Abstract
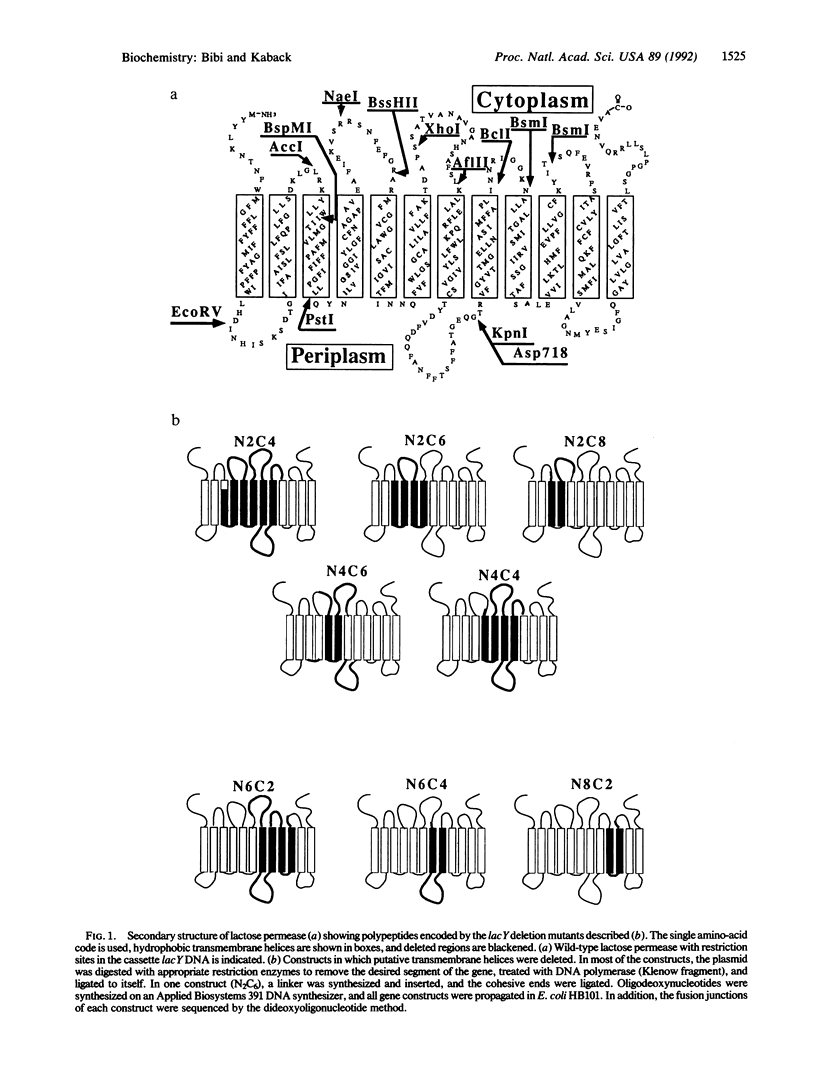
Using the lactose permease of Escherichia coli, a well-characterized membrane protein with 12 transmembrane domains, we demonstrated that certain paired in-frame deletion constructs complement each other functionally. Although cells expressing the deletion mutants individually are unable to catalyze active lactose accumulation, cells simultaneously expressing specific pairs of deletions catalyze transport up to 60% as do cells expressing wild-type permease. Moreover, complementation clearly does not occur at the level of DNA but probably occurs at the protein level. Remarkably, functional complementation is observed only with pairs of permease molecules containing large deletions and is not observed with missense mutations or point deletions. Although the mechanism of functional complementation is obscure, the findings indicate that certain pairs of permease molecules containing specific internal deletions can interact to form a functional complex in the same way phenomenologically as do independently expressed polypeptides corresponding to different N- and C-terminal portions of the permease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibi E., Kaback H. R. In vivo expression of the lacY gene in two segments leads to functional lac permease. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4325–4329. doi: 10.1073/pnas.87.11.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibi E., Verner G., Chang C. Y., Kaback H. R. Organization and stability of a polytopic membrane protein: deletion analysis of the lactose permease of Escherichia coli. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7271–7275. doi: 10.1073/pnas.88.16.7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamia J., Manoil C. lac permease of Escherichia coli: topology and sequence elements promoting membrane insertion. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4937–4941. doi: 10.1073/pnas.87.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco N., Herzlinger D., Mitchell R., DeChiara S., Danho W., Gabriel T. F., Kaback H. R. Intramolecular dislocation of the COOH terminus of the lac carrier protein in reconstituted proteoliposomes. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4672–4676. doi: 10.1073/pnas.81.15.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco N., Püttner I. B., Antes L. M., Lee J. A., Larigan J. D., Lolkema J. S., Roepe P. D., Kaback H. R. Characterization of site-directed mutants in the lac permease of Escherichia coli. 2. Glutamate-325 replacements. Biochemistry. 1989 Mar 21;28(6):2533–2539. doi: 10.1021/bi00432a028. [DOI] [PubMed] [Google Scholar]
- Carrasco N., Tahara S. M., Patel L., Goldkorn T., Kaback H. R. Preparation, characterization, and properties of monoclonal antibodies against the lac carrier protein from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6894–6898. doi: 10.1073/pnas.79.22.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco N., Viitanen P., Herzlinger D., Kaback H. R. Monoclonal antibodies against the lac carrier protein from Escherichia coli. 1. Functional studies. Biochemistry. 1984 Jul 31;23(16):3681–3687. doi: 10.1021/bi00311a017. [DOI] [PubMed] [Google Scholar]
- Costello M. J., Escaig J., Matsushita K., Viitanen P. V., Menick D. R., Kaback H. R. Purified lac permease and cytochrome o oxidase are functional as monomers. J Biol Chem. 1987 Dec 15;262(35):17072–17082. [PubMed] [Google Scholar]
