Abstract
A broad group of structurally diverse small organofluorine inhibitors have been synthesized and evaluated in the self-assembly of amyloid β. The major goal was to generate a diverse library of compounds with the same functional group and observe general structural features that characterize the oligomer and fibril inhibitors, and ultimately find lead structures for further, focused inhibitor design. The common structural motifs in these compounds were the CF3-C-OH or CF3-C-NH groups that were proposed to be a binding unit in our earlier studies. A broad range of potential small molecule inhibitors were synthesized by adding different carbocyclic and heteroaromatic rings with an array of substituents, overall 106 molecules. The compounds were tested by standard methods, such as thioflavine T-fluorescence spectroscopy for following fibril formation, biotinyl-Aβ(1–42) single-site streptavidin-based assay for observing oligomer formation and atomic force microscopy for morphological studies. These assays yielded a number of structures that showed significant inhibition against either fibril or oligomer formation. A detailed analysis on the structure activity relationship of anti-fibril and -oligomer properties is provided. In addition, these data present further experimental evidence for the distinct nature of the fibril vs oligomer formation and that the interaction of the Aβ peptide with chiral small molecules is not stereospecific in nature.
Keywords: alzheimer’s disease, amyloid beta, chiral inhibitors, fibrils, heterocycles, oligomers, organofluorine compounds
Graphical Abstract

Introduction
Protein deposits in the form of neurofibrillary tangles and amyloid plaques are the hallmarks of Alzheimer’s disease (AD).[1, 2] The major component of the extracellular amyloid plaques is the amyloid-beta peptide (Aβ).[3] One of the suggested therapeutic strategies for AD is the inhibition of the amyloid cascade,[4] and many inhibitors of the Aβ self-assembly have been identified. These include small organic molecules, peptides, peptidomimetics and proteins.[5] Recent studies have indicated that the soluble oligomeric aggregates of Aβ were more neurotoxic than the fibrillar end-products of the process.[6] Therefore, it has become imperative to distinguish between molecules that inhibit oligomerization, fibril formation, or both. Many small molecule anti-amyloidogenic compounds have been categorized and the underlying oligomer structures characterized using conformation-specific antibodies.[7–9] Several accounts[10–12] on the development of inhibitors active against fibrillogenesis and oligomer assembly serve as an excellent source of information. However, one cannot overlook the fact that the literature is far from systematic regarding the chemical nature of inhibitors. Most original studies focus on a single compound, or a small group of compounds with no clear indication why the compounds were selected. Also, rational extended structure-activity relationship studies outside of the pharmaceutical industry are quite rare.[13, 14] Although, the target (fibril, oligomers etc.) is usually specified, frequently there is little indication what type of interaction occurs between the inhibitor and the peptide.
Our chemistry-based approach is intended to fill this gap. While many approaches in searching for potential inhibitors were discovery based, our design of a core structure was based on literature data. In an earlier study we described a new class of organofluorine molecules as Aβ fibrillogenesis inhibitors.[15] These compounds have been found to be active in the disassembly of the preformed fibrils, as well.[16] In a study including chiral isomer pairs of the same compounds it was also observed that individual chirality did not appear to result in significant difference in the action of these compounds.[17] However, while providing interesting information and effective anti-fibril compounds, these previous studies were limited in scope, only a few compounds with closely related structural features were included. In continuation of our work on anti-Alzheimer’s compounds we designed and synthesized a broad range of organofluorine compounds with one common motif (CF3-C-XH, where X=O, N) but considerable structural diversity. This functionality was found to be of crucial importance in possessing activity; the removal of either the CF3 or OH groups resulted in completely inactive compounds.[15] The compounds were evaluated in fibril and oligomer inhibition and disassembly assays. Herein, we describe a broad structure-activity relationship study of these organofluorine compounds as potential anti-Alzheimer’s agents.
Results and Discussion
Results
Various substituted/unsubstituted monocyclic/bicyclic aromatic/heteroaromatic molecules like benzene, pyrrole, furan and indole were derivatized using commercially available trifluoromethyl-hydroxyalkylating agents; ethyl trifluoropyruvate (TFP), ethyl trifluoroacetoacetate (TFAA), hexafluoroacetone (HFA) and trifluoro acetaldehyde ethylhemiacetal (TFAE). The basic synthetic procedures for the preparation of these compounds are summarized in Figure 1.
Figure 1.
One-step syntheses of a diverse group of aryl CF3-C-OH group containing compounds from commercially available reagents.
It is common in all syntheses in Figure 1 that every process occurred in one step, using commercially available hydroxyalkylating agents. In some cases, we used our own earlier methods,[18–20] in the case of the remaining products literature analogies were applied.[21] A few selected chiral compounds were also synthesized by a cinchona alkaloid-catalyzed hydroxy-alkylation,[20, 22] to confirm the effect of inhibitor stereochemistry on the anti-aggregation potency, especially since in our earlier report only the anti-fibril effect was described.[17]
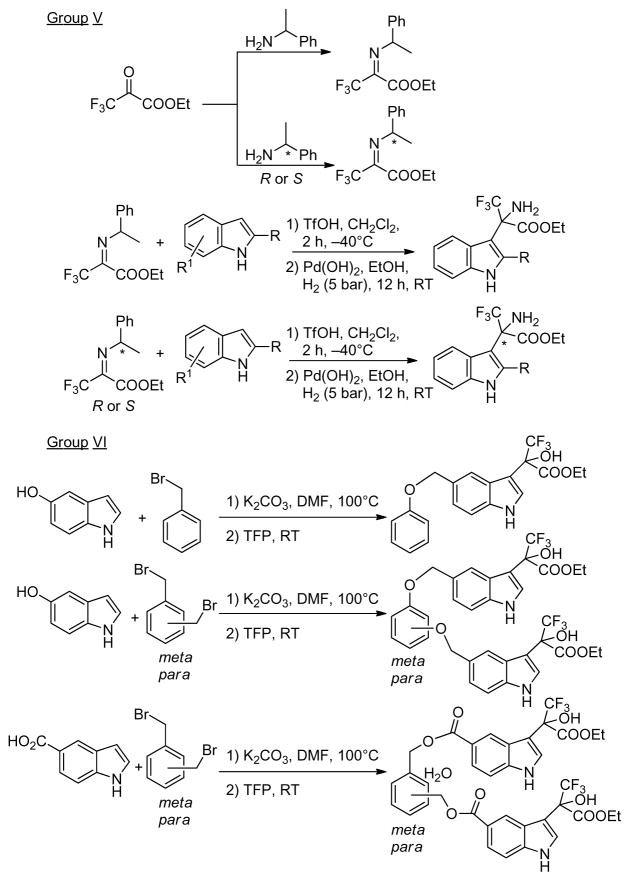
For the synthesis of trifluoromethyl amino acid esters and additional larger compounds multistep methods were applied.[23, 24] These procedures are summarized in Figure 2.
