Abstract
Lead-zinc deposits are often difficult to classify because clear criteria are lacking. In recent years, new tools, such as Cd and Zn isotopes, have been used to better understand the ore-formation processes and to classify Pb-Zn deposits. Herein, we investigate Cd concentrations, Cd isotope systematics and Zn/Cd ratios in sphalerite from nine Pb-Zn deposits divided into high-temperature systems (e.g., porphyry), low-temperature systems (e.g., Mississippi Valley type [MVT]) and exhalative systems (e.g., sedimentary exhalative [SEDEX]). Our results showed little evidence of fractionation in the high-temperature systems. In the low-temperature systems, Cd concentrations were the highest, but were also highly variable, a result consistent with the higher fractionation of Cd at low temperatures. The δ114/110Cd values in low-temperature systems were enriched in heavier isotopes (mean of 0.32 ± 0.31‰). Exhalative systems had the lowest Cd concentrations, with a mean δ114/110Cd value of 0.12 ± 0.50‰. We thus conclude that different ore-formation systems result in different characteristic Cd concentrations and fraction levels and that low-temperature processes lead to the most significant fractionation of Cd. Therefore, Cd distribution and isotopic studies can support better understanding of the geochemistry of ore-formation processes and the classification of Pb-Zn deposits.
Lead-zinc (Pb-Zn) deposits are typically classified by their ore-formation processes and geological settings. Such deposits can be formed by the discharge of deep sedimentary brine onto the sea floor (sedimentary exhalative; SEDEX) or by replacement of limestone (Mississippi Valley type; MVT)1,2, whereas some are associated with submarine volcanoes (volcanogenic massive sulfide; VMS) or are found in the aureoles of sub-volcanic intrusions of granite (e.g., skarn and porphyry deposits)3. Tectonically, SEDEX deposits mainly form in passive margins or Proterozoic rift/sag basins, whereas MVT deposits form in carbonate platform sequences at passive margins1,4,5,6. Although the classification appears to be clearly defined, it remains difficult to implement in practice. For example, most deposits classified as SEDEX lack unequivocal evidence of an exhalite in the ore or an alteration component7. Although the presence of laminated sulfides parallel to bedding is usually accepted as evidence of an exhalative ore, some MVT deposits also contain extensive (kilometer-scale) laminated and stratiform ores formed by highly selective carbonate replacement millions of years after the deposition of the host rocks1. Furthermore, some vein-type Pb-Zn deposits associated with magmatism are difficult to recognize because the intrusion is often concealed. Indeed, many ore deposits may be formed by one or more of the basic processes mentioned above, thus resulting in ambiguous classifications and substantial controversy and conjecture. Therefore, much effort has been invested in the discrimination and classification of Pb-Zn deposits on the basis of geochemical proxies (e.g., fluid-inclusion studies and S-Pb-C-O-H isotopes). In recent years, new isotope systematics, such as Cd and Zn isotopes, have been used to better understand ore-formation processes8,9,10.
Cd has eight stable isotopes and is typically hosted in sphalerite in Pb-Zn deposits at concentrations of several hundred to several thousand ppm11,12. Many studies have shown that Cd isotope fractionation occurs when evaporation and condensation processes occur, resulting in δ114/110Cd values between −8‰ and +16‰ in meteorite samples13,14,15,16,17. Lacan et al.18 have studied Cd cycling in the ocean and have shown that phytoplankton preferentially take up light Cd isotopes from seawater. The biological fractionation of Cd isotopes (giving a total δ114/110Cd range of 4.4‰) in surface seawater has been found to be primarily governed by Rayleigh fractionation during the near-quantitative uptake of dissolved Cd by phytoplankton18,19,20,21,22. Significant variations in Cd isotopic ratios have been found in natural terrestrial samples with a total fractionation of approximately 1.5‰ in δ114/110Cd values for samples from mid-ocean ridge basalt (MORB) and ocean island basalt (OIB) rocks, loess, sediments, and sulfides10,23,24. Thus, different geological processes result in distinct Cd isotope fractionation, which might possibly be used to effectively track formation processes in ore deposit geology. In this paper, we report the systematic measurements of Cd isotopes in different types of Pb-Zn deposits and provide direct evidence for the effective use of isotopic studies in ore-formation processes and the classification of Pb-Zn deposits.
