Abstract
Introduction
The usefulness of B-type natriuretic peptide (BNP) as a marker of ischemia is controversial. BNP levels have predicted arrhythmias in various settings, but it is unknown whether they are related to exercise-induced ischemic ventricular arrhythmias.
Material and methods
We analyzed in 63 patients (64 ±14 years, 65% male, 62% with known coronary disease) undergoing exercise stress single-photon emission computed tomography (SPECT) the association between plasma BNP values (before and 15 min after exercise) and the occurrence of ischemia or ventricular arrhythmias during the test.
Results
Exercise test (8.1 ±2.7 min, 7.4 ±8.1 metabolic equivalents, 82 ±12% of maximal predicted heart rate) induced reversible perfusion defects in 23 (36%) patients. Eight (13%) patients presented significant arrhythmias (≥ 7 ventricular premature complexes/min, couplets, or non-sustained ventricular tachycardia during exercise or in the first minute of recovery). Median baseline BNP levels were 17.5 (12.4–66.4) pg/ml in patients developing scintigraphic ischemia and 45.6 (13.2–107.4) pg/ml in those without ischemia (p = 0.137). The BNP levels increased after exercise (34.4 (15.3–65.4)% increment over baseline, p < 0.001), but the magnitude of this increase was not related to SPECT positivity (35.7 (18.8–65.4)% vs. 27.9 (5.6–64.0)% in patients with and without ischemia, respectively, p = 0.304). No significant association was found between BNP values (at baseline or their change during the test) and ventricular arrhythmias.
Conclusions
Plasma BNP values – at baseline or after exercise – were not associated with myocardial ischemia or with ventricular arrhythmia during exercise SPECT. These results highlight the limited usefulness of this biomarker to assess acute ischemia.
Keywords: biomarkers, exercise stress test, ischemia, natriuretic peptides
Introduction
B-type natriuretic peptide (BNP) is secreted in response to myocardial wall stress [1] and has become an established diagnostic and prognostic marker in patients with heart failure [2, 3]. In ischemic heart disease, increased BNP levels have been associated with a worse outcome in patients with ST-elevation acute myocardial infarction [4, 5], non-ST elevation acute coronary syndromes [6], or stable coronary artery disease [7].
Several studies have analyzed the changes in circulating levels of BNP or the amino-terminal portion of the prohormone (NT-proBNP) in response to exercise-induced myocardial ischemia. Whereas some found a direct association between ischemia and the post-ischemic change in BNP levels [8, 9], this association was not observed by others [10]. This issue is clinically relevant since a potentially useful application of BNP could be its measurement as an adjunct to exercise ECG to increase its sensitivity or in the emergency room to help guide management in patients with chest pain of uncertain coronary origin after standard evaluation.
On the other hand, myocardial stretch is arrhythmogenic [11] and wall stress in the ischemic region following coronary occlusion has been related to an increased incidence of ventricular arrhythmias [12–14]. BNP levels have predicted the occurrence of supraventricular or ventricular arrhythmias in various clinical settings [15–17], but their association with ischemic ventricular arrhythmias remains unexplored.
We assessed whether BNP levels are related to the occurrence of ischemia or ventricular arrhythmias during exercise stress test with myocardial perfusion imaging.
Material and methods
Patients
Sixty-three consecutive patients undergoing single-photon emission computed tomography (SPECT) stress testing for diagnostic (n = 24, 38%) or prognostic (n = 39, 62%) purposes were included. Our local Ethics Committee approved the study and all patients provided informed consent. Data were obtained on patients’ age, sex, risk factors for coronary artery disease, co-morbidities, previous cardiac disease and ongoing medications.
Ergometry
Patients underwent standard symptom-limited treadmill testing with continuous 12-lead ECG monitoring. If they failed to attain 80% of maximum heart rate in the absence of symptoms or ST-segment depression, an intravenous bolus of dipyridamole was administered at peak exercise [18]. Antianginal medications were not discontinued for prognostic tests. The occurrence of angina or ST-segment changes and the occurrence, number and severity of arrhythmias during exercise and during the first minute of recovery were recorded. Significant ventricular arrhythmia was defined as the occurrence of ≥ 7 premature ventricular premature complexes (VPCs)/min either during exercise or in the recovery phase or the presence of couplets or runs of ventricular tachycardia [19].
