Abstract
Pterospartum tridentatum and Mentha pulegium are largely used in Portuguese folk medicine to treat several human disorders and inflammatory processes but without any consistent evidence for those beneficial pointed properties. Thus, the aim of the current work is to evaluate its benefits and phytochemicals related to those beneficial properties. A distinct polyphenol profile between P. tridentatum and M. pulegium was found. Taxifolin, myricetin, ginestin, ginestein, and ginestein derivatives, biochanin A-glucoside, and biochanin A were identified in P. tridentatum, whilst in M. pulegium the luteolin-7-rutinoside, diosmin, and apigenin and respective derivatives were most representative polyphenols. These variations had implications in the antiradical and antibacterial activity and the P. tridentatum exhibited the highest antibacterial activity against methicillin-resistant and methicillin-sensitive Staphylococcus aureus MSSA, which was mainly dose-dependent. This antibacterial activity seems to be related to high content of flavonols, flavones, and isoflavones, which can act synergistically with each other against this type of bacteria. Our results showed consistent evidence that Pterospartum tridentatum and Mentha pulegium are an important reservoir of phytochemicals with antiradical activity and antibacterial capacity and thus they might be used in a preventive way or in a combined pharmaceutical and antibiotic therapy against pathogenic bacteria.
1. Introduction
Nowadays, one key problem in human health is the less effectiveness of commercial antibiotics against several pathogenic bacterial isolates. One of them is the Staphylococcus aureus, a gram-positive bacterium from Staphylococcaceae family, and considered one of the world's most important infectious agents causing disease outbreaks related to food consumption, badly treated wounds, and hospital-associated infections [1, 2]. S. aureus is often reported as being for a variety of human and animal diseases and its epidemiological relevance is mainly due to their ability of becoming highly resistant to common antimicrobials such as tetracycline, vancomycin, penicillin G, and methicillin [3, 4] and to a less degree to oxacillin, lincomycin, clindamycin, erythromycin, streptomycin, cefoxitin, kanamycin, chloramphenicol, and gentamicin [5, 6].
In the last decades, evolution of resistance, for example, to methicillin, has become an enormous problem for treatment of S. aureus infections. Thus, the health authorities have increased the research programs to develop new and more effective antimicrobial molecules and several plants have been used in different ways to extract potential antimicrobial compounds. Different authors have shown that plants have naturally bioactive compounds that could act alone or in synergy with antibiotics against bacterial isolates [7, 8] and the aromatic and medicinal plants have been one of the most studied plants and found useful as antibacterial, antifungal, and antihelminthic [9–11] among other beneficial properties. However there is still a lack of information about either their phytochemical composition, their antimicrobial activity, or even how the phytochemicals act against microorganisms. Two common plants highly present in native flora of Mediterranean areas particularly in the Iberian Peninsula are the Mentha pulegium, normally called European pennyroyal, and Pterospartum tridentatum (L.) W. K. & Lge. (Syn.: Genista tridentata L. subsp. cantabrica (Spach) Nyman), frequently named as “Carqueja.” M. pulegium is an aromatic herb that belongs to the family Lamiaceae, naturalized in America, and thrives in western, southern, and central Europe, Asia, Iran, Arab countries, and Ethiopia [12]. Its essential oil and dry parts have been traditionally used in medicine (digestive, liver, and gallbladder disorders, amenorrhea, gout, colds, increased micturition, skin diseases, and abortifacient), gastronomy (culinary herb), aromatherapy, and cosmetics [13]. P. tridentatum is a small shrub belonging to the Leguminosae family and Papilionoideae subfamily [14], and its flowers are traditionally used in folk medicine as depurative and hypoglycaemic and for throat irritation conditions [15]. Most studies performed so far on M. pulegium and related Mentha species were carried out with their essential oil in different regions of the world, including Tunisia [16], Greece [17], Turkey [18], and Portugal [19], and focused mainly on their chemical composition. There is still a lack of information about their phytochemical composition related to functional capacity and antimicrobial activity. Thus, we set this study in which we evaluate the phytochemical composition of P. tridentatum and M. pulegium and its effect on the antioxidant activity and antimicrobial potential against different isolates of Staphylococcus aureus, an important pathogen highly associated with outbreak diseases and antibiotic resistance phenomena.
2. Material and Methods
2.1. Sampling
The sampling process was done according to previous works conducted in our lab and already published [20], but with the same modifications. One kilogram of fresh Pterospartum tridentatum and Mentha pulegium was collected in natural open fields in Portugal. The P. tridentatum (Carqueja) samples were collected in Vila Real Region (altitude of 400 meters) near the Natural Park of Alvão (Northern Portugal) (N 41°17′35.538′′, W 7°44′29.6268′′), whilst M. pulegium (pennyroyal) samples were collected in Santarem Region (Central Portugal). These species are largely present in open fields of Portugal as native flora, but in the northern region Carqueja is more common whilst in the south the predominance goes to Pennyroyal. After harvest, the samples were botanically identified by the Botany Services of University of Trás-os-Montes and Alto Douro (UTAD), Vila Real, Portugal. After this identification, fresh samples were dried in a freeze-dryer system (UltraDry Systems™, USA), milled and reduced to a fine powder, and stored in dark flasks at 4°C in a dark environment until extraction. Fresh and dry weights were registered and the level of dry matter was determined.
