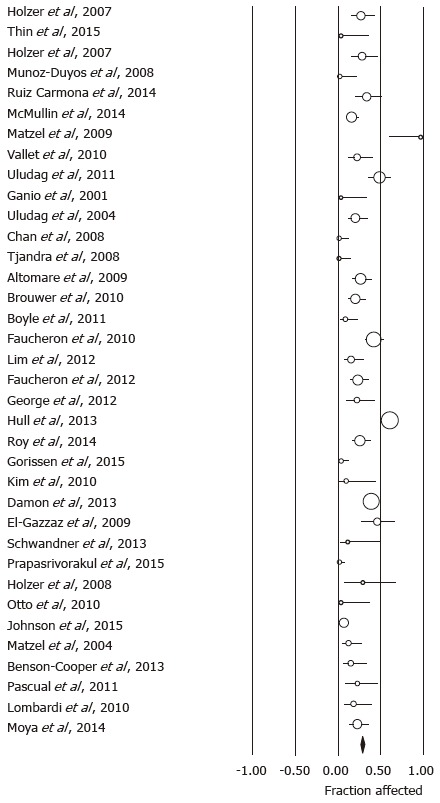
Using the predefined terms and filtering information based on English language of human studies and publication as full length article, the search yielded a total of 45 articles describing distinct patient cohorts from different institutions or collaborative groups, which provided information about adverse events and included more than 5 patients treated fecal incontinence a permanently implanted system (Table 1). The total number of patients was 1953 followed for a median time period of 27 mo (range: 1-117 mo). Definition and description of variables differed significantly between studies as did follow up times. The most detailed information was found in 2 studies that prospectively monitored a total of 201 patients and recorded 828 incident adverse events over a period 5 years, most of which were related to loss of benefit[8,14]. While most of these side effects responded to conservative therapy, one fifth of the cohorts had their devices explanted at the time of the last follow up with additional surgeries in a significant number of patients. Only five additional publications discussed adverse events with some detail, but reported a significantly lower incidence[15-19]. We therefore focused on serious adverse events that typically prompted secondary interventions and were more consistently described. We found information about infectious complications after implantation of a permanent device in 44 studies with 1953 patients. The pooled rate of infection was 5.1% (4.1-6.4) (Figure 1) without significant heterogeneity between trials (I2 = 0%). Device explants were largely due to infection, but were also caused by generator erosion through the skin or other local complications at the pocket site and lack of benefit, thus leading to a higher rate of reoperation. A total of 39 studies covering 1810 patients provided information about explant rates at the end of their follow up period, with of an average of 10.0% (7.8-12.7) (I2 = 54.0%; Figure 2) and a significant increase with the duration of follow up (Figure 3A). Lead complications, battery depletion or pain all contribute to additional intervention, with an overall re-operation rate of 18.6% (14.2-23.9) (I2 = 80.5%) based on cohorts with a total of 1784 patients (Figure 4). Reoperation rates rose with longer follow-up times (Figure 3B).
Table 1.
Studies included
| Sample | Follow-up1 | Response |
Adverse Events |
Ref. | ||
| Infection | Explant | Reoperation | ||||
| 61 | 13 | 0 | 1 | 2 | [60] | |
| 16 | 0 | 0 | 3 | [61] | ||
| 85 | 24 | 0 | 3 | 13 | [45] | |
| 39 | 12 | 2 | 2 | 2 | [62] | |
| 60 | 47 | 17 | 2 | 9 | 15 | [18] |
| 101 | 60 | 26 | 2 | 20 | 20 | [14] |
| 50 | 55.5 | 2 | 6 | 11 | [63] | |
| 42 | 0 | 2 | 5 | [64] | ||
| 23 | 114 | 2 | 3 | 5 | [65] | |
| 101 | 62 | 1 | 10 | 39 | [66] | |
| 37 | 17 | 27 | 0 | 0 | 3 | [50] |
| 41 | 51 | 0 | 0 | 6 | [67] | |
| 34 | 24 | 83 | 1 | 2 | 4 | [19] |
| 37 | 6 | 3 | 5 | 5 | [68] | |
| 58 | 74 | 37 | 0 | 6 | 15 | [49] |
| 12 | 117 | 0 | 3 | 14 | [69] | |
| 29 | 34 | 0 | 0 | 0 | [70] | |
| 7 | 32 | 1 | 1 | 2 | [71] | |
| 53 | 12 | 0 | 0 | 0 | [72] | |
| 10 | 0 | 0 | 1 | [73] | ||
| 29 | 35 | 2 | 2 | 8 | [74] | |
| 37 | 13 | 2 | 4 | 10 | [75] | |
| 14 | 6 | 0 | 0 | 0 | [76] | |
| 87 | 48.5 | 4 | 12 | 36 | [77] | |
| 40 | 3 | 3 | 7 | [78] | ||
| 15 | 6 | 10 | 0 | 0 | 0 | [47] |
| 85 | 12 | 31 | 0 | 1 | 1 | [54] |
| 145 | 12 | 137 | 5 | 6 | 15 | [6] |
| 29 | 0 | 1 | 1 | [79] | ||
| 27 | 10.7 | 3 | 1 | 4 | [80] | |
| 9 | 12 | 0 | 0 | 1 | [81] | |
| 120 | 60 | 90 | 12 | 30 | 72 | [8] |
| 57 | 63 | 2 | 6 | 12 | [82] | |
| 8 | 0 | 0 | 1 | [83] | ||
| 18 | 17 | 2 | 3 | 4 | [84] | |
| 50 | 74 | 4 | 11 | 24 | [85] | |
| 11 | 14 | 6 | 0 | 1 | 1 | [51] |
| 55 | 37 | 1 | 1 | 11 | [16] | |
| 23 | 38 | 0 | 0 | 4 | [15] | |
| 32 | 33 | 3 | 7 | 10 | [17] | |
| 53 | 12 | 0 | 0 | 0 | [7] | |
| 50 | 12 | 2 | 4 | 10 | [86] | |
| 10 | 29.5 | 10 | 2 | 8 | 8 | [53] |
| 33 | 27 | 0 | 5 | 11 | [87] | |
| 16 | 15.5 | 0 | 0 | 0 | [88] | |
Follow up in months.
Figure 1.
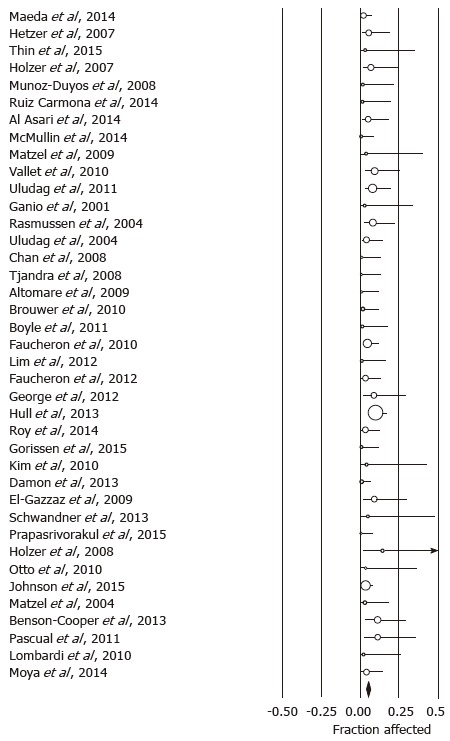
Forrest plot showing infection rates as fraction of the sample size in cohorts treated with sacral neurostimulation for fecal incontinence.
