Abstract
Evolutionary adaptation could assist organisms to cope with environmental changes, yet few experimental systems allow us to directly track evolutionary trajectory. Using experimental evolution, evolutionary tolerance to Microcystis aeruginosa was investigated in two cladocerans (Daphnia pulex and Simocephalus vetulus) to test the hypothesis that cladoceran grazers rapidly adapt to toxic cyanobacteria. After exposure for either three or six months, both grazers evolved a higher tolerance. The intrinsic rate of population increases in S. vetulus feeding on cyanobacteria was negatively correlated with that on green algae, which suggests that evolutionary adaptation in tolerance would carry a cost in the absence of cyanobacteria. However, the cyanobacterial selection resulted in a general increase in D. pulex when fed both cyanobacteria and green algae. Following a three-month relaxation of selection, S. vetulus in the selection line exhibited reverse evolution back to their original state when their diets were switched back to pure green algae. The present experimental evolution, both forwards and reverse, not only demonstrates the evolutionary responses of cladoceran grazers to toxic cyanobacterial cells in the laboratory, but also indicates that the grazer-cyanobacteria interaction would be an effective system to empirically study rapid evolution to environmental changes.
Environmental changes have shifted the growth/reproduction times of organisms, altered the geographic distributions of populations, changed the composition of communities, and shaped the nature of species interactions1,2. The magnitude and rate of some environmental changes are likely to override the potential responses of many organisms, leaving them susceptible to decline and extinction3. Extinction risk of some organisms can be reduced if they migrate to suitable habitats4, develop appreciate phenotypic plasticity5, or evolve adaptations to counter stressful conditions6.
A wave of recent studies have highlighted that adaptive phenotypic evolution can be rapid across a wide range of taxa7. These exciting results imply that adaptive evolution could assist organisms to cope with environmental changes to a greater extent than previously thought8. In some unconnected habitats, such as isolated lakes or ponds, adaptive evolution might be the only means for aquatic organisms to avoid extinction2. The need for evolutionary changes might be more urgent for aquatic organisms with limited swimming abilities, such as zooplankton, compared with nekton9. However, with few exception, adaptive evolution has rarely been considered when predicting biological responses to environmental changes and developing management strategies8.
In recent decades, eutrophication and climate changes have caused a rise in toxin-producing cyanobacterial harmful algal blooms (CyanoHABs) in freshwater and coastal systems worldwide10. Furthermore, increasing CyanoHABs are likely to accumulate more reactive oxygen species in calm surface waters and may form a positive-feedback loop favoring toxin-producing cyanobacteria11. CyanoHABs produce and release a wide variety of toxins or secondary compounds12, which can have harmful effects on aquatic grazers, such as impaired feeding, physiological dysfunction, depressed reproduction, and increased mortality13. Thus, CyanoHABs are likely to pose new selection pressures on aquatic grazers.
There is some evidence for spatial variation in genetic adaptation to toxic cyanobacteria. Somatic juvenile growth rates of Daphnia pulicaria from high-nutrient lakes are less inhibited by toxic cyanobacteria than those from low-nutrient lakes14. Tolerance to single-celled M. aeruginosa increases with increasing prevalence of CyanoHABs in large-bodied Daphnia pulex and small-bodied Chydorus sphaericus on a microgeographic level15. Spatial studies involving common garden experiments identify the direction of adaptive evolution and the pattern of local adaptation, but may not indicate the speed at which adaptation occurs.
Further, a few studies have explored evolutionary adaptation to toxic cyanobacteria on a temporal scale. By hatching resting eggs deposited over several decades, Hairston et al.16 showed that the tolerance of Daphnia galeata to toxic cyanobacteria increased significantly with eutrophication in Lake Constance. However, the direction of selection may not be very clear in such reconstructed populations because grazer performance integrates the adaptive evolution to toxic cyanobacteria during blooms, the reversal during non-blooms, and other selection forces, such as predation, competition and so on. Experimental evolution is a powerful approach for monitoring evolutionary processes occurring in experimental populations in simulated environments17. Gustafsson and Hansson18 reported that the ability of D. magna to deal with toxic cyanobacteria was improved after a four-week exposure to Microcystis. In the present study, we seek to extend this observation by asking the following questions: (1) Can cladoceran grazers rapidly adapt to toxic Microcystis within the time span of a typical bloom? (2) If so, is there a cost or trade-off for evolved tolerance? (3) Do they return to ancestral status during a non-bloom period?
