Abstract
An in vitro culture system of chicken primordial germ cells (PGCs) has been recently developed, but the growth factor involved in the proliferation of PGCs is largely unknown. In the present study, we investigated the growth effects of chicken stem cell factor (chSCF) on the in vitro proliferation of chicken PGCs. We established two feeder cell lines (buffalo rat liver cells; BRL cells) that stably express the putative secreted form of chSCF (chSCF1-BRL) and membrane bound form of chSCF (chSCF2-BRL). Cultured PGC lines were incubated on chSCF1 or chSCF2-BRL feeder cells with fibroblast growth factor 2 (FGF2), and growth effects of each chSCF isoform were investigated. The in vitro proliferation rate of the PGCs cultured on chSCF2-BRL at 20 days of culture was more than threefold higher than those cultured on chSCF1-BRL cells and more than fivefold higher than those cultured on normal BRL cells. Thus, use of chSCF2-BRL feeder layer was effective for in vitro proliferation of chicken PGCs. However, the acceleration of PGC proliferation on chSCF2-BRL was not observed without FGF2, suggesting that chSCF2 would act as a proliferation co-factor of FGF2. We transferred the PGCs cultured on chSCF2-BRL cells to recipient embryos, generated germline chimeric chickens and assessed the germline competency of cultured PGCs by progeny test. Donor-derived progenies were obtained, and the frequency of germline transmission was 3.39%. The results of this study demonstrate that chSCF2 induces hyperproliferation of chicken PGCs retaining germline competency in vitro in cooperation with FGF2.
Keywords: Chicken, Germline chimera, Primordial germ cells (PGCs), Stem cell factor (SCF)
Primordial germ cells (PGCs) act as the precursor of gametes and can transmit genetic information to the next generation through fertilization. In chickens, PGCs are efficient tools both for the cryopreservation of avian genetic resources and the production of transgenic chickens. In 2006, van de Lavoir et al. developed an efficient culture method for chicken PGCs using culture media containing basic fibroblast growth factor (bFGF, also known as FGF2), stem cell factor (SCF), and leukemia inhibitory factor [1]. These cultured PGCs differentiated into functional gametes when transplanted into recipient embryos and could be genetically modified. A recent study showed that FGF2 was one of the key factors involved in vitro proliferation of chicken PGCs [2], and the effectiveness of a PGC culture medium containing FGF2 was confirmed by several studies [3,4,5,6]. However, the growth factors involved in vitro proliferation of chicken PGCs other than FGF2 are largely unknown. Thus, identification of another growth factor that support the in vitro proliferation of chicken PGCs would contribute to the optimization of culture conditions.
In mice, c-KIT, a receptor of SCF, is expressed on the surface of PGCs. SCF is produced by gonadal somatic cells, and the interaction between c-KIT and SCF is required for germ cell proliferation, anti-apoptosis and migration [7,8,9,10,11,12]. Based on these data, SCF has been used as the supporting factor for the in vitro proliferation and survival of murine PGCs [7,8,9,10]. In chickens, SCF has also been used for the culture of PGCs in various studies [1,2,3,4,5, 13, 14], but several studies insist that SCF has no apparent function in PGC proliferation. However, those studies used mouse or human recombinant SCF proteins, and mammalian SCF may not support the proliferation of chicken PGCs in vitro, because the amino acid identity between mammalian SCF and chicken SCF (chSCF) is less than 60% [15, 16]. Previous reports suggest that administration of chSCF might have a positive impact on in vitro proliferation of chicken PGCs [17, 18]. Thus, we elucidated whether chSCF is required for in vitro culture of chicken PGCs.
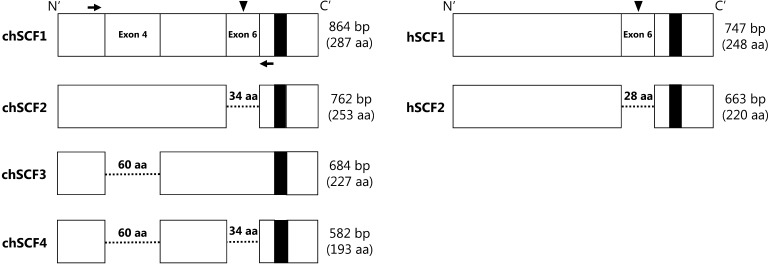
Mammalian SCF has two isoforms: a secreted form (longer form, SCF1) and a membrane-bound form (shorter form, SCF2) [19,20,21,22,23]. In mice, SCF1 has a limited ability for the survival of PGC cultures, but it is not involved in proliferation [7]. Meanwhile, SCF2 enhances the in vitro proliferation of PGCs, and use of SCF2-expressing feeder cells is effective for the long-term cultivation of murine PGCs [7,8,9,10]. Furthermore, proliferation and survival of endogenous PGCs is inhibited in the SCF2-lacking mice (Sl/Sld mutant mice) [11]. Therefore, SCF2 is an essential factor for proliferation and survival in vitro and in vivo. In chickens, chSCF has four reported isoforms (chSCF1–4) as alternative splice variants (Fig. 1) [24]. The longest form of chSCF, chSCF1 (Accession No. DQ870920, 287aa), contains a putative proteolytic cleavage site (195–196 aa) on exon 6, and thus chSCF1 is considered the secreted form [19, 20, 24]. chSCF2 (Accession No. DQ870921, 253 aa) lacks the exon 6 (34 aa) region, and hence chSCF2 is considered the membrane-bound form [21,22,23,24]. In addition, chSCF3 (Accession No. DQ870922, 227 aa) lacks exon 4 (60 aa), and chSCF4 (Accession No. DQ870923, 193 aa) lacks both exon 4 and exon 6 [24]. We chose chSCF1 and chSCF2 for the in vitro growth assay of chicken PGCs because chSCF1 and chSCF2 proteins are the closest orthologs of the secreted and membrane-bound forms of SCF in mammals, and the amino acid identities are highly conserved in various birds.
