Abstract
The oviduct is an active contractile tube that provides the proper environment for sperm transport, capacitation and survival. Oviductal contractions are regulated by autocrine/paracrine secretion of several factors, such as prostaglandins (PGs) and endothelin-1 (EDN-1). We have previously shown that during the preovulatory stage, sperm are exposed to polymorphonuclear neutrophils (PMNs) in the bovine oviduct, and the bovine oviduct epithelial cells (BOECs) secrete molecules including PGE2 that suppress sperm phagocytosis by PMNs in vitro. In this study, we investigated the possible effects of EDN-1 on the phagocytic activity of PMNs toward sperm. The local concentrations of EDN-1 in oviduct fluid and BOEC culture medium ranged from 10–10 to 10–11 M as determined by EIA. Phagocytosis and superoxide production were assayed by co-incubation of sperm pretreated to induce capacitation with PMNs exposed to EDN-1 (0, 10–11, 10–10, 10–9, and 10–8 M) for 2 h. EDN-1 suppressed dose dependently (10–11 to 10–8 M) the phagocytic activity for sperm and superoxide production of PMNs in response to capacitated sperm. Moreover, this suppression was eliminated by an ETB receptor antagonist (BQ-788). EDN-1 suppressed mRNA expression of EDN-1 and ETB but not ETA receptors in PMNs, suggesting the ETB receptor-mediated pathway. Scanning electron microscopic observation revealed that incubation of PMNs with EDN-1 (10–9 M) completely suppressed the formation of DNA-based neutrophil extracellular traps for sperm entanglement. The results provide evidence indicating that EDN-1 may be involved in the protection of sperm from phagocytosis by PMNs in the bovine oviduct, supporting sperm survival until fertilization.
Keywords: Endothelin-1, Oviduct, Phagocytosis, Polymorphonuclear neutrophils (PMN), Sperm
The oviduct plays a pivotal role in mammalian reproduction, providing an optimal environment for sperm capacitation, fertilization, and transport of gametes and embryos [1]. In order to fulfil these functions, the oviduct should have an efficient and strictly controlled immune system that can maintain optimal conditions for fertilization and early embryo development [2]. The local immune responses of the epithelial cells, regulated by their secretions, constitute the main part of the mucosal innate immunity inside the oviduct [3]. Once epithelial cells are stimulated either by microbial (i.e., pathogens) or nonmicrobial (i.e., stress or hormones) stimuli, they activate the innate immune responses and regulate the subsequent adaptive immune responses [4]. This process is mediated by the secretion of different molecules such as prostaglandins (PGs), chemokines and cytokines, which affect the conditioning of mucosal dendritic cells (DCs) [5].
We have previously reported that polymorphonuclear neutrophils (PMNs), the first line of defense against microorganisms, are present in the bovine oviduct lumen during the preovulatory stage under physiological conditions and that they serve as another major constituent of the local immune system inside the bovine oviduct [6]. Additionally, we proposed that the bovine oviduct epithelial cells (BOECs), through their secretions, i.e, PGE2, down-regulate the phagocytic activity of PMNs for sperm, maintaining sperm survival until fertilization [6].
The endothelins (EDNs) are a family of peptides, present as three isoforms (EDN-1, 2, 3) and encoded by three distinct endothelin-related genes [7]. The EDNs were first described as vasoconstrictor substances derived from vascular endothelial cells. However, it is now known to be produced in various tissues and different types of cells [8, 9]. EDNs exert their biological response by binding to one of two G protein-coupled receptor subtypes, endothelin receptor A (ETA) and ETB [10]. EDN-1 is secreted by the bovine oviduct and stimulates local oviductal contraction [11]. Luteinizing hormone (LH) enhances the in vitro secretion of PGE2, PGF2 and EDN-1, as well as the contractile amplitude of bovine oviducts from the follicular and postovulatory periods, but not those from the luteal period [12]. Furthermore, EDN-1 stimulates the release of PGE2 and PGF2α from the oviduct epithelial cells [13].
