Abstract
Introduction
Correct blood pressure (BP) measurement is crucial in the diagnosis of arterial hypertension (AH), and controversy exists whether supine BP should be treated as equal to sitting BP. The aim of this study was to evaluate the relation of supine BP to sitting BP and ambulatory BP with regard to identification of diagnostic cut-offs for hypertension.
Material and methods
This study included 280 patients with AH (mean age: 44.3 ±10.6 years). The following measurements of BP were performed and analyzed: 1) sitting office blood pressure measurement (OSBP and ODBP); 2) supine BP (supSBP and supDBP), measured automatically (5 times with a 2-minute interval) during evaluation by the Niccomo device (Medis, Germany); 3) 24-hour ambulatory blood pressure (ABP) monitoring.
Results
The mean supSBP and supDBP were found to be lower than OSBP and ODBP (130.9 ±14.2 vs. 136.6 ±15.5 mm Hg and 84.8 ±9.4 vs. 87.8 ±10.2 mm Hg, respectively; p < 0.000001). The correlations between ABP and supBP/OBP were moderate and strong (correlation coefficients in range 0.55–0.76). The ROC analysis revealed that mean supBP ≥ 130/80 mm Hg was more precise than OBP ≥ 140/90 mm Hg in diagnosing hypertension (AUC: 0.820 vs. 0.550; sensitivity 80.7% vs. 57.4%; specificity 83.2% vs. 52.7%; p < 0.0001) and the additive value derived mostly from its higher predictive power of identifying patients with increased night-time BP.
Conclusions
In young and middle-aged hypertensive patients the blood pressure during a 10-minute supine rest was lower than in the sitting position. The supine blood pressure ≥ 130/80 mm Hg was found to be a specific and sensitive threshold for hypertension.
Keywords: ambulatory blood pressure monitoring, office blood pressure, body position, impedance cardiography
Introduction
Correct blood pressure (BP) measurement is crucial in the diagnosis of arterial hypertension (AH), which is the main cardiovascular risk factor [1, 2]. The accuracy of office blood pressure (OBP) measurement depends on the observer's attention to technical details, the patient's momentary status and environmental circumstances. As a consequence, the standard of OBP measurement recommended by the international guidelines [1] is often lacking in everyday practice. Ambulatory blood pressure (ABP) monitoring includes many more measurements, providing a profile of BP during daytime activity and night-time rest. However, the use of ABP measurement is limited by its availability and discomfort reported by some patients, particularly at night. Moreover, the cost of ABP monitoring limits its use for repeated measurements [3, 4]. Even if performed according to the recommendations, OBP measurement and ABP show disagreement in some portion of patients [1, 5–8].
The diagnostic criteria of AH are defined for sitting OBP measurement, home blood pressure monitoring and ABP monitoring [1]. However, there is no consensus on the cut-off values for supine BP (supBP). It is still controversial whether supBP measurement is equal to, lower or higher than sitting BP [8–15]. Thus, it is not possible to adapt sitting BP diagnostic criteria for supBP. The need for cut-offs specific for supBP arises from the growing number of cardiac monitors equipped with a BP measuring module. They are used for continuous monitoring in intensive care wards and also in ambulatory practice. Their advantages are the repeated BP measurements, relatively stable environmental circumstances and lower staff involvement. However, it cannot be precisely utilized until the cut-offs are defined.
The justification of this study grew from our own empirical observation that the values of supBP, measured by the impedance cardiography (ICG) monitor, correspond to ABP but are not equal to OBP. We realized that we have at our disposal a simple and effective method of multiparametric hemodynamic monitoring but without the possibility to exactly identify patients with increased BP. Having in mind that ICG proved to be useful in the diagnosis and treatment of AH [16–18], we assumed that the identification of cut-offs of supBP for the diagnosis of AH could raise the diagnostic power of this method. In a broader sense, we aimed to equip methods providing supBP with additional, clinically relevant diagnostic functionality.
Therefore, the aim of this study was to evaluate the relation of supine blood pressure to sitting blood pressure and ambulatory blood pressure with regard to the identification of diagnostic cut-offs for hypertension.
Material and methods
Study population
This study included patients with at least 3-month history of AH defined according to European Society of Cardiology guidelines [1]. Exclusion criteria comprised: (1) confirmed secondary AH, (2) AH treated with three or more medicines before recruitment, (3) heart failure, (4) cardiomyopathy, (5) significant heart rhythm disorders, (6) significant valvular disease, (7) kidney failure (glomerular filtration rate (GFR) below 60 ml/min 1.73 m2), (8) chronic obstructive pulmonary disease, (9) diabetes, (10) polyneuropathy, (11) peripheral vascular disease, (12) age < 18 years and > 65 years. Subjects previously treated with hypotensive drugs (n = 60, 21.4%) were recommended to discontinue them at least 7 days before examination.
