Abstract
Diets deficient in protein often increase food consumption, body weight and fat mass; however, the underlying mechanisms remain poorly understood. We compared the effects of diets varying in protein concentrations on energy balance in obesity-prone rats. We demonstrate that protein-free (0% protein calories) diets decreased energy intake and increased energy expenditure, very low protein (5% protein) diets increased energy intake and expenditure, whereas moderately low protein (10% protein) diets increased energy intake without altering expenditure, relative to control diet (15% protein). These diet-induced alterations in energy expenditure are in part mediated through enhanced serotonergic and β-adrenergic signaling coupled with upregulation of key thermogenic markers in brown fat and skeletal muscle. The protein-free and very low protein diets decreased plasma concentrations of multiple essential amino acids, anorexigenic and metabolic hormones, but these diets increased the tissue expression and plasma concentrations of fibroblast growth factor-21. Protein-free and very low protein diets induced fatty liver, reduced energy digestibility, and decreased lean mass and body weight that persisted beyond the restriction period. In contrast, moderately low protein diets promoted gain in body weight and adiposity following the period of protein restriction. Together, our findings demonstrate that low protein diets produce divergent effects on energy balance.
The consumption of protein triggers adaptive responses in ingestive behavior, energy expenditure and metabolism that are under homeostatic controls. The ‘protein leverage’ hypothesis posits that protein intake is tightly regulated in several species including rats, mice and humans, which consume to acquire their required protein rather than meet requirements for fats and carbohydrates1,2,3. An increase in dietary protein density would decrease intake of carbohydrates and fats with consequent reduction in energy intake. There is substantial evidence that high protein diets promote satiety, weight loss and improve glycemic control4,5. A corollary to this hypothesis is that a reduction in the dietary protein concentration would increase total energy intake, due to overconsumption of carbohydrates and fat, in an effort to meet protein requirements. Consistent with this, moderately protein deficient diets were found to produce hyperphagia in rodents6,7,8,9 and in some1,10,11 but not all human studies12,13, whereas, severe protein restriction below a certain threshold leads to a reduction in food intake in rodents9,14. However, little is known of the underlying mechanisms by which moderate protein deficiency elicits such behavioural and metabolic adaptations and promotes positive energy balance with consequent predisposition to obesity and other metabolic disorders.
The hyperphagic effects of moderately low protein diets are purported to be through multiple mechanisms. These include imbalances in plasma and brain amino acid concentrations in rats15,16,17, modulation of energy sensors in the hypothalamus and anterior piriform cortex in rats18, and increased activity in the reward areas such as the orbitofrontal cortex and striatum in humans11. Low protein diets also enhance energy expenditure in rodents17,19,20,21,22, however, the underlying mechanisms are poorly understood. Potential mechanisms include increased sympathetic flux via β-adrenergic receptor (β-AR) signaling to brown adipose tissue (BAT) with consequent upregulation of mitochondrial uncoupling protein-1 (UCP1) expression19,21,22,23, as well as increased fibroblast growth factor-21 (FGF21) mediated thermogenesis17. However, the relative importance of these mechanisms, and whether gut-derived signals are associated with low protein induced hyperphagia and thermogenesis, are largely unknown.
The enteroendocrine cells of the gut secrete multiple hormones including peptide YY (PYY) which is postulated to play a role in the anorexigenic effects of high protein diets24; it is unknown whether gut hormones mediate the effects of low protein diets on energy balance. Apart from these hormones, gut-derived serotonin, which accounts for over 95% of total body serotonin25, has recently been shown to induce obesity due in part to reduced brown fat thermogenesis26,27, whereas brain-derived serotonin was reported to induce thermogenesis in brown fat28. The effects of serotonin are mediated by multiple receptor subtypes, of which 5-hydroxytryptamine (5HT3) receptors mediate the hypophagic effects of carbohydrate and fat29,30,31. It is unknown whether 5HT3 receptors mediate the effects of low protein diets on energy balance.
In addition to modulating energy intake and expenditure, moderate protein restriction in rats and mice has been consistently associated with alterations in body composition including reduction in lean mass, increased body fat content and development of fatty liver6,8,9,14,17,32,33. Protein restricted rats attempt to catch-up on body weight and adipose reserves following refeeding on a standard protein diet34,35,36,37,38. However, less is known of the temporal changes in body composition, energy intake and energy expenditure following realimentation of protein restricted animals to standard protein diets. Therefore, in this study, we investigated the effects of low protein diets varying in protein density on multiple metabolic parameters in obesity-prone rats. We demonstrate that protein deprivation decreased energy intake and increased energy expenditure. We also show that low to moderate protein deficiency increased energy intake with either an increase or no change in energy expenditure. Importantly, the effects on energy expenditure are mediated through enhanced serotonergic and β-AR signaling with concomitant upregulation of key thermogenic markers in BAT and skeletal muscle. Further, in contrast to the decreased lean mass and increased hepatic lipidosis observed with protein starvation or low protein diets, we provide evidence that moderate protein deficiency promoted gain in body weight and adipose reserves following the period of dietary protein restriction.
Results
Energy Intake, Energy Expenditure and Energy Digestibility
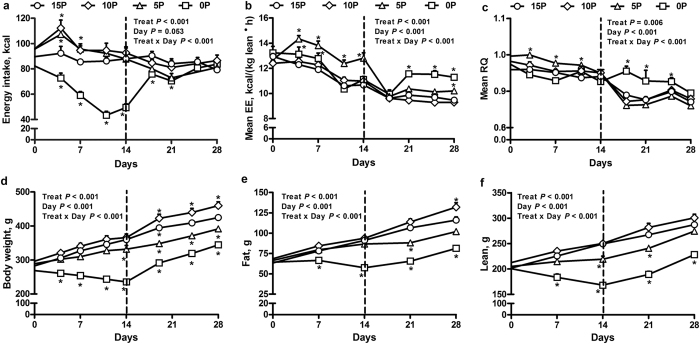
When compared to the daily energy intake of rats fed a control diet (15% protein, 15P; see Supplementary Table S1), in rats fed a protein-free diet (0P), energy intake was decreased by 13–49% during the 14-day restriction and by 14–54% during the 21-day restriction, with subsequent recovery to control levels after the first week of realimentation (Fig. 1a, see Supplementary Figs S1a,S2a,b and S3a–h). Relative to 15P, daily energy intake was increased by 12–16% and 11–21% during the first 7 days in rats fed very low protein diet (5% protein; 5P) and moderately low protein diet (10% protein; 10P), respectively (Fig. 1a, see Supplementary Figs S2a,b and S3a–d). Compared to 15P, the mean daily energy expenditure was increased by 7% for 7 days in 0P and by 16–20% for 14 days in the 5P (Fig. 1b, see Supplementary Figs S2c,d and S4a,b). The increased energy expenditure of 0P and 5P was persistent even when lean mass was used as a covariate (see Supplementary Fig. S2g–i). Relative to 15P, respiratory quotient (RQ) was decreased during restriction in 0P (see Supplementary Fig. S1c) but increased in the 5P from day (d) 4 until d 11 (Fig. 1c). Further, by d 7, relative to 15P, total energy digestibility was increased by 16% in 10P but decreased by 41% in 0P. By d 14, energy digestibility tended (P < 0.1) to be decreased by 12% in 5P and decreased by 42% in 0P compared with 15P (Supplementary Table S2). Interestingly, when the 0P rats were realimented to the 15P diet, the mean daily and dark period energy expenditure was increased from d 21 onwards, and mean daily RQ also increased from d 18 until d 25 (Fig. 1b,c, see Supplementary Figs S1b, S2c,d and S4e–h).
Figure 1. Effect of low protein diets on energy balance.
(a) Daily energy intake, (b) mean energy expenditure (EE), (c) mean respiratory quotient (RQ), (d) body weight, (e) body fat mass and (f) body lean mass of obesity-prone rats. The animals were fed either a control (15% protein; 15P), moderately low protein (10% protein; 10P), very low protein (5% protein; 5P) or protein-free (0% protein; 0P) isocaloric diet for 14 days, followed by a realimentation phase with ad libitum access to the control diet (15P) for another 14 days. Dotted line separates the restriction and recovery phases. Values are mean ± SEM, n = 13–16. *P < 0.05 vs 15P.