- Dornmair K., Corin A. F., Wright J. K., Jähnig F. The size of the lactose permease derived from rotational diffusion measurements. EMBO J. 1985 Dec 16;4(13A):3633–3638. doi: 10.1002/j.1460-2075.1985.tb04127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. L., Boublik M., Kaback H. R. Structure of the lac carrier protein of Escherichia coli. J Biol Chem. 1983 Jan 10;258(1):31–34. [PubMed] [Google Scholar]
- Ghazi A., Shechter E. Lactose transport in Escherichia coli cells. Dependence of kinetic parameters on the transmembrane electrical potential difference. Biochim Biophys Acta. 1981 Jun 22;644(2):305–315. doi: 10.1016/0005-2736(81)90388-6. [DOI] [PubMed] [Google Scholar]
- Goldkorn T., Rimon G., Kaback H. R. Topology of the lac carrier protein in the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3322–3326. doi: 10.1073/pnas.80.11.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldkorn T., Rimon G., Kempner E. S., Kaback H. R. Functional molecular weight of the lac carrier protein from Escherichia coli as studied by radiation inactivation analysis. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1021–1025. doi: 10.1073/pnas.81.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzlinger D., Viitanen P., Carrasco N., Kaback H. R. Monoclonal antibodies against the lac carrier protein from Escherichia coli. 2. Binding studies with membrane vesicles and proteoliposomes reconstituted with purified lac carrier protein. Biochemistry. 1984 Jul 31;23(16):3688–3693. doi: 10.1021/bi00311a018. [DOI] [PubMed] [Google Scholar]
- Houssin C., le Maire M., Aggerbeck L. P., Shechter E. The lactose permease of Escherichia coli: evidence in favor of a dimer. Arch Biochem Biophys. 1985 Aug 1;240(2):593–606. doi: 10.1016/0003-9861(85)90066-9. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Bibi E., Roepe P. D. Beta-galactoside transport in E. coli: a functional dissection of lac permease. Trends Biochem Sci. 1990 Aug;15(8):309–314. doi: 10.1016/0968-0004(90)90020-c. [DOI] [PubMed] [Google Scholar]
- König B., Sandermann H., Jr Beta-D-Galactoside transport in Escherichia coli: Mr determination of the transport protein in organic solvent. FEBS Lett. 1982 Oct 4;147(1):31–34. doi: 10.1016/0014-5793(82)81005-3. [DOI] [PubMed] [Google Scholar]
- Menick D. R., Carrasco N., Antes L., Patel L., Kaback H. R. lac permease of Escherichia coli: arginine-302 as a component of the postulated proton relay. Biochemistry. 1987 Oct 20;26(21):6638–6644. doi: 10.1021/bi00395a012. [DOI] [PubMed] [Google Scholar]
- Mieschendahl M., Büchel D., Bocklage H., Müller-Hill B. Mutations in the lacY gene of Escherichia coli define functional organization of lactose permease. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7652–7656. doi: 10.1073/pnas.78.12.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M. J., Foster D. L., Wilson T. H., Kaback H. R. Purification and reconstitution of functional lactose carrier from Escherichia coli. J Biol Chem. 1981 Nov 25;256(22):11804–11808. [PubMed] [Google Scholar]
- Page M. G., Rosenbusch J. P. Topography of lactose permease from Escherichia coli. J Biol Chem. 1988 Nov 5;263(31):15906–15914. [PubMed] [Google Scholar]
- Püttner I. B., Sarkar H. K., Padan E., Lolkema J. S., Kaback H. R. Characterization of site-directed mutants in the lac permease of Escherichia coli. 1. Replacement of histidine residues. Biochemistry. 1989 Mar 21;28(6):2525–2533. doi: 10.1021/bi00432a027. [DOI] [PubMed] [Google Scholar]
