Abstract
Many species of microalgae have been used as source of nutrient rich food, feed, and health promoting compounds. Among the commercially important microalgae, Haematococcus pluvialis is the richest source of natural astaxanthin which is considered as “super anti-oxidant.” Natural astaxanthin produced by H. pluvialis has significantly greater antioxidant capacity than the synthetic one. Astaxanthin has important applications in the nutraceuticals, cosmetics, food, and aquaculture industries. It is now evident that, astaxanthin can significantly reduce free radicals and oxidative stress and help human body maintain a healthy state. With extraordinary potency and increase in demand, astaxanthin is one of the high-value microalgal products of the future.This comprehensive review summarizes the most important aspects of the biology, biochemical composition, biosynthesis, and astaxanthin accumulation in the cells of H. pluvialis and its wide range of applications for humans and animals. In this paper, important and recent developments ranging from cultivation, harvest and postharvest bio-processing technologies to metabolic control and genetic engineering are reviewed in detail, focusing on biomass and astaxanthin production from this biotechnologically important microalga. Simultaneously, critical bottlenecks and major challenges in commercial scale production; current and prospective global market of H. pluvialis derived astaxanthin are also presented in a critical manner. A new biorefinery concept for H. pluvialis has been also suggested to guide toward economically sustainable approach for microalgae cultivation and processing. This report could serve as a useful guide to present current status of knowledge in the field and highlight key areas for future development of H. pluvialis astaxanthin technology and its large scale commercial implementation.
Keywords: Haematoccoccus pluvialis, astaxanthin, nutraceuticals, algae cultivation and processing, biorefinery
Introduction
“Green microalgae” comprise more than 7000 species growing in a variety of habitats. Haematococcus pluvialis (Chlorophyceae, Volvocales) is unicellular fresh water microalga distributed in many habitats worldwide. It is considered as the best natural source of astaxanthin and the main producing organism of this commercial product (Lorenz, 1999; Ranga Rao et al., 2010). Astaxanthin (3,3′-dihydroxy-ß-carotene-4,4′-dione) is a bright red secondary carotenoid from the same family as lycopene, lutein, and β-carotene, synthesized de novo by some microalgae, plants, yeast, bacteria, and present in many of our favored seafood including salmon, trout, red sea bream, shrimp, lobster, and fish eggs (Higuera-Ciapara et al., 2006; Ranga Rao et al., 2014). Astaxanthin contains two chiral centers and can exist in three different stereoizomers, (3S, 3′S); (3R, 3′S), and (3R, 3′R). The ratio of 1:2:1 of these isomers is obtained during chemical synthesis of this compound. H. pluvialis biosynthesizes predominantly the 3S, 3′S stereoisomer, the most valuable one (Yang et al., 2013; Al-Bulishi et al., 2015). Astaxanthin synthesis in H. pluvialis is directly correlated in space and time with deposition of cellular reserves in lipid droplets under conditions of cellular stress (Solovchenko, 2015). From biochemical perspective astaxanthin is synthesized through carotenoid pathway from glyceraldehyde-3-phosphate and pyruvate. Both of these compounds are products of photosynthesis and/or glycolysis depending on cultivation conditions. These two key metabolic intermediates then enter non-mevalonate (MEP) pathway to generate IPP—key intermediate for the synthesis of all carotenoids including astaxanthin. Astaxanthin has a wide range of applications in the food, feed, cosmetic, aquaculture, nutraceutical, and pharmaceutical industries because of its free radical scavenging capacity. In terms of antioxidant activity astaxanthin is 65 times more powerful than vitamin C, 54 times stronger than β-carotene, 10 times more potent than β-carotene, canthaxantin, zeaxanthin, and lutein; and 100 times more effective than α-tocopherol (Miki, 1991; Borowitzka, 2013; Koller et al., 2014; Pérez-López et al., 2014; Cyanotech, 2015). Currently, over 95% of the astaxanthin available in the market is produced synthetically; while H. pluvialis derived natural astaxanthin corresponds to < 1% of the commercialized quantity (Koller et al., 2014). Synthetic astaxanthin, synthesized from asta-C15 -triarylphosphonium salt and the C10 -dialdehyde in a Wittig reaction (Krause et al., 1997), has 20 times lower antioxidant capacity than its natural counterpart and to date has not been approved for human consumption (Lorenz and Cysewski, 2000; Koller et al., 2014). Moreover, there are concerns about the safety of using synthetic astaxanthin for direct human consumption due to both different stereochemistry and potential carryover of synthesis intermediates. These concerns make natural astaxanthin from H. pluvialis a preferred choice for high-end markets (Li et al., 2011). In addition, H. pluvialis has been already approved as a color additive in salmon feeds and as a dietary-supplement ingredient for human consumption in the USA, Japan, and several European countries (Yuan et al., 2011). Nevertheless, there is no EFSA (European Food Safety Authority) approval for the therapeutic application so far. Supercritical CO2 extracts from H. pluvialis have been granted “novel food” Status by the UK Food Standards Agency (FSA), whilst US FDA (Food and Drug Administration) granted astaxanthin from H. pluvialis “GRAS” status (Generally Recognized As Safe) (Grewe and Griehl, 2012; Capelli and Cysewski, 2013).
The increasing demand for natural astaxanthin and its high price raises interest in efficient systems to produce astaxanthin from H. pluvialis. Various cultivation and astaxanthin production methods in photoautotrophic, heterotrophic, and mixotrophic growth conditions, both indoors; in open raceway ponds or closed photobioreactors using batch or fed-batch approach have been reported (Kang et al., 2005, 2010; Kaewpintong et al., 2007; Ranjbar et al., 2008; García-Malea et al., 2009; Issarapayup et al., 2009; Zhang et al., 2009; Li et al., 2011; Han et al., 2013; Wang et al., 2013a,b). The most recent advances in H. pluvialis cultivation for astaxanthin production include a two-stage mixotrophic culture system (Park et al., 2014) and an “attached cultivation” system using the immobilized biofilm (Zhang et al., 2014). Along with the cultivation process, the induction of carotenoid synthesis in H. pluvialis has a direct correlation with the astaxanthin content of cells and total astaxanthin productivity. The accumulation of astaxanthin is affected by both environmental factors such as light (Saha et al., 2013; Park et al., 2014); temperature (Yoo et al., 2012); pH (Hata et al., 2001); salt concentration (Kobayashi et al., 1993); and nutritional stresses (Boussiba et al., 1999; Chekanov et al., 2014), as well as various plant hormones and their derivatives (Yu et al., 2015). Attempts were made to genetically enhance the capacity of H. pluvialis to produce astaxanthin using both classical mutagenesis (Hu et al., 2008), and more recently genetic engineering (Sharon-Gojman et al., 2015). Once biomass is successfully grown and achieved high cell density, efficient harvesting, cell disruption, dehydration, and recovery/extraction of astaxanthin from H. pluvialis biomass are important factors. Harvesting have been so far achieved through a combination of passive settling and subsequent centrifugation (Han et al., 2013; Pérez-López et al., 2014), or flotation and centrifugation (Panis, 2015). Mechanical processes such as expeller pressing and bead milling are commonly used cell disruption methods to enhance recovery of astaxanthin from H. pluvialis (Razon and Tan, 2011). For the dehydration of H. pluvialis biomass, spray drying (Li et al., 2011; Panis, 2015), freeze drying, lyophilization, and cryodesiccation (Milledge, 2013) methods have been utilized. There are several methods of astaxanthin extraction utilizing solvents, acids, edible oils, enzymes, pressurized liquids (Jaime et al., 2010; Zou et al., 2013; Dong et al., 2014), supercritical carbon dioxide (SC-CO2) (Wang et al., 2012; Reyes et al., 2014), and liquefied dimethyl ether (DME) (Boonnoun et al., 2014). Although many studies have explored various methods of extraction and downstream processing of astaxanthin from H. pluvialis biomass, more comprehensive investigation is required to take an advantage of the biological potential of this microalga and its highly valuable product. Since astaxanthin has a great potential in the global market (280 mt, $447 million in 2014 for both synthetic and natural astaxanthin) and high market value ($2500–7000/kg); (Koller et al., 2014; Pérez-López et al., 2014; Industry Experts, 2015), in depth investigation of H. pluvialis biology, physiology, efficient culture techniques, downstream bioprocessing, and product formation are highly desired for further development of this sector. Even though currently several commercial companies (Cyanotech Corporation, Mera Pharmaceuticals Inc, AIgatechnologies, Fuji Chemical Industry Co. Ltd etc.) are involved in large scale production of H. pluvialis and astaxanthin, the production capacity is far beyond the global demand of natural astaxanthin.
This review summarizes both classical knowledge and most recent advances in the cell biology, physiological, and biochemical characteristics, responses to environmental stresses, and their effect on astaxanthin accumulation, genetic engineering, growth conditions, and different cultivation techniques, harvesting, and post harvest downstream bioprocessing of H. pluvialis. The biorefinery concept, global market potential, challenges, and future direction for development of H. pluvialis and astaxanthin production in commercial scale also are discussed.
Biology of H. pluvialis
Taxonomy and occurrence
The freshwater unicellular biflagellate green microalgae H. pluvialis Flotow belongs to the class Chlorophyceae, order Volvocales and family Haematococcaseae (Bold and Wynne, 1985; Eom et al., 2006). It is also known as Haematococcus lacustris or Sphaerella lacustris. Haematococcus was first described by J. Von Flotow in 1844 and later in 1899 Tracy Elliot Hazen extensively presented its biology and life cycle (Hazen, 1899; Leonardi et al., 2011). H. pluvialis is common in small transient freshwater bodies and widely distributed in many habitats worldwide. It occurs primarily in temporary water bodies like ephemeral rain pools, artificial pools, natural and man-made ponds, and birdbaths (Czygan, 1970; Burchardt et al., 2006). This microalga can be usually found in temperate regions around the world and has been isolated from Europe, Africa, North America, and Himachal Pradeslv India (Pringsheim, 1966; Suseela and Toppo, 2006). It has been also found across diverse environmental and climate conditions: in brackish water on the rocks on the seashore (Chekanov et al., 2014); freshwater basin in the rock filled with melted snow on Blomstrandhalvøya Island (Norway) (Klochkova et al., 2013); dried fountain near Rozhen, Blagoevgrad in Bulgaria Gacheva et al., 2015, freshwater fishpond in Bihor, Romania (Dragos et al., 2010); rooftop surface of a building of KIOST in Seoul Korea (Kim et al., 2015). It is well suited for survival under conditions of expeditious and extreme in light, temperature, and salt concentration that would be deleterious to many other microalgae, due to its ability to encyst (become enclosed by thick membrane) in a rapid manner (Proctor, 1957).
Cellular morphology and life cycle
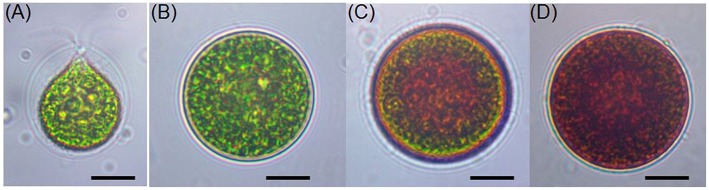
Cellular structure of H. pluvialis is similar to most of other members of volvocalean unicellular green algae. The life cycle of H. pluvialis consists of four types of distinguishable cellular morphologies: macrozooids (zoospores), microzooids, palmella, and hematocysts (aplanospores) (Hazen, 1899; Elliot, 1934). Macrozooids (zoospores), microzooids, and palmella stages are usually called “green vegetative phase” (Figures 1A,B). Hematocysts (aplanospores) are referred as “red nonmotile astaxanthin accumulated encysted phase” of the H. pluvialis life cycle (Figures 1C,D). Macrozooids (zoospores) are spherical, ellipsoidal, or pear-shaped cells with two flagella of equal length emerging from anterior end, and a cup-shaped chloroplast with numerous, scattered pyrenoids (Figure 1A). The macrozooid cells are between 8 and 20 μm long with a distinct gelatinous extracellular matrix of variable thickness. Numerous contractile vacuoles are irregularly distributed near the protoplast surface of the cell (Hagen et al., 2002). These flagellated fast-growing vegetative cells predominate under favorable culture conditions in the early vegetative growth stage (Figure 1A) Macrozooids may divide into 2–32 daughter cells by mitosis (Wayama et al., 2013) (Figures 2A,B). Under unfavorable environmental or culture conditions, macrozooids start losing flagella, and expand their cell size. They form an amorphous multilayered structure in the inner regions of the extracellular matrix or the primary cell wall as they develop into non-motile “palmella” and become resting vegetative cells (Hagen et al., 2002) (Figure 1B). With the continued environmental stress (i.e., nutrient deprivation, high light irradiance, high salinity) and cessation of cell division, “palmella” transform into the asexual “aplanospores” (Figures 1C,D). At this stage, cells contain two distinct structures, a thick and rigid trilaminar sheath, and secondary cell wall of acetolysis-resistant material. Such cells become resistant to prevailing extreme environmental conditions (Santos and Mesquita, 1984; Boussiba and Vonshak, 1991). Mature aplanospores; accumulate large amounts of secondary carotenoids, particularly astaxanthin, in lipid droplets deposited in the cytoplasm, which results in a characteristic bright red color of these cells (Hagen et al., 2002). Some H. pluvialis strains are reported to be capable of accumulating astaxanthin without forming aplanospores (Brinda et al., 2004). Once environmental or culture conditions return to optimal, red aplanospores germinate to form flagellated zoospores to initiate a new vegetative growth cycle (Figure 1A). In some cases, gametogenesis may occur in aplanospores (Figures 3A–D). Such process requires an exposure to extreme adverse conditions (freezing, desiccation, or nutrient starvation) followed by return to favorable culture conditions. During gametogenesis, aplanospore cells can produce up to 64 gametes which are known as microzooids. The microzooids are smaller in size (< 10 μm) compared to the zoospores (20–50 μm), and exhibit high-speed motility after their release from gametocysts. Sexual reproduction is rarely observed in H. pluvialis, and has been largely replaced by vegetative reproduction (Triki et al., 1997).
Figure 1.
Light microscopic images of H. pluvialis cells in life cycle. (A) Green vegetative motile cell; (B) Green vegetative palmella cell; (C) Astaxanthin accumulating palmella cell in transition to aplanospore; (D) Astaxanthin accumulated aplanospore cell. Scale bar: 10 μm.
Figure 2.
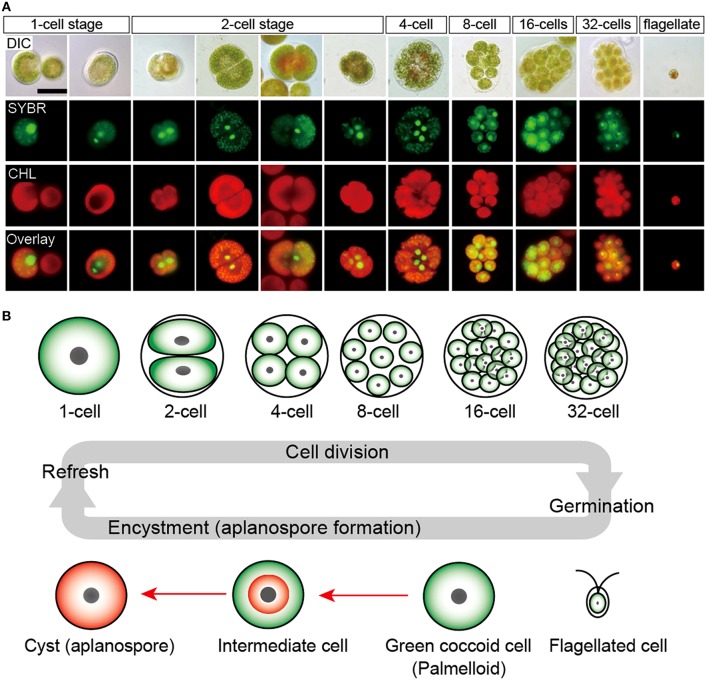
Life cycle of H. pluvialis. (A) Fluorescence microscopy images, showing the 1- to 32-cell stages, and the flagellated stage. DIC, differential interference contrast image; SYBR, SYBR Green I-stained cells (green); CHL, chlorophyll autofluorescence (red); and Overlay, overlaid images of SYBR and CHL. (B) Illustration of life cycle of H. pluvialis. Refresh, when old cultures are transplanted into fresh medium, coccoid cells undergo cell division to form flagellated cells within the mother cell wall. Germination, Flagellated cells settle and become coccoid cells. Continuous and/or strong light accelerate the accumulation of astaxanthin during encystment (red arrows). Figure reproduced from Wayama et al. (2013) distributed under the terms of the Creative Commons Attribution License.
Figure 3.
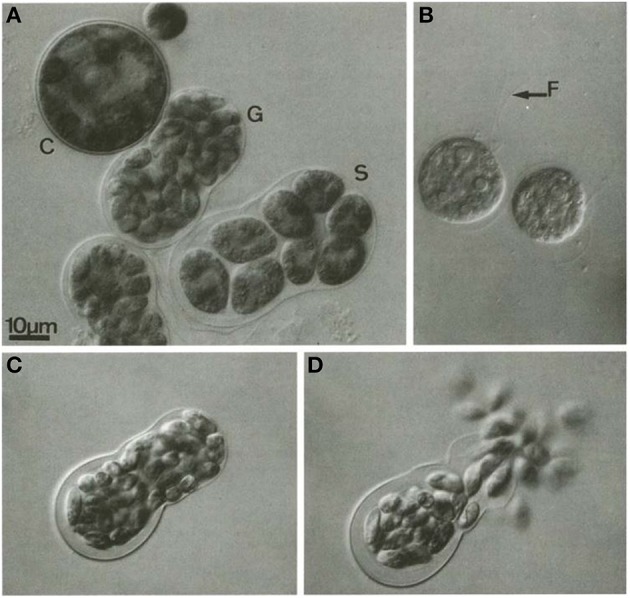
Gametogenesis in H. pluvialis. (A) C, cyst; G, gametocyst; S, a sporocyst; (B) vegetative zoospore, F, flagella (indicated by arrow); (C): gametocyst before releasing gametes; (D): release of gametes from gametocyst. Reproduced with permission from Triki et al., 1997, Phycologia, Allen Press Publishing Services. Copyright (1997) Allen Press Publishing Services.
Ultrastructural changes of H. pluvialis during the life cycle
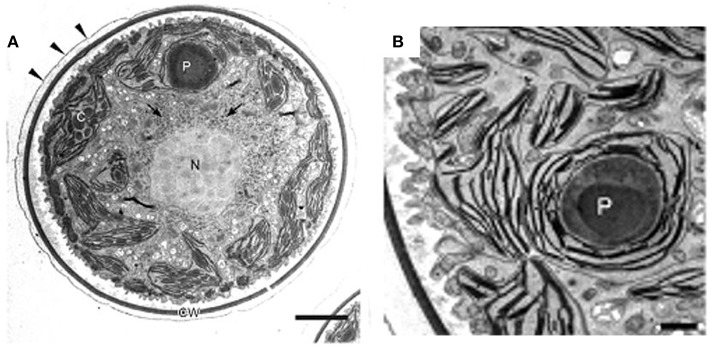
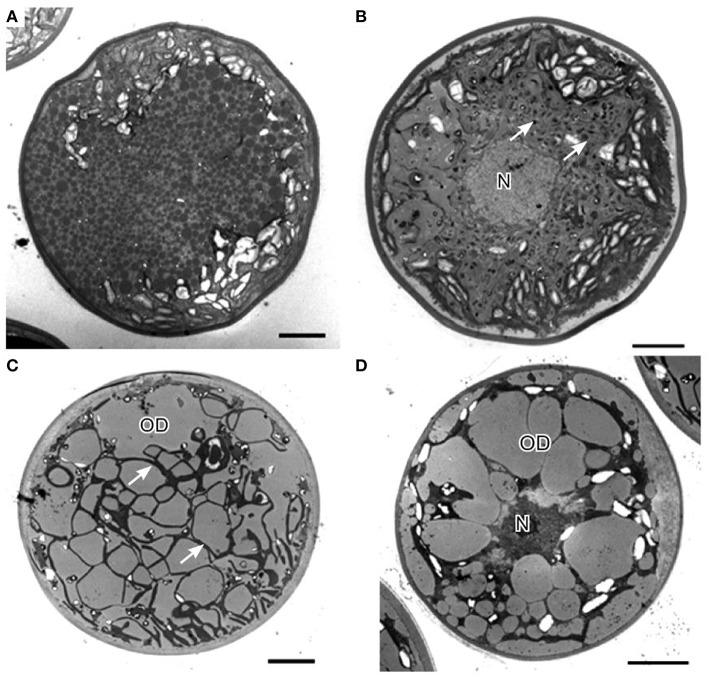
In H. pluvialis cells, large amount of ultrastructural changes occurs during their life cycle which is frequently associated with responses to stress conditions in the environment. In the green vegetative palmella cells, a thick cell wall surrounds the cell, and two layers of extracellular matrix present near the cell wall (Figure 4A). Nucleus is located in the center of the cell, and highly developed chloroplasts are located at the periphery (Figure 4A). Few astaxanthin granules are present which located around the nucleus (Figure 4A). Conspicuous pyrenoids with electron-dense matrix can be found in the stroma (Figures 4A,B) (Wayama et al., 2013). During the starting of encystment process, Haematococcus turned into greenish-orange cells (with some astaxanthin accumulation) which can be referred as intermediate stage cells. In this stage, conspicuous pyrenoids with electron-dense matrix located in the stroma, remain surrounded by thick starch capsules and many starch grains are located around the pyrenoids (Figure 5). Round oil droplets with various sizes, containing astaxanthin located around the nucleus (Figure 5). At this stage, thylakoids become partial degraded (Wayama et al., 2013). With the gradual accumulation of astaxanthin chloroplast reduce in volume but photosynthetic activity remains. In the aplanosopre or cyst stage, astaxanthin accumulates and cells form cysts. Oil droplets and astaxanthin accumulation patterns may differ among cyst cells. For example, electron-dense astaxanthin granules in oil droplets (Figures 6A,B) occurred in some cells. In other cells, relatively large oil droplets occurred throughout the cell (Figures 6C,D). Chloroplasts are degenerated and localized in the interspace between oil droplets (Wayama et al., 2013).