The group selected for the analysis comprised patients from two clinical studies performed in the Department of Cardiology and Internal Diseases of the Military Institute of Medicine from March 2008 to December 2013 (registered at www.nauka-polska – ID 227062 and ClinicalTrials.gov – NCT01996085). Both studies were conducted according to Good Clinical Practice guidelines and the Declaration of Helsinki, with the approval of the local ethics committee (no. 3/WIM/2008, no. 21/WIM/2011). Each patient provided written informed consent to participate in the study. The measurements were taken at two times: (1) baseline and (2) after 3 months of hypotensive treatment introduced at the baseline visit.
The clinical examination was performed in morning hours (7.30 a.m. – 8.30 a.m.) with consideration of cardiovascular risk factors and symptoms indicating a secondary cause of AH [1].
Office blood pressure measurement
The conventional OBP measurement was performed automatically (Omron M4 Plus, Japan) with the technique compliant with European Society of Cardiology guidelines [1]. Office systolic blood pressure (OSBP) and office diastolic blood pressure (ODBP) were measured in a quiet room in the presence of a trained physician or nurse (after a minimum 5 min of rest in a sitting position). The subjects were asked not to smoke or drink any drinks potentially increasing BP (e.g. alcohol, coffee, energizers) or take drugs influencing BP (e.g. pain relievers) within 12 h before measurement. They were seated comfortably with an arm supported and legs uncrossed. The average value of the two measurements was used as the final OSBP and ODBP reading. The BP category of AH was defined according to the European Society of Cardiology guidelines [1]: grade 1 – OSBP 140–159 mm Hg and/or ODBP 90–99 mm Hg, grade 2 – OSBP 160–179 mm Hg and/or ODBP 100–109 mm Hg, grade 3 – OSBP greater than 180 mm Hg and/or ODBP greater than 110 mm Hg.
Supine blood pressure measurement
After a 10-minute rest in a supine position after lying down, the supine BP was measured automatically during the evaluation by the Niccomo device (Medis, Ilmenau, Germany). This ICG monitor is dedicated to the noninvasive assessment of hemodynamic parameters (such as cardiac output, thoracic fluid content, systemic vascular resistance) including arm-cuff oscillometric BP measurements performed at user-defined periods of time. The left arm was lying freely on the examination bed. SupBP was registered 5 times with a 2-minute interval between the measurements and coded as follows: supSBP 1, supDBP 1, supSBP 2, supDBP 2, supSBP 3, supDBP 3, supSBP 4, supDBP 4, supSBP 5, supDBP 5. The average value of the 5 measurements was also calculated (mean supSBP, mean supDBP).
Ambulatory blood pressure (ABP) monitoring
Ambulatory blood pressure monitoring was started at about 1 h after supine BP measurement (Spacelabs 90207, Spacelabs, Medical Inc, Redmond, USA). The time from 6 a.m. to 10 p.m. was considered the daily activity period (daytime) with automatic blood pressure measurement in 10-minute intervals. During night rest (night-time: 10 p.m. – 6 a.m.) the measurement was performed every 30 min. The patients were recommended to adjust their circadian activity to those periods of time. The minimum correctness of BP measurement was defined as 70% for both daytime and night-time. The BP thresholds used to define AH were set according to the European Society of Cardiology guidelines [1]: a mean 24-hour SBP ≥ 130 mm Hg and/or DBP ≥ 80 mm Hg, a daytime SBP ≥ 135 mm Hg and/or DBP ≥ 85 mm Hg, or a night-time SBP ≥ 120 mm Hg and/or DBP ≥ 70 mm Hg. The mean values of 24-hour SBP, 24-hour DBP, daytime SBP, daytime DBP, night-time SBP, and night-time DBP were included in the analysis.
Laboratory tests
Laboratory tests included evaluation of renal function (creatinine, glomerular filtration rate) and metabolic disturbances (total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, fasting glucose). Metabolic syndrome was defined according to International Diabetes Federation criteria [19].
Statistical analysis
The statistical analysis was performed using Statistica 7.0 (StatSoft, Inc.). The distribution and normality of data were assessed by visual inspection and the Kolmogorov-Smirnov test. Continuous variables were presented as means ± standard deviations (SD) and categorical variables as absolute and relative frequencies (percentages). The daytime period of ABP monitoring was approved as the reference in comparing the methods (supBP, OBP measurement, ABP monitoring), based on the methodology of similar studies [5–8, 20]. The analysis of differences between absolute values of supine BP, OBP and daytime BP was performed using ANOVA. Then, the assessment of the relation between ABP and OBP/supBP was performed in univariate regression models. Bland-Altman plots were constructed to further evaluate agreement between supBP and daytime BP.
In the first step of defining clinically applicable cut-offs of supBP, receiver operating characteristic (ROC) analysis for continuous data was used. In the second step, the cut-offs of supSBP and supDBP of the highest classificatory power were rounded both down and up to obtain the clinically applicable thresholds for the diagnosis of hypertension (defined in reference to ABP monitoring as the presence of elevated BP in any period of ABP monitoring), which were appointed for final validation. A p value of < 0.05 was taken to indicate statistical significance.
Results
The study involved 280 patients, characterized in Table I. The analysis of BP relations measured by different techniques (OBP measurement, supBP and ABP monitoring) included 530 sets of data from 2 patients’ visits: [1] baseline (n = 280) and [2] after 3 months of hypotensive treatment (n = 250, 30 patients did not attend the follow-up visit or did not reach 70% of correct measurements in ABP monitoring).