Figure 2.
Syntheses of aryl trifluoromethyl amino acid esters and larger compounds with CF3-C-XH group (X=O, N) by multistep approaches.
The syntheses provided the products in good to excellent yields and in the case of the amino acid esters, the optical purity of the products were also high (up to 98 % ee). It is worth mentioning that several from the above synthesized molecules are new compounds, while the others were recently published by our group.[18–23] Therefore, other than the few compounds that appear in our previous publication on anti-fibrillogenesis compounds, none of these compounds have ever been tested and described in Aβ aggregation inhibition studies.
Overall we have completed the synthesis of 106 structurally related compounds. As a common feature, all of these products possess the CF3-C-OH or CF3-C-NH unit, the compounds otherwise are structurally diverse. It was our intention to observe the role of aromatic/heteroaromatic groups and their substituents may have in the course of fibril formation. In addition the effect of double substitution was also tested, namely whether having two CF3-C-OH units in one compound, is beneficial or disadvantageous for the biological effect.
After the syntheses initial biochemical tests were carried out. Since both the fibrillar aggregates and soluble oligomeric species of Aβ are neurotoxic, the inhibitory activity of the compounds has been determined against both forms of self-assembly products. Since Aβ(1–40) is the most abundant form of the peptide and readily forms fibrils it was our goal to use it. However, Aβ(1–42) was used for oligomers because 1–40 forms oligomers poorly at the low concentration (10 nM) used to avoid fibril formation unless a stimulant is applied. The efficacy of the inhibitors against fibrillogenesis has been evaluated by the commonly applied quantitative Thioflavin-T (THT) fluorescence spectroscopy assay.[25–27] The calculated intensity values were based on maximum fluorescence intensities in the 480–490 nm region (emission spectra) (λex=435 nm) after subtracting the background fluorescence of the starting solutions (0 hour). The samples were incubated for 4 days, ThT measurements were made at the plateau phase of fibril assembly and the data obtained with the inhibitor containing samples were compared to inhibitor-free controls. The assays were carried out using Aβ:inhibitor=0.1 ratio at 100 μM Aβ concentration, thus the original inhibitor concentration was at 1 mM (except compounds 89–93, which were tested at 1:1 molar ratio, thus at 100 μM concentration, due to solubility problems). The data along with the structures of the compounds are summarized in Figure 3. The anti-oligomer activity of the compounds were also determined using the quantitative biotinyl-Aβ(1–42) single-site streptavidin-based assay.[28, 29] The samples were incubated for 30 min in the assays. These assays were carried out at Aβ:inhibitor=0.0002 ratio at 0.01 μM Aβ concentration. The efficacy of the compounds was determined at these given concentrations. The measured intensities of the inhibitor containing samples (Isample) were normalized to the control sample (Icontrol) containing Aβ only. The percentile values with which a compound decreased the expected signal (control) are collected in Figure 3 as % inhibition:
| (16) |
Figure 3.
General structure of the compounds used in the current study and their activity (%) in Aβ aggregation (fibril and oligomer) assays
In some cases compounds promoted the self assembly, thus Isample>Icontrol, therefore negative inhibition percentile values are listed. Compounds with significant activity in the screening assay were titrated and the EC50 values were determined. The EC50 calculations were carried out as previously described.[15] Fluorescence intensity vs. molar ratio functions were used to determine the relative potency of inhibitors using a simple equation, similar to the analysis of the Michaelis–Menten kinetics or ligand binding to macromolecules (17).[15]
| (17) |
where ITHT is the fluorescence intensity of the inhibitor-containing sample expressed as a percentage of control, P is the inhibitor/Aβ molar ratio, EC50 is the median inhibitor constant and ECmax is the maximum inhibition. The double reciprocal plot of (17) allows the determination of EC50. Since inhibitor/Aβ molar ratios were applied in the formula, the EC50s were obtained as a ratio as well. Multiplying the obtained ratio with the Aβ concentration of 100 μM provided the values in concentration unit (μM).
The most active compounds and their EC50 data are collected in Table 1. The data show that group I and II compounds are the most effective fibrillogenesis inhibitors, while oligomer assembly inhibitors appear in several groups. A detailed analysis will be carried out in the Discussion to point out structural similarities among the active compounds. Atomic Force Microscopy (AFM) has been also used to confirm the THT data. Several illustrative AFM images of a control sample and inhibited samples are depicted in Figure 4.
Table 1.
EC50 data for the most active compounds in Aβ fibrillogenesis and oligomer assembly assays.[a]
| Compound | EC50,fibril [μM] | EC50, oligomer [μM] |
|---|---|---|
| 2 | 380±1.8 | >100 |
| 3 | 250±4.7 | N/A |
| 4 | 190±0.07 | NA |
| 22 | >1000 | 53±3.5 |
| 29 | 50[b] | >100 |
| 30 | 20[b] | >100 |
| 31 | 30[b] | >100 |
| 43 | >1000 | 28±2.8 |
| 64 | N/A | 15±1.4 |
| 79 | N/A | 60±10.6 |
| 90 | N/A | 19±5.1 |
| 92 | N/A | 23±4.9 |
N/A—either no inhibition or promoter;
ref. 15
Figure 4.
Atomic Force Microscopy images of Aβ1–40 samples incubated without (control) and with the inhibitor compounds for 4 days. The numbers above the images denote the inhibitor compounds as they are listed in Figure 3.