2.2. Extraction
Dried powder of each sample (200 g) was extracted in triplicate with methanol 70% (methanol : water, v/v) in a warm bath at 70°C in 30 minutes with intermittent agitation. After that, methanolic extracts were filtered (Whatman No. 1), centrifuged at 4000 rpm during 15 min 4°C (Kubota, 2000). Hydroalcoholic extracts were then evaporated until complete dryness in a rotary evaporator under vacuum (40°C, 178 mbar). Yields of extraction were calculated. The final concentration achieved was 5 mg·mL−1 dry weight. The concentration was prepared diluting the solid residue with dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Taufkirchen, Germany). These extracts were used for the phytochemical analysis and in vitro bioassays of antioxidant and antimicrobial activity.
2.3. Phytochemical Analysis HPLC-DAD-UV/VIS
Polyphenols generally named phenolic compounds are the group of secondary metabolites frequently found in extracts of aromatic and medicinal plants and they are often reported as having antimicrobial and antioxidant activity. The identification and quantification of phenolics in P. tridentatum and M. pulegium samples were performed using HPLC-DAD-UV/VIS. From the previous extracts, an aliquot of 1 mL was overnight evaporated until complete dryness under continuous nitrogen flux. The dried extracts were then resuspended with 1 mL of 70% methanol (methanol : water, v/v) and filtered (Spartan Ø 0.13) to HPLC amber vials (to avoid degradation by light) and stored at −20°C until injection in HPLC. The eluent was composed of water with 1% of trifluoroacetic acid (TFA) (solvent A) and acetonitrile with 1% TFA (solvent B). Elution was performed at a flow rate of solvent of 1 mL·min−1, with gradient starting with 100% of water, and the injection volume of 20 μL. Chromatograms were recorded at 280, 320, 370, and 520 nm with a C18 column (250 × 46 mm, 5 μm). Phytochemicals were identified using peak retention time, UV spectra, and UV max absorbance bands and through comparison with external commercial standards (Extrasynthese, France). The external standards were freshly prepared in 70% methanol (methanol : water) in a concentration of 1.0 mg·mL−1 and analysed by HPLC-DAD-UV immediately before the samples.. Methanol and acetonitrile were purchased from Panreac Chemistry (Lisbon, Portugal) and Sigma-Aldrich (Taufkirchen, Germany), respectively. The aqueous solutions were prepared using ultrapure water (Milli-Q, Millipore).
2.4. Evaluation of Functional Properties
2.4.1. Free Radical Scavenging of 2,1-Diphenyl-2-picrylhydrazyl Free Radical (DPPH•)
The scavenging capacity of DPPH of methanolic P. tridentatum and M. pulegium extracts was determined using a spectrophotometric 96-well microplate assay [21] with several modifications as follows: 20 μL of sample extract and 280 μL of 60 μM methanolic radical DPPH solution freshly prepared added to each well. Then the plate was left to stand at room temperature in 30 min. After that, the reduction in absorbance was measured at 517 nm with a spectrophotometer (Multiskan™ FC Microplate Photometer, USA). The free radical scavenging activity as expression of antioxidant activity (AA) was calculated as percentage inhibition of the DPPH radical, according to the following equation: I (%) = [((solvent absorbance − sample absorbance)/solvent absorbance) × 100]. The compound butylated hydroxytoluene (BHT) (Sigma-Aldrich, Taufkirchen, Germany), a synthetic analog of vitamin E, was used as positive control of AA in order to compare the results for the samples. Also the inhibition concentration at 50% inhibition (IC50) was determined in order to compare the AA between the extracts themselves and extracts and positive control, and lower IC50 means better free radical scavenging activity, thus higher AA. All determinations were performed in triplicate.
2.4.2. In Vitro Antibacterial Activity
(1) Bacterial Isolates. Seven gram-positive isolates of Staphylococcus aureus (3 methicillin-resistant Staphylococcus aureus and 3 methicillin-sensitive Staphylococcus aureus MSSA, plus one standard control strain from the American type culture collection (ATTC)) were obtained from Maria José Saavedra (Ph.D.) core collection located in Microbiology Laboratory of Veterinarian Science Department of UTAD (Table 1). The isolates were previously and properly identified by standard biochemical classification techniques [22] using API 20E, API 20NE, API Staph-Ident, and API Step (BioMerieux), according to the procedure previously described [23], followed by genetic identification through 16S rRNA sequencing. When tested, the isolates were prepared freshly, sowed in Petri plates (92 × 16 mm, Sarstedt, Germany) with BHI (Brain Heart Infusion, Oxoid, England) media, and incubated at 37°C overnight in order to obtain fresh and pure bacteria cultures. Then, bacterial suspension (5 × 106 cfu·mL−1) of each isolate was prepared adjusting to an optical density range of 0.5–1.0 measured at OD620 nm and used in the bioassays in order to obtain in each well 5 × 105 cfu·mL−1 inoculum final concentration.
Table 1.
Isolates of Staphylococcus aureus used in the in vitro antibacterial assay.