Table I.
Basic characteristics of study group
| Parameter | Study group (n = 280) |
|---|---|
| Age, mean ± SD [years] | 44.3 ±10.6 |
| Male, n (%) | 192 (68.6) |
| BMI, mean ± SD [kg/m2] | 28.7 ±4.2 |
| HR, mean ± SD [bpm] | 71.7 ±10.7 |
| OSBP, mean ± SD [mm Hg] | 143.8 ±14.3 |
| ODBP, mean ± SD [mm Hg] | 92.4 ±9.5 |
| AH grade 1, n (%) | 196 (70.0) |
| AH grade 2, n (%) | 67 (23.9) |
| AH grade 3, n (%) | 17 (6.1) |
| MS, n (%) | 153 (54.5) |
| Creatinine, mean ± SD [mg/dl] | 0.84 ±0.16 |
| eGFR, mean ± SD [ml/min/1.73 m2] | 100.1 ±20.8 |
| ABP monitoring, mean ± SD [mm Hg]: | |
| 24-hour BP | 140.8/88.1 ±11.5/8.1 |
| Daytime BP | 144.7/91.0 ±11.9/8.6 |
| Night-time BP | 128.2/77.8 ±12.9/9.0 |
BMI – body mass index, HR – heart rate, OSBP – office systolic blood pressure, ODBP – office diastolic blood pressure, AH – arterial hypertension, MS – metabolic syndrome, eGFR – estimated glomerular filtration rate, ABP – ambulatory blood pressure, BP – blood pressure.
Comparison of BP measurements with three methods
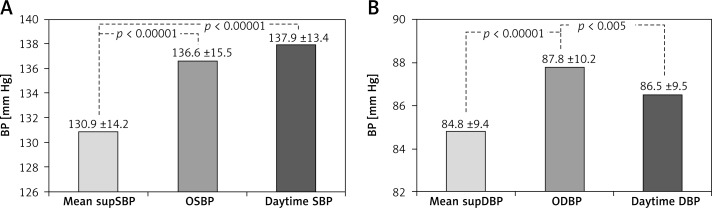
The values of supBP, as presented in Table II, were not equal to OBP and tended to decrease in the course of consecutive measurements. Mean supSBP and supDBP were lower than OSBP/ODBP and daytime SBP/DBP (Figure 1).
Table II.
Comparison of OBP with supBP (for 530 measurements)
| supSBP | OSBP (136.6 ±15.5) vs. | P-value |
|---|---|---|
| supSBP 1 | 135.5 ±16.0 | 0.254 |
| supSBP 2 | 131.3 ±14.6 | < 0.000001 |
| supSBP 3 | 129.6 ±14.4 | < 0.000001 |
| supSBP 4 | 129.2 ±14.7 | < 0.000001 |
| supSBP 5 | 129.0 ±14.7 | < 0.000001 |
| Mean supSBP | 130.9 ±14.2 | < 0.000001 |
| supDBP | ODBP (87.8 ±10.2) vs. | P -value |
| supDBP 1 | 86.3 ±9.7 | 0.011 |
| supDBP 2 | 85.1 ±9.7 | 0.000006 |
| supDBP 3 | 84.5 ±9.6 | < 0.000001 |
| supDBP 4 | 84.3 ±9.6 | < 0.000001 |
| supDBP 5 | 83.9 ±9.6 | < 0.000001 |
| Mean supDBP | 84.8 ±9.4 | 0.000001 |
Figure 1.
Comparison of BP (mm Hg) measured by three methods (for 530 measurements): A – mean supSBP vs. OSBP vs. daytime SBP; B – mean supDBP vs. ODBP vs. daytime DBP
Agreement between methods
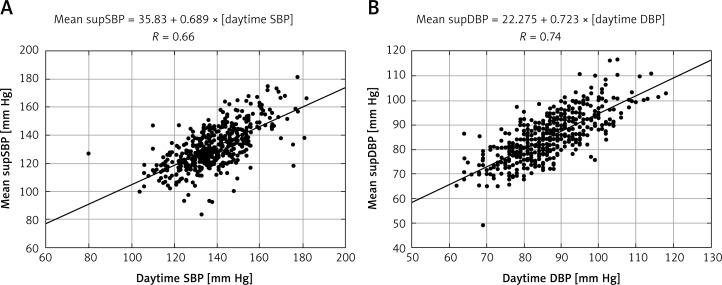
The correlations between ABP and OBP/supBP were moderate and strong (Tables III, IV, Figure 2). The strength of correlation tended to increase in the course of consecutive supBP measurements (with the strongest correlations for mean supBP). The biggest outlier was the first supBP measurement. The associations of ambulatory DBP with supDBP were stronger than with ODBP.
Table III.