Of note, EDN has been shown to exert immunomodulatory activity through stimulation of neutrophil migration via activation of protein kinase G [14] and suppression of respiratory burst and bacterial clearance from the blood and tissue in rabbits [15, 16]. Additionally, the suppressive effect of an LH-stimulated BOEC supernatant was partially but not completely inhibited by addition of an EP2 receptor antagonist for PGE2 to the medium [6]. This inidcates the possibile presence of other molecules secreted by BOECs that may further suppress PMN phagocytosis for sperm. Thus, in the present study, we investigated the possible role of EDN-1 secreted by BOECs in regulating sperm phagocytosis by PMNs in the bovine oviduct.
Materials and Methods
Bovine oviduct epithelial cell culture
The oviducts were transported in an ice box from a local slaughterhouse to the laboratory, with the oviducts immersed in phosphate buffer saline free of calcium and magnesium (PBS–/–, Cat. No. P3813-10PAK, Sigma, St. Louis, MO, USA) but with 0.3% gentamicin (Cat. No. g1264, Sigma) and amphotericin B (Cat. No. 15290-018, Life Technologies, Carlsbad, CA, USA). They were cut and separated from the connective tissue and washed twice with PBS–/–. The stage of the estrous cycle was determined macroscopically by ovarian morphology based on observed color, size and weight of the corpus luteum, as described previously [17]. Epithelial cells were isolated and cultured as previously described [6, 11]. In brief, the oviductal lumen was flushed with 15 ml PBS, and the epithelial cells were mechanically dislodged while being flushed with the same volume of PBS–/–. The isolated epithelial cells were cultured in culture medium (D-MEM/F12 Cat. No. 12400-024, Life Technologies, 0.1% gentamicin, 1% amphotericin and 2.2% NaHCO3 (Cat. No. 191-01305, Wako Pure Chemical Industries, Osaka, Japan) supplemented with 10% fetal calf serum (FCS; Cat. No. S1820, BioWhittaker, Walkersville, MD, USA) in 6-well culture dishes (Cat. No. 140675, Nalge Nunc International, Roskilde, Denmark) at 38ºC in 5% CO2 and 95% air. The following day, the BOEC culture was washed twice with PBS and incubated with culture medium supplemented with 5% FCS. After monolayer formation, cells were trypsinized (0.05% trypsin EDTA; Cat. No. 200-1395, Wako Pure Chemical Industries) until single cells appeared, and these cells were plated in 12-well culture dishes at a density of 1.5 × 104/ml and incubated at 38ºC in 5% CO2 and 95% air in culture medium supplemented with 5% FCS, until the growing BOEC monolayer covered up to 70–80% of the bottom of the culture plate. The growing BOEC monolayer was then incubated with culture medium supplemented with 0.1% FCS and incubated for 24 h with LH (10 ng/ml, USDA-bLH-B6, NHPP, Animal Hormone Program, Bethesda, MD, USA). The culture medium was collected and stored at –80ºC until used.
Measurements of EDN-1 concentrations
Oviducts were gently flushed with PBS–/– (0.2 ml /oviduct), and the resultant fluid was pooled in a sterile tube. EDN-1 concentrations were measured using a commercial EIA kit (Cayman, Ann Arbor, MI, USA) according to the manufacturer’s protocol. Seventeen oviducts (preovulatory, n = 6; postovulatory, n = 6; and mid-luteal; n = 5) and 12 BOEC supernatants (with LH stimulation, n = 6, and without LH stimulation, n = 6) were used in this experiment. The intra- and interassay coefficients of variation for EDN-1 were 7.8 and 10.7%. The median effective dose 50 (ED50) of the assay for EDN-1 was 115 pg/ml. The standard curve for EDN-1 ranged from 3.9 to 250 pg/ml.
