Abstract
Purpose
We evaluated quality-of-life changes (QoL) in 907 patients treated with either radical prostatectomy (open or laparoscopic), real-time planned conformal brachytherapy, or high-dose intensity-modulated radiotherapy (IMRT) on a prospective IRB-approved longitudinal study.
Methods
Validated questionnaires given pretreatment (baseline) and at 3, 6, 9, 12, 15, 18, 24, 36, and 48 months addressed urinary function, urinary bother, bowel function, bowel bother, sexual function, and sexual bother.
Results
At 48 months, surgery had significantly higher urinary incontinence than others (both P<.001), but fewer urinary irritation/obstruction symptoms (all P<.001). Very low levels of bowel dysfunction were observed and only small subsets in each group showed rectal bleeding. Brachytherapy and IMRT showed better sexual function than surgery accounting for baseline function and other factors (delta 14.29 of 100, 95% CI, 8.57–20.01; and delta 10.5, 95% CI, 3.78–17.88). Sexual bother was similar. Four-year outcomes showed persistent urinary incontinence for surgery with more obstructive urinary symptoms for radiotherapy. Using modern radiotherapy delivery, bowel function deterioration is less-often observed. Sexual function was strongly affected in all groups yet significantly less for radiotherapy.
Conclusions
Treatment selection should include patient preferences and balance predicted disease-free survival over a projected time vs potential impairment of QoL important for the patient.
Keywords: prostate cancer, radiotherapy, brachytherapy, surgery, quality of life
For patients with clinically localized prostate cancer, selecting the optimal treatment involves many considerations. There is retrospective evidence of difference in disease-free survival outcomes between treatments [1, 2] and changes in quality of life (QOL). Several longitudinal, prospective QOL assessments have shown differences between treatments for urinary incontinence, urinary bother, and bowel-related issues [3–11]. QOL symptoms improve or deteriorate with time; in most of these studies, the differences become less apparent with further time post-therapy. Understanding these temporal changes could provide valuable information to the clinician and patient that ultimately should play a large part in treatment selection.
We conducted a prospective longitudinal QOL study using a validated QOL tool. Patients from a single institution treated with state-of-the-art interventions of surgery, brachytherapy, or intensity-modulated radiotherapy (IMRT) were followed for 4 years. Several similar studies in prostate cancer have had various limitations, including smaller sample sizes, limited number of QOL assessments, cross-sectional study design, or comparing surgery to radiotherapy using less conformal radiotherapy techniques. With the advent of enhanced treatment-delivery approaches, such as enhanced surgical techniques or IMRT and real-time adaptive brachytherapy, less toxicity has been observed. The current study is unique in that it includes a large cohort of patients treated at a single institution with uniform state-of-the-art modern surgical and radiotherapy techniques who were evaluated at multiple times over an extended period.
Material and methods
We enrolled 907 patients with clinically localized prostate cancer from November 2002 to May 2009 on a prospective institutional review board–approved longitudinal QOL study who were treated with radical prostatectomy (RP), brachytherapy, or IMRT. Patients were included if they were diagnosed with localized, previously untreated prostate cancer, were able to read English, and agreed to complete the QOL assessments during follow-up. Patients were excluded if they were diagnosed with other cancers within the past 3 years (other than non-melanoma skin cancer), received prior chemotherapy or hormonal therapy within the past 3 years, or had received prior radiotherapy to the pelvis or pelvic surgery. Eligible patients including patients from regional network sites were approached by consenting professionals and invited to enroll.
Enrollment was open for surgery patients from November 2002 to May 2005. During this time, 1126 patients meeting eligibility criteria were treated with surgery, out of which 477 agreed to participate. Enrollment was open for radiation patients from November 2002 to May 2009, and of the 2001 radiation patients meeting eligibility criteria, 430 agreed to participate. Characteristics of non-enrolled and enrolled surgery and radiotherapy cohorts showed slightly higher percentage of Gleason 6 cancers in the surgery and radiotherapy cohorts, slightly lower baseline prostate-specific antigen in the enrolled radiotherapy cohort, and slightly more clinical T1 patients in the surgery cohorts (data not shown).
Cohort
Enrolled patients were given questionnaires before treatment (baseline) and at 3, 6, 9, 12, 15, 18, 24, 36, and 48 months after treatment. Of the 907 patients enrolled, 2 were missing the baseline questionnaire, 362 were missing the 48-month questionnaire, and 9 were missing both. These patients were omitted from analysis, leaving an evaluable cohort of 534 patients, of whom 210 were surgery, 171 were brachytherapy, and 153 were IMRT patients. Thirty radiotherapy patients had received neoadjuvant androgen-deprivation therapy (ADT).