5HT3 receptor blockade with Ondansetron
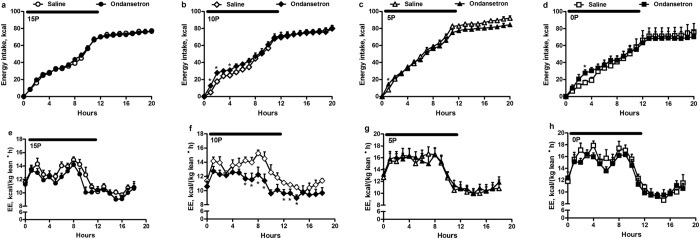
Ondansetron, a selective 5HT3 receptor blocker, was used to determine whether the serotonergic system mediates the effects of low protein diets on energy intake and expenditure. Ondansetron increased energy intake by 25–58% for the first 4 hours of dark period in 10P, by 82% at 1 hour (h) in 5P and by 68% at 3 h in 0P but not in 15P rats (Fig. 2a–d). Relative to vehicle, ondansetron reduced energy expenditure by 11–23% from 6 to 14 h in 10P, but not in 0P, 5P and 15P (Fig. 2e–h).
Figure 2. Effect of ondansetron on energy balance of rats fed low protein diets.
Energy intake (a–d) and energy expenditure (EE; (e–h) of obesity-prone rats fed either a control (15% protein; 15P), moderately low protein (10% protein; 10P), very low protein (5% protein; 5P) or protein-free (0% protein; 0P) isocaloric diet with injections of saline or ondansetron (1.0 mg/kg; IP). The analyses of data from the first 9 hours of dark period revealed that there were significant fixed effects of dietary treatment (P < 0.001), drug (P = 0.019) and time (P < 0.001) and interactions of dietary treatment × time (P < 0.001) and drug × time (P = 0.046) for calorie intake. Similarly, there were significant effects of time and dietary treatment (P < 0.001) and interaction of dietary treatment × time (P < 0.001) for energy expenditure. Values are mean ± SEM, n = 8. *P < 0.05 saline vs ondansetron.
β-AR blockade with Propranolol
To determine whether the sympathetic system mediates the effects of low protein diets on energy expenditure, we administered propranolol, a β1 and β2-AR blocker. Propranolol decreased energy expenditure in the 0P, 5P and 10P rats during the dark period by 18%, 14% and 8%, respectively (Fig. 3a–d). Further, area under the curve (AUC) analyses revealed that propranolol decreased energy expenditure to a greater extent in 0P (10%) and 5P (8%) than 15P.
Figure 3. Effect of propranolol on energy expenditure.
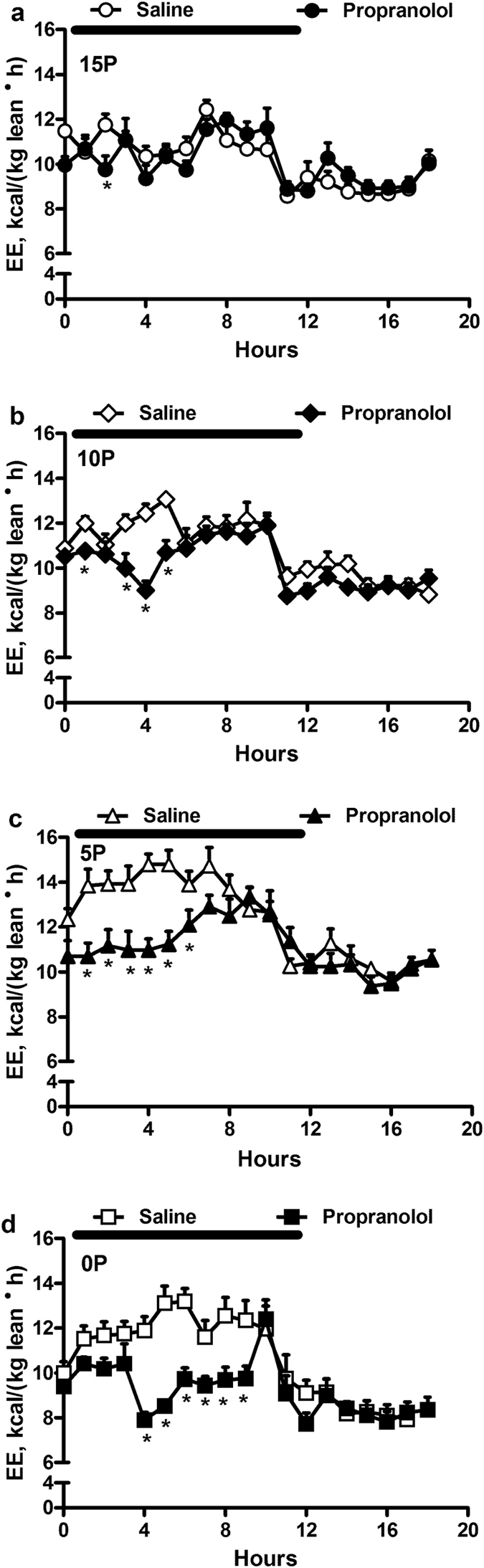
Obesity-prone rats were fed either a control (15% protein; 15P), moderately low protein (10% protein; 10P), very low protein (5% protein; 5P) or protein-free (0% protein; 0P) isocaloric diet with injections of saline or propranolol (10 mg/kg; SC). The analyses during the first 8 hours of dark period revealed that there were significant effects of time, drug, dietary treatment (P < 0.001), dietary treatment × time (P = 0.002), drug × time (P < 0.001), dietary treatment × drug (P = 0.026) and dietary treatment × drug × time (P = 0.009) for energy expenditure (EE). Values are mean ± SEM, n = 8. *P < 0.05 saline vs propranolol.
Body composition
Relative to 15P rats, the body weight of 0P decreased by 15–35% during the 14-day restriction, by 18–42% during the 21-day restriction, and remained lower by 19–26% during realimentation (Fig. 1d, see Supplementary Fig. S1d). Similarly, the body weight of 5P decreased by 8% by d 14 and remained lower by 8–12% compared to 15P during realimentation. The body weight of 10P did not change during restriction but increased by 7–8% during realimentation. Body composition analyses revealed that, relative to 15P, the fat mass of 0P decreased by 15–37% during the 14-day restriction, by 18–48% during the 21-day restriction, and was also reduced by 28–38% during realimentation (Fig. 1E, see Supplementary Figs S1e and S2e). During the 14-day restriction, though the fat mass of 5P and 10P were similar to 15P (Fig. 1e), the 5P had relatively greater fat% (see Supplementary Fig. S2e). During realimentation, the fat mass of 5P decreased by 17% on d 21 whereas the fat mass of 10P increased by 11% by d 28 (Fig. 1e); fat% also followed a similar pattern (see Supplementary Fig. S2e). The lean mass of 0P decreased by 19–33% during the 14-day restriction, by 18–39% during the 21-day restriction, and remained lower by 21–29% during realimentation when compared to 15P (Fig. 1f, see Supplementary Figs S1f and S2f). Relative to 15P, in 5P lean mass was 12% and 10% lower by d 14 and d 21, respectively (Fig. 1f), but lean% were relatively greater in 0P and 5P during realimentation (see Supplementary Fig. S2f). When expressed as proportions of body weight, the 0P had 26% greater heart weight, while 5P had 15% greater liver weight compared to 15P (Supplementary Table S2). Further, the 5P and 10P had 58% and 16% greater liver fat%, and the 0P tended (P = 0.06) to have 26% greater liver fat%, than 15P (Supplementary Table S2). Compared to 15P, the efficiency of converting energy consumed to body weight was greater in the 0P during d 8–14 of restriction and throughout realimentation, whereas the efficiency was greater for 5P and 10P during d 22–28 of realimentation (see Supplementary Table S4).