Fig. 1.
Isoforms of chSCF. Comparison of protein structures of chSCF1–4 and the secreted and membrane-bound forms of human SCF (hSCF1 and hSCF2, respectively). Dashed lines indicated deleted regions, and filled squares in the C’ region indicate the transmembrane domain. Arrowheads and black arrows indicate the putative proteolytic cleavage site and the forward and reverse primers used for Fig. 2B, respectively.
The purpose of this study was to confirm the growth effects of chSCF1 or chSCF2 on chicken PGCs in vitro using buffalo rat liver (BRL) feeder cells stably expressing chSCF1 (chSCF1-BRL) or chSCF2 (chSCF2-BRL). We showed that chSCF2-BRL cells significantly enhanced the in vitro proliferation rate of chicken PGCs, and the hyperproliferated PGCs on chSCF2-BRL cells were retained the germline competency.
Materials and Methods
Fertilized eggs and animal care
Fertilized eggs were obtained from White Leghorn (WL) and Barred Plymouth Rock (BPR) chickens, and were provided by the National Agriculture and Food Research Organization Institute of Livestock and Grassland Science (NILGS). All experiments in this study were performed in accordance with the Committee for the Care and Use of Experimental Animals at the NILGS.
RNA extraction
Total RNA was extracted from day 19 embryonic ovaries or cultured cells using an RNeasy Plus Micro Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Total RNA (1 μg) was reverse transcribed using the PrimeScript RT reagent Kit with gDNA Eraser (TaKaRa Bio, Otsu, Shiga, Japan). The synthesized cDNAs were used for the subsequent assays.
Establishment of feeder cells stably expressing chSCF
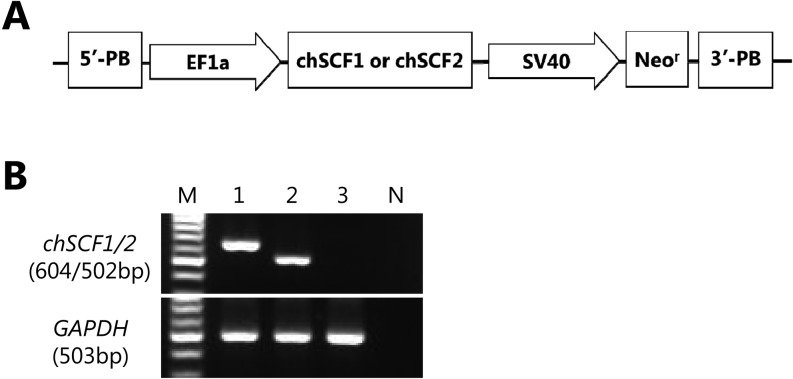
BRL cells were used as feeder cells (CRL-1441, provided by ATCC). BRL cells were cultured and maintained using Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA) containing 10% FBS (Biosera, Ringmer, UK). The cDNAs of chSCF1 (Accession No. DQ870920) and chSCF2 (Accession No. DQ870921) were isolated from the RNA of day 19 embryonic ovaries by RT-PCR using forward (5′-ttccttatgaagaaggcacaaact-3′) and reverse (5′-cagctacacttgtagatgttcttt-3′) primers and subcloned into pGEM-T Easy vector (Promega, Fitchburg, WI, USA). After sequencing confirmation, chSCF1 and chSCF2 cDNAs were cloned into a commercially available vector with piggyBac transposon (PB530A-2, System Biosciences, Mountain View, CA, USA) with an SV40 promoter-driven neomycin resistance (Neor) gene. The schema of vector construction is illustrated in Fig. 2A. These constructs were co-transfected with a piggyBac transposase expression vector into BRL cells using Lipofectamine 2000 (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. After transfection, cells were selected for more than 2 weeks using 10% FBS-DMEM containing 500 μg/ml of G-418 to establish BRL cell lines stably expressing chSCF1 and chSCF2 (chSCF1-BRL and chSCF2-BRL).
Fig. 2.
