Abstract
Suicide is a leading cause of death and a significant public health concern. Brain-derived neurotrophic factor (BDNF), a protein important to nervous system function, has been implicated in psychiatric disorders and suicidal behaviour. We investigated the association between serum levels of BDNF and attempted suicide in a sample of 281 participants using a case-control study design. Participants were recruited from clinical and community settings between March 2011 and November 2014. Cases (individuals who had attempted suicide) (n = 84) were matched on sex and age (within five years) to both psychiatric controls (n = 104) and community controls (n = 93) with no history of suicide attempts. We collected fasting blood samples, socio-demographic information, physical measurements, and detailed descriptions of suicide attempts. We used linear regression analysis to determine the association between BDNF level (dependent variable) and attempted suicide (key exposure variable), adjusting for age, sex, body mass index, current smoking status, and antidepressant use. 250 participants were included in this analysis. In the linear regression model, attempted suicide was not significantly associated with BDNF level (β = 0.28, SE = 1.20, P = 0.82). Our findings suggest that no significant association exists between attempted suicide and BDNF level. However, the findings need to be replicated in a larger cohort study.
Suicide claims nearly one million lives each year, making it a leading cause of death worldwide and a significant public health concern1. The devastating effects of suicide are felt at the family, community, and societal levels. Suicidal thoughts, plans, and acts intended to end one’s life all comprise the complex phenomenon of suicidal behaviour. Non-fatal suicidal behaviours are 10–20 times more common than completed suicide2. Attempted suicide is also an important risk factor for future completed suicide1.
Many risk factors are thought to contribute to the risk of suicidal behaviour. These include biological, psychological, social, and environmental factors3,4,5. Psychiatric disorders are highly predictive of suicidal behaviour, among which mood disorders pose significant risk5. Population level estimates suggest that 90% of attempted and completed suicides occur in the context of a psychiatric disorder3. Other known risk factors include chronic illness, substance use disorders, and demographic variables such as age and sex3. These biological and psychological factors point to a predisposition toward suicidal behaviour in some individuals. However, these factors alone do not predict suicidal behaviour. Social and environmental risk factors also play a role, and include unemployment, low educational attainment, unmarried status, and a lack of social support3. Incidents of suicidal behaviour likely result from the interaction between biological and psychosocial factors.
Brain-derived neurotrophic factor (BDNF) is the most abundant member of the neurotrophins, a family of proteins that regulate the survival, development, maintenance, and function of vertebral nervous systems6. BDNF is involved in many neural processes including neurogenesis, nerve growth, neuroplasticity, and neurotransmission7. Altered levels of BDNF have been associated with several psychiatric conditions.
Low blood levels of BDNF have been linked to depression8,9 and reduced BDNF expression in the brain has been linked to stress10,11. Both depression and stress are major risk factors for suicidal behaviour7. Since BDNF is intrinsic to optimal nervous system function, pathological changes in BDNF levels are a possible cause of neurobiological deficits that impair one’s ability to adapt to difficult situations7.
Recent research has examined the association between BDNF and suicidal behaviour12,13,14,15,16,17,18,19,20,21,22,23. The literature on this topic has been summarized and evaluated in a systematic review and meta-analysis by Eisen et al.24. Some studies have investigated postmortem levels of BDNF in the brains of suicide victims12,13,14, while other studies have measured peripheral BDNF levels in clinical samples15,16,17,18,19,20,21,22,23. Postmortem studies have found significantly lower BDNF levels in the hippocampus and prefrontal cortex in individuals who died by suicide compared to individuals who died of other causes12,13,14. Studies comparing peripheral levels of BDNF in individuals with and without a history of suicidal behaviour have shown conflicting results15,16,17,18,19,20. Some studies of serum levels of BDNF have shown significantly reduced levels in individuals with suicide attempts compared to both psychiatric and healthy controls15,16. However, other studies making the same comparison found no significant relationship17,18,19,20. Studies of plasma BDNF levels in individuals with depression with and without a history of suicidal behaviour are similarly conflicted in their findings21,22,23.
Studies of the association between BDNF levels and suicidal behaviour are few in number and limited in methodology. The sample sizes are generally small, with some comparison groups containing as few as 10 participants (for examples, see17,18). As well, nearly all previous studies conducted univariate analyses to compare BDNF levels among patient groups, and few studies adjusted for confounding variables in their analyses. Furthermore, many previous studies compare BDNF levels between groups of individuals with and without a lifetime history of attempted suicide (for examples, see15,18,22). In these studies, the BDNF measurements may not represent BDNF levels at the time of the attempt. Additional research is required to establish the relationship between BDNF levels and suicidal behaviour.