Results
Rapid evolution of tolerance
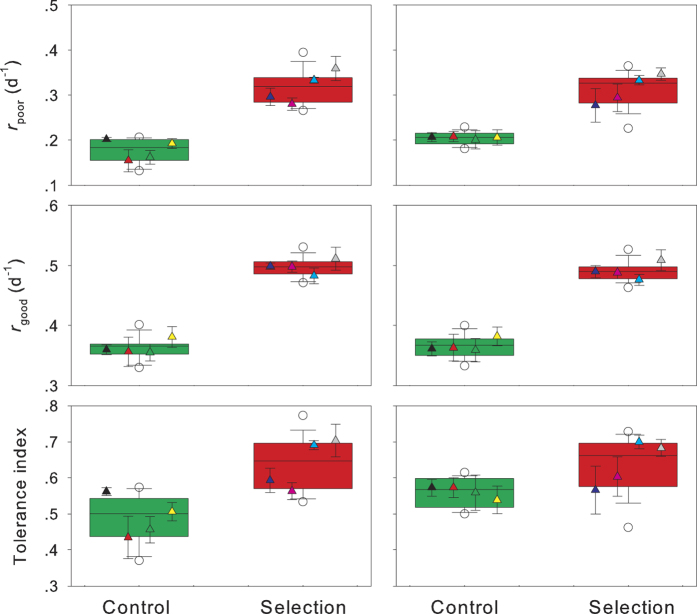
Although both the poor (50% Microcystis aeruginosa + 50% Chlorella pyrenoidosa) and good (100% C. pyrenoidosa) food supported positive growth of the large-bodied cladoceran D. pulex, the presence of toxic cyanobacteria in diet depressed their intrinsic rates of population increase (Fig. 1). After three months of exposure to toxic cyanobacteria, D. pulex in the selection line exhibited significantly higher rpoor, rgood, and tolerance index than their conspecifics in the control line (Fig. 1, two-way nest ANOVA, P < 0.05 for all). A similar pattern was observed when D. pulex were exposed to M. aeruginosa for six months (Fig. 1).
Figure 1. Intrinsic rate of population increase of Daphnia pulex feeding on poor (rpoor) or good (rgood) food, and their tolerance index (rpoor/rgood) to cyanobacteria when they were exposed to Microcystis aeruginosa for three (left panel) and six (right panel) months.
The selection experiment had two lines: the control (green boxplot) and selection (red boxplot), with four replicated tanks in each line. Box plots indicate the median, the 25th and 75th percentiles, with error bars for the 10th and 90th percentiles in grazer performance in an experimental line. Grazer performance in a tank was showed as mean ± s.d. for four replicates of 20 neonates.
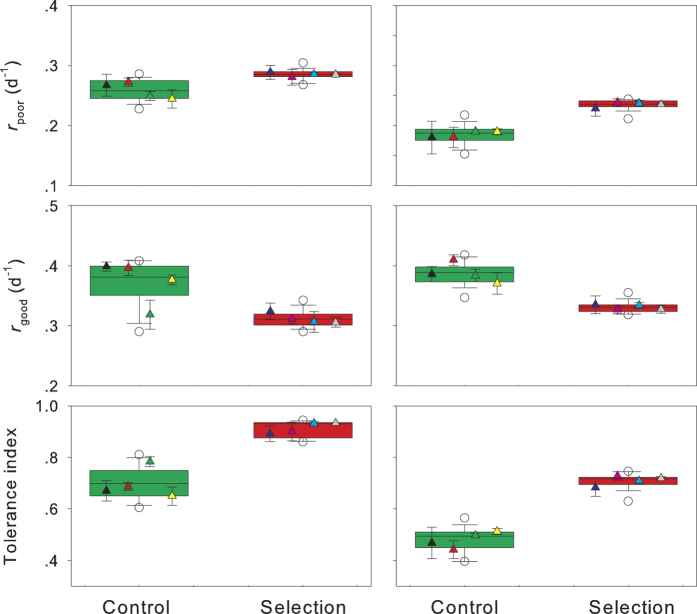
The medium-bodied cladoceran S. vetulus fed poor food also exhibited the lower intrinsic rates of population increase than the same set of populations fed good food (Fig. 2). After a three-month selection period for tolerance to toxic cyanobacteria, S. vetulus in the selection line had significantly higher rpoor and tolerance index (two-way nest ANOVA, P < 0.01 for both), but lower rgood (P = 0.02), than those in the control line (Fig. 2). When the selection time was extended from three to six months, S. vetulus exhibited a similar difference between the selection and the control lines (Fig. 2).