Establishment and characterization of chSCF1 or chSCF2-BRL cells. A) Schematic of the piggyBac chSCF1 and chSCF2 expression vectors. PB, piggyBac repetitive elements; EF1a, EF1a promoter; SV40, SV40 promoter; Neor, neomycin resistance. B) RT-PCR analysis of chSCF1-BRL cells, chSCF2-BRL cells and normal BRL cells. The 604-bp and 502-bp bands in the upper panel indicate chSCF1- or chSCF2-derived amplification products, respectively, and the 503-bp band in the lower panel indicates rat GAPDH-derived amplification products. M, Molecular size marker; 1, chSCF1-BRL cells; 2, chSCF2-BRL cells; 3, normal BRL cells; N, no cDNA control.
RT-PCR
To detect the chSCF isoforms in chSCF1-BRL cells or chSCF2-BRL cells, RT-PCR was performed using TaKaRa Ex Taq Hot Start Version (TaKaRa Bio). The PCR conditions were 95C for 2 min and then 40 cycles of 95ºC for 30 sec, 55°C for 30 sec and 72ºC for 30 sec, followed by 72ºC for 5 min. The sequences of the primers used were as follows: 5'-gtcaaagcccagagttcctg-3', forward, and 5'-ggctggagctgctaatgaag-3', reverse, for chSCF1 or chSCF2 and 5'-cagggctgccttctcttgtg-3', forward, and 5'-accagtggatgcagggatga-3', reverse, for rat GAPDH (Accession No. NM_017008).
Culture and maintenance of PGCs
Circulating PGCs were collected from male WL embryonic blood at Hamburger and Hamilton’s developmental stage 14 (HH14) [25]. Embryos were sexed by PCR using W chromosome-specific primers [26], and whole blood cells containing PGCs were grown on a BRL feeder layer. The PGC culture medium was prepared as described in previous studies with some modifications [1, 5]. The PGC culture medium contained KnockOut DMEM (KO-DMEM; Life Technologies), 50% BRL-conditioned KO-DMEM, 7.5% ES qualified FBS (Life Technologies), 2.5% chicken serum (Biowest, Nuaillé, France), 2 mM GlutaMAX (Life Technologies), 1 mM sodium pyruvate (Life Technologies), 1 × nonessential amino acids (Life Technologies), 1 × nucleosides (Millipore, Billerica, MA, USA), 0.5 mM monothioglycerol (Wako Pure Chemical Industries, Osaka, Japan) and 5 ng/ml human FGF2 (Wako Pure Chemical Industries). Half the volume of the culture medium was replaced every 2 days during the culture period for PGCs, and the cultured PGCs were passaged until 90% confluence. Several male PGCs were proliferated to large numbers, and could be maintained for more than 100 days. The established PGC lines were used for subsequent assays.
In vitro growth assay
Cultured PGCs (1.0 × 104 cells/ml) were seeded onto proliferation-inactivated chSCF1-BRL or chSCF2-BRL cells and subcultured for 20 days in PGC culture media containing 5 ng/ml human FGF2. For the control group, PGCs were cultured in a conventional culture system, namely on a normal BRL feeder layer using PGC culture media containing 5 ng/ml human FGF2. The experiment was repeated four times.
Immunocytochemistry
Circulating PGCs derived from HH14 embryonic blood or cultured PGCs were adhered onto a MAS-GP Type A coated glass slide (Matsunami Glass, Osaka, Japan) and fixed with 4% paraformaldehyde for 5 min at room temperature (RT). After several washes, cells were blocked with PBS containing 5% normal goat serum or Image-iT signal enhancer (Life Technologies) and incubated overnight at 4ºC with primary antibodies. Then, cells were incubated for 30 min or 1 h at RT with secondary antibodies. Subsequently, cells were counterstained with 1 µg/ml Hoechst 33342 (Dojindo, Kumamoto, Japan). Fluorescent images were captured using an Eclipse E1000 fluorescence upright microscope (Nikon, Tokyo, Japan), and these images were processed using Photoshop Elements (Adobe Systems, San Jose, CA, USA) for trimming and overlaying. Sources and dilution of used antibodies were as follows: rat anti-chicken vasa homolog (CVH) raised in our laboratory (1:10000) [27], mouse anti-stage specific embryonic antigen-1 (SSEA-1; 1:100, Developmental Studies Hybridoma Bank, Iowa City, IA, USA), mouse anti-chicken c-KIT (1:500, SouthernBiotech, Birmingham, AL, USA), goat anti-rat IgG conjugated with Alexa Fluor 488 (1:1000, Life Technologies) and goat anti-mouse IgG or IgM conjugated with Alexa Fluor 594 (1:1000, Life Technologies).