Myocardial perfusion imaging
At peak stress, 300 MBq of 99mTc-tetrofosmin was administered, and imaging was performed immediately afterwards in a dual-head 90° gamma camera (E.CAM, Siemens). Four hours later – 24 h for patients weighing > 90 kg – a second 800 MBq injection was administered and imaging was repeated. A 17-segment myocardial model was used for analysis, with a visual perfusion rating of 0–4 for each segment by two experienced nuclear cardiologists unaware of biomarker data [20]. The difference between stress and rest scores was calculated and scintigraphic ischemia was defined as a summed difference score ≥ 3. Rest gated SPECT was performed and end-systolic and end-diastolic indexed volumes were obtained and ejection fraction calculated [20].
Determination of B-type natriuretic peptide
Venous blood samples were obtained before and 15 min after stress testing. Plasma was stored at –80°C and aliquots were thawed for analysis. A commercially available immunoassay (98000XR, Inverness Medical) was used for measurement of serum BNP.
Statistical analysis
Categorical variables are reported as counts and percentages. Continuous variables are described as mean ± SD or as median (interquartile range) if their distribution follows a non-normal pattern. Comparison between categorical variables was performed by χ2 tests, and comparison between two continuous variables was performed by Student t-tests or by the Mann-Whitney test for non-normally distributed variables. Paired comparisons involving non-normally distributed variables were performed by the Wilcoxon rank sum test. The Spearman coefficient was used to assess the association between two continuous variables. P-values < 0.05 were considered significant.
Results
Baseline characteristics
Baseline patients’ characteristics with regard to the presence or absence of ischemia are shown in Table I. Patients had a significant prevalence of coronary risk factors and of documented ischemic heart disease. The use of antianginal drugs was frequent. Stress-induced myocardial ischemia was more frequent in males, in smokers and in those with known coronary artery disease. Echocardiography was available in 29 patients. Three had significant non-coronary heart disease (one obstructive cardiomyopathy, one moderate aortic regurgitation, and one prior arterial switch surgery for transposition of the great arteries) and 9 had non-significant valve disease. Systolic pulmonary artery pressure was calculated in 12 patients; it ranged between 21 mm Hg and 45 mm Hg and was > 30 mm Hg in five.
Table I.
Clinical characteristics
| Parameter | Overall (n = 63) | No ischemia (n = 40) | Ischemia (n = 23) | P-value |
|---|---|---|---|---|
| Age [years] | 64 ±14 | 64 ±14 | 63 ±14 | 0.725 |
| Female gender | 22 (35%) | 19 (48%) | 3 (13%) | 0.006 |
| Active smoker | 24 (40%) | 11 (28%) | 13 (65%) | 0.005 |
| Diabetes mellitus | 17 (28%) | 12 (30%) | 5 (24%) | 0.608 |
| Hypertension | 43 (70%) | 29 (74%) | 14 (64%) | 0.378 |
| Dyslipidemia | 37 (61%) | 22 (56%) | 15 (68%) | 0.366 |
| Peripheral vasculopathy | 5 (8%) | 2 (5%) | 3 (14%) | 0.341 |
| Previous stroke | 6 (10%) | 4 (10%) | 2 (9%) | 1.0 |
| Renal insufficiency | 5 (8%) | 3 (8%) | 2 (9%) | 1.0 |
| Previous heart failure | 5 (8%) | 4 (10%) | 1 (4%) | 0.647 |
| Previous myocardial infarction | 20 (34%) | 9 (24%) | 11 (50%) | 0.044 |
| Previous coronary revascularization | 27 (44%) | 13 (34%) | 14 (61%) | 0.042 |
| Antiplatelet use | 44 (72%) | 26 (68%) | 18 (82%) | 0.205 |
| β-blocker use | 27 (44%) | 15 (38%) | 12 (54%) | 0.225 |
| Calcium antagonist use | 12 (20%) | 8 (20%) | 4 (18%) | 1.0 |
| Nitrate use | 20 (32%) | 11 (28%) | 9 (39%) | 0.374 |
| ACEI/ARB use | 33 (54%) | 24 (62%) | 9 (41%) | 0.121 |
| Statin use | 35 (57%) | 24 (62%) | 11 (50%) | 0.382 |
ACEI/ARB – angiotensin converting enzyme inhibitor/angiotensin receptor blocker.