Figure 4.
Transmission electron micrographs of green vegetative cells of H. pluvialis. (A) General ultrastructure. The cell wall is surrounded by extracellular matrix (arrowheads). Arrows indicate astaxanthin granules. (B) Chloroplast and pyrenoid. C, chloroplast; CW, cell wall; N, nucleus; P, pyrenoid. Scale bars in (A,B): 5 and 1 μm, respectively. Figure reproduced from Wayama et al. (2013) distributed under the terms of the Creative Commons Attribution License.
Figure 5.
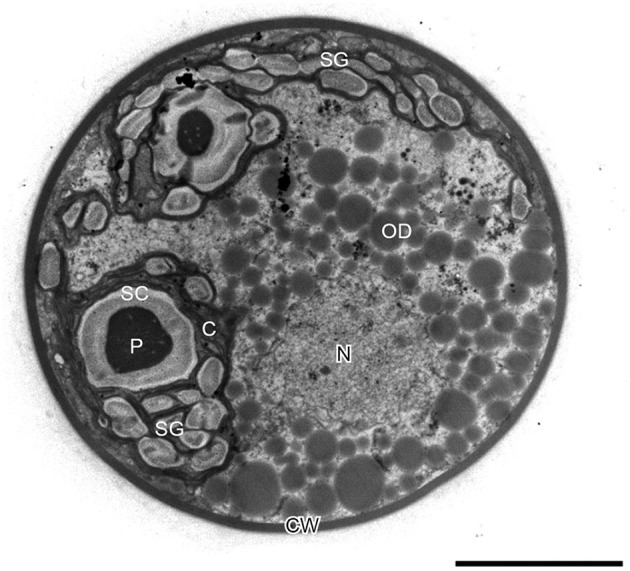
Transmission electron micrograph of intermediate H. pluvialis cell (general ultrastructure). C, chloroplast; CW, cell wall; N, nucleus; OD; oil droplet; P, pyrenoid; SC, starch capsule; SG, starch grain. Scale bar: 5 μm. Figure reproduced from Wayama et al. (2013) distributed under the terms of the Creative Commons Attribution License.
Figure 6.
Transmission electron micrographs of H. pluvialis cyst cells. (A) General ultrastructure of cyst cells, showing small granules that contain astaxanthin. (B) General ultrastructure of a cyst cell, showing astaxanthin accumulation in oil droplets. (C) General ultrastructure of a cyst cell, showing large oil droplets. Chloroplasts localize in the interspace between oil droplets (arrows). (D) Some oil droplets are fused. C, chloroplast; N, nucleus; OD, oil droplet. Scale bars in (A–D): 5 μm. Figure reproduced from Wayama et al. (2013) distributed under the terms of the Creative Commons Attribution License.
Biochemical composition of H. pluvialis
Because of the unique life cycle of H. pluvialis, cellular composition of this microalga varies tremendously between its “green” and “red” stages of cultivation (Table 1).
Table 1.
Typical composition of H. pluvialis biomass in green and red cultivation stages.
| Composition content (% of DW) | Green stage | Red stage |
|---|---|---|
| Proteins | 29–45 | 17–25 |
| Lipids (% of total) | 20–25 | 32–37 |
| Neutral lipids | 59 | 51.9–53.5 |
| Phospholipids | 23.7 | 20.6–21.1 |
| Glycolipids | 11.5 | 25.7–26.5 |
| Carbohydrates | 15–17 | 36–40 |
| Carotenoids (% of total) | 0.5 | 2–5 |
| Neoxanthin | 8.3 | n.d |
| Violaxanthin | 12.5 | n.d |
| β-carotene | 16.7 | 1.0 |
| Lutein | 56.3 | 0.5 |
| Zeaxanthin | 6.3 | n.d |
| Astaxanthin (including esters) | n.d | 81.2 |
| Adonixanthin | n.d | 0.4 |
| Adonirubin | n.d | 0.6 |
| Canthaxanthin | n.d | 5.1 |
| Echinenone | n.d | 0.2 |
| Chlorophylls | 1.5–2 | 0 |
Adapted from Grewe and Griehl (2012). n.d., no data.
Protein
In green stage, during favorable growth conditions most H. pluvialis strains are rich in protein (29–45) (Table 1), lower protein content (23.6%) have been however observed in a Bulgarian strain Haematococcus cf. pluvialis Rozhen-12 during green stage cultivation (Gacheva et al., 2015). It was estimated that proteins contribute to 21 (Kim et al., 2015) and 23% (Lorenz, 1999) of cellular content during red stage cultivation of H. pluvialis. Amino acid composition of proteins in the red stage indicated that proteins were mainly composed of aspartic acid, glutamic acid, alanine, and leucine with total amino acid content of 10.02/100 mg, 46.0% of which belonged to essential amino acids (Lorenz, 1999; Kim et al., 2015).
Carbohydrates
In green stage, carbohydrate content approximates to 15–17%, about a half of the red stage (Table 1). In the red stage, under conditions of stress (e.g., nutrient starvation, light stress, high acidity, temperature variations etc.), H. pluvialis accumulates higher content of carbohydrates (starch), for example 38 (Lorenz, 1999), 60 (Recht et al., 2012), and 74% (Boussiba and Vonshak, 1991). Under prolonged stress conditions starch is consumed in the cell.
Lipid
In green stage, total lipid content varies from 20 to 25%, with approximately 10% lipids composed predominantly of short (C16, C18) polyunsaturated fatty acids deposited in the chloroplasts. Neutral lipids are predominant lipid class in both green and red cells (Table 1). In red stage, prolonged stress conditions direct larger flux toward the synthesis of neutral lipids—triacylglycerols (TAG). Red stage cells can accumulate up to 40% of their cell weight as cytoplasmic lipid droplets (LD), and considerable amount of secondary metabolites including up to 4% of the ketocarotenoid astaxanthin (Boussiba et al., 1992, 1999; Saha et al., 2013). The phospholipid content does not change compared to the green stage, while the glycolipid fraction nearly doubles in red cells when compared with green vegetative cells (Damiani et al., 2010). The total fatty acid profile of H. pluvialis is relatively complex. Palmitic (16:0), linoleic (18:2), and linolenic (18:3) acids are predominant components of the profile with highly polyunsaturated species also present in considerable amounts (Table 2). Based on the comparative studies on fatty acids profile of two different H. pluvialis, it was revealed that both strains varied in composition, especially of palmitic (16:0), oleic (18:1), linoleic (18:2), and linolenic (18:3) acids. This variation might be associated with several factors such as culture environment, stress conditions, culture parameters, variation of strain origin, nutrient etc. Higher lipid content of H. pluvialis grown under nutrient starvation and the suitable profile of its fatty acids indicate a possibility of biodiesel production from this microalga (Damiani et al., 2010; Saha et al., 2013). The massive astaxanthin accumulation in H. pluvialis is a cellular response to stress conditions and is accompanied by the enhanced biosynthesis of triacylglycerols (TAG) (Zhekisheva et al., 2002, 2005; Cerón et al., 2007), and the reduction in photosynthetic activity of PSII, loss of cytochrome f, and subsequent reduction in electron transport, and increased respiration rate (Boussiba, 2000). During transition from green vegetative cells to red aplanospores after exposure to stress conditions astaxanthin start to accumulate as fatty acid mono- or diesters in cytoplasmic lipid droplets (LD) (Aflalo et al., 2007). As cells undergo transition to red stage, both chlorophyll and protein contents drop.
Table 2.
Comparison of fatty acid composition (%) of two different H. pluvialis strains.
| Fatty acids | Haematococcus sp. KORDI03 (Kim et al., 2015) | H. pluvialis (Lorenz, 1999) |
|---|---|---|
| C12:0 lauric | N/A | 0.1 |
| C14:0 myristic | 0.1 | 0.5 |
| C15:0 pentadecanoic acid | 0.1 | N/A |
| C16:0 palmitic | 13.7 | 29.0 |
| C16:1 palmitoleic | 0.5 | 0.6 |
| C16:2 | 0.4 | N/A |
| C16:3 | 3.5 | N/A |
| C16:4 | 3.3 | N/A |
| C17:0 margaric | N/A | 0.2 |
| C17:1 margaroleic | N/A | 1.3 |
| C18:0 stearic | 0.7 | 2.1 |
| C18:1 oleic | 4.9 | 25.9 |
| C18:2 linoleic | 24.9 | 20.8 |
| C18:3 linolenic | 39.7 | 12.8 |
| C18:4 octadecatetraenoic | 5.8 | 1.4 |
| C20:0 arachidic | N/A | 0.6 |
| C20:1 gadoleic | 0.5 | 0.3 |
| C20:2 eicosadenoic | N/A | 1.2 |
| C20:3 eicosatrienoic gamma | N/A | 0.5 |
| C20:4 arachidonic | 0.9 | 1.4 |
| C20:5 eicosapentaenoic | 0.6 | 0.6 |
| C22:0 behenic | N/A | 0.4 |
| C24:0 lignoceric | 0.3 | 0.2 |
| C24:1 nervonic acid | 0.1 | 0.1 |
| ∑ SFAs | 15.0 | 33.2 |
| ∑ MUFAs | 6.0 | 28.1 |
| ∑ PUFAs | 79.1 | 38.7 |
| Total | 100.0 | 100.0 |
Carotenoid
The carotenoid fraction of green vegetative cells consists of mostly lutein (75–80%), β-carotene (10–20%) and others, including chlorophyll a and b, primary carotenoids, violaxanthin, neoxanthin, lactucaxanthin, and zeaxanthin (Renstrøm et al., 1981; Harker et al., 1996a). In the red stage, the total carotenoid content is markedly enhanced, and the characteristic primary carotenoid pattern of vegetative stage is replaced by secondary carotenoids, mainly astaxanthin (80–99% of total carotenoids) (Harker et al., 1996a; Dragos et al., 2010). The ratio of carotenoids to chlorophylls is about 0.2 in the green stage and increases in the red stage by an order of magnitude and reaches about 2–9. The majority of astaxanthin is not deposited in its free form but it exists within the cell as fatty acid esters of astaxanthin, usually mono- or diesters of palmitic (16:0), oleic (18:1), or linoleic (18:2) acids. This type of modification is required for the deposition of this highly polar molecule within non-polar matrix of lipid droplets. Approximately 70% monoesters, 25% diesters, and only 5% of the free ketocarotenoid is present in the mature “red” cells of H. pluvialis (Zhekisheva et al., 2002; Solovchenko, 2015). Under certain conditions of stress H. pluvialis has been shown to accumulate up to 3–5% DW of astaxanthin (Han et al., 2013; Chekanov et al., 2014).
H. pluvialis-derived astaxanthin
H. pluvialis as a major source of astaxanthin
H. pluvialis can accumulate up to 5% DW of astaxanthin and is considered as the best natural source of this high-value carotenoid pigment (Wayama et al., 2013). Dietary supplements containing Haematococcus astaxanthin has proved to be safe to humans and widely used for over 15 years as a nutraceutical supplement with no adverse side-effects of its supplementation (Capelli and Cysewski, 2013; Yang et al., 2013). Natural astaxanthin from H. pluvialis or krill oil is available in the market as a dietary supplement in dosages from 3.8 to 7.6 mg per day due to potential health benefits (Yang et al., 2013). As societies nowadays are looking toward “green” solutions, natural astaxanthin form H. pluvialis seems to be more favorable than its synthetic counterpart due to structure, function, application, and security (Choubert and Heinrich, 1993; Capelli and Cysewski, 2013; Pérez-López et al., 2014).
Biosynthesis of astaxanthin in H. pluvialis
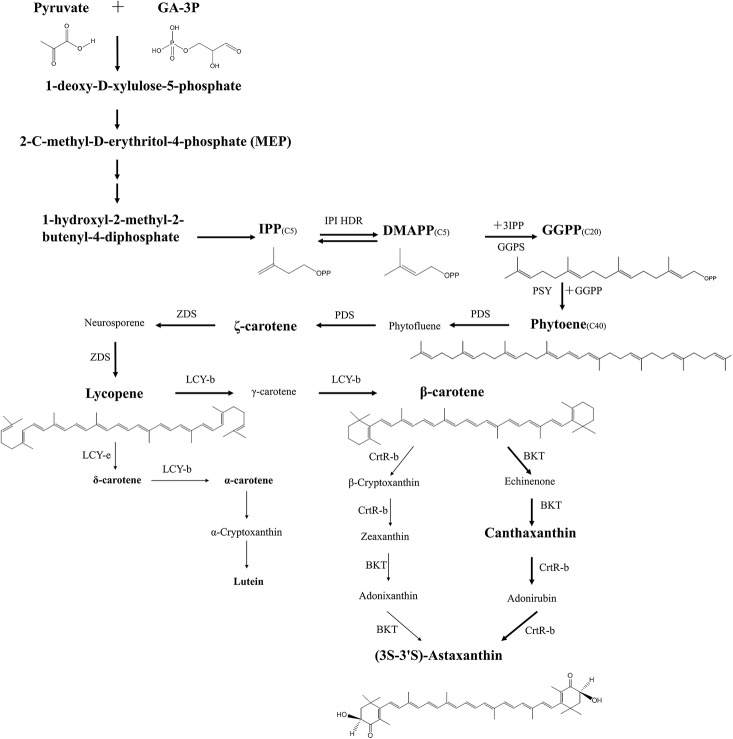
Biosynthesis of astaxanthin in H. pluvialis is a complex process that is highly up-regulated in conditions of stress and which coincides with the accumulation of triacylglycerols (TAGs). Both compounds are deposited in the cytosolic lipid bodies during the “red” stage of H. pluvialis cultivation. Astaxanthin belongs to carotenoids, a C40 tetraterpenes, synthesized from isoprene units. Isopentenyl pyrophosphate (IPP) is a key intermediate of carotenoid synthesis. In principle, IPP can originate from two dissimilar pathways: mevalonate pathway (MVA) located in cytosol and non-mevalonate (MEP) located in the chloroplast (Lichtenthaler et al., 1997; Lichtenthaler, 1999; Eisenreich et al., 2001). Alternative name for MEP is DOXP, due to the formation of 1-deoxy-D-xylulose-5-phosphate in the first stage of the pathway. Comparative transcriptomic analysis of astaxanthin biosynthesis in H pluvialis have shown that the key intermediate-IPP is most likely synthesized using the DOXP pathway. H. pluvialis lacks three key enzymes of the mevalonate pathway (MVA) catalyzing the formation of isopentenyl pyrophosphate (IPP) from acetoacetyl-CoA (Gwak et al., 2014). There has been numerous evidence of the full set of enzymes required for the conversion of photosynthesis-derived products i.e., pyruvate and glyceraldehyde-3-phosphate into isopentenyl pyrophosphate through DOXP pathway inside H. pluvialis chloroplasts (Gwak et al., 2014). It makes it the most likely source of IPP in H. pluvialis cells. The process of astaxanthin biosynthesis is presented on Figure 7. IPP derived from DOXP pathway is an initial building block of astaxanthin synthesis. In the subsequent step the IPP undergoes isomerization to dimethylallyl diphosphate (DMAPP). It has been long assumed that this conversion was catalyzed exclusively by isopentenyl pyrophosphate isomerase (IPI) (Sun et al., 1998; Lichtenthaler, 1999). However, recent transcriptomic studies suggest that neither of the two ipi genes of H pluvialis (ipi1 and ipi2IPI2) are up-regulated during cellular accumulation of astaxanthin (Gwak et al., 2014). Suggestions have been made that another enzyme of similar activity, 4-hydroxy-3-methylbut-2-enyl diphosphate reductase (HDR) was more likely to be responsible for catalyzing interconversion between IPP and DMAPP (Hoeffler et al., 2002; Rohdich et al., 2002; Gwak et al., 2014). Further studies are required to assess the contribution of these three enzymes to this key biosynthetic step of astaxanthin formation. Elongation of the isoprenoid chain is initiated with a molecule of DMAPP and a subsequent linear additions of three molecules of IPP catalyzed by an enzyme geranylgeranyl pyrophosphate synthase (GGPS) (Britton, 1993; Cunningham and Gantt, 1998). The final step of this process is the formation of a C20 compound, geranylgeranyl pyrophosphate (GGPP), a shared precursor with other isoprenoids. The first committed step of carotenoid synthesis is catalyzed by phytoene synthase (PSY) and results in a head-to-tail condensation of two GGPP molecules to form a C40 compound—phytoene that serves as a precursor for astaxanthin and other carotenoids (Cunningham and Gantt, 1998). The expression of the phytoene synthase gene (psy) was up-regulated in Haematococcus cells stressed with high light and undergoing transformation from “green” to “red” stage (Steinbrenner and Linden, 2001; Vidhyavathi et al., 2008; Gwak et al., 2014). The subsequent formation of highly unsaturated compound—lycopene proceeds through four desaturation steps catalyzed by two phytoene desaturases (PDS) and a ζ-carotene desaturase (ZDS) with two plastid terminal oxidase (PTOX 1, PTOX 2) acting as co-factors for electron transfer between C40 carotenoid intermediates, plastoquinone and final electron acceptor—oxygen (Li et al., 2010; Nawrocki et al., 2015). Of the two, PTOX 1 was found to be co-regulated with astaxanthin synthesis in H. pluvialis (Wang et al., 2009; Nawrocki et al., 2015). Desaturation reactions increase the number of conjugated carbon-carbon double bonds that form the chromophore in carotenoids and convert a colorless molecule of ζ-carotene to a pink colored lycopene (Cunningham and Gantt, 1998). Both termini of lycopene undergo cyclization catalyzed by lycopene cyclases (LCY-e and LCY-b). Cyclization is a branching point of the carotenoid biosynthesis in most organisms, yielding α-carotene (precursor of lutein) and β-carotene (precursor of other carotenoids including astaxanthin). In H. pluvialis vast majority of the carbon flux is directed into the latter (Gwak et al., 2014), and high level of LCY-b transcripts have been observed under stress conditions (Lorenz and Cysewski, 2000; Gwak et al., 2014). Final two oxygenation steps catalyzed by β-carotene ketolase (BKT) and β-carotene hydroxylase (CrtR-b) are rate limiting steps of astaxanthin synthesis (Linden, 1999; Steinbrenner and Linden, 2001; Vidhyavathi et al., 2008). Although in principle the reactions catalyzed by these two enzymes can proceed in any order, higher substrate specificity of BKT toward β-carotene than zeaxanthin favors initial addition of keto group before enantio-selective hydroxylation of canthaxanthin to astaxanthin is catalyzed by CrtR-b (Lotan and Hirschberg, 1995). Enantioselectivity of astaxanthin synthesis is of primary importance for the nutraceuticals market and the major advantage of H. pluvialis astaxanthin over its synthetic counterpart. Since astaxanthin has two identical chiral centers at the positions of 3 and 3′ it can exist in four different configurations which yield three different isomers: (3R, 3′S); (3R, 3′R); (3S, 3′S) depending on the spatial orientation of the hydroxyl (OH) groups in chiral carbon. During chemical synthesis these isomers are present in the ratio of 2:1:1, respectively, yielding only 25% of the naturally occurring (3S, 3′S) isoform. H. pluvialis synthesizes the (3S, 3′S) stereoisomer of astaxanthin and is therefore a much sought-off product in the nutraceutical market.
Figure 7.
Pathway of (3S-3′S)-astaxanthin biosynthesis in H. pluvialis. Major carbon flux during the red stage of H. pluvialis cultivation is indicated with thick arrows, minor products are indicated with thin arrows. Major intermediates of biosynthesis are indicated in large fonts, minor intermediates in small fonts. Enzyme abbreviations are as follows: IPI, Isopentenyl pyrophosphate isomerase; HDR, 4-hydroxy-3-methylbut-2-enyl diphosphate reductase; GGPS, geranylgeranyl pyrophosphate synthase; PSY, phytoenesysthase; PDS, phytoenedesaturase; ZDS, ζ-carotene desaturase; LCY-b, lycopene β-cyclase; LCY-e, lycopene ε-cyclase; BKT, β-carotene ketolase; CrtR-b, β-carotene 3,3′-hydroxylase; Intermediates: Phytofluene, Neurosporene, γ-carotene, β-Cryptoxanthin, Adonixanthin, Echinenone, Adonirubin.