Figure 2.
Forrest plot showing explant rates as fraction of the sample size in cohorts treated with sacral neurostimulation for fecal incontinence.
Figure 3.
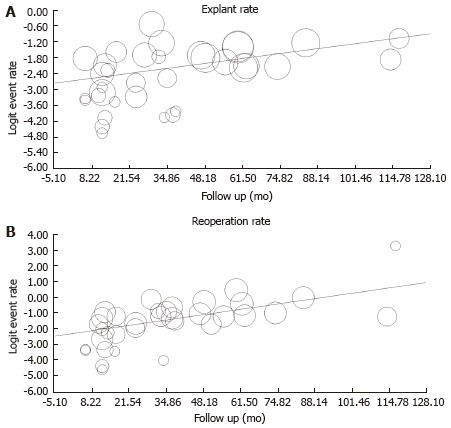
Meta-regression showing the time dependent increase in explant. A: Q = 9.35; P = 0.002 and reoperation panel; B: Q = 20.3; P < 0.001 rates after sacral neurostimulation initiation.
Figure 4.
Forrest plot showing reoperation rates as fraction of the sample size in cohorts treated with sacral neurostimulation for fecal incontinence.
MAUDE databank
Monthly reporting of adverse events related to the Interstim device ranged around 10 incidents per month in 2005, rose about tenfold within the next 3 years, and then remained stable until in the year prior to FDA approval of SNS for fecal incontinence [114.5 (65.2-163.8)]. As shown in Figure 5A, reported concerns more than tripled since. Focusing on the time from August to October 2015, the FDA received a total of 1684 reports of problems related to SNS, with 652 reports meeting the inclusion criteria as they mentioned GI as an indication for SNS therapy and did not focus on urinary symptoms. The narrative specifically referred to bowel dysfunction or fecal incontinence in 278 (42.6%) case forms. Within the same time period of 2014, only 4% of 1250 complaints listed GI or fecal incontinence as indication for stimulator implantation. In contrast to narratives reviewed for 2015, most reports did not include any information about treatment indications or targets, suggesting a change in recording of data as a potential confounder. The majority of adverse events were reported within the first two years after stimulator implantation without differences between the entire cohort and subgroup undergoing SNS for fecal incontinence (Figure 5B). The majority of reports came from patients, their partners or family members (71.6%). Company representatives (15.7%) or healthcare providers (12.7%) accounted for the remaining reports. Reports were classified as injury (65.0%) or malfunction (35.0%) with a significant overlap in adverse events described (Table 2).
Figure 5.
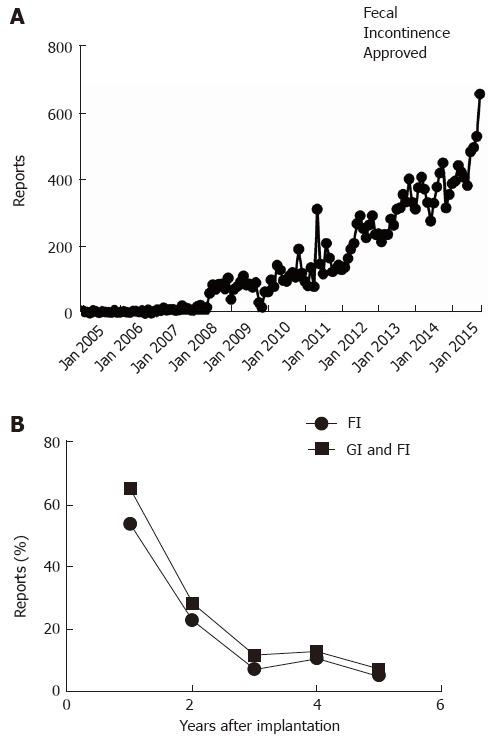
Manufacturer and user device experience databank. A: Reports about adverse events related to therapy with Interstim are plotted as a function of month of posting by the Federal Drug Administration; B: The lower panel depicts the percentage of reports mentioning gastrointestinal problems (squares) or specifically fecal incontinence (circles) as indication for sacral neurostimulation.
Table 2.
Reported problems based on classification of adverse effects
| Reported problem | Injury (n = 228) | Malfunction (n = 424) | P value |
| Battery depletion | 9 | 6 | 0.057 |
| Generator displacement | 11 | 5 | 0.007 |
| Pocket erosion | 9 | 0 | < 0.001 |
| Infection | 21 | 0 | < 0.001 |
| Pain | 41 | 102 | 0.091 |
| Lack of benefit | 70 | 243 | < 0.001 |
| Lead problems | 40 | 30 | < 0.001 |
| Other | 27 | 9 |
The identification of appropriate candidates for SNS proceeds in a two-step process with the initial phase utilizing insertion of temporary electrodes and an external device, followed by the implantation of a permanent stimulator only if patients experience at least 50% improvement during the 2 wk trial. Despite these stringent criteria, nearly half of the reported problems focused on lack or loss of benefit (49.7%). Results were similar in the cohort with fecal incontinence listed as the primary indication (51.8%). As was true for the overall pattern, 51.1% of the reports were filed within the first year after implantation, 24% in year 2, 8.6% in year 3, 10.8% in year 4 and 5.5% in year 5. Figure 6A shows the primary causes of concern. Pain or paresthesias accounted for 14.9% of the complaints (year 1: 78.4%; year 2: 3.1%; year 3: 13.4%; year 4: 5.1%), with 35.1% of these reports specifically referring to the generator site as affected area. Lead-related problems accounted for 10.7% of the reports and similarly surfaced primarily in the first year (62.9%), with a subsequent decrease over time (15.7% in year 2, 17.1% in year 3, 4.3% in year 4). Thirty reports (4.6%) described programming problems (year 1: 46.7%; year 2: 23.3%; year 3: 13.3%; year 4: 13.3%; year 5: 3.3%), which were related to the patient programmer in 43.3% of the cases. When reports listed a second concern (130; 19.9%), pain was most commonly brought up accounting for 40.8% of the problems, followed by limited benefit (16.1%) and concerns about possible electromagnetic interference (13.1%). Table 3 depicts secondary adverse events for the most common primary concerns.
Figure 6.