| Reference | Type | Source |
|---|---|---|
| MJMC021 | Methicillin-resistant strains | Clinical |
| MJMC024 | Methicillin-resistant strains | Clinical |
| MJMC026 | Methicillin-resistant strains | Clinical |
| MJMC025 | Methicillin-sensitive strains | Clinical |
| MJMC027 | Methicillin-sensitive strains | Clinical |
| MJMC029 | Methicillin-sensitive strains | Clinical |
(2) Determination of a Minimum Inhibitory Concentration (MIC). Phytochemicals are routinely classified as antimicrobials on the basis of susceptibility assays that produce a MIC within the range of 100–1000 μg·mL−1 [24] and the MIC was defined as the lowest concentration of an antimicrobial compound which can maintain or reduce the growth of a microorganism after 24 hours of incubation [25]. In the current study, the antibacterial activity was assessed by MIC and the P. tridentatum and M. pulegium methanolic extracts were prepared with a maximum concentration of 5000 μg·mL−1 dry weight in 10% DMSO in a microplate bioassay [26]. After that, 100 μL of each extract and 1000 μg of standard antibiotic were added into the first row of 96-well microplates, followed by serial dilutions on the additional wells containing 100 μL of nutrient broth. Positive and negative controls were included: a column with gentamicin (Oxoid, England) as positive control and three negative controls (a column without bioactive compounds, a column without the bacterial solution (20 μL of nutrient broth instead), and a column with DMSO solution). After this mixture the optical densities (OD) were measured at 620 nm (Multiskan FC Microplate Photometer, USA) that were automatically recorded. Then, the microplates were placed at 37°C during 24 hours and then the OD were measured again at 620 nm. These absorbance values were subtracted from those obtained before incubation to deplete the effect of color interference. The MIC was considered the lowest concentration in which the final OD was inferior to the initial OD. To classify the antibacterial effect we adopted the following scale: strong (+++), if MIC values ≤100 μg·mL−1, moderate (++) when 100 < MIC ≤ 500 μg·mL−1, weak (+) when 500 < MIC ≤ 1000 μg·mL−1, and null (−) (without effect) when MIC > 1000 μg·mL−1.
2.5. Statistical Analysis
The results were expressed as mean values and standard deviation (SD) of three replicates. The results were analyzed using one-way ANOVA followed by Duncan multiple range test, based on confidence level equal to or higher than 95% (p < 0.05). Software SPSS V.17 (SPSS-IBM, Orchard Road, Armonk, New York, NY, USA) was used to carry out this analysis.
3. Results and Discussion
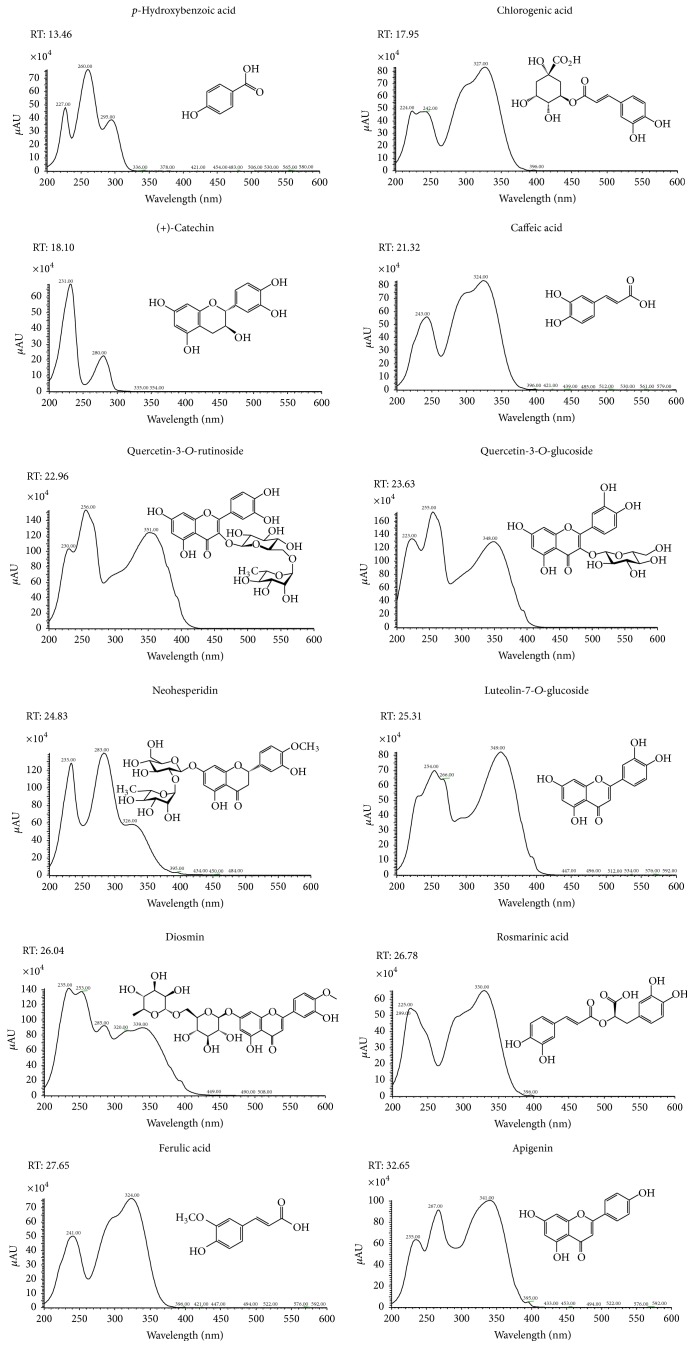
The polyphenol profile and respective chemical structures of phenolics identified in the current study of hydroalcoholic extracts from P. tridentatum and M. pulegium are presented in Figures 1 and 2. The respective data (retention time, λ max in the visible region) and average content of each polyphenol, expressed as mg·g−1 dry weight (dw), are presented in Tables 2 and 3. It was possible to assess that P. tridentatum had high content taxifolin, ginestin, ginestein, and ginestein derivatives, biochanin A-glucoside, and biochanin A, whilst M. pulegium exhibited higher diversity with luteolin-7-rutinoside, diosmin, and apigenin and respective derivatives have the most representative phenolics identified. P. tridentatum was richer in isoflavones, whilst M. pulegium present high but similar content in flavones and hydroxycinnamic acids. These results have shown an important class of phytochemicals in both plant species. The presence of high levels of taxifolin, ginestin, and ginestein, all frequently associated with antioxidant, antimicrobial, and anthelmintic activity [27–31], made this plant extract very interesting from bioactive point of view. Taxifolin, a flavanol subclass of flavonoids [28], is abundant in several types of plants and is an interesting potential component of dietary supplements or antioxidant-rich functional food [29]. Genistein is an isoflavone [28] known by its anti-inflammatory and antioxidant properties [30] and has been shown to interact with animal and human estrogenic receptors. This compound is often mentioned as responsible for wound healing properties [31], which is one of the main reasons because plants with high content of such compounds are largely used in traditional medicine, even if in the majority of the cases there is no scientific evidence for that. In fact, native floras have been used in folk medicine for thousands years, even if their biological effects and chemicals responsible for those properties were poorly understood. Only in the recent years, the therapeutic value of several herbs and the direct relation between their bioactive potential and their content in some specific essential oils (EOs), alkaloids, terpenoids, glucosinolates, and phenolics, among other compounds, were properly established. Our results showed that P. tridentatum and M. pulegium have important phenolics often associated with anti-inflammatory, antioxidant, and antimicrobial properties [32], reinforcing the scarce information available until now about these two herbs. Works about the phytochemical composition of these two native plants are very scarce but some of them [32, 33] seem to be in agreement with our results. Quercetin, genistein, and bioachanin-A and related isomers have been found by other authors in P. tridentatum [32], whilst in different types of Mentha [33] the presence of catechin and catechins derivatives, rosmarinic acid, quercetin, luteolin, and apigenin was detected as we found in this study. This difference could be related not only to genetic factors but also to agroclimatic conditions, since these herbs were collected in two different Portuguese regions: the P. tridentatum was collected in Northern Portugal (more temperate and wet) whilst M. pulegium was collected in Centre-South of Portugal (more dry and hot). As consequence, both profile and average content of phenolics of these two herbs were different. This difference may explain the difference noted in their bioactivity.