Production of germline chimeric chickens and progeny test
PGC lines derived from WL chickens were cultured on chSCF2-BRL cells to produce germline chimeric chickens. PGCs (1.0 × 103 cells) were injected into the dorsal aorta of HH14-16 BPR embryos. Manipulated embryos were incubated until hatching, and the chicks were grown until sexual maturity. Male putative germline chimeric chickens were crossed with female BPR chickens (i/i) by artificial insemination to test the donor WL chicken (I/I)-derived spermatogenesis. White offspring (I/i) indicated the progeny of cultured PGC (WL)-derived chicks, whereas black offspring (i/i) indicated the progeny of recipient (BPR)-derived chicks.
Statistical analysis
Statistical differences in the proliferation rate of PGCs were calculated by the Tukey-Kramer method or Welch’s t-test. Data were regarded as significant at P < 0.05.
Results
Establishment and characterization of chSCF-expressing feeder cells
To analyze the possible function of SCF in chicken PGCs, we attempted to isolate chicken orthologs of SCFs. Among the four chSCF alternative splice variants, chSCF1 and chSCF2 are putative orthologs of the mammalian SCF secreted form and membrane-bound form, respectively (Fig. 1). The cDNAs encoding chSCF1 and chSCF2 were first isolated from a day 19 embryonic ovary as 864 bp and 762 bp genes. The chSCF1 or chSCF2 expression vectors were then transfected into BRL cells (Fig. 2A), and these cells were selected with G-418. After selection, we examined chSCF1 and chSCF2 expression in chSCF1-BRL cells and chSCF2-BRL cells by RT-PCR. Amplification products derived from chSCF1 (604 bp) and chSCF2 (502 bp) were detected in chSCF1-BRL cells and chSCF2-BRL cells, respectively (Fig. 2B). By contrast, chSCF1 and chSCF2 were not expressed in normal BRL cells.
c-KIT expression in circulating and cultured PGCs
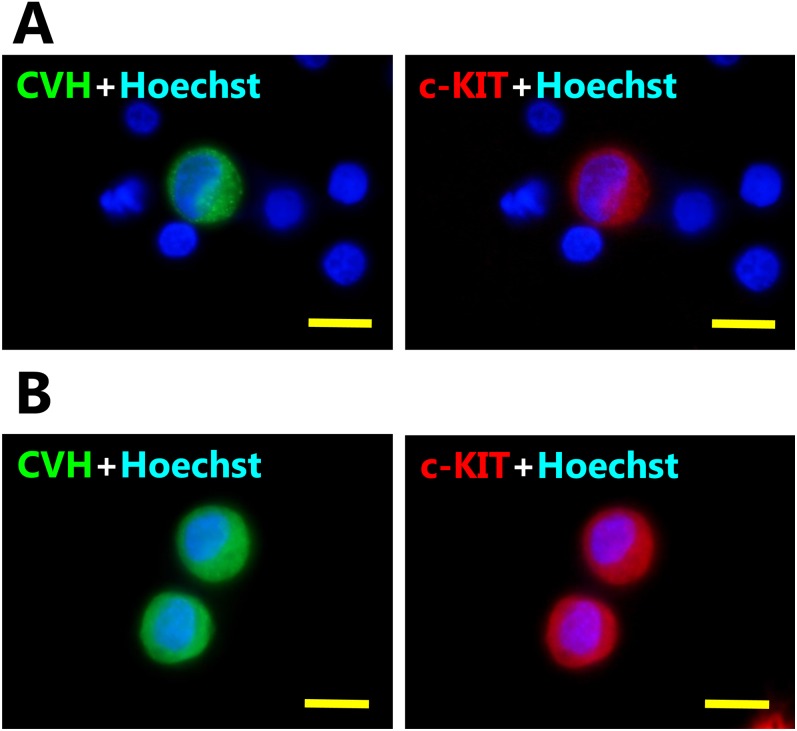
To examine the expression of the chSCF receptor c-KIT in chicken PGCs, we performed immunocytochemistry on circulating PGCs derived from embryonic blood at HH14 and cultured PGCs. Endogenous c-KIT proteins could be detected in the PGC-like large spheres with CVH, a pan-germ cell marker, but not in CVH-negative blood cells (Fig. 3A). Furthermore, cultured PGCs also co-expressed CVH and c-KIT (Fig. 3B). CVH protein was localized to the cytoplasm, and c-KIT protein was localized to the cytomembrane and cytoplasm.
Fig. 3.
Expression of c-KIT circulating and cultured chicken PGCs. A) Immunostaining of CVH (germ cell marker) and c-KIT (receptor of chSCF) in circulating PGCs derived from HH14 embryonic blood. B) Immunostaining of cultured PGCs stained with the same antibodies. CVH proteins were expressed in cytoplasm, and c-KIT proteins were expressed on the cytomembrane and cytoplasm, whereas red blood cells (CVH-negative cells) did not express CVH or c-KIT. Scale bars: 10 μm.