Zn/Cd ratios and Cd isotopic compositions from different types of Pb-Zn deposits
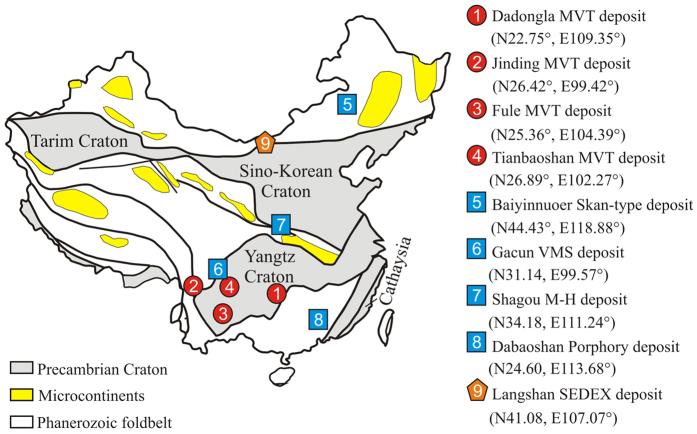
Nine Pb-Zn deposits in China were chosen, and their Cd isotopic compositions and relevant elemental ratios were investigated (Fig. 1)25. The deposits were divided into three categories: high-temperature systems, low-temperature systems and exhalative systems. Herein, the high-temperature systems referred to magma/volcanic-related deposits and included a porphyry deposit (Dabaoshan), magmatic hydrothermal deposit (Shagou), skarn deposit (Bayinnuoer) and VMS deposit (Gacun), all of which are closely associated with magmatism and formed at temperatures of approximately 200–250 °C26,27,28. The low-temperature systems referred to MVT deposits, including the Fule, Tianbaoshan, Jinding and Dadongla Pb-Zn deposits, which typically formed at temperatures <200 °C29. Finally, the exhalative systems were represented by the Langshan SEDEX deposit. The ore deposit geologies of these deposits are provided in the Supplementary materials.
Figure 1. Locations of the Pb-Zn deposits chosen in this study, modified from ref. 25.
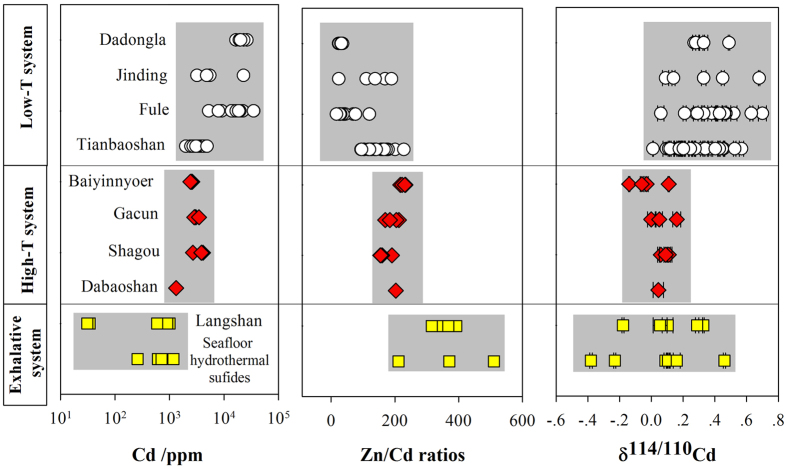
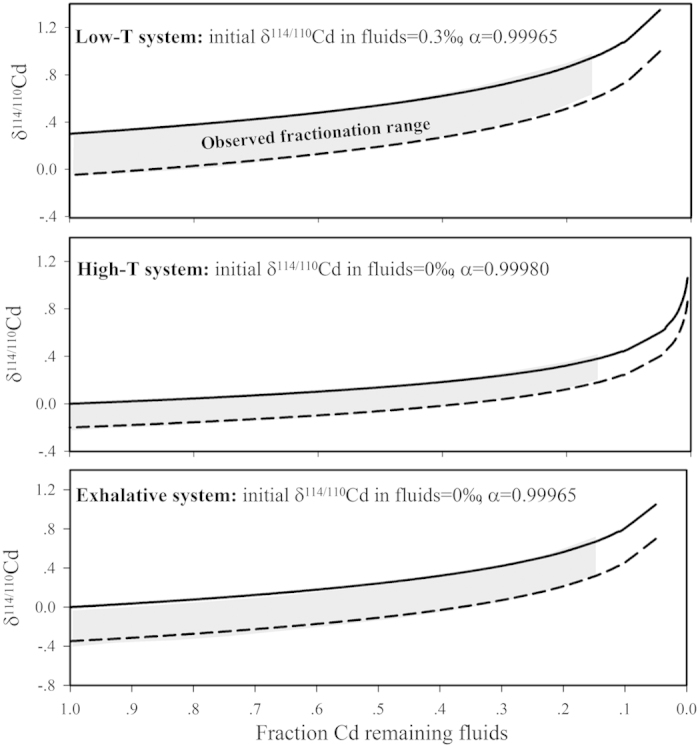
The analytical data and elemental analyses are presented in Supplementary Table 3, and the elemental enrichment ratios (Zn/Cd) and δ114/110Cd values are illustrated in Fig. 2.
Figure 2. Distribution of Cd concentrations, Zn/Cd ratios and Cd isotopes in Pb-Zn deposits; reference data for seafloor hydrothermal sulfides from Schmitt et al.24.