Effect of small molecules on astaxanthin synthesis
Astaxanthin is a secondary metabolite, a carotenoid synthesized by H. pluvialis in a response to stress conditions such as high light, salinity, or carbon to nitrogen ratio (Gao et al., 2012a). Regulation of these pathways can be affected by numerous small molecules like plant hormones or their analogs. An array of such molecules has been explored to modulate astaxanthin accumulation by H. pluvialis. Plant hormones that are usually associated with stress response mechanisms e.g., abscisic acid (ABA), jasmonic acid (JA), methyl jasmonate (MJ) or growth regulators like gibberellic acid (GA3), salicylic acid (SA) or brassinosteroid—2, 4-epibrassinolide (EBR) were found particularly promising in increasing astaxanthin accumulation in H. pluvialis (Kobayashi et al., 1997b; Gao et al., 2012a,b, 2013a,b; Yu et al., 2015). It was found that these compounds affect numerous genes involved in astaxanthin biosynthesis and result in their even six to 10 fold up-regulation. All of these compounds were tested in various concentrations and the highest improvement of astaxanthin accumulation was achieved with salicylic acid. At relatively low concentrations of the hormone 50 mg L−1 and low light 25 μmol photons m−2 s−1 the content of astaxanthin raised seven fold from 0.391 mg L−1 to 2.74 mg L−1. Higher levels of these hormones had however deleterious effect on both growth and astaxanthin accumulation (Gao et al., 2012a). Correlation between mRNA transcript levels of five key enzymes of astaxanthin synthesis pathway (ipi, psy, pds, crtO, and crtR-b encoding respectively isopentenyl-diphosphate δ-isomerase (IPI), phytoene synthase (PSY), phytoene desaturase (PDS), β-carotene oxygenase (CrtO), and β-carotene hydroxylase (CrtR-b) with alga growth and astaxanthin production suggested a complex, multiple regulatory mechanisms at transcriptional, translational, and post-translational levels controlling entire process of carotenoid synthesis in H. pluvialis (Li et al., 2010). Small molecules can have multiple effects on the regulation of each of these genes and more detailed investigation of the molecular responses to their application is required for both understanding gene regulation in H. pluvialis and enhancing its capacity as commercial astaxanthin producer.
Genetic improvement of H. pluvialis for astaxanthin production
Green eukaryotic microalgae are among the organisms that are notoriously difficult to genetically engineer. In principle, genetic engineering of microalgae has been reported for over 30 strains of various species, including Haematococcus (Rosenberg et al., 2008; Radakovits et al., 2010; Forján et al., 2015). In majority of these reports however, only a transient transgene expression has been achieved and a desired, stable, hereditary, and efficient genetic modification existed only for model species such as Chlamydomonas reinhardtii and Volvox carteri. Due to these constrains genetic improvement of H. pluvialis strains have been long limited to classical mutagenesis. Combination of mutagenic treatment and various screening methods resulted in the development of numerous interesting mutants of H. pluvialis including those of higher astaxanthin accumulation capacity (Tjahjono et al., 1994a; Chumpolkulwong et al., 1997; Tripathi et al., 2001; Chen et al., 2003; Hu et al., 2008; Hong et al., 2012; Gomez et al., 2013). Various mutagenic treatments have been tested, and can be broadly divided into UV and chemical mutagenesis. Chemical mutagenesis has been generally found to be more suitable for H. pluvialis due to alga's intrinsic capacity to limit the damage from light. Typical chemicals used for mutagenesis include ethyl methanesulphonate (EMS) and N-methyl-N-nitro-N-nitrosoguanidine (MNNG). In all studies their concentrations were adjusted to target 85–95% cell mortality. Successful mutants are usually screened using a combination of factors that promote identification of mutants capable of high astaxanthin accumulation. Typically, herbicides that affect carotenoid synthesis pathway such as norflurazon, fluridone, nicotine, compactin, or diphenylamine are used (Tjahjono et al., 1994a; Chumpolkulwong et al., 1997; Tripathi et al., 2001; Chen et al., 2003; Gomez et al., 2013). Screening relies on identifying colonies that are capable of surviving and/or growing well in the presence of inhibitory concentrations of these compounds and high illumination. Surviving strains should in principle exhibit better capacity to synthesize carotenoids and divert larger, or more efficient carbon flux toward these compounds. A number of successful mutants have been isolated this way and typical improvement of astaxanthin accumulation ranges from several percent (Gomez et al., 2013) to two or three fold improvement (Hu et al., 2008). In former case the mutated strain was tested in commercial scale cultivation system (120,000 L) and retained the improved capacity for astaxanthin production. An alternative approach to strain improvement relying on selection of photosensitive mutants was recently attempted (Hong et al., 2012). Photosensitive mutant with an ability to grow under hetero or mixotrophic conditions should be in principle advantageous over wild type strains due to faster growth rates and more efficient stress trigger. Screening for successful mutants was performed in a three stage process. Since photosensitivity is connected with impaired photosynthesis, these impaired mutants were selected in the first screening. Secondary screening tested for the ability of heterotrophic growth of these photosensitive isolates. Tertiary screening involved mixotrophic conditions with moderate illumination to obtain mixotrophic photosensitive strain that accumulated 4.7% (w/w) of astaxanthin under much shorter induction time (Hong et al., 2012). The mutated strain was stable for at least 1.5 year and is an interesting example of using classical mutagenesis for improvement of H. pluvialis. Mutagenic strain improvement can be expanded by breeding or creating hybrid strains from previous genetic improvement efforts. Technique of protoplast fusion has been successfully applied to H. pluvialis (Tjahjono et al., 1994a). Two mutagenized strains, norflurazon-resistant and nicotine-resistant have been fused to create a hybrid containing genetic material of two initial strains and showed 30% higher astaxanthin accumulation than the initial wild type strain, when neither of the fused strains showed such characteristics (Tjahjono et al., 1994a). Till very recently H. pluvialis was one of the organisms in which genetic engineering of its nuclear genome was considered difficult due to lack of suitable shuttle vectors and satisfactory transformation efficiencies (Sharon-Gojman et al., 2015). A number of unsuccessful approaches have been tried that included various transformation methods (particle bombardment, electroporation, Agrobacterium), vectors, promoters, and strains (Sharon-Gojman et al., 2015). To address these limitations and open a new array of possibilities in H. pluvialis and astaxanthin biology and technology new developments were required. In recent years these developments emerged and stable transformations of H. pluvialis chloroplast (Gutiérrez et al., 2012) and nuclear genomes (Sharon-Gojman et al., 2015) were achieved. Most recent nuclear transformation vectors are capable to transform one or two transgenes into the nuclear genome either 5′ or 3′ of the endogenous dominant selection marker, in the absence of any additional antibiotic resistance genes. The selection marker used in this system is a phytoene desaturase (pds) variant that confers resistance to a herbicide norflurazon due to a single point mutation (L504A). Successful transformation of H. pluvialis was obtained with particle bombardment and numerous constructs based on pds selection marker were delivered and incorporated to the genome showing stability of integration for over 16 months of subculturing (Sharon-Gojman et al., 2015). Genetic engineering of chloroplast genome of H. pluvialis have been also achieved relatively recently (Gutiérrez et al., 2012). So far these studies are limited to expressing exogenous antibiotic resistance gene (aadA cassette) between Internal Transcribed Spacer region and 16S gene of H. pluvialis chloroplast genome, but this technique may in the near future have significant impact on protein production in H. pluvialis (Gutiérrez et al., 2012) as higher protein yields are generally obtained during chloroplast expression of transgenes in other microalgal strains (Li et al., 2015). These new developments in genetic engineering of H. pluvialis can open a new chapter for the development of this organism as both industrial astaxanthin producer and an interesting model for carotenoid synthesis and accumulation studies.
Applications of H. pluvialis astaxanthin
Astaxanthin in human health and as nutraceutical
Astaxanthin possesses various human health benefits and nutraceutical applications and plenty of published information available with evidences, mainly from in vitro and animal models (Guerin et al., 2003; Chew et al., 2004; Higuera-Ciapara et al., 2006; Palozza et al., 2009; Yuan et al., 2011). The effect of Haematococcus derived astaxanthin on various physiological systems in animal and human subject is presented in Table 3. Astaxanthin is considered as “super anti-oxidant” which possesses one of the strongest known antioxidant effects. Its unique structure allows it to span biological membranes and act as an antioxidant by reducing and stabilizing free radicals (Hussein et al., 2006; Liu and Osawa, 2007; Ranga Rao et al., 2010). It is very good at protecting membrane phospholipids and other lipids against peroxidation (Naguib, 2000). There are several studies which showed high antioxidant activity of astaxanthin from H. pluvialis in rats supplemented with diet (Kamath et al., 2008; Ranga Rao et al., 2010, 2013; Augusti et al., 2012). Astaxanthin can terminate the induction of inflammation in biological systems. It can help to fight symptoms of ulcer disease from Helicobacter pylori (Liu and Lee, 2003); protect against gastric lesions (ulcers), improve gastrointestinal health (Nishikawa et al., 2005; Kamath et al., 2008); and treat gastrointestinal discomfort (Andersen et al., 2007; Kupcinskas et al., 2008). Astaxanthin offers protection against free radical damage to preserve immune-system defenses. The immunomodulating capacity of astaxanthin has been found to be superior to that of β-carotene and canthaxanthin (Chew and Park, 2004). Astaxanthin has shown significant effect on immune function in a number of in vitro and in vivo assays using both animal models (Chew et al., 2004) and humans (Park et al., 2010). Astaxanthin is a potential therapeutic agent against atherosclerotic cardiovascular disease (Fassett and Combes, 2011). Astaxanthin supplementation can be beneficial for people with enhanced risk for heart attacks. It is carried by VLDL, LDL, and HDL (high-density lipoprotein) in human blood and protects LDL-cholesterol against oxidation (Iwamoto et al., 2000); has a role in the reduction of blood plasma level (Karppi et al., 2007); and increases basal arterial blood flow (Miyawaki et al., 2008). Oxidative stress is a causative or at least ancillary factor in the pathogenesis of major neurodegenerative diseases (Alzheimer's, Huntington's, Parkinson's, and amyotrophic lateral sclerosis-ALS). Diets high in antioxidants offer the potential to lower the associated risks (Ferrante et al., 1997). Natural astaxanthin can cross the blood-brain barrier in mammals and can extend its antioxidant benefits beyond that barrier. Therefore, astaxanthin can help to alleviate the effects of Alzheimer's disease and other neurological diseases. Astaxanthin can improve respiratory and sympathetic nervous system activities (Nagata et al., 2006), inhibit the growth of fibrosarcoma, breast, and prostate cancer cells and embryonic fibroblasts (Palozza et al., 2009); cell death, cell proliferation and mammary tumors (Nakao et al., 2010). Astaxanthin supplementation can help to protect against UV-induced photooxidation; as an oral sun-protectant; can prevent skin thickening and reduce collagen reduction against UV induced skin damage (Ranga Rao et al., 2013) and can improve skin condition across its layers i.e., corneocyte, epidermis, basal, and dermis by combining oral supplementation and topical treatment (Seki et al., 2001; Yamashita, 2002; Tominaga et al., 2012). Results have shown that semen quality, pregnancy rate and sperm velocity in human subject can be improved (Elgarem et al., 2002; Comhaire et al., 2005) whereas idiopathic infertility can be decreased by astaxanthin (Andrisani et al., 2015).
Table 3.
Effect of H. pluvialis-derived astaxanthin on various physiological systems in human and animal subjects.
| Physiological System | Subject | Effect followed | Main outcome | References |
|---|---|---|---|---|
| Anti-oxidation | Rabbits | Thioredoxin reductase; Paraoxonase activity | Enhanced; No effect | Augusti et al., 2012 |
| Rats | Hepatoprotective and antioxidant activity | Improved | Ranga Rao et al., 2015 | |
| Rats | Antioxidant enzymes, catalase, superoxide dismutase, peroxidase, and lipid peroxidation in plasma and liver | Increased | Ranga Rao et al., 2010, 2013 | |
| Men (bilateral cataract) | Antioxidative effects through changes in superoxide scavenging activity, and hydroperoxides production in aqueous humor | Enhanced; Suppressed | Hashimoto et al., 2013 | |
| Eye function | 18 healthy men | Deep vision | Improved | Sawaki et al., 2002 |
| 10 healthy men | Eye function | Improved | Iwasaki and Tawara, 2006 | |
| 40 asthenopia patients | Eye accommodation power | Improved | Kenji et al., 2005 | |
| 49 healthy men | Uncorrected far visual acuity | Improved | Nakamura et al., 2004 | |
| 87 men (visual display terminal workers) | Eye accommodation amplitude (the adjustment in the lens of the eye that allows it to focus); | Improved; | Nagaki et al., 2006 | |
| Eye soreness, dryness, tiredness, and blurred vision | Reduced | Nagaki et al., 2002 | ||
| Skin | Healthy female or male | Skin wrinkle, corneocyte layer, epidermis, and dermis | Improved | Tominaga et al., 2012 |
| 46 healthy women | Skin elasticity and moisture | Improved | Seki et al., 2001; Yamashita, 2002 | |
| Immune response | 14 healthy women | Oxidative stress and inflammation markers; Immune response | Reduced; Improved | Park et al., 2010 |
| Inflammation | Rats | Gastrointestinal health | Improved | Nishikawa et al., 2005 |
| Gastric ulcer | H. pylori-infected mice | Bacterial load Gastric inflamation | Reduced | Liu and Lee, 2003 |
| Rats | Gastric ulcer markers | Reduced | Kamath et al., 2008 | |
| 44 patients with functional dyspepsia | Inflammatory markers; gastrointestinal discomfort | No effect; No effect | Andersen et al., 2007; Kupcinskas et al., 2008 | |
| Cardiovascular system | 20 adult men | Blood flow time | Improved | Miyawaki et al., 2008 |
| Men | Blood plasma levels | Reduced | Karppi et al., 2007 | |
| Muscle endurance | 16 non-trained men | Lactic acid accumulation after run | Reduced | Sawaki et al., 2002 |
| 19 non-trained men | Respiratory and sympathetic nervous system activities | Improved | Nagata et al., 2006 | |
| 20 non-trained men | Strength/explosiveness test; strength/endurance test | No effect; Improved | Lignell, 2001 | |
| 20 resistance-trained men | Markers of skeletal muscle injury | No effect | Bloomer et al., 2005 | |
| Cancer | Rats | Growth of colon cancer cells | Inhibited | Palozza et al., 2009 |
| Central nervous system | Healthy mice | Memory | Improved | Zhang et al., 2007 |
| 10 healthy men (50–69 years) | Response time and accuracy of several tasks | Improved | Satoh et al., 2009 | |
| Middle aged/elderly men and women | Cog Health battery scores (Nuropsychological memory test) | Improved | Katagiri et al., 2012 | |
| Male fertility | 20 sub-fertile men | Semen quality, pregnancy rate | Improved | Elgarem et al., 2002 |
| 30 sub-fertile men | Sperm velocity; oxidation markers; pregnancy rate | Improved; Reduced; Improved | Comhaire et al., 2005 | |
| 24 healthy men | Idiopathic infertility | Decreased | Andrisani et al., 2015 | |
| Metabolic Syndrome (MS) | Obese rats | Body weight; adipose tissue weight; MS markers | Reduced; Reduced; Improved | Ikeuchi et al., 2007 |
Astaxanthin in aquaculture and poultry industry
During last 20 years, synthetic astaxanthin has been widely used for pigmentation of fish. Haematococcus astaxanthin has great potential in aquaculture industry, due to increasing consumer demands for natural products and ability of Haematococcus astaxanthin to provide necessary supplementation for adequate growth and reproduction of commercially valuable fishes (Salmonid, Red sea bream), rainbow trouts, and shrimps. Microalgae- derived astaxanthin has been demonstrated as safe and effective compound for flesh pigmentation of these fish (Torrissen and Naevdal, 1984; Tolasa et al., 2005). Utilization of H. pluvialis meal for pigmenting has resulted in significant astaxanthin deposition in flesh and skin, flesh coloration enhancement, enhanced antioxidant system, fish egg quality, better growth and survival of fry of Salmonid, sea bream, and rainbow trout (Arai et al., 1987; Sommer et al., 1991; Choubert and Heinrich, 1993; Sheikhzadeh et al., 2012a,b), ornamental fishes (Ako and Tamaru, 1999), and shrimp (Arai et al., 1987; Parisenti et al., 2011). A recent study indicated that diets supplemented with H. pluvialis can improve large yellow croaker fish growth more than diets supplemented with synthetic astaxanthin (Li et al., 2014). H. pluvialis-derived natural astaxanthin has shown to be efficient in pigmentation of egg yolks, egg production (Elwinger et al., 1997) in hen and breast muscle tissue improvement and higher feed efficiency in broiler chicken (Inborr and Lignell, 1997; Inbbor, 1998). It has also been proved to improve health and fertility of chicken and to decrease their mortality (Lignell and Inborr, 1999, 2000).
Cultivation and processing of H. pluvialis for astaxanthin production
Culture conditions and requirements for cell growth and astaxanthin formation
Optimization of the various culture parameters, such as growth medium composition, light, pH, temperature etc. is necessary to achieve high biomass and astaxanthin production. Most of these parameters have different optima for biomass accumulation and astaxanthin production. For carotenogenesis induction, the stronger exposure to stress conditions, the higher astaxanthin accumulation. The origins of this stress can be diverse and successful astaxanthin accumulation has been induced with both, high levels of one stressor, or from a combination of multiple stress factors. In some cases, if cells are exposed to strong stress, cells growth completely ceases and cells begin to die in a relatively short time (Su et al., 2014). Various types of growth media are used for cultivation of H. pluvialis. The most commonly used media are BG- 11 (Rippka et al., 1979), BBM (Bischoff and Bold, 1963), OHM (Fábregas et al., 2000); KM1-basal medium with organic carbon sources in the form of sodium acetate (Kobayashi et al., 1993), and their modifications. An ideal composition of the medium to achieve high growth rate and biomass accumulation is different from ideal composition for high accumulation of astaxanthin. Sodium nitrate was found to be the most optimal inorganic nitrogen source (Sarada et al., 2002a), alternatively an organic source such as urea can be used. When culture is subjected to nutrient deficiency, it leads to accumulation of astaxanthin within the cells (Saha et al., 2013). Nitrogen limitation leads to approximately twice the rate of astaxanthin production than the limitation of phosphorus. It can be due to higher cellular damage resulting from a lack of nitrogen, which manifests in significant degradation of chlorophyll, compared to the phosphorus starvation (Boussiba et al., 1999). Micronutrients such as selenium and chromium result in an increased biomass and astaxanthin production (Tripathi et al., 1999; Fábregas et al., 2000; Domínguez-Bocanegra et al., 2004). Formation of astaxanthin can also be induced by adding NaCl (0.25–0.5% w/v) to the media. Also, when NaCl is added together with 2.2 mM sodium acetate, astaxanthin accumulation can be increased (Sarada et al., 2002b). Addition of 0.45 mM Fe2+ in the form of ferrous sulfate may significantly increase the biosynthesis of carotenoids in cysts due to formation of hydroxyl radicals. (Kobayashi et al., 1997b). This effect may be enhanced by combining Fe2+ treatment with an addition of sodium acetate and high temperature exposure (Kobayashi et al., 1993; Tjahjono et al., 1994b). According to most studies, the suitable temperature for the growth and astaxanthin accumulation of H. pluvialis is between 20 and 28°C (Fan et al., 1994; Hata et al., 2001; Lababpour et al., 2005; Kang et al., 2010; Yoo et al., 2012; Wan et al., 2014a). However, temperature above 30°C induces a transition from green vegetative stage to red stage and formation of red cysts can be observed within 2 days. This transition is combined with a significant slowdown in growth, while astaxanthin accumulation is 2–3 times higher than at 20°C. The increased temperature is likely to affect the synthesis of astaxanthin through stimulation of oxygen radicals formation and their higher reactivity (Tjahjono et al., 1994b). It is preferred that the temperature change takes place gradually, allowing better acclimation to the new conditions (Hata et al., 2001). pH can also significantly affect the cell growth and synthesis of chlorophyll and carotenoids in H. pluvialis. In terms of biomass and astaxanthin production optimal pH is within the range of 7.00–7.85 (Hata et al., 2001; Sarada et al., 2002a). The typical irradiation for H. pluvialis cultivation ranges between 40 and 50 μmol photons m−2s−1 (Hata et al., 2001; Chekanov et al., 2014; Park et al., 2014). Optimal irradiation to achieve a high growth rates tend to be higher, namely 70 (Zhang et al., 2014), 80 (Saha et al., 2013), 90 (Fan et al., 1994), or even up to 177 μmol photons m−2s−1 (Domínguez-Bocanegra et al., 2004). These different optimal values may be caused by other cultivation parameters such as media composition, temperature, or the strain of H. pluvialis. During vegetative stage cultivation of H. pluvialis, the regular cycles of alternating light and dark 12:12 or 16: 8 h are often used (Saha et al., 2013; Park et al., 2014), but the higher density cultures are achieved with continuous illumination (Domínguez-Bocanegra et al., 2004). The best practice to date appears to be white or blue LED lighting (Saha et al., 2013) or the mixture of both at the ratio of 3:1 at 7000 lx (~95 μmol photons m−2s−1). These conditions promote morphologic changes from green vegetative cells to red cyst cells (Sun et al., 2015). Carotenogenesis is induced in cells upon exposure to higher light intensity than the corresponding light saturation point (LSP). However, specific optimum value of LSP differ between studies. The lowest intensities that have been reported utilized irradiation of around 100–150 μmol photons m−2s−1 (Zhang et al., 2014) followed by 240 (Saha et al., 2013), 345 (Domínguez-Bocanegra et al., 2004), and 480 μmol photons m−2s−1 (Chekanov et al., 2014). Lower optimal irradiation was found to be influenced by other stress conditions, such as deficiency of nutrients (Saha et al., 2013; Zhang et al., 2014) or elevated temperature (Tjahjono et al., 1994b). It proves that for effective induction of carotenogenesis excessive irradiation may not be necessary if other stressors are present. With the reduced requirements of light for cultivation in photobioreactors, the costs of cultivation can be minimized which is essential for astaxanthin production in an industrial scale. Regarding the type of illumination, the highest carotenoid content was obtained by using a continuous PAR lighting (Saha et al., 2013). An interesting alternative to an immediate change in the radiation intensity to induce the transition from the vegetative phase to carotenoid production is gradually increasing the level of lighting. Gradual increase of light intensity can result in gradual transformation of cells to cysts and can also contribute to better accumulation of astaxanthin, because the cells are capable to cope with increasing higher levels of stress (Park et al., 2014).
