Abstract
Saffron is consumed as food and medicine to treat several illnesses. This study elucidates the saffron effectiveness on diabetic parameters in-vitro and combined with resistance exercise in-vivo. The antioxidant properties of saffron was examined. Insulin secretion and glucose uptake were examined by cultured RIN-5F and L6 myotubes cells. The expressions of GLUT2, GLUT4, and AMPKα were determined by Western blot. Diabetic and non-diabetic male rats were divided into: control, training, extract treatment, training + extract treatment and metformin. The exercise and 40 mg/kg/day saffron treatments were carried out for six weeks. The antioxidant capacity of saffron was higher compare to positive control (P < 0.01). High dose of saffron stimulated insulin release in RIN-5F cells and improved glucose uptake in L6 myotubes. GLUT4 and AMPKα expressions increased in both doses of saffron (P < 0.01), whereas GLUT2 not changed (p > 0.05). Serum glucose, cholesterol, triglyceride, low-density lipoprotein, very low-density lipoprotein, insulin resistance, and glycated hemoglobin levels decreased in treated rats compared to untreated (p < 0.01). However, no significant differences were observed in the high-density lipoprotein, insulin, adiponectin, and leptin concentration levels in all groups (p > 0.05). The findings suggest that saffron consuming alongside exercise could improve diabetic parameters through redox-mediated mechanisms and GLUT4/AMPK pathway to entrap glucose uptake.
Diabetes mellitus is a metabolic disorder characterized by high blood glucose level resulting from insufficient insulin production and/or action1. Diabetes is characterized by increased level of blood glucose and impaired carbohydrate, fat, and protein metabolisms. Chronic hyperglycemia in diabetics affects several major organs, including heart, blood vessels, nerves, eyes, and kidneys, thereby leading to diabetic complications, such as cardiac dysfunction, atherosclerosis, neuropathy, retinopathy, and nephropathy2,3. Peroxidation of membrane lipids induced by hyperglycemia contributes to the pathophysiology of cardiovascular disease and atherosclerotic foam cell formation in the arterial wall4. Diabetes, which is a silent disease, is the most prevalent disease in developed and underdeveloped countries, and its prevalence is increasing considerably. Diabetes has been considered as one of the fastest growing epidemic worldwide; the number of people with diabetes is estimated to increase from 381.8 million in 2013 to 591.9 million in 20355.
The metabolic disorder type 2 diabetes or non-insulin-dependent diabetes mellitus is also associated with abnormal levels of lipids and lipoproteins, including cholesterol, low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), high-density lipoprotein (HDL), triglyceride (TG), as well as leptin and adiponectin6. Intramyocellular lipid (IMCL), particularly long-chain fatty acyl-CoA and diacylglycerol, may possibly induce insulin resistance in the skeletal muscles through hexokinase activity inhibition or affect the insulin-signaling pathway. Accumulation of intramuscular lipid in sedentary people is associated with insulin resistance. Lipoprotein lipase (LPL) activity in skeletal muscles increases during heavy exercise activity, which is effective in reducing IMCL accumulation7. Leptin and Adiponectin, which are two major hormones produced by adipose tissue and associated with type 2 diabetes, normalize insulin action8. AMP-activated protein kinase (AMPK)-induced fat oxidation may mediate the effects of leptin and adiponectin, resulting in liver TG reduction and intramyocellular regulation or energy balance. Leptin stimulates insulin resistance whereas adiponectin elevates insulin sensitivity9.
Pharmacological studies have demonstrated the link between the presence of free radicals and degenerative disease. The crucial role of free radicals scavengers have been well explained10. At present, natural products or dietary phytochemicals have received significant interests as potential therapeutic agents to counter diabetes because of their valuable therapeutic properties, nontoxicity, and cost effectiveness11. Natural sources have strongly been suggested for curing diabetic patients instead of multiple insulin injections. However, anti-diabetic agents are required to regulate blood glucose levels in more serious conditions12,13. The potential therapeutic effects of the spice saffron have been documented over the last two decades. The body of evidence that saffron has possible anti-diabetic properties is growing. Saffron (Crocus sativus L.), belonging to the Iridaceae family, is commonly cultivated in Iran, Spain, India, and Greece. The phytochemical compounds of this plant include carotenoids (crocin and crocetin), glycoside (picrocrocin), and a volatile oil component (safranal). Crocin and crocetin, which are the most important carotenoids and major bioactive constituents of saffron, have a wide spectrum of biological activities14,15. Crocin, the main ingredient of saffron, has an anti-diabetic effect on male mice16. Crocetin reduces adiponectin expression suppression, insulin resistance caused by palmitate17, increase tumor necrosis factor alpha (TNF-α), and inhibit leptin expression increase in adipose tissue18.
Physical activity affects overall metabolism, especially of blood glucose and lipids. Therefore, it has a significant value in the treatment of type 2 diabetes, which is often managed by doing exercise modification. The interrelationships between physical activity and metabolic outcomes have been widely investigated19. For instance, lipid profiles and insulin sensitivity in type 2 diabetes have improved by doing aerobic exercise20. Moreover, exercise can also elevate Foxo1 expression, further reduce glycated hemoglobin (HbA1c), change blood lipid profiles, and further increase insulin sensitivity19. Physical activity has been proven to increase SIRT1 and Foxo1 levels by caloric restriction, leading to increased adiponectin levels21. Decreased fat mass with increased adiponectin levels because of physical activity has also been observed22. Exercise has been established to control or prevent diabetic disorder; however, the intensity, time, and mode of exercise programs for treatment have not been specified. Although scientific researchers provide many information on the biological properties of saffron but its acutely antidiabetic potential properties in-vitro and combined with exercise in-vivo have not yet been studied. Thus, the present study aims to establish the effectiveness of saffron on diabetic parameters in-vitro and combined with resistance exercise intervention in-vivo models.
Research Design and Method
Preparation of hydroalcoholic extract of saffron
The Saffron used in this study was obtained from Iranian premium Saffron provider (Saharkhiz Co.Mashhad-Iran) and identified by botanists in the herbarium with specimen number 829-537-06. To obtain and optimize the best quality technique for saffron compounds, different extractions method are performed23. Hydroalcoholic extract of saffron was prepared with 3.8 g of ground saffron added into 200 mL of 50% aqueous-alcoholic solution and soaked for 24 h. The prepared extract was filtered through a 0.2 mm filter and was concentrated by rotary evaporator to separate the hydroalcoholic solution and isolate the pure saffron extract. The resulting extract was concentrated under reduced pressure and stored at −20 °C until use. The 40 g of dried extract was dissolved in 1 mL of distilled water to arrive at the desired concentrations. The extraction method was repeated as much as needed.
Total phenolic content (TPC)
The total phenolic content was determined by the method described previously24. The procedure was carried out in triplicate and the total phenolic content of the samples were expressed in gallic acid equivalents (GAE) per gram of the sample (mg GAE/g dried weight).
Antioxidant activity
Ferric reducing power assay (FRAP)
The FRAP assay was used as a novel method for assessing “antioxidant power to measure the reduction of ferric to ferrous ion at low pH25. Positive controls were chosen to be ascorbic acid and the procedure was carried out in triplicate.
1,1-diphenyl-2- picrylhydrazyl (DPPH) radical scavenging assay
The assay of DPPH radical scavenging activity was carried according to the method adopted by Blois et al.26. The experiment was done in triplicates and ascorbic acid was used as the positive controls. The percentage of inhibition was determined according to the following equation: % Inhibition = Control OD-Sample OD/Control OD × 100.
In-vitro cell line studies
Cell culture
Rat pancreatic beta cell line (RIN-5F) and rat L6 myoblast cell line (L6) were used in this study. RIN-5F and L6 cells were purchased from the American Type Culture Collection (ATCC, USA). RIN-5F (ATCC, CRL- 2058) cell were cultured in RPMI-1640 (Sigma–Aldrich, St. Louis, MO, USA) and L6 cells (ATCC, CRL-1458) were grown in Dulbecco’s Modified Eagle Medium (DMEM, Life Technologies, Inc., Rockville, MD, USA). The cells were supplemented with 10% fetal bovine serum (FBS, Sigma–Aldrich, St. Louis, MO, USA) and 1% antibiotics (100 IU/mL of penicillin and 100 μg/mL of streptomycin (iDNA, South America) and were maintained in a humidified 5% CO2 incubator at 37 °C. Cells were seeded in a flask at the required density per well and incubated for the desired time prior to the experiments.
