Abstract
Introduction
New tests for improved diagnosis of diabetic peripheral neuropathy (DPN) are useful.
Material and methods
We evaluated the utility of automated nerve conduction study (NCS) of the sural nerve with a new portable device for the diagnosis of DPN in patients with type 2 diabetes mellitus (T2DM). This study included 114 T2DM patients (58 men) with mean age 64.60 ±8.61 years. Exclusion criteria were B12 depletion, alcohol abuse and other causes of peripheral neuropathy. The reference method was the Neuropathy Disability Score (NDS) with a threshold NDS ≥ 3. Sural nerve automated NCS was carried out with the portable NC-stat DPNCheck device. Sensory nerve conduction velocity and sensory nerve action potential amplitude were measured bilaterally. Automated NCS was considered abnormal when ≥ 1 of the two aforementioned neurophysiological parameters was abnormal in at least one leg.
Results
Examination with NC-stat DPNCheck exhibited 90.48% sensitivity, 86.11% specificity, 79.17% positive predictive value (PPV) and 93.94% negative predictive value (NPV). The positive likelihood ratio (LR+) was 6.51 and the negative likelihood ratio (LR–) was 0.11.
Conclusions
Sural nerve automated NCS with the NC-stat DPNCheck device exhibits high sensitivity and specificity for the diagnosis of DPN in T2DM.
Keywords: diabetes mellitus, diabetic neuropathy, diagnosis, nerve conduction study
Introduction
The abundance of new tests for diabetic peripheral neuropathy (DPN) suggests that there is a poorly met need to improve its diagnosis in everyday reality [1–3]. Some of these new tests are more suitable as screening tools, while others are appropriate for patient evaluation and follow-up in specialised centres [1–3]. Among the former, the indicator test Neuropad has been shown to exhibit a high sensitivity and negative predictive value as a screening tool for DPN [4–6]. A further approach to improve diagnosis includes automated nerve conduction study (NCS), which can be easily and quickly performed without specialised personnel [7, 8]. The first devices for automated NCS harboured a pre-arranged series of electrodes permitting automatic selection of the clearest electrical signal [7, 8]. With such devices, automated NCS was found to yield high sensitivity and specificity compared to established classical NCS [8]. More recently, a new, even simpler device has become commercially available, which offers automated NCS specifically of the sural nerve: the NC-stat DPNCheck (NeuroMetrix, Inc., Waltham, MA) [9]. This can be used by health care professionals after < 60 min training, and the examination takes approximately 2 min. A small study has shown that NC-stat DPNCheck yields high sensitivity and specificity in comparison with classical NCS, despite some slight over-estimation of nerve conduction velocity [9]. Importantly, very good intra- and inter-observer reproducibility for this examination has already been demonstrated [9, 10].
However, NC-stat DPNCheck has not been compared with standardised clinical examination. This comparison is useful, because traditional NCS is not widely available, in contrast to clinical examination [11, 12]. Given that NC-stat DPNCheck appears even simpler and can be used by diabetes nurses as well, we aimed to examine the diagnostic performance of this new device against standardised clinical examination for the diagnosis of DPN in patients with type 2 diabetes mellitus (T2DM).
Material and methods
This study included 114 T2DM patients (58 men, 56 women) who attended the Diabetes Clinic of the Second Department of Internal Medicine at Democritus University of Thrace, Greece. An age- and sex-matched group of 46 healthy controls (24 men, 22 women) was also included. The study was approved by the local institutional ethics committee and patients gave their informed consent.
Diagnosis of DPN was based on clinical examination by the standardised neuropathy disability score (NDS), according to Young et al. [11]. This evaluates ankle reflexes, as well as vibration, pain and temperature perception on the feet bilaterally [11], and is an established measure of the presence and severity of DPN [12]. An NDS ≥ 3 was considered diagnostic of DPN [11, 12]. Exclusion criteria were B12 depletion, alcohol abuse, lumbar spine disorders and other causes of peripheral neuropathy.