Exercise stress test and nuclear imaging
The results of the exercise stress test are summarized in Table II. Exercise duration averaged 8.1 ±2.7 min, maximum workload 7.4 ±2.8 metabolic equivalents, and percentage of maximum predicted heart rate 82 ±12%. Nine (14%) patients had angina, which was limiting in 6 (10%). Horizontal or downsloping ST-segment depression appeared in 13 (21%) patients. Five (8%) patients had ≥ 0.2 mV of ST depression during exercise. Reversible perfusion defects were observed in 23 (36%) patients, and 21 (33%) had fixed defects. Mean left ventricular ejection fraction was 57 ±12% and was ≤ 50% in 20 (32%) patients. Of these variables, exercise-induced ischemia was only associated with a greater prevalence of fixed defects.
Table II.
Exercise stress test characteristics
| Parameter | Overall (n = 63) | No ischemia (n = 40) | Ischemia (n = 23) | P-value |
|---|---|---|---|---|
| Total exercise time [min] | 8.1 ±2.7 | 7.9 ±2.4 | 8.4 ±3.1 | 0.469 |
| Maximum workload [METS] | 7.4 ±2.8 | 7.3 ±2.8 | 7.7 ±2.8 | 0.560 |
| Percentage of maximum predicted heart rate (%) | 82 ±12 | 83 ±12 | 80 ±11 | 0.464 |
| Baseline systolic arterial pressure [mm Hg] | 131 ±24 | 133 ±22 | 128 ±26 | 0.445 |
| Peak exercise systolic arterial pressure [mm Hg] | 157 ±21 | 158 ±21 | 155 ±22 | 0.555 |
| Peak exercise diastolic arterial pressure [mm Hg] | 80 ±9 | 79 ±9 | 82 ±8 | 0.162 |
| Exercise-induced angina | 9 (14%) | 6 (15%) | 3 (13%) | 1.0 |
| Exercise-induced ST depression 0.1 mV | 13 (21%) | 8 (20%) | 5 (22%) | 1.0 |
| Exercise-induced ST depression 0.2 mV | 5 (8%) | 3 (8%) | 2 (9%) | 1.0 |
| Myocardial necrosis | 21 (33%) | 9 (22%) | 12 (52%) | 0.016 |
| LV end-diastolic volume [ml/m2] | 86 (68–120) | 82 (60–102) | 94 (76–133) | 0.121 |
| LV end-systolic volume [ml/m2] | 38 (25–55) | 35 (18–53) | 44 (27–73) | 0.114 |
| LV ejection fraction (%) | 57 ±12 | 59 ±13 | 54 ±11 | 0.093 |
LV – left ventricle, METS – metabolic equivalents.
Ventricular arrhythmias
Twenty-three (36%) patients had ventricular ectopy during exercise. In these patients, the frequency of VPCs was low (2 [1–21]). Four patients (6%) had ≥ 7 VPCs/min during exercise and 4 (6%, 3 of those and 1 additional patient) had ≥ 7 VPCs during the first minute of recovery, 6 (10%) had ventricular couplets and 2 had runs of non-sustained ventricular tachycardia (lasting seven and 18 beats, respectively). Significant arrhythmia, as defined above, was present in 8 (13%) patients, with similar incidence in those with and without ischemia (13% vs. 12%, respectively, p = 1.0).
Plasma B-type natriuretic peptide levels
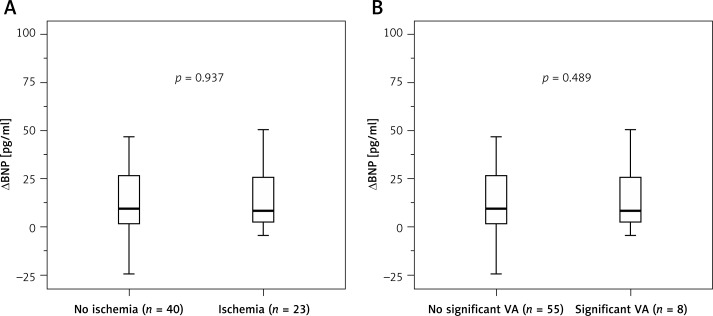
The BNP values in patients with and without ischemia are illustrated in Table III. The median value of plasma BNP before exercise was 33.7 (12.4–83.0) pg/ml, without differences among patients presenting or not presenting subsequent ischemia. BNP levels increased 15 min after exercise to 51.7 (14.2–96.5) pg/ml (p < 0.001). This increase was of similar magnitude in patients with and without ischemia, whether considered as an absolute increment (Figure 1 A) or as a relative increase over baseline. As compared to the remaining patients (n = 56), those with more severe ischemia (summed difference score ≥ 6, n = 7) also did not have a greater increase in BNP levels after exercise, either absolute (7.4 (1.8–9.0) vs. 10.7 (1.8–32.0) pg/ml, respectively, p = 0.266) or relative (34.4 (25.0–64.3) vs. 35.7 (14.5–66.5) pg/ml, respectively, p = 0.630).