Cell viability assay
The influence of saffron extract on RIN-5F and L6 cells was determined by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay27. Cells were seeded in a 96-well plate at a density of 5 × 103 cells/well and incubated at 37 °C and 5% CO2. After 24 h, medium was replaced with a fresh medium. Different concentrations (0, 100, 250, 500, and 1000 μg/mL) of saffron extract were prepared and transferred to the cells in the 96-well plate and incubated for an additional 48 h. Then, 20 μL of MTT solution (5 mg/mL MTT bromide in PBS) was added and the mixture was incubated for 4 h at 37 °C. Then, the medium was removed and the MTT formazan crystals formed by the metabolically viable cells were dissolved in 100 μL of dimethyl sulfoxide. The absorbance was measured at 595 nm. The assay was performed in triplicate.
Insulin secretion by the cultured RIN-5F pancreatic cell
RIN-5F cells were seeded in 24-well plates at 2 × 105 cells/well and incubated at 37 °C and 5% CO2. After incubation for 24 h, the medium was removed from the wells, and the cells were washed twice with fresh medium containing low glucose (6.25 mM) or high glucose (12.5 mM)28. Afterwards, the cells were incubated at 37 °C for 3 h with the low glucose or high glucose medium, supplemented with 1% FBS and treated with low or high concentrations of saffron extract (200 and 400 μg/mL). Then, the aliquots in all wells were collected to determine the concentration of insulin in the media with the use of ELISA kit (Insulin ELISA kit, Ab100578, Abcam, Cambridge, UK) according to the manufacturer’s instructions. The insulin secretion levels at different concentrations of saffron were assessed by comparing with the control insulin secretion level. The 0 concentration of extract (untreated cell) was considered as the control. The experiment was conducted in triplicate and data are presented as mean ± SD.
Determination of glucose uptake by cultured L6 myotubes
L6 myoblasts cells were subcultured in 24-well plates at 5 × 104 cells/well and allowed to proliferate for 11 days to form myotubes in 0.4 mL of 10% FBS/DMEM. The medium was refreshed every 3 days. Then the 11-day-old myotubes were kept for 2 h in Krebs–Henseleit buffer (pH 7.4, 0.141 g/L of MgSO4, 0.16 g/L of KH2PO4, 0.35 g/L of KCl, 6.9 g/L of NaCl, 0.373 g/L of CaCl2-2H2O and 2.1 g/L of NaHCO3) containing 0.1% bovine serum albumin (BSA), 10 mM Hepes, and 2 mM sodium pyruvate (KHH buffer). The myotubes were thereafter cultured in KHH buffer containing glucose (normal: 11 mM; high glucose: 25 mM) without or with saffron extract (0, 200, and 400 μg/mL) for another 4 h. Glucose concentrations in the KHH buffer were determined with a glucose assay kit and a microplate reader (Appliskan, Thermo Fisher Scientific Inc., Waltham, MA, USA) at 508 nm, and the consumed glucose levels were derived from the differences in glucose concentrations between before and after culturing29.
Protein extract and Western blot
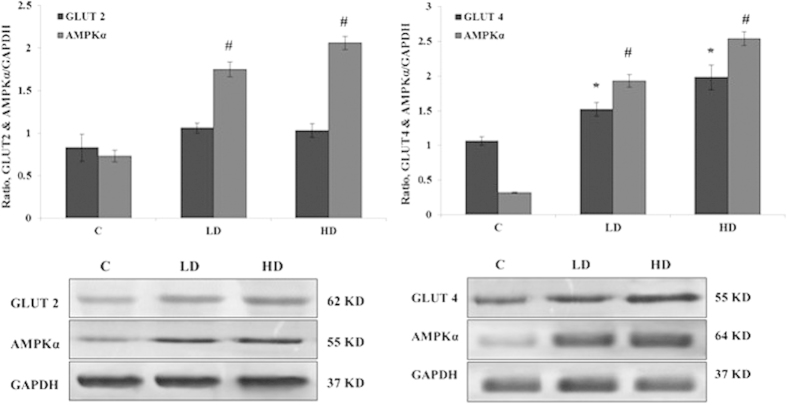
The expressions of GLUT2, GLUT4, and AMPKα were determined by Western blot. Briefly, total protein was extracted from RIN-5F and L6 myotube cells using lysis buffer (PRO-PREP Solution Kit, Intron, UK). The concentrations of the total extracted proteins were evaluated using a BSA standard kit (Thermo Scientific™ , US). With the use of 12% electrophoresis or sodium dodecyl sulphate polyacrylamide gel electrophoresis, the quantified proteins were separated to detect a specific protein and then transferred to a polyvinylidene fluoride membrane (BIORAD, UK) for immunoblot. The membranes were then incubated into specific primary and secondary antibodies (ABCAM, UK). The immunoreactive target protein bands were visualized using a colorimetric Opti-4CN TMS substrate kit. The captured target protein bands were analysed using Image J software (National Institutes of Health, USA). The ratio of each band over GAPDH was considered as the level of the target protein expression (Fig. 1).
Figure 1. Effect of saffron extract on glucose stimulated insulin release in RIN5 cells.
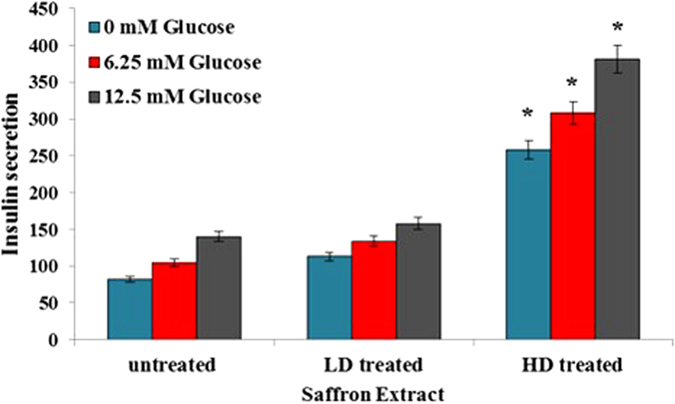
Data were expressed as mean ± SD for 6 replicates. LD; low dose of saffron-treated (200 μg/ml), HD; high dose of saffron-treated (400 μg/ml). *p value less than 0.05 considered as significant.
Experimental animals and ethical aspects
In this study, 70 male Sprague–Dawley rats 250 ± 15 g in weight, and 8 weeks of age were obtained from the animal center house of University Malaya and then transferred to the laboratory polycarbonate cages. They were reared at a temperature of 22 ± 2 °C and 55 ± 5% moisture under 12/12 h light/dark cycle. During the research process, the animals were fed with provided pellet food for animal breeding, reproduction, and stem cells. Water was allowed freely in special 500 mL bottles. All procedures involving animal experiments were approved and carried out in strict accordance with the United States Institute of Animal Research guidelines for the care and use of laboratory animals by the Animal Care and Use Committee (ACUC) University Malaya Institutional with ethics number: FIS/22/11/2011/FD(R).
In-vivo toxicity evaluation of saffron
In order to demonstrate the safety dosage of the plant extract, acute toxicity of the saffron was carried out in adult male and female rats. The experiment was conducted according to the guidelines of the Organization for Economic Co-operation and Development (OECD) No. 42030. Twenty male and female rats were assigned evenly into 2 groups and administered orally 200 and 400 mg/kg/day of the extract in a single dose, using intra-gastric tubes, or distilled water as vehicle. The animals were fasted overnight prior to the dosing (free access to water) and food was withheld for another 3 to 4 h after dosing. The animals were observed for 30 min and 2, 4, 8, 24 and 48 h following the administration to monitor any onset of clinical or toxicological symptoms. Any signs of toxicity, behavioral changes, and mortality were recorded over a period of 2 weeks. Sacrificed rats’ tissues (Liver & Kidney) were stained with hematoxylin and eosin, × 20, and then high-resolution images were captured.