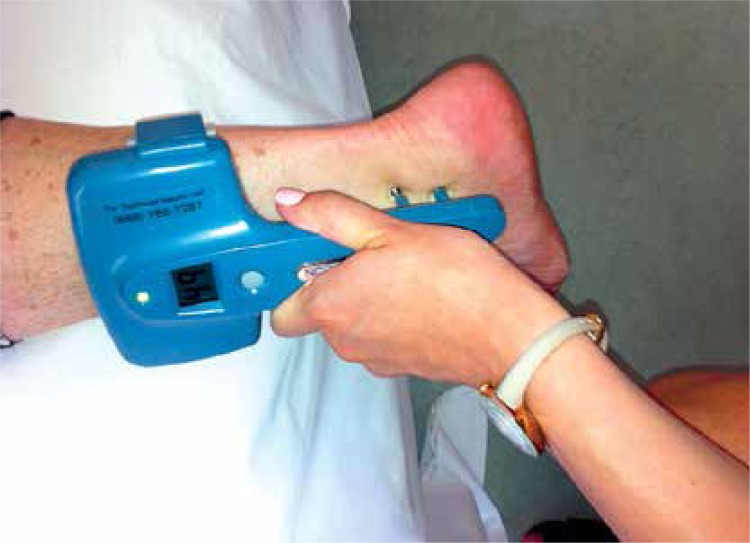
Examination with NC-stat DPNCheck was carried out at constant room temperature (20–25°C) as follows (Figure 1). Patients lay in the supine position and both sural nerves were examined. The device harbours two stimulating probes, a biosensor, a display screen, a button, and a battery. The probes were first immersed in gel to improve electrical conductance. The bigger probe was located by the examiner on the lateral side of the ankle in the middle of the lateral malleolus, and the biosensor was located in a straight line on the calf. Pressing the button, the examiner could choose the right or left limb and start measurements. On the display screen, sensory nerve conduction amplitude (SNAP) and sensory nerve conduction velocity (SNCV) were shown. Two measurements were performed in each limb, and the mean value of measured parameters was calculated. Automated NCS was considered abnormal when SNAP was < 4 µV in at least one leg and/or SNCV was < 40 m/s in at least one leg [13]. The examiner was blinded to the clinical NDS data.
Figure 1.
Examination with the NC-stat DPNCheck device
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of automated NCS against NDS were calculated by standard formulae. Data were expressed as mean ± SD. Continuous variables were compared by t-test. Likelihood ratios and Youden's J were calculated by standard formulae [14].
Results
Patient age was 64.60 ±8.61 years. Diabetic peripheral neuropathy was diagnosed in 42 (36.84%) patients by NDS. Patients with DPN had insignificantly higher age than those without DPN (66.40 ±8.96 vs. 63.56 ±8.29 years, p = 0.089), as well as significantly longer diabetes duration (16.14 ±7.28 vs. 11.82 ±7.89 years, p = 0.004) and higher glycated haemoglobin (HbA1c) (8.90 ±1.30 vs. 7.20 ±1.30%, p < 0.001).
In T2DM patients, examination with NC-stat DPNCheck exhibited 90.48% sensitivity and 86.11% specificity (Table I). The positive likelihood ratio (LR+) was 6.51 and the negative likelihood ratio (LR–) was 0.11. Youden's J was 0.77.
Table I.
Diagnostic performance of automated NCS vs. clinical examination (NDS)
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | LR+ | LR– | Youden's J |
|---|---|---|---|---|---|---|
| 90.48 | 86.11 | 79.17 | 93.94 | 6.51 | 0.11 | 0.77 |
LR+: positive likelihood ratio, LR–: negative likelihood ratio, NCS – nerve conduction study, NDS – neuropathy disability score, NPV – negative predictive value, PPV – positive predictive value.
In the control group, age was 64.19 ±8.82 years. The NDS was normal in all subjects, while automated NCS was abnormal in two subjects.