Treatment
Surgery patients were treated with RP using either an open (n=134; 64%) or a laparoscopic approach (n=76; 36%). For brachytherapy patients, conformal intraoperative planning was used to constrain dose to normal tissues, including urethra and rectum, and optimize accurate targeting of the prescription dose to the prostate. In general, permanent interstitial I-125 implantation was used. For monotherapy, the prescription dose was 144 Gy; for intermediate- and higher-risk disease, the dose was 110 Gy followed 2 months later by 45–50.4 Gy of supplemental IMRT. External beam radiotherapy (EBRT) patients received IMRT to 81–86 Gy directed to the prostate and seminal vesicles with a 1-cm margin, or a 6-mm margin at the prostate-rectum interface. In general, during this time, elective lymph node irradiation was not routinely employed, and patients did not undergo pretreatment fiducial marker placement for image-guidance for daily fractions.
Survey instrument
The questionnaire used in this study has been previously validated [12] and addresses six different domains: urinary function, urinary bother, bowel function, bowel bother, sexual function, and sexual bother. For each domain and subdomain, scores were summed and scaled to a percentage such that higher scores were consistent with better outcomes. If fewer than half the questions in a particular domain or subdomain were answered, the participant was considered to have missing scores for that domain or subdomain.
Primary endpoint
For each of these domains and subdomains, the 48-month scores of surgery patients were compared with those of IMRT and brachytherapy patients. A separate analysis included brachytherapy and IMRT patients who received ADT. A patient undergoing both brachytherapy and ADT, or IMRT and ADT, is therefore represented in two analyses. Patients who answered fewer than half the questions in a particular domain or subdomain were not included in the analysis. Differences between groups were estimated using multivariable linear regression. For all domains and subdomains, analysis was adjusted for baseline score and age at treatment. Additionally, for the domains of urinary function, urinary bother, bowel function, and bowel bother, and the associated subdomains, comparisons were adjusted for clinical T stage (T1 vs T2 vs T3), biopsy Gleason grade (≤6 vs 7 vs ≥8), and pretreatment prostate-specific antigen value. For the domains of sexual function, sexual bother, and the associated subdomains, comparisons were adjusted for categorized number of comorbidities (0 vs 1 vs 2 vs ≥3). Comorbidities included in this count were coronary artery disease, peripheral vascular disease, stroke, diabetes, hypertension, hypercholesterolemia, obesity, and smoking. Because sexual function is strongly binary, potency was defined as ≥22 (range 1–30) on the abbreviated International Index of Erectile Function (IIEF-6). Multivariable logistic regression models were adjusted for baseline IIEF-6 score, age at treatment, and categorized number of comorbidities.
Longitudinal analysis
We were also interested in evaluating the impact of treatment on QOL throughout follow-up. Thus, the analysis was repeated using a longitudinal measure of QOL as the outcome and a comparison of the groups 48 months after treatment. The longitudinal QOL, which can be thought of as an average function during follow-up, was estimated by calculating the area under the curve from 3-month to 48-month scores using the trapezoid rule. Patients missing a 3-month survey were dropped from the longitudinal analysis.