Plasma amino acids, glucose and hormones
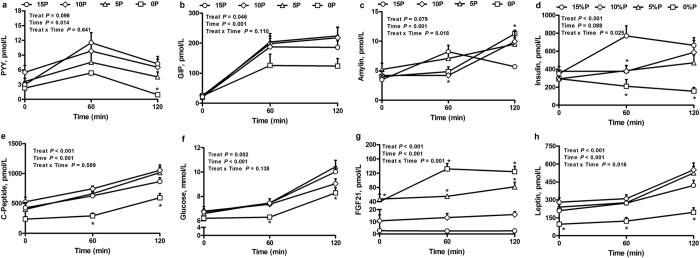
The 0P, 5P and 10P had lower postprandial plasma concentrations of the essential amino acids - threonine, tryptophan, valine, phenylalanine, leucine, isoleucine and lysine compared with 15P rats (see Supplementary Table S3). The concentration of histidine was decreased in 5P and 10P but not 0P, arginine was increased in 0P and methionine was decreased in the 0P and 5P. For non-essential amino acids, 0P, 5P and 10P had greater concentrations of serine and alanine, 0P and 5P had greater glycine but lower tyrosine, and 0P had lower ornithine, relative to 15P. Plasma concentrations of PYY, leptin, insulin, C-peptide and blood glucose were decreased, and amylin and glucose-dependent insulinotropic peptide (GIP) tended (P < 0.1) to be decreased, following a meal in 0P compared to 15P (Fig. 4). Plasma insulin concentrations in 5P and 10P, and glucose in 10P, were transiently decreased compared to 15P. Importantly, plasma FGF21 concentrations were increased in 0P and 5P, and tended in 10P (P < 0.1). Blood glucose concentrations and total glucose AUC following an intraperitoneal glucose tolerance test (IPGTT) did not differ among treatment groups (see Supplementary Fig. S5a,b).
Figure 4. Effect of low protein diets on plasma hormone concentrations.
(a) Peptide YY (PYY), (b) glucose-dependent insulinotropic peptide (GIP), (c) amylin, (d) insulin, (e) C-Peptide, (f) glucose, (g) fibroblast growth factor 21 (FGF21) and (h) leptin concentrations in obesity-prone rats. The animals were fed either a control (15% protein; 15P), moderately low protein (10% protein; 10P), very low protein (5% protein; 5P) or protein-free (0% protein; 0P) isocaloric diet for 14 days. Values are mean ± SEM, n = 5–9. *P < 0.05 vs 15P.
The mRNA and protein abundance of key molecules of energy metabolism in liver
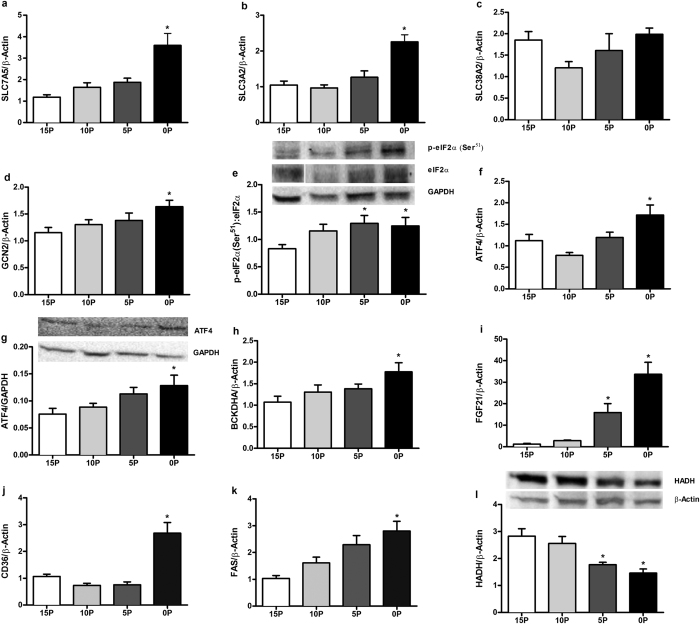
The mRNA abundance of molecules involved in amino acid uptake - solute carrier family 7 member 5 (SLC7A5), solute carrier family 3 member 2 (SLC3A2), amino acid sensing - general control non-depressible 2 (GCN2) and activating transcription factor 4 (ATF4), amino acid metabolism - branched chain keto acid dehydrogenase E1, alpha polypeptide (BCKDHA), fatty acid uptake - cluster of differentiation 36 (CD36), and fatty acid synthesis - fatty acid synthase (FAS) were all greater in 0P, and FAS tended (P < 0.1) to be greater in 5P, compared to 15P rats (Fig. 5a,b,d,f,h,j,k). Relative to 15P, the protein abundance of amino acid sensing molecules such as ATF4 in 0P and serine-51 phosphorylated eukaryotic initiation factor 2α (peIF2α (Ser51)):eIF2α ratio in 0P and 5P were greater, and the abundance of β-oxidation enzyme 3-hydroxyacyl-CoA dehydrogenase (HADH) were lower in 0P and 5P (Fig. 5e,g,l). Further, the 0P and 5P had greater mRNA abundance of FGF21 than 15P (Fig. 5i).
Figure 5. Effects of low protein diets on relative mRNA or protein abundance of key regulatory molecules of energy metabolism in liver.
(a) Solute carrier family 7 member 5 (SLC7A5), (b) solute carrier family 3 member 2 (SLC3A2), (c) solute carrier family 38 member 2 (SLC38A2), (d) general control non-depressible 2 (GCN2), (e) serine 51 phosphorylated eukaryotic initiation factor 2α (peIF2α (Ser51)):eIF2α ratio, (f,g) activating transcription factor 4 (ATF4), (h) branched chain keto acid dehydrogenase E1, alpha polypeptide (BCKDHA), (i) fibroblast growth factor 21 (FGF21), (j) cluster of differentiation 36 (CD36), (k) fatty acid synthase (FAS), (l) 3-hydroxyacyl-CoA dehydrogenase (HADH) in obesity-prone rats. The animals were fed a control (15% protein; 15P), moderately low protein (10% protein; 10P), very low protein (5% protein; 5P) or protein-free (0% protein; 0P) isocaloric diet for 14 days. The relative mRNA and protein abundance was determined by qPCR and immunoblot analysis. β-Actin or glyceraldehyde 3-phosphate dehydrogenase (GAPDH), were used as reference targets. The eIF2α, peIF2α (Ser51), and GAPDH were reprobed on the same blots. Values are mean ± SEM, n = 5–9. *P < 0.05 vs 15P.
The mRNA abundance of key molecules of energy metabolism in BAT
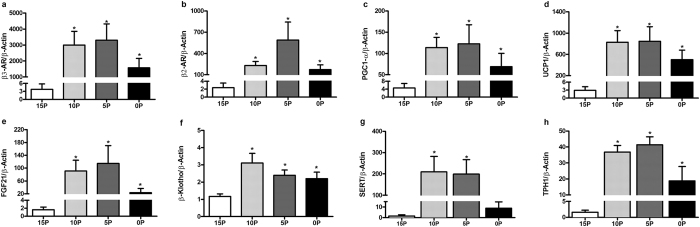
When compared to 15P rats, 0P, 5P and 10P had greater mRNA abundance of thermogenic genes encoding for β3-AR, β2-AR, peroxisome proliferator-activated receptor gamma coactivator 1 α (PGC1-α), UCP1, FGF21 and its co-receptor β-Klotho (Fig. 6a–f). Further, the transcript abundance of a key enzyme in serotonin synthesis - tryptophan hydroxylase 1 (TPH1) was increased in 0P, 5P, and 10P, and the serotonin reuptake transporter (SERT) was also increased in 5P and 10P, relative to 15P (Fig. 6g,h).
Figure 6. Effects of low protein diets on relative mRNA abundance of key regulatory molecules of thermogenesis in interscapular brown adipose tissue.
(a) β3-adrenergic receptors (β3-AR), (b) β2-adrenergic receptors (β2-AR), (c) peroxisome proliferator-activated receptor gamma coactivator 1 α (PGC1-α), (d) uncoupling protein 1 (UCP1), (e) fibroblast growth factor 21 (FGF21), (f) β-Klotho, (g) serotonin transporter (SERT) and (h) tryptophan hydroxylase 1 (TPH1) in obesity-prone rats. The animals were fed either a control (15% protein; 15P), moderately low protein (10% protein; 10P), very low protein (5% protein; 5P) or protein-free (0% protein; 0P) isocaloric diet for 14 days. The relative mRNA abundance was determined by qPCR using β-Actin as reference target. Values are mean ± SEM, n = 5–9. *P < 0.05 vs 15P.