High-temperature systems
Despite the existence of different ore deposit types (porphyry, magmatic hydrothermal, skarn, and VMS), the Cd concentrations, Zn/Cd ratios and Cd isotopic compositions were consistent. Cadmium concentrations from four deposits yielded a mean value of 2932 ppm and ranged from 2410 ppm to 4126 ppm, and the Zn/Cd ratios varied from 155 to 223 with a mean value of 195. No significant isotopic fractionation was found in these deposits, with values ranging from −0.14 to 0.16‰ and a mean δ114/110Cd of 0.04 ± 0.16‰ (2σ).
Low-temperature systems
Samples from the four low-temperature MVT deposits showed distinct distributions compared with those from the high-temperature systems. Cadmium concentrations were highest but varied in the 2415–34981 ppm range and had a mean value of 9399 ppm. Zn/Cd ratios ranged from 17 to 201 and had a mean value of 101. The δ114/110Cd values were highly variable and enriched in heavier isotopes, exhibiting a mean value of 0.32 ± 0.31‰ (2σ, from 0.09 to 0.70‰).
Exhalative systems
Sphalerite samples of the Langshan SEDEX deposit had the lowest Cd concentrations of the three categories and ranged from 595 to 996 ppm range (mean of 832 ppm) and exhibited tightly grouped Zn/Cd ratios in the 316–368 range (mean of 353). The deposit yielded an average δ114/110Cd value of 0.12 ± 0.50‰ (2σ) and ranged from −0.18 to 0.33‰. Measurements of seafloor hydrothermal sulfides, which are comparable to SEDEX deposits, revealed similar distributions with low Cd contents (259–1174 ppm, mean of 690 ppm) and high Zn/Cd ratios (211–510, mean of 366). The δ114/110Cd value is also typically highly variable, ranging from −0.38‰ to 0.46‰ (mean of 0.01 ± 0.27‰, 2σ)20.
Thermodynamic simulation of the Cd content and Zn/Cd ratios in sphalerite
The differences in the Cd concentrations and Zn/Cd ratios in sphalerite from the three studied systems were consistent with those in previous investigations11. Statistical analysis of 480 Pb-Zn deposits suggested that exhalative deposits had low Cd concentrations (mean of 2560 ppm for SEDEX deposits), that MVT deposits (mean of 4850 ppm) and veins in carbonate rock (mean of 7260 ppm) had high concentrations, and that Skarn deposits (mean of 3540 ppm) had intermediate concentrations. Thermodynamics is a useful tool for explaining the variations in the Cd content of sphalerite from various systems with different mineralization processes.
Cadmium is mainly involved in direct Zn2+ ↔ Cd2+ substitutions in sphalerite; therefore, the distributions of Cd and Zn between the liquid and solid phases can be approximated by the following equilibrium involving the end-member compositions of CdS and ZnS30,31,32:
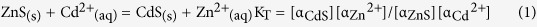 |
A rapidly decreasing trend was observed for the calculated thermodynamic distribution coefficient, KT, of this reaction (1) in the theoretical case of a complete absence of complexing ions as the temperature increases from 25 °C to 300 °C, as illustrated in Fig. 3 (Curve 1); thus, low temperatures favor Cd substitution in sphalerite. However, in hydrothermal fluids, most Zn and Cd exist as aqueous complexes, with Zn2+ and Cd2+ being subordinate33,34. The most important ligands are Cl−, HS− and OH− in hydrothermal fluids33,35,36. For example, aqueous Cd speciation is dominated by chloride species, CdClm(H2O)n2−m, over a wide range of temperatures (20 ≤ T ≤ 450 °C), acidities (1 ≤ pH ≤ 8) and chloride concentrations (0.04 ≤ mCl ≤ 18 mol/kg H2O). Therefore, in addition to the temperature, the presence of Cd complexes, salinity (Cl− concentration), concentration of reduced sulfur (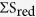 ) and pH value of hydrothermal fluids are also major factors that control the Cd distribution in sphalerite.
) and pH value of hydrothermal fluids are also major factors that control the Cd distribution in sphalerite.
Figure 3. Zn-Cd partitioning between liquid and coexisting sphalerite, based on logKT versus temperature under different hydrothermal conditions with Cl− activity, total reduced sulfur, and pH.