The data observed in the AFM images are in good correlation with the data obtained in the THT fluorescence measurements (Figure 3). The control sample (Figure 4 Control) shows the expected, well developed network of mature fibrils. Images obtained in the presence of 1, 5, 44, 46, respectively, indicate extensive fibril formation, hence little inhibition. This is exactly what we observed in the quantitative assay; these compounds practically do not inhibit fibril formation, the highest value, obtained with 1 (11 % inhibiton), is still negligible. 44 and 46 were found to be fibril formation promoters, which resulted in the visually denser fibrillar morphology. Compounds 2, 3, and 4 possess similar structure; all of them are in Group I (Figures 2, 3). These compounds exhibited significant inhibition in the THT assays in an increasing order (2–82 %, 3–93 %, 4–100 %), which can be followed in Figure 4, as well. Less and less fibrils appear in the images from 2 to 3 and there are practically no fibrils when 4 was used as an inhibitor. Other compounds (8, 9) showed moderate inhibition (34–47 %), and that is reflected in the AFM images; the dense fibrillar network characteristic for the control became less frequent, however, a significant amount of fibrils are still present in the images. The visual analysis of images obtained using other inhibitors also indicate strong inhibition (37, 42, 67, 69, 80, 81). It also reveals that in the presence of inhibitors aggregates with different morphology could form. In many cases the obtained fibrils are almost identical to those observed in the control sample, although less dense in appearance. In some cases (Figure 4. 3, 67, 69) it only means a few well identifiable fibrils. In other cases, however, the aggregates are short in appearance (Figure 4. 42, 80) or form round shaped deposits (Figure 4, 81) indicating that strong polymorphism could occur as a result of the presence of inhibitors.
Discussion
The analysis of the above data indicates that several compounds in the synthesized compound library showed strong activity against either fibril or oligomer formation. This phenomenon appears to support earlier suggestions that not all stable oligomers are obligatory precursors to the fibrils and that the two processes can occur in parallel pathways. Thus a certain compound may affect one or the other process and not necessarily both.[7–9] The structure activity relationship will be discussed separately for fibril and oligomer inhibition.
Fibrillogenesis inhibitors
The most potent fibril inhibitors were 2–4 and 6 from Group I having the (CF3)2-C-OH motif, while several CF3(COOEt)-C-OH containing compounds (31, 31-(S), 31-(R)) showed the strongest, nearly the same activity. Other groups usually exhibited weaker inhibitory potential with numerical values up to about 50 % inhibition. These data are consistent with our previous hypothesis that the acidity of the inhibitors is a crucial factor in the mode of action of these compounds and the more acidic the OH, stronger the potency. The order of the acid strength and by parallel the inhibitor activity of the several motifs used is illustrated in Figure 5.
Figure 5.

The acid strength order of the major types of inhibitors used.
The role of the aromatic groups also appears important. In every group of compounds tested, the indolyl derivatives were found to be the most active followed by the pyrrole-based compounds. The weakest (or no) effect was consistently observed for the simple carbocyclic molecules (benzene derivatives). This observation highlights the priority of the heterocyclic aryl group, particularly indole over carbocyclic rings. It is worth noting that while the carbocyclic derivatives of Group I (16–18) showed a weak inhibitory effect, similar compound from all other groups were found to be self-assembly promoters (44–48, 75–78). In summary, indole-based inhibitors showed strong fibril inhibition properties while other aromatic units were modest inhibitors at best and often acted as fibrillogenesis promoters.
Based on this observation the discussion on the role of individual substituents will focus on indole derivatives. Groups I and II compounds are the most promising fibril inhibitors, the general activity of other compounds, while consistent, show a steady decrease as the function of the acidity of the CF3-C-XH group (X=O, N, Figure 5). In the case of Group II compounds the 5-halogen substituted (R3 substituent) indoles were found to be the best inhibitors, in the order of F<Cl<Br<I. The same order was observed in the case of the newly synthesized Group I molecules. Compound 4 (R3=I) showed 100 % fibril inhibition under the experimental conditions, while the Br and Cl substituted inhibitors showed decreasing but still high efficacy (93 and 82 %). Therefore it appears that the presence of a larger halogen atom at R3 position of the indole ring increases the fibril inhibition. However, substitution of the halogens with bulky electron withdrawing groups such as -COOMe, -CN, -CONH2 at R3 position of indole molecules decreases the ability of the compound to inhibit the formation of fibrils. Substituents at the other positions, while showing a minor effect, do not appear to significantly alter the activity of the inhibitors.
Oligomerization inhibitors
The analysis of the molecular features that result in effective oligomer inhibitors leads to the observation that, in contrast to fibrillogenesis inhibition, the acidity of the CF3-C-OH does not appear to be of primary importance. Interestingly, the typically good fibril inhibitors (Groups I and II) show poor performance in oligomer inhibition assays, providing further evidence in support of the earlier findings that fibrils and stable oligomers do not form via the same pathways.[7, 8] In fact, in certain cases these compounds promote the oligomer formation. Effective oligomer inhibitors were found in all groups except group I ((CF3)2-C-OH derivatives). Interestingly, from the groups of typical fibril inhibitors (I and II) only one compound showed significant oligomer inhibition (22, EC50=53 μM) and this compounds was a weak fibril inhibitor. The most active inhibitors of oligomer assembly are those with multiple active CF3-C-OH substituents (43, 90, 92) showing better EC50 values (19–25 μM). Similar to the fibrillogenesis inhibitors, compounds with carbocyclic (benzene) rings are inactive in the inhibition of the oligomer assembly. All effective inhibitors possess heterocyclic rings. Based on the molecular structures listed in Table 1 for oligomer inhibitors, a single, well defined relationship cannot be made. However, the double CF3-C-OH units and in certain cases the larger size or more aromatic rings suggest that for oligomer inhibition the presence of aromatic groups and the possibility of π-π interactions[30–32] is more likely a decisive feature than the presence of acidic groups. It is also supported by data obtained with the carbocylic compounds. These compounds mostly showed fibril formation promoting effects (44–48, 75–78) in contrast to their mild to moderate (76–28 %, 77–40 %) inhibition in oligomer assembly highlighting the importance of π-π stacking.[30–32]
The effect of inhibitor chirality on the Aβ self-assembly inhibition has also attracted considerable attention.[17, 30, 33, 34] Earlier results obtained with peptide-based[30] versus small molecule inhibitors[17, 33] appear to be controversial. While the individual (amino acid) chirality is of crucial importance for peptide inhibitors[30, 34] it did not appear to so for small molecule inhibitors. In a recent work it was stated that chirality was an important feature of methoxytacripyrines as inhibitors.[35] Analyzing the published data, however, led us to the conclusion that the activity differences between the enantiomers or racemic mixtures, while considerably high for hAChE inhibition, were rather insignificant (<10 %) for Aβ assembly given the experimental error of the fibril growing and analytical processes. Chiral compounds in our current set of molecules are included in Groups II and V. The results obtained with these compounds support our earlier conclusions.[17] The differences between the inhibitory effect of enantiomers and racemic mixtures mostly fall within a 0–20 % range (29–5 %; 30–7 %; 31–2 %; 82–13 % and 83–18 %), although in a few cases it is larger than that. It is worth noting that similar observations were made in both fibrillogenesis and oligomer assembly inhibition. While clearly more data, using structurally diverse compounds, is needed to provide a definite answer to this problem, the present results suggest that the interaction of chiral small molecules with Aβ is, most likely, not stereo/enantiospecific.