Figure 2. Intrinsic rate of population increase of Simocephalus vetulus feeding on poor (rpoor) or good (rgood) food, and their tolerance index (rpoor/rgood) to cyanobacteria when they were exposed to Microcystis aeruginosa for three (left panel) and six (right panel) months.
Experimental design and figure items as Fig. 1.
Cost and reversibility of evolved tolerance
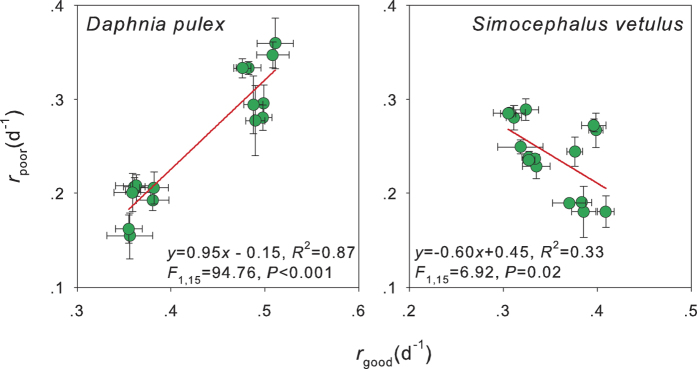
In the evolution of tolerance to toxic cyanobacteria, rpoor was positively correlated with rgood in D. pulex (Fig. 3). In contrast, rpoor was negatively correlated with rgood in S. vetulus (Fig. 3). The exposure to M. aeruginosa was halted when S. vetulus evolved higher tolerance in the selection line. After feeding on pure C. pyrenoidosa for three months, the differences in rpoor, rgood, and tolerance index were not significant between the selection and control lines (Fig. 4, two-way nest ANOVA, P > 0.05 for all).
Figure 3. Intrinsic rate of population increases of two grazers when fed poor food versus that of individuals fed good food.
Each data point (mean ± s.d.) represents the grazer performance in a tank either before or after the experimental evolution.
Figure 4. Intrinsic rate of population increase of Simocephalus vetulus feeding on poor (rpoor) or good (rgood) food, and their tolerance index (rpoor/rgood) to cyanobacteria when grazers obtained from the selection experiment (Fig. 2) were exposed to Chlorella pyrenoidosa for three months.
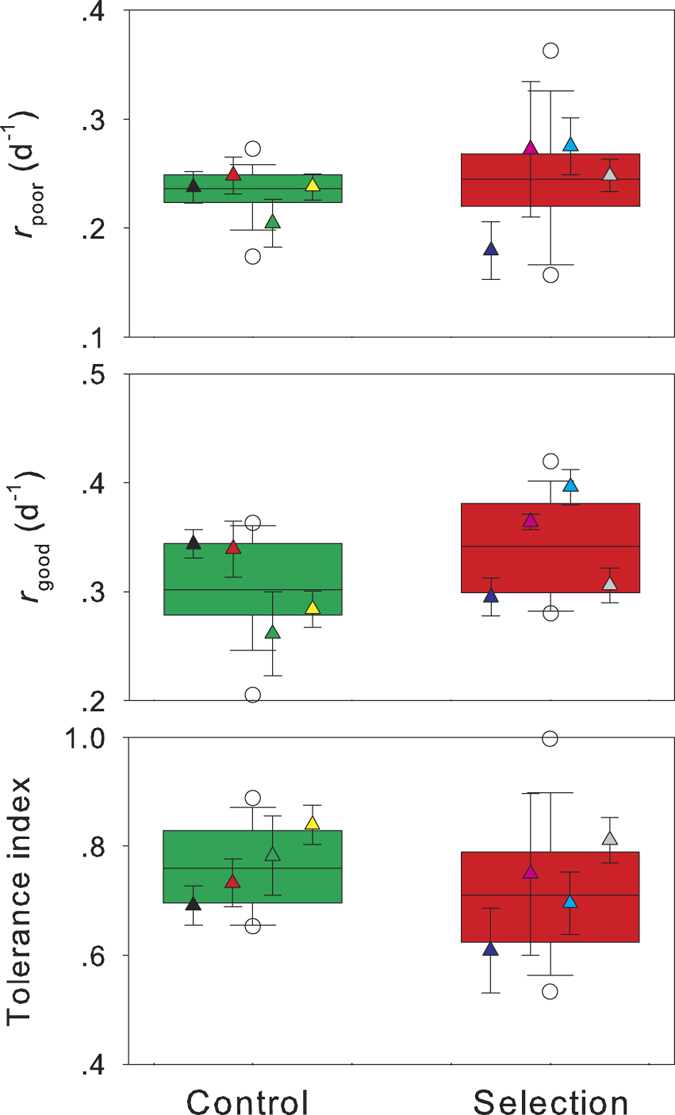
Figure items as Fig. 1.