Figure 1.
UV spectra of polyphenols detected in Pterospartum tridentatum.
Figure 2.
UV spectra of polyphenols detected in Mentha pulegium.
Table 2.
Average content of polyphenols and respective retention time (Rt) and wavelengths of maximum absorption in the visible region (λ max) in P. tridentatum extracts (by elution order)†.
| Polyphenols | Rt (min) | UV (nm) | UV-DAD/VIS bands (nm) in 70% methanol |
Class | Mg·g−1 dry weight |
|---|---|---|---|---|---|
| Taxifolin | 17.48 | 280 | 290, 327sh | Flavanol | 21.76 ± 0.030 |
| Genistin | 19.79 | 320 | 259, 323sh | Isoflavone | 16.75 ± 0.040 |
| Genistein | 22.73 | 320 | 259, 332sh | Isoflavone | 12.01 ± 0.030 |
| Quercetin-3-O-rutinoside | 23.88 | 370 | 256, 266sh, 351 | Flavonol | 1.58 ± 0.030 |
| Quercetin-3-O-glucoside | 23.99 | 370 | 255, 266sh, 348 | Flavonol | 1.23 ± 0.010 |
| Ferulic acid | 24.32 | 320 | 244, 296sh, 324 | Hydroxycinnamic acid | 0.27 ± 0.002 |
| Biochanin A-glucoside | 30.66 | 320 | 265, 322sh | Flavone | 1.37 ± 0.002 |
| Apigenin | 32.65 | 370 | 229, 267sh, 341 | Flavone | 0.44 ± 0.002 |
| Biochanin A | 34.88 | 320 | 265, 323sh | Flavone | 2.89 ± 0.004 |
|
| |||||
| Total of hydroxycinnamic acids | 0.27 ± 0.002 | ||||
| Total of flavonols | 24.57 ± 0.100 | ||||
| Total of flavone | 4.70 ± 0.008 | ||||
| Total of isoflavone | 28.76 ± 0.070 | ||||
| Total of polyphenols identified | 58.30 ± 0.180 | ||||
†Values expressed as mean ± standard deviation of three replicates.
Table 3.
Average content of polyphenols and respective retention time (Rt) and wavelengths of maximum absorption in the visible region (λ max) in M. pulegium extracts (by elution order)†.
| Polyphenols | Rt (min) | UV (nm) | UV-DAD/VIS bands (nm) in 70% methanol |
Class | Mg·g−1 dry weight |
|---|---|---|---|---|---|
| p-Hydroxybenzoic acid | 13.46 | 280 | 227, 260sh, 295 | Hydroxybenzoic acid | 0.212 ± 0.004 |
| Chlorogenic acid | 17.95 | 320 | 242, 300sh, 327 | Hydroxycinnamic acid | 0.387 ± 0.004 |
| (+)-Catechin | 18.10 | 280 | 231, 280 | Flavan-3-ols | 0.212 ± 0.004 |
| Caffeic acid | 21.32 | 320 | 243, 296sh, 324 | Hydroxycinnamic acid | 0.230 ± 0.001 |
| Quercetin-3-O-rutinoside | 22.96 | 370 | 256, 266sh, 351 | Flavonol | 0.144 ± 0.003 |
| Quercetin-3-O-glucoside | 23.63 | 370 | 255, 266sh, 348 | Flavonol | 0.140 ± 0.002 |
| Neohesperidin | 24.84 | 280 | 283, 326 | Flavanone | 0.628 ± 0.001 |
| Luteolin-7-O-glucoside | 25.31 | 370 | 254, 266, 349 | Flavone | 0.201 ± 0.004 |
| Diosmin | 26.04 | 370 | 253, 268, 339 | Flavone | 0.623 ± 0.006 |
| Rosmarinic acid | 26.78 | 320 | 249, 299sh, 330 | Hydroxycinnamic acid | 0.287 ± 0.006 |
| Ferulic acid | 27.55 | 320 | 241, 296sh, 324 | Hydroxycinnamic acid | 0.277 ± 0.003 |
| Apigenin | 32.65 | 370 | 235, 267sh, 341 | Flavone | 0.323 ± 0.002 |
|
| |||||
| Total of hydroxybenzoic acids | 0.212 ± 0.004 | ||||
| Total of hydroxycinnamic acids | 1.181 ± 0.014 | ||||
| Total of flavan-3-ols | 0.212 ± 0.004 | ||||
| Total of flavonols | 0.284 ± 0.005 | ||||
| Total of flavones | 1.147 ± 0.012 | ||||
| Total of flavanones | 0.628 ± 0.001 | ||||
| Total polyphenols identified | 3.664 ± 0.040 | ||||
†Values expressed as mean ± standard deviation of three replicates.