Table III.
Plasma BNP values in relation to exercise-induced ischemia or significant ventricular arrhythmia
| Parameter | Overall (n = 63) | No ischemia (n = 40) | Ischemia (n = 23) | P-value | No VA (n = 55) | VA (n = 8) | P-value |
|---|---|---|---|---|---|---|---|
| Baseline BNP [pg/ml] | 33.7 (12.4–83.0) | 45.6 (13.2–107.4) | 17.5 (12.4–66.4) | 0. 137 | 32.1 (12.2–83.0) | 59.4 (19.1–89.1) | 0.403 |
| 15-min post-exercise BNP [pg/ml] | 51.7 (14.2–96.5) | 60.4 (17.8–101.4) | 28.1 (14.2–85.6) | 0.233 | 46.8 (14.2–94.4) | 91.4 (25.8–110.5) | 0.269 |
| ΔBNP [pg/ml] | 9.3 (1.8–30.0) | 10.0 (1.9–31.0) | 8.5 (1.8–30.0) | 0.937 | 9.0 (1.8–28.0) | 24.8 (2.2–38.8) | 0.489 |
| Relative increase in BNP (%) | 34.4 (15.3–65.4) | 27.9 (5.6–64.0) | 35.7 (18.8–65.4) | 0.304 | 34.0 (15.3–66.5) | 36.3 (17.3–62.8) | 0.901 |
BNP – type-B natriuretic peptide, VA – ventricular arrhythmia.
Figure 1.
Absolute change in type-B natriuretic peptide levels with regard to the presence or absence of ischemia (A) or significant ventricular arrhythmia (B) during exercise test (ranges, medians, and 25th to 75th percentiles)
VA – ventricular arrhythmia.
Table III also summarizes BNP values in patients presenting and not presenting arrhythmias. Both groups had similar BNP levels at baseline and after exercise and also had similar absolute (Figure 1 B) or relative increments in BNP levels after exercise with respect to baseline values. The number of VPCs during exercise was not associated with BNP levels at baseline (r = 0.147, p = 0.249) or 15 min after exercise (r = 0.173, p = 0.175) nor with the post-exercise absolute (r = 0.057, p = 0.658) or relative (r = –0.119, p = 0.354) increase in BNP levels with respect to baseline values. The increase in BNP after exercise was not significantly different in the 2 patients presenting runs of non-sustained ventricular tachycardia during the test and in the remaining patients.
As Table IV shows, plasma BNP levels were not associated with other results of exercise SPECT, except for patients with fixed defects having significantly higher baseline BNP levels than those without fixed defects, although the increment in BNP value after exercise was comparable in both groups, and for patients with ≥ 0.2 mV ST depression having a greater relative increase in BNP levels. No significant correlation was found between plasma BNP value at baseline or its absolute increment after exercise and end-diastolic or end-systolic left ventricular volumes or ejection fraction.
Table IV.
Plasma BNP values in relation to other results of exercise SPECT
| Variable | Baseline BNP [pg/ml] | 15-min post-exercise BNP [pg/ml] | ΔBNP [pg/ml] | Relative increase in BNP (%) |
|---|---|---|---|---|
| Angina: | ||||
| No (n = 54) | 34.5 (12.1–81.7) | 54.3 (13.6–98.1) | 9.2 (1.8–30.5) | 35.8 (14.8–65.7) |
| Yes (n = 9) | 24.1 (14.8–116.8) | 50.5 (21.4–135.8) | 9.4 (2.4–33.0) | 24.4 (15.2–48.3) |
| P-value | 0.602 | 0.716 | 0.945 | 0.377 |
| 0.1 mV ST depression: | ||||
| No (n = 50) | 32.6 (12.1–83.7) | 42.8 (14.0–96.6) | 8.4 (1.8–28.0) | 27.9 (11.5–61.3) |
| Yes (n = 13) | 50.4 (19.9–90.7) | 72.1 (28.7–106.8) | 21.4 (3.8–42.7) | 56.0 (30.0–76.8) |
| P-value | 0.598 | 0.292 | 0.122 | 0.055 |
| 0.2 mV ST depression: | ||||
| No (n = 58) | 33.4 (12.4–83.7) | 51.1 (14.2–94.9) | 8.8 (1.8–28.0) | 30.1 (14.3–61.3) |
| Yes (n = 5) | 50.4 (13.6–105.2) | 89.4 (21.4–181.0) | 39.0 (7.8–75.8) | 70.1 (52.2–76.8) |
| P-value | 0.815 | 0.392 | 0.077 | 0.021 |
| Fixed perfusion defects: | ||||
| No (n = 42) | 26.0 (9.9–70.2) | 35.8 (13.0–93.6) | 8.4 (1.8–26.4) | 34.7 (15.8–58.6) |
| Yes (n = 21) | 66.4 (20.8–99.9) | 82.9 (28.2–123.5) | 14.4 (2.0–43.4) | 28.6 (2.8–69.6) |
| P-value | 0.031 | 0.052 | 0.287 | 0.838 |
BNP – type-B natriuretic peptide.