Discussion
The present results of experimental evolution are consistent with previous reports on the adaptive evolution of tolerance to toxic cyanobacteria from the viewpoints of local adaptation14,15 and paleolimnological records16. Furthermore, our results clearly demonstrated that this evolutionary process occurred within a short time span may be a general response to toxic cyanobacteria for cladoceran grazers via extending test animal from Daphnia18 to other species. After three months, both Daphnia pulex and Simocephalus vetulus developed a higher tolerance to Microcystis aeruginosa (30% increase). Most individuals of both grazer species mature and produce offspring on day 5 in our system (25 °C and 400 μg C L−1)19. Thus, grazer populations are expected to develop approximately 18 generations under the present experimental conditions. Such a rapid evolution of tolerance in grazers may occur during CyanoHABs in nature16. Selection intensity of toxic cyanobacteria in nature would be more intense due to their higher concentrations. Typical cell densities of cyanobacteria usually range from 105 to 106 cells mL−1 during blooms20, which greatly exceeds the level in the present selection line (3.4 × 104 cells mL−1). The strain of M. aeruginosa used in the present study produces microcystins with 24 fg cell−1, which translated to a microcystin level of 0.7 μg L−1 in the selection line15. Such a microcystin level is usually lower than field values during CyanoHABs21. However, Microcystis usually form colonies to reduce feeding activities by zooplankton grazers13. High cell densities and long persistence of cyanobacterial blooms may not certainly imply high selective pressure on zooplankton grazers. On the other hand, field populations of aquatic grazers usually harbor large genetic variation for tolerance to toxic cyanobacteria, which serves as the basis for rapid evolution. Large clonal variation in tolerance to toxic M. aeruginosa has been observed in both D. pulex19 and S. vetulus22, even within a population. In addition, field populations of grazers are subject to strong gene flow, which may homogenize adaptive evolution9. Given the variations in selective force and genetic structure, one must take care in extrapolating rapid evolution in laboratory studies to field populations. Further studies that use field populations of grazers and cyanobacteria in laboratory experiments or directly track evolutionary trajectory in nature are necessary and would provide stronger evidence for rapid evolution of zooplankton tolerance to CyanoHABs.