Analysis by baseline function
Because differences between treatment groups may be suppressed by the inclusion of patients who had poor performance at baseline, we repeated the urinary analyses comparing only patients who were continent at baseline according to the urinary section of the QOL Survey with ≥17 of 21 points. We also repeated the sexual analyses comparing only those patients who were potent at baseline according to the IIEF-6 with ≥22 of 30 points.
Results
Patient characteristics
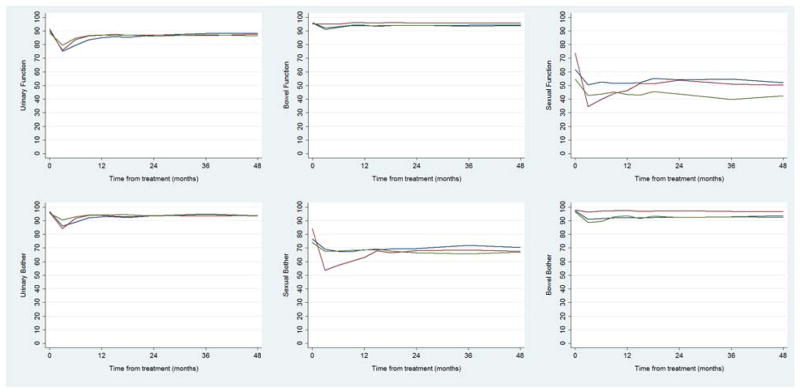
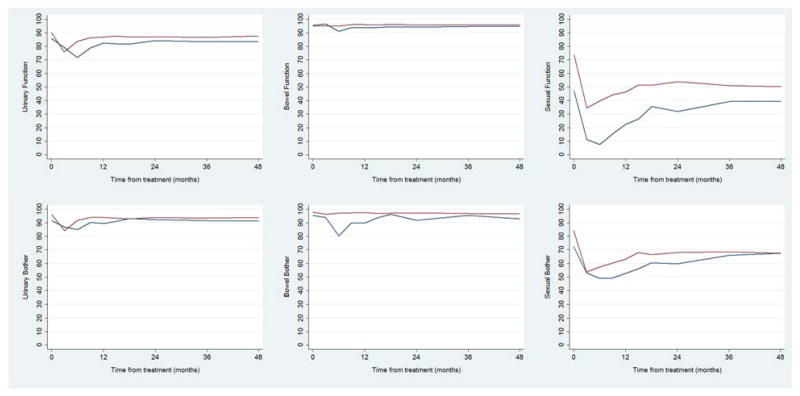
Patient characteristics are shown in Table 1. Surgery patients were significantly younger than brachytherapy, IMRT, or radiotherapy plus ADT patients (median age, 60 years vs. 67, 70, and 70 years, respectively; all P<.001) and had fewer comorbidities than brachytherapy patients (39% with ≥2 vs. 60%, P=.001). Despite some significant differences between cohorts, pretreatment prostate-specific antigen and clinical stage were generally similar. Baseline and 48-month scores for each domain and subdomain for each of the treatment groups are shown in Table 2. These mean scores were calculated from patients who provided answers to both the baseline and the 48-month questionnaire for each specific domain or subdomain. Most function and bother domain scores at 48 months were lower than baseline. The mean, unadjusted scores in each domain from baseline to 48 months are shown in Table 2 for patients in each treatment group (see Fig. 1 for mean adjusted scores across the treatment groups and Fig. 2 for mean adjusted scores comparing prostatectomy with patients treated with ADT and radiotherapy).
Table 1.
Patient characteristics.a
| Radical prostatectomy (n = 210; 39%) | Brachytherapy (n = 171; 32%) | IMRT (n = 153; 29%) | Androgen deprivationb (n = 30; 5.6%) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| P | P | P | |||||
| Age at treatment, y | 60 (56, 64) | 67 (61, 72) | <.001 | 70 (64, 74) | <.001 | 70 (66, 73) | <.001 |
| PSA before treatment | 5.3 (4.2, 7.6) | 5.8 (4.4, 7.4) | 0.3 | 6.1 (4.4, 8.8) | 0.017 | 8.3 (5.1, 11.1) | .002 |
| Clinical stage | .079 | .030 | .001 | ||||
| T1 | 142 (68) | 124 (73) | 88 (58) | 17 (57) | |||
| T2 | 66 (31) | 41 (24) | 58 (38) | 8 (27) | |||
| T3 | 2 (1.0) | 6 (3.5) | 7 (4.6) | 5 (17) | |||
| Biopsy Gleason score | .3 | <.001 | .027 | ||||
| ≤6 | 136 (65) | 117 (68) | 56 (37) | 13 (43) | |||
| 7 | 64 (30) | 51 (30) | 84 (55) | 13 (43) | |||
| ≥8 | 10 (4.8) | 3 (1.8) | 13 (8.5) | 4 (13) | |||
| Comorbidities | .001 | .2 | .5 | ||||
| 0 | 52 (25) | 23 (13) | 32 (21) | 6 (20) | |||
| 1 | 76 (36) | 47 (27) | 46 (30) | 8 (27) | |||
| 2 | 44 (21) | 59 (35) | 33 (22) | 8 (27) | |||
| ≥3 | 38 (18) | 42 (25) | 42 (27) | 8 (27) | |||
Abbreviations: IMRT = intensity-modulated radiotherapy, PSA = prostate-specific antigen.
Data presented as medians with interquartile ranges in parentheses or number of instances with percentages in parentheses, using Fisher’s exact test and Wilcoxon rank sum test, respectively. Radical prostatectomy (open or laparoscopic) is the reference group in all comparisons.
The androgen-deprivation group includes radiotherapy patients (external beam or brachytherapy) who also receive adjuvant or neoadjuvant hormones of any duration. Patients undergoing radiotherapy plus hormonal therapy are thus represented in both columns.
Table 2.