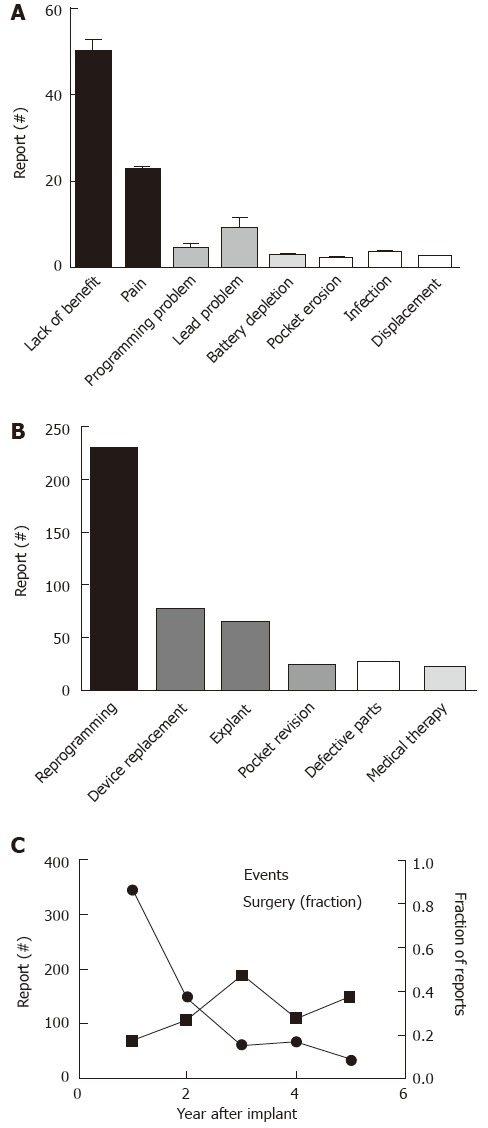
Concerns and interventions related to Interstim treatment and described in manufacturer and user device experience. A: Depicts the number of reported events sorted based on the primary concerns; B: Shows the main corrective steps taken (“Defective Parts” refers to intra-operative problems with device components requiring exchange); C: The proportions of operatively treated interventions is plotted in relation to the overall number of reported adverse events.
Table 3.
Relationship between common primary and secondary concerns
| Primary concern | No. of reports | Secondary concern |
| Lack of benefit | 74 | Battery depletion: n = 2 Generator displacement: n = 3 Electromagnetic interference: n = 7 Lead problem: n = 3 Pain: n = 43 Programming problems: n = 14 Other: n = 2 |
| Pain or discomfort | 32 | Generator displacement: n = 1 Electromagnetic interference: n = 8 Lack of benefit: n = 11 Lead problem: n = 1 Pain (second type): n = 4 Programming problems: n = 5 Other: n = 2 |
| Pocket site complications | 8 | Lack of benefit: n = 1 Lead problem: n = 2 Pain: n = 5 |
| Lead problem | 5 | Generator displacement: n = 1 Lack of benefit: n = 3 Pain: n = 1 |
| Programming problem | 4 | Electromagnetic interference: n = 2 Lack of benefit: n = 1 Pain: n = 1 |
The narrative did not include any information about corrective actions in 270 cases. As depicted in Figure 6B, most reported problems prompted adjustment of stimulation parameter (35.0%). Exactly one quarter of the concerns led to secondary operative interventions (n = 163; year 1: 37.4%; year 2: 24.5%; year 3: 17.8%; year 4: 11.7%; year 5: 8.6%). Re-interventions were nearly equally split between explants (39.3%) and replacements (41.7%). Isolated lead replacement accounted for only 4.3% of the reports. Pocket revisions were responsible for 14.1%, and minor operative revisions in 0.6%. Table 4 lists steps taken in relation to the reported primary concerns. While the majority of reports fell within the first two years after the original implant, the relative likelihood of undergoing secondary operative interventions increased significantly over time (χ2 = 18.5, P < 0.001; Figure 6C).
Table 4.
Reported interventions based on patient problems
| Primary concern | Sample | Conservative therapy | Operative therapy |
| Lack of benefit | 325 | Stimulation adjusted: 160 Medication: 3 System check: 7 | Explant: 22 Replacement: 17 Pocket revision: 3 |
| Pain or discomfort | 97 | Stimulation adjusted: 34 Medication: 4 System check: 2 | Explant: 8 Replacement: 1 Pocket revision: 7 |
| Lead problem | 70 | Stimulation adjusted: 3 System check: 2 | Explant: 1 Replacement: 36 |
| Programming problems | 30 | Stimulation adjusted: 11 | Replacement: 1 |
The most commonly described corrective actions are listed for key concerns addressed in the narratives. Limited or lacking information accounts for the differences between total sample size of the subgroups defined by their primary concerns.
The narratives included some information about the outcome in 249 cases (38.2%). Dichotomizing assessments as persisting or solved, all 27 intraoperative problems had been successfully addressed. Reports described adjustments of stimulation parameters in 176 cases, with sufficient benefit in 35.2%. Secondary surgeries solved problems in 17 (68%) of 25 patients based on follow up information. In the remaining cases, medications (n = 7 of 9), exchange of the personal programmer (n = 2 of 2) or no specific intervention (n = 3 of 9) led to resolution of reported problems.
DISCUSSION
Fecal incontinence carries a significant burden with impaired quality of life due to stigmatization and subjective fears about embarrassing situation, leading to withdrawal and isolation[20]. Dietary, medical and surgical intervention often leave patients with significant residual problems[21-24], thus prompting the need to look for novel and more effective treatments. Sacral nerve stimulation for urinary incontinence had been introduced more than 20 years ago[25] and led to systematic studies in fecal incontinence soon afterwards[26]. More than 10 years after FDA approval, Laudano et al[27] observed a significant increase in implantations for urological indications in Medicare recipients. During this time period, more providers adopted this technique, suggesting more widespread acceptance of SNS as an appropriate treatment modality[28]. This apparent rise correlated with an increase in the number of adverse events listed in MAUDE. A similar pattern may have contributed to the tripling in concerns received by the FDA since approval of SNS for fecal incontinence as shown in this study. However, narratives only recently include information about indications for SNS therapy, thus not allowing us to address to what extend fecal incontinence therapy contributed to these changes.
Even though response to temporary stimulation is a prerequisite for permanent stimulator implantation, most of the concerns focused on lack or loss of benefit, which accounted for half of the primary problems described in the narrative. Conceptually, one may question whether lack or loss of benefit is truly an adverse event. Adjustment of stimulation parameters effectively resolved many of the reported problems, which could thus be seen as analogous to dosing changes in pharmacotherapy. Similarly, any treatment based on electrical stimulation will require energy and will, therefore, deplete the battery over time. Unless the need for battery replacement surfaces very early after stimulator implantation, it may also be considered routine maintenance of electrotherapy. The inclusion of such anticipated and often correctable problems as adverse events obviously inflates the number of side effects and may paint a more negative picture of SNS. As already indicated, the clinical relevance of some of the episodes registered was limited, whether it was transient loss of benefit due to programming issues or an expired lead that had been discovered and replaced during surgery. Despite this theoretical shortcoming, we chose an approach that still accepts these occurrences as adverse events, as they were specifically reported to the FDA as an unanticipated problem that was sufficiently relevant to the affected individual to contact the agency. Especially in treatments that target quality of life, such as management of fecal incontinence, the perceived distress of an undesired outcome should count. Such a strategy also falls in line with operational definitions of side effects used in prospective studies. One large cohort study that followed 101 patients over 5 years reported more than 500 adverse events, most of which revolved around loss efficacy and prompted changes in stimulation parameters in about 80%[14]. Interestingly, only about one third of the changes in stimulation parameters led to a resolution of concerns, which correlates well with our results. Similarly high rates of adverse events were seen in a large consortium study of SNS for fecal incontinence[8] and in a cohort treated with mixed indications[29]. During 5 years of follow up, 46 of 120 patients underwent 72 additional operative interventions[8]. These numbers are significantly higher than data posted by Medtronics based on a prospective registry following 490 patients treated for urinary incontinence with a total number of 134 adverse events[30]. This apparent discrepancy may in part be due to the relatively short period of observation in the device maker’s registry. Consistent with this interpretation, preliminary data on lead survival in this cohort suggest rising rates of problems, which exceed 20% after 33 mo. Interestingly, physicians assessed details of the adverse events in this registry and judged less than 30% as device-related. This classification was based on the analysis of device components rather than the nature of the adverse event and, for example, considered infections or other localized complications at the implant site as unrelated, if the explanted device was functional.
