Abstract
Defaunation is causing declines of large-seeded animal-dispersed trees in tropical forests worldwide, but whether and how these declines will affect carbon storage across this biome is unclear. Here we show, using a pan-tropical data set, that simulated declines of large-seeded animal-dispersed trees have contrasting effects on aboveground carbon stocks across Earth's tropical forests. In our simulations, African, American and South Asian forests, which have high proportions of animal-dispersed species, consistently show carbon losses (2–12%), but Southeast Asian and Australian forests, where there are more abiotically dispersed species, show little to no carbon losses or marginal gains (±1%). These patterns result primarily from changes in wood volume, and are underlain by consistent relationships in our empirical data (∼2,100 species), wherein, large-seeded animal-dispersed species are larger as adults than small-seeded animal-dispersed species, but are smaller than abiotically dispersed species. Thus, floristic differences and distinct dispersal mode–seed size–adult size combinations can drive contrasting regional responses to defaunation.
 Defaunation is linked to the decline of tree species that depend on large animals for seed dispersal, but it is unclear if this affects carbon storage. Here the authors show that defaunation effects on carbon storage vary across continents, driven by relationships between seed dispersal strategies and adult tree size.
Defaunation is linked to the decline of tree species that depend on large animals for seed dispersal, but it is unclear if this affects carbon storage. Here the authors show that defaunation effects on carbon storage vary across continents, driven by relationships between seed dispersal strategies and adult tree size.
Anthropocene defaunation is amongst the most pervasive drivers of Earth's ongoing biodiversity crisis1,2,3,4. The rates at which the tropics are losing vertebrate species are amongst the highest globally, with larger species being particularly vulnerable to population declines and extirpations1,5,6,7. Declines of large vertebrates can have widespread, cascading effects on community- and ecosystem-level processes, because smaller organisms are often unable to perform the ecological roles of larger vertebrates1,2. In tropical forests, where the majority of tree species depend on vertebrate frugivores for seed dispersal8, theory and empirical evidence indicate that defaunation can substantially alter the composition of tree communities, through effects on seed dispersal processes9,10,11 and cause tree recruitment to shift towards smaller-seeded animal-dispersed and abiotically dispersed species12,13,14,15. Declines of large vertebrate frugivores can result in reductions of up to 60% in the abundance of tree species that depend on them for seed dispersal16, with resultant declines of over 25% in average seed sizes of adult tree communities13. With tropical forests representing one of the largest terrestrial carbon pools and playing a critical role in regulating global climate17,18, understanding whether and how such defaunation-driven declines in large-seeded species can affect carbon storage in tropical forests across the globe is critical19,20.
The consequences of defaunation-driven shifts in tree community composition for carbon stocks will depend on how small-seeded and abiotically dispersed species differ from large-seeded animal-dispersed species in their potential to store carbon19. Recent work from Amazonian and Atlantic forests suggest that defaunation has the potential to drive aboveground carbon losses through shifts towards tree communities with less-dense wood because small-seeded animal-dispersed species in Neotropical forests tend to have lower wood densities than larger-seeded species19,20. However, whether defaunation has similar effects on the carbon storage potential of other tropical forest communities that have different floristic compositions, and the potential mechanisms underlying such effects at the global scale, remain unknown.
Here, we present a pan-tropical assessment of the potential effects of defaunation on aboveground carbon storage by simulating extirpations of large-seeded animal-dispersed species from 10 relatively undisturbed tropical forest tree communities spanning four continents. These sites span a broad range of floristic types and form a gradient in the prevalence of animal-mediated seed dispersal from highest in tropical Africa, India and the Americas, to lowest in the more wind-dispersed assemblages of Australia and Southeast Asia. Our data set includes stem measurements and functional traits of over 25,000 trees of ∼2,500 species representing at least 785 genera and 155 families.
We examined the potential effects of defaunation on aboveground carbon storage by simulating declines in relative abundances of large-seeded animal-dispersed species within tree communities at each site. In our simulations, where we held the total basal area of each site constant, we removed individuals of large-seeded animal-dispersed species and replaced the lost basal area through a random draw of individuals from the remaining pool of individuals within each community21 (Methods section). We simulated four levels of defaunation-driven losses of large-seeded animal-dispersed species by removing 25, 50, 75 and 100% of these individuals from each of our sites. This, on average, reduced community-weighted seed size of animal-dispersed species across sites by 6, 14, 23 and 33%, respectively. Aboveground carbon stocks of the original and simulated communities were then estimated using data on tree diameters and species wood density in a general biomass equation for tropical forest trees22.
In addition to the above simulations, we also simulated two control scenarios. First, because natural tree communities typically contain far greater numbers of smaller trees than larger individuals, removal of large individuals followed by replacement using random draw from the remaining pool to make up original basal areas, as in our simulations, can reduce carbon stocks simply as a result of larger individuals being replaced by multiple smaller individuals. To distinguish the effects of losing individuals of large-seeded species from such random ‘sampling' effects, we also simulated a set of ‘individual-based' control scenarios in which equal numbers of individuals as those lost from defaunation scenarios were removed at random from original tree communities. The lost basal areas were replaced through random draw of individuals from the remaining pool, as was done in the defaunation scenarios. Second, removing individuals can sometimes lead to incidental species extinctions and reduce overall species richness, which in turn, can reduce the carbon storage potential of forest stands21. To distinguish the effects of defaunation-driven losses of large-seeded species from those of random species loss per se, we also simulated a second set of ‘species-based' control scenarios in which equal numbers of species as those lost in the defaunation scenarios were removed based on random selection from the overall pool of species, and lost basal areas replaced through random selection of individuals from the remaining pool (Methods section).