Growth effects of chSCF isoforms
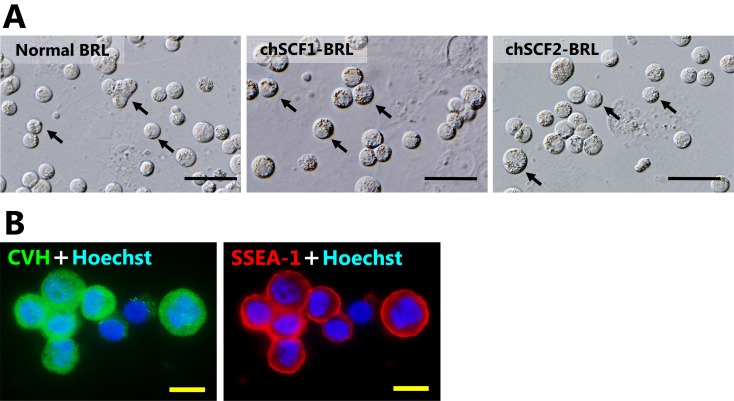
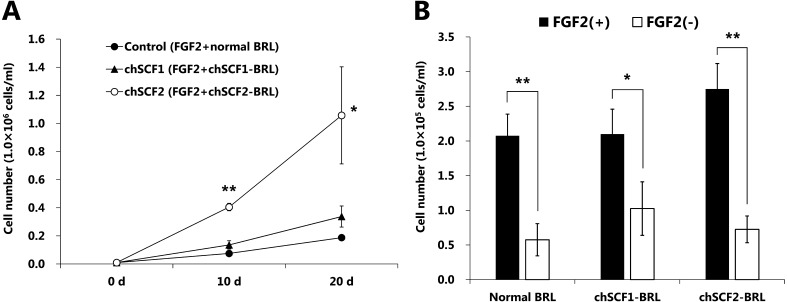
To elucidate the growth effects of chSCF1 and chSCF2 on PGCs, WL-derived PGC lines (obtained from male embryos) were seeded onto chSCF1-BRL or chSCF2-BRL feeder layers using a PGC culture medium containing FGF2 (5 ng/ml). In vitro growth assays were started from 1.0 × 104 cells/ml of PGCs, and the cells were cultured for 20 days. Cultured PGCs were maintained as floating or weakly adhesive cells under every culture condition. The cultured PGCs were shaped like large spheres and had large nuclei and many lipids in their cytoplasm, and their morphological characteristics were not different from those of normal chicken PGCs (Fig. 4A) [5]. In addition, various diameters of cultured PGCs were observed under every culture conditions (6–20 μm). Cultured PGCs co-expressed CVH and undifferentiated cell marker SSEA-1 under each condition (Fig. 4B). CVH protein was localized in the cytoplasm, and SSEA-1 was localized on the cytomembrane, respectively. Thus, the CVH and SSEA-1 expression indicated that the cultured PGCs were maintained as germ cells in undifferentiated state. PGCs showed significant proliferation on the chSCF2-BRL feeder layer compared with the normal BRL and chSCF1-BRL feeder layers (P < 0.05). The cell numbers at 20 days after culture start were 1.81 ± 0.83 × 105 cells/ml (control), 3.38 ± 0.75 × 105 cells/ml (chSCF1-BRL) and 1.06 ± 0.35 × 106 cells/ml (chSCF2-BRL) (Fig. 5A). These data indicate that use of chSCF2-BRL cells can induce a more than fivefold increase in proliferation of PGCs compared with conventional culture conditions. To investigate whether chSCF1 and chSCF2 are sufficient for PGC proliferation, we performed a proliferation assay using a culture medium without FGF2. The results showed that PGCs did not sufficiently proliferate on feeder layers compared with the proliferation observed under culture conditions with FGF2 (Fig. 5B).
Fig. 4.
Characterization of cultured PGCs. A) Morphological characteristics of PGCs cultured on chSCF1-BRL, chSCF2-BRL, and normal BRL feeder layers at 10 days of culture. Cultured PGCs had the same morphological characteristics as normal PGCs; they were shaped like large spheres, had large nuclei and had many lipids in their cytoplasm. The arrows in each panel indicate the typically cultured PGCs. Scale bars: 50 μm. B) Immunofluorescence of PGCs cultured on chSCF2-BRL cells using CVH and SSEA-1 antibodies. The CVH protein was localized in the cytoplasm, and SSEA-1 was localized on the cytomembrane. Scale bars: 10 μm.
Fig. 5.
Proliferation effects of chSCF isoforms. A) Growth curves of PGCs under several culture conditions. PGCs (1.0 × 104 cells/ml) were seeded onto feeder cells and cultured for 20 days in PGC culture medium containing 5 ng/ml FGF2. Data are presented as the mean ± SEM. Asterisks indicate statistical significance based on the Tukey-Kramer method (*P < 0.05; **P < 0.01). B) PGCs (1.0 × 104 cells/ml) were seeded onto feeder cells and cultured for 8 days using medium with or without FGF2. Data are presented as the mean ± SEM. Asterisks indicate statistical significance based on Welch’s t-test (*P < 0.05; **P < 0.01).