Data captured in MAUDE do not enable us to estimate incidence rates, which requires sufficient information about the total number of devices implanted for the indication of interest. In addition, data are de-identified and do not allow to differentiate repeat concerns by a single individual from similar concerns coming from multiple persons. Despite this caveat, our findings with descriptions of reoperation accounting for 25% of the reports highlight the potentially significant burden of SNS. This number falls into the range seen in larger cohort studies of spinal cord stimulators or gastric electrical stimulators with sufficient follow up[31-33] and corresponds with the calculated annual rate of repeat surgeries after cardiac pacemaker implantation of about 5%[34]. Large cohort studies of SNS for urinary problems showed even higher rates of about 40%[35,36]. A more detailed assessment of indications for re-interventions demonstrated comparable infection rates that require removal in 1%-4% of patients treated with spinal stimulators, intrathecal drug delivery systems, cardiac devices or gastric electrical stimulators[33,37-40], lead fractures or related problems in 2%-4% of cardiac devices[41] or a need for replacement due to device malfunction with annual replacement rates around 2% for implantable defibrillators[42]. Battery depletion and replacement played a relatively minor role in our analysis, largely due to the relatively recent approval of SNS for fecal incontinence and the expected battery lifetime of more than 4 years[43,44].
Operative re-intervention are obviously costly. However, even if patient concerns do not necessitate additional surgical steps, the required evaluations and treatment changes come with a significant financial burden[45]. This burden needs to be seen in the context of the perceived benefit. Consistent with a recently published meta-analysis of SNS for urinary and gastrointestinal problems, the lack of control groups, poorly defined or differing endpoints and limited information about the variance of reported results do not allow a sufficiently detailed meta-analysis[10]. Results typically used a more than 50% improvement of incontinence rating scales as cutoff for treatment success, which also functions as response definitions during temporary stimulation and was supported by a recently published analysis of patient data[46]. Accepting a 50% improvement or less stringent definitions for symptom improvement as response definition[6,8,18,19,47-54], we found improvement rates for fecal incontinence of about 60% (data not shown). Despite the general acceptance of this threshold definition, a comparison of overall treatment satisfaction with changes in symptom severity scales revealed only minor differences between 25%-50% and 50%-75% improvements, but a significant increase in global satisfaction above this range in a large cohort of SNS treated patients[55]. Especially assessments relying primarily on event frequency may underestimate disgust and emotional factors which contribute significantly to the subjective disease burden[56]. Finally, a small study demonstrated continued benefit after temporary discontinuation of SNS in about 50%[57], raising questions about non-specific or placebo effects of this treatment. None of these points question the benefit many patients experience. Nonetheless, the potential for improvement has to be weighed against a high likelihood of at least some residual symptoms and a high chance of adverse effects with secondary surgeries. Knowing about the less serious, but common unanticipated or undesired effects may also guide us as we can educate patients about such problems and their often successful solutions. Beyond open discussions with patients, we need controlled trials using less invasive approaches, such as medications, biofeedback or the transcutaneously applied stimulation[58,59].
Our study has several important limitations. Any study that relies on databanks collecting reports of adverse events will by definition paint a negative image of the intervention studied. In addition, relying on the MAUDE data repository cannot truly define the incidence of adverse events. However, we tried to address these important caveats by systematically examining published trials and cohort studies of SNS for fecal incontinence. Findings were strikingly similar, supporting the validity of our conclusions. As described above, the variable and at times incompletely described endpoints did not enable us to systematically assess the benefit in SNS for fecal incontinence. In addition, we need to keep in mind that the reported response rates of about 60% are skewed as they typically exclude patients who failed treatment trials with temporary stimulation. The retrospective design of many studies leaves questions about the reliable recording of adverse effects. We therefore limited our systematic analysis to more easily defined endpoints that required secondary interventions. Finally, we tried to gain more detailed insight into the time course of adverse event recordings related to SNS for fecal incontinence. However, more than 2 years after FDA approval, only 2%-4% of the narratives provided information about the underlying indication for SNS, thereby limiting our ability to extend our analysis to earlier times.
Taken together, our findings highlight the high rate of adverse events after SNS with common need for reoperations, which should be clearly discussed with patients considering this treatment. This point is even more relevant as criteria defining treatment responses may leave patients with potentially significant residual symptoms. Considering the emergence of less invasive stimulation paradigms, comparative effectiveness trials are essential to define true therapeutic gains of permanently implanted devices. Lastly, the results show the important insight we can gain by analyzing reports about adverse events received by the FDA.
COMMENTS
Background
Fecal incontinence is a stigmatizing and unfortunately at times difficult to treat problem. After the recent approval by regulatory agencies in the United States, electrical stimulation of nerves that control anal closing muscle control has become a treatment option for patients not responding to other therapies. It does involve surgery and requires ongoing use of an implanted device, thus increasing the risk of complications and the potential need for repeat operations.
Research frontiers
The key questions in such more invasive treatments relate to ratios of risk vs benefit. In the context of a treatment that targets the symptom of fecal incontinence, how does improvement of perceived benefit compare to the frequency and severity of perceived problems or even harm due to this very treatment?
Innovations and breakthroughs
This study took a different approach than previously published reports, which typically described side effects in patients treated by the various investigators. The data presented here are based on problems reported to the regulatory agencies in the United States. The results show that complaints are common. While many of the perceived problems may be correctable through reprogramming or other steps, they were disturbing enough to the affected persons to convey their concerns to federal regulators. Beyond such temporary problems, repeat surgeries are common and often involve device removal or replacement. Knowing about these adverse effects will help patients and healthcare providers when weighing decisions related to the use of this treatment.
Terminology
Fecal incontinence is defined as involuntary loss of fecal matter. Sacral neuromodulation is a surgical treatment of incontinence. It includes the implantation of electrodes, connected to a stimulator, which then electrically activates nerves that control anal closing muscle function.
Peer-review
The reviewers emphasized the fact that many of the perceived side effects represented expected problems that are part of any treatment. If stimulation intensity is too high or too low, patients may feel shocks or experience no or little benefits. Both scenarios can often be managed by changing stimulation parameter. Similarly, battery replacement should be expected in any therapy that relies on electrical stimulation. While perhaps more complex in electrotherapy, the reviewers emphasized that these steps may well be equivalent to changes in medication dosing or prescription refills.