The ultimate cause of rapid evolution of tolerance to toxic cyanobacteria in two grazers is not clear. Evolution with environmental changes in organisms usually depends on pre-existing genetic variation, de novo mutation for new alleles, or immigration of adaptive individuals23. The present experimental design did not consider grazer dispersal, which clearly ruled out the possibility of immigration of adaptive individuals. De novo mutations for new alleles is also not likely to account for the rapid evolution of tolerance in both grazers, not only because the experimental time (three months or 18 generations) is usually too short for mutation, but also because the increased tolerance occurred in all four independent tanks in the selection line rather than in a random one. Rapid evolution of tolerance in both grazers within three months may stem from selection on pre-existing genetic variation. Toxic cyanobacteria may select tolerant genotypes and change the gene frequency of grazers, which would enhance grazer tolerance to toxic cyanobacteria at the population level. Interestingly, we did not observe more divergence between the selection and control lines when the selection experiment was extended from three months to six months. The moderate divergence in tolerance may reflect the limited genetic variation for tolerance and relatively weak selection pressure in our experimental systems since evolutionary rate is expected to be related to the amount of genetic variation in populations and the strength of selection force24. Each experimental tank received an initial inoculum of approximately 200 individuals. Furthermore, these individuals were exposed to a relative low level of toxic cyanobacteria (3.4 × 104 cells mL−1). Compared with wild populations, the limited population size and the relatively low concentration of cyanobacteria might serve as constraints on the evolution of greater tolerance. The genetic analyses on experimental populations exposed to toxic cyanobacteria may provide more rigorous understandings for processes and mechanisms of increased tolerance in cladoceran grazers. The further studies on zooplankton abundance, fitness, genetic structure, and their correlations with the development of cyanobacterial blooms would decipher zooplankton responses into physiological or evolutionary adaptations, determine the magnitude and rate of adaptive evolution to increasing cyanobacteria in nature, and provide new insights on zooplankton population dynamics and their grazing control on cyanobacterial blooms. Regardless of the precise mechanisms, increased tolerance to toxic cyanobacteria via rapid evolution may buffer mortality risk and enhance grazing pressure in grazers, and consequently affect the dynamics of CyanoHABs.
All adaptations that enhance fitness essentially carry costs to organisms because additional resources are invested to activate necessary gene expression and biochemical processes5. These costs, however, have not been well documented. Better estimations of benefits and costs of evolutionary tolerance in terms of individual performance and especially fitness will be critical for any serious attempt to predict evolutionary trajectory of this trait6. The genotypes of a marine copepod with greater tolerance to a toxic dinoflagellate carry a substantial reproductive cost when feeding on nutritious algae, which would prevent the fixation of tolerant alleles in natural populations25. Rapid evolution of tolerance enhanced the intrinsic rates of population increase of S. vetulus in the presence of cyanobacteria, but depressed them in the absence of cyanobacteria, when compared with individuals in the control line. This fitness cost in the absence of cyanobacteria would serve as a constraint on the evolution of tolerance. The evolved tolerance to toxic M. aeruginosa in S. vetulus was completely lost following a three-month relaxation of selection.
Interestingly, the intrinsic rate of population increases of D. pulex on poor food was positively correlated with that on good food. Exposure to toxic M. aeruginosa resulted in a general increase in grazer performance on both poor and good food, rather than a trade-off between two conditions. This result is consistent with the previous finding that the juvenile growth rate of D. galeata feeding on toxic cyanobacteria is positively correlated with that on nutritious green algae26. Aquatic grazers with high growth rates usually have large adult body size, making them more vulnerable to predation27. Because actual growth is depressed in all genotypes during CyanoHABs, the combined selection by toxic cyanobacteria and fish predation in turn would favor the dominance of fast-growing genotypes of daphnids26. Compared with the large bodied D. pulex (2.17 ± 0.12 mm), the medium-bodied S. vetulus (1.33 ± 0.03 mm) is less vulnerable to predation, either by a fish predator Gambusia affinis (51.0 ± 6.6 vs. 26.9 ± 4.6 prey ind.−1 h−1, authors’ unpublished data) or by a coelenterate predator Hydra oligactis28, due to its relatively small size and less movement. The difference in vulnerability to predation in these two grazers may make them suffer different selection pressures in nature and contribute to their differences in the evolutionary trajectory of life history traits. Most studies on zooplankton responses to cyanobacteria have focused on a few Daphnia species, ignoring the high diversity in zooplankton13. The similar, but not same, evolutionary responses in two cladocerans suggest that interspecific variation should be incorporated when predicting adaptive evolution to toxic cyanobacteria in grazers.
Aquatic grazers are faced with ever-changing CyanoHABs. A particularly interesting question is whether evolved tolerance in grazers would be reversed when CyanoHABs disappear. Reverse evolution is defined as the reacquisition by derived populations of the same character states as those of ancestor populations, which is neither inevitable nor impossible29. S. vetulus in the selection line, which evolved high tolerance after six months of exposure to M. aeruginosa, exhibited reverse evolution back to their original state when their diets were switched back to pure green algae. Similar results have been obtained in experimental studies of evolutionary tolerance to toxic dinoflagellate in a marine copepod30. Some ecological variables, such as water temperature, predators, and competitors, usually vary when CyanoHABs disappear with season12. Aquatic grazers may face not only the relaxation of cyanobacterial selection, but also emerging selective forces. Testing the reversal evolution under field conditions is expected to bring more insights into evolutionary processes of aquatic grazers.