Four-year follow-up scores.a
| Radical prostatectomy | Brachytherapy | IMRT | Androgen deprivationb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| No. | Score (SD) | No. | Score (SD) | No. | Score (SD) | No. | Score (SD) | |||||
|
|
||||||||||||
| Domain | 0 mo | 48 mo | 0 mo | 48 mo | 0 mo | 48 mo | 0 mo | 48 mo | ||||
| Urinary function | 203 | 90 (11) | 88 (11) | 170 | 92 (7) | 88 (11) | 153 | 89 (9) | 86 (11) | 30 | 86 (10) | 84 (10) |
| Incontinence | 202 | 95 (10) | 82 (19) | 165 | 96 (8) | 91 (16) | 151 | 93 (10) | 89 (15) | 29 | 90 (10) | 84 (17) |
| Irritation or obstruction | 200 | 87 (14) | 92 (9) | 171 | 88 (9) | 86 (12) | 153 | 85 (12) | 84 (12) | 30 | 83 (13) | 83 (12) |
| Urinary bother | 200 | 96 (10) | 94 (13) | 171 | 97 (8) | 94 (13) | 153 | 95 (9) | 93 (12) | 30 | 92 (12) | 91 (13) |
| Bowel function | 204 | 95 (7) | 96 (7) | 170 | 96 (6) | 94 (7) | 150 | 96 (5) | 94 (7) | 29 | 95 (5) | 95 (5) |
| Pain or constipation | 204 | 96 (10) | 96 (11) | 170 | 96 (9) | 96 (9) | 149 | 94 (11) | 96 (9) | 29 | 93 (14) | 95 (9) |
| Diarrhea or frequency | 204 | 93 (9) | 95 (9) | 170 | 95 (6) | 93 (9) | 150 | 95 (7) | 93 (9) | 29 | 96 (5) | 94 (6) |
| Rectal bleeding | 204 | 97 (11) | 97 (9) | 168 | 97 (11) | 95 (12) | 147 | 99 (5) | 94 (14) | 27 | 98 (10) | 95 (14) |
| Bowel bother | 204 | 98 (8) | 96 (9) | 167 | 97 (8) | 94 (15) | 148 | 96 (10) | 92 (15) | 27 | 95 (9) | 93 (15) |
| Sexual function | 202 | 74 (28) | 50 (31) | 165 | 63 (33) | 52 (32) | 144 | 56 (35) | 42 (34) | 28 | 50 (39) | 39 (32) |
| Sexual desire | 202 | 68 (22) | 52 (25) | 166 | 62 (28) | 55 (26) | 143 | 57 (30) | 45 (29) | 28 | 48 (34) | 43 (28) |
| Erectile function | 207 | 78 (33) | 49 (38) | 165 | 63 (39) | 50 (39) | 144 | 56 (40) | 40 (39) | 28 | 51 (44) | 36 (38) |
| Sexual bother | 201 | 84 (18) | 67 (24) | 161 | 77 (22) | 70 (23) | 141 | 75 (22) | 67 (23) | 28 | 73 (23) | 67 (22) |
| Intercourse bother | 199 | 92 (15) | 78 (24) | 160 | 86 (19) | 83 (21) | 141 | 88 (18) | 82 (20) | 28 | 88 (17) | 86 (14) |
| Intercourse satisfaction | 196 | 73 (35) | 49 (39) | 160 | 62 (40) | 49 (39) | 142 | 52 (44) | 40 (41) | 28 | 49 (47) | 38 (43) |
Abbreviations: IMRT = intensity-modulated radiotherapy, SD = standard deviation.
Mean scores in each domain and subdomain, standard deviations given in parentheses for patients who provided scores at baseline and at 48 months after treatment.
The androgen-deprivation group includes radiotherapy patients (external beam or brachytherapy) who also received adjuvant or neoadjuvant hormones of any duration. Patients undergoing radiotherapy plus hormonal therapy are thus represented in both columns.
Fig. 1.
Mean scores in each domain throughout follow-up for patients treated with radical prostatectomy (red line), brachytherapy (blue line), and IMRT (green line).
Fig. 2.
Mean scores in each domain throughout follow-up for patients treated with radical prostatectomy (red line) and androgen-deprivation therapy (blue line).
Functional outcomes at 4 years
The results of the 48-month analysis are shown in Table 3. There was no significant difference between groups for overall urinary function or bother, but surgery was associated with greater incontinence but less urinary irritation and obstruction than the other groups.
Table 3.