Footnotes
Conflict-of-interest statement: The author has no conflict of interest.
Data sharing statement: Technical appendix and dataset are available from the corresponding author at bielefeldtk@upmc.edu.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 22, 2015
First decision: February 22, 2016
P- Reviewer: Carter D, Falletto E, Santoro GA S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
References
- 1.Ng KS, Sivakumaran Y, Nassar N, Gladman MA. Fecal Incontinence: Community Prevalence and Associated Factors--A Systematic Review. Dis Colon Rectum. 2015;58:1194–1209. doi: 10.1097/DCR.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 2.Norton C, Chelvanayagam S, Wilson-Barnett J, Redfern S, Kamm MA. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology. 2003;125:1320–1329. doi: 10.1016/j.gastro.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 3.Heymen S, Scarlett Y, Jones K, Ringel Y, Drossman D, Whitehead WE. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52:1730–1737. doi: 10.1007/DCR.0b013e3181b55455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler CJ. The perspective of a neurologist on treatment-related research in fecal and urinary incontinence. Gastroenterology. 2004;126:S172–S174. doi: 10.1053/j.gastro.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Rao SS. Diagnosis and management of fecal incontinence. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 2004;99:1585–1604. doi: 10.1111/j.1572-0241.2004.40105.x. [DOI] [PubMed] [Google Scholar]
- 6.Johnson BL, Abodeely A, Ferguson MA, Davis BR, Rafferty JF, Paquette IM. Is sacral neuromodulation here to stay? Clinical outcomes of a new treatment for fecal incontinence. J Gastrointest Surg. 2015;19:15–19; discussion 19-20. doi: 10.1007/s11605-014-2611-4. [DOI] [PubMed] [Google Scholar]
- 7.Tjandra JJ, Chan MK, Yeh CH, Murray-Green C. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum. 2008;51:494–502. doi: 10.1007/s10350-007-9103-5. [DOI] [PubMed] [Google Scholar]
- 8.Hull T, Giese C, Wexner SD, Mellgren A, Devroede G, Madoff RD, Stromberg K, Coller JA. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56:234–245. doi: 10.1097/DCR.0b013e318276b24c. [DOI] [PubMed] [Google Scholar]
- 9.Herbison GP, Arnold EP. Sacral neuromodulation with implanted devices for urinary storage and voiding dysfunction in adults. Cochrane Database Syst Rev. 2009;(2):CD004202. doi: 10.1002/14651858.CD004202.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Mirbagheri N, Sivakumaran Y, Nassar N, Gladman MA. Systematic review of the impact of sacral neuromodulation on clinical symptoms and gastrointestinal physiology. ANZ J Surg. 2016;86:232–236. doi: 10.1111/ans.13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Overbey DM, Townsend NT, Chapman BC, Bennett DT, Foley LS, Rau AS, Yi JA, Jones EL, Stiegmann GV, Robinson TN. Surgical Energy-Based Device Injuries and Fatalities Reported to the Food and Drug Administration. J Am Coll Surg. 2015;221:197–205.e1. doi: 10.1016/j.jamcollsurg.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Naumann RW, Brown J. Complications of Electromechanical Morcellation Reported in the Manufacturer and User Facility Device Experience (MAUDE) Database. J Minim Invasive Gynecol. 1989;22:1018–1021. doi: 10.1016/j.jmig.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Tremaine AM, Avram MM. FDA MAUDE data on complications with lasers, light sources, and energy-based devices. Lasers Surg Med. 2015;47:133–140. doi: 10.1002/lsm.22328. [DOI] [PubMed] [Google Scholar]
- 14.Maeda Y, Lundby L, Buntzen S, Laurberg S. Outcome of sacral nerve stimulation for fecal incontinence at 5 years. Ann Surg. 2014;259:1126–1131. doi: 10.1097/SLA.0b013e31829d3969. [DOI] [PubMed] [Google Scholar]
- 15.Lombardi G, Del Popolo G, Cecconi F, Surrenti E, Macchiarella A. Clinical outcome of sacral neuromodulation in incomplete spinal cord-injured patients suffering from neurogenic bowel dysfunctions. Spinal Cord. 2010;48:154–159. doi: 10.1038/sc.2009.101. [DOI] [PubMed] [Google Scholar]
- 16.Brouwer R, Duthie G. Sacral nerve neuromodulation is effective treatment for fecal incontinence in the presence of a sphincter defect, pudendal neuropathy, or previous sphincter repair. Dis Colon Rectum. 2010;53:273–278. doi: 10.1007/DCR.0b013e3181ceeb22. [DOI] [PubMed] [Google Scholar]
- 17.Vallet C, Parc Y, Lupinacci R, Shields C, Parc R, Tiret E. Sacral nerve stimulation for faecal incontinence: response rate, satisfaction and the value of preoperative investigation in patient selection. Colorectal Dis. 2010;12:247–253. doi: 10.1111/j.1463-1318.2009.01899.x. [DOI] [PubMed] [Google Scholar]
- 18.Roy AL, Gourcerol G, Menard JF, Michot F, Leroi AM, Bridoux V. Predictive factors for successful sacral nerve stimulation in the treatment of fecal incontinence: lessons from a comprehensive treatment assessment. Dis Colon Rectum. 2014;57:772–780. doi: 10.1097/DCR.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 19.Matzel KE, Kamm MA, Stösser M, Baeten CG, Christiansen J, Madoff R, Mellgren A, Nicholls RJ, Rius J, Rosen H. Sacral spinal nerve stimulation for faecal incontinence: multicentre study. Lancet. 2004;363:1270–1276. doi: 10.1016/S0140-6736(04)15999-0. [DOI] [PubMed] [Google Scholar]
- 20.Bharucha AE, Zinsmeister AR, Locke GR, Seide BM, McKeon K, Schleck CD, Melton LJ. Prevalence and burden of fecal incontinence: a population-based study in women. Gastroenterology. 2005;129:42–49. doi: 10.1053/j.gastro.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Madoff RD. Surgical treatment options for fecal incontinence. Gastroenterology. 2004;126(Suppl 1):S48–S54. doi: 10.1053/j.gastro.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Enck P, Van der Voort IR, Klosterhalfen S. Biofeedback therapy in fecal incontinence and constipation. Neurogastroenterol Motil. 2009;21:1133–1141. doi: 10.1111/j.1365-2982.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 23.Markland AD, Burgio KL, Whitehead WE, Richter HE, Wilcox CM, Redden DT, Beasley TM, Goode PS. Loperamide Versus Psyllium Fiber for Treatment of Fecal Incontinence: The Fecal Incontinence Prescription (Rx) Management (FIRM) Randomized Clinical Trial. Dis Colon Rectum. 2015;58:983–993. doi: 10.1097/DCR.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 24.Norton C, Cody JD, Hosker G. Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults. Cochr Data Syst Rev. 2006;(3):CD002111. doi: 10.1002/14651858.CD002111.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Dijkema HE, Weil EH, Mijs PT, Janknegt RA. Neuromodulation of sacral nerves for incontinence and voiding dysfunctions. Clinical results and complications. Eur Urol. 1993;24:72–76. doi: 10.1159/000474266. [DOI] [PubMed] [Google Scholar]
- 26.Vaizey CJ, Kamm MA, Turner IC, Nicholls RJ, Woloszko J. Effects of short term sacral nerve stimulation on anal and rectal function in patients with anal incontinence. Gut. 1999;44:407–412. doi: 10.1136/gut.44.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laudano MA, Seklehner S, Sandhu J, Reynolds WS, Garrett KA, Milsom JW, Te AE, Kaplan SA, Chughtai B, Lee RK. Disparities in the Use of Sacral Neuromodulation among Medicare Beneficiaries. J Urol. 2015;194:449–453. doi: 10.1016/j.juro.2015.03.111. [DOI] [PubMed] [Google Scholar]
- 28.Suskind AM, Clemens JQ, Zhang Y, Hollenbeck BK. Physician Use of Sacral Neuromodulation Among Medicare Beneficiaries With Overactive Bladder and Urinary Retention. Urology. 2015;86:30–34. doi: 10.1016/j.urology.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leroi AM, Lenne X, Dervaux B, Chartier-Kastler E, Mauroy B, Normand LL, Grise P, Faucheron JL, Parc Y, Lehur PA, et al. Outcome and cost analysis of sacral nerve modulation for treating urinary and/or fecal incontinence. Ann Surg. 2011;253:720–732. doi: 10.1097/SLA.0b013e318210f1f4. [DOI] [PubMed] [Google Scholar]
- 30.Leroi AM Medtronic. Medtronic Product Performance Report 2014. Available from: http://professional.medtronic.com/wcm/groups/mdtcom_sg/@mdt/@neuro/documents/documents/mdt_product_performance_2014.pdf.