Phenotypic variance in tolerance did not vary significantly when selection was extended from three to six months, which suggests that there may be still sufficient genetic variation for recovery. Reversion to low tolerance in S. vetulus implies that the tolerant genotype may carry a fitness cost in the absence of cyanobacteria, i.e., the lower intrinsic rates of population increase in the absence of M. aeruginosa. The experimental evolution, forwards or reverse, in this study suggests that the harmful effects of toxic cyanobacteria cannot always be eliminated, although natural selection can lessen these consequences by promoting adaptation. Additionally, the present study shows that grazer-cyanobacteria interactions could be a good system for empirical study on evolutionary adaptation to environmental changes over short time spans.
Methods
Collection and culture of organisms
The microcystin-producing cyanobacterium Microcystis aeruginosa (FACHB-905) and the nutritious green alga Chlorella pyrenoidosa (FACHB−15) were provided by the Freshwater Algae Culture Collection at the Institute of Hydrobiology, the Chinese Academy of Sciences. They were grown at 25 °C under fluorescent lights at 50 μmol m−2 s−1 on a 12:12 h light:dark cycle. Phytoplankton cultures were incubated in exponential growth phases with frequent transfers into BG-11 medium. M. aeruginosa grow as single or paired cells under the laboratory conditions, which can minimize their mechanical interferences with zooplankton13. The carbon contents of M. aeruginosa and C. pyrenoidosa are 3.81 and 2.59 pg cell−1, respectively, estimated by their cell volumes15. Phytoplankton suspension for grazers were prepared by measuring the light absorption of a culture in a spectotrophotometer and diluting it with 0.45-μm-filtered tap water with a pre-established linear regression between light absorption and carbon content.
Using a 200-μm mesh net, zooplankton samples were collected from Shangyi Pond (31°01′38″N, 121°26′52″W), Shanghai, China. The low value of microcystins in the sediments (0.30 ± 0.08 μg g−1) indicates that cyanobacteria are present, but rarely form blooms in the sampling site15. Approximately 1600 adults of either Daphnia pulex or Simocephalus vetulus were isolated from zooplankton samples.
Selection experiments
Grazers were randomly split into two lines, the selection line and the control line. For each line, there were four independent tanks filled with 5 L of 0.45-μm-filtered tap water. Individuals in the control lines were fed pure C. pyrenoidosa daily during the entire experiment, while those in the selection line were offered a mixed diet of 75% C. pyrenoidosa and 25% M. aeruginosa, at a food concentration of 400 μg C L−1. The relative low cell density of M. aeruginosa (3.4 × 104 cells mL−1) ensured the population persistence of grazers in the selection experiments, and represented the initial phase of a bloom in which single cells of cyanobacteria are common12. They were raised at 25 °C under dim light and half of water in tanks were renewed every four days. Their population density was 40 ind. L−1 (200 individual per tank) at the beginning of experiments and was kept under a level of 100 ind. L−1 to avoid crowding by randomly discarding individuals when necessary.
Given that CyanoHABs usually persist for several months in the field20, the selection experiments ran for either three or six months. At each time point, life table experiments were carried out to measure the intrinsic rate of population increase of grazers.
Life table experiments
Approximately 50 pre-matured individuals were randomly isolated from each tank and were provided with 400 μg C L−1 C. pyrenoidosa. Their neonates from the third brood within 24 h of birth were collected to initiate a life table experiment with four replicates (20 neonates in a 500-mL beaker). They were provided with either poor food (50% M. aeruginosa + 50% C. pyrenoidosa) or good food (100% C. pyrenoidosa) at 400 μg C L−1. This food concentration represents a typical biomass of phytoplankton in eutrophic lakes, especially in summer when CyanoHABs commonly occur20. The presence of 50% M. aeruginosa in the diet (6.8 × 104 cells mL−1) mimics the phytoplankton community in initial phase of CyanoHABs20, and seriously depresses the growth and reproduction of D. pulex15 and S. vetulus22. Food selection may be slight because both grazers are generalist feeders31,32 and both prey organisms have similar shape and diameter size (3.71 ± 0.42 μm for M. aeruginosa and 3.28 ± 0.52 μm for C. pyrenoidosa) under the present conditions. The number of survivors and their newborn neonates were counted and half of algal suspension was renewed daily. Life table experiments were conducted for ten days at 25 °C because the extension of experimental period contributes a little to the estimation for intrinsic rate of population increase in the present system (approximately 3%, authors’ unpublished data).