The mRNA abundance of key molecules of energy metabolism in muscle
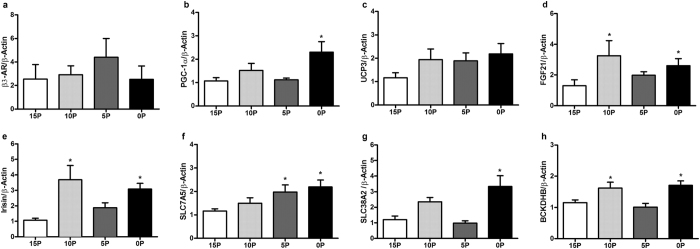
In the skeletal muscle, the mRNA abundance of thermogenic genes such as PGC1-α was greater in 0P, and FGF21 and irisin were greater in 0P and 10P compared with 15P rats (Fig. 7a–e). Further, key regulatory transcripts in amino acid metabolism that were upregulated include solute carrier family 38 member 2 (SLC38A2) in 0P, SLC7A5 in 0P and 5P, and branched chain keto acid dehydrogenase E1, beta polypeptide (BCKDHB) in 0P and 10P (Fig. 7f–h).
Figure 7. Effects of low protein diets on relative mRNA abundance of key regulatory molecules of energy metabolism in skeletal muscle.
(a) β3-AR (β3-adrenergic receptors), (b) peroxisome proliferator-activated receptor gamma coactivator 1 α (PGC1-α), (c) uncoupling protein 3 (UCP3), (d) fibroblast growth factor 21 (FGF21), (e) irisin, (f) solute carrier family 7 member 5 (SLC7A5), (g) solute carrier family 38 member 2 (SLC38A2) and (h) branched chain keto acid dehydrogenase E1, beta polypeptide (BCKDHB) in obesity-prone rats. The animals were fed either a control (15% protein; 15P), moderately low protein (10% protein; 10P), very low protein (5% protein; 5P) or protein-free (0% protein; 0P) isocaloric diet for 14 days. The relative mRNA abundance was determined by qPCR using β-Actin as reference target. Values are mean ± SEM, n = 5–9. *P < 0.05 vs 15P.
Discussion
We provide evidence that isocaloric diets with graded doses of protein produce divergent effects on energy intake, energy expenditure, plasma amino acids and gut hormones, and metabolic markers in peripheral tissues in obesity-prone rats. First, a protein-free diet (0P) decreased energy intake and increased energy expenditure, very low protein diet (5P) increased intake and expenditure, whereas moderately low protein diet (10P) increased intake with no change in expenditure. On realimentation to control diet (15P), 0P had transient anorexia but increased energy expenditure. Second, enhanced serotonergic and sympathetic signaling mediated the differential effects of low protein diets on energy balance. Blockade of 5HT3 receptors with ondansetron produced hyperphagia in 0P, 5P and 10P, but decreased energy expenditure in 10P, indicative of a role for higher serotonergic tone in inhibiting energy intake across these groups but in increasing energy expenditure in the 10P group. Propranolol (β-AR antagonist) decreased energy expenditure in 0P, 5P and 10P which, in part, is supportive of enhanced sympathetic drive in the metabolic adaptations to low protein diets. However, the increased energy expenditure is likely mediated through parallel or interdependent mechanisms because the mRNA abundance of key thermogenic markers in BAT (β2-AR, β3-AR, PGC1-α, UCP1, FGF21, β-Klotho, SERT, TPH1) and skeletal muscle (PGC1-α, FGF21, irisin) were increased with variable degrees of protein restriction. Third, 0P and 5P decreased plasma concentrations of multiple essential amino acids, 0P decreased anorexigenic hormones (PYY, leptin, insulin), but 0P and 5P increased the tissue expression and plasma concentrations of the metabolic hormone FGF21. Fourth, dietary protein deficiency produced disparate effects on body weight and composition. During protein restriction, body weight, fat and lean mass were decreased in 0P, body weight and lean mass were reduced in 5P, whereas these body compartments were unaltered in 10P. However, during the realimentation period, 10P gained weight and fat mass whereas the reduction in weight, fat and lean mass were sustained in the 0P and 5P groups. Further, the increased hepatic lipid content in 0P and 5P is supported by an increase in mRNA abundance of key lipogenic markers (CD36, FAS) and a reciprocal decrease in abundance of a lipolytic protein (HADH). Thus, these data demonstrate that dietary protein deficiency differentially modulates energy balance and metabolism.
The hypophagic effect of protein-free diets, and hyperphagic effect of very low protein and moderately low protein diets, in the current study are consistent with the ‘protein leverage’ hypothesis2, as well as previous studies on protein restriction in rats, mice and humans1,6,7,8,9,14,17,22. Although the low protein-induced hyperphagia is associated with a central orexigenic drive11,15,16,17,18, the peripheral signals that transmit information to central neural networks are poorly defined. To discern potential peripheral mediators, we focussed on circulating concentrations of amino acids, anorexigenic gut hormones, and peripheral serotonergic 5HT3, β-adrenergic and FGF21 signaling. Similar to other reports16,17, in the current study, protein restriction produced a dose-dependent decrease in majority of the essential amino acids. Interestingly, arginine, serine and glycine increased with concomitant anorexia in the 0P group. Protein deprivation decreased plasma concentrations of PYY and GIP, due in part to consumption of less food by animals in this group; hence, it is unlikely that these hormones mediate the anorexic effects of protein-free diets. Previous studies have shown that gut serotonergic signaling at peripheral 5HT3 receptor mediates the acute hypophagic effects of dietary carbohydrate in rats29,30,31. We extend these findings and demonstrate that ondansetron, a selective 5HT3 receptor antagonist, increased energy intake in rats that were fed isocaloric diets containing 0%, 5% and 10% protein with 67%, 62% and 57% carbohydrate calories, respectively. Therefore, as gut is a major source (~95%) of serotonin25, enhanced endogenous serotonin primarily of gut origin acting via 5HT3 receptors mediates the effects of diets low in protein, but comparatively high in carbohydrate, on energy intake.
There is limited evidence on the temporal changes in energy expenditure and substrate utilization with dietary protein deficiency. In the current study, the mean daily energy expenditure was greater in the 5P rats which was coincidental with an earlier hyperphagia during restriction. Despite an initial increase in energy expenditure, this effect did not persist in the 0P likely due to the sustained anorexia and weight loss. Previous studies have shown that an increased sympathetic influx to BAT is required for the thermogenic effects of low protein diets19,20,21,22,23. In the present study, using propranolol, a β-AR antagonist, we demonstrate that energy expenditure was dose-dependently attenuated in animals fed low protein diets with maximal attenuation in protein deprived rats. In support of enhanced sympathetic signaling, there was increased transcript abundance of β2-AR, β3-AR, PGC1-α and UCP1 in the BAT of low protein animals. Interestingly, majority of the BAT transcripts exhibited a non-linear parabolic response to the reduction in protein content with maximal responses between 10P and 5P and an attenuation with 0P. There are striking similarities between our findings and what others have shown with beta adrenergic signaling under cold acclimation. Chronic exposure to cold, and associated increase in sympathetic drive, has been shown to cause β-adrenergic receptor desensitization with a reduction in β3 transcripts in brown fat39,40,41,42. Because propranolol produced maximal attenuation of energy expenditure in 0P, it is likely that the greater and chronic sympathetic drive in the 0P lead to an adaptive attenuation of an increase in transcripts for β-adrenergic receptor and their downstream effectors PGC1α and UCP1. In addition, we also found a nearly dose-dependent upregulation of transcripts for FGF21 in the liver and muscle, increased plasma FGF21, and increased mRNA abundance for both FGF21 and its co-receptor β-Klotho in the BAT with low protein diets. Since the sympathetic system increases FGF21 expression and secretion from brown fat43, and FGF21 enhances sympathetic drive to brown fat44, the observed changes in FGF21 and β-klotho transcripts could likely be due to a reciprocal local or systemic feed-forward mechanism between FGF21 and sympathetic systems. Apart from adrenergic control, FGF21 expression and secretion could also be regulated by amino acids. Previously, dietary restriction of methionine45 or leucine46 has been shown to upregulate the tissue expression and circulating FGF21 concentrations in mice. We found greater plasma FGF21 concentrations in 0P and 5P rats which also had a concurrent decrease in plasma methionine and tyrosine but increase in glycine concentrations. In addition, the 10P had greater transcript abundance of FGF21 in brown fat and muscle together with a reduction in plasma threonine, tryptophan, valine, phenylalanine, leucine, isoleucine and lysine and an increase in serine and alanine. Whether alterations in these amino acids, either alone or together, contribute to the observed low protein diet-induced changes in FGF21 expression in multiple tissues remains to be determined. The relatively greater increase in FGF21 transcript in the liver than brown fat in 0P suggests that the source of FGF21 may shift from brown fat to the liver depending on the degree of protein restriction. Although liver-derived FGF21 may act through an endocrine mechanism to enhance BAT thermogenesis17,47, our data suggest that enhanced paracrine or autocrine FGF21 signaling in the BAT is also important for the effects of low protein diets on thermogenesis.