Conclusions
In conclusion, the synthesis and activity evaluation of a set of 106 structurally diverse compounds with one same motif (CF3-C-X, X=OH, NH2) resulted in valuable information for the further design of Aβ self-assembly inhibitors. An earlier observation regarding the importance of acidity of the OH group in fibrillogenesis inhibition was confirmed and new lead compounds (2–4) were identified. It was also observed that the acidity was a relatively unimportant characteristic of the compounds in oligomer assembly inhibition. The active oligomer inhibitors rather feature dual binding groups and more electron rich aromatic units, emphasizing that ability to participate in π-π interactions is also a dominant aspect of these compounds.
Experimental Section
Methods
General Information—Syntheses
The cinchona alkaloids were purchased from Fluka and used without further purification. Indole derivatives and ethyl 3,3,3-trifluoropyruvate, hexafluoroacetone hydrate, trifluoroacetaldehyde ethyl hemiacetal, substituted anilines and benzaldehydes were Aldrich products. CDCl3 used as a solvent (99.8 %) for the NMR studies was a Cambridge Isotope Laboratories product. 19F NMR reference compound CFCl3 was purchased from Aldrich. Other solvents used in synthesis with minimum purity of 99.5 % were Fisher products. K-10 montmorillonite, a solid acid used as catalyst, was obtained from Fluka. The mass spectrometric identification of the products were carried out by an Agilent 6850 gas chromatograph - 5973 mass spectrometer system (70 eV electron impact ionization) using a 30 m long DB-5 column (J&W Scientific). The 1H, 13C and 19F NMR spectra were obtained on a 300 MHz superconducting Varian Gemini 300 NMR spectrometer, in CDCl3 solvent with tetramethylsilane and CCl3F as internal standards. The temperature was 25±1 °C controlled by Varian temperature control unit. The determination of the enantiomeric excesses was carried out by chiral HPLC analysis using a Jasco PU-2080 HPLC coupled with a PU-2075 UV-VIS detector. The samples were analyzed in a hexane/isopropyl alcohol=95/5 mobile phase using a Chiralcel OJ-H (Daicel) analytical column at 260 nm wavelength.
General Information—Biochemical assays
Fibril assays—Sodium dihydrogenphosphate, disodium hydrogenphosphate, sodium azide, sodium hydroxide, sodiumchloride, glycine, dimethylsulfoxide and thioflavin-T were obtained from Sigma–Aldrich. Lyophilized Aβ(1–40) peptide (purity >95 %) was purchased from Anaspec. Mica sheets for AFM measurements were obtained from Alfa Aesar. Oligomer assays—1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP), dimethylsulfoxide (DMSO), fluorescamine, ultrapure Tween 20, tetramethylbenzidine (free base), N,N-dimethylacetamide, tetrabutylammonium borohydride, and 30 % (w/w) H2O2 were obtained from Sigma–Aldrich. N-α-Biotinyl-Aβ(1–42) (bio-Aβ42) was purchased from AnaSpec. Fatty acid-free fraction V bovine serum albumin was obtained from Boehringer-Mannheim. Streptavidin-HRP (SA-HRP) was obtained from Rockland. NeutrAvidin (NA) was obtained from Pierce. High-binding 9018 ELISA plates were purchased from Costar.
Synthesis of Inhibitor Candidates
Compounds used in this study were synthesized using literature methods[18–24] or as described below. In each case the compounds were purified by flash chromatography or preparative thin layer chromatography. The identification and purity determination were carried out by gas chromatography-mass spectrometry, and NMR spectroscopy (1H, 13C and 19F when applicable). The known compounds showed identical NMR and MS spectral characteristics to literature data (see Supporting Information).
General procedure for the synthesis of 1,1,1,3,3,3-hexafluoro-2-(indol-3-yl)-propan-2-ols. (1–12)
A microwave reaction vial containing molecular sieves (4 Å, 200 mg) was charged with 1 mmol of indole and 1.5 mmol (205 μL) of hexafluoroacetone trihydrate. The contents of the vial were irradiated in a CEM Discover microwave reactor for 10 min at 100 °C. The progress of the reaction was monitored by GC-MS. After the completion of the reaction, CH2Cl2 was added to the reaction mixture. The content of the vial was filtered into a round bottom flask and concentrated in vacuo. The products were isolated as crystals or oils and purified by flash chromatography if the GC-MS purity was >98 %.
General procedure for the synthesis of 1,1,1,3,3,3-hexafluoro-2-(1 H-pyrrol-2-yl)propan-2-ol (13–15)
A microwave reaction vial containing molecular sieves (4 Å, 200 mg) was charged with 1 mmol of pyrrole and 1.5 mmol (205 μL) of hexafluoroacetone trihydrate. The content of the vial was irradiated in a CEM Discover microwave reactor for 10 min at 80 °C. The progress of the reaction was monitored by GC-MS. After the completion of the reaction, CH2Cl2 was added to the reaction mixture. The content of the vial was filtered into a round bottom flask and concentrated in vacuo. The products were isolated as crystals or oils and purified by flash chromatography if the GC-MS purity was >98 %.
General procedure for the synthesis of 1,1,1,3,3,3-hexafluoro-2-phenylpropan-2-ol (16–18)
1 mmol arene and 1.5 mmol ethyl trifluoropyruvate were added to a dry pressure tube containing 3 mL dichloromethane under N2 atmosphere. Triflic acid (50 mol %) was added to the reaction mixture dropwise. The reaction mixture was stirred for 16 h after which the contents were poured into 5 mL of water and extracted with dichloromethane. After evaporation of solvents, the residue was purified by flash chromatography to give the final product.