In the present study we examine the relationship between serum BDNF levels and recent suicide attempts (within the past three months) in a large clinical sample using a case-control study design.
Results
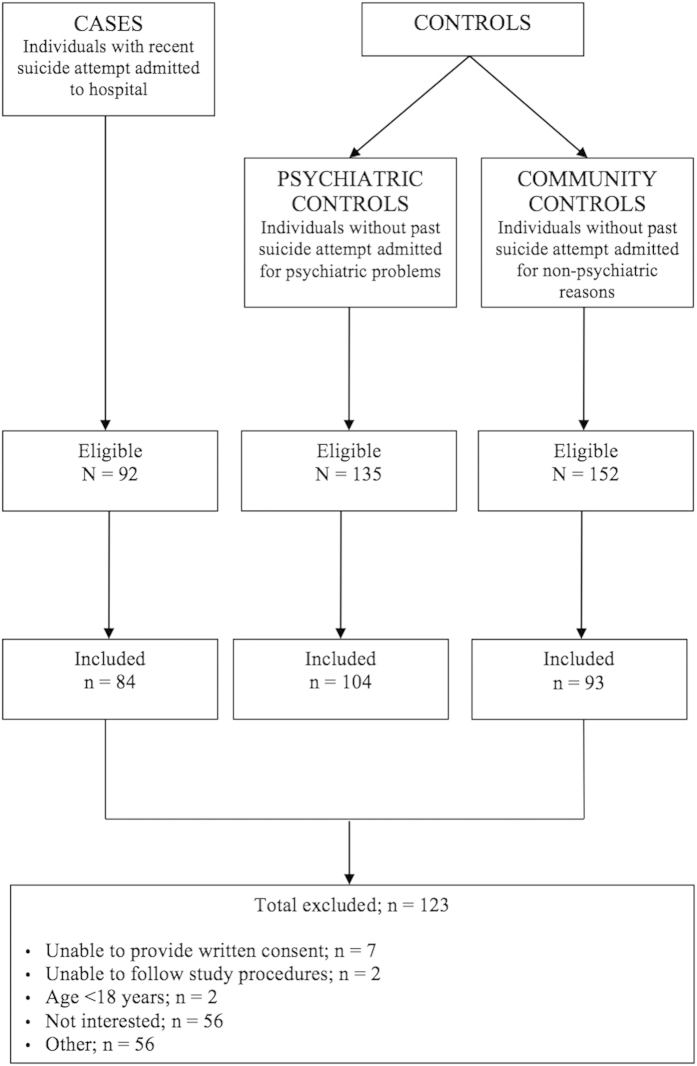
A total of 281 participants were recruited, including 84 cases (individuals who had attempted suicide), 104 psychiatric controls, and 93 community controls (see Fig. 1 for flow diagram of recruitment). The mean age of the sample was 43.7 years (standard deviation [SD] = 14.6, range: 18–73). The sample contained approximate equal numbers of males and female participants (52.2% female). The three groups differed significantly on a number of demographic variables including education level and employment status, with the case group containing the fewest individuals with secondary education and current employment. Several psychiatric disorders were most common in the case group, including major depressive disorder, anxiety disorders, alcohol and substance abuse, and antisocial personality disorder. The mean BDNF level was 24.21 ng/ml (SD = 7.19) in the case group, 23.88 ng/ml (SD = 6.95) in the psychiatric control group, and 24.77 ng/ml (SD = 7.01) in the community control group (see Fig. 2). The sample characteristics are summarized in Table 1.
Figure 1. Flow diagram for participants included in the DISCOVER Study.
Figure 2. Serum BDNF Level by Group.