The results for the evaluation of functional properties expressed as free radical (2,1-diphenyl-2-picrylhydrazyl free radical (DPPH•)) activity and in vitro antibacterial activity are presented in Figure 3 and in Table 4, respectively. We observed that biological P. tridentatum and M. pulegium have high levels of AA, even higher than the positive control (BHT) used in this assay (Figure 3). Despite this similarity, a different trend in the phenolic influence on AA was observed. In the P. tridentatum the high AA seems to be explained by the high content of isoflavones and flavanols (49 and 42% of total phenolics identified, resp.), whilst in M. pulegium the AA seems to be explained by the synergism between phenolics acids (32% of total phenolics), flavones, and flavanones (31 and 17% of total phenolics, resp.). This difference is understandable since all of these compounds are often reported [27, 29, 30, 32, 33] as having important antioxidant activities. The presence of several phenolic acids, flavones, isoflavones, and flavanols in both extracts, with potential to scavenge free radicals such as superoxide and nitric oxide [29], can thus explain the high antioxidant potential exhibited by these two herbs. However, based on the average content of phenolics (Tables 2 and 3) the expectation was to have higher AA in P. tridentatum extracts but was not the case. In fact, based on the values of IC50 (Figure 3) the AA in M. pulegium was higher compared to P. tridentatum, which seems to contradict the previous finding, but we must be aware that other compounds, besides the phenolics such as pigments, alkaloids, and carotenoids (not determined in this work), often reported as being present in high concentrations in these two herbs [34], might have contributed to high levels of AA observed in this type of extract. In addition, the higher presence of phenolic acids such as hydroxybenzoic acid, chlorogenic acid, caffeic acid, ferulic acid, and rosmarinic acid, all based on –CH=CH–COOH groups, widely recognizable for forming easily complexes with DPPH [35] seems to be one of the main reasons for M. pulegium extracts having the lowest IC50 and thus the highest AA. Therefore, based on the current work it seems that the higher proportion of phenolic compounds with hydroxyl groups on the aromatic ring is responsible for the higher AA exhibited by M. pulegium extracts, which is in agreement with the previous findings [35] in which the positions of hydroxyl groups were found extremely important for the bioactivity of polyphenols, including their antioxidant capacity. Despite these differences, the high AA found for both extracts are critical and determinant for their therapeutic value and this may be in part responsible for their reputation as anti-inflammatory, hypotensive, hypoglycemic, and depurative agent. It seems that they can reduce oxidative stress, a key factor in the progression of chronical inflammatory diseases. Further investigations should be done in order to determine the action keys on the pathways of the inflammatory mechanisms. Based on these results it seems very clear that the traditional usage of P. tridentatum and M. pulegium as medicinal plants associated with anti-inflammatory and depurative processes is correct and thereby these plants serve as natural sources of antioxidants for food and medicinal purposes.
Figure 3.
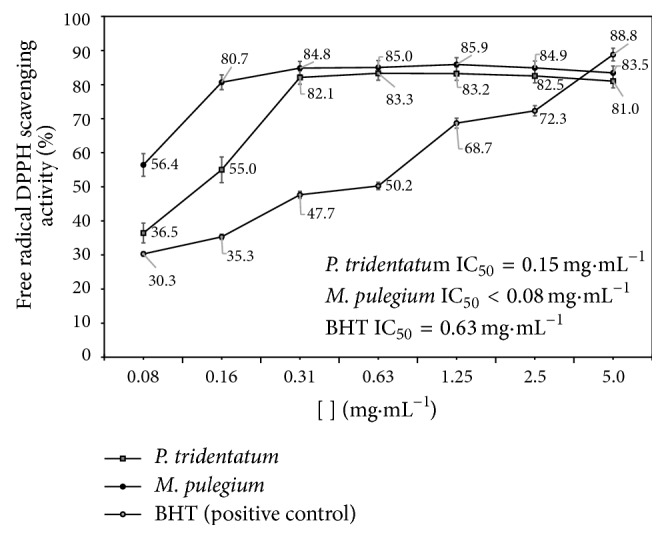
% free radical DPPH scavenging activity of Pterospartum tridentatum and Mentha pulegium methanolic extracts.
Table 4.
Minimum inhibitory concentration (MIC) of P. tridentatum and M. pulegium aqueous and methanolic extracts expressed as µg · mL−1 †.
| Isolate | Reference | Type | Gentamicin (commercial antibiotic) | P. tridentatum | M. pulegium |
|---|---|---|---|---|---|
| S. aureus | ATCC 13565 | Standard | <39 (+++) | 312.5 (++) | 2500 (−) |
| S. aureus | MJMC021 | MRSA | <39 (+++) | 78.1 (+++) | 2500 (−) |
| S. aureus | MJMC024 | MRSA | <39 (+++) | 78.1 (+++) | 2500 (−) |
| S. aureus | MJMC026 | MRSA | <39 (+++) | 78.1 (+++) | 2500 (−) |
| S. aureus | MJMC025 | MSSA | <39 (+++) | 39.1 (+++) | 39.1 (+++) |
| S. aureus | MJMC027 | MSSA | <39 (+++) | 39.1 (+++) | 78.1 (+++) |
| S. aureus | MJMC029 | MSSA | <39 (+++) | 39.1 (+++) | 39.1 (+++) |
†Inside brackets there is the classification of the antibacterial activity effect: strong (+++), if MIC values ≤ 100 μg·mL−1, moderate (++) when 100 < MIC ≤ 500 μg·mL−1, weak (+) when 500 < MIC ≤ 1000 μg·mL−1, and null (−) (without effect) when MIC > 1000 μg·mL−1.