Isolation and preparation of PMNs
PMNs were isolated as previously described [18]. Blood samples were collected at the Field Center of Animal Science and Agriculture of Obihiro University, and all experimental procedures complied with the guidelines for the care and use of agricultural animals at Obihiro University (license number 25-101). Heparinized blood from a multiparous Holstein cow during the luteal stage was collected and mixed with an equal volume of PBS–/–, slowly layered over Ficoll-Paque solution (Lymphoprep, Cat. No. 1114547, Cosmo Bio, Tokyo, Japan), and centrifuged at 1000 × g for 30 min at 10ºC. The PMN layer was mixed with ammonium chloride lysis buffer (composed of 155 mM NH4Cl, KHCO3 3.4 mM (Cat. No. 017-02995 and Cat. No 166-03275 respectively, Wako Pure Chemical Industries) and 96.7 µM EDTA (Cat. No. 1.08418.100, Merck, Kgaa, 6427, Darmstadt, Germany) for 10 sec, and then centrifuged at 500 × g for 10 min at 10ºC in order to purify PMNs from red blood cells. After centrifugation, the cell pellet was washed twice with PBS–/–. The purity of PMNs as evaluated by flow cytometry was > 98%, and the viability was around 99% as assessed by Trypan blue staining. Prior to phagocytosis assay, PMNs were suspended at a density of 1 × 107 cells/ml and cultured in 0.5 ml of D-MEM/F12 supplemented with EDN-1 (Cat. No. 4198-s, Peptide Institute, Osaka, Japan) at various concentrations (0, 10–8, 10–9, 10–10 or 10–11 M). PMNs were incubated for 2 h at 38.5ºC in 5% CO2 and 95% air with gentle shaking. After PMN incubation, PMNs were washed 2 times with PBS–/– and used for a phagocytosis assay.
Preparation of sperm
In parallel with PMN preparation, sperm preparation was performed as previously described [6]. Frozen semen straws were obtained from three highly fertile Holstein bulls of Genetics Hokkaido Association (Hokkaido, Japan). In vitro capacitation of bull sperm, obtained from 2 semen straws for each bull, was induced by using modified Tyrode’s albumin, lactate, and pyruvate medium (Sp-TALP), according to the method previously described [19, 20]. Semen samples were thawed, allowed to swim up for 1 h and then capacitated by 4 h incubation in Sp-TALP medium supplemented with 10 µg/ml heparin. Capacitation was confirmed by induction of acrosome reactions by using 100 µg/ml lysophosphatidylcholine for 15 min. Acrosome reactions were detected by performing a dual staining procedure with Trypan-blue supravital stain and Giemsa stain (TBG) as previously reported [21]. After capacitation, sperm were washed and suspended in Tyrode’s medium containing lactate, pyruvate, and HEPES (TL-HEPES) [22, 23], and then were used for a phagocytosis assay. The term, treated sperm, is used throughout the manuscript to refer to sperm treated to induce capacitation.
Phagocytosis assay
Assay of phagocytosis of sperm by PMNs was performed according to previous studies [6, 24] with minor modifications. Briefly, the 2 h incubated PMN were suspended in TL-HEPES. PMNs suspension was mixed with sperm suspension and serum in a 96-well untreated polystyrene microtest plate (Cat. No. 269787, Nalge Nunc International) and incubated for 60 min with gentle swirling on a test-plate shaker. The final concentrations of PMNs, sperm and fresh serum were 10 × 106 cells/ml, 10 × 106 cells/ml, and 12% (v/v), respectively, and the total volume was 100 µl. After incubation, an equal volume of heparin (Cat. No. H3149-100ku, Sigma, 40 mg/ml in TL-HEPES) was added to facilitate dissociation of agglutinated PMNs. Subsamples of 75 µl were fixed by adding 25 µl of 2% (v/v) glutaraldehyde. The fixed samples were mounted on glass slides and examined at × 400 magnification under a phase-contrast microscope connected to a digital camera and a computer system (Leica Application Suite, Leica microsystems, Wetzlar, Germany). A minimum of 400 PMNs were counted in different areas of a specimen. The percentage of PMNs with phagocytized sperm was recorded as the phagocytosis rate. Quantification of the number of PMNs with phagocytized sperm was performed independently by 2 observers.