General procedure for the synthesis of 3,3,3-trifluoro-2-hydroxyl-2-(indol-3-yl)-propionic acid ethyl esters (19–34)
The compounds were synhesized based on a literature method. Indole (0.75 mmol), ethyl 3,3,3-trifluoropyruvate (TFP, 1.125 mmol) and 500 mg of K-10 montmorillonite were mixed in 3 mL of toluene in a teflon screw cap pressure tube. The reaction mixture was heated and stirred at 60 °C and the progress of reaction was monitored by thin-layer chromatography (TLC). After satisfactory conversion, the product mixture was separated from catalyst by filtration. The solvent and excess of TFP were removed under vacuum. The products were isolated as crystals or oils and purified by flash chromatography. Pyrrole derivatives (35–43) were prepared using the same method.
General procedure for the synthesis of ethyl 3,3,3-trifluoro-2-hydroxy-2-(1 H-pyrrol-2-yl)propanoate (35–43)
Pyrrole (0.5 mmol), ethyl 3,3,3-trifluoropyruvate (TFP, 0.51 mmol) were mixed in round bottomed flask. The reaction mixture was stirred at room temperature and the progress of reaction was monitored by gas chromatography (GC). After satisfactory conversion, the product mixture was extracted into dichloromethane. The solvent and excess of TFP were removed under vacuum. The product was then purified by flash chromatography.
General procedure for the synthesis of enantiomeric 3,3,3-trifluoro-2-hydroxyl-2-(indol-3-yl)-propionic acid ethyl esters (29–31, (S) and (R))
The enantiomers have been prepared by an earlier cinchona alkaloid-catalyzed organocatalytic method. Indole (0.5 mmol) and cinchonidine (for (S) products) or cinchonine (for (R) products) (0.0375 mmol) were placed into a glass reaction vessel and 3 mL Et2O was added. The mixture was stirred at −8 °C (salt-ice cooling bath) for 30 min. 0.75 mmol of ethyl 3,3,3-trifluoropyruvate was then added and the mixture was stirred at −8 °C (salt-ice cooling bath) for an additional 3 h and the progress was monitored by TLC. After the reaction was completed, the solvent and excess ethyl trifluoropyruvate were removed by evaporation. The mixture was then dissolved in ether and the cinchona catalyst was removed by a treatment with 500 mg of K-10 montmorillonite (a solid acid). After the treatment the alkaloid-K-10 complex was removed by filtration and the solvent was evaporated. A colorless solid was obtained in 98 % yield. The enantiomeric excess of the product was determined by HPLC (see below). The product purity was >98 % (86–93 % ee).
General procedure for the synthesis of ethyl 3,3,3-trifluoro-2-hydroxy-2-phenylpropanoate (44–48)
1 mmol arene and 1.5 mmol hexafluoroacetone trihydrate were added to a pressure tube containing 3 mL dichloromethane under N2 atmosphere. Triflic acid (2 equiv) was added to the reaction mixture dropwise. The reaction mixture was stirred for 16 h after which the contents were poured into 5 mL of water and extracted into dichloromethane. After evaporation of solvents, the residue was purified by flash chromatography to give the final product.
General procedure for the synthesis of ethyl 4,4,4-trifluoro-3-hydroxy-3-(1H-indol-3-yl)butanoate (49–59)
Solution of indole (0.5 mmol), ethyl 4,4,4-trifluoro-3-oxobutanoate (TFAA) (0.5 mmol) and K-10 montmorillonite were mixed in 3 mL of toluene in a teflon screw cap pressure tube. The reaction mixture was heated and stirred at 60 °C and the progress of reaction was monitored by thin-layer chromatography (TLC). After satisfactory conversion, the product mixture was separated from catalyst by filtration. The product was purified by flash chromatography and isolated as crystals or oils.
General procedure for the synthesis of ethyl 4,4,4-trifluoro-3-hydroxy-3-(1H-pyrrol-2-yl)butanoates (60–66)
Solution of pyrrole (0.5 mmol), ethyl 4,4,4-trifluoro-3-oxobutanoate (TFAA) (0.5 mmol) and K-10 montmorillonite were mixed in 3 mL of toluene in a teflon screw cap pressure tube. The reaction mixture was heated and stirred at 60 °C and the progress of reaction was monitored by thin-layer chromatography (TLC). After satisfactory conversion, the product mixture was separated from catalyst by filtration. The product was purified by flash chromatography and isolated as crystals or oils.
General procedure for the synthesis of 1-(1H-indol-1-yl)-2,2,2-trifluoroethanols (67–73)
A solution of indole (0.5 mmol), trifluoroacetaldehyde ethyl hemiacetal (2 mmol) and triethylamine (0.05 mmol) in 0.25 mL of DMF was irradiated in a CEM Discover microwave reactor for 20 min at 150 °C. Then reaction mixture was quenched with 10 mL of water and the product was extracted with ethyl acetate (3×10 mL). The combined organic extracts were dried over anhydrous sodium sulfate, evaporated and the product was isolated and purified by preparative TLC or column chromatography.
Synthesis of 1-(5-bromo-1H-indol-3-yl)-2,2,2-trifluoroethanol (74)
Mixture of 5-bromoindole (0.5 mmol) and trifluoroacetaldehyde methyl hemiacetal (1 mmol) was irradiated in the above microwave reactor for 10 min at 100 °C. Then reaction mixture was directly subjected to preparative TLC for purification and product isolation. Isolated yield 72 %; Colorless solid; M.P.: 113–115 °C.1H NMR (300.128 MHz, CDCl3), δ=7.76 (m, 1 H), 7.33–7.37 (m, 3 H), 6.57 (d, J=3.3 Hz, 1 H), 6.09 (p, J=4.8 Hz, 1 H), 3.88 ppm (d, J=4.8 Hz, 1 H); 13C NMR (75.474 MHz, CDCl3), δ=130.68, 125.69, 125.58, 123.82, 120.30, 116.96, 114.13, 111.16, 104.23, 76.65 ppm (q, J=36 Hz); 19F NMR (300.128 MHz, CDCl3): −77.63 (d, J=4.8 Hz); MS-C10H7BrF3NO (294) m/z (%): 293 (M+, 100), 295 (M+, 98), 214 (30), 175 (25).
General procedure for the synthesis of 2,2,2-trifluoro-1-phenylethanols (75–78)
1 mmol arene and 1.5 mmol trifuoroacetaldehyde hemiacetal were added to a pressure tube containing 3 mL dichloromethane under N2 atmosphere. Triflic acid (2 equiv) was added to the reaction mixture dropwise. The reaction mixture was stirred for 16 h after which the contents were poured into 5 mL of water and extracted with dichloromethane. After evaporation of solvents, the residue was purified by flash chromatography to give the final product.