Our simulations of declines in large-seeded species in tropical forests across four continents suggest that (i) defaunation effects on aboveground carbon storage are likely to differ between regions, contingent on floristic composition. Carbon losses are expected in forests of the Americas, Africa and South Asia where tree communities are dominated by animal-dispersed species, but not in forests of Southeast Asia and Australia where large, abiotically dispersed species are more prevalent; (ii) at the pan-tropical scale, changes in aboveground carbon storage are driven by changes in stand volume rather than wood density; (iii) shifts in carbon storage following defaunation are underlain by globally consistent relationships between species' dispersal mode, seed size and potential adult size, wherein large-seeded animal-dispersed species attain larger adult sizes and therefore have more volume as adults than small-seeded animal-dispersed species, but are smaller than abiotically dispersed species. As a growing proportion of tropical forests are facing defauntion due to hunting, fragmentation, logging and other anthropogenic disturbances, conserving large tree species and their seed dispersers will be important for climate regulation.
Results
Defaunation scenarios and aboveground carbon storage
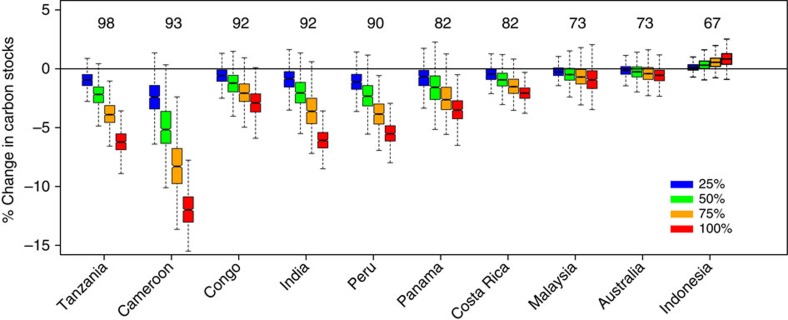
Simulated losses of large-seeded, animal-dispersed tree species decreased estimated aboveground carbon stocks by up to 5% under the 50% removal scenario (half of the individuals of large-seeded species lost from communities), and by as much as 12% under the 100% removal scenario, that is, complete extirpation of all large-seeded animal-dispersed species in communities (Fig. 1, Supplementary Tables 1 and 2). In contrast, carbon storage was largely unaffected in the individual- and species-based control scenarios, with shifts in carbon stocks ranging between −0.4% and +0.1% at the highest extirpation levels across the 10 sites (Supplementary Fig. 1, Supplementary Tables 1 and 2), suggesting that changes in carbon stocks in the defaunation scenarios were indeed primarily driven by reductions of large-seeded animal-dispersed tree species. Overall, simulated losses of large-seeded species reduced carbon stocks in the African, Indian and American tree communities, which had relatively high abundances (>80% of all individuals) of animal-dispersed species (Fig. 1 and Supplementary Table 1). Aboveground carbon stocks in these communities decreased by 1–5% under the 50% removal scenario, and by 2–12% under the 100% extirpation scenario (Fig. 1 and Supplementary Table 1). In Australia and Southeast Asia, where abundances of animal-dispersed tree species are lower (<75% of all individuals), carbon stocks either decreased or increased marginally (Fig. 1 and Supplementary Table 1). These results are in accordance with the limited empirical evidence that currently exists, which shows carbon losses associated with defaunation in an African forest23 but not in a Southeast Asian site15. These patterns remained unchanged when we used fixed, rather than site-specific cutoffs (75th percentile; Methods section) to classify large-seeded species for our simulations (Supplementary Fig. 2).
Figure 1. Effects of simulated removal of large-seeded animal-dispersed species on aboveground carbon storage.
Box-and-whisker plots depicting changes relative to original carbon stocks for 1,000 simulation runs following removal of 25, 50, 75 and 100% of individuals belonging to large-seeded animal-dispersed tree species. Numbers above boxes represent the relative abundances (%) of animal-dispersed species at each site.
Wood density and woody volume
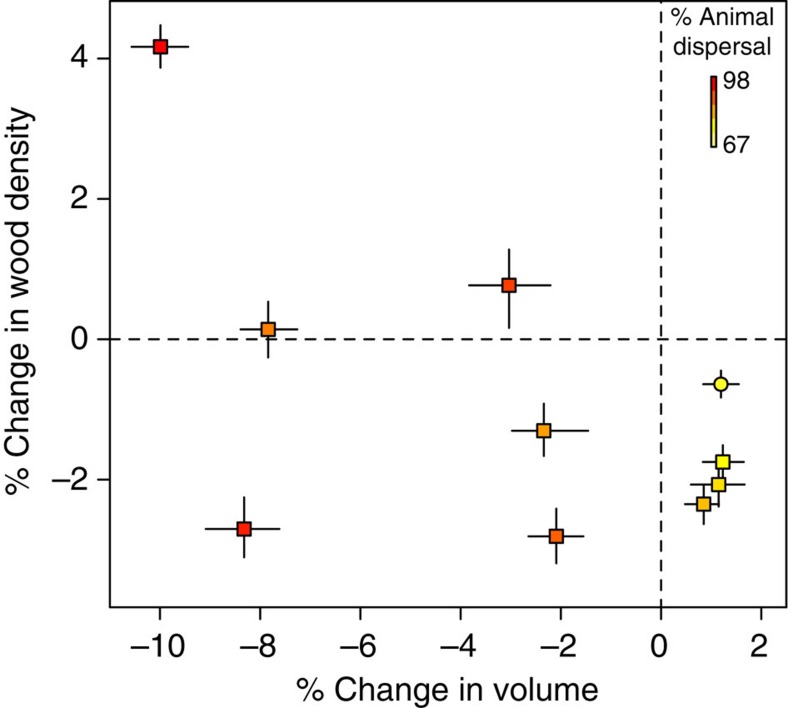
Theoretically, for a given basal area, changes in aboveground carbon storage can result from shifts in either wood density or stand volume, or both24. We related changes in carbon stocks to overall changes in stand wood densities and volumes by assessing the independent responses of their respective terms within the allometric equation used for biomass estimation (Methods section). Because our plot data sets only contain measurements of tree diameters and not heights, we estimated tree heights from diameters using a model derived from a large pan-tropical data set of tree measurements, with height increasing monotonically with diameter22. In our simulations, changes in carbon stocks following the loss of large-seeded species were strongly correlated to shifts in stand volumes (Pearson correlation Rp=0.84, P<0.01), but not stand-level wood densities (Rp=−0.04, P>0.90). Stand volumes decreased at sites dominated by animal-dispersed species (for example, Africa) while they increased at sites with relatively higher abundances of abiotically dispersed species (for example, Southeast Asia) (Fig. 2, Supplementary Fig. 3a, Supplementary Table 1). In contrast, wood density showed no consistent response at sites dominated by animal-dispersed species, but decreased at sites with relatively higher abundances of abiotically dispersed species (Fig. 2, Supplementary Fig. 3b, Supplementary Table 1).