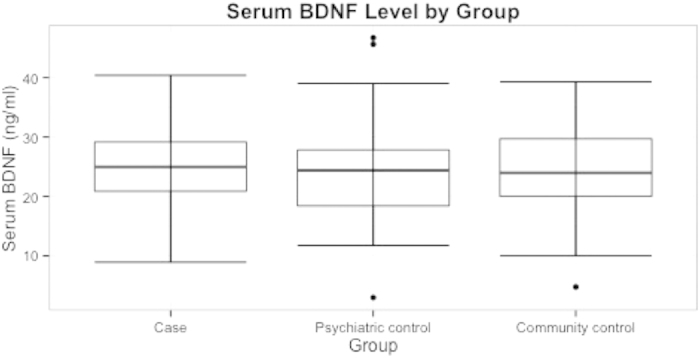
The mean serum BDNF level was 24.21 (7.19) ng/ml in the case group, 23.88 (6.95) ng/ml in the psychiatric control group, and 24.77 (7.19) ng/ml in the community control group. No significant differences were found among groups (P = 0.59).
Table 1. Comparison of Baseline Characteristics.
| Cases (n = 84) | Psychiatric controls (n = 104) | Community controls (n = 93) | Univariate differences1 | |
|---|---|---|---|---|
| Demographic Variables | ||||
| Mean Age (SD) (years) | 43.01 (14.03) | 45.01 (14.23) | 46.36 (17.81) | F = 1.05, P = 0.35 |
| Sex (% female) | 44 (52.38) | 52 (50.00) | 46 (49.46) | χ2 = 0.17, P = 0.92 |
| Completed secondary education (%) | 35 (42.68) | 51 (50.49) | 69 (74.19) | χ2 = 19.53, P < 0.001 |
| Currently employed (%) | 22 (26.51) | 33 (32.67) | 55 (59.14) | χ2 = 22.80, P < 0.001 |
| Marital status | χ2 = 24.20, P < 0.001 | |||
| Never married (%) | 27 (32.53) | 43 (41.75) | 28 (30.11) | |
| Married/common law (%) | 21 (25.30) | 31 (30.10) | 50 (53.76) | |
| Widowed/separated/divorced (%) | 35 (42.17) | 29 (28.16) | 15 (16.13) | |
| Prognostic Factors | ||||
| Currently smoking (%) | 35 (43.21) | 35 (35.35) | 11 (11.83) | χ2 = 22.84, P < 0.001 |
| Taking antidepressants (%) | 40 (47.61) | 32 (30.77) | 14 (15.05) | χ2 = 22.04, P < 0.001 |
| Mean serum BDNF (SD) (ng/ml) | 24.21 (7.19) | 23.88 (6.95) | 24.77 (7.19) | F = 0.297, P = 0.59 |
| Mean body mass index (SD) (kg/m2) | 27.88 (7.28) | 29.78 (9.80) | 27.73 (6.18) | F = 0.045, P = 0.83 |
| Psychiatric Diagnoses2 | ||||
| Major depressive disorder (%) | 42 (63.63) | 38 (41.30) | 20 (21.74) | χ2 = 28.21, P < 0.001 |
| Mood disorder (%) | 63 (95.45) | 76 (82.60) | 26 (28.26) | χ2 = 95.22, P < 0.001 |
| Anxiety disorder (%) | 43 (65.15) | 49 (53.26) | 9 (9.78) | χ2 = 58.93, P < 0.001 |
| Alcohol abuse (%) | 18 (27.27) | 13 (14.13) | 4 (4.35) | P < 0.001 |
| Substance abuse (%) | 11 (16.67) | 11 (11.96) | 2 (2.17) | P = 0.003 |
| Eating disorder (%) | 5 (75.76) | 5 (5.43) | 1 (1.09) | P = 0.11 |
| Psychotic disorder (%) | 3 (4.55) | 6 (6.52) | 0 (0) | P = 0.024 |
| Antisocial personality disorder (%) | 15 (22.73) | 0 (0) | 2 (2.17) | P < 0.001 |
1Analysis of variance tests (ANOVA) were used to compare means for continuous variables. Chi-square tests were used to compare proportions for categorical variables. Fisher’s exact test was used for categorical variables when one or more values in the contingency table were below 5.
2Since not all participants underwent the Mini-International Neuropsychiatric Interview (MINI), the group sizes for this section of the table are as follows: 66 cases, 92 psychiatric controls, 92 community controls. Abbreviations: SD, standard deviation; BDNF, brain-derived neurotrophic factor.
Mean BDNF levels for the largest psychiatric disorder categories (mood disorders and anxiety disorders) were compared using ANOVA. In both categories, no significant differences were found in mean BDNF level among cases, psychiatric controls, and community controls (F = 0.55, P = 0.58 for mood disorder; F = 0.20, P = 0.82 for anxiety disorder).