Diabetes induction
After a week of acclimatizing the rats to the laboratory standard conditions, streptozotocin (STZ, Sigma–Aldrich, St. Louis, MO, USA) was injected intraperitoneally (55 mg/kg) to induce diabetes in rats11. Distilled citrate buffer was used to prepare the injection solution. Rats fasted 14 hours before injection. The animals in non-diabetic group were received citrate buffer injection as vehicle. A few days after STZ injection concentration 300 mg/dl were considered as diabetic. Blood samples were collected from the tail vein to measure the rats’ blood glucose using a glucometer (Bionime GM300). After one week of diabetes induction, the animals were transferred to conduct the treatment and experimental part.
Experimental animal groups
For accurate comparison of study data, animal models were grouped into two: A) diabetic and B) non-diabetic groups. In diabetic group (A), animals were randomly distributed to five subgroups: 1) control (cn), 2) training (tn), 3) extract treatment (ext), 4) training + extract treatment (tn + ext) and 5) treated with metformin 100 mg/kg (met) and served as reference group. In non-diabetic group (B), animals were randomly distributed to four subgroups: 1) control (cn), 2) training (tn), 3) extract treatment (ext), and 4) training + extract treatment (tn + ext). Both non-diabetic and diabetic subgroups comprise 5 and 10 rats, respectively. Treated animals received intra-gastric hydroalcoholic extract of saffron at 40 mg/kg daily31.
Resistance training protocol
After a week of acclimatization, the animals were first trained to climb the vertical ladder as the exercise for this study. The climbing familiarization sessions were conducted three to four replicates every day for a week without weights attached. Resistance exercise was performed from 4–8 p.m., and the extracted saffron treatment was administered at 10–11 a.m. with the use of a special gavage manufactured by Iran Supa Company. For 6 weeks, the training groups underwent 5 exercise sessions of climbing the ladder with attached weights weighing 30–100% of body weight per week. A specific ladder for rodents with a height of 1 m, with steps that are 4 cm apart and inclined at 85° angle was used. As warm-up for the training program, the animals climbed the ladder thrice without weights attached. The animals then performed their training twice with a weight attached to their tails, and then new weights were added. The training load consisted of four climbs with 50, 75, 90, and 100% of the previous maximum carrying load the animals were able to raise32.
Blood sampling and determination of biochemical variables
Animals from the trained and sedentary treated groups were euthanized at the end of six weeks of resistance training and gavage procedure. The animals from the trained groups were decapitated 24 hours after the last training session. Animals were anaesthetized with ketamine/xylazine (80/8 mg/kg i.p.) and sacrificed by cervical dislocation. Scarifies were performed at the same period and all efforts were made to minimize suffering. Blood sample was collected through a heart puncture under similar conditions. In this study, insulin level was measured in micro-international units per milliliter (mcIU/mL). Insulin resistance was obtained using the previously validated homeostasis model assessment of insulin resistance (HOMA-IR); HOMA‐IR = fasting glucose (mmol/L) × fasting insulin (μU/mL)/22.533. Serum glucose and total cholesterol were measured using an enzymatic glucose oxidase assay with a digital spectrophotometer (Spectronic, US), whereas fasting insulin level was measured using ELISA (enzyme-linked immunosorbent assay) kit. Adiponectin and leptin levels were measured by using ELISA Kit (Abcam-Cambridge, UK). LDL was calculated using the Friedewald equation34, and VLDL by VLDL = TG/5 formula. To measure HDL, HDL-C diagnosis kits were used following the photometric method.
Statistical Analysis
All data are presented as mean ± standard deviation. Shapiro–Wilk test was conducted to determine the data are normally distributed. Leven’s test for homogeneity was conducted, and the data displayed a homogenous characteristic with p > 0.05. Analysis of variance (ANOVA) was performed using SPSS version 18 and applied to measures of central tendency and dispersion. Two-way ANOVA was used for comparing the effect of exercise and saffron extract and the combination of them on diabetic parameters. A Tukey post-hoc analysis was used to check for significant differences among the main effects of each dependent variable. Statistical significance was considered when p value was less than 0.05.
Results
Antioxidant properties of hydroalcoholic extract of saffron
As shown in Table 1, the total phenolic content of the samples are expressed in gallic acid equivalents (GAE) per gram of the sample (mg GAE/g dried weight). It indicates that the extract has the high TPC contents (9.02 ± 0.69 mg GAE/g DW) which is in line with previous study reported by35. Table 1 also shows the power of the hydroalcoholic extract of saffron in reducing ferric tripyridyl (Fe3+) to ferrous form (Fe2+). In fact, the reduction of the ferric tripyridyltriazine (Fe III TPTZ) complex to its ferrous form, which has an intense blue colour, can be monitored by measuring the change in the absorbance at 593 nm. The FRAP value obtained of the saffron extract (1.4 ± 0.21 mmol Fe2+/g of dried weight) was lower than that obtained of Ascorbic acid as positive control (5.1 ± 0.45 mmol Fe2+/g of dried weight). The results of correlations analysis calculated by SPSS, shows that there was a strong positive correlation between total phenolic and FRAP in the saffron extract (r = 0.998, p < 0.01). The anti-oxidant activity of the extracts was considered by using FRAP correlated significantly and positively with the total phenolic content (r = 0.998, p < 0.01). The results showed that the DPPH radical scavenging activity of the hydroalcoholic extract of saffron increases in a dose-dependent manner. The IC50 value obtained of the extract was 189 ± 7.6 μg/ml, though not as potent as ascorbic acid used as positive control (16 ± 5.84). IC50 of DPPH radical scavenging activity and total phenolic content were analysed by Pearson’s method. There was highly and negative correlation between total phenolic content in saffron extract with the IC50 of DPPH scavenging capacities (r = −0.996, p < 0.01). In the other words, negatively high correlation coefficient means that the higher amount of total phenolic content is related to lower IC50 of DPPH scavenging capacity. In fact, the highly and negative correlation between TPC with IC50 DPPH expressed better antioxidant activity. The results from this study have revealed the potential health benefit of saffron extract by scavenging free radicals.
Table 1. Antioxidant activities of hydroalcoholic extract of saffron. Results are expressed as means ± SD of triplicate (n = 3).
| Assays | TPC (mg gallic acid equivalent/g DW) | IC50 of DPPH Radical Scavenging Activity (μg/ml) | FRAP (mmol Fe2+/g DW) |
|---|---|---|---|
| Hydroalcoholic extract of saffron | 9.02 ± 0.69 | 189 ± 7.6 | 1.4 ± 0.21 |
The IC50 values were calculated from linear regression analysis which indicates the effective concentration of samples used to reduce 50% of the reagent colour.
Cell proliferation assay
To assess the non-cytotoxic concentration of saffron, the viability of RIN-5F and L6 cells were evaluated at doses ranging between 0 and 1000 μg/mL, using MTT assay. Within the tested concentrations, saffron only showed negligible cytotoxicity at 800–900 μg/mL in both tested cell lines (data not shown), and concentrations up to 400 μg/mL of saffron were used in subsequent experiments.
Insulin secretion by cultured RIN-5F pancreatic cells
As shown in Fig. 1, saffron extract markedly increased insulin secretion in a dose-dependent manner at glucose concentrations of 6.25 and 12.5 mM. A slight induction of insulin secretion was observed in the RIN-5F cells treated with saffron extract at a concentration of as low as 200 μg/mL. The induction level obtained at higher concentrations (400 μg/mL) was significant compared with that at a lower concentration (200 μg/mL) or not treated.
Determination of glucose uptake by cultured L6 myotubes
As shown in Fig. 2, the glucose uptake of myotubes was considerably stimulated by the treatment of saffron in a concentration-dependent manner at concentrations of 200 and 400 μg/mL under normal glucose (11.1 mM) and high glucose (25 mM) conditions of the present study. This result suggests that saffron may act on the proteins associated with glucose uptake signaling pathways in muscle cells.
Figure 2. Effect of saffron extract on glucose uptake in L6 myotubes.