Discussion
In the present study, plasma BNP values – at baseline or their increment induced by exercise – were not associated with inducible myocardial ischemia or with the occurrence of ventricular arrhythmias during exercise stress SPECT.
Plasma B-type natriuretic peptide levels and exercise-induced ischemia
In part because of their association with depressed ventricular function and/or heart failure, increased BNP or NT-proBNP levels predict a worse outcome across the entire spectrum of ischemic heart disease [4–7]. NT-proBNP has also been shown to contain prognostic information in patients with suspected non-ST-segment elevation acute coronary syndrome [21] and in those arriving in the emergency room with a normal or nearly normal ECG and no troponin elevation [22, 23]. However, BNP and NT-proBNP mainly predict long-term events [21, 23]; it is unclear that they are useful to drive patients’ management [24, 25], and their value in the chest pain unit has been challenged when high-sensitive assays are used for troponin detection [26].
In the present study, BNP levels increased shortly after exercise, which concurs with previous observations in healthy people [8, 27] or in patients with suspected or known ischemic heart disease [8–10]. Baseline BNP levels were related to the existence of fixed defects indicating myocardial necrosis. However, neither baseline BNP values nor their increment after exercise were associated with myocardial ischemia. Previous studies that have analyzed the changes in plasma BNP or NT-proBNP in relation to exercise-induced ischemia have obtained conflicting results. Foote et al. described in 74 patients with coronary artery disease referred for exercise SPECT that the increment in plasma BNP or NT-proBNP 1 min after exercise was higher in patients with ischemia than in those without ischemia, and suggested that measuring the change in BNPs might be used to increase the sensitivity of exercise ECG for detecting ischemia [8]. Sabatine et al. found in 112 patients undergoing exercise SPECT that both baseline BNP values and their increment after exercise were related to the presence and severity of ischemia, this association being less pronounced for NT-proBNP [9]. In contrast, van der Zee et al. found in 101 patients undergoing exercise SPECT that, although baseline NT-proBNP values were higher in those subsequently developing ischemia, their increase after exercise was of similar magnitude in patients with and without ischemia [10]. Our results are at variance with those two former studies [8, 9] but, overall, concur with van der Zee's results [10].
The reason for the discrepancy among these otherwise similar studies is unclear. Although differences in populations studied, methodology, results of exercise SPECT or BNP measurement may have played a role, it is likely that the relatively reduced size of the samples and the significant variability of BNP determinations [28] have contributed to these conflicting results. Anyway, the lack of a consistent increase in BNP values associated with acute myocardial ischemia challenges the clinical usefulness of measuring this biomarker during exercise ECG test or in chest pain units. Increased baseline BNP or NT-proBNP levels in patients with inducible myocardial ischemia [9, 10, 29–31] or admitted to the emergency room [32] may just reflect increased wall stress due to impaired systolic and/or diastolic function rather than myocardial ischemia.
Plasma B-type natriuretic peptide levels and ischemic arrhythmias
BNP or NT-proBNP levels have predicted atrial arrhythmias after non-cardiac surgery [15] or after electrical cardioversion [16] and ventricular arrhythmias and sudden death in patients at risk for these arrhythmias [17]. Increased wall stress has been related to ischemic ventricular arrhythmias [12–14], but whether BNP values differ among patients with and without exercise-induced, ischemic arrhythmias is unknown. In the present study, the incidence of ventricular arrhythmias during or after the test was low and comparable to that reported previously [19, 33, 34]. BNP values were not significantly related to the presence or the frequency of VPCs or more complex arrhythmias during exercise, which is not surprising given the rarity of VPCs and arrhythmias and the lack of a significant association between BNP values and ischemia in our series.