Differences between groups at 4 years.a
| Domain | Radical prostatectomy vs. brachytherapy | Radical prostatectomy vs. IMRT | Radical prostatectomy vs. Androgen deprivationb | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Delta (95% CI) | P | Delta (95% CI) | P | Delta (95% CI) | P | |
| Urinary unction | .83 (−1.59, 3.26) | .5 | 1.06 (−1.64, 3.75) | .4 | −1.53 (−6.45, 3.39) | .5 |
| Incontinence | 10.13 (6.27, 13.98) | <.001 | 11.03 (6.70, 15.37) | <0.001 | 7.30 (−1.11, 15.72) | .089 |
| Irritation or obstruction | −6·36 (−8·55, −4·16) | <0·001 | −6·83 (−9·28, −4·38) | <0·001 | −8·30 (−12·17, −4·43) | <0·001 |
| Urinary bother | −.18 (−3.00, 2.63) | .9 | 1.17 (−1.96, 4.31) | .5 | −1.82 (−7.61, 3.96) | .5 |
| Bowel function | −1.08 (−2.46, .31) | .13 | −1.30 (−2.81, .20) | .090 | −.00 (−2.52, 2.52) | 1 |
| Pain or constipation | .89 (−1.15, 2.92) | .4 | 1.48 (−.92, 3.87) | .2 | 2.44 (−1.95, 6.82) | .3 |
| Diarrhea or frequency | −1.26 (−3.08, .56) | .2 | −0·78 (−2·83, 1·28) | .5 | 1.11 (−2.36, 4.59) | .5 |
| Rectal bleeding | −1.39 (−3.51, .73) | .2 | −4.80 (−7.59, −2.01) | .001 | −3.97 (−7.92, −.01) | .049 |
| Bowel bother | −1.97 (−4.58, .64) | .14 | −2.65 (−5.52, .22) | .071 | −3.02 (−7.32, 1.28) | .2 |
| Sexual function | 14.29 (8.57, 20.01) | <.001 | 10.55 (3.78, 17.33) | .002 | 11.30 (−0.55, 23.15) | .061 |
| Sexual desire | 10.30 (5.61, 14.99) | <.001 | 4.27 (−1.37, 9.90) | .14 | 5.89 (−3.95, 15.73) | .2 |
| Erectile unction | 16.51 (9.43, 23.60) | <.001 | 14.99 (6.75, 23.23) | <.001 | 15.51 (1.06, 29.95) | .035 |
| Sexual bother | 8.40 (3.50, 13.31) | .001 | 7.29 (1.89, 12.68) | .008 | 8.35 (−1.48, 18.19) | .10 |
| Intercourse bother | 7.44 (2.48, 12.41) | 003 | 6.53 (1.19, 11.88) | .017 | 8.76 (−1.02, 18.55) | .079 |
| Intercourse satisfaction | 11.69 (4.10, 19.28) | .003 | 10.08 (1.21, 18.95) | .026 | 10.59 (−4.87, 26.04) | .2 |
Abbreviations: CI = confidence interval, IMRT = intensity-modulated radiotherapy.
Difference between means (delta) for scores at 48 months in each domain and subdomain for the treatments specified, with 95% confidence intervals in parentheses. Radical prostatectomy (open or laparoscopic) is the reference group in all comparisons. A positive score means better function in radiotherapy compared with surgery.
The androgen-deprivation group includes radiotherapy patients (external beam or brachytherapy) who also received adjuvant or neoadjuvant hormones of any duration. Patients undergoing radiotherapy plus hormonal therapy are thus represented in both columns.
As shown in Table 2, there were very low levels of bowel dysfunction overall and only a small subset of patients in each group showed problems in the subdomain of rectal bleeding. Most patients had good to excellent scores in this subdomain, with 95% of surgery, 91% of brachytherapy, 88% of IMRT, and 93% of ADT patients scoring >80 points before adjustment. Table 3 shows that the only statistically significant difference between groups for bowel-related domains is that IMRT and ADT had slightly worse rectal bleeding than surgery.
All patient groups had low mean sexual function scores at 48 months (Table 2), though means of 40–50 mask wide variation. At 48 months after treatment, 22% and 25% of surgery patients had unadjusted scores <20 and >80, respectively, as did 22% and 29% of brachytherapy patients, 38% and 21% of IMRT patients, and 46% and 11% of ADT patients. Although groups had similar scores at the 48-month follow-up, brachytherapy and IMRT patients had significantly better sexual function after adjusting for baseline scores. In the simplest terms, this can be thought of as indicating a greater decrease in scores for surgery. Similar results were found for sexual bother. Differences between surgery and radiotherapy patients receiving hormonal therapy were similar in magnitude but were not statistically significant except in the subdomain of erectile function (P=.035).
These results are consistent with the binary outcome (yes/no) of potency at 48 months using the IIEF-6. Brachytherapy patients, IMRT patients, and radiotherapy patients who received ADT were all more likely to be potent at 48 months than surgery patients (P=.001, P<.001, and P=.015, respectively). According to these analyses, a patient 58 years old at time of treatment with 0 comorbidities and potent at baseline with an IIEF of 28 points has a 4-year potency probability of 47% if treated with surgery, 70% if treated with brachytherapy, and 76% if treated with IMRT.
Functional outcomes during follow-up
Longitudinal analysis results are shown in Table 4. Findings for urinary scores followed an overall pattern similar to the 48-month endpoint, with similar bother and overall scores reflecting higher incontinence with surgery and more irritative symptoms with radiotherapy. One exception was that IMRT patients experienced more bother but significantly better urinary function scores throughout follow-up assessments compared with surgery patients, suggesting that reduced dose exposure to normal tissue may influence long-term QOL outcomes.
Table 4.