Correlations: OSBP/supSBP vs. ABP
| Parameter | OSBP | supSBP 1 | supSBP 2 | supSBP 3 | supSBP 4 | supSBP 5 | Mean supSBP |
|---|---|---|---|---|---|---|---|
| Daytime SBP | 0.67 | 0.62 | 0.64 | 0.64 | 0.63 | 0.66 | 0.66 |
| Night-time SBP | 0.61 | 0.55 | 0.57 | 0.56 | 0.58 | 0.60 | 0.60 |
| 24-hour SBP | 0.66 | 0.63 | 0.65 | 0.65 | 0.65 | 0.67 | 0.68 |
All p < 0.00001; no statistically significant differences between correlation coefficients.
Table IV.
Correlations: ODBP/supDBP vs. ABP
| Parameter | ODBP | supDBP 1 | supDBP 2 | supDBP 3 | supDBP 4 | supDBP 5 | Mean supDBP |
|---|---|---|---|---|---|---|---|
| Daytime DBP | 0.68 | 0.72 | 0.72 | 0.72 | 0.72 | 0.73 | 0.74* |
| Night-time DBP | 0.61 | 0.63 | 0.63 | 0.64 | 0.65 | 0.65 | 0.66 |
| 24-hour DBP | 0.68 | 0.73 | 0.73 | 0.73 | 0.74* | 0.74* | 0.76** |
All p < 0.00001; differences between correlation coefficients statistically significant:
p = 0.04
p = 0.005.
Figure 2.
Correlation plots: A – mean supSBP versus daytime SBP; B – mean supDBP vs. daytime DBP
According to the formulas of calculation derived from the linear regression models, the daytime SBP of 135 mm Hg was equal to mean supSBP of 128.8 mm Hg and daytime DBP of 85 mm Hg to mean supDBP of 83.7 mm Hg.
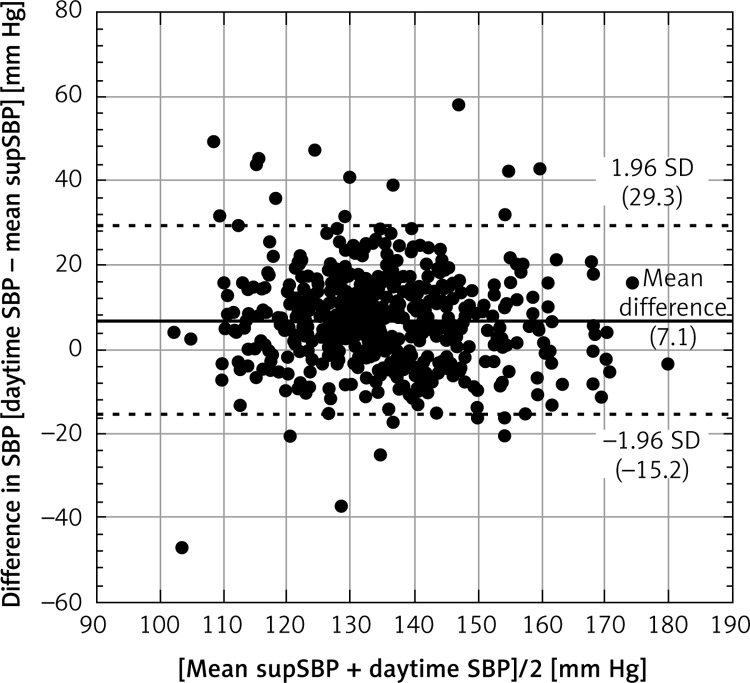
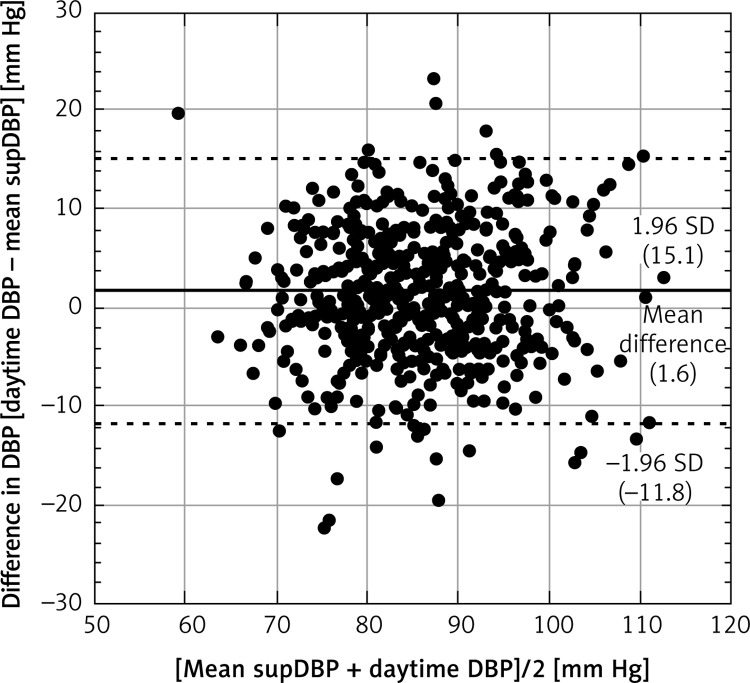
The Bland-Altman plot analysis confirmed the agreement between supBP and daytime BP (Figures 3 and 4). The mean difference for daytime SBP and mean supSBP was 7.1 mm Hg, and for daytime DBP and supDBP it was 1.6 mm Hg. The limits of agreement were acceptable and confirmed that the results are clinically relevant.