Germline transmission of cultured PGCs on chSCF2-BRL cells
To further assess whether the PGCs that hyperproliferated on chSCF2-BRL cells could differentiate into functional gametes, we produced germline chimeric chickens following transplantation of these cells and performed progeny tests. We analyzed male chimeric chickens, because cultured chicken PGCs could not complete normal gametogenesis in the gonads of the opposite sex [3, 28, 29]. Three male chimeric chickens were produced, and two chimeras were grown to sexual maturity. Two donor-derived white progenies (I/i) were generated from one germline chimeric chicken (ID 121), and the frequency of germline transmission was 3.39% (Fig. 6, Table 1). Thus, these data demonstrated that expanded PGCs following enhanced proliferation with chSCF2-BRL cells could differentiate into functional spermatozoa.
Fig. 6.

Germline transmission of PGCs cultured on chSCF2-BRL cells. Donor (injected PGCs)-derived offspring from injected PGCs cultured on chSCF2-BRL cells. The white feather chick is a WL donor PGC-derived offspring (I/i), and the black feather chicks are recipient-derived offspring (i/i).
Table 1. Frequency of germline transmission of PGCs cultured with chSCF2-BRL cells.
| ID | Eggs set | No. of hatched chicks |
No. of recipient PGC-derived chicks (i/i) |
No. of cultured PGC-derived chicks (I/i) |
% of cultured PGC-derived chicks |
| 103 | 32 | 29 | 29 | 0 | 0 |
| 121 | 65 | 32 | 30 | 2 | 6.25 |
| Total | 97 | 61 | 59 | 2 | 3.39 |
Discussion
In the present study, we first demonstrated that chSCF2 positively regulates PGC proliferation in vitro and that the hyperproliferated PGCs retained germline competency. We also clarified that the proliferative effect of chSCF2 requires FGF2, indicating that these two cytokines functionally interact and enhance chicken PGC proliferation.
SCF is an essential cytokine for the proliferation, survival, and migration of mice PGCs, and the SCF signal is also required for in vitro culture [7,8,9,10,11,12]. In mice, SCF1 has a limited effect on survival of PGCs, and thus SCF1 does not enhance the in vitro proliferation of PGCs [7]. Meanwhile, use of SCF2-expressing feeder cells is effective for long-term proliferation of mouse PGCs compared with use of medium supplemented with SCF1 [10]. In chickens, several studies reported that chSCF1 was an important factor for proliferation or differentiation in various cells including embryonic stem cells, normal erythroid progenitor cells and osteoblasts [30,31,32]. However, how SCF functions in chicken PGCs has been unknown. This study revealed that c-KIT, a receptor of chSCF, was expressed in proliferative PGCs in vivo, suggesting that chSCF functions as a growth factor that enables expansion of endogenous PGCs in chickens. Our data showed that chSCF2-BRL feeder cells improved the in vitro proliferation rate of chicken PGCs by fivefold compared with normal BRL feeder cells. Meanwhile, our data also demonstrated that chSCF1-BRL feeder cells did not sufficiently supported the in vitro proliferation of chicken PGCs, and this result was consistent with the previous studies [17, 33]. Thus, chSCF2 was considered one of the essential factor for the in vitro proliferation of chicken PGCs. On the other hand, another group showed that SCF2 sustained the tyrosine kinase activity of the c-KIT receptor in a human myeloid cell line more than SCF1 [34]. This report and our data suggest that the chSCF2 might have more potent or prolonged effects on PGC proliferation via c-KIT mediated growth signals compared with chSCF1.
Our data demonstrated that neither chSCF1 nor chSCF2 is sufficient for PGC proliferation in conventional medium without FGF2. Previous studies reported that FGF2 treatment was sufficient for in vitro proliferation and survival of chicken PGCs [2, 3, 5]. Our results are consistent with these findings and clarified that chSCF1 and chSCF2 cannot induce PGC proliferation as a substitute for FGF2. On the other hand, we also showed that the use of chSCF2-BRL feeder cells enhanced the in vitro proliferation rate of chicken PGCs under culture conditions that contained FGF2. These findings suggested that chSCF2 is the proliferation co-factor of FGF2.
Several studies reported that chicken PGCs cultured for a long term tend to lose their characteristics as endogenous PGCs, including their gonadal migration activity, undifferentiated state and normal gametic differentiation ability [5, 13]. In the present study, we obtained live offspring derived from PGCs cultured on the chSCF2-BRL feeder layer. We found that PGCs cultured on chSCF2-BRL cells completed differentiation into functional spermatozoa in the recipient germline.
Together, these results indicate that chSCF2 induces hyperproliferation of chicken PGCs in cooperation with FGF2, and those hyperproliferated PGCs retain germline competency. chSCF2 has a high amino acid identity among various birds such as the quail (98%, Coturnix japonica, AAC59934), Japanese ibis (95%, Nipponia nippon, XP_009462259), and duck (94%, Anas platyrhynchos, XP_012960092). Thus, this PGC culture system combined with chSCF2 and FGF2 would be useful for the in vitro culture of PGCs derived from these birds. Furthermore, the present culture system would be extremely valuable for the cell based systems for cryopreservation of avian genetic resources and the generation of transgenic chickens.