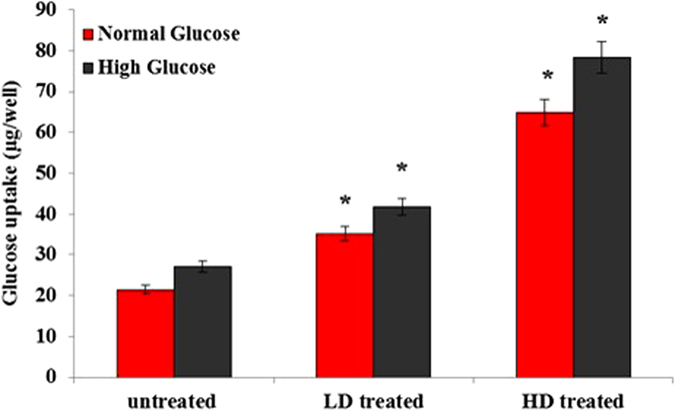
Data were expressed as mean ± SD for 6 replicates. LD; low dose of saffron-treated (200 μg/ml), HD; high dose of saffron-treated (400 μg/ml). *p value less than 0.05 considered as significant.
GLUT2, GLUT4, and AMPKα protein expression quantification
The protein blot expression analysis by Image J scanning (Fig. 3) indicated that GLUT2 expression approximately increased insignificantly by 0.2-fold in the pancreatic cells under high- or low-dose saffron treatment compared with the untreated RIN-5F pancreatic cell. GLUT4 expression increased significantly by 0.46 and 0.92-fold in the pancreatic cells under high or low-dose saffron treatment compare with the untreated L6 myotubes cell. AMPKα expression increased significantly by 1.02- and 1.33-fold, 1.61- and 2.22 fold in the RIN-5F pancreatic or L6 myotubes cell, respectively, under high- or low-dose saffron treatment compared with the untreated cells.
Figure 3. Western blot analysis for GLUT2, GLUT4, and AMPKα protein expression form of RIN-5F pancreatic cell and L6 myotubes cell with high or low dose of saffron treated compare to untreated.
Data were expressed as mean ± SD. C; control, LD; low dose of saffron-treated (200 μg/ml), HD; high dose of saffron-treated (400 μg/ml). #&*P value less than 0.05 considered as significant.
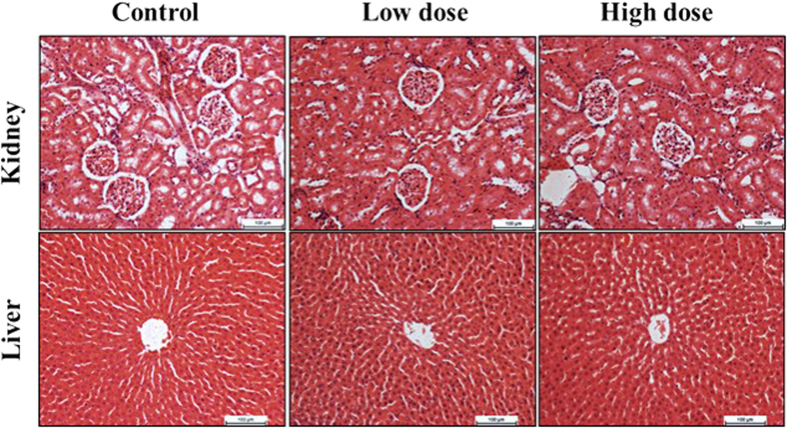
Acute toxicity
Figure 4 presents the results of histological evaluation of liver and kidney in acute toxicity study. The result of the histopathologic examination confirmed that saffron is highly biocompatible for clinical applications. All of the animals were healthy without any sign of toxicity at the tested dose and during the experiment no mortality was recorded.
Figure 4. In-vivo toxicity evaluation of saffron on rat kidney and liver.
Histological evaluation of rats’ kidney and liver tissues stained with H & E × 20 (Haematoxylin and Eosin). Data were collected from rats sacrificed at 7 days of saffron (low & high doses, 20 & 60 mg/kg/day) administration. The H & E investigation confirmed that there are no inflammation, abnormality, and organs lesions throughout the toxicity administration period.
Animal body weight in both diabetic and non-diabetic rats
The effects of Saffron or in combination with exercise on animal weight within 6 weeks are presented in Table 2. Rats in control diabetics groups were lost body mass as a result of the streptozotocin (STZ) throughout 6 weeks while tn, ext, and tn + ext groups had slightly increased of body weight at first 4 weeks then was significantly increased after 6 weeks in two groups of ext, and tn + ext. Further, over the six weeks of saffron treatment and exercise in non-diabetic groups no significant changes of body weight have observed during experimental study.
Table 2. Comparison of the effect of Saffron and Training on changes in both A) diabetic and B) Non-diabetic rat’s weight.
| A) Diabetic groups | Control (cn) | Training (tn) | Extract treated (ext) | Training + extract treated (tn + ext) | Metformin (Met) |
|---|---|---|---|---|---|
| Day 1 | 251.2 ± 19.5 | 260.1 ± 16.4 | 258.2 ± 13.9 | 252.9 ± 11.8 | 255.3 ± 17.6 |
| Week 2 | 243.7 ± 14.7 | 260.2 ± 18.7 | 259.9 ± 17.3 | 254.1 ± 15.4 | 258.3 ± 14.2 |
| Week 4 | 231.9 ± 11.8 | 263.7 ± 19.9 | 265.6 ± 15.7 | 264.7 ± 19.5 | 261.3 ± 19.1 |
| Week 6 | 223.1 ± 11.5 | 264.5 ± 20.2 | 271.7 ± 14.5* | 272.8 ± 21.6* | 270.9 ± 24.3 |
| B) Non-diabetic groups | |||||
| Day 1 | 259.4 ± 13.3 | 255.7 ± 11.8 | 260.5 ± 16.1 | 258.1 ± 17.2 | |
| Week 2 | 263.1 ± 12.5 | 251.4 ± 16.7 | 259.8 ± 14.7 | 250.6 ± 17.4 | |
| Week 4 | 270.5 ± 12.4 | 244.3 ± 13.5 | 255.8 ± 19.2 | 241.2 ± 14.8 | |
| Week 6 | 282.9 ± 18.5 | 244.5 ± 10.8 | 250.6 ± 18.3 | 232.1 ± 13.3 | |
Biochemical analysis
The Saffron and training effects on blood biochemical variables changes in A) diabetic and B) Non-diabetic groups are presented in Table 3.
Table 3. Comparison of the effect of Saffron and Training on changes of biochemical parameters in both A) diabetic and B) Non-diabetic groups.