Culture systems
Astaxanthin-producing H. pluvialis is capable of growing in photoautotrophic, heterotrophic, or mixotrophic growth conditions in indoors, open raceway ponds or closed photobioreactors in batch, fed batch, or continuous modes.
Photoautotrophic culture
Photoautotrophic culture of H. pluvialis is mainly carried out in open raceway ponds or closed photobioreactors. Typical photobioreactors used for its cultivation include tubular, bubble column and airlift photobioreactors. As the culture conditions for maximum growth and maximum astaxanthin content are mutually exclusive, a two-step cultivation strategy is commonly adopted for the commercial production. The first step, green vegetative growth phase (“green stage”) is to promote algal growth under favorable culture conditions (e.g., low light and nitrogen-replete) (Boussiba, 2000; Aflalo et al., 2007; Del Rio et al., 2007). When high cell density is reached, the culture enters into the second step, reddish inductive production phase (“red stage”), where algal cells are subjected to stress conditions such as high light intensity and nitrogen depletion, excess acetate addition, pH or salt stress, phosphate deficiency, or the addition of specific cell division inhibitors. These stress factors (either one or combination of more) induce the astaxanthin production in H. pluvialis (Fábregas et al., 2001; Torzillo et al., 2003; Orosa et al., 2005; He et al., 2007; Hu et al., 2008; Li et al., 2010; Choi et al., 2011). Therefore, carotenoid induction method has a direct correlation with both the astaxanthin content and total astaxanthin productivity. The optimal environmental and nutritional conditions for each stage are quite different (Del Rio et al., 2007). The reported biomass productivities in green stage and red stage ranged from 0.01 to 0.5 g L−1 d−1 and 0.01 to 4.8 g L−1 d−1, respectively. Astaxanthin productivity and astaxanthin content ranged from 0.44 to 21 mg L−1 d−1 and 0.8 to 4.8% of DW, respectively (Table 4). Astaxanthin can be also produced efficiently by H. pluvialis using a simpler “one-step strategy.” This strategy involves the administration of nitrate starvation and specific average irradiance in the culture medium, resulting in simultaneous algal cell growth and astaxanthin accumulation (Del Río et al., 2005; Del Rio et al., 2007; Del Río et al., 2008; García-Malea et al., 2009). At the laboratory scale and under continuous illumination, mean astaxanthin productivity of 20.8 mg L−1 d−1 has been reported for the one-step method (Del Río et al., 2008). The technical feasibility of this approach at a pilot scale was demonstrated in an outdoor tubular photobioreactor, which resulted in biomass and astaxanthin productivities of 0.7 g L−1 d−1 and 8 mg L−1 d−1, respectively (García-Malea et al., 2009). One-stage cultivation seems attractive, since it is less complicated than the two-stage process and the production of astaxanthin takes place in a continuous mode. It has however two serious drawbacks. First, the actual astaxanthin production is significantly lower compared to the two-stage approach. Second, this cultivation is unsuitable for outdoor setting, since it requires incessant illumination during night as well what makes the process too expensive (Aflalo et al., 2007). An “attached cultivation” approach was successfully applied in the induction of H. pluvialis for astaxanthin production. In this method green cells are cultured in the conventional water column and then deposited on the membrane to increase light stress surface area in the second phase of cultivation. Under the optimized conditions, biomass, and astaxanthin productivities in the attached cultivation system were 2.8 (3.7 g m−2 d −1) and 2.4-fold (65.8 mg m−2 d−1) higher than those of the suspended bioreactor, respectively (Wan et al., 2014b). Other studies that used similar approach have reported higher astaxanthin productivities of 124 mg m−2 d−1 (Yin et al., 2015) and 164.5 mg m−2 d−1 (Zhang et al., 2014). Attached cultivation approach is superior to suspended induction in several aspects such as, lower water consumption and smaller risk of contamination. This indicates that attached induction approach can provide a promising way to boost economic benefits and considerably reduce production cost of astaxanthin from H. pluvialis (Zhang et al., 2014; Wan et al., 2014b). Recently, Park et al. (2014) established a two-stage “perfusion culture” system for H. pluvialis combining it with stepwise increase of light irradiance. Approach is based on repeated replacement of the growth medium. Cells are grown in a photobioreactor and are periodically retained in the cell settling chamber whilst growth medium is being replaced in the photobioreactor. Cells are later recycled to the bioreactor and can efficiently utilize fresh growth medium which is free of inhibitory metabolic by-products. Perfusion culture can provide high biomass productivities of 0.18 g L−1 d−1. Under stepwise increased light irradiance (150–450 μE/m2/s), cellular density of 12.3 g L−1 of have been obtained which is 3.09 and 1.67 times higher than batch and fed-batch processes, respectively whilst the productivity of astaxanthin reached 602 mg L−1 (Park et al., 2014).
Table 4.
Summary of various methods of H. pluvialis biomass cultivation and corresponding astaxanthin productivities.
| PBRs type | Outdoor/Indoor | Mode | Culture medium* | Biomass productivity in Green stage (g L−1 d−1) | Biomass productivity in Red stage (g L−1 d−1) | Astaxanthin content (%, DW) | Astaxanthin productivity (mg L−1 d−1) | References |
|---|---|---|---|---|---|---|---|---|
| Airlift column (30 L) | Indoor | Batch | Modified Bold's Basal medium | 0.03 | 0.01 | 2.7 | 0.44b | Harker et al., 1996b |
| Tubular /open pond (25,000 L) | Outdoor | – | Modified Bold's Basal Medium | 0.036–0.052 | N/A | 2.8–3.0 | N/A | Olaizola, 2000 |
| Tubular (50 L) | Indoor | Semi continuous | BG-11 medium | N/A | 0.05 | 3.6 | 7.2c, d | Torzillo et al., 2003 |
| Bubbling column (1.8) | Indoor | Batch | Basal inorganic culture medium | N/A | 0.6 | 0.8 | 5.6a | Del Río et al., 2005 |
| Airlift Tubular (55 L) | – | Inorganic medium free of acetate | N/A | 0.41 | 1.1 | 4.4a | López et al., 2006 | |
| Bubbling column (0.5 L) | Indoor | Batch | BG-11 medium | 0.5 | 0.21 | 4 | 11.5b | Aflalo et al., 2007 |
| Tubular (200 L) | Outdoor | Batch | BG-11 medium | 0.37 | 0.21 | 3.8 | 10.1b, c | Aflalo et al., 2007 |
| Bubbling column (1.8 L) | Indoor | Batch | Basal inorganic culture medium | N/A | 1.9 | 1.1 | 21a | Del Río et al., 2008 |
| Bubbling column (1 L) | Indoor | Batch | Standard inorganic medium | 0.36 | 0.14 | 3.6 | 12b | Ranjbar et al., 2008 |
| Tubular (1.8 L), outdoor, | Outdoor | Continuous | Standard inorganic medium | N/A | 0.7 | 1 | 8a | García-Malea et al., 2009 |
| Open pond | Indoor | Batch | BG-11 medium | N/A | 0.15 | 2.79 | 4.3a | Zhang et al., 2009 |
| Flat type (1 L) | Indoor | Fed batch | NIES-C medium | 0.33 | 0.44 | 4.8 | 14b | Kang et al., 2010 |
| Airlift column | Indoor | Batch | Haematococcus medium (OHM) | N/A | 0.14 | N/A | 3.3a | Choi et al., 2011 |
| Bubbling column (6 L) | Indoor | Batch | NIES-C medium | N/A | 0.047 | N/A | 1.4a | Yoo et al., 2012 |
| Bubbling column (0.6 L) | Outdoor | Batch | BG-11 medium | N/A | 0.58 | 2.7 | 17.1b, d | Wang et al., 2013a |
| Bubbling column (0.6 L) | Outdoor | Batch | BG-11 medium | N/A | 0.30 | 3.8 | 16.0b, d | Wang et al., 2013b |
Obtained from one-step culture process.
Obtained from two-step culture process. Productivity value was calculated based on total time required by the “green stage” and “red stage” of cultivation.
Induction of astaxanthin was performed outdoors;
Obtained from a two-step process in which astaxanthin productivity was calculated based on time spent on the “red stage” only.
Detail medium composition can be found in relevant references.
Heterotrophic and mixotrophic culture
High light irradiance is often employed for enhancing astaxanthin formation in H. pluvialis cultures. However, light absorption and scattering caused by mutual shading of cells in large-scale cultures severely affects the productivity and quality of algal biomass and products. The high cost of illumination is another problem hindering the commercialization of Haematococcus products. To overcome this drawback, heterotrophic culture approach may be considered. Under heterotrophic growth conditions light is not needed as organic substrates serve as carbon and energy sources for growth and synthesis of secondary metabolites. Also, since lipid accumulation and astaxanthin biosynthesis are connected in space and time the effect of carbon source on lipid accumulation can have significant effect on overall productivity. It has been shown in Haematococcus and other microalgae lipid content and lipid profiles of microalgae are dependent on the cultivation conditions with various stress factors such as starvation or salt stress are efficient triggers of lipid accumulation, and can result in the alteration of fatty acid profiles due to cellular adjustment to particular stressor (Damiani et al., 2010; Lei et al., 2012; Saha et al., 2013; Chen et al., 2015). Various types of organic carbon sources have been used for heterotrophic cultivation and induction using acetate has been found effective for Haematococcus encystment and initiation of astaxanthin production (Kobayashi et al., 1991; Kakizono et al., 1992; Orosa et al., 2000; Hata et al., 2001; Kang et al., 2005). However, unlike many microalgae in which oversupply of easily accessible carbon in combination with nitrogen limitation yields diversion of the carbon flux toward lipid accumulation (Miao and Wu, 2006; Jia et al., 2014), H. pluvialis grows at a relatively low rate (0.22 d−1) and accumulates negligible amount of astaxanthin, too low to be considered for commercial scale production if single step cultivation is considered (Kobayashi et al., 1992, 1997a; Moya et al., 1997). Additionally, heterotrophic cultivation of Haematococcus increases the risk of bacterial or fungal contamination (Hata et al., 2001; Olguín et al., 2012). H. pluvialis can be also produced indoors mixotrophically employing an organic acid (e.g., acetate) or carbohydrates as an additional carbon and energy source (Kobayashi et al., 1993). Studies have shown that both growth and astaxanthin production can be enhanced under mixotrophic culture conditions. A final cell density of 0.9–2.65 g L−1 and a maximum astaxanthin content of 1–2% DW were obtained from mixotrophic cultures of H. pluvialis (Chen et al., 1997; Zhang et al., 1999; Wang et al., 2003). A sequential, heterophotric-photoautotrophic culture mode was also explored. Heterotrophic culture was used in the green stage to produce algal biomass, while astaxanthin production was induced in photoautotrophic culture conditions. The induction of astaxanthin accumulation was performed under nitrogen deprivation conditions and whilst using bicarbonate or CO2 as carbon sources. As a result, a very high cellular astaxanthin content of 7% (DW) was achieved, 3.4-fold higher than heterotrophic induction whilst astaxanthin productivity of 6.25 mg L−1 d−1 was obtained (Kang et al., 2005). Results indicate that photoautotrophic induction of astaxanthin production in H. pluvialis is more effective than heterotrophic one. Based upon the information obtained thus far, heterotrophic and mixotrophic culture modes are less cost-effective than the photoautotrophic one for Haematococcus mass culture.
Microbial contamination and possible control measures
Since interest in commercial microalgae cultivation is increasing, microbial contaminants that hamper production by resulting in reduced biomass yield and quality received great attention recently. Mass culture of H. pluvialis is reported to be contaminated by fungal parasites and zooplanktonic predators (e.g., amoebas, ciliates, and rotifers), as well as other microalgae and cyanobacteria (Han et al., 2013). A parasitic chytrid/blastoclad fungus Paraphysoderma sedebokerenses is found to be responsible for reduced astaxanthin productivity and frequent culture collapses in commercial Haematococcus cultivation facilities (Hoffman et al., 2008; Strittmatter et al., 2015). Detection of contaminants is prerequisite for preventing and controlling of microbial contamination in mass microalgal culture. Methods of detection usually include microscopy and staining, flow cytometry, molecular based detection and monitoring. In order to cope with microalgae culture contamination, the techniques that are generally used include abiotic stresses such as limitation, pH stress, temperature stress, light stress, toxic substances, and shear forces. There are some other techniques for parasite removal including salvage harvest, chemical agents (abscisic acid, copper sulfate), physical methods, biological methods (selective breeding and biological agents) (Carney and Lane, 2014). Recently, several patent applications relating to control of fungus P. sedebokerenses have been developed in the USA and China to protect production losses in commercial Haematococcus culture facilities across the world (McBride et al., 2013; Zhang et al., 2013; Carney and Sorensen, 2015).
Harvesting
Harvesting remains one of the most challenging issues and a limiting factor for commercial algal biomass production. Harvesting of H. pluvialis refers to the selection of appropriate techniques to recover the “red” biomass, after the accumulation of astaxanthin in the cells and also that can facilitate cost-efficient astaxanthin extraction in the extraction phase. For the large scale harvesting of H. pluvialis centrifugation is the most common method and combined with other processes. Usually haematocysts are separated from the water through passive settling and subsequently concentrated with centrifugation (Lorenz and Cysewski, 2000; Olaizola, 2000; Li et al., 2011; Han et al., 2013; Pérez-López et al., 2014). Through the combination of these processes total suspended solid of 13.5% in the algal cake is achieved (Li et al., 2011). Flotation and disk-stack centrifugation have been also reported as another alternative for H. pluvialis harvest. Both showed more than 95% biomass recovery efficiency (Panis, 2015).
Cell disruption
Different techniques have been developed in order to disrupt the algal cell and recover the intracellular metabolites. The most appropriate cell disruption methods to enhance recovery of astaxanthin from H. pluvialis at a commercial scale involve mechanical processes and more specifically expeller pressing and bead milling (Lorenz and Cysewski, 2000; Olaizola, 2003; Mercer and Armenta, 2011; Razon and Tan, 2011). During pressing (pulverization) microalgae cells are squeezed under high pressure in order to rupture the thick sporopollenin wall. Main advantage of expeller pressing is simple operation and minimization of contamination from external sources. Algal oil recovery efficiency of 75% can be achieved in a single step. Bead milling utilizes vessels filled with tiny glass, ceramic or steel beads that are agitated at high speeds. The dried biomass is fed in these vessels, where continuous exposure of biomass to the grinding media (beads) leads to cell-wall rupture, and subsequent release of intracellular compounds. This method is most effective when biomass concentration in the algal cake after harvesting is between 100 and 200 g/l (Greenwell et al., 2010). Both methods are reliable and widely applied for the H. pluvialis cells disruption at a commercial scale.
Dehydration
In commercial scale astaxanthin production, dehydration (drying) ensures the quality of the pigment and leads to the formulation of the final product (Mata et al., 2010; Li et al., 2011). After algal cell walls have been disrupted, biomass must be processed rapidly within few hours to avoid spoilage. Thus, dehydration is a process applied prior to recovery of the desired metabolite, in order to extend the shelf-life of the algal biomass (Mata et al., 2010). The most known dehydration techniques that have been employed on microalgae are solar drying, spray drying, and freeze drying (Molina Grima et al., 2003; Brennan and Owende, 2010; Milledge, 2013). Spray drying has been considered as the most appropriate method to dry high-value microalgal products including H. pluvialis astaxanthin (Leach et al., 1998; Brennan and Owende, 2010; Li et al., 2011; Han et al., 2013; Milledge, 2013; Panis, 2015). The recovery efficiency of dry biomass (in powder) using this method exceeds 95% and in some occasions may approach 100% (Leach et al., 1998). After spray drying, the moisture content in “red” biomass is lowered to about 5% (Pérez-López et al., 2014). The main drawbacks of spray drying include high operational costs and the risk of microalgae pigments deterioration (Molina Grima et al., 2003). Freeze drying (lyophilization or cryodesiccation), involves the freezing of algal cake, the technique causes less damage than spray drying, but it is even more expensive, especially on a commercial scale (Milledge, 2013).
Recovery of astaxanthin
Once the cell wall is disrupted and the biomass is fully dried, the recovery of the desired product is possible. Astaxanthin is a lipophilic compound and can be dissolved in solvents and oils. There is an abundance of astaxanthin extraction methods from H. pluvialis utilizing solvents, acids, edible oils, supercritical carbon dioxide (SC-CO2) as well as microwave-assisted and enzyme-assisted approaches. Among the recovery methods used solvent extraction and supercritical carbon dioxide (SC-CO2) extraction are considered as the most efficient, compatible, and widely used methods for astaxanthin extraction from H. pluvialis. The summary of various extraction methods of astaxanthin from H. pluvialis with recent updates is presented in Table 5 Supercritical carbon dioxide (SC- CO2) extraction has been widely used for industrial applications due to its many processing advantages. Due to low critical temperature of carbon dioxide, the SC- CO2 system can be operated at moderate temperatures, preventing the degradation of valuable substances (Machmudah et al., 2006). Several studies have reported experiments on supercritical CO2 extraction for the recovery of astaxanthin from H. pluvialis. Considering astaxanthin quality as the most important criterion, supercritical CO2 extraction is the most favorable option. Supercritical CO2 provides shorter extraction time and limits the use of toxic organic solvents. By contrast to most solvents, CO2 is relatively cheap, chemically inert, non-toxic, and stable (Guedes et al., 2011). Supercritical fluid extraction has also been tested with Haematococcus, aiming at improving the extraction efficiency. For instance, supercritical carbon dioxide (SC- CO2) coupled with ethanol or vegetable oil as a co-solvent can further increase the extraction efficiency of astaxanthin (80–90%) (Nobre et al., 2006; Krichnavaruk et al., 2008). There is an array of alternative approaches that can assist astaxanthin extraction from H. pluvialis such as solvents, acids, edible oils, enzymes, or pressurized liquids (Sarada et al., 2006; Kang and Sim, 2008; In, 2009; Jaime et al., 2010; Zou et al., 2013; Dong et al., 2014) Pressurized liquid extraction has several advantages over traditional solvent extraction. PLE requires shorter time, can be automated, uses less solvent, and retains the sample in an oxygen-free and light-free environment in contrast to traditional organic solvent extraction (Jaime et al., 2010). Recently, a simple method for the direct extraction of lipids from high moisture H. pluvialis microalgae was successfully achieved using liquefied dimethyl ether (Boonnoun et al., 2014).
Table 5.