Primary Analysis
The linear regression analysis did not demonstrate a significant association between attempted suicide and BDNF level (β = 0.28, standard error [SE] = 1.20, P = 0.82). Of the covariates included (age, sex, smoking status, body mass index, and antidepressant use), only antidepressant use was significantly associated with BDNF level (β = −2.50, SE = 0.99, P = 0.012). The analysis included 250 out of 281 participants (11% missing data). The assumptions of linearity, independence, normality, and homoscedasticity were tested and all were satisfied. The variance inflation factors (VIF) for all variables were below 1, indicating that multicollinearity was not a concern. See Table 2 for linear regression model.
Table 2. Association between BDNF and Key Baseline Characteristics.
| Univariate Analysis |
Multivariable Analysis1 |
|||||
|---|---|---|---|---|---|---|
| Variables | β Estimate | Standard Error | P-value | β Estimate | Standard Error | P-value |
| Group | ||||||
| Suicide attempt | −0.56 | 1.09 | 0.611 | 0.28 | 1.20 | 0.818 |
| Psychiatric control | −0.89 | 1.04 | 0.391 | −0.82 | 1.10 | 0.455 |
| Community control | 0 (ref.) | – | – | 0 (ref.) | – | – |
| Age (years) | −0.02 | 0.03 | 0.388 | −0.02 | 0.03 | 0.578 |
| Sex (female) | 1.29 | 0.87 | 0.138 | 1.51 | 0.89 | 0.090 |
| Currently smoking (yes) | 1.08 | 0.97 | 0.268 | 1.43 | 1.02 | 0.163 |
| Body mass index (kg/m2) | −0.03 | 0.05 | 0.613 | −0.01 | 0.05 | 0.864 |
| Taking antidepressant (yes) | −2.06 | 0.92 | 0.026* | −2.50 | 0.99 | 0.012* |
1Adjusted R-squared: 0.022. P-value: 0.09. N = 250.
When this analysis was repeated with the inclusion of mood disorders and anxiety disorders as covariates, neither was significantly associated with BDNF level β = −0.79, P = 0.54 for mood disorder; β = 2.20, P = 0.07 for anxiety disorder). The overall results were the same as those of the primary analysis.
Sensitivity Analysis
Linear regression analyses were conducted to examine the relationship between attempted suicide and BDNF level when cases are compared to each control group individually. In the first model cases were compared to psychiatric controls, and in the second model cases were compared to community controls. In both models, attempted suicide was not significantly associated with BDNF level (β = 0.85, SE = 1.13, P = 0.46 in the first model; β = 0.20, SE = 1.27, P = 0.87 in the second model).
Discussion
Upon examining the association between serum BDNF level and attempted suicide using a case-control design, our findings demonstrate that serum BDNF level was not significantly associated with attempted suicide. The association was true regardless of comparison group.
Our study’s findings are not in accordance with the hypothesis that lower levels of BDNF are associated with suicidal behaviour. Our findings conflict with previous literature on this relationship, including a study by Dawood et al. that examined the association between internal jugular venous BDNF and suicidal behaviour25. Dawood et al. found a negative correlation between suicide risk and BDNF concentration, which supports the notion that reduced brain levels of BDNF are involved in the pathogenesis of depression and suicide. However, the study’s small sample size (16 participants) and unadjusted statistical analyses may have resulted in a biased estimate.
Only six previous studies have compared serum BDNF levels in groups with and without suicidal behaviour. While two of these studies found a significant association between serum BDNF and attempted suicide15,16, the remaining studies found no significant association17,18,19,20. While our study’s findings are not in accordance with the hypothesis that lower levels of BDNF are associated with depression and suicidal behaviour, they are consistent with other studies of this relationship.
This study has a number of strengths that distinguish it from other studies in this area. Our sample of 281 participants was larger than most previous studies, some of which included only 40 participants in total. As well, since our case group comprised individuals with recent suicide attempts (within three months of recruitment), the BDNF measurement was taken within a consistently short period of time relative to the attempt. Other studies have included participants with a lifetime history of suicide attempts. In these studies, the BDNF measurements may not represent BDNF levels near the time of the attempt. An additional strength of this study is the inclusion of two control groups, a psychiatric control group and a community control group. Since the majority of suicide attempts occur in the context of a psychiatric disorder3, it is important to include both comparisons in studies of suicidal behaviour. Finally, unlike most previous studies, we performed adjusted analyses to examine the relationship between BDNF level and attempted suicide. We adjusted for a number of variables known to be associated with altered BDNF level. The results of other studies may have been influenced by confounding variables such as age, smoking status, and body mass index.