Figure 3.
Bland-Altman plot for mean supSBP and daytime SBP. The horizontal solid line shows the mean difference between methods and the dashed lines show the limit of agreement (±1.96 standard deviation of the difference between daytime SBP and mean supSBP)
Figure 4.
Bland-Altman plot for mean supDBP and daytime DBP. The horizontal solid line shows the mean difference between methods and the dashed lines show the limit of agreement (±1.96 standard deviation of the difference between daytime DBP and mean supDBP)
Calculation of cut-off values
The significant difference between OBP and supBP motivated further analysis to define cut-off values of supBP in reference to the gold standard method (ABP monitoring). Therefore, the ROC analysis was performed. The analysis for continuous data identified mean supSBP of 126 mm Hg (AUC = 0.814) and mean supDBP of 84 mm Hg (AUC = 0.857) as the most precise cut-off points for daytime SBP ≥ 135 mm Hg and daytime DBP ≥ 85 mm Hg, respectively.
Preliminary validation
Based on the above results, as well as taking into consideration possible clinical application of the BP values, the following rounded cut-off values were selected for validation: mean supSBP ≥ 130 mm Hg, mean supSBP ≥ 125 mm Hg and mean supDBP ≥ 80 mm Hg, mean supDBP ≥ 85 mm Hg.
The mean supSBP ≥ 130 mm Hg showed similar predictive value for daytime SBP ≥ 135 mm Hg (AUC = 0.726) as supSBP ≥ 125 mm Hg (AUC = 0.722, p = NS) and OSBP ≥ 140 mm Hg (AUC = 0.729, p = NS). The accuracy of mean supDBP ≥ 85 mm Hg for daytime DBP ≥ 85 mm Hg (AUC = 0.772) was slightly more distinctive in comparison with mean supSBP ≥ 80 mm Hg (AUC = 0.729, p = NS) and OSBP ≥ 90 mm Hg (AUC = 0.742, p = NS).
Final validation
On the basis of the preliminary validation, the mean supBP ≥ 130/80 mm Hg (mean supSBP ≥ 130 mm Hg and/or mean supDBP ≥ 80 mm Hg) and mean supBP ≥ 130/85 mm Hg were verified as the criteria for diagnosing hypertension. The ROC analysis presented in Table V revealed that mean supBP ≥ 130/80 mm Hg was more precise than mean supBP ≥ 130/85 mm Hg and OBP ≥ 140/90 mm Hg. The additive predictive value derived mostly from higher predictive power of identifying patients with increased night-time BP despite the fact that OBP measurement was more accurate with elevated daytime BP.
Table V.
ROC analysis results
| AUC | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|
| Reference to elevated mean BP in any period of ABP monitoring: | |||||
| OBP ≥ 140/90 mm Hg | 0.550 | 0.574 | 0.527 | 0.787 | 0.289 |
| supBP ≥ 130/80 mm Hg | 0.820* | 0.807 | 0.832 | 0.936 | 0.586 |
| supBP ≥ 130/85 mm Hg | 0.791*# | 0.667 | 0.916 | 0.960 | 0.474 |
| Reference to elevated daytime BP: | |||||
| OBP ≥ 140/90 mm Hg | 0.753 | 0.711 | 0.794 | 0.880 | 0.565 |
| SupBP ≥ 130/80 mm Hg | 0.631* | 0.733 | 0.529 | 0.767 | 0.484 |
| supBP ≥ 130/85 mm Hg | 0.655* | 0.622 | 0.688 | 0.809 | 0.462 |
| Reference to elevated night-time BP: | |||||
| OBP ≥ 140/90 mm Hg | 0.556 | 0.588 | 0.524 | 0.698 | 0.406 |
| SupBP ≥ 130/80 mm Hg | 0.791* | 0.852 | 0.730 | 0.855 | 0.726 |
| SupBP ≥ 130/85 mm Hg | 0.769* | 0.710 | 0.827 | 0.884 | 0.605 |
Statistically significant difference between supBP and OBP: p < 0.0001.
Statistically significant difference between supBP ≥ 130/80 mm Hg and ≥ 130/85 mm Hg: p = 0.026. AUC – area under curve, PPV – positive predictive value, other abbreviations explained in text, NPV – negative predictive value.
Discussion
The results of our study revealed that the supBP is not equal to standard sitting OBP and depends on the duration of the rest period, decreasing in the course of relaxed lying. Moreover, the precise cut-offs of supBP in the diagnosis of hypertension were derived. According to our knowledge, this is the first study concluding with clinically applicable diagnostic thresholds of supBP.
Sitting versus supine blood pressure measurement
In our analysis the supBP was found to be lower than OBP. The relation between sitting and supBP is not clearly defined, and the opinions of researchers are inconsistent in this area.