Determination of superoxide generation
Superoxide generation was determined by a luminol-based luminescence method as previously described [25]. Briefly, 10 µl 5-amino-2,3-dihydrophthalazine-1,4-dione (luminol, Sigma) was injected into a 96-well FluoroNunc plate (Cat. No. 269787, Nunc, Denmark). Next, 100 µl of 2 h incubated PMNs (0.5 × 106 cells/ml) and treated sperm (1 × 106 cells/ml) were suspended in the 96-well FluoroNunc plate. Superoxide generation was detected at 425 nm using an AB-2350 Phelios (ATTO, Japan).
RNA extraction and real-time polymerase chain reaction (real-time PCR)
PMNs were stimulated with EDN-1 (0, 10–11 and 10–10 M) for 2 h. Total RNA was extracted from PMNs by using TRIzol reagent (Cat. No. 15596-018, Life Technologies), and reverse transcription was carried out as previously described [26]. DNase treatment was carried out using an RQ1 RNase-Free DNase kit (Promega, Madison, WI, USA). Two microliters of the extracted RNA was incubated for 30 min at 37ºC with 1 μl RQ1 RNase-free DNase 10 × reaction buffer and 2 μl of 1 μg/μl RNase-free DNase. To halt the reaction, 1 μl RQ1 DNase Stop solution (20 mM EDTA) was added to the sample, and the mixture was incubated for 10 min at 65ºC. First-strand cDNA synthesis was conducted according to the commercial protocol described in the SuperScript™ II Reverse Transcriptase kit (Cat. No.18064-014, Life Technologies). The first cocktail was prepared using 2 μl total RNA extracted from the BOEC sample, 1.5 μl of 50 ng/μl random primer (Life Technologies), 1.5 μl of 10 mM PCR Nucleotide Mix (dNTP, Cat. No. 11814362001, Roche Diagnostics, Indianapolis, IN, USA) and 12 μl H2O, resulting in a final volume of 18 μl per tube. This cocktail was then incubated at 65ºC for 5 min in a thermal cycler (Bio-Rad, Munich, Germany). The samples were kept on ice while the second cocktail comprised of 3 μl of 0.1 M DTT (Cat. No. 18064-014, Life Technologies), 1.5 μl of 40 units/μl RNase Inhibitor Recombinant (Cat. No. SIN-201, Toyobo, Osaka, Japan), and 6 μl of 5 × First-Strand Buffer, was added to each tube. The samples were incubated for 2 min at 42ºC, and 0.2 μl of 200 units/μl SuperScript™ II Reverse Transcriptase was added to each tube. The thermal cycler was programmed at 25ºC for 10 min, 42ºC for 50 min, and then 70ºC for 15 min. The synthesized cDNA was stored at –30°C. We analyzed the following genes: EDN-1, ETA and ETB receptor, and β-actin (Table 1). Quantifications of mRNA expression were performed using synthesized cDNA via real-time PCR [3] with a LightCycler (Roche Diagnostics, Mannheim, Germany) using a QuantiTect™ SYBR Green PCR Master Mix (Cat. No. 204145, QIAGEN, Hilden, Germany). The primers were designed using Primer3 based on bovine sequences. The primers used for real-time PCR are listed in Table 1. The amplification program consisted of 15 min activation at 95ºC, followed by 40 cycles of PCR (15 sec denaturation at 95ºC, 30 sec annealing at 54–58ºC and 20 sec extension at 72ºC). The values of mRNA expression were assayed by normalization to β-actin as an internal control. The expression of β-actin was stable in all experiments, and no significant difference was detected in the levels of β-actin expression between treatments.
Table 1. Bovine primers used in real-time PCR.
| Gene | Sequence of nucleotide (5'–3')* | Accession No. | |
| EDN-1 | F | CAAATGCATCCTGCCTGGTC | X52740 |
| R | ATTGCCACCCCCATAGAGGA | ||
| EDNAR | F | GCATCCAGTGGAAGAACCAT | X57765 |
| R | AACCAGTCAACCCTTCAACG | ||
| EDNBR | F | GCTCCATCCCACTCAGAAAA | D90456 |
| R | GCCAACACAGAGCAAAGACA | ||
| β-actin | F | CCAAGGCCAACCGTGAGAAAAT | K00622 |
| R | CCACATTCCGTGAGGATCTTCA |
* F, forward; R, reverse.