Difference between groups during the 4-year follow-up.a
| Radical Prostatectomy vs Brachytherapy | Radical Prostatectomy vs IMRT | Radical Prostatectomy vs Androgen Deprivationb | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Domain | Delta (95% CI) | P | Delta (95% CI) | P | Delta (95% CI) | P |
| Urinary function | −.12 (−2.52, 2.27) | .9 | 3.18 (.54, 5.81) | .019 | −.85 (−6.00, 4.30) | .7 |
| Incontinence | 11.89 (8.15, 15.63) | <.001 | 14.08 (9.81, 18.35) | <.001 | 11.29 (2.69, 19.88) | .010 |
| Irritation or obstruction | −9.26 (−11.36, −7.16) | <.001 | −5.42 (−7.64, −3.19) | <.001 | −10.29 (−14.06, −6.52) | <.001 |
| Urinary bother | 0.56 (−2.06, 3.18) | .7 | 3.35 (0.42, 6.28) | .025 | −0.18 (−6.14, 5.78) | 1 |
| Bowel function | −1.39 (−2.57, −0.21) | .022 | −1.42 (−2.74, −0.09) | .036 | 0.33 (−1.76, 2.43) | .8 |
| Pain or constipation | −0.52 (−2.15, 1.11) | .5 | .80 (−1.18, 2.77) | .4 | 1.92 (−1.63, 5.48) | .3 |
| Diarrhea or frequency | −1.74 (−3.25, −0.23) | .024 | −2.35 (−4.08, −.62) | .008 | −.01 (−2.74, 2.71) | 1 |
| Rectal bleeding | −1.37 (−3.19, .45) | .14 | −2.92 (−5.13, −.71) | .010 | −.96 (−4.21, 2.29) | .6 |
| Bowel bother | −2.43 (−4.37, −0.50) | .014 | −3.05 (−5.16, −.93) | .005 | −2.19 (−5.29, .91) | .2 |
| Sexual function | 17.45 (12.78, 22.11) | <.001 | 13.51 (7.80, 19.23) | <.001 | 3.75 (−6.19, 13.69) | .5 |
| Sexual desire | 10.66 (6.94, 14.39) | <.001 | 5.56 (1.00, 10.13) | .017 | −2.43 (−10.35, 5.49) | .5 |
| Erectile function | 22.23 (16.36, 28.10) | <.001 | 19.34 (12.14, 26.54) | <.001 | 8·57 (−4.3, 21.17) | .2 |
| Sexual bother | 11.94 (7.81, 16.07) | <.001 | 11.19 (6.43, 15.96) | <.001 | 3·11 (−5.66, 11.89) | .5 |
| Intercourse bother | 8.83 (4.53, 13.14) | <.001 | 8.56 (3.65, 13.47) | .001 | 3·42 (−5.69, 12.53) | .5 |
| Intercourse satisfaction | 17.02 (11.08, 22.96) | <.001 | 14.84 (7.57, 22.10) | <.001 | 2·19 (−10.30, 14.69) | .7 |
Abbreviations: CI = confidence interval, IMRT = intensity-modulated radiotherapy.
Between means (delta) for area under the curve from 3 to 48 months in each domain and subdomain for the treatments specified, with 95% confidence intervals in parentheses. Radical prostatectomy (open or laparoscopic) is the reference group in all comparisons. A positive score means better function in radiotherapy compared to surgery.
The androgen-deprivation group includes radiotherapy patients (external beam or brachytherapy) who also received adjuvant or neoadjuvant hormones of any duration. Patients undergoing radiotherapy plus hormonal therapy are thus represented in both columns.
For bowel scores, IMRT patients had slightly worse scores for rectal bleeding compared with surgery patients and slightly worse scores in diarrhea and frequency. Accordingly, IMRT patients had significantly worse bowel bother scores than surgery patients, though effect sizes are small. Similar results were found on longitudinal analysis comparing brachytherapy and ADT patients to surgery patients, though results were only statistically significant for the brachytherapy comparison in diarrhea/frequency and bowel bother.
The results of the longitudinal analysis of sexual QOL scores were similar to those for the 48-month analysis. Brachytherapy and EBRT patients experienced significantly better sexual function and less sexual bother than surgery patients. Similar differences were again found comparing surgery patients to ADT patients but were not significant.
Discussion
Our prospective longitudinal assessment of long-term QOL in prostate cancer patients is unique in that patients were treated with state-of-the-art therapies including laparoscopic prostatectomy, dose-escalated IMRT, and real-time intra-operative brachytherapy techniques by experienced surgeons and radiation oncologists at a major cancer center. The findings are similar to prior community-based studies regarding temporal QOL changes after prostate cancer treatment [3–6, 9, 11]. This suggests that even if the absolute level of dysfunction varies between community and specialist centers, the relative difference between treatments is comparable.
One of our key findings is that, although urinary incontinence improves after the expected high dysfunction rates shortly after surgery, urinary incontinence scores remained significantly worse than IMRT or brachytherapy even at a 4-year follow-up. In contrast, urinary irritative or obstructive symptoms were more prevalent especially for brachytherapy and IMRT at 4 years after treatment compared with surgery. In general, these different types of urinary dysfunction lead to similar overall rates of urinary function and bother. Although statistically significant, differences in function and bother on the longitudinal analysis, with better scores in IMRT compared to surgery, were small.