Our observations are contrary to those presented in the study by Lu et al. [9] performed in a large cohort of hypertensive patients (n = 1487), where SBP and DBP in the supine position was higher than in the sitting position (2.9 and 0.9 mm Hg, respectively, p < 0.001). Likewise, Wei et al. [13] reported that in healthy persons BP in the supine was higher than in the sitting position. Netea et al. [11] examined diabetic patients and observed that both SBP and DBP were lower if the patient was sitting (by 7.4 mm Hg and 6.6 mm Hg, respectively, p < 0.01).
There are also reports presenting similar results to ours. Aoki and Sato [14] related reduced SBP and DBP to the supine position, and these phenomena were more pronounced in hypertensives than in normotensives. The results of Turjanmaa et al. [21] also suggest that the supBP readings are lower than the sitting ones. Kruszewski et al. [22] observed that postprandial BP during supine rest was about 10–11 mm Hg lower than during activity. Still others [15, 23] did not reveal any difference in hemodynamic status in the supine and upright position.
The inconsistency of the cited reports may derive from different characteristics of populations. Salice et al. [24] proved that relations between OBP and ABP differ with age. Sex, body height and body mass index are other influencing factors [9, 10]. These discrepancies may also be related to the method of measurement, including different devices, time periods, number of repetitions, arm position and environment [1, 12]. Inability to standardize all aspects of supBP measurement substantiates the aim to define the cut-offs specific for the method and patient population.
The lower supBP observed in our study may be explained by a 10-minute period of rest before the evaluation and several consecutive measurements performed during a further 10 min of relaxed laying. Such an approach provides stabilization of BP that was confirmed before. Van der Wel et al. [20] observed that BP measured automatically every 5 min declined substantially in the first 15 min and then reached a plateau. Mancia et al. [25] emphasized that stress-provoked BP overestimations may be minimized by 10-minute relaxation.
Agreement between methods
The analysis of relations between OBP, supBP and ABP confirmed incomplete agreement between incidental and 24-hour BP evaluation. The correlation coefficients of OSBP vs. ambulatory SBP and ODBP vs. ambulatory DBP were comparable. However, they were stronger for supBP vs. DBP than supBP vs. SBP (0.72–0.76 and 0.62–0.68, respectively). Additionally, supDBP correlated better with ambulatory DBP than ODBP. Our results remain in general agreement with previous studies. Myers et al. [26] reported higher coefficients of correlation between office DBP vs. diastolic ABP than office SBP vs. systolic ABP, measured by the automated (0.72 and 0.62, respectively) and manual method (0.48 and 0.32, respectively). On the other hand, Zachariach et al. [27] observed better correlations of SBP (sitting and supine) than DBP with ABP (0.76–0.82 and 0.60–0.69, respectively).
Cut-offs of supine blood pressure in diagnosis of hypertension
The clinical value of cut-offs derived from ROC analysis were found to be relevant. The mean supBP ≥ 130/80 mm Hg provided a more accurate estimate of elevated ABP than OBP ≥ 140/90 mm Hg (sensitivity 80.7% vs. 57.4% and specificity 83.2% vs. 52.7%, respectively). The fact that the accuracy of OBP ≥ 140/90 mm Hg was lower than in the previous systematic review [7] (mean sensitivity 74.6% and specificity 74.6%) may be explained by poor correspondence of OBP measurement thresholds with elevated night-time BP (Table V). In fact, OBP was a good classifier of elevated mean daytime BP (sensitivity 71.1% and specificity 79.4%), even better than supBP. However, from the clinical point of view diagnostic accuracy with the gold standard criterion of AH (elevated BP in any period of ABP monitoring) is of higher priority.
The relatively strong correlation of supBP with nocturnal BP may be of clinical importance. Increased night-time BP was found to be a predictor of worse cardiovascular outcomes, associated with target organ damage [1]. The OBP measurement fails to quantify asleep BP level well, misclassifying up to 50% of patients [28]. Our results agree with that expert opinion. In a study performed in a similar population (182 patients with untreated AH) Chatzistamatiou et al. [29] observed even lower correlation coefficients between OBP and night-time BP than in our cohort (0.291 and 0.248 for SBP and DBP, respectively). If supBP were confirmed as a surrogate of nocturnal BP in future studies, it would be an additional advantage of this method of measurement.
We must emphasize that the justification of this retrospective analysis grew from the clinical need to improve diagnostic power of noninvasive hemodynamic assessment by ICG. The defined cut-offs of supBP raise the practical convenience of the method that is perceived as prospective in diagnosis and treatment of AH [30]. We are aware that several BP measurements during a 10-minute rest in the supine position are undoubtedly more difficult to perform and the supBP will not replace standard sitting BP measurement. However, the possibility to evaluate BP control during ICG measurement could limit the costs of repeated BP monitoring. We hope that this additional, clinically relevant diagnostic functionality of ICG will encourage wider use of this method in clinical practice. It is especially important in view of the fact that the accuracy of blood pressure measurement is crucial for prognosis of high risk patients, including those with metabolic syndrome and cerebrovascular events [31, 32].