Discussion
To the best of our knowledge, this is the first study examining the diagnostic performance of automated NCS by the NC-stat DPNCheck device against standardised clinical examination. It was found that NC-stat DPNCheck exhibited a very good diagnostic performance, as reflected in the high Youden's J. Its sensitivity and NPV were excellent, and its specificity and PPV were high. In particular, its LR+ was good [14], suggesting that a positive result is of value in ruling in DPN. Most importantly, its LR- was very low, indicating that the test is especially reliable as a screening tool to rule out DPN.
Lee et al. [9] recently demonstrated generally small differences in electrophysiological parameters between NC-stat DPNCheck and classical NCS. Against the latter, sensitivity and specificity of the former were: 88% and 94%, respectively for SNAP; 94% and 82%, respectively for SNCV; 95% and 71%, respectively for ≥ 1 abnormal electrophysiological parameter [9]. These data are very important in the validation of measurements with the new device. Although their study addressed a different question from ours, the findings of the two works together provide evidence for the diagnostic utility of the new test.
The practical implications of our findings are that the new automated NCS device merits further use in everyday clinical practice. In an endeavour to simplify classical NCS, we have previously shown that the sural sensory/radial motor amplitude ratio has high diagnostic sensitivity compared with the complete neurophysiological examination, but it is less time-demanding, thereby having the potential to enable examination of more patients [15]. NC-stat DPNCheck is a step further ahead: its application may be expected to increase patient examination rates for DPN, which remains a priority in order to reduce diabetic foot morbidity [16, 17]. Indeed, the new test may be used by diabetes nurses and other health care professionals after minimal training. Therefore, NC-stat DPNCheck may be widely employed as a screening tool, with particular value in exclusion of DPN. A disadvantage is, certainly, the cost for purchase of the device and disposable biosensor electrodes, so a cost-utility analysis should be designed.
The strengths of the present study are the inclusion of a relatively adequate patient series, the use of the established NDS, the simple, straightforward neurophysiological criteria permitting widespread use of the new device as a screening tool, and the clear message. Its limitations may be outlined as follows. First, we included patients from a tertiary care setting, and therefore the results may not be directly applicable to the general diabetic population. A second limitation may be that we did not confirm the diagnosis of DPN by classical NCS. However, this was beyond the scope of our study. Instead, we chose to compare the new device with standardised clinical examination, which is more widely available. For this purpose, we used the established NDS [6, 11, 12]. Finally, we only studied patients with T2DM, and so more experience with type 1 diabetes is needed.
In conclusion, the findings of this study suggest that sural nerve automated NCS with the NC-stat DPNCheck device exhibits high sensitivity and specificity for the diagnosis of clinical DPN in T2DM. This high diagnostic performance suggests that the test may prove useful as a screening tool of DPN, with a particular utility in exclusion of this condition. The present results add to the increasing appreciation of the importance that automated NCS may have in improving diagnosis of DPN, including the primary health care setting [7–10]. In view of the need to increase our knowledge in DPN [17–19], they encourage further use of the new device in clinical practice.
Conflicts of interest
Nikolaos Papanas has served as a member in the scientific advisory board of TrigoCare International, distributor of Neuropad.