Scanning electron microscopy (SEM)
Neutrophils (1 × 107 cells/ml) were incubated in culture medium supplemented with EDN-1 (10–9 M) for 2 h, and then phagocytosis was assayed. For SEM, each sample after phagocytosis was put on cover glass coated by 0.1% neoprene in toluene, dried at room temperature and fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (PB, pH 7.4). After fixation, the samples were washed in PB, postfixed in 1% osmium tetroxide in PB and dehydrated in graded series of ethanol. The specimens were then freeze-dried with t-butyl alcohol in a freeze dryer (ES-2030, Hitachi, Tokyo, Japan). Each dried sample was mounted on a specimen stub with cover glass and sputter coated with platinum (Pt) (Ion sputter coater E-1045, Hitachi). The specimens were observed by scanning electron microscope (S3400N, Hitachi) at an accelerating voltage of 5 kV.
Experimental design
Effect of EDN-1 on phagocytic activity for sperm and superoxide generation by PMNs in response sperm: Depending on the local concentrations of EDN-1 detected in the oviduct fluid and BOEC culture, dose-dependent experiments were conducted. PMNs were incubated for 2 h with EDN-1 (10–11, 10–10, 10–9 and 10–8 M) prior to phagocytosis or superoxide production assays.
Effect of receptor antagonists for EDN-1 on phagocytic activity of PMNs for sperm: Mammalian cells possess 2 types of receptors for EDN-1, the so-called type A (ETA) and type B (ETB) receptors. [27]. Therefore, ETA receptor antagonist (LU135252) and ETB receptor antagonist (BQ-788) were used to identify by which receptor EDN-1 interacts with PMNs to produce its physiological responses. PMNs (1 × 107/ml) were incubated for 2 h with EDN-1 (10–9 M) together with an ETA receptor antagonist (LU135252, 10–7 M, Darusentan, Knoll, Ludwigshafen, Germany) or ETB receptor antagonist (BQ-788, 10–7 M, Sigma-Aldrich, Tokyo, Japan). Then, phagocytosis was assayed.
Statistical analysis
Data are presented as the mean ± SEM of 4–5 experiments. Statistical analyses were performed with StatView 5.0 (SAS Institute). Statistical significance between groups was examined by the t test (for 2 groups) or one-way ANOVA followed by multiple comparison test (Fisher’s test for 3 groups, and Bonferroni’s test for more than 3 groups), and all results were considered to be statistically significant at P < 0.05.
Results
EDN-1 concentrations in oviduct fluid and BOEC culture
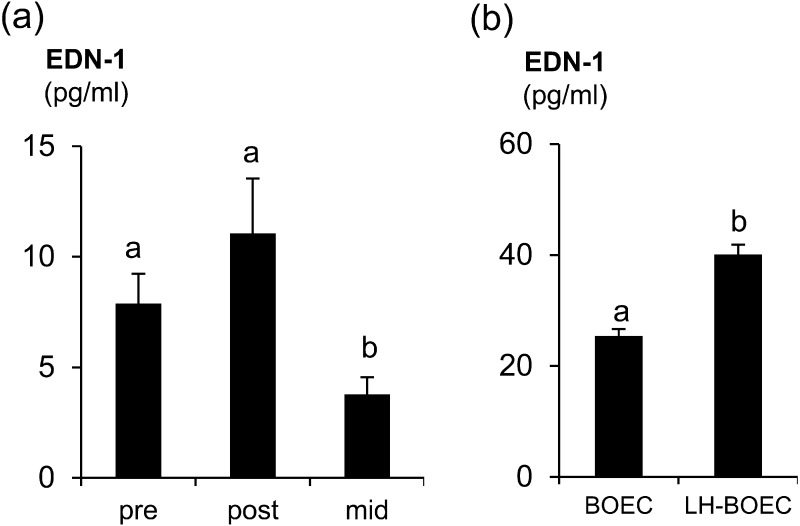
The EDN-1 concentrations in oviduct fluid and BOEC culture were around 10–11 M to 10–10 M (24.9 pg/ml). Additionally, the EDN-1 concentrations during the preovulatory (P < 0.05) and postovulatory (P < 0.04) phases were higher than that during the mid-luteal phase (Fig. 1a). In the BOEC supernatants, LH stimulation significantly (P < 0.05) enhanced EDN-1 secretions by BOECs (Fig. 1b).