The limitation of our study is that the sequence of BP measurements was not randomly assigned and the protocol was not set prospectively but analyzed from the retrospective view. We used three different tools to evaluate BP in different diagnostic settings, which can cause a potential bias of disagreement between methods of measurement. However, the supine BP (measured by the Niccomo) was compared with automated, well-validated devices (according to www.dableducational.org), and OBP and ABP monitoring were performed according to the current guidelines [1]. Furthermore, we did not use patients’ diary reports of activity, assuming they would follow the recommendation of adjusting their activity to the ABP monitor settings of the night-time period. We must also mention that BP was evaluated before and on hypotensive therapy (case to case), but it can be assumed that the influence of pharmacotherapy on evaluated BP relations is negligible. Moreover, our results refer to a specific population of patients without cardiovascular disease other than AH, mostly young and middle aged. Thus, the extrapolation of these observations to the general population should be carefully considered.
In conclusion, the present results show that in young and middle-aged hypertensive patients without other cardiovascular diseases, blood pressure values in the sitting and supine position are not equivalent. The blood pressure measured automatically during a 10-minute supine rest is lower than sitting blood pressure. We suggest supine blood pressure ≥ 130/80 mm Hg as a specific and sensitive threshold for diagnosis of hypertension. The proposed diagnostic criteria can be used in clinical practice and provide additional cost savings.
Acknowledgments
The study was supported by the Minister of Science and Higher Education/Military Institute of Medicine, Warsaw, Poland (grant no. 148/WIM). There is no authors’ conflict of interest to disclose.
We would like to thank the medical staff of the Department of Cardiology and Internal Diseases of Military Institute of Medicine, especially: Prof. Andrzej Skrobowski for assistance in patient care and organizational supervision, Drs Robert Wierzbowski, Beata Uziębło-Życzkowska, Małgorzata Kurpaska, Katarzyna Hałas, Magdalena Potapowicz-Krysztofiak, Agnieszka Jaguś-Jamioła, Łukasz Michalczyk, Anna Kazimierczak, Agnieszka Jurek, Jarosław Kowal, Kalina Niedolaz, and Agata Galas, for assistance in patient care and data collection, and Lidia Wojda and Lidia Latosek for nursing care and data collection.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Mancia G, Fagard R, Narkiewicz K, et al. Task Force Members ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 2.Aronow WS. Commentary on recent guidelines for treating hypertension. Arch Med Sci. 2014;10:1069–72. doi: 10.5114/aoms.2014.47818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Brien E, Parati G, Stergiou G, et al. European Society of Hypertension Working Group on Blood Pressure Monitoring European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–68. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 4.Tholl U, Forstner K, Anlauf M. Measuring blood pressure: pitfalls and recommendations. Nephrol Dial Transplant. 2004;19:766–70. doi: 10.1093/ndt/gfg602. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa J, Nasothimiou EG, Karpettas N, et al. Automatic office blood pressure measured without doctors or nurses present. Blood Press Monit. 2012;17:96–102. doi: 10.1097/MBP.0b013e328352ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giles TD, Oparil S, Ofili EO, et al. The role of ambulatory blood pressure monitoring compared with clinic and home blood pressure measures in evaluating moderate versus intensive treatment of hypertension with amlodipine/valsartan for patients uncontrolled on angiotensin receptor blocker monotherapy. Blood Press Monit. 2011;16:87–95. doi: 10.1097/MBP.0b013e328344c713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodgkinson J, Mant J, Martin U, et al. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ. 2011;342:d3621. doi: 10.1136/bmj.d3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin U, Holder R, Hodgkinson J, McManus R. Inter-arm blood pressure differences compared with ambulatory monitoring: a manifestation of the ‘white-coat’ effect? Br J Gen Pract. 2013;63:e97–103. doi: 10.3399/bjgp13X663055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu LC, Wei TM, Li S, Ye XL, Zeng CL, Wang LX. Differences in blood pressure readings between supine and sitting positions in hypertensive patients. Acta Cardiol. 2008;63:707–11. doi: 10.2143/AC.63.6.2033387. [DOI] [PubMed] [Google Scholar]
- 10.Netea RT, Smits P, Lenders JW, Thien T. Does it matter whether blood pressure measurements are taken with subjects sitting or supine? J Hypertens. 1998;16:263–8. doi: 10.1097/00004872-199816030-00002. [DOI] [PubMed] [Google Scholar]
- 11.Netea RT, Elving LD, Lutterman JA, Thien T. Body position and blood pressure measurement in patients with diabetes mellitus. J Intern Med. 2002;251:393–9. doi: 10.1046/j.1365-2796.2002.00958.x. [DOI] [PubMed] [Google Scholar]