In addition to enhanced sympathetic drive, we provide evidence that increased serotonergic signaling acting via 5HT3 receptors is also important for the increased expenditure. Previously, it was shown that metergoline, a non-selective 5HT receptor antagonist and dopamine agonist, reduced resting VO2 in animals fed 8% protein diets48. In our study, the selective 5HT3 receptor antagonist ondansetron decreased energy expenditure particularly in the 10P group. Given that about 15% of circulating ondansetron gains access to the brain49, the effects of the antagonist on expenditure in the current study are likely mediated via both peripheral and central mechanisms. Although brain-derived serotonin may play a role in thermogenesis28, the increased transcript abundance of TPH1 and SERT in the BAT of 10P and 5P rats is suggestive of an upregulation of local serotonin turnover, which in turn might act via a paracrine or autocrine manner to enhance thermogenesis. Together, these findings suggest that increased adaptive thermogenesis of low protein diets is likely mediated through the convergence of parallel or interdependent sympathetic, serotonergic and FGF21 signaling pathways.
In the current study, reduction in body weight gain and tissue reserves in animals fed 0 to 5% protein diets is in agreement with previous studies6,8,9,14,17,34. As expected, the 0P with the lowest dietary ratios of protein to carbohydrate (0:67) or fat (0:33) had the lowest fat and lean tissue mass during restriction. The anorexia with consequent increase in lipid utilization, coupled with increased energy expenditure, likely contributed to a sustained reduction in weight gain, fat and lean mass in 0P. The lower ratio of protein to carbohydrate (5:62) in the 5P diet led to a reduction in lean mass without changes in fat mass, which contributed to an apparent increase in fat% during restriction. The reduction in weight and lean mass in 5P is likely due to increased energy expenditure that is not compensated for by the hyperphagia to maintain energy balance. Interestingly, the 5P animals appear to protect their fat mass while shifting their substrate oxidation from fats towards carbohydrates. The preferential oxidation of carbohydrates in 5P is reflective of the higher dietary carbohydrate content and is consistent with other reports50, and the partitioning of dietary fat towards adipose reserves is also similar to other studies with adipogenic effects of low protein-high carbohydrate diets in mice51. Previous studies have shown that following protein restriction, rats attempt to regain body weight and adipose reserves on refeeding with a standard protein diet34,35,37,38; however, the time-course of changes in tissue compartments and substrate utilization was relatively unknown. We demonstrate that during the realimentation period, 0P and 5P had decreased body weight, body fat and lean mass. Interestingly, on realimentation, 0P exhibited a robust preference for carbohydrate utilization, despite all groups being fed a common diet, and the greater lean% in 0P and 5P suggest that both groups rapidly partition dietary protein towards replenishing protein reserves. Importantly, 10P had increased body weight and body fat content on realimentation to a standard protein diet despite a lack of body composition differences during restriction. The greater feed efficiency of these animals during the realimentation resulted in increased weight gain with excess calories being partitioned towards adipose reserves. Thus, prior protein restriction exerts divergent long-term effects on body composition and substrate utilization with severe protein restriction delaying fat and lean accretion and enhancing carbohydrate use during realimentation, whereas moderate protein restriction predisposes to weight gain and obesity. These findings have important implications for long-term consequences of protein restriction on adiposity.
Despite lack of alterations in total body fat in 5P and 10P during protein restriction, we found that these animals, together with the 0P, had greater liver fat% indicative of hepatic lipidosis. In support of fat accumulation in the liver, we found that 0P rats had decreased protein abundance of the hepatic lipolytic marker HADH, with a reciprocal increase in the mRNA abundance of the key lipogenic markers CD36 and FAS. In our study, the low protein diets contained 67–57% carbohydrates with 33% fat. Others reported that diets relatively high in carbohydrate and fat, similar to the ranges in our study, promoted development of fatty liver and impaired glucose tolerance in both rats52,53,54 and mice51, whereas low protein-high carbohydrate diets increased adiposity and fatty liver with paradoxical improvement in glucose tolerance in mice6,32,33. Therefore, despite weight loss with 0P and 5P diets, the increased hepatic lipidosis likely negated any improvements in glucose tolerance. We next focused on key markers of amino acid metabolism in the liver and skeletal muscle. As expected, protein deprivation lead to an upregulation of the hepatic amino acid sensor GCN2 and its downstream targets peIF2α (Ser51):eIF2α and ATF4. We also observed distinct changes in key regulators of amino acid metabolism. The upregulation of transcripts for the amino acid transporters SLC7A5 and SLC3A2, and the rate-limiting enzyme in branched chain amino acid catabolism in the liver and muscle BCKDH, of particularly the 0P rats, is indicative of enhanced uptake and metabolism of branched chain amino acids by these tissues.
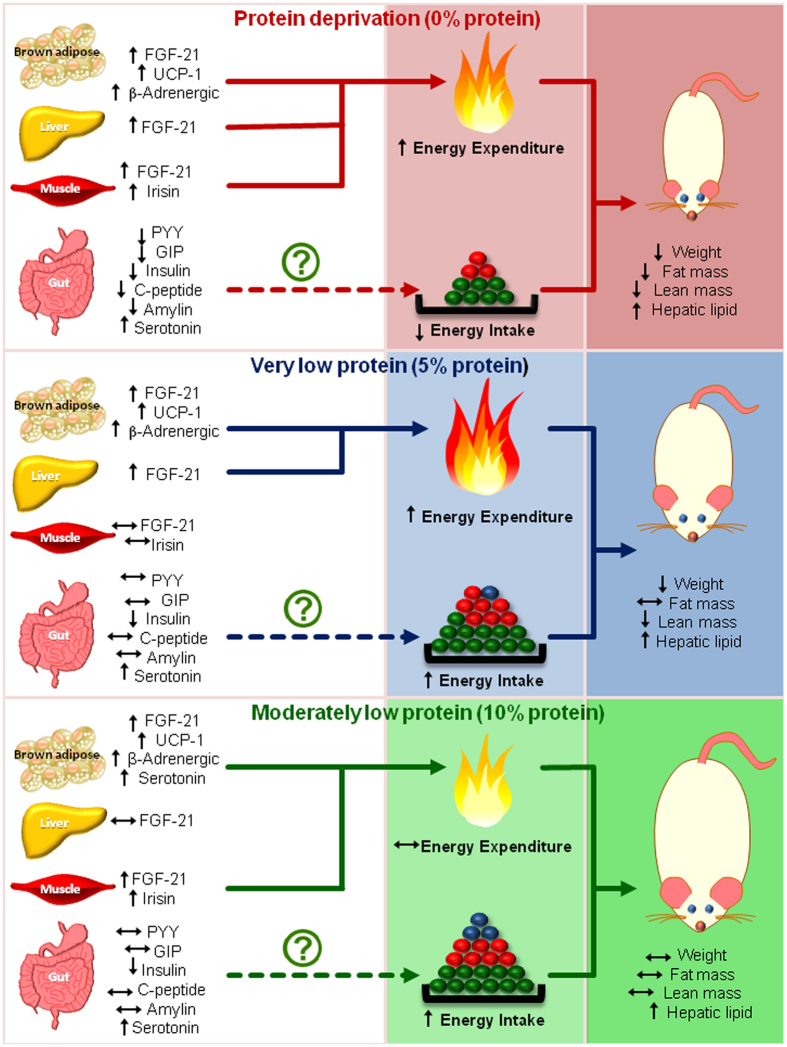
A potential caveat with our model is that the obesity prone OP-CD rats, which were originally developed from Sprague Dawley rats, have undergone multiple generations of breeding, and we did not test the effects of low protein diets in the control obesity-resistant strain. However, it is noteworthy that the hyperphagia, augmented thermogenesis and reduction in weight gain with our 0P and 5P, and the hyperphagia with 10P, is consistent with numerous other studies reporting similar findings in normal lean Sprague Dawley rats7,8,9,19,21,55,56. Although, the thermogenic activity of brown adipose tissue to dietary stimuli is often weak in obese animals57, the augmented thermogenesis with dietary protein restriction in our OP-CD rats suggests that effects of low protein diets on energy balance might be conserved in both lean and obese phenotypes. The potential mechanisms by which low protein diets modulate energy balance are depicted in Fig. 8. We provide evidence that severe protein deprivation produces a state of negative energy balance that persists beyond the period of deprivation primarily due to a decrease in energy intake and an increase in energy expenditure. In contrast, moderate protein deficiency produces hyperphagia without altering energy expenditure and predisposes to weight gain, adiposity and hepatic lipidosis. We also demonstrate that protein deficiency engages sympathetic and serotonergic signaling primarily in BAT to induce thermogenesis. Together, our findings demonstrate that dietary protein deficiency exerts divergent effects on multiple metabolic parameters in obesity-prone rats. Given that moderately low protein diets promote hyperphagia in humans1,10,11, our data, with an animal model that better represents human obesity, indicate that such diets could exacerbate pre-existing susceptibility to weight gain and obesity.