Within all groups, there was a significant decrement in sexual function compared with baseline, which seems to be the area of QOL most affected by any of the interventions. However, radiation-treated and in particular brachytherapy-treated patients experienced significantly less decline in sexual function at 4 years compared with surgery. Similar findings were reported by Ferrer et al [7] using the Expanded Prostate Cancer Index Composite, a longitudinal evaluation of 704 patients over 5 years. The greatest deterioration of sexual function was with surgery (delta −19.1; 95% CI, −25.1, +13.1), with moderate decrement of function after EBRT (delta −7.5; 95% CI, −12.5, +2·5); and the least change was with brachytherapy (delta −2.1; 95% CI, −5.5, +1.3). At 5 years after treatment, 84% of the surgery patients, 81% of EBRT patients, and 52% of brachytherapy patients stated that erections were not firm enough for penetration. Penile-rehabilitation strategies such as daily sildenafil citrate may show promise for these patients, as our recent experience has suggested [13].
A decrement in bowel function was seen in the radiation patients and in particular among the EBRT patients. This manifested as proctitis and intermittent rectal bleeding. Diarrhea, loose stools, or bowel pain, however, were not major QOL issues across interventions. In a QOL longitudinal assessment out to 52 months after treatment, investigators from the Medical Center of Rotterdam noted that, among 314 surgery or EBRT (median dose, 74 Gy) patients, 2% of the RP cohort experienced loose or liquid stools compared with 8% for EBRT [8]. In that report, overall bowel function and bother scores for the RP and EBRT cohorts at 52 months after treatment were 89 ± 13, 95 ± 14 and 84 ± 19, 81 ± 29, respectively. Similar decrement of bowel function related to urgency, frequency, and fecal incontinence for EBRT and brachytherapy compared with surgery was observed by Sanda et al [4]; however, these findings have not consistently been observed by others [11, 14]. In addition, in the Sanda et al report, assessments were made up until 24 months after treatment, so the time course of the symptoms is unclear.
Our apparent lower rates of bowel dysfunction may be related to IMRT and tighter treatment margins, as well as further improvement of brachytherapy techniques, which have evolved since the prior reports. We have previously reported significant reductions in proctitis symptoms including rectal bleeding with IMRT. With reduced volume of rectum exposed to the high dose of irradiation, a substantial decrement in rectal bleeding was observed, from 15% to 2% at 10 years after therapy [15]. Our data continued to show low rates of proctitis with IMRT at 15 years out from treatment compared with conventional 3-dimensional treatment planning or two-dimensional planned therapy. These data represent proof of the principal that enhancements in radiotherapy delivery have importantly affected patient QOL. Also, we recently observed that with the use of image-guided brachytherapy or EBRT, reduced dose levels can be achieved at the bladder neck region, which appear to be associated with long-term urinary irritative or obstructive symptoms [16]. This should likely lead to further improvements in urinary QOL outcomes for treated patients in the future.
Published QOL analyses have limitations, including that the outcomes represent a conglomeration of assessments from large patient cohorts with various comorbidities, ages, psychological preferences, and preconceived notions as to how to best address their disease, as well as varying perceptions of bothersome typical side effects. Some patients may be terribly bothered by nocturia twice nightly, while some may consider it a minor annoyance. At our institution we employ a clinical tool that captures real-time QOL assessments at each routine follow-up visit and allows the physician and healthcare team to address in real time the individual patient’s specific concerns with interventions and symptom-directed strategies; the efficacy of these interventions can be tracked to determine if symptoms are being appropriately addressed. Such real-time QOL assessments may be the most effective way of using QOL information for the patient’s benefit, which could help physicians address their concerns and symptoms.
In conclusion, our findings evaluating longitudinal QOL changes after prostate cancer therapies over a 4-year period experienced greater sexual dysfunction than the other cohorts. Urinary symptoms are similarly prevalent, with greater long-term incontinence after RP and more urinary bother symptoms for radiotherapy. Fewer bowel problems were experienced with radiotherapy compared with prior reports, likely due to the use of more targeted radiotherapy techniques. Our findings suggest that the decision to choose a particular treatment intervention can be informed by the importance the patient places on sexual dysfunction vs the various forms of urinary problems that could be experienced. Also, with the current use of IMRT and targeted therapies, bowel-related issues should represent less of a QOL issue.
Ultimately, treatment selection would best incorporate a balance between the predicted disease-free survival outcomes over a projected period vs potential impairment of various aspects of QOL considered important for the individual patient. Physicians should discuss with patients the potential advantage of nonsurgical interventions that may be associated with less sexual dysfunction compared with surgery, and that with the use of more sophisticated radiotherapy delivery systems such as IMRT, QOL declines for rectal function are no longer as prevalent compared with what has been previously reported. Clearly, the physician must be able to incorporate the patient’s personal preferences in selecting treatment, rather than imposing their personal biases, which has been previously shown to weigh heavily on treatment selection [17].