Methodological considerations and limitations
The relatively small sample size represents a limitation, particularly for the assessment of the association between BNP levels and arrhythmias. However, the sample size is similar to that of previous studies showing an association between BNP changes and ischemia [8], and the lack of a trend in the association between BNP levels and the occurrence of ischemia or arrhythmias suggest that the results would not have been different with a larger sample size. The limited sample size also made it impossible to explore whether there were differences in BNP values across subgroups of patients with increasing severity of ischemia. Although the results of BNP measurements in the few patients with more severe scintigraphic ischemia argue against this hypothesis, the significantly larger increment in circulating BNP levels in patients with greater exercise-induced ST-segment depression might suggest that severe ischemia could affect BNP levels. Our results cannot be extrapolated to a scenario of prolonged ischemia with significant myocardial injury, where other pathways play a more prominent role [35, 36]. Our patients were all-comers undergoing a SPECT exercise test for diagnostic or prognostic purposes. The results might have been different with more stringent inclusion criteria, but then the clinical relevance would have been lower. BNP values were measured only once after the exercise test, because in previous studies sequential determinations had not provided additional information [9, 10]. Another limitation is that only BNP was analyzed. Combining several biomarkers, particularly high-sensitive troponin [37], or assessing other ECG characteristics exercise [38] might have provided more insight in the study of predictors of ischemia and arrhythmias.
In conclusion, our findings argue against the hypothesis that BNP levels can predict the presence of ischemia and its extension during exercise testing. We also have not found any correlation between BNP levels and the incidence of arrhythmia during the test. Measuring BNP levels probably has little diagnostic utility in patients undergoing exercise ECG or in those evaluated in the emergency room for suspected acute coronary syndrome.
Acknowledgments
This study was funded by a grant of the Spanish Government (FIS PI12/01844).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–8. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 2.Maisel A, Mueller C, Adams K, Jr, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10:824–39. doi: 10.1016/j.ejheart.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Stachowiak P, Kornacewicz-Jach Z, Safranow K. Prognostic role of troponin and natriuretic peptides as biomarkers for deterioration of left ventricular ejection fraction after chemotherapy. Arch Med Sci. 2014;10:1007–18. doi: 10.5114/aoms.2013.34987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arakawa N, Nakamura M, Aoki H, Hiramori K. Plasma brain natriuretic peptide concentrations predict survival after acute myocardial infarction. J Am Coll Cardiol. 1996;27:1656–61. doi: 10.1016/0735-1097(96)00067-8. [DOI] [PubMed] [Google Scholar]
- 5.Omland T, Aakvaag A, Bonarjee VV, et al. Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation. 1996;93:1963–9. doi: 10.1161/01.cir.93.11.1963. [DOI] [PubMed] [Google Scholar]
- 6.de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345:1014–21. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 7.Omland T, Richards AM, Wergeland R, Vik-Mo H. B-type natriuretic peptide and long-term survival in patients with stable coronary artery disease. Am J Cardiol. 2005;95:24–8. doi: 10.1016/j.amjcard.2004.08.058. [DOI] [PubMed] [Google Scholar]
- 8.Foote RS, Pearlman JD, Siegel AH, Yeo KT. Detection of exercise-induced ischemia by changes in B-type natriuretic peptides. J Am Coll Cardiol. 2004;44:1980–7. doi: 10.1016/j.jacc.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 9.Sabatine MS, Morrow DA, de Lemos JA, et al. Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. J Am Coll Cardiol. 2004;44:1988–95. doi: 10.1016/j.jacc.2004.07.057. [DOI] [PubMed] [Google Scholar]