Although the results with DPPH• free radical scavenging activity have shown that P. tridentatum and M. pulegium have similar values of AA, the antibacterial activity was very different. P. tridentatum exhibited the highest antibacterial activity due to lower minimum inhibitory concentration levels found. The MSSA isolates were more affected than MRSA isolates, as we expected. This activity was mainly dose-dependent and in general according to the classification criteria adopted (Table 4) the antibacterial activity was strong and in some isolates similar to the antibacterial activity observed for the antibiotic used as positive control. As similar to AA, the higher antimicrobial activity for P. tridentatum can be the consequence of two effects: (i) the higher content of flavonols and isoflavones and (ii) the additive effect of different types of phenolics, which seems to boost the antimicrobial efficacy of such extracts. Isoflavones such as genistin and genistein (and respective isomers) have been reported as having anti-inflammatory, antiproliferative, and antibacterial effects [36]. Also, phenolics like rutin, isoquercetin, and quercetin can play an important role as antimicrobial agents due to their capacity of interference on bacterial mechanisms of nucleic acid synthesis, cytoplasmic membrane, energy metabolisms, and being particularly effective against gram-positive bacteria [37] such as S. aureus studied in the current work. The antimicrobial activity of P. tridentatum extracts and in a less extension M. pulegium might be due to one of the mechanisms of action mentioned above. The richness of flavonols and isoflavones on P. tridentatum can be responsible for the increment in antibacterial activity exhibited for this extract compared to the M. pulegium. In fact, the high presence of compounds such as taxifolin, genistin, and biochanin often reported as having antibacterial activity [38] can be responsible for depletion of bacteria resistance mechanisms leading to increment in their susceptibility to these compounds. Also, it was observed that taxifolin and respective isomers extracted from Hypericum japonicum Thunb. ex. Murray (Guttiferae) delayed the protein synthesis of S. aureus (including the MRSA strains), affecting the synthesis of nucleic acids and enzymatic systems needed for bacteria growth [39]. This action is responsible for increasing the membranes permeability to drugs, leading to a decrease in bacteria survival, suggesting that these compounds might have a bacteriostatic effect rather than a bactericidal activity. Moreover it was noted that, in general, flavonoids and oligomers of flavonoids, particularly those with high grade of hydroxylation (such as flavonols, flavones, and flavanones), have a strong ability to link with bacteria cell walls from complexes [40, 41], affecting the bacteria growth and survival. Thus, plant extracts with high content of such compounds, like P. tridentatum, can be very useful when used in a complementary therapy with commercial drugs due to their bacteriostatic effect.
The majority of the studies available in literature about the antimicrobial activity of P. tridentatum and M. pulegium report mainly the effects of their essential oils (EOs) [19, 39–41] and very few about the effect of their hydroalcoholic extracts [41–43], and thus they attribute their antibacterial efficacy essentially to the EOs, and fewer conclusions are made about the importance of other bioactive compounds such as polyphenols. Moreover, their effects against S. aureus MSSA and MRSA have been scarcely explored. Thus, our results seem to be important because they not only reinforce the idea that richness of polyphenols is also critical for the antimicrobial capacity of any plant, but also show the bioactivities of these two herbs, proving that they can be used to extract bioactive compounds with antimicrobial activity against MSSA and MRSA.
4. Conclusion
P. tridentatum and M. pulegium are two important herbs from Mediterranean native flora and might be used to extract important and effective bioactive compounds against epidemiological important pathogenic bacteria, particularly against S. aureus, one of the most important pathogenic bacteria, often associated with foodborne outbreak diseases and hospital/clinical environment infections. Our results have shown that both extracts can be effective against MRSA and MSSA due to high content of different class of flavonoids, particularly flavonols, flavones, and isoflavones compounds, which can act synergistically with each other against those bacteria. Further research is needed to elucidate accurately the pathways and mechanisms used by these compounds against bacteria.
Acknowledgments
The authors acknowledge the financial support provided by the Portuguese Foundation for Science and Technology (FCT) (Alfredo Aires-SFRH/BPD/65029/2009) under Project UID/AGR/04033/2013.
Competing Interests
The authors declare no conflict of interests.