References
- 1.Papanas N, Ziegler D. New vistas in the diagnosis of diabetic polyneuropathy. Endocrine. 2014;47:690–8. doi: 10.1007/s12020-014-0285-z. [DOI] [PubMed] [Google Scholar]
- 2.Papanas N, Boulton AJ, Malik RA, et al. A simple new non-invasive sweat indicator test for the diagnosis of diabetic neuropathy. Diabet Med. 2013;30:525–34. doi: 10.1111/dme.12000. [DOI] [PubMed] [Google Scholar]
- 3.Papanas N, Ziegler D. Corneal confocal microscopy: a new technique for early detection of diabetic neuropathy. Curr Diab Rep. 2013;13:488–99. doi: 10.1007/s11892-013-0390-z. [DOI] [PubMed] [Google Scholar]
- 4.Tsapas A, Liakos A, Paschos P, et al. A simple plaster for screening for diabetic neuropathy: a diagnostic test accuracy systematic review and meta-analysis. Metabolism. 2014;63:584–92. doi: 10.1016/j.metabol.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Papanas N, Papatheodorou K, Papazoglou D, Monastiriotis C, Christakidis D, Maltezos E. A comparison of the new indicator test for sudomotor function (Neuropad) with the vibration perception threshold and the clinical examination in the diagnosis of peripheral neuropathy in subjects with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2008;116:135–8. doi: 10.1055/s-2007-984455. [DOI] [PubMed] [Google Scholar]
- 6.Manes C, Papanas N, Exiara T, et al. The indicator test Neuropad in the assessment of small and overall nerve fibre dysfunction in patients with type 2 diabetes: a large multicentre study. Exp Clin Endocrinol Diabetes. 2014;122:195–9. doi: 10.1055/s-0034-1367061. [DOI] [PubMed] [Google Scholar]
- 7.Vinik AI, Kong X, Megerian JT, Gozani SN. Diabetic nerve conduction abnormalities in the primary care setting. Diabetes Technol Ther. 2006;8:654–62. doi: 10.1089/dia.2006.8.654. [DOI] [PubMed] [Google Scholar]
- 8.Perkins BA, Grewal J, Ng E, Ngo M, Bril V. Validation of a novel point-of-care nerve conduction device for the detection of diabetic sensorimotor polyneuropathy. Diabetes Care. 2007;29:2023–7. doi: 10.2337/dc08-0500. [DOI] [PubMed] [Google Scholar]
- 9.Lee A, Halpern EM, Lovblom LE, Yeung E, Bril V, Perkins BA. Reliability and validity of a point-of-care sural nerve conduction device for identification of diabetic neuropathy. PLoS One. 2014;9:e86515. doi: 10.1371/journal.pone.0086515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong X, Boettcher B, Snow K, Gozani S. Reproducibility of a rapid point-of-care sural nerve conduction test. Diabetes. 2012;61:595–P. [Google Scholar]
- 11.Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PA. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36:150–4. doi: 10.1007/BF00400697. [DOI] [PubMed] [Google Scholar]
- 12.Papanas N, Paschos P, Papazoglou D, et al. Accuracy of the Neuropad test for the diagnosis of distal symmetric polyneuropathy in type 2 diabetes. Diabetes Care. 2011;34:1378–82. doi: 10.2337/dc10-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neurometrix Inc. NC-stat DPNCheck instructions; 2013. [Google Scholar]
- 14.Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ. 2004;329:168–9. doi: 10.1136/bmj.329.7458.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papanas N, Trypsianis G, Giassakis G, et al. The sural sensory/radial motor amplitude ratio for the diagnosis of peripheral neuropathy in type 2 diabetic patients. Hippokratia. 2010;14:198–202. [PMC free article] [PubMed] [Google Scholar]
- 16.Apelqvist J, Bakker K, van Houtum WH, Schaper NC, International Working Group on the Diabetic Foot (IWGDF) Editorial Board Practical guidelines on the management and prevention of the diabetic foot: based upon the International Consensus on the Diabetic Foot (2007) prepared by the International Working Group on the Diabetic Foot. Diabetes Metab Res Rev. 2008;24(Suppl. 1):S181–7. doi: 10.1002/dmrr.848. [DOI] [PubMed] [Google Scholar]
- 17.Kasznicki J. Advances in the diagnosis and management of diabetic distal symmetric polyneuropathy. Arch Med Sci. 2014;10:345–54. doi: 10.5114/aoms.2014.42588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monastiriotis C, Papanas N, Veletza S, Maltezos E. APOE gene polymorphisms and diabetic peripheral neuropathy. Arch Med Sci. 2012;8:583–8. doi: 10.5114/aoms.2012.30279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amer MA, Ghattas MH, Abo-Elmatty DM, Abou-El-Ela SH. Evaluation of glutathione S-transferase P1 genetic variants affecting type-2 diabetes susceptibility and glycemic control. Arch Med Sci. 2012;8:631–6. doi: 10.5114/aoms.2012.30286. [DOI] [PMC free article] [PubMed] [Google Scholar]