Acknowledgments
This study was supported by a Research Fellowship for Young Scientists from the Japan Society for the Promotion Science (JSPS) to DM (14J10343). This work was also supported in part by the Industrial Technology Research Grant Program from of NEDO, Japan (to IO), JSPS KAKENHI Grant Number 25450481 (to IO) and Takeda Science Foundation (to IO). The authors thank the staff of the Poultry Management Section of the NILGS for taking care of the birds and providing the fertilized eggs. We thank Takafumi Mori for technical assistance in establishing the cultured PGC lines. We also thank Yoshiaki Nakamura, Kumiko Takeda, Eiji Kobayashi and Keijiro Nirasawa for kind support and discussion.
References
- 1.van de Lavoir MC, Diamond JH, Leighton PA, Mather-Love C, Heyer BS, Bradshaw R, Kerchner A, Hooi LT, Gessaro TM, Swanberg SE, Delany ME, Etches RJ. Germline transmission of genetically modified primordial germ cells. Nature 2006; 441: 766–769. [DOI] [PubMed] [Google Scholar]
- 2.Choi JW, Kim S, Kim TM, Kim YM, Seo HW, Park TS, Jeong JW, Song G, Han JY. Basic fibroblast growth factor activates MEK/ERK cell signaling pathway and stimulates the proliferation of chicken primordial germ cells. PLoS ONE 2010; 5: e12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macdonald J, Glover JD, Taylor L, Sang HM, McGrew MJ. Characterisation and germline transmission of cultured avian primordial germ cells. PLoS ONE 2010; 5: e15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song Y, Duraisamy S, Ali J, Kizhakkayil J, Jacob VD, Mohammed MA, Eltigani MA, Amisetty S, Shukla MK, Etches RJ, de Lavoir MC. Characteristics of long-term cultures of avian primordial germ cells and gonocytes. Biol Reprod 2014; 90: 15. [DOI] [PubMed] [Google Scholar]
- 5.Miyahara D, Mori T, Makino R, Nakamura Y, Oishi I, Ono T, Nirasawa K, Tagami T, Kagami H. Culture conditions for maintain propagation, long-term survival and germline transmission of chicken primordial germ cell-like cells. Jpn Poult Sci 2014; 51: 87–95. [Google Scholar]
- 6.Naito M, Harumi T, Kuwana T. Long-term culture of chicken primordial germ cells isolated from embryonic blood and production of germline chimaeric chickens. Anim Reprod Sci 2015; 153: 50–61. [DOI] [PubMed] [Google Scholar]
- 7.Dolci S, Williams DE, Ernst MK, Resnick JL, Brannan CI, Lock LF, Lyman SD, Boswell HS, Donovan PJ. Requirement for mast cell growth factor for primordial germ cell survival in culture. Nature 1991; 352: 809–811. [DOI] [PubMed] [Google Scholar]
- 8.De Felici M, Dolci S, Pesce M. Cellular and molecular aspects of mouse primordial germ cell migration and proliferation in culture. Int J Dev Biol 1992; 36: 205–213. [PubMed] [Google Scholar]
- 9.Matsui Y, Toksoz D, Nishikawa S, Nishikawa S, Williams D, Zsebo K, Hogan BL. Effect of Steel factor and leukaemia inhibitory factor on murine primordial germ cells in culture. Nature 1991; 353: 750–752. [DOI] [PubMed] [Google Scholar]
- 10.Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell 1992; 70: 841–847. [DOI] [PubMed] [Google Scholar]
- 11.Runyan C, Schaible K, Molyneaux K, Wang Z, Levin L, Wylie C. Steel factor controls midline cell death of primordial germ cells and is essential for their normal proliferation and migration. Development 2006; 133: 4861–4869. [DOI] [PubMed] [Google Scholar]
- 12.Gu Y, Runyan C, Shoemaker A, Surani A, Wylie C. Steel factor controls primordial germ cell survival and motility from the time of their specification in the allantois, and provides a continuous niche throughout their migration. Development 2009; 136: 1295–1303. [DOI] [PubMed] [Google Scholar]
- 13.Naito M, Harumi T, Kuwana T. Expression of GFP gene in cultured PGCs isolated from embryonic blood and incorporation into gonads of recipient embryos. Jpn Poult Sci 2012; 49: 116–123. [Google Scholar]
- 14.Tonus C, Cloquette K, Ectors F, Piret J, Gillet L, Antoine N, Desmecht D, Vanderplasschen A, Waroux O, Grobet L. Long term-cultured and cryopreserved primordial germ cells from various chicken breeds retain high proliferative potential and gonadal colonisation competency. Reprod Fertil Dev 2014; DOI 10.1071/RD14194 . [DOI] [PubMed] [Google Scholar]
- 15.Zhou JH, Ohtaki M, Sakurai M. Sequence of a cDNA encoding chicken stem cell factor. Gene 1993; 127: 269–270. [DOI] [PubMed] [Google Scholar]