Figure 2. Changes in stand volume and wood density following simulated extirpations of large-seeded animal-dispersed species.
Points represent medians of percentage change in stand volumes and community-weighted wood densities in response to simulated extirpation of all large-seeded animal-dispersed species. Corresponding inter-quantile ranges of change in volume and wood density across 1,000 simulation runs at each site are depicted using lines. Sites that show decreases and increases in carbon stocks are depicted with squares and circles, respectively. The colour gradient depicts variation in the relative abundances of animal-dispersed tree species ranging from yellow (low) to red (high).
Seed dispersal-woody trait relationships
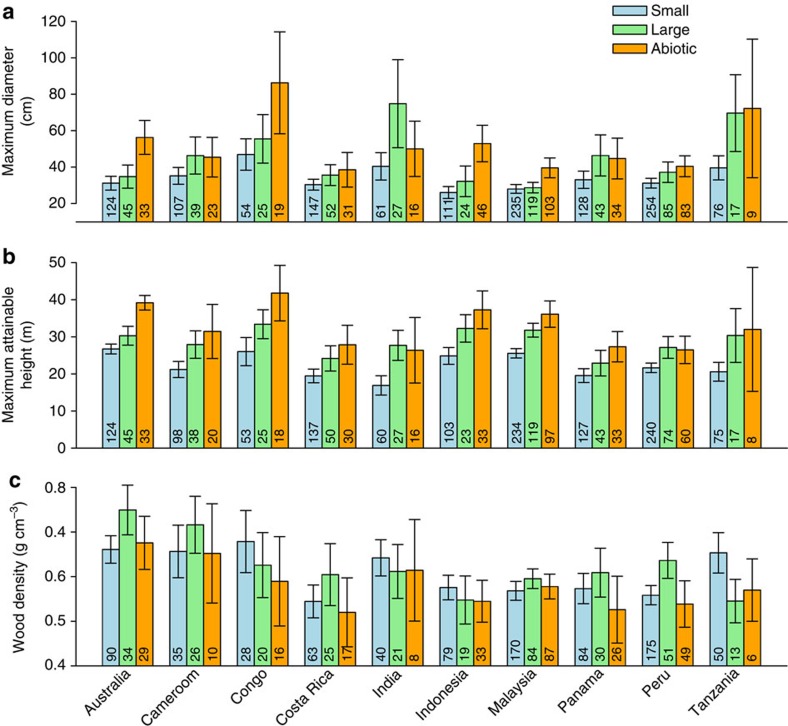
When basal area is constant, changes in stand volumes can result from shifts in tree size class distributions, because individuals with larger basal diameters, and correspondingly greater heights, contain more volume than an equal basal area made up of smaller individuals (Supplementary Fig. 4). In our simulations, losses of large-seeded species were accompanied by reductions in relative abundances of large trees (trees of basal diameter ≥70 cm (ref. 25)), and thus stand volumes, in communities dominated by animal-dispersed species, but not in communities with more abiotically dispersed species, or in the control scenarios (Supplementary Fig. 3a,c, Supplementary Table 1). These patterns are explained by consistent empirical relationships between species' seed dispersal traits and potential adult size, as indexed by maximum diameter and maximum attainable height, across ∼2,100 of the 2,500 species in our data set (Fig. 3a,b). Large-seeded animal-dispersed species in our plots had 24% greater maximum diameters on average than did small-seeded animal-dispersed species, but were 14% smaller on average than abiotically dispersed species across sites (Fig. 3a, Supplementary Table 3). We also found a very similar relationship between seed dispersal traits and maximum attainable height, collated from secondary sources (Methods section), wherein maximum attainable heights were 26% greater for large-seeded animal-dispersed species than for small-seeded animal-dispersed species, but 12% lower than for abiotically dispersed species (Fig. 3b, Supplementary Table 3). Extirpations of large-seeded species, particularly where abiotically dispersed species are rare, can therefore reduce the representation of species that attain relatively large adult sizes, and thus drive declines in stand volume and carbon stocks in these communities26. In regions where large, abiotically dispersed species are more abundant, such as the Dipterocarp forests of Southeast Asia27, increased abundances of these species following losses of large-seeded, animal-dispersed species can potentially maintain or even increase stand volumes and carbon storage15.
Figure 3. Relationships of potential adult size and wood density with seed size and dispersal mode.
Large-seeded animal- and abiotically dispersed tree species have greater maximum diameters (a) and maximum attainable heights (b) than small-seeded animal-dispersed species, consistently across sites. (c) Wood densities do not consistently differ with seed sizes of animal-dispersed species and are lowest among abiotically dispersed species (Supplementary Table 3). Bars represent means and error bars 95% confidence intervals. The numbers of species belonging to each category are reported within bars. Species' maximum diameters are obtained from the plot data set while maximum attainable height and wood density are collated from secondary sources (Methods section).
In contrast to potential adult size, wood density showed little variation across seed sizes of animal-dispersed species and was lowest among abiotically dispersed species in our data set (Fig. 3c, Supplementary Table 3). At a pan-tropical scale, extirpations of large-seeded animal-dispersed species are therefore unlikely to consistently reduce stand wood densities (Fig. 2, Supplementary Table 1). Thus, although declines in large-seeded animal-dispersed species may reduce carbon stocks by promoting species with lower wood densities in some tropical forests20,28, the importance of wood density as a factor driving defaunation effects on carbon storage across the tropical forest biome appears to be limited29.