| A) Diabetic groups | Control (cn) | Training (tn) | Extract treated (ext) | Training + extract treated (tn + ext) | Metformin (Met) |
|---|---|---|---|---|---|
| Glucose mg/dl | 392.20 ± 7.95 | 316.42 ± 9.04 * | 305.28 ± 1.82* | 292.28 ± 4.65* | 162.9 ± 6.85* |
| Insulin mcIU/mL | 6.04 ± 0.36 | 6.07 ± 0.36 | 6.15 ± 0.15 | 6.28 ± 0.30 | 5.98 ± 1.05 |
| Insulin resistance μU/ml | 6.25 ± 0.37 | 4.69 ± 0.31* | 4.64 ± 0.13* | 4.94 ± 0.21* | 3.54 ± 0.62* |
| Glycated haemoglobin% | 9.53 ± 0.35 | 7.40 ± 0.19* | 7.02 ± 0.08* | 6.96 ± 0.11* | 6.32 ± 0.82* |
| Cholesterol mg/dl | 122.85 ± 1.24 | 84.14 ± 1.63* | 85.00 ± 0.57* | 82.50 ± 1.49* | 78.43 ± 2.02* |
| Triglyceride mg/dl | 201.70 ± 4.48 | 152.28 ± 4.66* | 143.00 ± 0.57* | 138.33 ± 1.68* | 119.37 ± 2.5* |
| HDL mmol/L | 17.85 ± 0.73 | 19.42 ± 0.64 | 19.5 ± 0.15 | 18.93 ± 0.47 | 19.03 ± 1.29 |
| LDL mg/dl | 64.65 ± 1.08 | 37.25 ± 1.42* | 36.90 ± 0.56* | 34.50 ± 1.62* | 40.84 ± 1.67 |
| VLDL mg/dl | 40.34 ± 0.89 | 30.45 ± 0.93* | 28.60 ± 0.11* | 27.67 ± 0.33* | 29.62 ± 1.03* |
| Leptin ng/mL | 3.28 ± 0.06 | 3.04 ± 0.29 | 2.78 ± 0.14 | 2.76 ± 0.08 | 2.13 ± 0.17* |
| Adiponectin ng/mL | 6.31 ± 0.23 | 6.57 ± 0.70 | 6.50 ± 0.32 | 6.63 ± 0.23 | 7.91 ± 0.48* |
| B) Non-diabetic groups | |||||
| Glucose mg/dl | 107.4 ± 2.33 | 102.8 ± 1.45 | 107.33 ± 1.81 | 97.00 ± 1.62* | |
| Insulin mcIU/mL | 15.30 ± 0.15 | 15.29 ± 0.17 | 15.47 ± 0.15 | 15.27 ± 0.13 | |
| Insulin resistance μU/ml | 4.06 ± 0.12 | 3.88 ± 0.09 | 4.10 ± 0.09 | 3.66 ± 0.08 | |
| Glycated haemoglobin% | 1.98 ± 0.08 | 1.89 ± 0.07 | 1.86 ± 0.07 | 1.95 ± 0.07 | |
| Cholesterol mg/dl | 70.85 ± 1.47 | 66.02 ± 1.69 | 68.33 ± 0.98 | 59.45 ± 0.93 | |
| Triglyceride mg/dl | 75.14 ± 2.62 | 61.30 ± 1.57 | 65.88 ± 1.94 | 55.18 ± 1.31 | |
| HDL mmol/L | 31.87 ± 1.88 | 33.70 ± 1.26 | 33.22 ± 1.53 | 32.72 ± 1.36 | |
| LDL mg/dl | 24.22 ± 2.54 | 18.70 ± 2.50* | 18.93 ± 2.02* | 14.69 ± 1.97* | |
| VLDL mg/dl | 15.02 ± 0.52 | 12.26 ± 0.31* | 11.77 ± 0.38* | 11.03 ± 0.26* | |
| Leptin ng/mL | 3.05 ± 0.08 | 2.91 ± 0.05 | 2.76 ± 0.09 | 2.77 ± 0.02 | |
| Adiponectin ng/mL | 6.23 ± 0.28 | 6.12 ± 0.06 | 5.39 ± 0.22 | 5.23 ± 0.25 | |
Glucose
The blood glucose concentration levels in the tn, ext, tn + ext subgroups were significantly lower than that in the cn subgroups of both diabetic or non-diabetic groups (p < 0.05), and among the subgroups, the tn + ext subgroup exhibited the most reduction albeit not significant. In non-diabetic groups, the blood glucose concentration levels was significantly decreased in tn + ext group while there was no significant changes observed in tn and ext groups compared to control.
Insulin and insulin resistance
No significant differences exist among the diabetic and non-diabetic groups in terms of insulin concentration level. However, the insulin level insignificantly increased in the ext and tn + ext subgroups.
Insulin resistance levels were markedly decreased in the tn, ext, tn + ext subgroups compared with that in the cn diabetic subgroup, although no significant change was observed in non-diabetic groups.
Glycated hemoglobin (HbA1c)
The concentration level of HbA1c were significantly lower in the tn, ext, tn + ext subgroups compared with that in the cn diabetic subgroup, and among the subgroups, the tn + ext subgroup exhibited the most reduction albeit not significant. While, there was no statistically change in non-diabetic group.
Cholesterol and TG
The concentration levels of cholesterol was significantly lower in the tn, ext, tn + ext subgroups of both diabetic and non-diabetic groups, and among the subgroups, the tn + ext exhibited the most reduction albeit not significant. The level of TG concentration was markedly decreased in diabetic group compere to diabetic control while there was insignificant decrease in no-diabetic sub-groups in comparison with non-diabetic control.
HDL
The HDL concentration levels in all the groups (non-diabetic and diabetic) are not significantly different.
LDL and VLDL
The concentration levels of LDL and VLDL were significantly lower in both diabetic and non-diabetic groups compared with respected cn group. Meanwhile among the subgroups, the tn + ext subgroup exhibited the most reduction in both group albeit not significant.
Leptin
The leptin concentration levels in the both groups (non-diabetic and diabetic) are not significantly different.
Adiponectin
No significant differences were observed in the adiponectin concentration levels in both diabetic and non-diabetic groups.
Discussion
The control and treatment of diabetes and its complications mainly depend on chemical or biochemical agents; however, a case of total recovery from diabetes has never been reported36,37. The data from the present study indicated that saffron extract increased insulin secretion by the RIN-5F cells. The stimulation of insulin secretion was significant in high-dose treatment. In addition, saffron could significantly stimulate glucose uptake in the L6 myoblast cells under hyperglycemic and normoglycemic conditions. Insulin secretion and glucose absorption were stimulated by saffron in a dose-dependent manner, which was more pronounced in high dose saffron treated cells. The result of the in vivo study showed that fasting glucose decreased in diabetic rats after six weeks of resistance exercise and/or saffron treatment which was in consist with in-vitro finding. With respect to the antioxidant results of saffron the possibility that it acts as redox-mediated mechanisms on insulin secretion and glucose uptake.
HbA1c reduction was observed after six weeks of resistance exercise and saffron treatment. Blood glucose level has a positive correlation with glycosylation of erythrocytes and HbA1C level. The beneficial effect of exercise on HbA1C reduction has been proved by multiple studies7,18,38,39. Moreover, fluctuations in body composition and weight loss can be the reasons for fasting blood glucose reduction, which is caused by metabolic marker improvement. Significant decreases in serum glucose, HbA1c39, and lipid profile in diabetic patients have been observed through exercise40,41. The data of the present study showed that the six-week consumption of the aqueous saffron extract reduced fasting glucose in diabetic rats. Furthermore, saffron supplementation combined with resistance training was more effective than either extract or resistance training (non-significant) alone in reducing fasting glucose. These findings are consistent with the results of previous studies on fasting blood glucose reduction with crocetin. Therefore, low levels of serum insulin with crocetin consumption show that crocetin can be considered as an effective treatment for treating insulin resistance or related diseases17,42,43. Medicinal properties of crocetin have been indicated to be associated with antioxidant activity as an important factor in the treated of many degenerative diseases44,45.
Hyperinsulinemia and enhancement of insulin resistance cause renal sodium retention, increased sympathetic tone, and vascular endothelium smooth muscle hypertrophy46. Six weeks of resistance training had no significant effects on insulin reduction in diabetic rats, which is contradictory to findings of several studies40,47,48. Such deviation can be ascribed to the subjects and diabetes induction process in rats. In some cases, streptozotocin has been shown to further damage pancreatic β-cells, whereas in other cases it is less destructive. However, this streptozotocin causes oxidative stress, apoptosis, and reduced production and release of insulin. The effect of exercise on insulin levels can vary. Therefore, whether exercise contributes to insulin or streptozotocin reduction cannot be determined definitively.
The findings of this study showed that six weeks of resistance training combined with saffron treatment resulted in insulin resistance reduction. These data results are consistent with previous findings39,41. The positive effects of resistance exercise on improved insulin resistance can be achieved through insulin receptor enhancement in muscle cells or an increase in the number of glucose transporter proteins in the skeletal muscle cells. GLUT4 from the glucose transporter proteins family is insulin-responsive and benefits glucose transportation through muscle contraction and insulin in the muscles. Hence, the increase in total muscle mass because of resistance exercise leads to an increased glucose uptake through insulin mediation41. Moreover, muscle contraction under metabolic stress conditions cause a rapid depletion of glycogen and change the binding of various proteins linked to glycogen. This function requires the considerable amount of muscle fibers to refill their carbohydrate reserves. Reconstruction of glycogen stores with changes in molecular structure and non-esterified fatty acids concentration reduction contribute to insulin sensitivity regulation in skeletal muscles after exercise training49. Therefore, an increase in lean body mass after resistance exercise may be an important mediator in the improvement of glycemic control. Probably because of resistance training, the muscle mass even without altering the intrinsic capacity of the muscle to respond to insulin improves glucose disposal39. The data of the in-vitro study through Western blot confirmed no significant increase in the GLUT2 and AMPKα expression in the RIN-5F cells, which is in line with in-vivo results.