- 16.Petitte JN, Kulik MJ. Cloning and characterization of cDNAs encoding two forms of avian stem cell factor. Biochim Biophys Acta 1996; 1307: 149–151. [DOI] [PubMed] [Google Scholar]
- 17.Karagenç L, Petitte JN. Soluble factors and the emergence of chick primordial germ cells in vitro. Poult Sci 2000; 79: 80–85. [DOI] [PubMed] [Google Scholar]
- 18.Glover JD, McGrew MJ. Primordial germ cell technologies for avian germplasm cryopreservation and investigating germ cell development. Jpn Poult Sci 2012; 49: 155–162. [Google Scholar]
- 19.Zsebo KM, Wypych J, McNiece IK, Lu HS, Smith KA, Karkare SB, Sachdev RK, Yuschenkoff VN, Birkett NC, Williams LR, Satyagal VN, Tung W, Bosselman RA, Mendiaz EA, Langley KE. Identification, purification, and biological characterization of hematopoietic stem cell factor from buffalo rat liver—conditioned medium. Cell 1990; 63: 195–201. [DOI] [PubMed] [Google Scholar]
- 20.Anderson DM, Lyman SD, Baird A, Wignall JM, Eisenman J, Rauch C, March CJ, Boswell HS, Gimpel SD, Cosman D, Williams DE. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell 1990; 63: 235–243. [DOI] [PubMed] [Google Scholar]
- 21.Flanagan JG, Chan DC, Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell 1991; 64: 1025–1035. [DOI] [PubMed] [Google Scholar]
- 22.Toksoz D, Zsebo KM, Smith KA, Hu S, Brankow D, Suggs SV, Martin FH, Williams DA. Support of human hematopoiesis in long-term bone marrow cultures by murine stromal cells selectively expressing the membrane-bound and secreted forms of the human homolog of the steel gene product, stem cell factor. Proc Natl Acad Sci USA 1992; 89: 7350–7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNiece IK, Briddell RA. Stem cell factor. J Leukoc Biol 1995; 58: 14–22. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Li J, Ying Wang C, Yan Kwok AH, Leung FC. Epidermal growth factor (EGF) receptor ligands in the chicken ovary: I. Evidence for heparin-binding EGF-like growth factor (HB-EGF) as a potential oocyte-derived signal to control granulosa cell proliferation and HB-EGF and kit ligand expression. Endocrinology 2007; 148: 3426–3440. [DOI] [PubMed] [Google Scholar]
- 25.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol 1951; 88: 49–92. [PubMed] [Google Scholar]
- 26.Clinton M, Haines L, Belloir B, McBride D. Sexing chick embryos: a rapid and simple protocol. Br Poult Sci 2001; 42: 134–138. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura Y, Yamamoto Y, Usui F, Mushika T, Ono T, Setioko AR, Takeda K, Nirasawa K, Kagami H, Tagami T. Migration and proliferation of primordial germ cells in the early chicken embryo. Poult Sci 2007; 86: 2182–2193. [DOI] [PubMed] [Google Scholar]
- 28.Tagami T, Matsubara Y, Hanada H, Naito M. Differentiation of female chicken primordial germ cells into spermatozoa in male gonads. Dev Growth Differ 1997; 39: 267–271. [DOI] [PubMed] [Google Scholar]
- 29.Tagami T, Kagami H, Matsubara Y, Harumi T, Naito M, Takeda K, Hanada H, Nirasawa K. Differentiation of female primordial germ cells in the male testes of chicken (Gallus gallus domesticus). Mol Reprod Dev 2007; 74: 68–75. [DOI] [PubMed] [Google Scholar]
- 30.Pain B, Clark ME, Shen M, Nakazawa H, Sakurai M, Samarut J, Etches RJ. Long-term in vitro culture and characterisation of avian embryonic stem cells with multiple morphogenetic potentialities. Development 1996; 122: 2339–2348. [DOI] [PubMed] [Google Scholar]
- 31.Hayman MJ, Meyer S, Martin F, Steinlein P, Beug H. Self-renewal and differentiation of normal avian erythroid progenitor cells: regulatory roles of the TGF alpha/c-ErbB and SCF/c-kit receptors. Cell 1993; 74: 157–169. [DOI] [PubMed] [Google Scholar]
- 32.van’t Hof RJ, von Lindern M, Nijweide PJ, Beug H. Stem cell factor stimulates chicken osteoclast activity in vitro. FASEB J 1997; 11: 287–293. [DOI] [PubMed] [Google Scholar]
- 33.Yang G, Fujiwara N. Survival and proliferation of refined circulating primordial germ cells cultured in vitro. J Reprod Dev 1999; 45: 177–181. [Google Scholar]
- 34.Miyazawa K, Williams DA, Gotoh A, Nishimaki J, Broxmeyer HE, Toyama K. Membrane-bound Steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood 1995; 85: 641–649. [PubMed] [Google Scholar]