Discussion
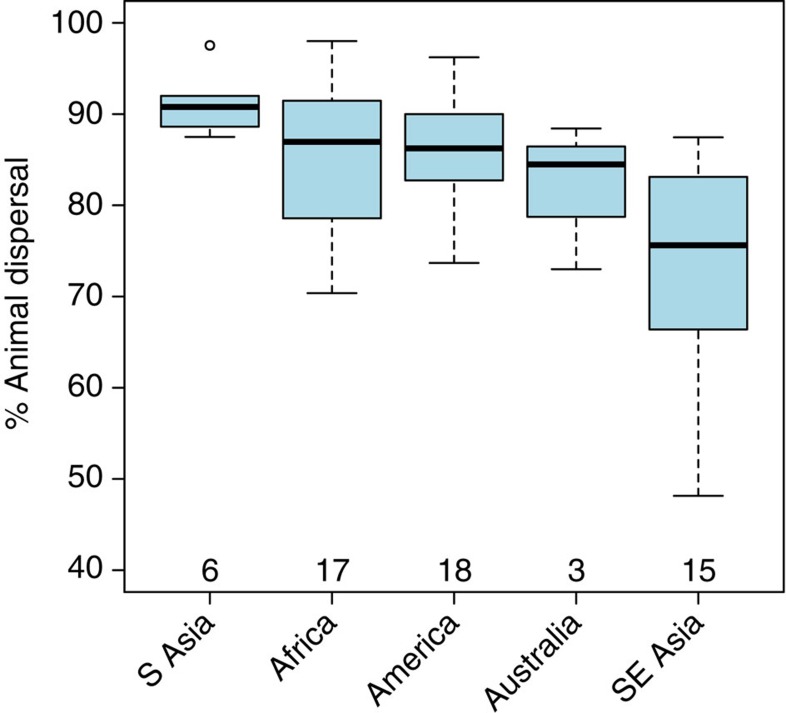
Presently, an estimated 88% of all tropical forests face the threat of defaunation through the combined effects of hunting, habitat fragmentation, selective logging and other forms of anthropogenic disturbance3, with animals that disperse large-seeded tree species amongst the most vulnerable30. Our results suggest that declines of these large-bodied dispersers can have contrasting effects on aboveground carbon storage across tropical forests, contingent on local species pools, relative abundances of animal-dispersed species in communities and the relationships between dispersal mode, seed size and adult size. Given that tropical forests of the Americas, Africa and South Asia, which together account for over 75% of all forests within the biome31, are primarily composed of animal-dispersed tree species (Fig. 4, Supplementary Fig. 5), our results suggest that defaunation could have marked negative effects on aboveground carbon storage in tropical forests overall. Although accurate extrapolation of our results to all tropical forests is not possible at this time, a rough estimate of the overall magnitude of likely carbon losses due to defaunation does provide some perspective. If tropical America and Africa lose 2.1% of their 124 Pg aboveground carbon stocks32, as predicted on average by our 50% extirpation scenarios (Supplementary Table 1), this would release ∼2.6 Pg of carbon, or 14 years' worth of Amazonian deforestation33, to the atmosphere. Such losses can be further exacerbated when defaunation additionally favours other carbon-poor woody life forms such as lianas and palms29,34,35.
Figure 4. Variation in relative abundances of animal-dispersed species across major tropical forest regions.
The box-and-whisker plots show that tropical forest tree communities in most regions have high-relative abundances of animal-dispersed species, with the exception of Southeast Asia where some communities comprise over 50% abiotically dispersed species. Numbers below boxes represent the number of sites from which data were obtained for each continent (see Supplementary Fig. 5 and Supplementary References 1–44 for more details).
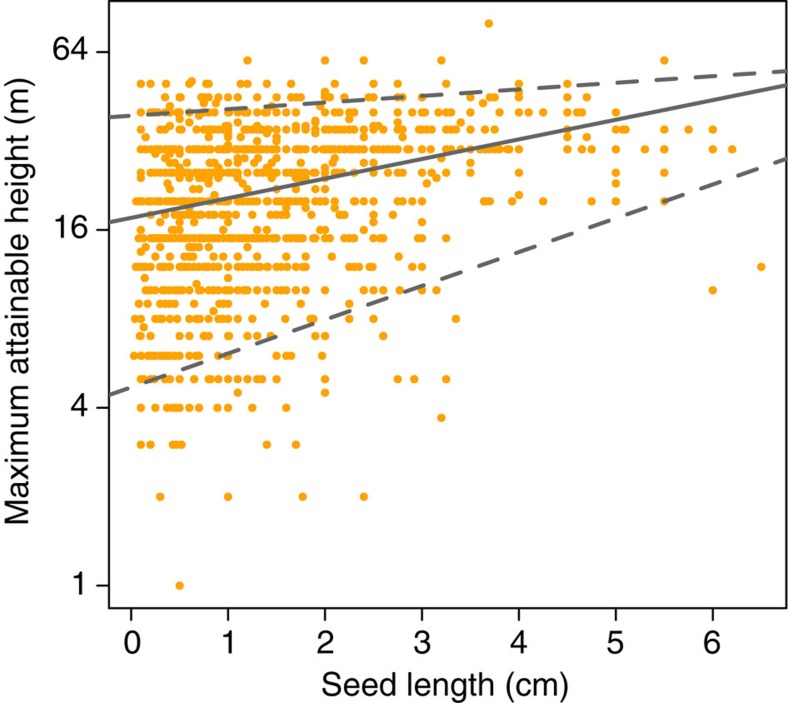
Ultimately, the magnitude of aboveground carbon losses will depend on the identity of tree species that replace the individuals lost in defaunated forests. For example, although small-seeded species are considerably shorter on average as adults than large-seeded ones (Fig. 3b), the tallest species of both groups have similar heights, resulting in the characteristic wedge shape of seed size-maximum height relationships (Fig. 5) (ref. 36). While our estimates are based on all trees having an equal probability of replacing large-seeded species, carbon losses could be mitigated if the taller among small-seeded species were to disproportionately benefit in defaunated forests. However, our understanding of such processes is currently limited by a paucity of empirical data on forest dynamics in defaunated forests. Changes in aboveground species compositions are also likely to affect carbon cycling and storage in leaf litter and soils37,38, but the effects of defaunation on these important components of the tropical forest carbon cycle are virtually unknown. Likewise, the time frame over which such changes may occur is unknown at this time, but is likely to be much longer than more immediate drivers of carbon losses such as deforestation or logging. More long-term empirical data on tree community dynamics and carbon budgets of defaunated forests, combined with temporally explicit models39, are urgently needed to better predict magnitudes of these changes and the time frames over which they may occur. Our results highlight that defaunation can have contrasting effects on carbon storage across the global tropics, contingent on the local species compositions of tree communities. Whereas previous discussions have focused on shifts in wood densities in some regions20,28, we illustrate that declines of large tree species and concomitant reductions in stand volumes may be the primary mode of defaunation-driven carbon losses in tropical forests at the global scale. Given the disproportionate contributions of large trees to aboveground carbon stocks25, conserving their seed dispersers across the tropics is likely to be important for climate regulation.