Upregulation GLUT4 and AMPKα were observed in both low and high doses of saffron treated in L6 myoblast cell line. Saffron intensely stimulates phosphorylation of in both cell lines. AMPK performs many functions in fat or lipid synthesis of hepatic or skeletal muscle tissues, such as stimulating fatty acid oxidation, inhibiting cholesterol synthesis, and modulating insulin, adipocyte lipolysis, and muscle glucose uptake43. AMPK decrease production of glucose through various mediators e.g. by inhibiting gluconeogenic enzymes phosphoenolpyruvate carboxykinase (PEPCK) or translocating of GLUT-4 leads to stimulating glucose uptake. The anti-diabetic effects of natural products through increasing expression and promoting translocation of GLUT-4 via PI3K/AKT, CAP/Cb1/TC10 and AMPK pathways have been proven in previous studies. The data confirm previous findings on Saffron extracts in regards to strongly enhance glucose uptake50, as well as involvement of AMPK into this exact process in diabetic rats51. The results of current study suggest that the GLUT4/AMPK translocation and insulin resistance reduction may confirm a crosstalk between insulin metabolism, saffron compounds, and resistance training. Consequently, saffron may have a direct role in stimulating AMPK and GLUT4 via redox-mediated mechanisms for insulin sensitivity or glucose uptake in skeletal muscle cells.
No significant differences were observed in the adiponectin levels in diabetic rats after six weeks of resistance training and saffron consumption. Studies on the effect of saffron extract on adiponectin level are very limited. A study reported increased adiponectin levels in rats consuming 40 mg/kg of crocetin, which is consistent with the present data; such deviation may be caused by differences in saffron dosage18,43. Adiponectin enhancement by doing physical activity has also been reported52,53. The type of physical activity can be the cause for such inconsistency. Studies have shown that aerobic exercise has greater beneficial effect on adiponectin level compared with resistance training. Hypoadiponectinemia leads to greater insulin resistance and increased risk of type 2 diabetes54. Unlike most other adipokines, adiponectin levels in obese individuals are lower compared with those in lean people55,56,57. Nevertheless the fragmentation of adiponectin molecules is likely to be elevated in obese patients58. TNF-α decreases adipocyte-derived adiponectin expression in-vitro59 leading to insulin resistance53.
Furthermore, six weeks of resistance training and saffron consumption had a significant reduction in the total cholesterol of diabetic rats. Most researchers investigated the effect of saffron, with crocetin as the main efficient substance, on lipids and glycemic index16,42,43,60,61. Lower cholesterol after physical training can be due to changes in fat regulator enzymes and increased use of fats as fuel source during exercise. Crocin appears to indirectly inhibit the absorption of fat and cholesterol by preventing fat hydrolysis through the inhibition of pancreatic lipase. Crocin also increases the amount of fecal excretion of fat and cholesterol but had no effect on bile acids. A direct effect of crocin is the inhibition of lymphatic absorption of cholesterol62 as its roles as antioxidant agents have confirmed63. The present study showed saffron extract consumption had a significant reduction effect on TG, LDL, and VLDL. Studies have proved the positive influence of saffron on improved lipid profiles62,64. Crocin contained in saffron possibly causes an increased activity of lecithin–cholesterol acyltransferase (LCAT), which is involved in the regulation of blood lipid metabolism. LCAT also performs a vital function in the binding of free cholesterol to HDL62. Therefore, saffron and its constituents reduce lipid peroxidation65.
No significant differences in the HDL levels because of saffron consumption were observed in the present study. The findings are also inconsistent with the results of other studies. Asdaq and Inamdar reported an increase in HDL levels because of the use of saffron extract. Such an inconsistency could be partially related to different doses of saffron. In the present study, 40 mg/kg was the dose used; it might not have sufficient effect on the HDL compared with the doses used in previous research64. The present data also demonstrated no significant differences in the HDL levels after six weeks of resistance training, which is consistent with the findings of some previous studies66 but inconsistent with those of other studies67,68. However, this inconsistency might be related to the type, intensity, and duration of the exercise program. Jiméneza et al.67 conducted 8 weeks of resistance training, whereas other study set an endurance training of 90 min/day biking at 50% of peak68.
In the present study, a marked decrease was observed in the TG69, VLDL68,70 and LDL54 levels after six weeks of resistance training, which is supported by previous studies. Considering the positive changes in the lipid profile, physical activity protocols are recommended to increase lipolysis, decrease TG, and improve the ratio of oxidant to antioxidant, change LDL-C synthesis or plasma LDL-C elimination by tissue. Some investigations have suggested that changes in lipid profile by practice are possibly associated with a change in fat mass71. Physical activity causes an increase in LDL and LCAT and decrease in LTP (lipid transfer protein), resulting in HDL elevation and TG or VLDL reduction72. Greater LPL activity because of exercise performance and decrease in TG concentration create an increase in the LDL peak particle size73.
No significant changes were observed in the leptin levels in diabetic rats after six weeks of resistance training and saffron consumption. These findings are inconsistent with recent work74 but have been defended in several studies75,76. Leptin is an important factor involved in various processes, such as appetite regulation, metabolic function rate, reproduction, and immunity76. A higher leptin level is independently associated with increased risk of insulin resistance syndrome60 and is inversely related with insulin sensitivity77 and inverse relationship with insulin sensitivity18. Obese people generally have higher leptin concentrations. In these cases, leptin resistance could be induced by blood–brain barrier dysfunction, defects in leptin receptor signaling, or interference of neuropeptide Y78. Physical activities consequently reduce fat mass by creating a negative energy balance, which is mainly ascribed to the control of leptin. Despite the remarkable effect of physical activity, other factors may also be involved in leptin regulation. Subjects with lower fat mass have significantly lower response to plasma leptin caused by exercise compared with people with higher fat mass. In addition, the association between leptin and fat distribution possibly change with age75. Exercise training seems to improve leptin activity and sensitivity78.
Conclusion
To sum up, the findings of the present study indicated that the consumption of the herbal plant saffron combined with resistance exercise is a strong therapeutic effective factor on diabetic parameters in-vivo. Our data were supported by in-vitro results. It was suggested that our two factors may improve diabetic parameters via GLUT4/AMPK pathway and may acts as redox-mediated mechanisms to regulate insulin or glucose. Thus, with the benefits of resistance training and concurrent saffron consumption on reducing health risk factors, diabetic patients are advised to exploit this combination of factors to control and manage their disease. Our study provides a key insight into diabetes, but significant evidences for the benefits of the combined factors have not been obtained. However, further investigations are warranted to identify the proper dose and role of specific compounds of saffron on diabetic parameters at the molecular level.
Additional Information
How to cite this article: Dehghan, F. et al. Saffron with resistance exercise improves diabetic parameters through the GLUT4/AMPK pathway in-vitro and in-vivo. Sci. Rep. 6, 25139; doi: 10.1038/srep25139 (2016).
Acknowledgments
The authors wish to sincerely thank University of Malaya for High Impact Research grant UM.C/HIR/MOHE/MED/11 and research grant TC003-2012 for supporting this research.
Footnotes
Author Contributions Conceived and designed the study: F.D., M.A.A. and A.Y. Performed the experiments: F.D., F.H., L.Z.A.S., S.H., S.A.H. and M.A.A. Analyzed the data: F.D., A.Y. and M.A.A. Contributed reagents/materials/analysis tools: F.D., A.Y., M.A.A. and S.M. Wrote the manuscript: F.D., A.Y. and M.A.A.