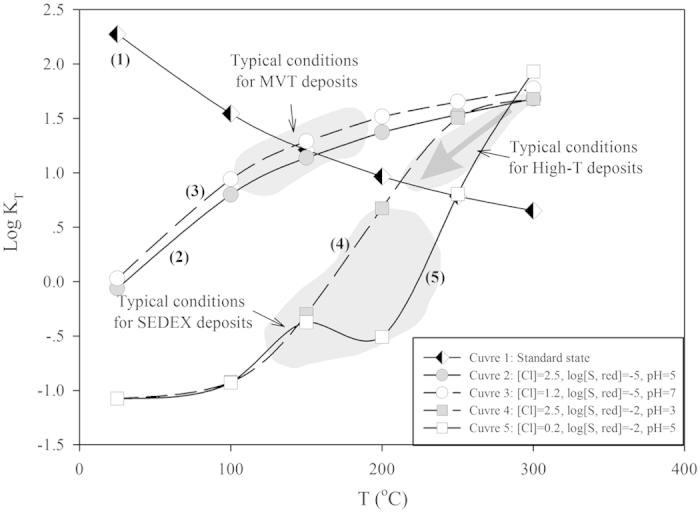
Calculated thermodynamic data were obtained from Schwartz (2010) and analyzed with SUPCRT92 software.
As shown in Curves 2–5 in Fig. 3, in contrast with Curve 1, this trend was reversed if the effect of complexing agents was considered. Curves 2 through 5 have several important implications. For hydrothermal fluids with similar reduced sulfur (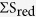 ) concentrations and pH values, there was no obvious difference in the distribution coefficient, KT, at the same temperature (see Curves 2 and 3 or 4 and 5), if only the salinity (Cl− concentration) was considered. However, the reduced sulfur (
) concentrations and pH values, there was no obvious difference in the distribution coefficient, KT, at the same temperature (see Curves 2 and 3 or 4 and 5), if only the salinity (Cl− concentration) was considered. However, the reduced sulfur (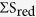 ) concentrations of ore-forming fluids affected the Cd distribution in sphalerite, whereas the salinity and pH did not. High
) concentrations of ore-forming fluids affected the Cd distribution in sphalerite, whereas the salinity and pH did not. High 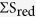 activities shifted KT to lower values, favoring the formation of Cd-poor sphalerite (Curves 4 and 5 in Fig. 3), whereas low
activities shifted KT to lower values, favoring the formation of Cd-poor sphalerite (Curves 4 and 5 in Fig. 3), whereas low 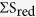 activities favored the formation of Cd-rich sphalerite (Curves 2 and 3 in Fig. 3). This result was consistent with the ore-forming conditions of MVT and SEDEX deposits. MVT deposits commonly form at temperatures <250 °C (typically between 90 and 150 °C) and salinities of 15–35% in oxidized (SO42−-dominant) and acidic to near-neutral brines evolved from carbonate-dominated sedimentary basins2. In contrast, SEDEX deposits commonly form at temperatures of 120–250 °C and variable salinities (10–30 wt%) from acidic, reduced (H2S-predominant) connate brines evolved from reduced siliciclastic and shale basins7. Therefore, it is reasonable that fundamental differences in the chemistry of the mineralizing fluid for MVT and SEDEX deposits will result in distinct Cd distributions in sphalerite.
activities favored the formation of Cd-rich sphalerite (Curves 2 and 3 in Fig. 3). This result was consistent with the ore-forming conditions of MVT and SEDEX deposits. MVT deposits commonly form at temperatures <250 °C (typically between 90 and 150 °C) and salinities of 15–35% in oxidized (SO42−-dominant) and acidic to near-neutral brines evolved from carbonate-dominated sedimentary basins2. In contrast, SEDEX deposits commonly form at temperatures of 120–250 °C and variable salinities (10–30 wt%) from acidic, reduced (H2S-predominant) connate brines evolved from reduced siliciclastic and shale basins7. Therefore, it is reasonable that fundamental differences in the chemistry of the mineralizing fluid for MVT and SEDEX deposits will result in distinct Cd distributions in sphalerite.
The highest KT values were found to be nearly identical for all physico-chemical conditions, especially when the temperature reached 300 °C, indicating that high temperatures favor Cd substitution in sphalerite under hydrothermal conditions. However, studies on the solubility of sphalerite have shown that temperature may be one of most important factors controlling Zn deposition. For example, Tagirov and Seward34 have found that the solubility of sphalerite at 300 °C is four orders of magnitude higher than that at 150 °C in a solution with pH = 4 and m(NaCl) = 2, thus indicating that even in high-temperature systems, substantial sphalerite accumulation will occur only when the temperature decreases, as shown in Fig. 3.
Therefore, the distribution of Cd in sphalerite from various types of Pb-Zn deposits is the result of the competing influences of various parameters, according to the thermodynamic theory on the liquid-solid partitioning of Cd and Zn. The inherent differences in the physico-chemical conditions under which hydrothermal fluids form various types of Pb-Zn deposits are key factors resulting in different Cd concentrations and Zn/Cd ratios in sphalerite.
Mechanism underlying Cd isotopic variations in Pb-Zn deposits:
Because of their similar chemical and crystallographic properties, Cd closely follows Zn in geochemical cycling11, and thus, the isotope fractionation of these two elements may be similar37. Therefore, several causes analogous to those for Zn isotopes are proposed to explain the mass-dependent Cd isotopic variations in different systems, including (1) the equilibrium effect, (2) the kinetic effect, and (3) the Cd source.