- 31.Hayek SM, Veizi E, Hanes M. Treatment-Limiting Complications of Percutaneous Spinal Cord Stimulator Implants: A Review of Eight Years of Experience From an Academic Center Database. Neuromodulation. 2015;18:603–608; discussion 608-609. doi: 10.1111/ner.12312. [DOI] [PubMed] [Google Scholar]
- 32.Abell T, McCallum R, Hocking M, Koch K, Abrahamsson H, Leblanc I, Lindberg G, Konturek J, Nowak T, Quigley EM, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 33.Zehetner J, Ravari F, Ayazi S, Skibba A, Darehzereshki A, Pelipad D, Mason RJ, Katkhouda N, Lipham JC. Minimally invasive surgical approach for the treatment of gastroparesis. Surg Endosc. 2013;27:61–66. doi: 10.1007/s00464-012-2407-0. [DOI] [PubMed] [Google Scholar]
- 34.Duray GZ, Schmitt J, Cicek-Hartvig S, Hohnloser SH, Israel CW. Complications leading to surgical revision in implantable cardioverter defibrillator patients: comparison of patients with single-chamber, dual-chamber, and biventricular devices. Europace. 2009;11:297–302. doi: 10.1093/europace/eun322. [DOI] [PubMed] [Google Scholar]
- 35.Peeters K, Sahai A, De Ridder D, Van Der Aa F. Long-term follow-up of sacral neuromodulation for lower urinary tract dysfunction. BJU Int. 2014;113:789–794. doi: 10.1111/bju.12571. [DOI] [PubMed] [Google Scholar]
- 36.Al-zahrani AA, Elzayat EA, Gajewski JB. Long-term outcome and surgical interventions after sacral neuromodulation implant for lower urinary tract symptoms: 14-year experience at 1 center. J Urol. 2011;185:981–986. doi: 10.1016/j.juro.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 37.Engle MP, Vinh BP, Harun N, Koyyalagunta D. Infectious complications related to intrathecal drug delivery system and spinal cord stimulator system implantations at a comprehensive cancer pain center. Pain Physician. 2013;16:251–257. [PubMed] [Google Scholar]
- 38.Kahlow H, Olivecrona M. Complications of vagal nerve stimulation for drug-resistant epilepsy: a single center longitudinal study of 143 patients. Seizure. 2013;22:827–833. doi: 10.1016/j.seizure.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Palmisano P, Accogli M, Zaccaria M, Luzzi G, Nacci F, Anaclerio M, Favale S. Rate, causes, and impact on patient outcome of implantable device complications requiring surgical revision: large population survey from two centres in Italy. Europace. 2013;15:531–540. doi: 10.1093/europace/eus337. [DOI] [PubMed] [Google Scholar]
- 40.Krahn AD, Lee DS, Birnie D, Healey JS, Crystal E, Dorian P, Simpson CS, Khaykin Y, Cameron D, Janmohamed A, et al. Predictors of short-term complications after implantable cardioverter-defibrillator replacement: results from the Ontario ICD Database. Circ Arrhythm Electrophysiol. 2011;4:136–142. doi: 10.1161/CIRCEP.110.959791. [DOI] [PubMed] [Google Scholar]
- 41.Armaganijan LV, Toff WD, Nielsen JC, Andersen HR, Connolly SJ, Ellenbogen KA, Healey JS. Are elderly patients at increased risk of complications following pacemaker implantation? A meta-analysis of randomized trials. Pacing Clin Electrophysiol. 2012;35:131–134. doi: 10.1111/j.1540-8159.2011.03240.x. [DOI] [PubMed] [Google Scholar]
- 42.Maisel WH, Moynahan M, Zuckerman BD, Gross TP, Tovar OH, Tillman DB, Schultz DB. Pacemaker and ICD generator malfunctions: analysis of Food and Drug Administration annual reports. JAMA. 2006;295:1901–1906. doi: 10.1001/jama.295.16.1901. [DOI] [PubMed] [Google Scholar]
- 43.Blandon RE, Gebhart JB, Lightner DJ, Klingele CJ. Re-operation rates after permanent sacral nerve stimulation for refractory voiding dysfunction in women. BJU Int. 2008;101:1119–1123. doi: 10.1111/j.1464-410X.2007.07426.x. [DOI] [PubMed] [Google Scholar]
- 44.North RB, Brigham DD, Khalessi A, Calkins SK, Piantadosi S, Campbell DS, Daly MJ, Dey PB, Barolat G, Taylor R. Spinal cord stimulator adjustment to maximize implanted battery longevity: a randomized, controlled trial using a computerized, patient-interactive programmer. Neuromodulation. 2004;7:13–25. doi: 10.1111/j.1525-1403.2004.04002.x. [DOI] [PubMed] [Google Scholar]
- 45.McMullin CM, Jadav AM, Hanwell C, Brown SR. Resource implications of running a sacral neuromodulation service: a 10-year experience. Colorectal Dis. 2014;16:719–722. doi: 10.1111/codi.12686. [DOI] [PubMed] [Google Scholar]
- 46.Jelovsek JE, Chen Z, Markland AD, Brubaker L, Dyer KY, Meikle S, Rahn DD, Siddiqui NY, Tuteja A, Barber MD. Minimum important differences for scales assessing symptom severity and quality of life in patients with fecal incontinence. Female Pelvic Med Reconstr Surg. 2014;20:342–348. doi: 10.1097/SPV.0000000000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thin NN, Taylor SJ, Bremner SA, Emmanuel AV, Hounsome N, Williams NS, Knowles CH. Randomized clinical trial of sacral versus percutaneous tibial nerve stimulation in patients with faecal incontinence. Br J Surg. 2015;102:349–358. doi: 10.1002/bjs.9695. [DOI] [PubMed] [Google Scholar]
- 48.Mishra A, Prapasrivorakul S, Gosselink MP, Gorissen KJ, Hompes R, Jones O, Cunningham C, Matzel KE, Lindsey I. Sacral neuromodulation for persistent faecal incontinence after laparoscopic ventral rectopexy for high-grade internal rectal prolapse? Colorectal Dis. 2016;18:273–278. doi: 10.1111/codi.13125. [DOI] [PubMed] [Google Scholar]