Summary of astaxanthin extraction methods from H. pluvialis.
| Astaxanthin Extraction/Purification method | Astaxanthin yield/Extraction efficiency | References |
|---|---|---|
| SC-CO2 at 20 MPa, 55°C and13% (w/w) ethanol for 120 min of extraction time. | 83% recovery | Reyes et al., 2014 |
| CO2 expanded ethanol (50% %w/w ethanol), 7 MPa, 45°C, 120 min of extraction time. | 124.2% recovery | Reyes et al., 2014 |
| SC- CO2 at at 20 MPa, 60°C, 2 ml of ethanol for 1 h of extraction time | 2.45 mg/g DW | Fujii, 2012 |
| SC- CO2, co-solvent 0.154–1% (v/v) ethanol, 7–34 MPa, 30–80°C, 0–100 min | 74% recovery | Pan et al., 2012 |
| SC- CO2, co-solvent 1.25–8.75% (v/v) ethanol, 30–50 MPa, 35–75°C, 210 min | 87.4% recovery | Wang et al., 2012 |
| SC- CO2, co-solvent 0–12% (v/v) vegetable oils, 30–50 MPa 50–80°C 300 min | 51% recovery | Krichnavaruk et al., 2008 |
| SC- CO2, co-solvent 30–50 MPa 40–80°C 60–240 min | 84% recovery | Thana et al., 2008 |
| SC- CO2, co-solvent, 1.67–7.5% (v/v) ethanol 20–55 MPa 40–80°C, 240 min | 80% recovery | Machmudah et al., 2006 |
| SC- CO2, co-solvent 0, 10% (v/v) ethanol 20–30 MPa, 40–60°C | 90% recovery | Nobre et al., 2006 |
| SC- CO2, co-solvent 0, 9.4% (w/w) ethanol, 30 MPa at 60°C | 97% recovery | Valderrama et al., 2003 |
| Cell germination (12 h), Ionic liquid (1-ethyl-3- methylimidazolium ethylsulfate) (24 h), | 32.5 pg/cell | Praveenkumar et al., 2015 |
| Direct extraction using liquefid dimethyl ether (DME) at 0.59 MPa and 25°C, without drying, cell disruption, or heating, | 1 (mg/g cell) | Boonnoun et al., 2014 |
| HCl:acetone (5:5), 70°C, 20 min | 19.8 mg/g cell | Dong et al., 2014 |
| Ultrasound in solvent (EtOH and EA), 16 min, 41°C, 40 kHz, 200 W, EtOH: ethyl acetate (20:1) | 28 (mg/g) | Zou et al., 2013 |
| Grinding three repetitions, pressurized hexane (10.3 Mpa) | 35 (mg/g cell) | Jaime et al., 2010 |
| Treating with enzymes (Viscozyme, Alcalase) at 50°C, 2 h | 2649 ± 359 μg/g cell | In, 2009 |
| Dodecane mixing 48 h, saponification with methanolic NaOH (0.02 M), sedimentation in darkness at 4°C, 12 h. | 85% efficiency | Kang and Sim, 2008 |
| Acid digestion, 2 N HCl, 70°C. Acetone extraction for 1 h | 87% efficiency | Sarada et al., 2006 |
| NaOH 30 min, Acetone (16 h) | 7 (mg/g cell) | Mendes-Pinto et al., 2001 |
| 40% (v/v) acetone for 2 min at 80°C, followed by lyophilization or treatment with specific lytic enzymes | 70% recovery | Kobayashi et al., 1997c |
SC, supercritical; EA, ethyl acetate.
Biorefinery approach for H. pluvialis
Microalgae have been often proposed as third generation feedstock for biofuel production that does not compete for freshwater or land resources (Daroch et al., 2013a). However, despite significant advances in recent years it becomes apparent that cultivation of microalgae for the sole purpose of biofuel production is unlikely to be possible unless a major low-energy breakthrough technologies in algae cultivation, dewatering, and harvesting are developed (Li et al., 2015). In the meantime, microalgae are extremely important producers of many high-value nutraceutical compounds such as polyunsaturated fatty acids or astaxanthin that can justify high cost of microalgae cultivation and processing technologies. Integration of simultaneous production of numerous compounds within one system maximizing the benefits and limiting the costs is called biorefining (Li et al., 2015). Taking into consideration these findings H. pluvialis emerges as a very useful organism for the development of a dedicated microalgal biorefinery. It fits numerous requirements of for the development of first microalgal biorefineries especially the “high value product first” principle (Li et al., 2015). First, H. pluvialis is the best-known producer of astaxanthin-high value product worth in excess of several thousand US $ per kilogram. This product itself can easily justify expensive cultivation systems required for this organism. Second, H. pluvialis grown under nutrient starvation conditions induces both carotenogenesis (astaxanthin formation) and deposition of storage materials (triglycerides). It has been shown that these two responses are closely related and coincide in both space and time and triglycerides are essential for deposition of astaxanthin inside lipid bodies to confer its protective function (Solovchenko, 2015). In traditional approaches of microalgae to biofuels starvation-induced lipid accumulation is considered as significant challenge for commercialization of these systems as the overall lipid productivity of culture can drop significantly due to impaired growth rates under starvation conditions (Daroch et al., 2013b). In case of high value product like astaxanthin this drop becomes much less of the burden as the high value of the main product will compensate for this delay in final product formation. Due to the coexistence of astaxanthin and triglycerides in space and time it is possible to simultaneously obtain high value product (astaxanthin) and a biofuel feedstock (triglycerides) from a single algal feedstock. Since fatty acid content in the astaxanthin-containing ‘red’ cells can be as high as 30–60% of algae dry weight (Solovchenko, 2015) making H. pluvialis a very good candidate for biorefining strain. The fatty acid profiles of the alga have been evaluated by several studies and are summarized in Table 2, indicating that fatty acid profiles of the algae are suitable for biodiesel production (Damiani et al., 2010). Third, H. pluvialis have been found to be a mixotrophic alga what is highly advantageous for development of microalgae biorefineries. H. pluvialis is capable of utilizing carbon dioxide, carbonates, and carbohydrates as carbon sources, this opens a possibilities of lowering cultivation costs and/or speeding up the cultivation of the strain through using various waste streams like flue gasses or waste streams containing carbon and nutrient compounds (Wu et al., 2013). Auto-, hetero-, and mixo-trophic cultivation modes require energy and nutrients, both of which can be to an extent recycled from anaerobic digestion process. Carbon sources vary depending on cultivation mode. Photoautotrophic cultivation requires CO2 that can be recycled from energy production at anaerobic digestion stage. Heterotrophic cultivation requires reduced carbon source—such as carbohydrates or acetate which need to be supplied from alternative source. These compounds can also originate from waste streams. For example, food industry is rich in carbohydrate-rich waste streams that can be used in heterotrophic cultivation of H. pluvialis (Wang, 2014). Mixotrophic cultivation can take advantage of both sources of carbon. After simultaneous extraction of both high value product-astaxanthin and biofuel product-triglycerides algal cake composed of residual biomass can be utilized as a supplementary feedstock for biogas production using anaerobic digestion that would further assist in extraction of residual energy from this integrated bioprocess. These three features of H. pluvialis make it a suitable strain for the development of algal biorefineries producing high value product (astaxanthin) and biofuel molecule (biodiesel and/or biogas). The proposed biorefinery scheme is presented on Figure 8 and employs a classical two stage cultivation of H. pluvialis in green and red stage.
Figure 8.
Scheme of two stage cultivation H. pluvialis biorefinery producing high value compound astaxanthin and either edible oil or biofuel compound-biodiesel. Green stage of cultivation can be performed using either photoautotrophic cultivation (deep green section) or hetero/mixotrophic cultivation (pale green section) systems. Red stage cultivation (red section) takes place after green stage of H. pluvialis cultivation and is aimed to maximize astaxanthin content Recycling of waste is performed through anaerobic digestion process. Following annotations are used: solid arrows—subsequent steps; dashed arrows—optional steps; double lines—final products; double arrows—inputs; dotted lines—opportunities for recycling resources.
Current global market and market players of H. pluvialis astaxanthin
Synthetic astaxanthin dominates current commercial market, of the total value exceeding $200 million, corresponding to 130 metric tons of product per year (Li et al., 2011). According to recent reports, microalgae-derived astaxanthin corresponds to less than 1% of the commercialized quantity due to much lower price of synthetic astaxanthin and technological problems associated with large-scale algae cultivation (Koller et al., 2014; Pérez-López et al., 2014). In recent years, there has been a growing trend toward using natural ingredients in food, nutraceutical, and cosmetic markets, resulting from increasing concerns for consumer safety and regulatory issues over the introduction of synthetic chemicals into the human food chain. The demand for natural astaxanthin derived from H. pluvialis in the global market has been “sky-rocketing” in recent years owing to increasing consumer awareness of its health benefits. Global market for both synthetic and natural source astaxanthin in aquaculture feed, nutraceuticals, cosmetics, and food and beverages is estimated at 280 metric tons valued at $447 million in 2014. It is further projected to reach 670 metric tons valued at $1.1 billion by 2020 (Industry Experts, 2015; Panis, 2015). Synthetic astaxanthin, astaxanthin rich Phaffia yeast, and Paracoccus bacteria are predominantly used in the aquaculture sector, while the astaxanthin derived from H. pluvialis is the main source for human applications such as dietary supplements, cosmetics, and food and beverages. Nowadays, the estimated market value of astaxanthin depending on products' purity varies from $2500–7000/kg to about $15,000/kg pigment from H. pluvialis in some cases (Borowitzka, 2013; Koller et al., 2014; Pérez-López et al., 2014; Industry Experts, 2015), while the production cost is estimated at about $1000 per kg of astaxanthin from H. pluvialis (Li et al., 2011). Natural astaxanthin is three to four times more valuable than the synthetic alternative in nutraceutical and pharmaceutical markets (Han et al., 2013). Since there is growing market demand for natural astaxanthin for specific commercial applications (e.g., the nutraceuticals market) to replace the synthetic astaxanthin, mass cultivation of H. pluvialis in industrial scale has great potential and attractive business opportunity. However, current market demand for natural astaxanthin is not met. It is expected that in the foreseeable future after the optimization of the production technology, the production costs of the natural astaxanthin from H. pluvialis should be more competitive to these of the synthetic alternative (Pérez-López et al., 2014). Since the mid 1990's, several leading companies are successfully producing H. pluvialis at commercial scale and marketing natural astaxanthin from H. pluvialis worldwide. The list of the leading companies with their products is presented in Table 6. The size of the nutraceutical astaxanthin market is growing day by day and this market is very attractive to Haematococcus astaxanthin producers since the price of these products is significantly higher than those of feed applications. Haematococcus producers need to invest their attention for increasing astaxanthin production capacity to meet the global demand. It is worthy to mention that several manufacturers already have doubled the cultivation capacities in recent years. Apart from an increase in existing players' capacities, new producers, such as BGG in China, have entered the market with significant production capacities.
Table 6.
Leading commercial companies and their H. pluvialis-derived astaxanthin and related products in the world market.
| Company Name | Country | Brand Name | Product particulars |
|---|---|---|---|
| Cyanotech Corporation (www.cyanotech.com) | USA | BioAstin® | Astaxanthin extract packaged in soft gel, beadlets; dietary supplement |
| Naturose™ | Algae meal; pigmentation source for ornamental fish and animals | ||
| Mera Pharmaceutigals Inc. (www.merapharma.com) | USA | AstaFactor® | Astaxanthin packaged as soft gel; dietary supplement |
| Stazen Inc. (www.stazen.com) | USA | Stazen® | Dietary supplement containing algae crushed and dried algae meal |
| Valensa International (www.valensa.com) | USA | Zanthin® | Astaxanthin extract, soft gel, beadlets |
| AIgatechnologies Ltd. (www.algatech.com) | Israel | AstaPure™ | Dry algal biomass, astaxanthin beadlets, and oleoresin |
| Fuji Chemical Industry Co. Ltd. (AstaReal Co Tld) (www.fujichemical.co.jp; www.astareal.com) | Japan, Sweden, USA | AstaREAL® | Astaxanthin oleoresin products, water dispersible, and soluble powders |
| BioReaI (Sweden) AB (subsidiary of Fuji Chemical) (www.bioreal.se) | Sweden | AstaXine® AstaCaroxe® | Dietary supplement containing algae crushed, and dried algae meal |
| AstaEquus® | Astaxanthin extract feed supplement for horses | ||
| Novaasta® | Astaxanthin extract feed supplement for animals | ||
| Britannia Health Products Ltd. (www.britanniahealth.co.uk) | UK | Britaxan® | Astaxanthin complex with other carotenoids packaged as capsule- dietary supplement |
| Supreme Biotechnologies NZ Ltd. (www.supremebiotech.com) | New Zealand | AstaSupreme® | Algal biomass, Oleoresins, beadlets, and soft gels of Astaxanthin |
| Atacama Bio Natural (www.atacamabionatural.com) | Chile | Supreme Asta Oil™ Supreme Asta powder™ | Oleoresin for food, nutraceutic, and cosmetic products, and powder for animal feed supplement |
| Jingzhou Natural Astaxanthin Inc. (www.asta.cn) | China | NaturAsta™ | Dry algal biomass and astaxanthin soft gel |
| Kunming Biogenic Co. Ltd. (www.bgenic.com) | China | AstaBio® | Algal biomass, oleoresins, beadlets, and soft gels of Astaxanthin |
| Beijing Ginko Group (BGG) Biological Technology Co. Ltd. (www.gingkogroup.com.cn) | China | AstaZine® | Astaxanthin oil, powder, and beadlets |
| Wefirst Biotechnology Co. Ltd. (www.astawefirst.com) | China | AstaFirst™ | Dried algal powder, Astaxanthin oleoresin, and soft gel |
| Algaetech International SDN BHD (www.algaetech.com.my) | Malaysia | Astaxanthin Premia-Ex | Algae biomass, oleoresin, and soft gel |
| Parry Nutraceuticals Ltd. (EID Parry) (www.eidparry.com/) | India | Zanthin® | Astaxanthin oleoresins, beadlets, and soft gel |
Major challenges for the improvement of H. pluvialis biomass and astaxanthin production
There are many challenges and problems for the development of large scale production of biomass and astaxanthin from H. pluvialis. Due to these obstacles the productivity can be hampered and in some cases a failure of the production system can make the production process economically unsustainable. Following issues are considered as most important challenges for the development of H. pluvialis astaxanthin production process:
Lack of effective solution to prevent or treat microbial contaminations of mass cultures in a commercial scale.
Slow cell growth rate, sensitivity of the cells to hydrodynamic stress, and changes in cell morphology under various environmental conditions.
Inadequate and cost ineffective cultivation, drying, and astaxanthin extraction technologies at the commercial scale.
Unavailability of genetically improved/engineered strains of H. pluvialis and genetic transformation tools for engineering astaxanthin biosynthesis pathways in this organism for improved astaxanthin production.
Lack of sufficient number of skilled workers in production farms and insufficient collaboration between universities and commercial enterprises.
Lack of adequate scientific research on the economic performance and viability of commercial scale astaxanthin production process.
Conclusion and perspectives
This review provides an insight about the latest scientific and technological advancements in various aspects of astaxanthin-producing microalga H. pluvialis such as cell biology, reproduction, biosynthesis pathway, stress mechanism, biomass production, and downstream processing. It also contemplates a broader image including potential benefits, global market opportunities and integration of astaxanthin production into biorefining. In recent years there is an increased interest for natural astaxanthin from green microalga H. pluvialis. Wide ranges of scientific improvements have been achieved during the last decade in terms of productivity and bioprocessing in order to obtain a refined astaxanthin product. Yet its commercial production, especially for low-end markets is too expensive for mass adoption of natural astaxanthin over its synthetic counterpart. H. pluvialis has been shown to be cultured in photoautotrophic, heterotrophic or mixotrophic growth conditions in various culture systems. Research have been conducted on the optimization of the various culture parameters, such as growth medium composition, light, pH, temperature etc. to achieve high biomass and astaxanthin production. Most of these parameters have been optimized and found different for biomass accumulation and astaxanthin production. Little can be done to address this limitatiation as it is funadamentally connected with the life cycle of this microalgae. We believe there exist three key areas where further improvements are required and interesting novel approaches have been recently developed: cultivation efficiency and cost; good cultivation practice and predator control; and astaxanthin isolation and purification.
First, due to complex life-cycle of H. pluvialis it is important to maximase cell densities of alga at “green stage” of cultivation to maximize astaxanthin yield from the “red stage.” We think that a number of recent developments can make significant impact in maximizing cell densities. Especially attached cultivation approach and a two-stage “perfusion culture” system can be considered most promising due to the capability of producing several fold higher biomass and astaxanthin productivity and some other benefits such as lower water consumption and smaller risk of contamination. These improvements may boost economic benefits and reduce production cost of astaxanthin from H. pluvialis (Park et al., 2014; Wan et al., 2014b; Zhang et al., 2014). Alternatively, utilization of supplementary carbon source and adoption a two-stage sequential heterotrophic-photoautotrophic approach could improve biomass and astaxanthin production. Especially utilization of waste carbon and nutrient sources in biorefinery setup could help to decrease cultivation costs. Unfortunately, these researches are still in laboratory stage and need to be tested in large-scale commercial production for further validation.
Second, control of contaminants, parasites, and predators remains to be primary concern for Haematococcus growers and major issue in culture stability and astaxanthin productivity. Since there is very little that can be done once contamination takes place it is important to limit the possibility of such disruption and identify it as soon as possible, and avoid spreading to other parts of culture. Traditional detection methods such as microscopy and staining can be used to visualize algal parasites, however this technique may be too labor intensive to perform on a routine basis for most commercial operations. For routine detection, more automated systems such as flow cytometry would be ideal. Alternatively, molecular-based techniques that are considered as the most informative and sensitive for the detection and identification of parasites. Following techniques are worth further exploring DNA sequencing (Sanger, shotgun, or next generation) and then monitoring for these specifically using qPCR or phylochip technology. Decreasing costs of next generation DNA sequencing can make DNA sequencing for culture diagnostic purposes more accessible in the near future.
Third, combination of low cell densities and robust trilayer cell walls of astaxanthin-containing aplanospores make isolation of astaxanthin difficlut and expensive. Currentlly harvesting by centrifugation, cell wall disruption by expeller pressing and bead milling are the most common described methods for commercial scale astaxanthin production from H. pluvialis. After cell walls disruption, biomass is usually processed by spray drying or freeze drying. A number of astaxanthin extraction methods such as (solvents, acids, edible oils, supercritical carbon dioxide, microwave-assisted, and enzyme-assisted approaches have been reported for H. pluvialis and supercritical carbon dioxide (SC- CO2) extraction has been widely used for industrial applications. Two recently developed methods allow efficient extraction of astaxanthin-containing lipids from wet biomass at yields comparable to conventional drying-solvent extraction method. Efficient extraction of astaxanthin from wet H. pluvialis biomass was achieved with liquefied dimethyl ether (Boonnoun et al., 2014) and also cell germination process in conjunction with ionic liquids treatment (Praveenkumar et al., 2015).
Despite significant advances in research and development of H. pluvialis astaxanthin production is still in laboratory stage and often faces difficulties to become implemented in large-scale commercial production. There is a number of other areas of improvement that will contribute to the expansion of Haematococcus production capacity, lowering the production cost, and increasing market penetration at low end applications. These include: next-generation culture systems along with advanced management practices; better understanding of astaxanthin biosynthesis, metabolic pathways and their regulation, genetic engineering, and omics-scale understanding of astaxanthin accumulation; development of genetic manipulation toolbox; exploration of integration of H. pluvialis cultivation with other processes. Yet, we firmly believe that three key areas of focus should be: cultivation efficiency and cost; good cultivation practice and predator control; and astaxanthin isolation and purification. Further developments in these fields can have a profound effect on the commercial deployment of H. pluvialis astaxanthin products and can act as a catalyst for the development of an entire microalgae industry in the near future.
Author contributions
MS, collected data, participated in preparation of draft manuscript, participated in assembly and editing of the final manuscript; YML, collected data, participated in preparation of draft manuscript; JJC, participated in assembly and editing of the final manuscript; MD, collected data, participated in preparation of draft manuscript, participated in assembly and editing of the final manuscript.