Many studies have shown that evaporation and condensation processes can yield large Cd isotopic fractionation in meteorite samples with δ114/110Cd values ranging from −8‰ to +16‰16,17. Recently, large Cd isotopic fractionations have been reported for partly evaporated, industrially produced Cd metal, yielding a total δ114/110Cd fractionation of approximately 1‰38,39. However, these processes are associated with the gas transformation of solid Cd minerals, and homogeneous isotopic compositions of samples from high-temperature systems suggest that gas transformation may not occur during mineralization. Yang et al.37 have reported a systematic approach using quantum chemical calculations (density functional theory) of Cd isotope reduced partition function ratios to understand the fractionation properties of Cd species in hydrothermal fluids. Considering cadmium hydrate, chloride, hydroxide, nitrate and hydrosulfide complexes, Yang et al. have found that Cd isotopes show higher fractionation at low temperatures than at high temperatures, at which light isotopes are enriched in the solid phase. This result strongly suggests that low-temperature processes lead to significant isotopic fractionation of Cd in nature, in agreement with the Cd variation in the three systems considered here.
The Zn isotopes of Pb-Zn deposits, such as the Alexandrinka (VHMS-type), Irish Midlands (Irish-type), Red Dog (SEDEX-type) and Navan (Irish-type), have been studied9,40,41 and explained by Rayleigh distillation. However, only a few studies have considered Cd isotopes. A recent study21 has demonstrated that Cd readily replaces Ca during Cd sorption onto calcite. This process can be explained by Rayleigh fractionation using a fractionation factor ranging from 0.99943 to 0.99967. Such a process may occur during ore formation. A detailed study of the Fule MVT Pb-Zn deposit has revealed that Cd in early stages of sphalerite formation is enriched in light isotopes, whereas heavy isotopes become enriched in later stages (Fig. 4). In addition, at the same stage, the first precipitated sphalerite, enclosed in galena, is enriched with light Cd isotopes, in contrast to black sphalerite, which is crystallized a second time in galena. The reddish-brown sphalerite is the last to precipitate and presents the heaviest Cd isotope composition. Preferentially removing light Cd isotopes from the fluids may reveal a kinetic effect; however, the observed restricted fractionation favored a strong equilibrium effect as illustrated in Fig. 4.
Figure 4. Cd isotopic variations in different early- to late-stage samples from the No. 78 orebody in the Fule MVT Pb-Zn deposit.

Besides isotope fractionation during chemical reaction, the source-rock δ144/110Cd value may be another factor in Cd isotopic variability. Metals in the high-temperature system (porphyry, skarn and VMS deposits) are closely associated with magmatism, and although limited data exist, several igneous MORB and OIB basalt samples have revealed homogenous Cd isotopic compositions (δ114/110Cd value of 0.01 ± 0.06‰)20,23. Thus, the initial Cd isotopic compositions for high-temperature system fluids may be small or near zero. Sedimentary strata providing a source of metals in exhalative systems carry metal ions trapped within clay and phyllosilicate minerals and adsorbed onto mineral surfaces. These characteristics are consistent with shale-hosted SEDEX deposits having a large Cd isotopic variation: δ114/110Cd values of −0.48–0.12‰23. Finally, the sources of metals in MVT deposits may be complicated, but carbonate, which hosted MVT deposits, may provide an important contribution. Experimental studies have shown that Cd isotopic compositions of marine carbonates record a seawater Cd signature21. The global deep-ocean is typically enriched in heavy Cd isotopes with a mean δ114/110Cd value of 0.3‰18,19,22,42. Therefore, it is reasonable to assume that carbonate sources have heavy Cd isotopic compositions.
Combining all the above factors, the Cd variation of the three systems can be explained using a Rayleigh distillation model, as illustrated in Fig. 5. The fractionation factor estimated herein is comparable to those determined previously21,23, and the high-temperature system probably provides a slightly lower fractionation factor, consistently with quantum chemical calculations (density functional theory), resulting in a restricted Cd isotope variation range37. The low-temperature and exhalative systems provide the same fractionation factor with a large dispersion of the Cd isotopic composition, whereas the δ114/110Cd values of the low-temperature systems are heavier, reflecting the difference in the original fluids resulting from their different Cd sources.
Figure 5. Evolution of δ144/110Cd during the deposition of aqueous Cd in different hydrothermal fluids.
Dashed lines represent the evolution of the deposited minerals, and solid lines represent the evolution of residual aqueous Cd. The grey fields represent the observed fractionation range of the sphalerite samples.