Figure 5. Seed size-maximum attainable height relationship.
The relationship of maximum attainable height (log scale) with seed length for animal-dispersed tree species across all 10 study sites, with fitted lines representing median (solid line) and 95 and 5% regression quantiles (dashed lines). The wedge shape of this point cloud is characteristic for seed size maximum-attainable height relationships36.
Methods
Vegetation plot data
Tree community data consisting of measurements of all trees ≥10 cm diameter at breast height (DBH) were obtained from 58 vegetation plots with a total sampled area of 55 ha from 10 sites spanning the humid tropics (Supplementary Table 4). Data were obtained from three sources: (a) 1 ha plot data available from the Tropical Ecology Assessment & Monitoring Network (TEAM) open data portal (http://www.teamnetwork.org/), (b) 1 ha plot data from an open access data set published by Ramesh et al.40 and (c) 0.5 ha plot data from an open access data set published by Bradford et al.41. For multi-temporal data sets containing repeated measures, only data from single surveys conducted during 2011–2013 were considered. Species names were checked and standardized in accordance with currently accepted taxonomies42. Tree DBH values were cross-checked against measurements from previous survey periods, when available, to detect and correct obvious errors. Entries for conifers (0.03% of all individuals recorded in plots) and woody vegetation other than trees, such as lianas (0.09%) and palms (5%), were excluded because the general allometric equations used for biomass estimation (see below) are not applicable to these groups. However, in the case of palms which are quite common in some tropical forests, we performed a comparison between simulations run with palms excluded and simulations with palms included, using the same biomass equation for palms as for trees43 and found that excluding palms had little influence on overall carbon responses (Supplementary Fig. 6). The data set comprised 26,512 trees, including 23,822 trees identified to the species level and 1,660 identified only to the genus level. The data set also comprised 1,030 trees that were not identified to the genus level, which were excluded from subsequent analyses.
Functional traits data
Data were collected on seed dispersal mode, seed length, wood density and maximum attainable height for the species in the vegetation plot data sets (key secondary data sources listed in Supplementary References 45–77). First, species were classified based on seed dispersal mode into four categories: (a) animal (vertebrates and invertebrates), (b) wind, (c) unassisted (explosive dehiscence, gravity and water) and (d) multiple (modes combining a with b and c). Groups a and d were then aggregated under the category of animal-dispersed species while groups b and c were grouped as abiotically dispersed species. Data on seed dispersal modes were primarily obtained from the Seed Information Database of Kew Gardens44, as well as floras and scientific studies focusing on seed dispersal in tropical forests. In the absence of species-level classifications, dispersal mode was assigned based on data from congeners or using resources which provide genus-level classifications45,46.
Species' seed sizes were estimated as the average length (cm) of the longest seed axis. This metric was selected to index seed sizes in this study because (a) it is the most widely reported measure of seed size, and (b) it shares generally consistent relationships with dimensions of other seed axes13,47. Comparison of 295 tropical tree species in our database showed that seed lengths were closely correlated with seed dry weights (Spearman correlation Rs=0.87, P<0.0001), another trait widely used to index seed size in studies of animal seed dispersal. Seed lengths were obtained from direct measurements, as well as from regional floras accessed through online databases, the Biodiversity Heritage Library (http://www.biodiversitylibrary.org/), journal articles and digital images of specimens. In case of images, seed lengths were estimated using the reference scales provided. Large-seeded animal-dispersed species were classified as those larger than the 75th percentile seed length amongst all animal-dispersed species within each community (Supplementary Data 1). This approach to defining large-seeded species was used because although size distributions of both seeds and their dispersers are known to vary considerably across tropical regions46, guideline values for defining large-seeded species are not available for all sites. However, where such values were available from other sites in the Americas (1.5 cm) (refs 48, 49), Africa (1.8 cm) (ref. 13) and the Orient (1.5–2.0 cm; refs 15, 50), we found them to be similar to the 75th percentile cutoffs used in this study. When species-level seed lengths were not available for defining large-seeded species, proxies derived from genus-level information were used to classify species for the simulations. These values were obtained from the following sources in decreasing order of preference:
Seed length as a function of fruit length: data on fruit lengths are generally reported alongside seed lengths in most flora resources, and are often available even when seed lengths are not reported. We developed genus-wise linear models of seed length as a function of fruit length, and observed a strong fit amongst models (mean R 2=0.92) based on an assessment of 982 species across 139 genera. Thereafter, in genera that had at least three species contributing to the model, these models were used to predict seed lengths for congeners which only had data on fruit lengths.
Genus-level seed length: genus-level seed lengths were obtained from Dennis et al. 45. Based on a comparison of 1,125 species across 325 genera, we observed that seed lengths at the species and genus levels are strongly positively correlated (R p=0.69, P<0.0001).
Wood density (g cm−3) data were obtained from primary sources and from the Global Wood Density Database51,52. When using secondary sources, only data that were collected in the same continent as the location of the target species were used, that is, for example, only records from Africa contributed to wood density estimates for African species, and so on. In cases where species-level wood densities were not available, average values across members of the same genus or family were assigned. These genus- and family-level estimates were used only in the simulations, and not for assessing relationships with other species traits.