References
- Association A. D. Diagnosis and classification of diabetes mellitus. Diabetes care 33, S62–S69 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini G. P., Iori E., Marescotti M. C., de Kreutzenberg S. V. & Avogaro A. Insulin-induced glucose control improves HDL cholesterol levels but not reverse cholesterol transport in type 2 diabetic patients. Atherosclerosis 235, 415–417 (2014). [DOI] [PubMed] [Google Scholar]
- Patiño M. N. et al. Caries, periodontal disease and tooth loss in patients with diabetes mellitus types 1 and 2. Acta Odontol Latinoam 21, 127–133 (2007). [PubMed] [Google Scholar]
- Watala C. & Winocour P. The relationship of chemical modification of membrane proteins and plasma lipoproteins to reduced membrane fluidity of erythrocytes from diabetic subjects. Clin Chem Lab Med 30, 513–520 (1992). [DOI] [PubMed] [Google Scholar]
- Guglani R., Shenoy S. & Sandhu J. S. Role of Pedometer Based Walking in the Management of Type 2 Diabetes: A Review. Inter J Sci Res 3, 449–452 (2014). [Google Scholar]
- Krauss R. M. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes care 27, 1496–1504 (2004). [DOI] [PubMed] [Google Scholar]
- Goodpaster B. H., He J., Watkins S. & Kelley D. E. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86, 5755–5761 (2001). [DOI] [PubMed] [Google Scholar]
- Inoue M., Maehata E., Yano M., Taniyama M. & Suzuki S. Correlation between the adiponectin-leptin ratio and parameters of insulin resistance in patients with type 2 diabetes. Metabolism 54, 281–286 (2005). [DOI] [PubMed] [Google Scholar]
- Havel P. J. Update on adipocyte hormones regulation of energy balance and carbohydrate/lipid metabolism. Diabetes 53, S143–S151 (2004). [DOI] [PubMed] [Google Scholar]
- Lipinski B. Hydroxyl radical and its scavengers in health and disease. Oxid Med Cell Longev 2011, doi: http://dx.doi.org/10.1155/2011/809696 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshadi S. et al. Evaluation of Trigonella foenum-graecum extract in combination with swimming exercise compared to glibenclamide consumption on type 2 Diabetic rodents. Food Nutr Res 59, 29717, doi: 10.3402/fnr.v59.29717 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. J. & Day C. Traditional plant medicines as treatments for diabetes. Diabetes care 12, 553–564 (1989). [DOI] [PubMed] [Google Scholar]
- Patel D., Kumar R., Laloo D. & Hemalatha S. Natural medicines from plant source used for therapy of diabetes mellitus: An overview of its pharmacological aspects. Asian Pac J Trop Dis 2, 239–250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung N. H. & Shoyama Y. New minor glycoside components from saffron. J Nat Med 67, 672–676 (2013). [DOI] [PubMed] [Google Scholar]
- Bhattacharjee B., Vijayasarathy S., Karunakar P. & Chatterjee J. Comparative reverse screening approach to identify potential anti-neoplastic targets of saffron functional components and binding mode. Asian Pac J Cancer Prev 13, 5605–5611 (2012). [DOI] [PubMed] [Google Scholar]
- Asai A., Nakano T., Takahashi M. & Nagao A. Orally administered crocetin and crocins are absorbed into blood plasma as crocetin and its glucuronide conjugates in mice. J Agric Food Chem 53, 7302–7306 (2005). [DOI] [PubMed] [Google Scholar]
- Yang L. et al. Inhibitory effect on protein kinase Cθ by Crocetin attenuates palmitate-induced insulin insensitivity in 3T3-L1 adipocytes. Eur J Pharmacol 642, 47–55 (2010). [DOI] [PubMed] [Google Scholar]
- Xi L. et al. Beneficial impact of crocetin, a carotenoid from saffron, on insulin sensitivity in fructose-fed rats. J Nutr Biochem 18, 64–72 (2007). [DOI] [PubMed] [Google Scholar]
- Zanuso S., Jimenez A., Pugliese G., Corigliano G. & Balducci S. Exercise for the management of type 2 diabetes: a review of the evidence. Acta Diabetol 47, 15–22 (2010). [DOI] [PubMed] [Google Scholar]
- Hills A. P. et al. Resistance training for obese, type 2 diabetic adults: a review of the evidence. Obes Res 11, 740–749 (2010). [DOI] [PubMed] [Google Scholar]
- Qiao L. & Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein α transcriptional complex. J Biol Chem 281, 39915–39924 (2006). [DOI] [PubMed] [Google Scholar]
- Rokling-Andersen M. H. et al. Effects of long-term exercise and diet intervention on plasma adipokine concentrations. Am J Clin Nutr 86, 1293–1301 (2007). [DOI] [PubMed] [Google Scholar]
- Heydari S. & Haghayegh G. H. Extraction and Microextraction Techniques for the Determination of Compounds from Saffron. Can Chem Trans 2, 221–247 (2014). [Google Scholar]
- Pinelo M., Rubilar M., Sineiro J. & Núñez M. J. Extraction of antioxidant phenolics from almond hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster). Food Chem 85, 267–273 (2004). [Google Scholar]
- Benzie I. F. & Strain J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239, 70–76 (1996). [DOI] [PubMed] [Google Scholar]
- Blois M. S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 181, 1199–1200 (1958). [Google Scholar]
- Plumb J. A., Milroy R. & Kaye S. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer res 49, 4435–4440 (1989). [PubMed] [Google Scholar]
- Arya A., Looi C. Y., Cheah S. C., Mustafa M. R. & Mohd M. A. Anti-diabetic effects of Centratherum anthelminticum seeds methanolic fraction on pancreatic cells, β-TC6 and its alleviating role in type 2 diabetic rats. J Ethnopharmacol 144, 22–32 (2012). [DOI] [PubMed] [Google Scholar]
- Subash-Babu P., Ignacimuthu S. & Alshatwi A. Nymphayol increases glucose-stimulated insulin secretion by RIN-5F cells and GLUT4-mediated insulin sensitization in type 2 diabetic rat liver. Chem Biol Interact 226, 72–81 (2015). [DOI] [PubMed] [Google Scholar]
- OECD. Test No. 420: Acute Oral Toxicity - Fixed Dose Procedure. (OECD Publishing).