References
- 1.Tong S. Y. C., Chen L. F., Fowler V. G., Jr. Colonization, pathogenicity, host susceptibility, and therapeutics for Staphylococcus aureus: what is the clinical relevance? Seminars in Immunopathology. 2012;34(2):185–200. doi: 10.1007/s00281-011-0300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fetsch A., Contzen M., Hartelt K., et al. Staphylococcus aureus food-poisoning outbreak associated with the consumption of ice-cream. International Journal of Food Microbiology. 2014;187:1–6. doi: 10.1016/j.ijfoodmicro.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 3.Gao J., Ferreri M., Yu F., et al. Molecular types and antibiotic resistance of Staphylococcus aureus isolates from bovine mastitis in a single herd in China. Veterinary Journal. 2012;192(3):550–552. doi: 10.1016/j.tvjl.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 4.Jamali H., Paydar M., Radmehr B., Ismail S., Dadrasnia A. Prevalence and antimicrobial resistance of Staphylococcus aureus isolated from raw milk and dairy products. Food Control. 2015;54:383–388. doi: 10.1016/j.foodcont.2015.02.013. [DOI] [Google Scholar]
- 5.Dhand A., Sakoulas G. Daptomycin in combination with other antibiotics for the treatment of complicated Methicillin-resistant Staphylococcus aureus bacteremia. Clinical Therapeutics. 2014;36(10):1303–1316. doi: 10.1016/j.clinthera.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Mandal S. M., Ghosh A. K., Pati B. R. Dissemination of antibiotic resistance in methicillin-resistant Staphylococcus aureus and vancomycin-resistant S aureus strains isolated from hospital effluents. American Journal of Infection Control. 2015;43(12):e87–e88. doi: 10.1016/j.ajic.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Chusri S., Siriyong T., Na-Phatthalung P., Voravuthikunchai S. P. Synergistic effects of ethnomedicinal plants of Apocynaceae family and antibiotics against clinical isolates of Acinetobacter baumannii . Asian Pacific Journal of Tropical Medicine. 2014;7(6):456–461. doi: 10.1016/s1995-7645(14)60074-2. [DOI] [PubMed] [Google Scholar]
- 8.Bellio P., Segatore B., Mancini A., et al. Interaction between lichen secondary metabolites and antibiotics against clinical isolates methicillin-resistant Staphylococcus aureus strains. Phytomedicine. 2015;22(2):223–230. doi: 10.1016/j.phymed.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Badawy M. E. I., Abdelgaleil S. A. M. Composition and antimicrobial activity of essential oils isolated from Egyptian plants against plant pathogenic bacteria and fungi. Industrial Crops and Products. 2014;52:776–782. doi: 10.1016/j.indcrop.2013.12.003. [DOI] [Google Scholar]
- 10.Zhang X. P., Li W. X., Ai T. S., Zou H., Wu S. G., Wang G. T. The efficacy of four common anthelmintic drugs and traditional Chinese medicinal plant extracts to control Dactylogyrus vastator (Monogenea) Aquaculture. 2014;420-421:302–307. doi: 10.1016/j.aquaculture.2013.09.022. [DOI] [Google Scholar]
- 11.Chen Z., He B., Zhou J., He D., Deng J., Zeng R. Chemical compositions and antibacterial activities of essential oils extracted from Alpinia guilinensis against selected foodborne pathogens. Journal of Ethnopharmacology. 2016;178:125–136. doi: 10.1016/j.jep.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Gruenwald J., Brendler T., Jaenicke C. PDR for Herbal Medicines. Montvale, NJ, USA: Medical Economic Company; 2000. [Google Scholar]
- 13.Agnihotri V. K., Agarwal S. G., Dhar P. L., et al. Essential oil composition of Mentha pulegium L. growing wild in the north-western Himalayas India. Flavour and Fragrance Journal. 2005;20(6):607–610. doi: 10.1002/ffj.1497. [DOI] [Google Scholar]
- 14.Talavera S. Flora Iberica. Vol. 7. Madrid, Spain: CSIC; 1999. [Google Scholar]
- 15.Pardo-de-Santayana M., Tardío J., Blanco E., et al. Traditional knowledge of wild edible plants used in the northwest of the Iberian Peninsula (Spain and Portugal): a comparative study. Journal of Ethnobiology and Ethnomedicine. 2007;3, article 27 doi: 10.1186/1746-4269-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riahi L., Elferchichi M., Ghazghazi H., et al. Phytochemistry, antioxidant and antimicrobial activities of the essential oils of Mentha rotundifolia L. in Tunisia. Industrial Crops and Products. 2013;49:883–889. doi: 10.1016/j.indcrop.2013.06.032. [DOI] [Google Scholar]
- 17.Alexopoulos A., Kimbaris A. C., Plessas S., et al. Antibacterial activities of essential oils from eight Greek aromatic plants against clinical isolates of Staphylococcus aureus . Anaerobe. 2011;17(6):399–402. doi: 10.1016/j.anaerobe.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Erhan M. K., Bölükbaşi Ş. C., Ürüşan H. Biological activities of pennyroyal (Mentha pulegium L.) in broilers. Livestock Science. 2012;146(2-3):189–192. doi: 10.1016/j.livsci.2012.01.014. [DOI] [Google Scholar]
- 19.Teixeira B., Marques A., Ramos C., et al. European pennyroyal (Mentha pulegium) from Portugal: chemical composition of essential oil and antioxidant and antimicrobial properties of extracts and essential oil. Industrial Crops and Products. 2012;36(1):81–87. doi: 10.1016/j.indcrop.2011.08.011. [DOI] [Google Scholar]
- 20.Freitas E., Aires A., Rosa E. A. D. S., Saavedra M. J. Antibacterial activity and synergistic effect between watercress extracts, 2-phenylethyl isothiocyanate and antibiotics against 11 isolates of Escherichia coli from clinical and animal source. Letters in Applied Microbiology. 2013;57(4):266–273. doi: 10.1111/lam.12105. [DOI] [PubMed] [Google Scholar]