General procedure for the synthesis of ethyl 2-amino-3,3,3-trifluoro-2-(-1H-indol-3-yl)propanoate (79–88)
Step 1 - General procedure for the preparation of ethyl 3,3,3 trifluoro-2-(1-phenylethylimino) propanoate: Montmorillonite K-10 (4 g) and 20 mL of toluene were placed into a round-bottomed flask equipped with a stir bar, a reflux condenser and a dry tube. Ethyl trifluoropyruvate (6.24 mL, 0.047 mol) and α-methyl benzylamine (5 mL, 0.039 mol) (racemic or R or S) was dissolved in 5 mL of toluene and this solution was added to the above mixture. The reaction mixture was stirred at 100 °C for 4 h and the progress was monitored by TLC. After the reaction was completed, the resulting reaction mixture was filtered through a sintered glass funnel and washed with CH2Cl2. The solvent and excess ethyl trifluoropyruvate were removed in vacuo to obtain the brown oil. This oil was later subjected to column chromatography (hexane/ethyl acetate 90/10) to obtain a colorless liquid with 92 % isolated yield.
General procedure for synthesis of racemic 3, 3, 3-Trifluoro-2-(1H-indol-3-yl)-2-(1-phenylethyl amino)propanoates. (Step 2)
Racemic trifluoro imine, synthesized in step 1 (300 mg, 1.09 mmol) and indole (0.98 mmol) were dissolved in 2 mL CH2Cl2. The reaction vessel was placed into an ice bath and the mixture was stirred at 0 °C for 5 min. Trifluoromethanesulfonic acid (0.40 mmol, 20 % solution in CH2Cl2) was added drop wise to the reaction mixture over the period of 15 min. After the complete addition, the reaction mixture was stirred at 0 °C for another 2 h and the progress was followed by TLC. After the reaction was completed, 5 mL of water was added to reaction mixture and stirred at room temperature for 5 min to quench the acid. The resulting mixture was extracted with CH2Cl2, and the organic layer was washed with water three times. The organic layers were combined, dried over sodium sulfate and filtered. The solvent was removed by evaporation and the resulting crude mixture was purified by column chromatography.
General procedure for synthesis of chiral 3, 3, 3-Trifluoro-2-(1H-indol-3-yl)-2-(1-phenylethyl amino)propanoates
(R) or (S) Trifluoromethylated-imine (300 mg, 1.09 mmol) and indole (0.98 mmol) were dissolved in 2 mL CH2Cl2. The reaction vessel was placed into a cooling bath (EtOH/dry ice mixture) and the mixture was stirred at −40 °C for 15 min. Trifluoromethanesulfonic acid (0.40 mmol, 20 % solution in CH2Cl2) was added dropwise to the reaction mixture over the period of 15 min. After the complete addition, the reaction mixture was stirred at −40 °C for another 2 h and the progress was followed by TLC. After the reaction was complete, 5 mL of water was added to the reaction mixture and stirred at room temperature for 5 min to quench the acid. The resulting mixture was extracted into CH2Cl2 and the organic layer was washed three times with water. The organic layers were combined, dried over sodium sulphate, and filtered. The solvent was removed by evaporation and the resulting crude mixture was purified by column chromatography.
General procedure for synthesis of ethyl 2-amino-3, 3, 3-trifluoro-2-(1H-indol-3-yl) propanoate. (Hydrogenolysis) (Step 3)
3,3,3-Trifluoro-2-(1H-indol-3-yl)-2-(1-phenylethylamino)propanoate (150 mg, 0.38 mmol) was dissolved in 2 mL EtOH along with Pd(OH)2 (Pearlman’s catalyst) (75 mg). The mixture was stirred under 5 bar H2 pressure at room temp for 12 h. After the reaction was complete, the catalyst was separated by filtration. The resulting filtrate was concentrated in vacuo and purified by column chromatography.
Synthesis of 3,3,3-trifluoro-2-hydroxyl-2-(5-benzyloxy-indol-3-yl)-propionic acid ethyl ester (89)
The solution of 5-hydroxyindole (66.5 mg, 0.5 mmol), benzyl bromide (65 μL, 0.55 mmol) and potassium carbonate (276 mg, 2 mmol) in 0.5 mL of DMF was stirred for 24 h. Then ethyl trifluoropyruvate (192 μL, 1.4 mmol) was added and stirred continuously for another 24 h. The reaction was diluted with 10 mL of water and extracted with 3 portions of 5 mL of dichloromethane. Combined organic extracts were dried over anhydrous sodium sulfate and the solvent was removed in vacuo. The residue was then subjected to column chromatography affording pure product (170 mg, 87 % yield).
General procedure for the synthesis of 1,3- and 1,4-phenylenebis(methylene)bis(3-(3-ethoxy-1,1,1-trifluoro-2-hydroxy-3-oxopropan-2-yl)-1H-indole-5-carboxylates) (90 and 93)
The solution of 5-indole carboxylic acid (80.5 mg, 0.5 mmol), α,α′-dibromo-xylene (meta or para, 66 mg, 0.25 mmol) and potassium carbonate (276 mg, 2 mmol) in 0.5 mL of DMF was stirred for 24 h. Then ethyl trifluropyruvate (192 μL, 1.4 mmol) was added and stirred for 24 h. The reaction was quenched with 10 mL of water and the mixture was extracted with dichloromethane (3×5 mL). The combined organic extracts were dried over anhydrous sodium sulfate and the solvent was evaporated. The residue was purified by column chromatography affording pure 90 (130 mg, 68 % yield) or 93 (135 mg, 71 % yield).
General procedure for the synthesis of diethyl 2,2′-(5,5′-(1,3- and 1,4-phenylenebis(methylene))bis(oxy)bis(1H-indole-5,3-diyl))bis (3,3,3-trifluoro-2-hydroxypropanoates) (91 and 92)
The solution of 5-hydroxyindole (66.5 mg, 0.5 mmol), α,α′-dibromo-xylene (meta or para, 66 mg, 0.25 mmol) and potassium carbonate (276 mg, 2 mmol) in 0.5 mL of acetonitrile was stirred for 24 h. Then ethyl trifluropyruvate (192 μL, 1.4 mmol) was added and the stirring was continued for another 24 h. The reaction was diluted with 10 mL of water and extracted with 3 portions of 5 mL of dichloromethane. The combined organic extracts were dried over anhydrous sodium sulfate and the solvent was removed in vacuo. The raw products were subjected to column chromatography yielding pure 91 (143 mg, 81 % yield) or 92 (138 mg, 78 % yield).