Comparing BDNF levels among the three groups (cases, psychiatric controls, and community controls) within categories of psychiatric disorders (mood disorders and anxiety disorders) revealed that mean BDNF levels did not differ significantly within either the mood disorder or anxiety disorder categories. As well, when each of these diagnosis categories was include in the linear regression model, neither was significantly associated with BDNF level. While there is evidence to suggest that BDNF levels are related to several psychiatric disorders, including depression8,9, bipolar disorder26,27, schizophrenia28, and substance use disorder29,30, as of yet, it is unknown whether the relationship between BDNF and suicidal behaviour depends on the presence of an underlying psychiatric disorder. Future research should explore this topic.
Our finding that antidepressant use was associated with decreased BDNF level is contradictory to most previous evidence. In a meta-analysis conducted by Molendijk et al. in 2013, serum BDNF levels were found to be increased in antidepressant-treated patients with depression compared to untreated patients with depression31. The meta-analysis included 28 comparisons, of which only four found the opposite effect, with increased BDNF levels in untreated groups with depression32,33,34,35. These studies generally concluded that the effects of antidepressants on BDNF concentrations depends on the type of antidepressant used, and even within the same category of antidepressant (selective serotonin reuptake inhibitors, for instance), different antidepressants vary in their effects on BDNF level. Since all types of antidepressants were combined into one variable in our analysis, this could explain our unexpected result. Molendijk et al.’s meta-analysis also concluded that most studies of BDNF levels in antidepressant-treated and untreated individuals with depression are underpowered, and when publication bias is accounted for, the effect size is smaller than previously thought31. This puts into question the notion that high BDNF levels are in fact associated with antidepressant use. Another important point to consider is that of the 80 individuals in our sample who were taking antidepressants, 41% did not meet the requirements for major depressive disorder according to the Mini-International Neuropsychiatric Interview. These individuals could have been taking antidepressant medications for various reasons, including treatment of anxiety, chronic pain, or insomnia. This may partly explain our finding, since other factors may be responsible for the low BDNF levels in patients on antidepressants in our sample.
Future studies should aim to replicate these findings using a cohort study design. Additional research should also explore other potential biomarkers for suicidal behaviour. Since there is evidence for a connection between hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis and suicidal behaviour2, potential biomarkers could include cortisol or pro-inflammatory cytokines.
Conclusion
Our case-control study shows no significant association between serum BDNF level and attempted suicide. However, the finding will need to be replicated in a larger cohort study.
Methods
Data Collection
The data used in this study were collected from the Study of Determinants of Suicide Conventional and Emergent Risk (DISCOVER), an observational matched case control study that aims to understand the risk factors involved in suicidal behaviour36. Cases (participants hospitalized for a suicide attempt) were matched on age and sex to two control groups (psychiatric patients and community controls). Data were collected at St. Joseph’s Healthcare Hamilton, Hamilton Health Sciences Hospitals, and the Hamilton City community, in Ontario, Canada. Data collection began in March 2011 and ended in November 2014. The study was approved by the Hamilton Integrated Research Ethics Board (HiREB) (number 10–661 for St. Joseph’s Healthcare Hamilton and 11–3479 for Hamilton Health Sciences Hospitals). The methods of this study were performed in accordance with the HiREB guidelines. This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines37.
Inclusion and Exclusion Criteria
The study included men and women 18 years and older who were able to provide written informed consent, communicate in English, and follow study procedures. Cases were defined as individuals who made a serious suicide attempt (defined as self-directed injury with intent to die) within in the last three months, were admitted to hospital, and required medical or psychiatric intervention. Cases were matched based on sex and age (within five years) to both psychiatric and community control groups. The psychiatric control group consisted of individuals with psychiatric disorders with no history of suicide attempts, who were admitted to hospital within the same time frame as the cases. The community control group consisted of individuals with no history of suicide attempts who were recruited from community and non-psychiatric clinical areas. Participants were excluded if they were unable to provide informed consent or follow study procedures.