Funding
Authors would like to acknowledge the support of National Natural Science Foundation of China for Young International Scientists Grant no. 31450110424 and 31550110497, Shenzhen Municipal Government for Special Innovation Fund for Shenzhen Overseas High-level Personnel KQCX20140521150255300 and Shenzhen Knowledge and Innovation Basic Research Grant JCYJ20150626110855791, State Ocean Administration Grant 201305022, and National Thousand People Plan Grant of Jay Jiayang Cheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Aflalo C., Meshulam Y., Zarka A., Boussiba S. (2007). On the relative efficiency of two- vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnol. Bioeng. 98, 300–305. 10.1002/bit.21391 [DOI] [PubMed] [Google Scholar]
- Ako H., Tamaru C. S. (1999). Are feeds for food fish practical for aquarium fish? Int. Aqua Feeds 2, 30–36. [Google Scholar]
- Al-Bulishi M. S. M., Changhu X., Tang Q. J. (2015). Health aspects of astaxanthin: a review. Canad. J. Clin. Nutr. 3, 71–78. 10.14206/canad.j.clin.nutr.2015.02.08 [DOI] [Google Scholar]
- Andersen L. P., Holck S., Kupcinskas L., Kiudelis G., Jonaitis L., Janciauskas D., et al. (2007). Gastric inflmmatory markers and interleukins in patients with functional dyspepsia treated with astaxanthin. FEMS Immunol. Med. Microbiol. 50, 244–248. 10.1111/j.1574-695X.2007.00257.x [DOI] [PubMed] [Google Scholar]
- Andrisani A., Donà G., Tibaldi E., Brunati A. M., Sabbadin C., Armanini D., et al. (2015). Astaxanthin improves human sperm capacitation by inducing lyn displacement and activation. Mar. Drugs 13, 5533–5551. 10.3390/md13095533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S., Mori T., Miki W., Yamaguchi K., Konosu S., Satake M., et al. (1987). Pigmentation of juvenile coho salmon with carotenoid oil extracted from Antarctic krill. Aquaculture 66, 255–264. 10.1016/0044-8486(87)90111-6 [DOI] [Google Scholar]
- Augusti P. R., Quatrin A., Somacal S., Conterato G. M., Sobieskim R., Ruviaro A. R., et al. (2012). Astaxanthin prevents changes in the activities of thioredoxin reductase and paraoxonase in hypercholesterolemic rabbits. J. Clin. Biochem. Nutr. 51, 42–49. 10.3164/jcbn.11-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff H. W., Bold H. C. (1963). Phycological studies IV, in Some Soil Algae from Enchanted Rock and Related Algal Species. (Austin, TX: University of Texas Publications; ), 95. [Google Scholar]
- Bloomer R. J., Fry A., Schilling B., Chiu L., Hori N., Weiss L. (2005). Astaxanthin supplementation does not attenuate muscle injury following eccentric exercise in resistance-trained men. Int. J. Sport Nutr. Exerc. Metab. 15, 401–412. [DOI] [PubMed] [Google Scholar]
- Bold H. C., Wynne M. J. (1985). Introduction to the Algae. Texas: Prentice-Hall Inc. [Google Scholar]
- Boonnoun P., Kurita Y., Kamo Y., Machmudah S., Okita Y., Ohashi E., et al. (2014). Wet extraction of lipids and astaxanthin from Haematococcus pluvialis by liquefied dimethyl ether. J. Nutr. Food Sci. 4:305 10.4172/2155-9600.1000305 [DOI] [Google Scholar]
- Borowitzka M. A. (2013). High-value products from microalgae—their development and commercialisation. J. Appl. Phycol. 25, 743–756. 10.1007/s10811-013-9983-9 [DOI] [Google Scholar]
- Boussiba S. (2000). Carotenogenesis in the green alga Haematococcus pluvialis: cellular physiology and stress response. Physiol. Plantarum 108, 111–117. 10.1034/j.1399-3054.2000.108002111.x [DOI] [Google Scholar]
- Boussiba S., Vonshak A. (1991). Astaxanthin accumulation in the green alga Haematococcus pluvialis. Plant Cell Physiol. 32, 1077–1082. [Google Scholar]
- Boussiba S., Bing W., Yuan J. -P., Zarka A., Chen F. (1999). Changes in pigments profie in the green alga Haeamtococcus pluvialis exposed to environmental stresses. Biotechnol. Lett. 21, 601–604. 10.1023/A:1005507514694 [DOI] [Google Scholar]
- Boussiba S., Fan L., Vonshak A. (1992). Enhancement and determination of astaxanthin accumulation in green alga Haematococcus pluvialis in Carotenoids Part A: Chemistry, Separation, Quantitation, and Antioxidation, Enzymology (Academic Press; ), 386–391. [Google Scholar]
- Brennan L., Owende P. (2010). Biofuels from microalgae-A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sust. Energ. Rev. 14, 557–577. 10.1016/j.rser.2009.10.009 [DOI] [Google Scholar]
- Brinda B. R., Sarada R., Kamath B. S., Ravishankar G. A. (2004). Accumulation of astaxanthin in flgellated cells of Haematococcus pluvialis - cultural and regulatory aspects. Curr. Sci. 87, 1290–1294. [Google Scholar]
- Britton G. (1993). Biosynthesis of carotenoids, in Carotenoids in Photosynthesis SE - 4, eds Young A., Britton G. (London: Springer; ), 96–126. 10.1007/978-94-011-2124-8_4 [DOI] [Google Scholar]
- Burchardt L., Balcerkiewicz S., Kokocinski M., Samardakiewicz S., Adamski Z. (2006). Occurrence of Haematococcus pluvialis Flotow emend. Wille in a small artificial pool on the university campus of the collegium biologicum in poznan (Poland). Biodiv. Res. Conserv. 1, 163–166. [Google Scholar]
- Capelli G. C., Cysewski G. (2013). The Worlds' Best Kept Health Secret Natural Astaxanthin. Kailua-Kona, HI: Cyanotech Corporation. [Google Scholar]
- Carney L. T., Lane T. W. (2014). Parasites in algae mass culture. Front. Microbiol. 5:278. 10.3389/fmicb.2014.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney L. T., Sorensen K. (2015). Methods for Treating a Culture of Haematococcus pluvialis for Contamination using Hydrogen Peroxide. US Patent 9113607 B1. [Google Scholar]
- Cerón M. C., García-Malea M. C., Rivas J., Acien F. G., Fernandez J. M., Del Río E., et al. (2007). Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content. Appl. Microbiol. Biotechnol. 74, 1112–1119. 10.1007/s00253-006-0743-5 [DOI] [PubMed] [Google Scholar]
- Chekanov K., Lobakova E., Selyakh I., Semenova L., Sidorov R., Solovchenko A. (2014). Accumulation of astaxanthin by a new Haematococcus pluvialis strain BM1 from the White Sea coastal rocks (Russia). Mar. Drugs. 12, 4504–4520. 10.3390/md12084504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Chen H., Gong X. (1997). Mixotrophic and heterotrophic growth of Haematococcus lacustris and rheological behaviour of the cell suspensions. Bioresour. Technol. 62, 19–24. 10.1016/S0960-8524(97)00115-6 [DOI] [Google Scholar]
- Chen G., Wang B., Han D., Sommerfeld M., Lu Y., Chen F., et al. (2015). Molecular mechanisms of the coordination between astaxanthin and fatty acid biosynthesis in Haematococcus pluvialis (Chlorophyceae). Plant J. 81, 95–107. 10.1111/tpj.12713 [DOI] [PubMed] [Google Scholar]
- Chen Y., Li D., Lu W., Xing J., Hui B., Han Y. (2003). Screening and characterization of astaxanthin-hyperproducing mutants of Haematococcus pluvialis. Biotechnol. Lett. 25, 527–529. 10.1023/A:1022877703008 [DOI] [PubMed] [Google Scholar]
- Chew B. P., Park J. S., Hayek M. G., Massimino S., Reinhart G. A. (2004). Dietary astaxanthin stimulates cell-mediated and humoral immune response in cats. FASEB J. 18, 533. [DOI] [PubMed] [Google Scholar]
- Chew B. P., Park J. S. (2004). Carotenoid action on the immune reponse. J. Nutr. 134, 257–261. [DOI] [PubMed] [Google Scholar]
- Choi Y. E., Yun Y. S., Park J. M., Yang J. W. (2011). Determination of the time transferring cells for astaxanthin production considering two-stage process of Haematococcus pluvialis cultivation. Bioresour. Technol. 102, 11249–11253. 10.1016/j.biortech.2011.09.092 [DOI] [PubMed] [Google Scholar]
- Choubert G., Heinrich O. (1993). Carotenoid pigments of the green alga Haematococcus pluvialis: assay on rainbow trout, Oncorhynchus mykiss, pigmentation in comparison with synthetic astaxanthin and canthaxanthin. Aquaculture 112, 217–226. 10.1016/0044-8486(93)90447-7 [DOI] [Google Scholar]
- Chumpolkulwong N., Kakizono T., Handa T., Nishio N. (1997). Isolation and characterization of compactin resistant mutants of an astaxanthin synthesizing green alga Haematococcus pluvialis. Biotechnol. Lett. 19, 299–302. 10.1023/A:1018330329357 [DOI] [Google Scholar]
- Comhaire F. H., El Garem Y., Mahmoud A., Eertmans F., Schoonjans F. (2005). Combined conventional/ antioxidant “Astaxanthin” treatment for male infertility: a double blind randomized trial. Asian J. Androl. 7, 257–262. 10.1111/j.1745-7262.2005.00047.x [DOI] [PubMed] [Google Scholar]
- Cunningham F. X., Gantt E. (1998). Genes and enzymes of carotenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 557–583. 10.1146/annurev.arplant.49.1.557 [DOI] [PubMed] [Google Scholar]
- Cyanotech (2015). BioAstin - Natural Astaxanthin. Available online at: http://www.cyanotech.com/bioastin.html (Retrieved 21 October, 2015).
- Czygan F. C. (1970). Blood-rain and blood-snow: nitrogendefiient cells of Haematococcus pluvialis and Chlamydomonas nivalis. Arch. Mikrobiol. 74, 69–76. 10.1007/BF00408689 [DOI] [PubMed] [Google Scholar]
- Damiani M. C., Popovich C. A., Constenla D., Leonardi P. I. (2010). Lipid analysis in Haematococcus pluvialis to assess its potential use as a biodiesel feedstock. Bioresour. Technol. 101, 3801–3807. 10.1016/j.biortech.2009.12.136 [DOI] [PubMed] [Google Scholar]
- Daroch M., Geng S., Wang G. (2013a). Recent advances in liquid biofuel production from algal feedstocks. Appl. Energy 102, 1371–1381. 10.1016/j.apenergy.2012.07.031 [DOI] [Google Scholar]
- Daroch M., Shao C., Liu Y., Geng S., Cheng J. J. (2013b). Induction of lipids and resultant FAME profiles of microalgae from coastal waters of pearl river delta. Bioresour. Technol. 146, 192–199. 10.1016/j.biortech.2013.07.048 [DOI] [PubMed] [Google Scholar]
- Del Río E., Acién F. G., García-Malea M. C., Rivas J., Del Rio E., Acién F. G., et al. (2005). Efficient one-step production of astaxanthin by the microalga Haematococcus pluvialis in continuous culture. Biotechnol. Bioeng. 91, 808–815. 10.1002/bit.20547 [DOI] [PubMed] [Google Scholar]
- Del Rio E., Acien F. G., Garcia-Malea M. C., Rivas J., Molina-Grima E., Guerrero M. G. (2007). Efficiency assessment of the one-step production of astaxanthin by the microalga Haematococcus pluvialis. Biotechnol. Bioeng. 100, 397–402. 10.1002/bit.21770 [DOI] [PubMed] [Google Scholar]
- Del Río E., Acién F. G., García-Malea M. C., Rivas J., Molina-Grima E., Guerrero M. G. (2008). Efficiency assessment of the one-step production of astaxanthin by the microalga Haematococcus pluvialis. Biotechnol. Bioeng. 100, 397–402. 10.1002/bit.21770 [DOI] [PubMed] [Google Scholar]
- Domínguez-Bocanegra A. R., Guerrero Legarreta I., Martinez Jeronimo F., Tomasini Campocosio A. (2004). Influence of environmental and nutritional factors in the production of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 92, 209–214. 10.1016/j.biortech.2003.04.001 [DOI] [PubMed] [Google Scholar]
- Dong S., Huang Y., Zhang R., Wang S., Liu Y. (2014). Four different methods comparison for extraction of astaxanthin from green alga Haematococcus pluvialis. Sci. World J. 7, 694–705. 10.1155/2014/694305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragos N., Bercea V., Bica A., Druga B., Nicoara A., Coman C. (2010). Astaxanthin production from a new strain of Haematococcus pluvialis grown in batch culture. Ann. Roman. Soc. Cell Biol. 15, 353–361. [Google Scholar]
- Eisenreich W., Rohdich F., Bacher A. (2001). Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci. 6, 78–84. 10.1016/S1360-1385(00)01812-4 [DOI] [PubMed] [Google Scholar]
- Elgarem Y., Lignell A., Comhaire F. H. (2002). Supplementation with Astaxanthin (Astacarox) improves semen quality in infertile men, in Proceedings of the 13th International Carotenoid Symposium (Honolulu, HI: ), 180–197. [Google Scholar]
- Elliot A. M. (1934). Morphology and life history of Haematococcus pluvialis. Arch. Protistenk. 82, 250–272. [Google Scholar]
- Elwinger K., Lignell A., Wilhelmson M. (1997). Astaxanthin rich algal meal (Haematococcus pluvialis) as carotenoid source in feed for laying hens in Proceedings of the VII European Symposium on the Quality of Eggs and Egg Products (Poznan: ), 52–59. [Google Scholar]
- Eom H., Lee C. G., Jin E. (2006). Gene expression profile analysis in astaxanthin-induced Haematococcus pluvialis using a cDNA microarray. Planta 223, 1231–1242. 10.1007/s00425-005-0171-2 [DOI] [PubMed] [Google Scholar]
- Fábregas J., Domínguez A., Regueiro M., Maseda A., Otero A. (2000). Optimization of culture medium for the continuous cultivation of the microalga Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 53, 530–535. 10.1007/s002530051652 [DOI] [PubMed] [Google Scholar]
- Fábregas J., Otero A., Maseda A., Domínguez A. (2001). Two-stage cultures for the production of astaxanthin from Haematococcus pluvialis. J. Biotechnol. 89, 65–71. 10.1016/S0168-1656(01)00289-9 [DOI] [PubMed] [Google Scholar]
- Fan L., Vonshak A., Boussiba S. (1994). Effect of temperature and irradiance on growth of Haematococcus pluvialis (Chlorophyceae). J. Phycol. 30, 829–833. 10.1111/j.0022-3646.1994.00829.x [DOI] [Google Scholar]
- Fassett R. G., Combes J. S. (2011). Astaxanthin: a potential therapeutic agent in cardiovascular disease. Mar. Drugs 9, 447–465. 10.3390/md9030447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante R. J., Browne S. E., Shinobu L. A., Bowling A. C., Baik M. J., MacGarvey U., et al. (1997). Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J. Neurochem. 69, 2064–2074. 10.1046/j.1471-4159.1997.69052064.x [DOI] [PubMed] [Google Scholar]
- Forján E., Navarro F., Cuaresma M., Vaquero I., Ruíz-Domínguez M. C., Gojkovic Z., et al. (2015). Microalgae: fast-growth sustainable green factories. Crit. Rev. Environ. Sci. Technol. 45, 1705–1755. 10.1080/10643389.2014.966426 [DOI] [Google Scholar]
- Fujii K. (2012). Process integration of supercritical carbon dioxide extraction and acid treatment for astaxanthin extraction from a vegetative microalga. Food Bioprod. Process. 90, 762–766. 10.1016/j.fbp.2012.01.006 [DOI] [Google Scholar]
- Gacheva G., Dimitrova P., Pilarski P. (2015). New strain Haematococcus cf. pluvialis Rozhen-12 - growth, biochemical characteristics and future perspectives. Genet. Plant Physiol. 5, 29–38. [Google Scholar]
- Gao Z., Meng C., Gao H., Li Y., Zhang X., Xu D., et al. (2013b). Carotenoid genes transcriptional regulation for astaxanthin accumulation in fresh water unicellular alga Haematococcus pluvialis by gibberellin A3 (GA3). Ind. J. Biochem. Biophys. 50, 548–553. [PubMed] [Google Scholar]
- Gao Z., Meng C., Gao H., Zhang X., Xu D., Su Y., et al. (2013a). Analysis of mRNA expression profi les of carotenogenesis and astaxanthin production of Haematococcus pluvialis under exogenous. Biol. Res. 46, 201–206. 10.4067/S0716-97602013000200012 [DOI] [PubMed] [Google Scholar]
- Gao Z., Meng C., Zhang X., Xu D., Miao X., Wang Y., et al. (2012a). Induction of salicylic acid (SA) on transcriptional expression of eight carotenoid genes and astaxanthin accumulation in Haematococcus pluvialis. Enzyme Microb. Technol. 51, 225–230. 10.1016/j.enzmictec.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Gao Z., Meng C., Zhang X., Xu D., Zhao Y., Wang Y., et al. (2012b). Differential expression of carotenogenic genes, associated changes on astaxanthin production and photosynthesis features induced by JA in H. pluvialis. PLoS ONE 7:e42243. 10.1371/journal.pone.0042243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Malea M. C., Acién F. G., Del Río E., Fernández J. M., Cerón M. C., Guerrero M. G., et al. (2009). Production of astaxanthin by Haematococcus pluvialis: taking the one-step system outdoors. Biotechnol. Bioeng. 102, 651–657. 10.1002/bit.22076 [DOI] [PubMed] [Google Scholar]
- Gomez P. I., Inostroza I., Pizarro M., Perez J. (2013). From genetic improvement to commercial-scale mass culture of a Chilean strain of the green microalga Haematococcus pluvialis with enhanced productivity of the red ketocarotenoid astaxanthin. AoB Plants 5:plt026. 10.1093/aobpla/plt026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell H. C., Laurens L. M. L., Shields R. J., Lovitt R. W., Flynn K. J. (2010). Placing microalgae on the biofuels priority list: a review of the technological challenges. J. Royal Soc. Int. 7, 703–726. 10.1098/rsif.2009.0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe C. B., Griehl C. (2012). The carotenoid astaxanthin from Haematococcus pluvialis, in Microalgal Biotechnology: Integration and Economy eds Posten C., Walter C. (Berlin; Boston, MA: De Gruyter; ), 129–144. 10.1515/9783110298321.129 [DOI] [Google Scholar]
- Guedes A. C., Amaro H. M., Malcata F. X. (2011). Microalgae as sources of carotenoids. Mar. Drugs 9, 625–644. 10.3390/md9040625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin M., Huntley M. E., Olaizola M. (2003). Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. 21, 210–216. 10.1016/S0167-7799(03)00078-7 [DOI] [PubMed] [Google Scholar]
- Gutiérrez C. L., Gimpel J., Escobar C., Marshall S. H., Henríquez V. (2012). Chloroplast genetic tool for the green microalgae Haematococcus pluvialis (Chlorophyceae, Volvocales)1. J. Phycol. 48, 976–983. 10.1111/j.1529-8817.2012.01178.x [DOI] [PubMed] [Google Scholar]
- Gwak Y., Hwang Y. S., Wang B., Kim M., Jeong J., Lee C. G., et al. (2014). Comparative analyses of lipidomes and transcriptomes reveal a concerted action of multiple defensive systems against photooxidative stress in Haematococcus pluvialis. J. Exp. Bot. 65, 4317–4334. 10.1093/jxb/eru206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen C., Siegmund S., Braune W. (2002). Ultrastructural and chemical changes in the cell wall of Haematococcus pluvialis (Volvocales, Chlorophyta) during aplanospore formation. Eur. J. Phycol. 37, 217–226. 10.1017/S0967026202003669 [DOI] [Google Scholar]