- Robertson D. E., Kaczorowski G. J., Garcia M. L., Kaback H. R. Active transport in membrane vesicles from Escherichia coli: the electrochemical proton gradient alters the distribution of the lac carrier between two different kinetic states. Biochemistry. 1980 Dec 9;19(25):5692–5702. doi: 10.1021/bi00566a005. [DOI] [PubMed] [Google Scholar]
- Rubin R. A., Levy S. B. Tet protein domains interact productively to mediate tetracycline resistance when present on separate polypeptides. J Bacteriol. 1991 Jul;173(14):4503–4509. doi: 10.1128/jb.173.14.4503-4509.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckler R., Möröy T., Wright J. K., Overath P. Anti-peptide antibodies and proteases as structural probes for the lactose/H+ transporter of Escherichia coli: a loop around amino acid residue 130 faces the cytoplasmic side of the membrane. Biochemistry. 1986 May 6;25(9):2403–2409. doi: 10.1021/bi00357a016. [DOI] [PubMed] [Google Scholar]
- Seckler R., Wright J. K., Overath P. Peptide-specific antibody locates the COOH terminus of the lactose carrier of Escherichia coli on the cytoplasmic side of the plasma membrane. J Biol Chem. 1983 Sep 25;258(18):10817–10820. [PubMed] [Google Scholar]
- Seckler R., Wright J. K. Sidedness of native membrane vesicles of Escherichia coli and orientation of the reconstituted lactose: H+ carrier. Eur J Biochem. 1984 Jul 16;142(2):269–279. doi: 10.1111/j.1432-1033.1984.tb08281.x. [DOI] [PubMed] [Google Scholar]
- Stochaj U., Bieseler B., Ehring R. Limited proteolysis of lactose permease from Escherichia coli. Eur J Biochem. 1986 Jul 15;158(2):423–428. doi: 10.1111/j.1432-1033.1986.tb09770.x. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi H., Anfinsen C. B. An experimental approach to the study of the folding of staphylococcal nuclease. J Biol Chem. 1969 Jul 25;244(14):3864–3875. [PubMed] [Google Scholar]
- Trumble W. R., Viitanen P. V., Sarkar H. K., Poonian M. S., Kaback H. R. Site-directed mutagenesis of cys148 in the lac carrier protein of Escherichia coli. Biochem Biophys Res Commun. 1984 Mar 30;119(3):860–867. doi: 10.1016/0006-291x(84)90853-2. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Jacob F., Monod J. Characterization by in vitro complementation of a peptide corresponding to an operator-proximal segment of the beta-galactosidase structural gene of Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):339–343. doi: 10.1016/0022-2836(67)90341-5. [DOI] [PubMed] [Google Scholar]
- Viitanen P., Garcia M. L., Kaback H. R. Purified reconstituted lac carrier protein from Escherichia coli is fully functional. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1629–1633. doi: 10.1073/pnas.81.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H., Wright J. K., Jähnig F. The structure of the lactose permease derived from Raman spectroscopy and prediction methods. EMBO J. 1985 Dec 16;4(13A):3625–3631. doi: 10.1002/j.1460-2075.1985.tb04126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. K., Weigel U., Lustig A., Bocklage H., Mieschendahl M., Müller-Hill B., Overath P. Does the lactose carrier of Escherichia coli function as a monomer? FEBS Lett. 1983 Oct 3;162(1):11–15. doi: 10.1016/0014-5793(83)81039-4. [DOI] [PubMed] [Google Scholar]
- Wrubel W., Stochaj U., Sonnewald U., Theres C., Ehring R. Reconstitution of an active lactose carrier in vivo by simultaneous synthesis of two complementary protein fragments. J Bacteriol. 1990 Sep;172(9):5374–5381. doi: 10.1128/jb.172.9.5374-5381.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