Maximum attainable heights (m) were obtained from regional floras accessed through online databases and the Biodiversity Heritage Library (http://www.biodiversitylibrary.org/). Among species for which data on maximum height and maximum diameter were available from floras, there was a strong and positive correlation between these two indices of adult size (Rs=0.75, P<0.0001, N=813).
Carbon storage estimation
Carbon stored by individual trees was estimated using the following equation developed by Chave et al.22 for moist forests:
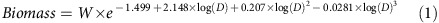 |
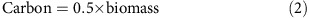 |
where W is wood density in g cm−3, and D is DBH in cm.
This equation was selected because (a) it is developed based on a large pan-tropical data set of tree measurements, and (b) height measurements are not required for carbon estimation (but height-diameter relationships are implicit), which is crucial because the plot data sets used here did not contain information on tree heights. Moreover, the structure of equation 1 permits ready deconstruction into its wood density (W, term 1) and volume (term 2 comprising the DBH-related terms within the exponent) components. Stand-level carbon stocks and volume estimates were obtained by summing over all individuals within each site, and dividing by the total sampled area for per-hectare estimates. Stand-level wood density was estimated as the average wood density across species, weighted by basal area25.
Although the allometric equation used here is known to overestimate aboveground biomass53, we make no between-site comparisons of aboveground biomass, and thus, do not expect our choice of equation to bias our results in any way.
Simulations
For the simulations, data from plots within sites were pooled to create single community data sets per site, ranging in sampled area from 3 to 6 ha (Supplementary Table 4). Shifts in tree community composition and aboveground carbon storage were simulated using a two-step procedure, comprising a removal, followed by a recovery step. In the removal step, a certain number of individuals (NRem) were removed from tree communities following a set of rules (see below), which resulted in a reduction of total basal area (BALoss), as well as incidental reductions in species richness (SLoss). In the recovery step, BALoss was recovered (with a target accuracy of ±1%) by repopulating the community with individuals (NRec) selected through a random draw, with replacement, from the remaining pool of individuals21. As the target was to recover lost basal area, and not tree densities, NRem and NRec could differ in value.
Three sets of scenarios were simulated for each community, namely (1) a defaunation scenario in which declines of large-seeded animal-dispersed species were simulated, and two control scenarios to distinguish defaunation effects from (2) simulation artefacts resulting from removing and replacing individuals, and (3) the effects of species loss per se.
In the defaunation scenario, declines of species dispersed by large animals were simulated by randomly removing individuals belonging to large-seeded animal-dispersed (>75th percentile seed length) tree species and repopulating the community with individuals drawn at random from the remaining pool, to recover original total basal areas. Four levels of defaunation-driven losses were simulated, with NRem equalling 25, 50, 75 and 100% of individuals belonging to large-seeded animal-dispersed species, respectively. One thousand iterations were simulated for each of the four levels at each site.
The first control scenario was simulated to distinguish the effects of defaunation on aboveground carbon stocks from numerical effects arising solely from the removal and replacement of individuals in the simulations. One control scenario was simulated for every iteration of the defaunation scenario, with NRem values matching those of the corresponding defaunation scenario, but with removal of individuals through a random draw from the overall pool, irrespective of seed dispersal category. Recovery of lost basal areas was simulated through random draws of individuals from the remaining community.
The second control scenario was simulated to control for the effects of species loss per se, which could accompany the removal of individuals in the defaunation scenarios, and is bound to occur in the 100% removal scenario. One control scenario was simulated for every iteration of the defaunation scenario, with SLoss values matching those of corresponding defaunation scenarios, but with removal of species through a random draw from the overall pool. Recovery of lost basal area was simulated through random draw of individuals from the remaining community.
The R code used to run the simulations has been uploaded to a GitHub repository and is available here: https://github.com/aosuri/defaunation_carbon_project.
Analysis
Before analysis, each response variable was recalculated as the percentage change from the corresponding value for the original community. The distributions of percentage change in carbon stocks, stand volumes, basal area-weighted wood densities and relative abundances of large trees (≥70 cm DBH, following Slik et al.25) in the defaunation and control scenario simulations are graphically represented using boxplots. We considered cases where the entire inter-quantile range of a response in any given scenario does not overlap with zero to indicate consistent effects of species/individual extirpations. The percentage of simulation runs in each scenario that showed declines in aboveground carbon stocks was also recorded. Defaunation effects were estimated as the difference in median percentage change between the defaunation and control scenarios for each response variable. We present these effect sizes without conducting statistical significance tests to estimate P values, as recommended by recent papers on the inappropriateness of significance testing in simulation studies54,55.
The R statistical environment version 3.0.2 (ref. 56) was used for running simulations, analyses and preparation of figures.
Additional information
How to cite this article: Osuri, A. M. et al. Contrasting effects of defaunation on aboveground carbon storage across the global tropics. Nat. Commun. 7:11351 doi: 10.1038/ncomms11351 (2016).