Conclusions
Overall, this study provides some clarity regarding the mechanism by which Cd concentrations vary among Pb-Zn deposit types and variation in Zn/Cd ratios. Furthermore, the isotopic fraction outcomes of different geological and geochemical conditions are presented, and a schematic diagram is provided to develop the use of Cd isotopes as a proxy for the classification of Pb-Zn deposits (Fig. 6). Most importantly, theoretical considerations and field measurements are combined provide the first direct evidence that Cd distribution and isotopic fractionation in sphalerite, which are strongly dependent on the Cd source and the geochemical conditions of the ore-forming fluid (temperature, ΣSred activities, pH and salinity), can be used as potential geochemical proxies to classify the corresponding Pb-Zn deposits.
Figure 6. Distribution of Zn/Cd ratios versus Cd isotopic compositions in different mineralization systems.
Methods
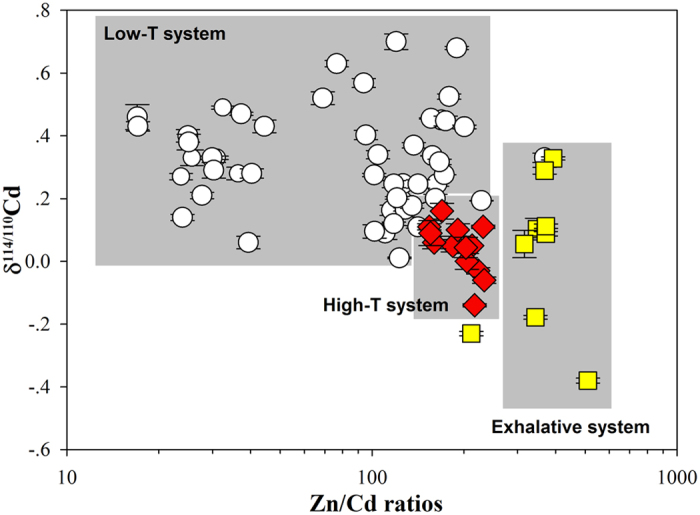
A total of 70 sphalerite samples were collected from these deposits and separated, and their Zn and Cd concentrations, and Cd isotopes were analyzed. The Zn and Cd concentrations were determined by inductively coupled plasma optical emission spectrometry (ICP-OES, Varian Vista MPX). The Cd isotope measurements were performed at the State Key Laboratory of Ore Deposit Geochemistry at the Institute of Geochemistry, Chinese Academy of Sciences, using a Neptune Plus multi collector ICP-MS instrument. An anion-exchange resin column was used to separate the Cd from the matrix. The method resulted in a mean Cd recovery of 99.8%. Elements that could potentially interfere with the determination of Cd isotopes, such as Sn, In, Zn, and Pb, were found at negligible concentrations relative to Cd. The standard–sample bracketing (SSB) method was used to calculate the delta values43. The delta values for Cd isotopes are expressed as: 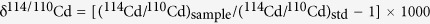 , where “std” is the internal reference standard (Spex solution)12. Further details about the analytical methods and data are provided in the Supplementary materials.
, where “std” is the internal reference standard (Spex solution)12. Further details about the analytical methods and data are provided in the Supplementary materials.
Additional Information
How to cite this article: Wen, H. et al. Zn/Cd ratios and cadmium isotope evidence for the classification of lead-zinc deposits. Sci. Rep. 6, 25273; doi: 10.1038/srep25273 (2016).
Supplementary Material
Acknowledgments
This project was financially supported by the 973 Program (2014CB44090×4), the National Natural Science Foundation of China (Grant Nos 40930425 and 41173026), the CAS/SAFEA International Partnership Program for Creative Research Teams (KZZD-EW-TZ-20), and the 12th Five-Year Plan project of State Key Laboratory of Ore-deposit Geochemistry, Chinese Academy of Sciences (SKLODG-ZY125-07). C.C. acknowledges the ANR-10-LABX-21-01 project. We are grateful for the anonymous comments that helped us to improve this manuscript.
Footnotes
Author Contributions H.J.W. and C.C. proposed and organized the project. H.J.W., C.C., H.F.F. and C.W.Z. discussed and designed the experiments. C.W.Z. and Y.X.Z. performed the experiments. H.J.W., C.C., C.W.Z., H.F.F. and S.H.F. analyzed and interpreted the data. H.J.W. wrote the main manuscript. C.C. revised the main manuscript. All the authors discussed the research.