- Imenshahidi M. et al. The effect of chronic administration of saffron (Crocus sativus) stigma aqueous extract on systolic blood pressure in rats. Jundishapur J Nat Pharm Prod 8, 175–179 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite R. D. et al. Effects of ovariectomy and resistance training on lipid content in skeletal muscle, liver, and heart; fat depots; and lipid profile. Appl Physiol Nutr Metab 34, 1079–1086 (2009). [DOI] [PubMed] [Google Scholar]
- Matthews D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985). [DOI] [PubMed] [Google Scholar]
- Friedewald W. T., Levy R. I. & Fredrickson D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18, 499–502 (1972). [PubMed] [Google Scholar]
- Baba S. A. et al. Phytochemical analysis and antioxidant activity of different tissue types of Crocus sativus and oxidative stress alleviating potential of saffron extract in plants, bacteria, and yeast. S Afr J Bot 99, 80–87 (2015). [Google Scholar]
- Li W., Zheng H., Bukuru J. & De Kimpe N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol 92, 1–21 (2004). [DOI] [PubMed] [Google Scholar]
- Ivorra M., Paya M. & Villar A. A review of natural products and plants as potential antidiabetic drugs. J Ethnopharmacol 27, 243–275 (1989). [DOI] [PubMed] [Google Scholar]
- Wintrobe M. M. & Greer J. P. Wintrobe’s clinical hematology. Vol. 1 (Lippincott Williams & Wilkins, 2009). [Google Scholar]
- Dunstan D. W. et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes care 25, 1729–1736 (2002). [DOI] [PubMed] [Google Scholar]
- Dunstan D. W. et al. Community center–based resistance training for the maintenance of glycemic control in adults with type 2 diabetes. Diabetes care 29, 2586–2591 (2006). [DOI] [PubMed] [Google Scholar]
- Cauza E. et al. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehabil 86, 1527–1533 (2005). [DOI] [PubMed] [Google Scholar]
- Arasteh A. et al. Effects of hydromethanolic extract of saffron (Crocus sativus) on serum glucose, insulin and cholesterol levels in healthy male rats. J Med Plant Res 4, 397–402 (2010). [Google Scholar]
- Aruoma O. I., Neergheen V. S., Bahorun T. & Jen L.-S. Free radicals, antioxidants and diabetes: embryopathy, retinopathy, neuropathy, nephropathy and cardiovascular complications. Neuroembryol Aging 4, 117–137 (2007). [Google Scholar]
- Yoshino F., Yoshida A., Umigai N., Kubo K. & Lee M. C. Crocetin reduces the oxidative stress induced reactive oxygen species in the stroke-prone spontaneously hypertensive rats (SHRSPs) brain. J Clin Biochem Nutr 49, 182–187 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X. C. & Qian Z. Y. Effects of crocetin on antioxidant enzymatic activities in cardiac hypertrophy induced by norepinephrine in rats. Pharmazie 61, 348–352 (2006). [PubMed] [Google Scholar]
- Abdulla M. H., Sattar M. A. & Johns E. J. The relation between fructose-induced metabolic syndrome and altered renal haemodynamic and excretory function in the rat. Int J Nephrol 2011, doi: http://dx.doi.org/10.4061/2011/934659 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorana A., O’Driscoll G., Goodman C., Taylor R. & Green D. Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diabetes Res Clin Pract 56, 115–123 (2002). [DOI] [PubMed] [Google Scholar]
- Castaneda C. et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes care 25, 2335–2341 (2002). [DOI] [PubMed] [Google Scholar]
- Babraj J. A. et al. Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC Endocr Disord 9, 3, doi: 10.1186/1472-6823-9-3 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajeri D., Mousavi G. & Doustar Y. Antihyperglycemic and pancreas-protective effects of Crocus sativus L.(Saffron) stigma ethanolic extract on rats with alloxan-induced diabetes. J Biol Sci 9, 302–310 (2009). [Google Scholar]
- Kang C. et al. Saffron (Crocus sativus, L.) increases glucose uptake and insulin sensitivity in muscle cells via multipathway mechanisms. Food chem 135, 2350–2358 (2012). [DOI] [PubMed] [Google Scholar]
- Moran C. N. et al. Effects of diabetes family history and exercise training on the expression of adiponectin and leptin and their receptors. Metabolism 60, 206–214 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A. S. et al. Depot-specific modulation of adipokine levels in rat adipose tissue by diet-induced obesity: the effect of aerobic training and energy restriction. Cytokine 52, 168–174 (2010). [DOI] [PubMed] [Google Scholar]
- Hara K. et al. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes 51, 536–540 (2002). [DOI] [PubMed] [Google Scholar]
- Weyer C. et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86, 1930–1935 (2001). [DOI] [PubMed] [Google Scholar]
- Kumada M. et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 23, 85–89 (2003). [DOI] [PubMed] [Google Scholar]
- Hotamisligil G. Molecular mechanisms of insulin resistance and the role of the adipocyte. Int J Obes Relat Metab Disord 24, S23–S27 (2000). [DOI] [PubMed] [Google Scholar]
- Hoffstedt J., Arvidsson E., Sjölin E., Wåhlén K. & Arner P. Adipose tissue adiponectin production and adiponectin serum concentration in human obesity and insulin resistance.J Clin Endocrinol Metab 89, 1391–1396 (2004). [DOI] [PubMed] [Google Scholar]
- Wang B., Jenkins J. R. & Trayhurn P. Expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture: integrated response to TNF-α. Am J Physiol Endocrinol Metab 288, E731–E740 (2005). [DOI] [PubMed] [Google Scholar]
- Yu Y.-H. & Zhu H. Chronological changes in metabolism and functions of cultured adipocytes: a hypothesis for cell aging in mature adipocytes. Am J Physiol Endocrinol Metab 286, E402–E410 (2004). [DOI] [PubMed] [Google Scholar]
- Giaccio M. Crocetin from saffron: an active component of an ancient spice. Crit Rev Food sci Nutr 44, 155–172 (2004). [DOI] [PubMed] [Google Scholar]
- Sheng L., Qian Z., Zheng S. & Xi L. Mechanism of hypolipidemic effect of crocin in rats: crocin inhibits pancreatic lipase. Eur J Pharmacol 543, 116–122 (2006). [DOI] [PubMed] [Google Scholar]
- Chen Y. et al. Antioxidant potential of crocins and ethanol extracts of Gardenia jasminoides ELLIS and Crocus sativus L.: A relationship investigation between antioxidant activity and crocin contents. Food chem 109, 484–492 (2008). [Google Scholar]
- Asdaq S. M. B. & Inamdar M. N. Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl Biochem Biotechnol 162, 358–372 (2010). [DOI] [PubMed] [Google Scholar]
- Hasani-Ranjbar S., Larijani B. & Abdollahi M. A systematic review of the potential herbal sources of future drugs effective in oxidant-related diseases. Inflamm Allergy Drug Targets 8, 2–10 (2009). [DOI] [PubMed] [Google Scholar]
- Yoshida H. et al. We-P13: 370 Clinical relevance of VLDL cholesterol reduction to increased serum adiponectin when assessing serum lipid amelioration achieved by exercise training. Atheroscler Suppl 7, 428 (2006). [Google Scholar]
- Jiménez Ó. H. & Ramírez-Vélez R. Strength training improves insulin sensitivity and plasma lipid levels without altering body composition in overweight and obese subjects. Endocrinol Nutr 58, 169–174 (2011). [DOI] [PubMed] [Google Scholar]
- Søndergaard E., Poulsen M. K., Jensen M. D. & Nielsen S. Acute changes in lipoprotein subclasses during exercise. Metabolism 63, 61–68 (2014). [DOI] [PubMed] [Google Scholar]
- Karanth J. & Jeevaratnam K. Effect of dietary lipid, carnitine and exercise on lipid profile in rat blood, liver and muscle. Indian J Exp Biol 47, 748–753 (2009). [PubMed] [Google Scholar]
- Dekker M. J., Graham T. E., Ooi T. & Robinson L. E. Exercise prior to fat ingestion lowers fasting and postprandial VLDL and decreases adipose tissue IL-6 and GIP receptor mRNA in hypertriacylglycerolemic men. J Nutr Biochem 21, 983–990 (2010). [DOI] [PubMed] [Google Scholar]
- Barzegari A. & Mahdirejei H. A. Effects of 8 weeks resistance training on plasma vaspin and lipid profile levels in adult men with type 2 diabetes. Caspian J Intern Med 5, 103–108 (2014). [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Quesada J. L. et al. LDL from aerobically-trained subjects shows higher resistance to oxidative modification than LDL from sedentary subjects. Atherosclerosis 132, 207–213 (1997). [DOI] [PubMed] [Google Scholar]
- Wooten J. S., Biggerstaff K. D. & Ben-Ezra V. Responses of LDL and HDL particle size and distribution to omega-3 fatty acid supplementation and aerobic exercise. J Appl Physiol 107, 794–800 (2009). [DOI] [PubMed] [Google Scholar]
- Jafari M. et. al The effect of eight weeks endurance training and high-fat diet on appetite-regulating hormones in rat plasma. ran J Basic Med Sci. 2014. Apr; 17(4): 237–243. [PMC free article] [PubMed] [Google Scholar]
- Houmard J. A., Cox J. H., MacLean P. S. & Barakat H. A. Effect of short-term exercise training on leptin and insulin action. Metabolism 49, 858–861 (2000). [DOI] [PubMed] [Google Scholar]
- Hosoda H., Kojima M. & Kangawa K. Ghrelin and the regulation of food intake and energy balance. Mol Interv 2, 494–503 (2002). [DOI] [PubMed] [Google Scholar]
- Hulver M. W. et al. Adiponectin is not altered with exercise training despite enhanced insulin action. Am J Physiol Endocrinol Metab 283, E861–E865 (2002). [DOI] [PubMed] [Google Scholar]
- Pasman W., Westerterp-Plantenga M. & Saris W. The effect of exercise training on leptin levels in obese males. Am J Physiol Endocrinol Metab 274, E280–E286 (1998). [DOI] [PubMed] [Google Scholar]