Recruitment
Upon recruitment, fasting blood and urine samples were collected and a structured interview was conducted. Data were obtained on socio-demographic variables (age, sex, ethnicity, religion, marital status, education, employment, and social support), medical history and current medications, psychopathology, physical measurements, and suicidal behaviour. The study questionnaires were compiled using previously validated diagnostic and assessment tools including the Mini-International Neuropsychiatric Interview38 and the Beck Suicide Intent Scale39. For participants in the case group, a detailed description of the suicide attempt was recorded. All assessments were administered in hospital or community by trained research staff.
Laboratory Analysis
12-hour fasting blood samples were collected, processed, and stored at the Clinical Research and Clinical Trials Laboratory at Hamilton General Hospital. Samples were processed within two hours of collection. After 30 minutes of clotting time, samples were spun at 1500 × g (3000 rpm) for 15 minutes until blood was well separated. Samples were then aliquotted into 2 mL cryovials and stored in liquid nitrogen for future analyses. Serum BDNF level was assayed using Quantikine® ELISA Human BDNF Immunoassay (R&D Systems Inc.). All analyses were conducted blindly according to standard procedures.
Statistical Analysis
We used descriptive statistics to summarize baseline characteristics of the sample. Means and standard deviations (SD) were reported for continuous variables, and counts and percentages were reported for categorical variables. Analysis of variance tests (ANOVA) were used to compare means for continuous variables and chi-square tests were used to compare proportions for categorical variables.
We employed a linear regression to model the association between attempted suicide and serum BDNF level. The confounders included in the model were selected a priori based on previous literature (age, sex, smoking status, body mass index, and antidepressant use). Serum BDNF level was the outcome variable, and case/control group (suicide attempt, psychiatric control, or community control) was the independent variable of interest. We chose to perform a linear regression analysis with BDNF level as the dependent variable because, while it is unknown whether BDNF is related to suicidal behaviour in a causal manner, it is known that many factors can influence BDNF levels. This analysis allowed us to investigate the relationship between attempted suicide and BDNF level in the context of other factors known to affect BDNF level.
We performed a sensitivity analysis using linear regression to explore the association between attempted suicide and serum BDNF level by comparing the case group to each control group individually. The same confounders listed above were included in the sensitivity analyses models. All analyses were performed using R version 3.0.240.
Additional Information
How to cite this article: Eisen, R.B. et al. Exploring the Association between Serum BDNF and Attempted Suicide. Sci. Rep. 6, 25229; doi: 10.1038/srep25229 (2016).
Acknowledgments
This work was supported by the Brain and Behavior Research Foundation Young Investigator Grant (# 19058). The Brain and Behavior Research Foundation has no role in the design of the study or publication of the results.
Footnotes
Author Contributions R.B.E. developed the research question, analyzed and interpreted the data, and wrote and critically revised the manuscript. S.P. contributed to data analysis and interpretation, and critically revised the manuscript. M.B. and B.D. assisted with recruitment and critically revised the manuscript. W.E.-S. was the research assistant for this study and was responsible for recruitment, interviews, and data collection. J.D. was the study coordinator responsible for the daily running of the study, training research assistants and study personnel on study-related procedures. S.R. was the study project manager responsible for the study conduct; with Z.S. she designed the study protocol, provided supervision of study personnel, and coordinated the overall study management. J.V., H.S., N.H. and E.I. are nurses specializing in psychiatry; they recruited hospitalized study patients, conducted interviews, collected blood samples, and provided feedback on participants’ recruitment and study procedures. P.M. was responsible for database management, quality and edits, checks of data, and provision of reports, including age and sex matching status, to the study team for the weekly study meetings. S.I. was responsible for data management and preparation of data reports to the team. M.D. designed the dietary tool and analyzed the dietary data for the DISCOVER study. J.B. provided advice on study procedures including recruitment and the inclusion of psychiatric controls based on her expertise in emergency psychiatry and suicide prevention. R.A. critically revised the manuscript. L.M. contributed to data analysis and interpretation, and critically revised the manuscript. L.T. was responsible for the case-control study design, and overall methodological and statistical aspects of the study. Z.S. conceived the study and was principally responsible for the conduct of the study and obtaining funding. Z.S. also developed the research question, contributed to data analysis and interpretation, and critically revised the manuscript. All authors have reviewed and approved the final manuscript.