- Han D., Li Y., Hu Q. (2013). Biology and commercial aspects of Haematococcus pluvialis, in Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd Edn., eds Richmond A., Hu Q. (Hoboken, NJ: Blackwell; ), 388–405. 10.1002/9781118567166.ch20 [DOI] [Google Scholar]
- Harker M., Tsavalos A. J., Young A. J. (1996a). Factors responsible for astaxanthin formation in the chlorophyte Haematococcus pluvialis. Bioresour. Technol. 55, 207–214. 10.1016/0960-8524(95)00002-X [DOI] [Google Scholar]
- Harker M., Tsavalos A. J., Young A. J. (1996b). Autotrophic growth and carotenoid production of Haematococcus pluvialis in a 30 liter air-lift photobioreactor. J. Ferment. Bioeng. 82, 113–118. 10.1016/0922-338X(96)85031-8 [DOI] [Google Scholar]
- Hashimoto H., Arai K., Hayashi S., Okamoto H., Takahashi J., Chikuda M., et al. (2013). Effects of astaxanthin on antioxidation in human aqueous humor. J. Clin. Biochem. Nutr. 53, 1–7. 10.3164/jcbn.13-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata N., Ogbonna J. C., Hasegawa Y., Taroda H., Tanaka H. (2001). Production of astaxanthin by Haematococcus pluvialis in a sequential heterotrophic-photoautotrophic culture. J. Appl. Phycol. 13, 395–402. 10.1023/A:1011921329568 [DOI] [Google Scholar]
- Hazen T. E. (1899). The life history of Sphaerella lacustris. Mem. Torrey Bot. Club 6, 211–244. [Google Scholar]
- He P., Duncan J., Barber J. (2007). Astaxanthin accumulation in the green alga Haematococcus pluvialis: effects of cultivation parameters. J. Integr. Plant. Biol. 49, 447–451. 10.1111/j.1744-7909.2007.00468.x [DOI] [Google Scholar]
- Higuera-Ciapara I., Felix-Valenzuela L., Goycoolea F. M. (2006). Astaxanthin: a review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 46, 185–196. 10.1080/10408690590957188 [DOI] [PubMed] [Google Scholar]
- Hoeffler J. F., Hemmerlin A., Grosdemange-Billiard C., Bach T. J., Rohmer M. (2002). Isoprenoid biosynthesis in higher plants and in Escherichia coli: on the branching in the methylerythritol phosphate pathway and the independent biosynthesis of isopentenyl diphosphate and dimethylallyl diphosphate. Biochem. J. 366, 573–583. 10.1042/bj20020337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman Y., Aflalo C., Zarka A., Gutman J., James T. Y., Boussiba S. (2008). Isolation and characterization of a novel chytrid species (phylum Blastocladiomycota), parasitic on the green alga Haematococcus. Mycol. Res. 112, 70–81. 10.1016/j.mycres.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Hong M.-E., Choi S. P., Park Y.-I., Kim Y.-K., Chang W. S., Kim B. W., et al. (2012). Astaxanthin production by a highly photosensitive Haematococcus mutant. Process. Biochem. 47, 1972–1979. 10.1016/j.procbio.2012.07.007 [DOI] [Google Scholar]
- Hu Z., Li Y., Sommerfeld M., Chen F., Hu Q. (2008). Enhanced protection against oxidative stress in an astaxanthin-overproduction Haematococcus mutant (Chlorophyceae). Eur. J. Phycol. 43, 365–376. 10.1080/09670260802227736 [DOI] [Google Scholar]
- Hussein G., Sankawa U., Goto H., Matsumoto K., Watanabe H. (2006). Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 69, 443–449. 10.1021/np050354+ [DOI] [PubMed] [Google Scholar]
- Ikeuchi M., Koyama T., Takahashi J., Yazawa K. (2007). Effects of astaxanthin in obese mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 71, 893–899. 10.1271/bbb.60521 [DOI] [PubMed] [Google Scholar]
- In M. (2009). Effect of enzyme treatments on the extraction efficacy and antioxidant activity of haematococcus extract from Haematococcus pluvialis. J. Korea Acad. Indus. Cooperat. Soc. 10, 194–199. 10.5762/KAIS.2009.10.1.194 [DOI] [Google Scholar]
- Inbbor J. (1998). Haematococcus, the poultry pigmentor. Feed Mix. 6, 31–34. [Google Scholar]
- Inborr J., Lignell Å. (1997). Effect of feeding astaxanthin-rich algae meal (Haematococcus pluvialis) on performance and carotenoid concentration of different tissues of broiler chickens, in Proceedings of the XIII WPSA Conference on Poultry Meat Quality in Poznan (Poland: Session M1; ), 39–43. [Google Scholar]
- Industry Experts (2015). Global Astaxanthin Market – Sources, Technologies and Applications. Available online at: http://industry-experts.com/verticals/healthcare-and-pharma/global-astaxanthin-market-sources-technologies-and-applications
- Issarapayup K., Powtongsook S., Pavasant P. (2009). Flat panel airlift photobioreactors for cultivation of vegetative cells of microalga Haematococcus pluvialis. J. Biotechnol. 142, 227–232. 10.1016/j.jbiotec.2009.04.014 [DOI] [PubMed] [Google Scholar]
- Iwamoto T., Hosoda K., Hirano R., Kurata H., Matsumoto A., Miki W., et al. (2000). Inhibition of low-density lipoprotein oxidation by astaxanthin. J. Atheroscler. Thromb. 7, 216–222. 10.5551/jat1994.7.216 [DOI] [PubMed] [Google Scholar]
- Iwasaki T., Tawara A. (2006). Effects of astaxanthin on eyestrain induced by accommodative dysfunction. J. Eye (Atarashii Ganka). 6, 829–834. [Google Scholar]
- Jaime L., RodrIguez-Meizoso I., Cifuentes A., Santoyo S., Suarez S., Ibánez E. (2010). Pressurized liquids as an alternative process to antioxidant carotenoids extraction from Haematococcus pluvialis microalgae. LWT Food Sci. Tech. 43, 105–112. 10.1016/j.lwt.2009.06.023 [DOI] [Google Scholar]
- Jia Z., Liu Y., Daroch M., Geng S., Cheng J. J. (2014). Screening, growth medium optimisation and heterotrophic cultivation of microalgae for biodiesel production. Appl. Biochem. Biotechnol. 173, 1667–1679. 10.1007/s12010-014-0954-7 [DOI] [PubMed] [Google Scholar]
- Kaewpintong K., Shotipruk A., Powtongsook S., Pavasant P. (2007). Photoautotrophic high-density cultivation of vegetative cells of Haematococcus pluvialis in airlift bioreactor. Bioresour. Technol. 98, 288–295. 10.1016/j.biortech.2006.01.011 [DOI] [PubMed] [Google Scholar]
- Kakizono T., Kobayashi M., Nagai S. (1992). Effect of carbon/nitrogen ratio on encystment accompanied with astaxanthin formation in a green alga Haematococcus pluvialis. J. Ferment. Bioeng. 74, 403–405. [Google Scholar]
- Kamath B. S., Srikanta B. M., Dharmesh S. M., Sarada R., Ravishankar G. A. (2008). Ulcer preventive and antioxidative properties of astaxanthin from Haematococcus pluvialis. Eur. J. Pharmacol. 590, 387–395. 10.1016/j.ejphar.2008.06.042 [DOI] [PubMed] [Google Scholar]
- Kang C. D., Sim S. J. (2008). Direct extraction of astaxanthin from Haematococcus culture using vegetable oils. Biotechnol. Lett. 30, 441–444. 10.1007/s10529-007-9578-0 [DOI] [PubMed] [Google Scholar]
- Kang C. D., Han S. J., Choi S. P., Sim S. J. (2010). Fed-batch culture of astaxanthin-rich Haematococcus pluvialis by exponential nutrient feeding and stepwise light supplementation. Bioproc. Biosys. Eng. 33, 133–139. 10.1007/s00449-009-0362-5 [DOI] [PubMed] [Google Scholar]
- Kang C. D., Lee J. S., Park T. H., Sim S. J. (2005). Comparison of heterotrophic and photoautotrophic induction on astaxanthin production by Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 68, 237–241. 10.1007/s00253-005-1889-2 [DOI] [PubMed] [Google Scholar]
- Karppi J., Rissanen T. H., Nyyssönen K., Kaikkonen J., Olsson A. G., Voutilainen S., et al. (2007). Effects of astaxanthin supplementation on lipid peroxidation. Int. J. Vitam. Nutr. Res. 77, 3–11. 10.1024/0300-9831.77.1.3 [DOI] [PubMed] [Google Scholar]
- Katagiri M., Satoh A., Tsuji S., Shirasawa T. (2012). Effects of astaxanthin-rich Haematococcus pluvialis extract on cognitive function: a randomised, double-blind, placebo-controlled study. J. Clinic. Biochem. Nutr. 51, 102–107. 10.3164/jcbn.D-11-00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenji S., Kazuhiro O., Takuya N., Yasuhiro S., Shinki C., Kazuhiko Y., et al. (2005). Effect of astaxanthin on accommodation and asthenopia-efficacy -identification study in healthy volunteers. J. Clin. Ther. Med. 21, 637–650. [Google Scholar]
- Kim J. H., Affan M. A., Jang J., Kang M. H., Ko A. R., Jeon S. M., et al. (2015). Morphological, molecular, and biochemical characterization of astaxanthin-producin g green microalga Haematococcus sp. KORDI03 Haematococcaceae, Chlorophyta) isolated from Korea. J. Microbiol. Biotechnol. 25, 238–246. 10.4014/jmb.1410.10032 [DOI] [PubMed] [Google Scholar]
- Klochkova T. A., Kwak M. S., Han J. W., Motomura T., Nagasato C., Kim G. H. (2013). Cold-tolerant strain of Haematococcus pluvialis (Haematococcaceae, Chlorophyta) from Blomstrandhalvoya (Svalbard). Algae 28, 185–192. 10.4490/algae.2013.28.2.185 [DOI] [Google Scholar]
- Kobayashi M., Hirai N., Kurimura Y., Ohigashi H., Tsuji Y. (1997b). Abscisic acid-dependent algal morphogenesis in the unicellular green alga Haematococcus pluvialis. Plant Growth Regul. 22, 79–85. 10.1023/A:1005862809711 [DOI] [Google Scholar]
- Kobayashi M., Kakizono T., Nagai S. (1993). Enhanced carotenoid biosynthesis by oxidative stress in acetateinduced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl. Environ. Microbiol. 59, 867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Kakizono T., Nagai S. (1991). Astaxanthin production by a green alga, Haematococcus pluvialis accompanied with morphological changes in acetate media. J. Ferment. Bioeng. 71, 335–339. 10.1016/0922-338X(91)90346-I [DOI] [Google Scholar]
- Kobayashi M., Kakizono T., Yamaguchi K., Nishio N., Nagai S. (1992). Growth and astaxanthin formation of Haematococcus pluvialis in heterotrophic and mixotrophic conditions. J. Ferment. Bioeng. 74, 17–20. 10.1016/0922-338X(92)90261-R [DOI] [Google Scholar]
- Kobayashi M., Kurimura Y., Tsuji Y. (1997a). Light independent, astaxanthin production by the green microalga Haematococcus pluvialis under salt stress. Biotechnol. Lett. 19, 507–509. 10.1023/A:1018372900649 [DOI] [Google Scholar]
- Kobayashi M., Kurimura Y., Sakamoto Y., Tsuji Y. (1997c). Selective extraction of astaxanthin and chlorophyll from the green alga Haematococcus pluvialis. Biotechnol. Technol. 11, 657–660. 10.1023/A:1018455209445 [DOI] [Google Scholar]
- Koller M., Muhr A., Braunegg G. (2014). Microalgae as versatile cellular factories for valued products. Algal Res. 6, 52–63. 10.1016/j.algal.2014.09.002 [DOI] [Google Scholar]
- Krause W., Henrich K., Paust J., Ernst H. (1997). Preparation of Astaxanthin. Available online at: https://www.google.com/patents/US5654488
- Krichnavaruk S., Shotipruk A., Goto M., Pavasant P. (2008). Supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis with vegetable oils as co-solvent. Bioresour. Technol. 99, 5556–5560. 10.1016/j.biortech.2007.10.049 [DOI] [PubMed] [Google Scholar]
- Kupcinskas L., Lafolie P., Lignell A., Kiudelis G., Jonaitis L., Adamonis K., et al. (2008). Efficacy of the natural antioxidant astaxanthin in the treatment of functional dyspepsia in patients with or without Helicobacter pylori infection: a prospective, randomized, double blind, and placebo-controlled study. Phytomedicine 15, 391–399. 10.1016/j.phymed.2008.04.004 [DOI] [PubMed] [Google Scholar]
- Lababpour A., Shimahara K., Hada K., Kyoui Y., Katsuda T., Katoh S. (2005). Fedbatch culture under illumination with blue light emitting diodes (LEDs) for astaxanthin production by Haematococcus pluvialis. J. Biosci. Bioeng. 100, 339–342. 10.1263/jbb.100.339 [DOI] [PubMed] [Google Scholar]
- Leach M., Hamilton L. C., Olbrich A., Wray G. M., Thiemermann C. (1998). Effects of inhibitors of the activity of cyclooxygenase-2 on the hypotension and multiple organ dysfunction caused by endotoxin: a comparison with dexa-methasone. Br. J. Pharmacol. 124, 586–592. 10.1038/sj.bjp.0701869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei A., Chen H., Shen G., Hu Z., Chen L., Wang J. (2012). Expression of fatty acid synthesis genes and fatty acid accumulation in Haematococcus pluvialis under different stressors. Biotechnol. Biofuels 5:18. 10.1186/1754-6834-5-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi P. I., Popovich C. A., Damiani M. C. (2011). Feedstocks for second-generation biodiesel: microalgae's biology and oil composition, in Economic Effects of Biofuel Production, ed Bernardes M. A. d. S. (InTech; ), 317–347. 10.5772/23125 [DOI] [Google Scholar]
- Li J., Liu Y., Cheng J. J., Mos M., Daroch M. (2015). Biological potential of microalgae in China for biorefinery-based production of biofuels and high value compounds. N. Biotechnol. 32, 588–596. 10.1016/j.nbt.2015.02.001 [DOI] [PubMed] [Google Scholar]
- Li J., Zhu D. L., Niu J., Shen S. D., Wang G. (2011). An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 29, 568–574. 10.1016/j.biotechadv.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Li M., Wu W., Zhou P., Xie F., Zhou Q., Kangsen Mai K. (2014). Comparison effect of dietary astaxanthin and Haematococcus pluvialis on growth performance, antioxidant status and immune response of large yellow croaker Pseudosciaena crocea. Aquaculture 434, 227–232. 10.1016/j.aquaculture.2014.08.022 [DOI] [Google Scholar]
- Li Y., Sommerfeld M., Chen F., Hu Q. (2010). Effect of photon flux densities on regulation of carotenogenesis and cell viability of Haematococcus pluvialis (Chlorophyceae). J. Appl. Phycol. 22, 253–263. 10.1007/s10811-009-9453-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler H. K. (1999). the 1-Deoxy-D-Xylulose-5-Phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 47–65. 10.1146/annurev.arplant.50.1.47 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler H. K., Rohmer M., Schwender J. (1997). Two independent biochemical pathways for isopentenyl diphosphate and isoprenoid biosynthesis in higher plants. Plant Physiol. 101, 643–652. 10.1111/j.1399-3054.1997.tb01049.x [DOI] [Google Scholar]
- Lignell A. (2001). Medicament for Improvement of Duration of Muscle Function or Treatment of Muscle Disorders or Diseased. Varmdo: US patent # 6,245,818 Astacarotene AB. [Google Scholar]
- Lignell A., Inborr J. (1999). Agent for Increasing the Production of/in Breeding and Production Mammals. Varmdo: European patent. EP0912106. [Google Scholar]
- Lignell A. N. G. K., Inborr J. (2000). Agent for Increasing the Production of/in Breeding and Production Mammals. Varmdo: United States patent and trademark office granted patent. WO97/35491. [Google Scholar]
- Linden H. (1999). Carotenoid hydroxylase from Haematococcus pluvialis: cDNA sequence, regulation and functional complementation. Biochim. Biophys. Acta Gene Struct. Expr. 1446, 203–212. 10.1016/S0167-4781(99)00088-3 [DOI] [PubMed] [Google Scholar]
- Liu B. H., Lee Y. K. (2003). Effect of total secondary carotenoids extracts from Chlorococcum sp. on Helicobacter pylori-infected BALB/c mice. Int. Immunopharmacol. 3, 979–986. 10.1016/S1567-5769(03)00096-1 [DOI] [PubMed] [Google Scholar]
- Liu X., Osawa T. (2007). Cis astaxanthin and especially 9-cis astaxant hin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochem. Biophys. Res. Commun. 357, 187–193. 10.1016/j.bbrc.2007.03.120 [DOI] [PubMed] [Google Scholar]
- López M. C. G.-M., Sanchez E. D. R., López J. L. C., Fernández F. G. A., Sevilla J. M. F., Rivas J., et al. (2006). Comparative analysis of the outdoor culture of Haematococcus pluvialis in tubular and bubble column photobioreactors. J. Biotechnol. 123, 329–342. 10.1016/j.jbiotec.2005.11.010 [DOI] [PubMed] [Google Scholar]
- Lorenz R. T. (1999). A Technical Review of Haematococcus Algae. NatuRose™ Technical Bulletin #060 (Kailua-Kona, HI: Cyanotech Corporation; ). [Google Scholar]
- Lorenz R. T., Cysewski G. R. (2000). Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 18, 160–167. 10.1016/S0167-7799(00)01433-5 [DOI] [PubMed] [Google Scholar]
- Lotan T., Hirschberg J. (1995). Cloning and expression in Escherichia coli of the gene encoding beta-C-4-oxygenase, that converts beta-carotene to the ketocarotenoid canthaxanthin in Haematococcus pluvialis. FEBS Lett. 364, 125–128. 10.1016/0014-5793(95)00368-J [DOI] [PubMed] [Google Scholar]
- Machmudah S., Shotipruk A., Goto M., Sasaki M., Hirose T. (2006). Extraction of astaxanthin from Haematococcus pluvialis using supercritical CO2 and ethanol as entrainer. Ind. Eng. Chem. Res. Dev. 45, 3652–3657. 10.1021/ie051357k [DOI] [Google Scholar]
- Mata T. M., Martins A. A., Caetano N. S. (2010). Microalgae for biodiesel production and other applications: a review. Renew. Sust. Energ. Rev. 14, 217–232. 10.1016/j.rser.2009.07.020 [DOI] [Google Scholar]
- McBride R., Behnke C., Botsch K., Heaps N., Meenach C. (2013). Use of Fungicides in Liquid Systems. Merryfield Row San Diego, CA: PCT Patent Application WO2013056166 A1. [Google Scholar]
- Mendes-Pinto M. M., Raposo M. F. J., Bowen J., Young A. J., Morais R. (2001). Evaluation of different cell disruption processes on encysted cells of Haematococcus pluvialis: effects on astaxanthin recovery and implications for bioavailability. J. Appl. Phycol. 13, 19–24. 10.1023/A:1008183429747 [DOI] [Google Scholar]
- Mercer P., Armenta R. E. (2011). Developments in oil extraction from microalgae. Eur. J. Lipid Sci. Tech. 113, 539–547. 10.1002/ejlt.201000455 [DOI] [Google Scholar]
- Miao X., Wu Q. (2006). Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 97, 841–846. 10.1016/j.biortech.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Miki W. (1991). Biological functions and activities of animal carotenoids. Pure Appl. Chem. 63, 141–146. 10.1351/pac199163010141 [DOI] [Google Scholar]
- Milledge J. J. (2013). Energy Balance and Techno-economic Assessment of Algal Biofuel Production Systems. Doctoral Thesis, University of Southampton, Faculty of Engineering and the Environment.