- 49.Altomare DF, Ratto C, Ganio E, Lolli P, Masin A, Villani RD. Long-term outcome of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2009;52:11–17. doi: 10.1007/DCR.0b013e3181974444. [DOI] [PubMed] [Google Scholar]
- 50.Boyle DJ, Murphy J, Gooneratne ML, Grimmer K, Allison ME, Chan CL, Williams NS. Efficacy of sacral nerve stimulation for the treatment of fecal incontinence. Dis Colon Rectum. 2011;54:1271–1278. doi: 10.1097/DCR.0b013e3182270af1. [DOI] [PubMed] [Google Scholar]
- 51.Kim DH, Faruqui N, Ghoniem GM. Sacral neuromodulation outcomes in patients with urge urinary incontinence and concomitant urge fecal incontinence. Female Pelvic Med Reconstr Surg. 2010;16:171–178. doi: 10.1097/SPV.0b013e3181d67c24. [DOI] [PubMed] [Google Scholar]
- 52.Duelund-Jakobsen J, van Wunnik B, Buntzen S, Lundby L, Laurberg S, Baeten C. Baseline factors predictive of patient satisfaction with sacral neuromodulation for idiopathic fecal incontinence. Int J Colorectal Dis. 2014;29:793–798. doi: 10.1007/s00384-014-1870-6. [DOI] [PubMed] [Google Scholar]
- 53.El-Gazzaz G, Zutshi M, Salcedo L, Hammel J, Rackley R, Hull T. Sacral neuromodulation for the treatment of fecal incontinence and urinary incontinence in female patients: long-term follow-up. Int J Colorectal Dis. 2009;24:1377–1381. doi: 10.1007/s00384-009-0745-8. [DOI] [PubMed] [Google Scholar]
- 54.Prapasrivorakul S, Gosselink MP, Gorissen KJ, Fourie S, Hompes R, Jones OM, Cunningham C, Lindsey I. Sacral neuromodulation for faecal incontinence: is the outcome compromised in patients with high-grade internal rectal prolapse? Int J Colorectal Dis. 2015;30:229–234. doi: 10.1007/s00384-014-2078-5. [DOI] [PubMed] [Google Scholar]
- 55.Duelund-Jakobsen J, van Wunnik B, Buntzen S, Lundby L, Baeten C, Laurberg S. Functional results and patient satisfaction with sacral nerve stimulation for idiopathic faecal incontinence. Colorectal Dis. 2012;14:753–759. doi: 10.1111/j.1463-1318.2011.02800.x. [DOI] [PubMed] [Google Scholar]
- 56.Reynolds LM, Bissett IP, Consedine NS. Predicting the patients who will struggle with anal incontinence: sensitivity to disgust matters. Colorectal Dis. 2015;17:73–80. doi: 10.1111/codi.12781. [DOI] [PubMed] [Google Scholar]
- 57.Altomare DF, Giannini I, Giuratrabocchetta S, Digennaro R. The effects of sacral nerve stimulation on continence are temporarily maintained after turning the stimulator off. Colorectal Dis. 2013;15:e741–e748. doi: 10.1111/codi.12418. [DOI] [PubMed] [Google Scholar]
- 58.Rimmer CJ, Knowles CH, Lamparelli M, Durdey P, Lindsey I, Hunt L, Nugent K, Gill KA. Short-term Outcomes of a Randomized Pilot Trial of 2 Treatment Regimens of Transcutaneous Tibial Nerve Stimulation for Fecal Incontinence. Dis Colon Rectum. 2015;58:974–982. doi: 10.1097/DCR.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 59.Thomas GP, Norton C, Nicholls RJ, Vaizey CJ. A pilot study of transcutaneous sacral nerve stimulation for faecal incontinence. Colorectal Dis. 2013;15:1406–1409. doi: 10.1111/codi.12371. [DOI] [PubMed] [Google Scholar]
- 60.Gorissen KJ, Bloemendaal AL, Prapasrivorakul S, Gosselink MP, Jones OM, Cunningham C, Lindsey I, Hompes R. Dynamic Article: Permanent Sacral Nerve Stimulation Under Local Anesthesia: Feasibility, Best Practice, and Patient Satisfaction. Dis Colon Rectum. 2015;58:1182–1185. doi: 10.1097/DCR.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 61.Kahlke V, Topic H, Peleikis HG, Jongen J. Sacral nerve modulation for fecal incontinence: results of a prospective single-center randomized crossover study. Dis Colon Rectum. 2015;58:235–240. doi: 10.1097/DCR.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 62.Al Asari S, Meurette G, Mantoo S, Kubis C, Wyart V, Lehur PA. Percutaneous tibial nerve stimulation vs sacral nerve stimulation for faecal incontinence: a comparative case-matched study. Colorectal Dis. 2014;16:O393–O399. doi: 10.1111/codi.12680. [DOI] [PubMed] [Google Scholar]
- 63.Moya P, Arroyo A, Lacueva J, Candela F, Soriano-Irigaray L, López A, Gómez MA, Galindo I, Calpena R. Sacral nerve stimulation in the treatment of severe faecal incontinence: long-term clinical, manometric and quality of life results. Tech Coloproctol. 2014;18:179–185. doi: 10.1007/s10151-013-1022-y. [DOI] [PubMed] [Google Scholar]
- 64.Norderval S, Behrenbruch C, Brouwer R, Keck JO. Efficacy of cyclic sacral nerve stimulation for faecal incontinence. Tech Coloproctol. 2013;17:511–516. doi: 10.1007/s10151-013-0999-6. [DOI] [PubMed] [Google Scholar]
- 65.George AT, Kalmar K, Panarese A, Dudding TC, Nicholls RJ, Vaizey CJ. Long-term outcomes of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2012;55:302–306. doi: 10.1097/DCR.0b013e3182401ecd. [DOI] [PubMed] [Google Scholar]
- 66.Damon H, Barth X, Roman S, Mion F. Sacral nerve stimulation for fecal incontinence improves symptoms, quality of life and patients’ satisfaction: results of a monocentric series of 119 patients. Int J Colorectal Dis. 2013;28:227–233. doi: 10.1007/s00384-012-1558-8. [DOI] [PubMed] [Google Scholar]
- 67.Lim JT, Hastie IA, Hiscock RJ, Shedda SM. Sacral nerve stimulation for fecal incontinence: long-term outcomes. Dis Colon Rectum. 2011;54:969–974. doi: 10.1097/DCR.0b013e31821e57c2. [DOI] [PubMed] [Google Scholar]
- 68.Rasmussen OO, Buntzen S, Sørensen M, Laurberg S, Christiansen J. Sacral nerve stimulation in fecal incontinence. Dis Colon Rectum. 2004;47:1158–1162; discussion 1162-1163. doi: 10.1007/s10350-004-0553-8. [DOI] [PubMed] [Google Scholar]