Figure 8. Model of potential mechanisms by which low protein diets modulate energy balance.
Protein-free diets decrease energy intake and enhance energy expenditure resulting in loss of body weight, fat and lean mass, whereas very low protein diets promote hyperphagia and thermogenesis with resultant reduction in weight and lean mass, and moderately low protein diets are hyperphagic without altering energy expenditure and body fat and lean mass. Enhanced sympathetic, serotonergic and fibroblast growth factor-21 (FGF21) secretion and signalling likely contribute to the thermogenic effects of protein-free and very low protein diets. It is unlikely that anorexigenic gut peptides play a role in modulating intake, however, enhanced serotonergic signalling, likely of gut origin, mediates the effects of low protein diets on food intake. Further, dietary protein deficiency promotes hepatic lipidosis. Arrows pointing upwards, downwards or horizontally indicate an increase, decrease or no change, respectively. Pathways that need further validation are indicated by dashed lines. The colored circles represent relative proportions of protein (blue), carbohydrate (green) and fat (red) in the diet, respectively.
Methods
Animals, housing and treatments
The animal experiments were approved by the University of Calgary Animal Care Committee (#AC12–0033). Male obesity-prone Sprague Dawley rats (~155 g, 6 weeks old; Crl: OP-CD, Strain 463; Charles River, Montreal, QC, Canada) were selected as they capture the hallmarks of human obesity including polygenic inheritance, glucose intolerance and obesity58,59,60; hence, they would have a greater translational significance for testing the obesogenic effects of low protein diets. They were housed individually in metabolic cages of the Comprehensive Lab Animal Monitoring System (CLAMS®, Columbus Instruments; Columbus, OH, USA) under standard temperature (23–24 °C) and lighting conditions (12 hours light-dark cycle; lights off at 1100 h). The general maintenance and husbandry (Supplementary Methods) was according to our previously published procedures5.
Prior to testing, animals were acclimatized to the environment and experimental conditions for 2 weeks. During the acclimatization period, they received a standard chow diet (25% protein, 62% carbohydrate, 13% fat, energy density 4.07 kcal/g; PicoLab® Rodent Diet 20; LabDiet, St. Louis, MO, USA) for 4 days, followed by a high-fat control diet for 10 days (Supplementary Table S1). The rats (279 ± 3 g body weight) were then weight-matched and randomly allocated to four isocaloric high-fat diets (4.4 kcal/g; 33% fat calories) with protein contributing to 15% (control; 15P), 10% (10P), 5% (5P) or 0% (0P) calories. These diets represent arbitrary states of protein starvation or total deprivation (0P), very low (5P) and moderately low (10P) dietary protein, relative to recommended control (15P) requirements61. Diets were made in-house (Supplementary Table S1) using ingredients from Dyets, Inc. (Bethlehem, PA, USA). Three experiments were conducted. In experiment 1, to determine the metabolic responses to the duration of protein deprivation, rats (n = 8/group) were randomized to 0P for 14 days, 0P for 21 days, or 15P, during the restriction phase followed by a realimentation phase with feeding on 15P diet for 28 or 21 days. Since the metabolic responses were similar between 14 vs 21 days of protein deprivation, in experiment 2, rats (n = 4–8/group) were randomized to either 15P, 10P, 5P or 0P for 14 days of restriction followed by 14 days of realimentation on the 15P diet. Multiple energy balance parameters were measured in both phases. In experiment 3, the rats (n = 8–10/group) were randomly assigned to identical treatment groups as indicated above for experiment-2, and fed the test diets for 14 days followed by a meal challenge and subsequent tissue sampling.
Metabolic measurements
Food intake and energy expenditure were recorded daily using CLAMS® throughout the study (Supplementary Methods) as we reported previously5. The rats were weighed twice a week and body composition was measured weekly using a Minispec LF110® NMR Analyzer (Bruker Corporation, Milton, ON, Canada). IPGTT was performed in all animals at d 10–13 as we previously described5,62. Gross energy content (kilocalories per gram) of fecal samples collected towards the end of the first and second week of the study were analyzed by bomb calorimetry (1341 Plain Jacket Bomb Calorimeter, Parr Instrument Company, Moline, IL, USA) and energy digestibility calculated from the differences between total energy intake and fecal energy output.
Blockade of 5HT3 receptors and β-AR
To assess the role of 5HT3 receptors and β-AR in energy balance, ondansetron (Ondansetron hydrochloride, Tocris, Burlington, ON, Canada, #2891) a selective 5HT3 receptor antagonist, and propranolol (Propranolol hydrochloride; Sigma-Aldrich, Oakville, ON, Canada, #P8688) a β1 and β2-AR blocker, were administered on d 6–8 and 15–17, respectively. In a cross-over design, following an overnight fast, each animal received intraperitoneal injection of saline or ondansetron (0.5 ml; 1 mg/kg in sterile 0.9% saline)29,30,31 at 1030 h (30 min before the onset of dark period) with ~48 h between injections. Similarly, overnight fasted rats received subcutaneous injection of either saline or propranolol (0.5 ml; 10 mg/kg in sterile 0.9% saline)20,63.
Meal test and tissue harvesting
In experiment 3, on d 19, following an overnight fast, rats were allowed to freely consume their usual treatment diet for 1 h after dark onset (1100 h). Blood samples were obtained from the saphenous vein before (0 min) and at 60 and 120 min after onset of food access, plasma separated, and various tissues sampled at termination (Supplementary Materials and Methods). Blood glucose concentrations were measured using a glucometer at the above mentioned time points (Accu-Chek®; Roche Diagnostics, QC, Canada).
Plasma hormones and amino acids
Plasma concentrations of PYY, GIP, amylin, insulin, C-peptide and leptin were measured in duplicate using a Milliplex® Map rat gut hormone panel (Millipore, Luminex Corp., Austin, TX; RGT 88 K) on a Luminex® platform (Bio-Plex 200) following our published procedures5,62. Plasma FGF21 concentrations were measured using a commercially available rat/mouse FGF21 ELISA kit (EMD Millipore Corporation, Saint Charles, MO, USA, #EZRMFGF21–26 K). The intra-assay coefficient of variation for PYY, GIP, amylin, insulin, C-peptide, leptin and FGF21 were 8.42, 19.21, 11.45, 3.46, 5.03, 8.13 and 3.75%, respectively. Terminal postprandial samples were used for measuring plasma amino acid concentrations (Supplementary Materials and Methods).
Immunoblot and reverse transcription semi-quantitative real-time polymerase chain reaction (RT-qPCR) analyses
Immunoblotting was performed (see Supplementary Methods and Supplementary Table S5) for eIF2α, peIF2α (Ser51), ATF4, and HADH in liver following our published procedures5,62,64. RT-qPCR was performed (see Supplementary Methods and Supplementary Table S6) for GCN2, ATF4, FGF21, β-Klotho, SLC38A2, SLC7A5, SLC3A2, BCKDH, CD36, FAS, CPT1, SERT, TPH1, β3-AR, UCP1, UCP3, irisin, and PGC1-α in BAT, muscle and liver following our published procedures5,62,64.