- 10.van der Zee PM, Verberne HJ, van Spijker RC, et al. Relation of N-terminal pro B-type natriuretic peptide levels after symptom-limited exercise to baseline and ischemia levels. Am J Cardiol. 2009;103:604–10. doi: 10.1016/j.amjcard.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Calvo D, Jalife J. Mechanoelectric feedback in the ischemic myocardium: an interplay that modulates susceptibility to fibrillation. Rev Esp Cardiol. 2013;66:168–70. doi: 10.1016/j.rec.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Barrabés JA, Garcia-Dorado D, González MA, et al. Regional expansion during myocardial ischemia predicts ventricular fibrillation and coronary reocclusion. Am J Physiol. 1998;274:H1767–75. doi: 10.1152/ajpheart.1998.274.5.H1767. [DOI] [PubMed] [Google Scholar]
- 13.Coronel R, Wilms-Schopman FJG, deGroot JR. Origin of ischemia-induced phase 1b ventricular arrhythmias in pig hearts. J Am Coll Cardiol. 2002;39:166–76. doi: 10.1016/s0735-1097(01)01686-2. [DOI] [PubMed] [Google Scholar]
- 14.Barrabés JA, Figueras J, Candell-Riera J, Agulló L, Inserte J, Garcia-Dorado D. Distension of the ischemic region predicts increased ventricular fibrillation inducibility following coronary occlusion in swine. Rev Esp Cardiol. 2013;66:171–6. doi: 10.1016/j.rec.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Cardinale D, Colombo A, Sandri MT, et al. Increased perioperative N-terminal pro-B-type natriuretic peptide levels predict atrial fibrillation after thoracic surgery for lung cancer. Circulation. 2007;115:1339–44. doi: 10.1161/CIRCULATIONAHA.106.647008. [DOI] [PubMed] [Google Scholar]
- 16.Zografos T, Maniotis C, Katsivas A, Katritsis D. Relationship between brain natriuretic peptides and recurrence of atrial fibrillation after successful direct current cardioversion: a meta-analysis. Pacing Clin Electrophysiol. 2014;37:1530–7. doi: 10.1111/pace.12477. [DOI] [PubMed] [Google Scholar]
- 17.Scott PA, Barry J, Roberts PR, Morgan JM. Brain natriuretic peptide for the prediction of sudden cardiac death and ventricular arrhythmias: a meta-analysis. Eur J Heart Fail. 2009;11:958–66. doi: 10.1093/eurjhf/hfp123. [DOI] [PubMed] [Google Scholar]
- 18.Candell-Riera J, Santana-Boado C, Castell-Conesa J, Aguadé-Bruix S, Bermejo-Fraile B, Soler-Soler J. Dipyridamole administration at the end of an insufficient exercise Tc-99m MIBI SPECT improves detection of multivessel coronary artery disease in patients with previous myocardial infarction. Am J Cardiol. 2000;85:532–5. doi: 10.1016/s0002-9149(99)00806-1. [DOI] [PubMed] [Google Scholar]
- 19.Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med. 2003;348:781–90. doi: 10.1056/NEJMoa022353. Erratum in: N Engl J Med 2003; 348: 1508. [DOI] [PubMed] [Google Scholar]
- 20.Candell-Riera J, Ferreira-González I, Marsal JR, et al. Usefulness of exercise test and myocardial perfusion-gated single photon emission computed tomography to improve the prediction of major events. Circ Cardiovasc Imaging. 2013;6:531–41. doi: 10.1161/CIRCIMAGING.112.000158. [DOI] [PubMed] [Google Scholar]
- 21.Jernberg T, Stridsberg M, Venge P, Lindahl B. N-terminal pro brain natriuretic peptide on admission for early risk stratification of patients with chest pain and no ST-segment elevation. J Am Coll Cardiol. 2002;40:437–45. doi: 10.1016/s0735-1097(02)01986-1. [DOI] [PubMed] [Google Scholar]
- 22.Sanchis J, Bosch X, Bodí V, et al. Combination of clinical risk profile, early exercise testing and circulating biomarkers for evaluation of patients with acute chest pain without ST-segment deviation or troponin elevation. Heart. 2008;94:311–5. doi: 10.1136/hrt.2007.115626. [DOI] [PubMed] [Google Scholar]
- 23.van der Zee PM, Cornel JH, Bholasingh R, Fischer JC, van Straalen JP, De Winter RJ. N-terminal pro B-type natriuretic peptide identifies patients with chest pain at high long-term cardiovascular risk. Am J Med. 2011;124:961–9. doi: 10.1016/j.amjmed.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Sanchis J, Bosch X, Bodí V, et al. Randomized comparison between clinical evaluation plus N-terminal pro-B-type natriuretic peptide versus exercise testing for decision making in acute chest pain of uncertain origin. Am Heart J. 2010;159:176–82. doi: 10.1016/j.ahj.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Scirica BM, Morrow DA, Bode C, et al. Patients with acute coronary syndromes and elevated levels of natriuretic peptides: the results of the AVANT GARDE-TIMI 43 Trial. Eur Heart J. 2010;31:1993–2005. doi: 10.1093/eurheartj/ehq190. [DOI] [PubMed] [Google Scholar]