- 21.Dudonné S., Vitrac X., Coutiére P., Woillez M., Mérillon J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. Journal of Agricultural and Food Chemistry. 2009;57(5):1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 22.Murray P. R., Baron E. J., Pfaller M. A., Tenover F. C., Yolken R. H., editors. Manual of Clinical Microbiology. 6th. Washington, DC, USA: American Society for Microbiology; 1995. [Google Scholar]
- 23.Reller L. B., Weinstein M., Jorgensen J. H., Ferraro M. J. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clinical Infectious Diseases. 2009;49(11):1749–1755. doi: 10.1086/647952. [DOI] [PubMed] [Google Scholar]
- 24.Simões M., Bennett R. N., Rosa E. A. S. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Natural Product Reports. 2009;26(6):746–757. doi: 10.1039/b821648g. [DOI] [PubMed] [Google Scholar]
- 25.Andrews J. M. Determination of minimum inhibitory concentrations. Journal of Antimicrobial Chemotherapy. 2001;48(supplement 1):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 26.Sarker S. D., Nahar L., Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42(4):321–324. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vrba J., Kren V., Vacek J., Papouskova B., Ulrichova J. Quercetin, quercetin glycosides and taxifolin differ in their ability to induce AhR activation and cyp1a1 expression in HepG2 cells. Phytotherapy Research. 2012;26(11):1746–1752. doi: 10.1002/ptr.4637. [DOI] [PubMed] [Google Scholar]
- 28.Rice-Evans C. A., Miller N. J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine. 1996;20(7):933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 29.Topal F., Nar M., Gocer H., et al. Antioxidant activity of taxifolin: an activity–structure relationship. Journal of Enzyme Inhibition and Medicinal Chemistry. 2015:1–10. doi: 10.3109/14756366.2015.1057723. [DOI] [PubMed] [Google Scholar]
- 30.Sung N.-Y., Byun E.-B., Song D.-S., et al. Anti-inflammatory action of γ-irradiated genistein in murine peritoneal macrophage. Radiation Physics and Chemistry. 2014;105:17–21. doi: 10.1016/j.radphyschem.2014.05.029. [DOI] [Google Scholar]
- 31.Ndhlala A. R., Ghebrehiwot H. M., Ncube B., et al. Antimicrobial, anthelmintic activities and characterisation of functional phenolic acids of achyranthes aspera linn.: a medicinal plant used for the treatment of wounds and Ringworm in East Africa. Frontiers in Pharmacology. 2015;6, article 274 doi: 10.3389/fphar.2015.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roriz C. L., Barros L., Carvalho A. M., Santos-Buelga C., Ferreira I. C. F. R. Scientific validation of synergistic antioxidant effects in commercialised mixtures of Cymbopogon citratus and Pterospartum tridentatum or Gomphrena globosa for infusions preparation. Food Chemistry. 2015;185:16–24. doi: 10.1016/j.foodchem.2015.03.136. [DOI] [PubMed] [Google Scholar]
- 33.Moldovan R. I., Oprean R., Benedec D., et al. LC-MS analysis, antioxidant and antimicrobial activities for five species of Mentha cultivated in Romania. Digest Journal of Nanomaterials and Biostructures. 2014;9(2):559–566. [Google Scholar]
- 34.Candan N., Tarhan L. Relationship among chlorophyll-carotenoid content, antioxidant enzyme activities and lipid peroxidation levels by Mg2+ deficiency in the Mentha pulegium leaves. Plant Physiology and Biochemistry. 2003;41(1):35–40. doi: 10.1016/s0981-9428(02)00006-2. [DOI] [Google Scholar]
- 35.Maurya D. K., Devasagayam T. P. A. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food and Chemical Toxicology. 2010;48(12):3369–3373. doi: 10.1016/j.fct.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Hong H., Landauer M. R., Foriska M. A., Ledney G. D. Antibacterial activity of the soy isoflavone genistein. Journal of Basic Microbiology. 2006;46(4):329–335. doi: 10.1002/jobm.200510073. [DOI] [PubMed] [Google Scholar]
- 37.Kuspradini H., Mitsunaga T., Ohashi H. Antimicrobial activity against Streptococcus sobrinus and glucosyltransferase inhibitory activity of taxifolin and some flavanonol rhamnosides from kempas (Koompassia malaccensis) extracts. Journal of Wood Science. 2009;55(4):308–313. doi: 10.1007/s10086-009-1026-4. [DOI] [Google Scholar]
- 38.An J., Zuo G. Y., Hao X. Y., Wang G. C., Li Z. S. Antibacterial and synergy of a flavanonol rhamnoside with antibiotics against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) Phytomedicine. 2011;18(11):990–993. doi: 10.1016/j.phymed.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Grosso A. C., Costa M. M., Ganço L., et al. Essential oil composition of Pterospartum tridentatum grown in Portugal. Food Chemistry. 2007;102(4):1083–1088. doi: 10.1016/j.foodchem.2006.06.049. [DOI] [Google Scholar]
- 40.Mahboubi M., Haghi G. Antimicrobial activity and chemical composition of Mentha pulegium L. essential oil. Journal of Ethnopharmacology. 2008;119(2):325–327. doi: 10.1016/j.jep.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 41.Morteza-Semnani K., Saeedi M., Akbarzadeh M. Chemical composition and antimicrobial activity of the essential oil of mentha pulegium L. Journal of Essential Oil-Bearing Plants. 2011;14(2):208–213. doi: 10.1080/0972060X.2011.10643923. [DOI] [Google Scholar]
- 42.Coelho M. T., Gonçalves J. C., Alves V., Martins M. M. Antioxidant activity and phenolic content of extracts from different Pterospartum tridentatum populations growing in Portugal. Procedia Food Science. 2011;1:1454–1458. doi: 10.1016/j.profoo.2011.09.215. [DOI] [Google Scholar]
- 43.Ghazghazi H., Chedia A., Weslati M., et al. Chemical composition and in vitro antimicrobial activities of mentha pulegium leaves extracts against foodborne pathogens. Journal of Food Safety. 2013;33(3):239–246. doi: 10.1111/jfs.12045. [DOI] [Google Scholar]