Statistical analysis
Repeated measures on energy intake, energy expenditure, body composition, body weight, IPGTT and plasma hormones were analyzed by linear mixed models using SPSS (IBM® SPSS® Statistics Version 22, Armonk, NY, USA). Metabolic measurements during the 14-day protein restriction phase of experiments 1 to 3 were combined prior to analyses. The fixed effects of dietary treatment, time and the interaction of dietary treatment and time were included in the model. In addition, energy expenditure was also analyzed by incorporating lean mass as a covariate in the above model, followed by ANCOVA at each time point. For ondansetron and propranolol effects on energy intake and energy expenditure, data were modeled to include fixed effects of dietary treatment, drug, time and interactions of dietary treatment, drug and time. Animal nested in dietary treatment was the random variable on which repeated measures were taken and covariance structures modeled either as compound symmetry, heterogenous compound symmetry, first-order antedependence, autoregressive, heterogenous autoregressive or toeplitz. Discrete data on plasma amino acids, AUC for drug effects on energy expenditure, digestible energy, feed efficiency, and protein and mRNA abundance of tissue markers, were analyzed by one-way ANOVA with dietary treatment as a between-subject factor. Means were separated by Dunnett’s post hoc test with 15P dietary treatment as the control. For drug effects within groups, paired t-test was used to separate means. Data are presented as the mean ± standard error of the mean (SEM). P values < 0.05 were considered to declare significant difference and trends were indicated at P values < 0.10.
Additional Information
How to cite this article: Pezeshki, A. et al. Low protein diets produce divergent effects on energy balance. Sci. Rep. 6, 25145; doi: 10.1038/srep25145 (2016).
Supplementary Material
Acknowledgments
This study was funded by the Heart and Stroke Foundation of Canada Postdoctoral Fellowship to A.P., Markin USRP Scholarship to N.J.Y. and by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (Application ID 355993-2013), Koopmans Memorial Research Fund, Canada Foundation for Innovation (Project# 18617) and Alberta Advanced Education and Technology (Project# URSI-09-008-SEG) to P.K.C.
Footnotes
Author Contributions A.P. and P.K.C. designed the study; A.P., P.K.C., N.J.Y., A.S. and R.C.Z. performed animal experiments; A.P., R.C.Z., A.S. and N.J.Y. performed the tissue and other laboratory analyses; A.P., P.K.C. and R.C.Z. analyzed the data, A.P. drafted the article; P.K.C., R.C.Z., A.S. and N.J.Y. contributed to writing and editing of the article; P.K.C. had primary responsibility for final content. All authors have read and approved the final manuscript.
References
- Gosby A. K. et al. Testing protein leverage in lean humans: a randomised controlled experimental study. PLos. One 6, e25929 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S. J. & Raubenheimer D. Obesity: the protein leverage hypothesis. Obes. Rev 6, 133–142 (2005). [DOI] [PubMed] [Google Scholar]
- Gosby A. K., Conigrave A. D., Raubenheimer D. & Simpson S. J. Protein leverage and energy intake. Obes. Rev 15, 183–191 (2014). [DOI] [PubMed] [Google Scholar]
- Leidy H. J. et al. The role of protein in weight loss and maintenance. Am. J. Clin. Nutr (2015). [DOI] [PubMed] [Google Scholar]
- Pezeshki A., Fahim A. & Chelikani P. K. Dietary Whey and Casein Differentially Affect Energy Balance, Gut Hormones, Glucose Metabolism, and Taste Preference in Diet-Induced Obese Rats. J. Nutr 145, 2236–2244 (2015). [DOI] [PubMed] [Google Scholar]
- Solon-Biet S. M. et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 19, 418–430 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. D., He B., Dean R. G. & Martin R. J. Low protein diets increase neuropeptide Y gene expression in the basomedial hypothalamus of rats. J. Nutr 124, 1152–1160 (1994). [DOI] [PubMed] [Google Scholar]
- White B. D., Porter M. H. & Martin R. J. Effects of age on the feeding response to moderately low dietary protein in rats. Physiol Behav 68, 673–681 (2000). [DOI] [PubMed] [Google Scholar]
- Whitedouble dagger B. D., Porter M. H. & Martin R. J. Protein selection, food intake, and body composition in response to the amount of dietary protein. Physiol Behav 69, 383–389 (2000). [DOI] [PubMed] [Google Scholar]
- Griffioen-Roose S. et al. Protein status elicits compensatory changes in food intake and food preferences. Am. J. Clin. Nutr 95, 32–38 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffioen-Roose S. et al. Human protein status modulates brain reward responses to food cues. Am. J. Clin. Nutr 100, 113–122 (2014). [DOI] [PubMed] [Google Scholar]
- Martens E. A., Tan S. Y., Dunlop M. V., Mattes R. D. & Westerterp-Plantenga M. S. Protein leverage effects of beef protein on energy intake in humans. Am. J. Clin. Nutr 99, 1397–1406 (2014). [DOI] [PubMed] [Google Scholar]
- Martens E. A., Tan S. Y., Mattes R. D. & Westerterp-Plantenga M. S. No protein intake compensation for insufficient indispensable amino acid intake with a low-protein diet for 12 days. Nutr. Metab (Lond) 11, 38 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F., Higginbotham D. A. & White B. D. Food intake, energy balance and serum leptin concentrations in rats fed low-protein diets. J. Nutr 130, 514–521 (2000). [DOI] [PubMed] [Google Scholar]
- Peng Y. S., Meliza L. L., Vavich M. G. & Kemmerer A. R. Changes in food intake and nitrogen metabolism of rats while adapting to a low or high protein diet. J. Nutr 104, 1008–1017 (1974). [DOI] [PubMed] [Google Scholar]
- Kalhan S. C. et al. Metabolic and genomic response to dietary isocaloric protein restriction in the rat. J. Biol. Chem 286, 5266–5277 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T. et al. FGF21 is an endocrine signal of protein restriction. J. Clin. Invest 124, 3913–3922 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony T. G. & Gietzen D. W. Detection of amino acid deprivation in the central nervous system. Curr. Opin. Clin. Nutr. Metab Care 16, 96–101 (2013). [DOI] [PubMed] [Google Scholar]
- Rothwell N. J. & Stock M. J. Influence of carbohydrate and fat intake on diet-induced thermogenesis and brown fat activity in rats fed low protein diets. J. Nutr 117, 1721–1726 (1987). [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J. & Tyzbir R. S. Energy balance and mitochondrial function in liver and brown fat of rats fed “cafeteria” diets of varying protein content. J. Nutr 112, 1663–1672 (1982). [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J. & Tyzbir R. S. Mechanisms of thermogenesis induced by low protein diets. Metabolism 32, 257–261 (1983). [DOI] [PubMed] [Google Scholar]
- parecida de F. S. et al. Low protein diet changes the energetic balance and sympathetic activity in brown adipose tissue of growing rats. Nutrition 25, 1186–1192 (2009). [DOI] [PubMed] [Google Scholar]
- Glick Z., Teague R. J. & Bray G. A. Brown adipose tissue: thermic response increased by a single low protein, high carbohydrate meal. Science 213, 1125–1127 (1981). [DOI] [PubMed] [Google Scholar]
- Reidelberger R., Haver A. & Chelikani P. K. Role of peptide YY(3-36) in the satiety produced by gastric delivery of macronutrients in rats. Am. J. Physiol Endocrinol. Metab 304, E944–E950 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon M. D. & Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414 (2007). [DOI] [PubMed] [Google Scholar]
- Crane J. D. et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat. Med 21, 166–172 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh C. M. et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat. Commun 6, 6794 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashon J. M. et al. Central serotonergic neurons activate and recruit thermogenic brown and beige fat and regulate glucose and lipid homeostasis. Cell Metab 21, 692–705 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savastano D. M., Carelle M. & Covasa M. Serotonin-type 3 receptors mediate intestinal Polycose- and glucose-induced suppression of intake. Am. J. Physiol Regul. Integr. Comp Physiol 288, R1499–R1508 (2005). [DOI] [PubMed] [Google Scholar]