Relaxation experiments
When evolved tolerance in S. vetulus was evident in the selection line after the six-month exposure to M. aeruginosa, their diet was switched to 100% C. pyrenoidosa. After three months, grazer performance on two types of food was measured using life table experiments, to test whether evolved tolerance is reversible.
Data analysis
Intrinsic rate of population increase (r) was calculated iteratively by the Euler-Lotka equation:
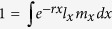 |
where lx is the survival proportion at day x, and mx is the number of newborn neonates produced at day x. Intrinsic rate of population increase integrates both survival and reproduction and is a more reliable proxy for population fitness. A tolerance index (T) to toxic cyanobacteria was calculated as the relative change in intrinsic rate of population increase on poor food (rpoor) compared to that on good food (rgood): T = rpoor/rgood16. A higher value of this index indicates greater tolerance to toxic cyanobacteria in grazers15.
Grazer performance between the treatment lines was compared using two-way nested ANOVA. The tank was nested within the treatment line and was treated as a random effect. Data homogeneity and normality were confirmed by Leven’s test and Kolmogorov-Smirnov test prior to ANOVA. A linear regression was used to explore the relationship between rpoor and rgood. All statistical analyses were performed using the Statistical Product and Service Solution (SPSS) 16.0 statistical package.
Additional Information
How to cite this article: Jiang, X. et al. Rapid evolution of tolerance to toxic Microcystis in two cladoceran grazers. Sci. Rep. 6, 25319; doi: 10.1038/srep25319 (2016).
Acknowledgments
This work was supported by the Special Fund for Agro-scientific Research in the Public Interest (201203065); the National Nature Science Foundation of China (31272307 and 31471983); Innovation Program of Shanghai Municipal Education Commission (15ZZ024); and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
Footnotes
Author Contributions X.J. designed the research plan and wrote the manuscript. H.G., L.Z., H.L. and X.Z. performed the experiments. All authors reviewed the manuscript.
References
- Walther G. et al. Ecological responses to recent climate change. Nature 416, 389–395 (2002). [DOI] [PubMed] [Google Scholar]
- Alberti M. Eco-evolutionary dynamics in an urbanizing planet. Trends Ecol. Evol. 30, 114–126 (2015). [DOI] [PubMed] [Google Scholar]
- McCauley D. J. et al. Marine defaunation: Animal loss in the global ocean. Science 347, doi: 10.1126/science.1255641 (2015). [DOI] [PubMed] [Google Scholar]
- Burrows M. T. et al. The pace of shifting climate in marine and terrestrial ecosystems. Science 334, 652–655 (2011). [DOI] [PubMed] [Google Scholar]
- Agrawal A. A. Phenotypic plasticity in the interactions and evolution of species. Science 294, 321–326 (2001). [DOI] [PubMed] [Google Scholar]
- Dam H. G. Evolutionary adaptation of marine zooplankton to global change. Annu. Rev. Mar. Sci. 5, 349‒370 (2013). [DOI] [PubMed] [Google Scholar]
- Hoffmann A. A. & Sgrò C. M. Climate change and evolutionary adaptation. Nature 470, 479–485 (2011). [DOI] [PubMed] [Google Scholar]
- Carroll S. P. et al. Applying evolutionary biology to address global challenges. Science 346, doi: 10.1126/science.1245993 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel J. E. & Shurin J. B. Mechanisms, effects, and scales of dispersal in freshwater zooplankton. Limnol. Oceanogr. 49, 1229–1238 (2004). [Google Scholar]
- Paerl H. W. & Huisman J. Blooms like it hot. Science 320, 57–58 (2008). [DOI] [PubMed] [Google Scholar]