References
- Leach D. L. et al. Sediment-Hosted Lead-Zinc Deposits in Earth History. Econ. Geol. 105, 593–625 (2010). [Google Scholar]
- Basuki N. I. & Spooner E. T. C. A review of fluid inclusion temperatures and salinities in Mississippi Valley-type Zn-Pb deposits: Identifying thresholds for metal transport. Explor. Mining Geol. 11, 1–17 (2002). [Google Scholar]
- Barrie C. T. & Hannington M. D. Classification of volcanic-associated massive sulfide deposits based on host-rock composition. Revs. Econ. Geol. 8, 1–11 (1999). [Google Scholar]
- Betts P. G., Giles D. & Lister G. S. Tectonic Environment of Shale-Hosted Massive Sulfide Pb-Zn-Ag Deposits of Proterozoic Northeastern Australia. Econ. Geol. 98, 557–576 (2003). [Google Scholar]
- Bradley D. C. & Leach D. L. Tectonic controls of Mississippi Valley-type lead-zinc mineralization in orogenic forelands. Miner. Deposita 38, 652–667 (2003). [Google Scholar]
- Huston D. L., Pehrsson S., Eglington B. M. & Zaw K. The Geology and Metallogeny of Volcanic-Hosted Massive Sulfide Deposits: Variations through Geologic Time and with Tectonic Setting. Econ. Geol. 105, 571–591 (2010). [Google Scholar]
- Leach D. L. et al. Nature of Hydrothermal Fluids at the Shale-Hosted Red Dog Zn-Pb-Ag Deposits, Brooks Range, Alaska. Econ. Geol. 99, 1449–1480 (2004). [Google Scholar]
- Fujii T., Moynier F., Pons M.-L. & Albarède F. The origin of Zn isotope fractionation in sulfides. Geochim. Cosmochim. Acta 75, 7632–7643 (2011). [Google Scholar]
- Kelley K. D., Wilkinson J. J., Chapman J. B., Crowther H. L. & Weiss D. J. Zinc Isotopes in Sphalerite from Base Metal Deposits in the Red Dog District, Northern Alaska. Econ. Geol. 104, 767–773 (2009). [Google Scholar]
- Zhu C. W. et al. Characteristics of Cd isotopic compositions and their genetic significance in the lead-zinc deposits of SW China. Sci. China-Earth Sciences 56, 2056–2065 (2013). [Google Scholar]
- Schwartz M. O. Cadmium in Zinc Deposits: Economic Geology of a Polluting Element. Int. Geol. Rev. 42, 445–469 (2000). [Google Scholar]
- Cloquet C., Rouxel O., Carignan J. & Libourel G. Natural Cadmium isotopic variations in eight geological reference materials (NIST 2711, BCR 176, GSS 1, GXR 1, GXR 2, GSD 12, Nod P1, Nod A1) and anthropogenic samples, measured by MC-ICP MS. Geost. Geoan. Res. 29, 95–106 (2005). [Google Scholar]
- Rosman K. J. R. & De Laeter J. R. Isotopic fractionation in meteoritic cadmium. Nature 261, 216–218 (1976). [Google Scholar]
- Rosman K. J. R. & De Laeter J. R. Cadmium mass fractionation in unequilibrated ordinary chondrites. Earth Planet. Sci. Lett. 89, 163–169 (1988). [Google Scholar]
- Wombacher F., Rehkamper M., Mezger K., Munker C. & Bischoff A. Stable isotope compositions of Cadmium in stony meteorites. Geochim. Cosmochim. Acta 66, A844 (2002). [Google Scholar]
- Wombacher F., Rehkamper M. & Mezger K. Determination of the mass-dependence of cadmium isotope fractionation during evaporation. Geochim. Cosmochim. Acta 68, 2349–2357 (2004). [Google Scholar]
- Wombacher F., Rehkamper M., Mezger K., Bischoff A. & Munker C. Cadmium stable isotope cosmochemistry. Geochim. Cosmochim. Acta 72, 646–667 (2008). [Google Scholar]
- Lacan F., Francois R., Ji Y. C. & Sherrell R. M. Cadmium isotopic composition in the ocean. Geochim. Cosmochim. Acta 70, 5104–5118 (2006). [Google Scholar]
- Ripperger S., Rehkamper M., Porcelli D. & Halliday A. N. Cadmium isotope fractionation in seawater - A signature of biological activity. Earth Planet. Sci. Lett. 261, 670–684 (2007). [Google Scholar]
- Schmitt A. D., Galer S. J. G. & Abouchami W. Mass-dependent cadmium isotopic variations in nature with emphasis on the marine environment. Earth Planet. Sci. Lett. 277, 262–272 (2009). [Google Scholar]
- Horner T. J., Rickaby R. E. M. & Henderson G. M. Isotopic fractionation of cadmium into calcite. Earth Planet. Sci. Lett. 312, 243–253 (2011). [Google Scholar]
- Abouchami W. et al. Modulation of the Southern Ocean cadmium isotope signature by ocean circulation and primary productivity. Earth Planet. Sci. Lett. 305, 83–91 (2011). [Google Scholar]