- 26.Sanchis J, Bardají A, Bosch X, et al. N-terminal pro-brain natriuretic peptide and high-sensitivity troponin in the evaluation of acute chest pain of uncertain etiology. A PITAGORAS substudy. Rev Esp Cardiol (Engl Ed) 2013;66:532–8. doi: 10.1016/j.rec.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 27.McNairy M, Gardetto N, Clopton P, et al. Stability of B-type natriuretic peptide levels during exercise in patients with congestive heart failure: implications for outpatient monitoring with B-type natriuretic peptide. Am Heart J. 2002;143:406–11. doi: 10.1067/mhj.2002.120148. [DOI] [PubMed] [Google Scholar]
- 28.Wu AH, Smith A, Wieczorek S, et al. Biological variation for N-terminal pro- and B-type natriuretic peptides and implications for therapeutic monitoring of patients with congestive heart failure. Am J Cardiol. 2003;92:628–31. doi: 10.1016/s0002-9149(03)00741-0. [DOI] [PubMed] [Google Scholar]
- 29.Bibbins-Domingo K, Ansari M, Schiller NB, Massie B, Whooley MA. B-type natriuretic peptide and ischemia in patients with stable coronary disease: data from the Heart and Soul study. Circulation. 2003;108:2987–92. doi: 10.1161/01.CIR.0000103681.04726.9C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asada J, Tsuji H, Iwasaka T, Thomas JD, Lauer MS. Usefulness of plasma brain natriuretic peptide levels in predicting dobutamine-induced myocardial ischemia. Am J Cardiol. 2004;93:702–4. doi: 10.1016/j.amjcard.2003.11.051. [DOI] [PubMed] [Google Scholar]
- 31.Palumbo B, Siepi D, Lupattelli G, et al. Usefulness of brain natriuretic peptide levels to discriminate patients with stable angina pectoris without and with electrocardiographic myocardial ischemia and patients with healed myocardial infarction. Am J Cardiol. 2004;94:780–3. doi: 10.1016/j.amjcard.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Möckel M, Müller R, Vollert JO, et al. Role of N-terminal pro-B-type natriuretic peptide in risk stratification in patients presenting in the emergency room. Clin Chem. 2005;51:1624–31. doi: 10.1373/clinchem.2005.049155. [DOI] [PubMed] [Google Scholar]
- 33.Elhendy A, Chandrasekaran K, Gersh BJ, Mahoney D, Burger KN, Pellikka PA. Functional and prognostic significance of exercise-induced ventricular arrhythmias in patients with suspected coronary artery disease. Am J Cardiol. 2002;90:95–100. doi: 10.1016/s0002-9149(02)02428-1. [DOI] [PubMed] [Google Scholar]
- 34.Partington S, Myers J, Cho S, Froelicher V, Chun S. Prevalence and prognostic value of exercise-induced ventricular arrhythmias. Am Heart J. 2003;145:139–46. doi: 10.1067/mhj.2003.60. [DOI] [PubMed] [Google Scholar]
- 35.Kasprzak MP, Iskra M, Majewski W, et al. PON1 status evaluation in patients with chronic arterial occlusion of lower limbs due to atherosclerosis obliterans. Arch Med Sci. 2014;10:1101–8. doi: 10.5114/aoms.2014.41348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang S, Li H, Tang L, et al. Apelin-13 protects the heart against ischemia-reperfusion injury through the RISK-GSK-3β-mPTP pathway. Arch Med Sci. 2015;11:1065–73. doi: 10.5114/aoms.2015.54863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabatine MS, Morrow DA, de Lemos JA, Jarolim P, Braunwald E. Detection of acute changes in circulating troponin in the setting of transient stress test-induced myocardial ischaemia using an ultrasensitive assay: results from TIMI 35. Eur Heart J. 2009;30:162–9. doi: 10.1093/eurheartj/ehn504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janusek D, Kania M, Zaczek R, et al. Evaluation of T-wave alternans in high-resolution ECG maps recorded during the stress test in patients after myocardial infarction. Arch Med Sci. 2015;11:99–105. doi: 10.5114/aoms.2013.39939. [DOI] [PMC free article] [PubMed] [Google Scholar]