- Paerl H. W. & Otten T. G. Blooms bite the hand that feeds them. Science 342, 433–434 (2013). [DOI] [PubMed] [Google Scholar]
- O’Neil J. M., Davis T. W., Burford M. A. & Gobler C. J. The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change. Harmful Algae 14, 313–334 (2012). [Google Scholar]
- Ger K. A., Hansson L. A. & Lürling M. Understanding cyanobacteria-zooplankton interactions in a more eutrophic world. Freshwater Biol. 59, 1783‒1798 (2014). [Google Scholar]
- Sarnelle O. & Wilson A. E. Local adaptation of Daphnia pulicaria to toxic cyanobacteria. Limnol. Oceanogr. 50, 1565‒1570 (2005). [Google Scholar]
- Jiang X., Liang H., Chen Y., Xu Y. & Huang D. Microgeographic adaptation to toxic cyanobacteria in two aquatic grazers. Limnol. Oceanogr. 60, 947–956 (2015). [Google Scholar]
- Hairston N. G. Jr et al. Rapid evolution revealed by dormant eggs. Nature 401, 446 (1999). [Google Scholar]
- Kawecki T. J. et al. Experimental evolution. Trends Ecol. Evol. 27, 547–560 (2012). [DOI] [PubMed] [Google Scholar]
- Jiang X. et al. Resistance variation within a Daphnia pulex population against toxic cyanobacteria. J. Plankton Res. 35, 1177–1181 (2013). [Google Scholar]
- Gustafsson S. & Hansson L. A. Development of tolerance against toxic cyanobacteria in Daphnia. Aquat. Ecol. 38, 37–44 (2004). [Google Scholar]
- Deng J. et al. Earlier and warmer springs increase cyanobacterial (Microcystis spp.) blooms in subtropical Lake Taihu, China. Freshwater Biol. 59, 1079–1085 (2014). [Google Scholar]
- Wang X. et al. Differences in microcystin production and genotype composition among Microcystis colonies of different sizes in Lake Taihu. Water Res. 47, 5659–5669 (2013). [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhang L. & Jiang X. Fine-scale local adaptation of cyanobacterial tolerance in Simocephalus vetulus (Crustacea: Cladocera). J. Plankton Res. 37, 764–772 (2015). [Google Scholar]
- Barrett R. D. H. & Schluter D. Adaptation from standing genetic variations. Trends Ecol. Evol. 23, 38–44 (2008). [DOI] [PubMed] [Google Scholar]
- Lanfear R., Kokko H. & Eyre-Walker A. Population size and the rate of evolution. Trends Ecol. Evol. 29, 33–41 (2014). [DOI] [PubMed] [Google Scholar]
- Avery D. E. & Dam H. G. Newly discovered reproductive phenotypes of a marine copepod reveal the costs and advantages of resistance to a toxic dinoflagellate. Limnol. Oceanogr. 52, 2099–2108 (2007). [Google Scholar]
- Hairston N. G. Jr et al. Natural selection for grazer resistance to toxic cyanobacteria: evolution of phenotypic plasticity? Evolution 55, 2203‒2214 (2001). [DOI] [PubMed] [Google Scholar]
- Brooks J. L. & Dodson S. I. Predation, body size, and composition of plankton. Science 150, 28–35 (1965). [DOI] [PubMed] [Google Scholar]
- Jiang X., Yang W., Zhang L., Chen L. & Niu Y. Predation and cyanobacteria jointly facilitate competitive dominance of small-bodied cladocerans. J. Plankton Res. 36, 956–965 (2014). [Google Scholar]
- Tetónio H. & Rose M. R. Variation in the reversibility of evolution. Nature 408, 463–466 (2000). [DOI] [PubMed] [Google Scholar]
- Jiang X., Lonsdale D. J. & Gobler C. J. Rapid gain and loss of evolutionary resistance to the harmful dinoflagellate Cochlodinium polykrikoides in the copepod Acartia tonsa. Limnol. Oceanogr. 56, 947–954 (2011). [Google Scholar]
- DeMott W. R. Feeding selectivities and relative ingestion rates of Daphnia and Bosmina. Limnol. Oceanogr. 27, 518–527 (1982). [Google Scholar]
- Fernandez R., Nandini S. & Sarma S. S. S. A comparative study on the ability of tropical micro-crustaceans to feed and grow on cyanobacterial diets. J. Plankton Res. 34, 719–731 (2012). [Google Scholar]