Supplementary Material
Supplementary Figures 1-6, Supplementary Tables 1-4 and Supplementary References
Species details table, including data on site, family, species name in database, accepted species name (according to http://www.theplantlist.org/) and seed dispersal category assigned. Seed dispersal categories include large-seeded animal-dispersed (L), small-seeded animaldispersed (S) and abiotically-dispersed (A)
Acknowledgments
This study includes data obtained from the Tropical Ecology Assessment and Monitoring (TEAM) open data Network and open data sets published by Commonwealth Scientific and Industrial Research Organisation (CSIRO) and Institut Français de Pondichéry (IFP). TEAM is a collaboration between Conservation International, the Smithsonian Institution and the Wildlife Conservation Society, and partially funded by these institutions, the Gordon and Betty Moore Foundation and other donors. We thank Jorge Ahumada, Dan Metcalfe, Andrew Ford, Helen Murphy, Moses Sainge, Peter Mambo, George Chuyong, Duncan Thomas, Richard Condit, Rolando Perez, Salomón Aguilar, Meyner Nusalawo, William Marthy, Patrick Boundja, Sarah Yoga, Pebou Stevy, Poka Tecle, Emanuel H. Martin, Simon Lewis, Jon Lovett, Pantaleo Munishi, John Terborgh, Fernando Cornejo, M.H. Swaminath, Santoshgouda V. Patil, Dasappa, Raphaël Pélissier, P. Dilip Venugopal, S. Aravajy, Claire Elouard, S. Ramalingam and all field technicians for helping establish and maintain the tree plots used in this study. We acknowledge CSIRO, Terrestrial Ecosystem Research Network (TERN), University of Buea, WWF-Cameroon, Ministry of Forest and Wildlife, Cameroon, Smithsonian Tropical Research Institute, Ministry of Environment and Forestry—Republic of Indonesia, Bukit Barisan Selatan National Park, Wildlife Conservation Society-Indonesia Program, Forest Research Institute Malaysia, Organization for Tropical Studies, La Selva Biological Station, Wildlife Conservation Society, Leverhulme Trust (c/o Valuing the Arc project), MUSE—Science Museum of Trento (Italy), Tanzania National Parks, SERNANP, Servicio Nacional de Areas Protegidas del Peru, Department of Science and Technology, Government of India (SERB/SR/SO/PS/78/2012), Rufford Small Grants for Nature Conservation, National Centre for Biological Sciences, Nature Conservation Foundation and Karnataka Forest Department for funding, institutional support and research permits. We thank William Bond, T.R. Shankar Raman and Hari Sridhar for comments on the manuscript. A.M.O. thanks James Maccarthy, Patrick Boundja and William Marthy for advising on and facilitating use of TEAM data. A.M.O. was supported by a fellowship from National Centre for Biological Sciences.
Footnotes
Author contributions A.M.O., J.R., V.V. and M.S. developed ideas, designed the study and led preparation of the manuscript. A.M.O. programmed the simulations with inputs from V.V.; A.M.O. collated species traits data with inputs from P.A.-L. and M.B.; P.A.-L., J.H.A., M.B., C.F., M.N.-H., P.A.J., D.K., A.R.M., B.R.R. and F.R. contributed tree plot data and provided feedback on drafts of the manuscript.
References
- Dirzo R. et al. Defaunation in the anthropocene. Science 345, 401–406 (2014). [DOI] [PubMed] [Google Scholar]
- Galetti M. & Dirzo R. Ecological and evolutionary consequences of living in a defaunated world. Biol. Conserv. 163, 1–6 (2013). [Google Scholar]
- Malhi Y., Gardner T. A., Goldsmith G. R., Silman M. R. & Zelazowski P. Tropical forests in the Anthropocene. Annu. Rev. Environ. Resour. 39, 125–159 (2014). [Google Scholar]
- Laurance W. F. et al. Averting biodiversity collapse in tropical forest protected areas. Nature 489, 290–294 (2012). [DOI] [PubMed] [Google Scholar]
- Harrison R. D. Emptying the forest: hunting and the extirpation of wildlife from tropical nature reserves. Bioscience 61, 919–924 (2011). [Google Scholar]
- Abernethy K. A., Coad L., Taylor G., Lee M. E. & Maisels F. Extent and ecological consequences of hunting in Central African rainforests in the twenty-first century. Philos. Trans. R. Soc. London B Biol. Sci. 368, 20120303 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. J. et al. The plight of large animals in tropical forests and the consequences for plant regeneration. Biotropica 39, 289–291 (2007). [Google Scholar]
- Howe H. F. & Smallwood J. Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 13, 201–228 (1982). [Google Scholar]
- da Silva J. M. C. & Tabarelli M. Tree species impoverishment and the future flora of the Atlantic forest of northeast Brazil. Nature 404, 72–74 (2000). [DOI] [PubMed] [Google Scholar]
- Terborgh J. Using Janzen–Connell to predict the consequences of defaunation and other disturbances of tropical forests. Biol. Conserv. 163, 7–12 (2013). [Google Scholar]
- Wright S. J. The myriad consequences of hunting for vertebrates and plants in tropical forests. Perspect. Plant Ecol. Evol. Syst. 6, 73–86 (2003). [Google Scholar]
- Terborgh J. et al. Tree recruitment in an empty forest. Ecology 89, 1757–1768 (2008). [DOI] [PubMed] [Google Scholar]
- Vanthomme H., Bellé B. & Forget P. Bushmeat hunting alters recruitment of large-seeded plant species in central Africa. Biotropica 42, 672–679 (2010). [Google Scholar]
- Effiom E. O., Nuñez-Iturri G., Smith H. G., Ottosson U. & Olsson O. Bushmeat hunting changes regeneration of African rainforests. Proc. R. Soc. B Biol. Sci. 280, 20130246 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. D. et al. Consequences of defaunation for a tropical tree community. Ecol. Lett. 16, 687–694 (2013). [DOI] [PubMed] [Google Scholar]
- Nuñez-Iturri G. & Howe H. F. Bushmeat and the fate of trees with seeds dispersed by large primates in a lowland rain forest in western Amazonia. Biotropica 39, 348–354 (2007). [Google Scholar]
- Malhi Y., Baldocchi D. D. & Jarvis P. G. The carbon balance of tropical, temperate and boreal forests. Plant Cell Environ. 22, 715–740 (1999). [Google Scholar]
- Saatchi S. S. et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl Acad. Sci. 108, 9899–9904 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello C. et al. Defaunation affects carbon storage in tropical forests. Sci. Adv. 1, e1501105 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres C. A., Emilio T., Schietti J., Desmoulière S. J. M. & Levi T. Dispersal limitation induces long-term biomass collapse in overhunted Amazonian forests. Proc. Natl Acad. Sci. 113, 892–897 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker D. E. et al. Species loss and aboveground carbon storage in a tropical forest. Science 310, 1029–1031 (2005). [DOI] [PubMed] [Google Scholar]