Supplementary Material
Acknowledgments
Supported by: the NIH/NCI via the P50 CA092629 and the P30 CA008748; the Sidney Kimmel Center for Prostate and Urologic Cancers; and David H. Koch through the Prostate Cancer Foundation.
Abbreviations
- ADT
androgen-deprivation therapy
- CI
confidence interval
- EBRT
external beam
- IIEF-6
International Index of Erectile Function
- IMRT
intensity-modulated radiotherapy
- PSA
prostate-specific antigen
- QoL
quality of life
- RP
radical prostatectomy
- SD
standard deviation
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
- 1.Kibel AS, Ciezki JP, Klein EA, et al. Survival among men with clinically localized prostate cancer treated with radical prostatectomy or radiation therapy in the prostate specific antigen era. J Urol. 2012;187:1259–65. doi: 10.1016/j.juro.2011.11.084. [DOI] [PubMed] [Google Scholar]
- 2.Zelefsky MJ, Eastham JA, Cronin AM, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol. 2010;28:1508–13. doi: 10.1200/JCO.2009.22.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton AS, Stanford JL, Gilliland FD, et al. Health outcomes after external-beam radiation therapy for clinically localized prostate cancer: results from the Prostate Cancer Outcomes Study. J Clin Oncol. 2001;19:2517–26. doi: 10.1200/JCO.2001.19.9.2517. [DOI] [PubMed] [Google Scholar]
- 4.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 5.Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109:2239–47. doi: 10.1002/cncr.22676. [DOI] [PubMed] [Google Scholar]
- 6.Schaake W, de Groot M, Krijnen WP, Langendijk JA, van den Bergh AC. Quality of life among prostate cancer patients: a prospective longitudinal population-based study. Radiother Oncol. 2013;108:299–305. doi: 10.1016/j.radonc.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 7.Ferrer M, Guedea F, Suarez JF, et al. Quality of life impact of treatments for localized prostate cancer: cohort study with a 5 year follow-up. Radiother Oncol. 2013;108:306–13. doi: 10.1016/j.radonc.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 8.Korfage IJ, Essink-Bot ML, Borsboom GJ, et al. Five-year follow-up of health-related quality of life after primary treatment of localized prostate cancer. Int J Cancer. 2005;116:291–6. doi: 10.1002/ijc.21043. [DOI] [PubMed] [Google Scholar]
- 9.Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23:2772–80. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- 10.Resnick MJ, Guzzo TJ, Cowan JE, Knight SJ, Carroll PR, Penson DF. Factors associated with satisfaction with prostate cancer care: results from Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) BJU Int. 2012;111:213–20. doi: 10.1111/j.1464-410X.2012.11423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank SJ, Pisters LL, Davis J, Lee AK, Bassett R, Kuban DA. An assessment of quality of life following radical prostatectomy, high dose external beam radiation therapy and brachytherapy iodine implantation as monotherapies for localized prostate cancer. J Urol. 2007;177:2151–6. doi: 10.1016/j.juro.2007.01.134. discussion 6. [DOI] [PubMed] [Google Scholar]
- 12.Befort CA, Zelefsky MJ, Scardino PT, Borrayo E, Giesler RB, Kattan MW. A measure of health-related quality of life among patients with localized prostate cancer: results from ongoing scale development. Clin Prostate Cancer. 2005;4:100–8. doi: 10.3816/cgc.2005.n.017. [DOI] [PubMed] [Google Scholar]
- 13.Zelefsky MJ, Shasha D, Branco RD, et al. Prophylactic sildenafil citrate improves select aspects of sexual function in men treated with radiotherapy for prostate cancer. J Urol. 2014;192:868–74. doi: 10.1016/j.juro.2014.02.097. [DOI] [PubMed] [Google Scholar]
- 14.Pardo Y, Guedea F, Aguilo F, et al. Quality-of-life impact of primary treatments for localized prostate cancer in patients without hormonal treatment. J Clin Oncol. 2010;28:4687–96. doi: 10.1200/JCO.2009.25.3245. [DOI] [PubMed] [Google Scholar]
- 15.Alicikus ZA, Yamada Y, Zhang Z, et al. Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer. 2011;117:1429–37. doi: 10.1002/cncr.25467. [DOI] [PubMed] [Google Scholar]
- 16.Ghadjar P, Jackson A, Spratt DE, et al. Patterns and predictors of amelioration of genitourinary toxicity after high-dose intensity-modulated radiation therapy for localized prostate cancer: implications for defining postradiotherapy urinary toxicity. Eur Urol. 2013;64:931–8. doi: 10.1016/j.eururo.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang TL, Bekelman JE, Liu Y, et al. Physician visits prior to treatment for clinically localized prostate cancer. Arch Intern Med. 2010;170:440–50. doi: 10.1001/archinternmed.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.