Fig. 1.
(a) EDN-1 concentrations (pg/ml) in oviduct fluid during estrous cycle. Pre, preovulatory phase (n = 6); post, postovulatory phase (n = 6); and mid, mid-luteal phase (n = 5). (b) EDN-1 concentrations (pg/ml) in BOEC culture stimulated with 10 ng/ml LH (LH-BOEC) or without any stimulant (BOEC). Numerical values are presented as the mean ± SEM of 4–6 experiments. The letters a and b indicate significant differences between the treatments at P < 0.05 as determined by ANOVA followed by Bonferroni’s multiple comparison test or the t-test.
Effect of EDN-1 on phagocytic activity for sperm and superoxide generation by PMNs in response sperm
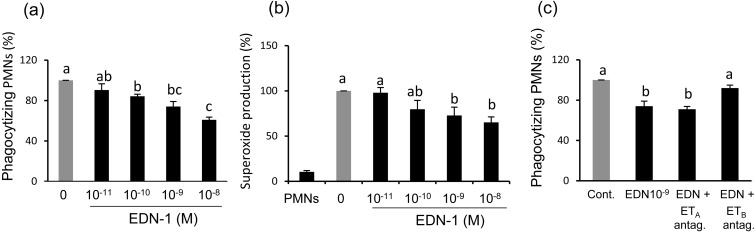
Incubation of PMNs with EDN-1 (10–11, 10–10, 10–9, and 10–8 M) for 2 h prior to phagocytosis or superoxide production assays, resulted in a dose-dependent decrease in phagocytic activity for sperm and superoxide generation by PMNs in response sperm (Fig. 2a and b).
Fig. 2.
(a) Dose-dependent effect EDN-1 on percentage of in vitro phagocytosis for sperm treated to induce capacitation by polymorphonuclear neutrophils (PMNs). PMNs were incubated for 2 h in a culture medium supplemented with different concentrations of EDN-1 (10–11 M = 24.9 pg/ml, 10–10, 10–9 and 10–8 M) before a phagocytosis assay (100% = 48.4 ± 3.5, means ± SEM). (b) Dose-dependent effect of EDN-1 on superoxide generation by PMNs for sperm. PMNs were incubated for 2 h in a culture medium supplemented with different concentrations of EDN-1 (10–11 M = 24.9 pg/ml, 10–10, 10–9 and 10–8 M) before a superoxide assay (100% = 56747.5 ± 5731, means ± SEM). (c) The effect of receptor antagonists for EDN-1 on PMN phagocytosis for sperm treated to induce capacitation in vitro. PMNs were incubated for 2 h with EDN-1 (10–9 M) together with an ETA receptor antagonist (LU135252) (10–7 M) or ETB receptor antagonist (BQ-788) (10–7 M) and then phagocytosis was assayed (100% = 50.2 ± 4.1, means ± SEM). Numerical values are presented as the mean ± SEM of 4–5 experiments. The letters a, b and c, indicate significant differences between the treatments at P < 0.05 as determined by ANOVA followed by Bonferroni’s multiple comparison test.
Effect of receptor antagonists for EDN-1 on phagocytic activity of PMNs for sperm
The results showed that the suppressive effect of EDN-1 on PMNs phagocytosis for sperm was removed (P < 0.05) only when the ETB receptor antagonist (BQ-788) was added to the culture medium (Fig. 2c). This means that EDN-1 exerts its suppressive effect on PMNs phagocytosis for sperm mainly via the ETB receptor.