- Chave J. et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145, 87–99 (2005). [DOI] [PubMed] [Google Scholar]
- Poulsen J. R., Clark C. J. & Palmer T. M. Ecological erosion of an Afrotropical forest and potential consequences for tree recruitment and forest biomass. Biol. Conserv. 163, 122–130 (2013). [Google Scholar]
- Stegen J. C., Swenson N. G., Valencia R., Enquist B. J. & Thompson J. Above-ground forest biomass is not consistently related to wood density in tropical forests. Glob. Ecol. Biogeogr. 18, 617–625 (2009). [Google Scholar]
- Slik J. W. et al. Large trees drive forest aboveground biomass variation in moist lowland forests across the tropics. Glob. Ecol. Biogeogr. 22, 1261–1271 (2013). [Google Scholar]
- Osuri A. M. & Sankaran M. Seed size predicts community composition and carbon storage potential of tree communities in rainforest fragments in India's Western Ghats. J. Appl. Ecol doi:10.1111/1365-2664.12626 (2016). [Google Scholar]
- Slik J. W. F. et al. Environmental correlates of tree biomass, basal area, wood specific gravity and stem density gradients in Borneo's tropical forests. Glob. Ecol. Biogeogr. 19, 50–60 (2010). [Google Scholar]
- Brodie J. F. & Gibbs H. K. Bushmeat hunting as climate threat. Science 326, 364–365 (2009). [DOI] [PubMed] [Google Scholar]
- Jansen P. A., Muller-Landau H. C. & Wright S. J. Bushmeat hunting and climate: an indirect link. Science 327, 30 (2010). [DOI] [PubMed] [Google Scholar]
- McConkey K. R. et al. Seed dispersal in changing landscapes. Biol. Conserv. 146, 1–13 (2012). [Google Scholar]
- Achard F. et al. Determination of deforestation rates of the world's humid tropical forests. Science 297, 999–1002 (2002). [DOI] [PubMed] [Google Scholar]
- Baccini A. et al. Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nat. Clim. Change 2, 182–185 (2012). [Google Scholar]
- Song X.-P., Huang C., Saatchi S. S., Hansen M. C. & Townshend J. R. Annual carbon emissions from deforestation in the Amazon Basin between 2000 and 2010. PLoS ONE 10, e0126754 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. J., Hernandéz A. & Condit R. The bushmeat harvest alters seedling banks by favoring lianas, large seeds, and seeds dispersed by bats, birds and wind. Biotropica 39, 363–371 (2007). [Google Scholar]
- van der Heijden G. M. F., Powers J. S. & Schnitzer S. A. Lianas reduce carbon accumulation and storage in tropical forests. Proc. Natl Acad. Sci. 112, 13267–13271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb P. J., Coomes D. A. & Metcalfe D. J. Comment on‘ a brief history of seed size'. Science 310, 783 (2005). [DOI] [PubMed] [Google Scholar]
- De Deyn G. B., Cornelissen J. H. C. & Bardgett R. D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 11, 516–531 (2008). [DOI] [PubMed] [Google Scholar]
- Cornwell W. K. et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 11, 1065–1071 (2008). [DOI] [PubMed] [Google Scholar]
- Caughlin T. T. et al. Loss of animal seed dispersal increases extinction risk in a tropical tree species due to pervasive negative density dependence across life stages. Proc. R. Soc. London B Biol. Sci. 282, 20142095 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh B. R. et al. Forest stand structure and composition in 96 sites along environmental gradients in the central Western Ghats of India: Ecological Archives E091-216. Ecology 91, 3118 (2010). [Google Scholar]
- Bradford M. G., Murphy H. T., Ford A. J., Hogan D. L. & Metcalfe D. J. Long-term stem inventory data from tropical rain forest plots in Australia: Ecological Archives E095-209. Ecology 95, 2362-000 (2014). [Google Scholar]
- The Plant List Version 1.1. The Plant List. Available at http://www.theplantlist.org/ (2013).
- Baker T. R. et al. Variation in wood density determines spatial patterns in Amazonian forest biomass. Glob. Change Biol. 10, 545–562 (2004). [Google Scholar]
- Royal Botanical Gardens Kew. Seed information database (SID). Version 7.1 http://data.kew.org/sid/ (2014).
- Dennis A. J., Schupp E. W., Green R. J. & Wescott D. A. Seed Dispersal: Theory And Its Application In A Changing World CABI (2007). [Google Scholar]
- Snow D. W. Tropical frugivorous birds and their food plants: a world survey. Biotropica 13, 1–14 (1981). [Google Scholar]
- Forget P. M. et al. in Seed Dispersal: Theory And Applications In A Changing World (eds Dennis, A.J. et al.) Ch.1 CABI (2007). [Google Scholar]
- Tabarelli M. & Peres C. A. Abiotic and vertebrate seed dispersal in the Brazilian Atlantic forest: implications for forest regeneration. Biol. Conserv. 106, 165–176 (2002). [Google Scholar]
- Santos B. A. et al. Drastic erosion in functional attributes of tree assemblages in Atlantic forest fragments of northeastern Brazil. Biol. Conserv. 141, 249–260 (2008). [Google Scholar]
- Kitamura S. et al. A botanical inventory of a tropical seasonal forest in Khao Yai National Park, Thailand: implications for fruit–frugivore interactions. Biodivers. Conserv. 14, 1241–1262 (2005). [Google Scholar]
- Zanne A. E. et al. Global wood density database. Dryad. Identifier: http://hdl. handle. net/10255/dryad (2009).
- Chave J. et al. Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366 (2009). [DOI] [PubMed] [Google Scholar]
- Feldpausch T. R. et al. Tree height integrated into pantropical forest biomass estimates. Biogeosciences 9, 3381–3403 (2012). [Google Scholar]
- White J. W., Rassweiler A., Samhouri J. F., Stier A. C. & White C. Ecologists should not use statistical significance tests to interpret simulation model results. Oikos 123, 385–388 (2014). [Google Scholar]
- Troitzsch K. G. Interdisciplinary Applications of Agent-based Social Simulation and ModelIng 88–105IGI Global (2014). [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. http://www. R-project. org (2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-6, Supplementary Tables 1-4 and Supplementary References
Species details table, including data on site, family, species name in database, accepted species name (according to http://www.theplantlist.org/) and seed dispersal category assigned. Seed dispersal categories include large-seeded animal-dispersed (L), small-seeded animaldispersed (S) and abiotically-dispersed (A)