- 12.Netea RT, Thien T. Blood pressure measurement: we should all do it better! Neth J Med. 2004;62:297–303. [PubMed] [Google Scholar]
- 13.Wei TM, Lu LC, Ye XL, Li S, Wang LX. Difference in blood pressure between supine and sitting positions in diabetic and non-diabetic subjects. Med Sci Monit. 2009;15:CR123–7. [PubMed] [Google Scholar]
- 14.Aoki K, Sato K. Decrease in blood pressure and increase in total peripheral vascular resistance in supine resting subjects with normotension or essential hypertension. Jpn Heart J. 1986;27:467–74. doi: 10.1536/ihj.27.467. [DOI] [PubMed] [Google Scholar]
- 15.Mizuno H, Yanagisawa A, Shigeyama T, et al. Continuous ambulatory radionuclide monitoring of left ventricular function: effect of body position during ergometer exercise. J Nucl Med. 1997;38:1669–72. [PubMed] [Google Scholar]
- 16.Krzesiński P, Gielerak GG, Kowal JJ. A “patient-tailored” treatment of hypertension with use of impedance cardiography: a randomized, prospective and controlled trial. Med Sci Monit. 2013;19:242–50. doi: 10.12659/MSM.883870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Packer M, Abraham WT, Mehra MR, et al. Prospective Evaluation and Identification of Cardiac Decompensation by ICG Test (PREDICT) Study Investigators and Coordinators. Prospective Evaluation and Identification of Cardiac Decompensation by ICG Test (PREDICT) Study Investigators and Coordinators Utility of Impedance Cardiography for the Identification of Short-Term Risk of Clinical Decompensation In Stable Patients With Chronic Heart Failure. J Am Coll Cardiol. 2006;47:2245–52. doi: 10.1016/j.jacc.2005.12.071. [DOI] [PubMed] [Google Scholar]
- 18.Siebert J, Gutknecht P, Molisz A, Trzeciak B, Nyka W. Hemodynamic findings in patients with brain stroke. Arch Med Sci. 2012;8:371–4. doi: 10.5114/aoms.2012.28567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group The metabolic syndrome: a new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 20.van der Wel MC, Buunk IE, van Weel C, Thien TA, Bakx JC. A novel approach to office blood pressure measurement: 30-minute office blood pressure vs daytime ambulatory blood pressure. Ann Fam Med. 2011;9:128–35. doi: 10.1370/afm.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turjanmaa MHV, Kalli TS, Uusitalo AJ. Blood pressure level changes caused by posture change and physical exercise: can they be determined accurately using a standard cuff method? J Hypertens. 1988;6:S79–81. doi: 10.1097/00004872-198812040-00021. [DOI] [PubMed] [Google Scholar]
- 22.Kruszewski P, Bieniszewski L, Neubauer-Geryk J, Swierblewska E, Krupa-Wojciechowska B. Supine body position is an important factor influencing postprandial ambulatory blood pressure. Med Sci Monit. 2003;9:CR34–41. [PubMed] [Google Scholar]
- 23.Vrachatis D, Papaioannou TG, Konstantopoulou A, et al. Deffect of supine versus sitting position on noninvasive assessment of aortic pressure waveform: a randomized cross-over study. J Hum Hypertens. 2014;28:236–41. doi: 10.1038/jhh.2013.101. [DOI] [PubMed] [Google Scholar]
- 24.Salice P, Ardissino G, Barbier P, et al. Differences between office and ambulatory blood pressures in children and adolescents attending a hospital hypertension clinic. J Hypertens. 2013;31:2165–75. doi: 10.1097/HJH.0b013e3283643361. [DOI] [PubMed] [Google Scholar]
- 25.Mancia G, Casadei R, Groppelli A, Parati G, Zanchetti A. Effect of stress on diagnosis of hypertension. Hypertension. 1991;17:III56–62. doi: 10.1161/01.hyp.17.4_suppl.iii56. [DOI] [PubMed] [Google Scholar]
- 26.Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27:280–6. doi: 10.1097/HJH.0b013e32831b9e6b. [DOI] [PubMed] [Google Scholar]
- 27.Zachariach PK, Sheps GS, Moore AG. Office blood pressures in supine, sitting and standing positions: correlation with ambulatory blood pressures. Int J Cardiol. 1990;28:353–60. doi: 10.1016/0167-5273(90)90319-z. [DOI] [PubMed] [Google Scholar]
- 28.Hermida RC, Ayala DE, Ríos MT, Fernández JR, Mojón A, Smolensky MH. Around-the-clock ambulatory blood pressure monitoring is required to properly diagnose resistant hypertension and assess associated vascular risk. Curr Hypertens Rep. 2014;16:445. doi: 10.1007/s11906-014-0445-9. [DOI] [PubMed] [Google Scholar]
- 29.Chatzistamatiou EI, Moustakas GN, Veioglanis S, et al. Nocturnal hypertension: poor correlation with office blood pressure but strong prognostic factor for target organ damage. Hellenic J Cardiol. 2012;53:263–72. [PubMed] [Google Scholar]
- 30.Taler SJ. Individualizing antihypertensive combination therapies: clinical and hemodynamic considerations. Curr Hypertens Rep. 2014;16:451. doi: 10.1007/s11906-014-0451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaki ME, El-Bassyouni HT, El-Gammal M, Kamal S. Indicators of the metabolic syndrome in obese adolescents. Arch Med Sci. 2015;11:92–8. doi: 10.5114/aoms.2015.49214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karliński M, Gluszkiewicz M, Członkowska A. The accuracy of prehospital diagnosis of acute cerebrovascular accidents: an observational study. Arch Med Sci. 2015;11:530–5. doi: 10.5114/aoms.2015.52355. [DOI] [PMC free article] [PubMed] [Google Scholar]