References
- World Health Organization. Preventing suicide: A global imperative. (World Health Organization, 2014). [Google Scholar]
- Mann J. J. Neurobiology of suicidal behaviour. Nat. Rev. Neurosci. 4, 819–828, doi: 10.1038/nrn1220 (2003). [DOI] [PubMed] [Google Scholar]
- Crump C., Sundquist K., Sundquist J. & Winkleby M. A. Sociodemographic, psychiatric and somatic risk factors for suicide: a Swedish national cohort study. Psychol. Med. 44, 279–289, doi: 10.1017/S0033291713000810 (2014). [DOI] [PubMed] [Google Scholar]
- Mann J. J. A current perspective of suicide and attempted suicide. Ann. Intern. Med. 136, 302–311 (2002). [DOI] [PubMed] [Google Scholar]
- Harris E. C. & Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br. J. Psychiatry: the journal of mental science 170, 205–228 (1997). [DOI] [PubMed] [Google Scholar]
- Huang E. J. & Reichardt L. F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci 24, 677–736, doi: 10.1146/annurev.neuro.24.1.677 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y. In The Neurobiological Basis of Suicide Frontiers in Neuroscience (ed. Dwivedi Y.) (CRC Press, 2012). [PubMed] [Google Scholar]
- Terracciano A. et al. Neuroticism, depressive symptoms, and serum BDNF. Psychosom. Med. 73, 638–642, doi: 10.1097/PSY.0b013e3182306a4f (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F. et al. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol. Psychiatry 57, 1068–1072, doi: 10.1016/j.biopsych.2005.01.008 (2005). [DOI] [PubMed] [Google Scholar]
- Fuchikami M., Morinobu S., Kurata A., Yamamoto S. & Yamawaki S. Single immobilization stress differentially alters the expression profile of transcripts of the brain-derived neurotrophic factor (BDNF) gene and histone acetylation at its promoters in the rat hippocampus. Int. J. Neuropsychopharmacol. 12, 73–82, doi: 10.1017/S1461145708008997 (2009). [DOI] [PubMed] [Google Scholar]
- Pizarro J. M. et al. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Res. 1025, 10–20, doi: 10.1016/j.brainres.2004.06.085 (2004). [DOI] [PubMed] [Google Scholar]
- Banerjee R., Ghosh A. K., Ghosh B., Bhattacharyya S. & Mondal A. C. Decreased mRNA and Protein Expression of BDNF, NGF, and their Receptors in the Hippocampus from Suicide: An Analysis in Human Postmortem Brain. Clin Med. Insights. Pathol. 6, 1–11, doi: 10.4137/CMPath.S12530 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F., Vaudan G., Schwald M., Perroud N. & La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res. Mol. Brain Res. 136, 29–37, doi: 10.1016/j.molbrainres.2004.12.020 (2005). [DOI] [PubMed] [Google Scholar]
- Dwivedi Y. et al. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Archives Gen. Psychiatry 60, 804–815, doi: 10.1001/archpsyc.60.8.804 (2003). [DOI] [PubMed] [Google Scholar]
- Grah M. et al. Brain-derived neurotrophic factor as a suicide factor in mental disorders. Acta neuropsychiatr. 1–8, doi: 10.1017/neu.2014.27 (2014). [DOI] [PubMed] [Google Scholar]
- Liang W., Zhang H.-M., Zhang H.-Y. & LV L.-X. Association of brain-derived neurotrophic factor in peripheral blood and gene expression to suicidal behaviour in patients with depression. Chin. Ment. Health J. 26, 5 (2012). [Google Scholar]
- Deveci A., Aydemir O., Taskin O., Taneli F. & Esen-Danaci A. Serum BDNF levels in suicide attempters related to psychosocial stressors: a comparative study with depression. Neuropsychobiology 56, 93–97, doi: 10.1159/000111539 (2007). [DOI] [PubMed] [Google Scholar]
- Huang T. L. & Lee C. T. Associations between serum brain-derived neurotrophic factor levels and clinical phenotypes in schizophrenia patients. J. Psychiatr. Res. 40, 664–668, doi: 10.1016/j.jpsychires.2005.11.004 (2006). [DOI] [PubMed] [Google Scholar]
- Park Y. M., Lee B. H., Um T. H. & Kim S. Serum BDNF levels in relation to illness severity, suicide attempts, and central serotonin activity in patients with major depressive disorder: a pilot study. Plos one 9, e91061, doi: 10.1371/journal.pone.0091061 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro R. T. et al. Brain-derived neurotrophic factor levels in women with postpartum affective disorder and suicidality. Neurochem. Res. 37, 2229–2234, doi: 10.1007/s11064-012-0851-9 (2012). [DOI] [PubMed] [Google Scholar]