Thioflavin-T fluorescence assay for the determination of inhibitor activity in Aβ fibrillogenesis
The Thioflavin-T fluorescence assay was carried out using a standard procedure.[25–27] The synthetic lyophilized Aβ 1–40 peptide was dissolved in 100 mM NaOH to a concentration of 40 mg mL−1 and diluted in 10 mM HEPES, 100 mM NaCl, 0.02 % NaN3 (pH 7.4) buffer to a final peptide concentration of 100 μM. Using NaOH as an initial solvent ensures that the isoelectronic point of Aβ is bypassed and the peptide will remain in monomeric form.[36, 37] The inhibitors were dissolved in dimethyl sulfoxide (DMSO) to achieve a concentration of 0.15 M and added to the Aβ samples in HEPES buffer (inhibitor/Aβ=10) to attain a final concentration of 1 mM. After 30 s of vigorous vortexing the solutions were incubated at 37 °C with gentle shaking (77 rpm) for seven days and the increase in fibril amount in each sample was followed by Thioflavin-T fluorescence using the peptide without any inhibitor as the control. The fluorescence measurements have been carried out using a Hitachi F-2500 fluorescence spectrophotometer. The incubated peptide solutions were briefly vortexed before each measurement, and then 3.5 μL aliquots of the suspended fibrils were withdrawn and added into 700 μL of 5 μM Thioflavin-T prepared freshly in 50 mM glycine-NaOH (pH 8.5) buffer. The maximum fluorescence intensity of these mixtures was measured at 484±5 nm emission wavelength with preset excitation wavelength of 435 nm. None of the inhibitor compounds showed fluorescence intensity in this region. For the purposes of a screening assay, the fibril signal generated under the conditions of the assay in the stopping the reaction with 100 μL of 1 % (v/v) H2SO4. For the purposes of a screening assay, the oligomer signal generated under the conditions of the assay in the presence of 1 % DMSO (solvent control) and absence of compound is taken as 100 %. The EC50 values of potent compounds were determined as described earlier.[15]
Atomic Force Microscopy of fibrils
The morphology of the incubated peptide samples were studied using atomic force microscopy (AFM).[38, 39] 2 μL aliquots were spotted on freshly cleaved mica sheets and air dried. The buffer salts were washed off with deionized water. AFM was carried out using a Quesant Q-Scope 250 microscope in non-contact mode.
Assay for inhibition of Aβ oligomer assembly
Biotinyl-Aβ(1–42) stored as a 1 mg mL−1 solution in HFIP at −75 °C is dried and treated with neat trifluoroacetic acid for 10 min at room temperature to disaggregate the peptide and dissolved to 500 nM (50×) in DMSO as described.[28, 29] Two microliters of peptide is dispensed into each well of a polypropylene 96-well plate and 100 μL of PBS containing the desired concentration of test compound and 1 % DMSO added to initiate oligomer formation at room temperature. After 30 min, 50 μL of 0.3 % v/v Tween 20 is added to stop oligomer assembly. Fifty microliters of this mixture is then assayed for oligomer content by single-site Streptavidin-based assay.
Biotinyl-Aβ(1–42) single-site Streptavidin-based assay for the determination of inhibitor activity in Aβ oligomer formation.[28, 29] Fifty μl of 1 μg mL−1 NA in 10 mM NaPi (pH 7.5) is coated per well overnight at 4 °C on Costar 9018 high-binding ELISA plates sealed with adhesive plastic film. The plates are blocked by the addition of 200 μL phosphate-buffered saline (PBS, 10 mM sodium phosphate, 150 mM NaCl [pH 7.5], 0.1 % v/v Tween 20 at room temperature for 1–2 h and stored at 4 °C. In the assay after removal of the blocking solution, a sample containing a mixture of oligomers and monomers of biotinylated peptide (50 μL containing up to 10 nM Aβ is bound for 2 h at room temperature. The wells are washed three times with TBST (20 mM Tris-HCl, 34 mM NaCl [pH 7.5], and 0.1 % v/v Tween 20) on a Biotek EL x 50 automated plate washer. After washing, 50 μL of 1:20 000 SA-HRP in PBS+0.1 % v/v Tween 20 is added, the plate sealed, and the incubation is continued for 1 h at room temperature. The plate is washed again with TBST, 100 μL of tetramethylbenzidine/H2O2 substrate solution is added, and the plate is incubated at room temperature for 5–10 min. The OD450 nm is determined on a Biotech Synergy HT plate reader after stopping the reaction with 100 μL of 1 % (v/v) H2SO4. For the purposes of a screening assay, the oligomer signal generated under the conditions of the assay in the presence of 1 % DMSO (solvent control) and absence of compound is taken as 100 %.
A diverse library of compounds with the same binding moiety was generated and the general structural features that characterize the oligomer and fibril inhibitors were observed with the ultimate goal of finding lead structures for further, focused inhibitor design. alzheimer’s disease amyloid beta chiral inhibitors fibrils heterocycles oligomers organofluorine compounds
Acknowledgments
Financial support provided by the University of Massachusetts Boston, and National Institute of Health (R-15 AG025777–03A1) and (R21AG028816-01 to H.L.) is gratefully acknowledged.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/cmdc.201100569.
Contributor Information
Prof. Béla Török, Email: bela.torok@umb.edu.
Prof. Harry LeVine, III, Email: hlevine@email.uky.edu.
Prof. Marianna Török, Email: marianna.torok@umb.edu.