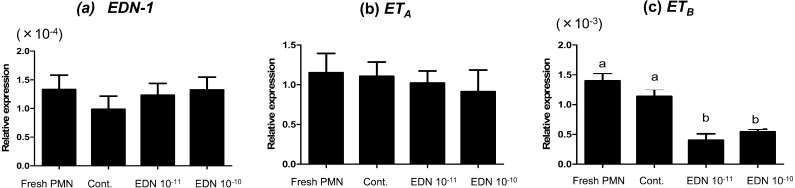
Effect of EDN-1 on mRNA expression in PMNs
Incubation of PMNs with EDN-1 (10–11 and 10–10 M) for 2 h suppressed the mRNA expressions of ETB receptor without any significant effect on the gene expression of ETA or EDN-1 (Fig 3a, b and c).
Fig. 3.
Relative mRNA expression of EDN-1 and the ETA and ETB receptors in the bovine PMNs stimulated with EDN-1 (0, 10–11, and 10–10 M) and harvested after 2 h. Numerical values are presented as the mean ± SEM of 5 experiments. Different letters indicate significant differences between the treatments at P < 0.05 as determined by ANOVA followed by Bonferroni’s multiple comparison test.
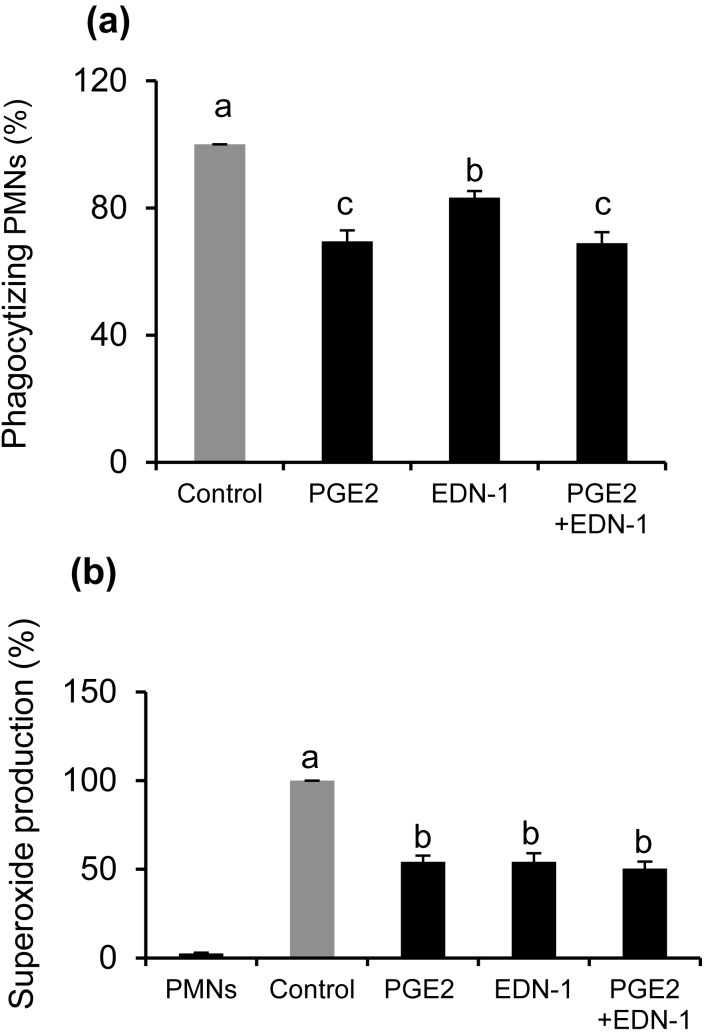
Effect of combinations of EDN-1 and PGE2 on phagocytic activity for sperm and superoxide generation by PMNs in response sperm
Incubation of PMNs for 2 h with both EDN-1 (10–10 M) and PGE2 (10–7 M) at concentrations detected in BOEC culture medium [6] resulted in the suppression of phagocytosis (P < 0.001) without any significant difference compared with PGE2 alone (Fig. 4a). Moreover, the individual suppressive effect of PGE2 on PMN phagocytosis for sperm was significantly stronger than that for EDN-1. Furthermore, EDN-1 and PGE2 together induced suppression of superoxide generation (P < 0.01) by PMNs in response to sperm (Fig 4b).