- Kim Y. K. et al. Low plasma BDNF is associated with suicidal behavior in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 31, 78–85, doi: 10.1016/j.pnpbp.2006.06.024 (2007). [DOI] [PubMed] [Google Scholar]
- Lee B. H., Kim H., Park S. H. & Kim Y. K. Decreased plasma BDNF level in depressive patients. J. Affect. Disord. 101, 239–244, doi: 10.1016/j.jad.2006.11.005 (2007). [DOI] [PubMed] [Google Scholar]
- Lee B. H. & Kim Y. K. Reduced platelet BDNF level in patients with major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 849–853, doi: 10.1016/j.pnpbp.2009.04.002 (2009). [DOI] [PubMed] [Google Scholar]
- Eisen R. B. et al. Association between BDNF levels and suicidal behaviour: a systematic review and meta-analysis. Syst. Rev 4, 1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood T. et al. Reduced overflow of BDNF from the brain is linked with suicide risk in depressive illness. Mol. psychiatry 12, 981–983 (2007). [DOI] [PubMed] [Google Scholar]
- Tunca Z. et al. Alterations in BDNF (brain derived neurotrophic factor) and GDNF (glial cell line-derived neurotrophic factor) serum levels in bipolar disorder: The role of lithium. J. Affect. Disord. 166, 193–200, doi: 10.1016/j.jad.2014.05.012 (2014). [DOI] [PubMed] [Google Scholar]
- Piccinni A. et al. Decreased plasma levels of brain-derived neurotrophic factor (BDNF) during mixed episodes of bipolar disorder. J. Affect. Disord. 171, 167–170 (2015). [DOI] [PubMed] [Google Scholar]
- Green M., Matheson S., Shepherd A., Weickert C. & Carr V. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol. psychiatry 16, 960–972 (2011). [DOI] [PubMed] [Google Scholar]
- Chen S.-L. et al. The BDNF Val66Met polymorphism and plasma brain-derived neurotrophic factor levels in Han Chinese heroin-dependent patients. Sci. Rep. 5, doi: 10.1038/srep08148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke X. et al. Serum brain-derived neurotrophic factor and nerve growth factor decreased in chronic ketamine abusers. Drug Alcohol Depend. 142, 290–294 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk M. L. et al. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N = 9484). Mol. psychiatry 19, 791–800, doi: 10.1038/mp.2013.105 (2014). [DOI] [PubMed] [Google Scholar]
- Hellweg R., Ziegenhorn A., Heuser I. & Deuschle M. Serum concentrations of nerve growth factor and brain-derived neurotrophic factor in depressed patients before and after antidepressant treatment. Pharmacopsychiatry 41, 66–71 (2008). [DOI] [PubMed] [Google Scholar]
- Molendijk M. L. et al. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state–trait issues, clinical features and pharmacological treatment. Mol. psychiatry 16, 1088–1095 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisciano F. et al. Changes in BDNF serum levels in patients with major depression disorder (MDD) after 6 months treatment with sertraline, escitalopram, or venlafaxine. J. Psychiatr. Res. 43, 247–254 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle M. et al. Changes of serum concentrations of brain-derived neurotrophic factor (BDNF) during treatment with venlafaxine and mirtazapine: role of medication and response to treatment. Depression 1, 2 (2013). [DOI] [PubMed] [Google Scholar]
- Samaan Z. et al. Exploring the Determinants of Suicidal Behavior: Conventional and Emergent Risk (DISCOVER): a feasibility study. Pilot and Feasibility Studies 1, doi: 10.1186/s40814-015-0012-4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke J. P. et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann. Intern. Med. 147, W-163–W-194 (2007). [DOI] [PubMed] [Google Scholar]
- Sheehan D. V. et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59, 22–33 (1998). [PubMed] [Google Scholar]
- Beck R. W., Morris J. B. & Beck A. T. Cross-validation of the suicidal intent scale. Psychol. Rep. 34, 445–446 (1974). [DOI] [PubMed] [Google Scholar]
- R: A language and environment for statistical computing v. 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria, 2014).