- Miyawaki H., Takahashi J., Tsukahara H., Takehara I. (2008). Effects of astaxanthin on human blood rheology. J. Clin. Biochem. Nutr. 43, 69–74. 10.3164/jcbn.2008048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina Grima E., Belarbi E. H., Acién Fernández F. G., Robles Medina A., Chisti Y. (2003). Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv. 20, 491–515. 10.1016/S0734-9750(02)00050-2 [DOI] [PubMed] [Google Scholar]
- Moya M. J., Sánchez-Guardamino M. L., Vilavella A., Barbera E. (1997). Growth of Haematococcus lacustris: a contribution to kinetic modelling. J. Chem. Technol. Biotechnol. 68, 303–309. [Google Scholar]
- Nagaki Y., Hayasaka S., Yamada T., Hayasaka Y., Sanada M., Uonomi T. (2002). Effects of astaxanthin on accommodation, critical flicker fusion, and pattern visual evoked potential in visual display terminal workers. J. Trad. Med. 19, 170–173. [Google Scholar]
- Nagaki Y., Mihara M., Tsukuhara H., Ohno S. (2006). The supplementation effect of astaxanthin on accommodation and asthenopia. J. Clin. Therap. Med. 22, 41–54. [Google Scholar]
- Nagata A., Tajima T., Takahashi J. (2006). Effect of astaxanthin 5 mg on anti fatigue and task performance of human. Carotenoid Sci. 10, 102–106. [Google Scholar]
- Naguib Y. M. A. (2000). Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 48, 1150–1154. 10.1021/jf991106k [DOI] [PubMed] [Google Scholar]
- Nakamura A., Isobe R., Otaka Y., Abematsu Y., Nakata D., Honma C., et al. (2004). Changes in visual function following peroral astaxanthin. Jpn. J. Clin. Opthal. 58, 1051–1054. [Google Scholar]
- Nakao R., Nelson O. L., Park J. S., Mathison B. D., Thompson P. A., Chew B. P. (2010). Effect of astaxanthin supplementation on inflammation and cardiac function in BALB/c mice. Anticancer Res. 30, 2721–2725. [PubMed] [Google Scholar]
- Nawrocki W. J., Tourasse N. J., Taly A., Rappaport F., Wollman F. A. (2015). The plastid terminal oxidase: its elusive function points to multiple contributions to plastid physiology. Annu. Rev. Plant Biol. 66, 49–74. 10.1146/annurev-arplant-043014-114744 [DOI] [PubMed] [Google Scholar]
- Nishikawa Y., Minenaka Y., Ichimura M., Tatsumi K., Nadamoto T., Urabe K. (2005). Effects of astaxanthin and vitamin C on the prevention of gastric ulcerations in stressed rats. J. Nutr. Sci. Vit. 51, 135–141. 10.3177/jnsv.51.135 [DOI] [PubMed] [Google Scholar]
- Nobre B., Marcelo F., Passos R., Beirao L., Palavra A., Gouveia L., et al. (2006). Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 223, 787–790. 10.1007/s00217-006-0270-8 [DOI] [Google Scholar]
- Olaizola M. (2000). Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J. Appl. Phycol. 12, 499–506. 10.1023/A:1008159127672 [DOI] [Google Scholar]
- Olaizola M. (2003). Commercial development of microalgal biotechnology: from the test tube to the marketplace. Biomol. Eng. 20, 459–466. 10.1016/S1389-0344(03)00076-5 [DOI] [PubMed] [Google Scholar]
- Olguín E. J., Giuliano G., Porro D., Tuberosa R., Salamin F. (2012). Biotechnology for a more sustainable world. Biotechnol. Adv. 30, 931–932. 10.1016/j.biotechadv.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Orosa M., Franqueira D., Cid A., Abalde J. (2005). Analysis and enhancement of astaxanthin accumulation in Haematococcus pluvialis. Bioresour. Technol. 96, 373–378. 10.1016/j.biortech.2004.04.006 [DOI] [PubMed] [Google Scholar]
- Orosa M., Torres E., Fidalgo P., Abalde J. (2000). Production and analysis of secondary carotenoids in green algae. J. Appl. Phycol. 12, 553–556. 10.1023/A:1008173807143 [DOI] [Google Scholar]
- Palozza P., Torelli C., Boninsegna A., Simone R., Catalano A., Mele M. C., et al. (2009). Growth-inhibitory effects of the astaxanthin-rich alga Haematococcus pluvialis in human colon cancer cells. Cancer Lett. 283, 108–117. 10.1016/j.canlet.2009.03.031 [DOI] [PubMed] [Google Scholar]
- Pan J. L., Wang H. M., Chen C. Y., Chang J. S. (2012). Extraction ofastaxanthin from Haematococcus pluvialis by supercritical carbon dioxide fluid with ethanol modifier. Eng. Life Sci. 12, 638–647. [Google Scholar]
- Panis G. (2015). Commercial Astaxanthin Production Derived by Green Alga Haematococcus pluvialis: A Microalgae Process Model and a Techno-Economic Assessment All Through Production Line. Master Thesis (45 EC), Utrecht University, Netherlands. [Google Scholar]
- Parisenti J., Beirao L. H., Maraschin M., Mourino J. L., Nascimento V.iera F., Do Nascimento Vieira F., Bedin L. H., et al. (2011). Pigmentation and carotenoid content of shrimp fed with Haematococcus pluvialis and soy lecithin. Aquacult. Nutr. 17, 530–535. 10.1111/j.1365-2095.2010.00794.x [DOI] [Google Scholar]
- Park J. C., Choi S. P., Hong M. E., Sim S. J. (2014). Enhanced astaxanthin production from microalga, Haematococcus pluvialis by two-stage perfusion culture with stepwise light irradiation. Bioprocess Biosyst. Eng. 37, 2039–2047. 10.1007/s00449-014-1180-y [DOI] [PubMed] [Google Scholar]
- Park J. S., Chyun J. H., Kim Y. K., Line L. L., Chew B. P. (2010). Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. (Lond). 7:18. 10.1186/1743-7075-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-López P., González-García S., Jeffryes C., Agathos S. N., McHugh E., Walsh D., et al. (2014). Life-cycle assessment of the production of the red antioxidant carotenoid astaxanthin by microalgae: from lab to pilot scale. J. Clea. Prod. 64, 332–344. 10.1016/j.jclepro.2013.07.011 [DOI] [Google Scholar]
- Praveenkumar R., Lee K., Lee J., Oh Y.-K. (2015). Breaking dormancy: an energy- efficient means of recovering astaxanthin from microalgae. Green Chem. 17, 1226–1234. 10.1039/C4GC01413H [DOI] [Google Scholar]
- Pringsheim E. G. (1966). Nutritional requirements of Haematococcus pluvialis and related species. J. Phycol. 2, 1–7. 10.1111/j.1529-8817.1966.tb04584.x [DOI] [PubMed] [Google Scholar]
- Proctor V. W. (1957). Some controlling factors in the distribution of Haematococcus pluvialis. Ecology 38, 457–462. 10.2307/1929890 [DOI] [Google Scholar]
- Radakovits R., Jinkerson R. E., Darzins A., Posewitz M. C. (2010). Genetic engineering of algae for enhanced biofuel production. Eukaryot. Cell 9, 486–501. 10.1128/EC.00364-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranga Rao A., Harshvardhan Reddy A., Aradhya S. M. (2010). Antibacterial properties of Spirulina platensis, Haematococcus pluvialis, Botryococcus braunii micro algal extracts. Curr. Trends Biotechnol. Pharm. 4, 809–819. [Google Scholar]
- Ranga Rao A., Sarada R., Shylaja M. D., Ravishankar G. A. (2015). Evaluation of hepatoprotective and antioxidant activity of astaxanthin and astaxanthin esters from microalga-Haematococcus pluvialis. J. Food Sci. Tech. 52, 6703–6710. 10.1007/s13197-015-1775-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranga Rao A., Siew Moi P., Ravi S., Aswathanarayana R. G. (2014). Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—a review. Mar. Drugs 12, 128–152. 10.3390/md12010128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranga Rao A., Sindhuja H. N., Dharmesh S. M., Sankar K. U., Sarada R., Ravishankar G. A. (2013). Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 61, 3842–3851. 10.1021/jf304609j [DOI] [PubMed] [Google Scholar]
- Ranjbar R., Inoue R., Shiraishi H., Katsuda T., Katoh S. (2008). High efficiency production of astaxanthin by autotrophic cultivation of Haematococcus pluvialis in a bubble column photobioreactor. Biochem. Eng. J. 39, 575–580. 10.1016/j.bej.2007.11.010 [DOI] [Google Scholar]
- Razon L. F., Tan R. R. (2011). Net energy analysis of the production of biodiesel and biogas from the microalgae: Haematococcus pluvialis and Nannochloropsis. Appl. Energ. 88, 3507–3514. 10.1016/j.apenergy.2010.12.052 [DOI] [Google Scholar]
- Recht L., Zarka A., Boussiba S. (2012). Patterns of carbohydrate and fatty acid changes under nitrogen starvation in the microalgae Haematococcus pluvialis and Nannochloropsis sp. Appl. Microbiol. Biotechnol. 94, 1495–1503. 10.1007/s00253-012-3940-4 [DOI] [PubMed] [Google Scholar]
- Renstrøm B., Borch G., Skulberg O. M., Liaaen-Jensen S. (1981). Optical purity of (3S,3′S)-astaxanthin from Haematococcus pluvialis. Phytochemistry 20, 2561–2564. 10.1016/0031-9422(81)83094-4 [DOI] [Google Scholar]
- Reyes F. A., Mendiola J. A., Ibanez E., del Valle J. M. (2014). Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J. Supercrit. Fluids 92, 75–83. 10.1016/j.supflu.2014.05.013 [DOI] [Google Scholar]
- Rippka R., Deruelles J., Waterbury M., Herdman M., Stanier R. (1979). Generic assignments, strain histories and properties of pure culture of cyanobacteria. J. Gen. Microbiol. 111, 1–61. 10.1099/00221287-111-1-1 [DOI] [Google Scholar]
- Rohdich F., Hecht S., Gärtner K., Adam P., Krieger C., Amslinger S., et al. (2002). Studies on the nonmevalonate terpene biosynthetic pathway: metabolic role of IspH (LytB) protein. Proc. Natl. Acad. Sci. U.S.A. 99, 1158–1163. 10.1073/pnas.032658999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg J. N., Oyler G. A., Wilkinson L., Betenbaugh M. J. (2008). A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr. Opin. Biotechnol. 19, 430–436. 10.1016/j.copbio.2008.07.008 [DOI] [PubMed] [Google Scholar]
- Saha S. K., McHugh E., Hayes J., Moane S., Walsh D., Murray P. (2013). Effect of various stressregulatory factors on biomass and lipid production in microalga Haematococcus pluvialis. Bioresour. Technol. 128, 118–124. 10.1016/j.biortech.2012.10.049 [DOI] [PubMed] [Google Scholar]
- Santos M. F., Mesquita J. F. (1984). Ultrastructural study of Haematococcus lacustris (Girod.) Rostafinski (Volvocales) I. Some aspects of carotenogenesis. Cytologia 49, 215–228. 10.1508/cytologia.49.215 [DOI] [Google Scholar]
- Sarada R., Bhattacharya S., Ravishankar G. A. (2002a). Optimization of culture conditions for growth of the green alga Haematococcus pluvialis. World J. Microbiol. Biotechnol. 18, 517–521. 10.1023/A:1016349828310 [DOI] [Google Scholar]
- Sarada R., Tripathi U., Ravishankar G. A. (2002b). Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process Biochem. 37, 623–627. 10.1016/S0032-9592(01)00246-1 [DOI] [Google Scholar]
- Sarada R., Vidhyavathi R., Usha D., Ravishankar G. A. (2006). An efficient method for extraction of astaxanthin from green alga Haematococcus pluvialis. J. Agric. Food Chem. 54, 7585–7588. 10.1021/jf060737t [DOI] [PubMed] [Google Scholar]
- Satoh A., Tsuji S., Okada Y., Murakami N., Urami M., Nakagawa K., et al. (2009). Preliminary clinical evaluation of toxicity and efficacy of a new astaxanthin-rich Haematococcus pluvialis extract. J. Clin. Biochem. Nutr. 44, 280–284. 10.3164/jcbn.08-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki K., Yoshigi H., Aoki K., Koikawa N., Azum ane A., Kaneko K., et al. (2002). Sports performance benefits from taking natural astaxanthin characterized by visual acuity and muscle fatigue improvements in humans. J. Clinic. Therap. Med. 18, 73–88. [Google Scholar]
- Seki T., Sueki H., Kono H., Suganuma K., Yamashita E. (2001). Effects of astaxanthin from Haematococcus pluvialis on human skin-patch test; skin repeated application test; effect on wrinkle reduction. Fragrance J. 12, 98–103. [Google Scholar]
- Sharon-Gojman R., Maimon E., Leu S., Zarka A., Boussiba S. (2015). Advanced methods for genetic engineering of Haematococcus pluvialis (Chlorophyceae, Volvocales). Algal Res. 10, 8–15. 10.1016/j.algal.2015.03.022 [DOI] [Google Scholar]
- Sheikhzadeh N., Panchah I. K., Asadpour R., Tayefi-Nasrabadi H., Mahmoudi H. (2012a). Effects of Haematococcus pluvialis in maternal diet on reproductive performance and egg quality in rainbow trout (Oncorhynchus mykiss). Anim. Reprod. Sci. 130, 119–123. 10.1016/j.anireprosci.2011.12.010 [DOI] [PubMed] [Google Scholar]
- Sheikhzadeh N., Tayefi-Nasrabadi H., Oushani A. K., Najafi Enferadi M. H. (2012b). Effects of Haematococcus pluvialis supplementation on antioxidant system and metabolism in rain- bow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 38, 413–419. 10.1007/s10695-011-9519-7 [DOI] [PubMed] [Google Scholar]
- Solovchenko A. E. (2015). Recent breakthroughs in the biology of astaxanthin accumulation by microalgal cell. Photosynth. Res. 125, 437–449. 10.1007/s11120-015-0156-3 [DOI] [PubMed] [Google Scholar]
- Sommer T. R., Potts W. T., Morrissy N. M. (1991). Utilization of microalgal astaxanthin by rainbow trout (Oncorhynchus mykiss). Aquaculture 94, 79–88. [Google Scholar]
- Steinbrenner J., Linden H. (2001). Regulation of two carotenoid biosynthesis genes coding for phytoene synthase and carotenoid hydroxylase during stress-induced astaxanthin formation in the green alga Haematococcus pluvialis. Plant Physiol. 125, 810–817. 10.1104/pp.125.2.810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter M., Guerra T., Silva J., Gachon C. M. M. (2015). A new flagellated dispersion stage in Paraphysoderma sedebokerense, a pathogen of Haematococcus pluvialis. J. Appl. Phycol. 27, 1–6. 10.1007/s10811-015-0700-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Wang J., Shi M., Niu X., Yu X., Gao L., et al. (2014). Metabolomic and network analysis of astaxanthin-producing Haematococcus pluvialis under various stress conditions. Bioresour. Technol. 170, 522–529. 10.1016/j.biortech.2014.08.018 [DOI] [PubMed] [Google Scholar]
- Sun H., Kong Q., Geng Z., Duan L., Yang M., Guan B. (2015). Enhancement of cell biomass and cell activity of astaxanthin-rich Haematococcus pluvialis. Bioresour. Technol. 186, 67–73. 10.1016/j.biortech.2015.02.101 [DOI] [PubMed] [Google Scholar]
- Sun Z., Cunningham F. X., Jr., Gantt E. (1998). Differential expression of two isopentenyl pyrophosphate isomerases and enhanced carotenoid accumulation in a unicellular chlorophyte. Proc. Natl. Acad. Sci. U.S.A. 95, 11482–11488. 10.1073/pnas.95.19.11482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suseela M. R., Toppo K. (2006). Haematococcus pluvialis—a green alga, richest natural source of astaxanthin. Curr. Sci. 90, 1602–1603. [Google Scholar]
- Thana P., Machmudah S., Goto M., Sasaki M., Pavasant P., Shotipruk A. (2008). Response surface methodology to supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 99, 3110–3115. 10.1016/j.biortech.2007.05.062 [DOI] [PubMed] [Google Scholar]
- Tjahjono A. E., Hayama Y., Kakizono T., Terada Y., Nishio N., Nagai S. (1994b). Hyper accumulation of astaxanthin in a green alga Haematococcus pluvialis at elevated temperatures. Biotechnol. Lett. 16, 133–138. 10.1007/BF01021659 [DOI] [Google Scholar]
- Tjahjono A. E., Kakizono T., Hayama Y., Nishio N., Nagai S. (1994a). Isolation of resistant mutants against carotenoid biosynthesis inhibitors for a green alga Haematococcus pluvialis, and their hybrid formation by protoplast fusion for breeding of higher astaxanthin producers. J. Ferment. Bioeng. 77, 352–357. 10.1016/0922-338X(94)90003-5 [DOI] [Google Scholar]
- Tolasa S., Cakli S., Ostermeyer U. (2005). Determination of astaxanthin and canthaxanthin in salmonid. Eur. Food. Res. Technol. 221, 787–791. 10.1007/s00217-005-0071-5 [DOI] [Google Scholar]
- Tominaga K., Hongo N., Karato M., Yamashita E. (2012). Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim Pol. 59, 43–47. [PubMed] [Google Scholar]
- Torrissen O. J., Naevdal G. (1984). Pigmentation of salmonids — Genetical variation in carotenoid deposition in rainbow trout. Aquaculture 38, 59–66. 10.1016/0044-8486(84)90137-6 [DOI] [Google Scholar]
- Torzillo G., Goksan T., Faraloni C., Kopecky J., Masojidek J. (2003). Interplay between photochemical activities and pigment composition in an outdoor culture of Haematococcus pluvialis during the shift from the green to red stage. J. Appl. Phycol. 15, 127–136. 10.1023/A:1023854904163 [DOI] [Google Scholar]
- Triki A., Maillard P., Gudin C. (1997). Gametogenesis in Haematococcus pluvialis Flotow (Volvocales, Chlorophyta). Phycologia 36, 190–194. 10.2216/i0031-8884-36-3-190.1 [DOI] [Google Scholar]
- Tripathi U., Sarada R., Ramachandra Rao S., Ravishankar G. A. (1999). Production of astaxanthin in Haematococcus pluvialis cultured in various media. Bioresour. Technol. 68, 197–199. 10.1016/S0960-8524(98)00143-6 [DOI] [Google Scholar]
- Tripathi U., Venkateshwaran G., Sarada R., Ravishankar G. A. (2001). Studies on Haematococcus pluvialis for improved production of astaxanthin by mutagenesis. World J. Microbiol. Biotechnol. 17, 143–148. 10.1023/A:1016609815405 [DOI] [Google Scholar]
- Valderrama J. O., Perrut M., Majewski W., Serena L. (2003). Extraction of Astaxantine and Phycocyanine from Microalgae with Supercritical Carbon Dioxide, J. Chem. Eng. Data 48, 827–830. 10.1021/je020128r [DOI] [Google Scholar]
- Vidhyavathi R., Venkatachalam L., Sarada R., Ravishankar G. A. (2008). Regulation of carotenoid biosynthetic genes expression and carotenoid accumulation in the green alga Haematococcus pluvialis under nutrient stress conditions. J. Exp. Bot. 59, 1409–1418. 10.1093/jxb/ern048 [DOI] [PubMed] [Google Scholar]
- Wan M., Hou D., Li Y., Fan J., Huang J., Liang S., et al. (2014b). The effective photoinduction of Haematococcus pluvialis for accumulating astaxanthin with attached cultivation. Bioresour. Technol. 163, 26–32. 10.1016/j.biortech.2014.04.017 [DOI] [PubMed] [Google Scholar]
- Wan M., Zhang J., Hou D., Fan J., Li Y., Huang J., et al. (2014a). The effect of temperature on cell growth and astaxanthin accumulation of Haematococcus pluvialis during a light–dark cyclic cultivation. Bioresour. Technol. 167, 276–283. 10.1016/j.biortech.2014.06.030 [DOI] [PubMed] [Google Scholar]
- Wang B., Zarka A., Trebst A., Boussiba S. (2003). Astaxanthin accumulation in Haematococcus pluvialis (Chlorophyceae) as an active photoprotective process under high irradiance. J. Phycol. 39, 1116–1124. 10.1111/j.0022-3646.2003.03-043.x [DOI] [Google Scholar]
- Wang J. F., Han D. X., Sommerfeld M. R., Lu C. M., Hu Q. (2013a). Effect of initial biomass density on growth and astaxanthin production of Haematococcus pluvialis in an outdoor photobioreactor. J. Appl. Phycol. 25, 253–260. 10.1007/s10811-012-9859-4 [DOI] [Google Scholar]
- Wang J. F., Sommerfeld M. R., Lu C. M., Hu Q. (2013b). Combined effect of initial biomass density and nitrogen concentration on growth and astaxanthin production of Haematococcus pluvialis (Chlorophyta) in outdoor cultivation. Algae 28, 193–202. 10.4490/algae.2013.28.2.193 [DOI] [Google Scholar]
- Wang J., Sommerfeld M., Hu Q. (2009). Occurrence and environmental stress responses of two plastid terminal oxidases in Haematococcus pluvialis (Chlorophyceae). Planta 230, 191–203. 10.1007/s00425-009-0932-4 [DOI] [PubMed] [Google Scholar]
- Wang L., Yang B., Yan B., Yao X. (2012). Supercritical fluid extraction of astaxanthin from Haematococcus pluvialis and its antioxidant potential in sunflower oil. Innovative Food Sci. Emerg. Technol. 13, 120–127. 10.1016/j.ifset.2011.09.004 [DOI] [Google Scholar]
- Wang P. (2014). Culture Medium and Culture Method for Culturing Haematococcus pluvialis by using Brewery Wastewater. Available online at: https://www.google.com/patents/CN103966103A?cl=en
- Wayama M., Ota S., Matsuura H., Nango N., Hirata A., Kawano S. (2013). Three-dimensional ultrastructural study of oil and astaxanthin accumulation during encystment in the green alga Haematococcus pluvialis. PLoS ONE 8:e53618. 10.1371/journal.pone.0053618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. H., Yang J., Hu H. Y., Yu Y. (2013). Lipid-rich microalgal biomass production and nutrient removal by Haematococcus pluvialis in domestic secondary effluent. Ecol. Eng. 60, 155–159. 10.1016/j.ecoleng.2013.07.066 [DOI] [Google Scholar]
- Yamashita E. (2002). Cosmetic benefi of dietary supplements containing astaxanthin and tocotrienol on human skin. Food Style 21, 112–117. [Google Scholar]
- Yang Y., Kim B., Lee J. Y. (2013). Astaxanthin structure, metabolism, and health benefits. J. Hum. Nutr. Food Sci. 1, 1003:1–1003:11. [Google Scholar]
- Yin S., Wang J., Chen L., Liu T. (2015). The water footprint of biofilm cultivation of Haematococcus pluvialis is greatly decreased by using sealed narrow chambers combined with slow aeration rate. Biotechnol. Lett. 37, 1819–1827. 10.1007/s10529-015-1864-7 [DOI] [PubMed] [Google Scholar]
- Yoo J. J., Choi S. P., Kim B. W., Sim S. J. (2012). Optimal design of scalable photobioreactor for phototropic culturing of Haematococcus pluvialis. Bioprocess Biosyst. Eng. 35, 309–315. 10.1007/s00449-011-0616-x [DOI] [PubMed] [Google Scholar]
- Yu X., Chen L., Zhang W. (2015). Chemicals to enhance microalgal growth and accumulation of high-value bioproducts. Front. Microbiol. 6:56. 10.3389/fmicb.2015.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J. P., Peng J., Yin K., Wang J. H. (2011). Potential health promoting effects of astaxanthin: a high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 55, 150–165. 10.1002/mnfr.201000414 [DOI] [PubMed] [Google Scholar]
- Zhang B., Geng Y., Li Z., Hu H., Li Y. (2009). Production of astaxanthin from Haematococcus in open pond by two-stage growth one-step process. Aquaculture 295, 275–281. 10.1016/j.aquaculture.2009.06.043 [DOI] [Google Scholar]
- Zhang J., Luo Z., Li J., Liu Z. (2013). Production method and device for preventing and treating contamination of Paraphysoderma sedebokerensis in Haematococcus pluvialis. PCT Patent Application WO2013127280 A1. [Google Scholar]
- Zhang W., Wang J., Wang J., Liu T. (2014). Bioresource Technology. Attached cultivation of Haematococcus pluvialis for astaxanthin production. Bioresour. Technol. 158, 329–335. 10.1016/j.biortech.2014.02.044 [DOI] [PubMed] [Google Scholar]
- Zhang X. W., Gong X. D., Chen F. (1999). Kinetic models for astaxanthin production by high cell density mixotrophic culture of the microalga Haematococcus pluvialis. J. Ind. Microbiol. Biotechnol. 23, 691–696. 10.1038/sj.jim.2900685 [DOI] [PubMed] [Google Scholar]
- Zhang X., Pan L., Wei X., Gao H., Liu J. (2007). Impact of astaxanthin-enriched algal powder of Haematococcus pluvialis on memory improvement in BALB/c mice. Environ. Geochem. Health 29, 483–489. 10.1007/s10653-007-9117-x [DOI] [PubMed] [Google Scholar]
- Zhekisheva M., Boussiba S., Khozin-Goldberg I., Zarka A., Cohen Z. (2002). Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters. J. Phycol. 38, 325–331. 10.1046/j.1529-8817.2002.01107.x [DOI] [Google Scholar]
- Zhekisheva M., Zarka A., Khozin-Goldberg I., Cohen Z., Boussiba S. (2005). Inhibition of astaxanthin synthesis under high irradiance does not abolish triacylglycerol accumulation in the green alga Haematococcus pluvialis (Chlorophyceae). J. Phycol. 41, 819–826. 10.1111/j.0022-3646.2005.05015.x [DOI] [Google Scholar]
- Zou T. B., Jia Q., Li H. W., Wang C.-X., Wu H.-F. (2013). Response surface methodology for ultrasound-assisted extraction of astaxanthin from Haematococcus pluvialis. Mar. Drugs 11, 1644–1655. 10.3390/md11051644 [DOI] [PMC free article] [PubMed] [Google Scholar]