- Savastano D. M. & Covasa M. Intestinal nutrients elicit satiation through concomitant activation of CCK(1) and 5-HT(3) receptors. Physiol Behav 92, 434–442 (2007). [DOI] [PubMed] [Google Scholar]
- Savastano D. M., Hayes M. R. & Covasa M. Serotonin-type 3 receptors mediate intestinal lipid-induced satiation and Fos-like immunoreactivity in the dorsal hindbrain. Am. J. Physiol Regul. Integr. Comp Physiol 292, R1063–R1070 (2007). [DOI] [PubMed] [Google Scholar]
- Huang X. et al. Effects of dietary protein to carbohydrate balance on energy intake, fat storage, and heat production in mice. Obesity. (Silver. Spring) 21, 85–92 (2013). [DOI] [PubMed] [Google Scholar]
- Solon-Biet S. M. et al. Dietary Protein to Carbohydrate Ratio and Caloric Restriction: Comparing Metabolic Outcomes in Mice. Cell Rep 11, 1529–1534 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alippi R. M. et al. Dynamics of recovery of morphometrical variables and pQCT-derived cortical bone properties after a short-term protein restriction in maturing rats. Growth Dev. Aging 65, 67–72 (2002). [PubMed] [Google Scholar]
- Boualga A., Bouchenak M. & Belleville J. Low-protein diet prevents tissue lipoprotein lipase activity increase in growing rats. Br. J. Nutr 84, 663–671 (2000). [DOI] [PubMed] [Google Scholar]
- Lamri M. Y., Meghelli-Bouchenak M., Boualga A., Belleville J. & Prost J. Rat plasma VLDL composition and concentration and hepatic lipase and lipoprotein lipase activities are impaired during two types of protein malnutrition and unaffected by balanced refeeding. J. Nutr 125, 2425–2434 (1995). [DOI] [PubMed] [Google Scholar]
- Beck B., Dollet J. M. & Max J. P. Refeeding after various times of ingestion of a low protein diet: effects on food intake and body weight in rats. Physiol Behav 45, 761–765 (1989). [DOI] [PubMed] [Google Scholar]
- Dulloo A. G. & Girardier L. Influence of dietary composition on energy expenditure during recovery of body weight in the rat: implications for catch-up growth and obesity relapse. Metabolism 41, 1336–1342 (1992). [DOI] [PubMed] [Google Scholar]
- Bengtsson T., Cannon B. & Nedergaard J. Differential adrenergic regulation of the gene expression of the beta-adrenoceptor subtypes beta1, beta2 and beta3 in brown adipocytes. Biochem. J 347 Pt 3, 643–651 (2000). [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Cannon B. & Nedergaard J. Thermogenesis is beta3- but not beta1-adrenergically mediated in rat brown fat cells, even after cold acclimation. Am. J. Physiol 275, R2002–R2011 (1998). [DOI] [PubMed] [Google Scholar]
- Bengtsson T., Redegren K., Strosberg A. D., Nedergaard J. & Cannon B. Down-regulation of beta3 adrenoreceptor gene expression in brown fat cells is transient and recovery is dependent upon a short-lived protein factor. J. Biol. Chem 271, 33366–33375 (1996). [DOI] [PubMed] [Google Scholar]
- Senault C., Le, C., V. & Portet, R. Characteristics of beta-adrenergic receptors in isolated cells and in crude membranes of brown adipose tissue. Biochimie 66, 573–578 (1984). [DOI] [PubMed] [Google Scholar]
- Hondares E. et al. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem 286, 12983–12990, doi: 10.1074/jbc.M110.215889 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douris N. et al. Central Fibroblast Growth Factor 21 Browns White Fat via Sympathetic Action in Male Mice. Endocrinology 156, 2470–2481, doi: 10.1210/en.2014-2001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees E. K. et al. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell 13, 817–827 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders D. et al. Metabolic responses to dietary leucine restriction involve remodeling of adipose tissue and enhanced hepatic insulin signaling. Biofactors 41, 391–402 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher F. M. et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26, 271–281 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell N. J. & Stock M. J. Effect of diet and fenfluramine on thermogenesis in the rat: possible involvement of serotonergic mechanisms. Int. J. Obes 11, 319–324 (1987). [PubMed] [Google Scholar]
- Simpson K. H., Murphy P., Colthup P. V. & Whelan P. Concentration of ondansetron in cerebrospinal fluid following oral dosing in volunteers. Psychopharmacology (Berl) 109, 497–498 (1992). [DOI] [PubMed] [Google Scholar]
- Miles-Chan J. L., Dulloo A. G. & Schutz Y. Fasting substrate oxidation at rest assessed by indirect calorimetry: is prior dietary macronutrient level and composition a confounder? Int. J. Obes. (Lond) 39, 1114–1117 (2015). [DOI] [PubMed] [Google Scholar]
- van Schothorst E. M., Bunschoten A., Schrauwen P., Mensink R. P. & Keijer J. Effects of a high-fat, low- versus high-glycemic index diet: retardation of insulin resistance involves adipose tissue modulation. FASEB J 23, 1092–1101, doi: 10.1096/fj.08-117119 (2009). [DOI] [PubMed] [Google Scholar]
- Pawlak D. B., Bryson J. M., Denyer G. S. & Brand-Miller J. C. High glycemic index starch promotes hypersecretion of insulin and higher body fat in rats without affecting insulin sensitivity. J Nutr 131, 99–104 (2001). [DOI] [PubMed] [Google Scholar]
- Aziz A. A., Kenney L. S., Goulet B. & Abdel-Aal el S. Dietary starch type affects body weight and glycemic control in freely fed but not energy-restricted obese rats. J Nutr 139, 1881–1889, doi: 10.3945/jn.109.110650 (2009). [DOI] [PubMed] [Google Scholar]
- Ble-Castillo J. L. et al. Differential effects of high-carbohydrate and high-fat diet composition on metabolic control and insulin resistance in normal rats. Int J Environ Res Public Health 9, 1663–1676, doi: 10.3390/ijerph9051663 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonney M. L., Arnold A. M., Ross D. A., Schaaf S. L. & Rounsaville T. R. Energetic efficiency of rats fed low or high protein diets and grown at controlled rates from 80 to 205 grams. J Nutr 121, 1397–1406 (1991). [DOI] [PubMed] [Google Scholar]
- Hillgartner F. B. & Romsos D. R. Iodothyronine 5’-deiodination in rats fed low protein diets: lack of correlation with energy balance. J Nutr 117, 368–375 (1987). [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J. & Cui J. Obesity: Dietary Factors and Control. (ed. Romsos D. R., Himms-Hagen J. & Suzuki M.). 81–95 (Basel, Karger, 1991). [Google Scholar]
- Tschop M. & Heiman M. L. Overview of rodent models for obesity research. Curr. Protoc. Neurosci Unit 9.1, 1–14 (2001). [DOI] [PubMed] [Google Scholar]
- Levin B. E., Dunn-Meynell A. A., Balkan B. & Keesey R. E. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am. J. Physiol 273, R725–R730 (1997). [DOI] [PubMed] [Google Scholar]
- Mercer J. G. & Archer Z. A. Putting the diet back into diet-induced obesity: diet-induced hypothalamic gene expression. Eur. J. Pharmacol 585, 31–37 (2008). [DOI] [PubMed] [Google Scholar]
- National Research Council (US). Subcommittee on Laboratory Animal Nutrition. Nutrient requirements of laboratory animals. 4th rev. edn, (National Academy of Sciences, 1995). [Google Scholar]
- Nausheen S., Shah I. H., Pezeshki A., Sigalet D. L. & Chelikani P. K. Effects of sleeve gastrectomy and ileal transposition, alone and in combination, on food intake, body weight, gut hormones, and glucose metabolism in rats. Am. J. Physiol Endocrinol. Metab 305, E507–E518 (2013). [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Saville M. E. & Stock M. J. Effects of feeding a “cafeteria” diet on energy balance and diet-induced thermogenesis in four strains of rat. J. Nutr 112, 1515–1524 (1982). [DOI] [PubMed] [Google Scholar]
- Pezeshki A. & Chelikani P. K. Effects of Roux-en-Y gastric bypass and ileal transposition surgeries on glucose and lipid metabolism in skeletal muscle and liver. Surg Obes Relat Dis 10, 217–228 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.