References
- 1.The American Alzheimer’s Association’s. [accessed October 2011]; website http://www.alz.org/
- 2.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 3.Chiti F, Dobson CM. Nat Chem Biol. 2009;5:15–22. doi: 10.1038/nchembio.131. [DOI] [PubMed] [Google Scholar]
- 4.Estrada LD, Soto C. Curr Top Med Chem. 2007;7:115–126. doi: 10.2174/156802607779318262. [DOI] [PubMed] [Google Scholar]
- 5.Stains C, Mondal K, Ghosh I. ChemMedChem. 2007;2:1674–1692. doi: 10.1002/cmdc.200700140. [DOI] [PubMed] [Google Scholar]
- 6.Walsh DM, Selkoe DJ. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 7.Necula M, Kayed R, Milton S, Glabe CG. J Biol Chem. 2007;282:10 311–10 324. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- 8.Glabe CG. J Biol Chem. 2008;283:29 639–29 643. [Google Scholar]
- 9.Nerelius C, Johansson J, Sandegren A. Front Biosci. 2009;14:1716–1729. doi: 10.2741/3335. [DOI] [PubMed] [Google Scholar]
- 10.Stains C, Mondal K, Ghosh I. ChemMedChem. 2007;2:1674–1692. doi: 10.1002/cmdc.200700140. same as ref. 5? [DOI] [PubMed] [Google Scholar]
- 11.Estrada LD, Soto C. Curr Top Med Chem. 2007;7:115–126. doi: 10.2174/156802607779318262. [DOI] [PubMed] [Google Scholar]
- 12.LeVine H., III Amyloid. 2007;14:185–197. doi: 10.1080/13506120701461020. [DOI] [PubMed] [Google Scholar]
- 13.Török B, Dasgupta S, Török M. Curr Bioact Comp. 2008;4:159–174. [Google Scholar]
- 14.Adamski-Werner SL, Palaninathan SK, Sacchettini JC, Kelly JW. J Med Chem. 2004;47:355–374. doi: 10.1021/jm030347n. [DOI] [PubMed] [Google Scholar]
- 15.Török M, Abid M, Mhadgut SC, Török B. Biochemistry. 2006;45:5377–5383. doi: 10.1021/bi0601104. [DOI] [PubMed] [Google Scholar]
- 16.Sood A, Abid M, Sauer C, Hailemichael S, Foster M, Török B, Török M. Bioorg Med Chem Lett. 2011;21:2044–2047. doi: 10.1016/j.bmcl.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sood A, Abid M, Hailemichael S, Foster M, Török B, Török M. Bioorg Med Chem Lett. 2009;19:6931–6934. doi: 10.1016/j.bmcl.2009.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abid M, Török B. Adv Synth Catal. 2005;347:1797–1803. [Google Scholar]
- 19.Landge SM, Borkin DA, Török B. Tetrahedron Lett. 2007;48:6372–6376. doi: 10.1016/j.tetlet.2007.06.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borkin D, Landge SM, Török B. Chirality. 2011;23:612–616. doi: 10.1002/chir.20982. [DOI] [PubMed] [Google Scholar]
- 21.Sridhar M, Narsaiah C, Ramanaiah BC, Ankathi VM, Pawar RB, Asthana SN. Tetrahedron Lett. 2009;50:1777–1779. [Google Scholar]
- 22.Török B, Abid M, London G, Esquibel J, Török M, Mhadgut SC, Yan P, Prakash GKS. Angew Chem. 2005;117:3146–3149. doi: 10.1002/anie.200462877. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:3086–3089. doi: 10.1002/anie.200462877. [DOI] [PubMed] [Google Scholar]
- 23.Abid M, Teixeira L, Török B. Org Lett. 2008;10:933–935. doi: 10.1021/ol703095d. [DOI] [PubMed] [Google Scholar]
- 24.Ban HS, Minegishi H, Shimizu K, Maruyama M, Yasui Y, Nakamura H. ChemMedChem. 2010;5:1236–1241. doi: 10.1002/cmdc.201000112. [DOI] [PubMed] [Google Scholar]
- 25.Naiki H, Higuchi K, Hosokawa M, Takeda T. Anal Biochem. 1989;177:244–249. doi: 10.1016/0003-2697(89)90046-8. [DOI] [PubMed] [Google Scholar]
- 26.LeVine H., III Protein Sci. 1993;2:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsson MR. Methods. 2004;34:151–160. doi: 10.1016/j.ymeth.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 28.LeVine H., III Anal Biochem. 2006;356:265–272. doi: 10.1016/j.ab.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 29.LeVine H, III, Ding Q, Walker JA, Voss RS, Augelli-Szafran CE. Neurosci Lett. 2009;465:99–103. doi: 10.1016/j.neulet.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sciaretta KL, Gordon DJ, Meredith SC. Methods Enzymol. 2006;413:273–312. doi: 10.1016/S0076-6879(06)13015-3. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi T, Mihara H. Acc Chem Res. 2008;41:1309–1318. doi: 10.1021/ar8000475. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong AH, Chen J, McKoy AF, Hecht MH. Biochemistry. 2011;50:4058–4067. doi: 10.1021/bi200268w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore SA, Huckerby TN, Gibson GL, Fullwood NJ, Turnbull ST, Tabner BJ, El-Agnaf OMA, Allsop D. Biochemistry. 2004;43:819–826. doi: 10.1021/bi035728h. [DOI] [PubMed] [Google Scholar]
- 34.Chalifour RJ, McLaughlin RW, Lavoie L, Morisette C, Tremblay N, Boule M, Sarazin P, Stea D, Tremblay P. J Biol Chem. 2003;278:34 874–34 881. doi: 10.1074/jbc.M212694200. [DOI] [PubMed] [Google Scholar]
- 35.Bartolini M, Pistolozzi M, Andrisano V, Egea J, Lopez MG, Iriepa I, Moraleda I, Galvez E, Marco-Contelles J, Samadi A. ChemMedChem. 2011;6:1990–1997. doi: 10.1002/cmdc.201100239. [DOI] [PubMed] [Google Scholar]
- 36.Fezoui Y, Hartley DM, Harper JD, Khurana R, Walsh DM, Condron MM, Selkoe DJ, Lansbury PT, Fink AL, Teplow DB. Amyloid. 2000;7:166–78. doi: 10.3109/13506120009146831. [DOI] [PubMed] [Google Scholar]
- 37.Bourhim M, Kruzel M, Srikrishnan T, Nicotera T. J Neurosci Methods. 2007;160:264–268. doi: 10.1016/j.jneumeth.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 38.Ding TT, Harper JD. Methods Enzymol. 1999;309:510–25. doi: 10.1016/s0076-6879(99)09035-7. [DOI] [PubMed] [Google Scholar]
- 39.Antzutkin ON. Magn Reson Chem. 2004;42:231–246. doi: 10.1002/mrc.1341. [DOI] [PubMed] [Google Scholar]