- Wombacher F., Rehkamper M., Mezger K. & Munker C. Stable isotope compositions of cadmium in geological materials and meteorites determined by multiple-collector ICPMS. Geochim. Cosmochim. Acta 67, 4639–4654 (2003). [Google Scholar]
- Schmitt A. D., Galer S. J. G. & Abouchami W. High-precision cadmium stable isotope measurements by double spike thermal ionisation mass spectrometry. J. Anal. Atom. Spectrom. 24, 1079–1088 (2009). [Google Scholar]
- Wen H. J. & Qiu Y. Z. Geology and Geochemistry of Se-Bearing Formations in Central China. Int. Geol. Rev. 44, 164–178 (2002). [Google Scholar]
- Hou Z. Q. Origin of the Gacun Volcanic-Hosted Massive Sulfide Deposit inSichuan, China: Fluid Inclusion and Oxygen Isotope Evidence. Econ. Geol. 96, 1491–1512 (2001). [Google Scholar]
- Han J.-S., Yao J.-M., Chen H.-Y., Deng X.-H. & Ding J.-Y. Fluid inclusion and stable isotope study of the Shagou Ag–Pb–Zn deposit, Luoning, Henan province, China: Implications for the genesis of an orogenic lode Ag–Pb–Zn system. Ore Geol. Rev. 62, 199–210 (2014). [Google Scholar]
- Li C.-Y. et al. The formation of the Dabaoshan porphyry molybdenum deposit induced by slab rollback. Lithos 150, 101–110 (2012). [Google Scholar]
- Zaw K., Peters S. G., Cromie P., Burrett C. & Hou Z. Nature, diversity of deposit types and metallogenic relations of South China. Ore Geol. Rev. 31, 3–47 (2007). [Google Scholar]
- Mookherjee A. Certain Aspects of the Geochemistry of Cadmium. Geochim. Cosmochim. Acta 26, 351–360 (1962). [Google Scholar]
- Tsusue A. & Holland H. D. The eoprecipitation of cations with CaCO3-III. The coprecipitation of Zn2+ with calcite between 50 and 250C. Geochim. Cosmochim. Acta 30, 439–453 (1966). [Google Scholar]
- Sverjensky D. A., Shock E. L. & Helgeson H. C. Prediction of the thermodynamic properties of aqueous metal complexes to 1000 °C and 5 kbar. Geochim. Cosmochim. Acta 61, 1359–1412 (1997). [DOI] [PubMed] [Google Scholar]
- Bazarkina E. F., Pokrovski G. S., Zotov A. V. & Hazemann J.-L. Structure and stability of cadmium chloride complexes in hydrothermal fluids. Chem. Geol. 276, 1–17 (2010). [Google Scholar]
- Tagirov B. R. & Seward T. M. Hydrosulfide/sulfide complexes of zinc to 250 °C and the thermodynamic properties of sphalerite. Chem. Geol. 269, 301–311 (2010). [Google Scholar]
- Giordano T. H. Transport of Pb and Zn by carboxylate complexes in basinal ore fluids and related petroleum-field brines at 100 °C: the influence of pH and oxygen fugacity. Geochem. T. 3, 56–72 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Y. et al. Zinc complexation in chloride-rich hydrothermal fluids (25–600 °C): A thermodynamic model derived from ab initio molecular dynamics. Geochim. Cosmochim. Acta 150, 265–284 (2015). [Google Scholar]
- Yang J. et al. Theoretical calculations of Cd isotope fractionation in hydrothermal fluids. Chem. Geol. 391, 74–82 (2015). [Google Scholar]
- Shiel A. E., Weis D. & Orians K. J. Evaluation of zinc, cadmium and lead isotope fractionation during smelting and refining. Sci. total environ. 408, 2357–2368 (2010). [DOI] [PubMed] [Google Scholar]
- Cloquet C., Carignan J., Libourel G., Sterckeman T. & Perdrix E. Tracing source pollution in soils using cadmium and lead isotopes. Environ. Sci. Tech. 40, 2525–2530 (2006). [DOI] [PubMed] [Google Scholar]
- Mason T. F. D. et al. Zn and Cu isotopic variability in the Alexandrinka volcanic-hosted massive sulphide (VHMS) ore deposit, Urals, Russia. Chem. Geol. 221, 170–187 (2005). [Google Scholar]
- Gagnevin D., Boyce A. J., Barrie C. D., Menuge J. F. & Blakeman R. J. Zn, Fe and S isotope fractionation in a large hydrothermal system. Geochim. Cosmochim. Acta 88, 183–198 (2012). [Google Scholar]
- Gault-Ringold M., Adu T., Stirling C. H., Frew R. D. & Hunter K. A. Anomalous biogeochemical behavior of cadmium in subantarctic surface waters: Mechanistic constraints from cadmium isotopes. Earth Planet. Sci. Lett. 341–344, 94–103 (2012). [Google Scholar]
- Wen H. J. et al. Tracing sources of pollution in soils from the Jinding Pb-Zn mining district in China using cadmium and lead isotopes. Appl. Geochem. 52, 147–154 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.