- 69.Matzel KE, Lux P, Heuer S, Besendörfer M, Zhang W. Sacral nerve stimulation for faecal incontinence: long-term outcome. Colorectal Dis. 2009;11:636–641. doi: 10.1111/j.1463-1318.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 70.Muñoz-Duyos A, Navarro-Luna A, Brosa M, Pando JA, Sitges-Serra A, Marco-Molina C. Clinical and cost effectiveness of sacral nerve stimulation for faecal incontinence. Br J Surg. 2008;95:1037–1043. doi: 10.1002/bjs.6140. [DOI] [PubMed] [Google Scholar]
- 71.Holzer B, Rosen HR, Zaglmaier W, Klug R, Beer B, Novi G, Schiessel R. Sacral nerve stimulation in patients after rectal resection--preliminary report. J Gastrointest Surg. 2008;12:921–925. doi: 10.1007/s11605-008-0485-z. [DOI] [PubMed] [Google Scholar]
- 72.Chan MK, Tjandra JJ. Sacral nerve stimulation for fecal incontinence: external anal sphincter defect vs. intact anal sphincter. Dis Colon Rectum. 2008;51:1015–1024; discussion 1024-1025. doi: 10.1007/s10350-008-9326-0. [DOI] [PubMed] [Google Scholar]
- 73.O’Riordan JM, Healy CF, McLoughlin D, Cassidy M, Brannigan AE, O’Connell PR. Sacral nerve stimulation for faecal incontinence. Ir J Med Sci. 2008;177:117–119. doi: 10.1007/s11845-008-0145-z. [DOI] [PubMed] [Google Scholar]
- 74.Mattioli S, Lugaresi ML, Costantini M, Del Genio A, Di Martino N, Fei L, Fumagalli U, Maffettone V, Monaco L, Morino M, et al. The short esophagus: intraoperative assessment of esophageal length. J Thorac Cardiovasc Surg. 2008;136:834–841. doi: 10.1016/j.jtcvs.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 75.Hetzer FH, Hahnloser D, Clavien PA, Demartines N. Quality of life and morbidity after permanent sacral nerve stimulation for fecal incontinence. Arch Surg. 2007;142:8–13. doi: 10.1001/archsurg.142.1.8. [DOI] [PubMed] [Google Scholar]
- 76.Otto SD, Burmeister S, Buhr HJ, Kroesen A. Sacral nerve stimulation induces changes in the pelvic floor and rectum that improve continence and quality of life. J Gastrointest Surg. 2010;14:636–644. doi: 10.1007/s11605-009-1122-1. [DOI] [PubMed] [Google Scholar]
- 77.Faucheron JL, Voirin D, Badic B. Sacral nerve stimulation for fecal incontinence: causes of surgical revision from a series of 87 consecutive patients operated on in a single institution. Dis Colon Rectum. 2010;53:1501–1507. doi: 10.1007/DCR.0b013e3181f1cf14. [DOI] [PubMed] [Google Scholar]
- 78.Rosen A, Taragano L, Condrea A, Sidi A, Ron Y, Ginath S. Effects of Sacral Neuromodulation on Urinary and Fecal Incontinence. Isr Med Assoc J. 2015;17:351–355. [PubMed] [Google Scholar]
- 79.McNevin MS, Moore M, Bax T. Outcomes associated with Interstim therapy for medically refractory fecal incontinence. Am J Surg. 2014;207:735–737; discussion 737-738. doi: 10.1016/j.amjsurg.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 80.Benson-Cooper S, Davenport E, Bissett IP. Introduction of sacral neuromodulation for the treatment of faecal incontinence. N Z Med J. 2013;126:47–53. [PubMed] [Google Scholar]
- 81.Schwandner O. Sacral neuromodulation for fecal incontinence and “low anterior resection syndrome” following neoadjuvant therapy for rectal cancer. Int J Colorectal Dis. 2013;28:665–669. doi: 10.1007/s00384-013-1687-8. [DOI] [PubMed] [Google Scholar]
- 82.Faucheron JL, Chodez M, Boillot B. Neuromodulation for fecal and urinary incontinence: functional results in 57 consecutive patients from a single institution. Dis Colon Rectum. 2012;55:1278–1283. doi: 10.1097/DCR.0b013e31826c7789. [DOI] [PubMed] [Google Scholar]
- 83.Simpson JA, Peacock J, Maxwell-Armstrong C. Use of a gentamicin-impregnated collagen sheet (Collatamp(®) ) following implantation of a sacral nerve stimulator for faecal incontinence. Colorectal Dis. 2012;14:e200–e202. doi: 10.1111/j.1463-1318.2011.02886.x. [DOI] [PubMed] [Google Scholar]
- 84.Pascual I, Gómez Cde C, Ortega R, Toscano MJ, Marijuán JL, Espadas ML, Cebrián JM, Olmo DG, Montero JA. Sacral nerve stimulation for fecal incontinence. Rev Esp Enferm Dig. 2011;103:355–359. doi: 10.4321/s1130-01082011000700004. [DOI] [PubMed] [Google Scholar]
- 85.Uludağ O, Melenhorst J, Koch SM, van Gemert WG, Dejong CH, Baeten CG. Sacral neuromodulation: long-term outcome and quality of life in patients with faecal incontinence. Colorectal Dis. 2011;13:1162–1166. doi: 10.1111/j.1463-1318.2010.02447.x. [DOI] [PubMed] [Google Scholar]
- 86.Uludağ O, Koch SM, van Gemert WG, Dejong CH, Baeten CG. Sacral neuromodulation in patients with fecal incontinence: a single-center study. Dis Colon Rectum. 2004;47:1350–1357. doi: 10.1007/s10350-004-0589-9. [DOI] [PubMed] [Google Scholar]
- 87.Ruiz Carmona MD, Martín Arévalo J, Moro Valdezate D, Plá Martí V, Checa Ayet F. Sacral nerve stimulation for the treatment of severe faecal incontinence: results after 10 years experience. Cir Esp. 2014;92:329–335. doi: 10.1016/j.ciresp.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 88.Ganio E, Ratto C, Masin A, Luc AR, Doglietto GB, Dodi G, Ripetti V, Arullani A, Frascio M, BertiRiboli E, et al. Neuromodulation for fecal incontinence: outcome in 16 patients with definitive implant. The initial Italian Sacral Neurostimulation Group (GINS) experience. Dis Colon Rectum. 2001;44:965–970. doi: 10.1007/BF02235484. [DOI] [PubMed] [Google Scholar]