Fig. 4.
Effect of combinations of EDN-1 and PGE2 on phagocytic activity for sperm and superoxide production by PMNs in response to sperm. PMNs were incubated for 2 h in a culture medium supplemented with EDN-1 (10–10 M), and PGE2 (10–7 M) at concentrations detected in BOEC culture medium and then phagocytosis (a) and superoxide generation (b) were assayed. Numerical values are presented as the mean ± SEM of 4 experiments. Different letters indicate significant differences between the treatments at P < 0.05 as determined by ANOVA followed by Bonferroni’s multiple comparison test.
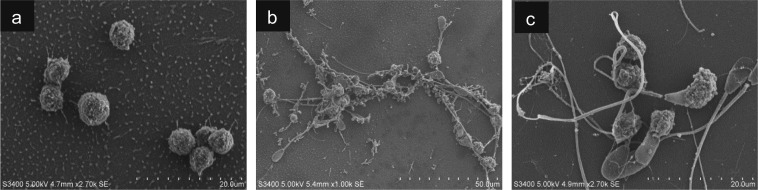
Investigation of neutrophil extracellular trap formation by SEM
Neutrophils either directly phagocytize sperm through cell-cell attachment or entrap them with neutrophil extracellular traps (NETs), and the latter method is mainly detected by SEM [28]. Therefore, SEM was used to investigate the effect of EDN-1 on NET formation by PMNs for sperm entanglement. During the phagocytosis assay, the addition of sperm to PMNs induced NET formation (Fig. 5b) compared with that without sperm addition (Fig. 5a). Incubation of PMNs with EDN-1 (10–9 M) prior to the phagocytosis assay, resulted in the prevention of NET formation by PMNs for sperm entanglement (Fig. 5c).
Fig. 5.
Scanning electron microscopic observation for sperm phagocytosis by polymorphonuclear neutrophils (PMNs). PMNs were incubated with culture medium supplemented with EDN-1 (10–9 M) for 2 h and then subjected to a 1 h phagocytosis assay. In the phagocytosis assay, addition of sperm to PMNs induced neutrophil extracellular trap (NET) formation (b) compared with that without sperm addition (a). Incubation of PMNs with EDN-1 (10–9 M) prior to the phagocytosis assay resulted in complete suppression of NET formation by PMNs for sperm entanglement (c).
Discussion
This study tested the hypothesis that EDN-1 may play a role in regulating sperm survival in the bovine oviduct through suppression of PMNs phagocytosis for sperm. It has been previously shown that EDN-1 is involved in regulating sperm transport inside the bovine oviduct through simulation of the contraction of oviductal smooth muscles [13]. Furthermore, we previously reported that PMNs are present in the bovine oviduct during the preovulatory stage under physiological conditions and that the bovine oviduct acts through its secretions, i.e, PGE2, to protect sperm from the phagocytic activity of these PMNs [6]. Moreover, PGE2 released into a BOEC culture medium partially but not completely suppressed the phagocytic activity of PMNs for sperm via interaction with EP2 receptors [6]. It has been shown that EDN-1 stimulates the release of PGE2 and PGF2α from the oviduct epithelial cells [12]. LH enhances the in vitro secretion of PGE2, PGF2α and EDN-1, as well as the contractile amplitude of bovine oviducts [13]. Therefore, in this study we investigated the effect of EDN-1 on the phagocytic activity of PMNs for sperm.
In the present study, EDN-1 dose-dependently suppressed the phagocytic activity for sperm and superoxide generation by PMNs in response to sperm. The suppressive effect of EDN-1 on PMN phagocytosis for sperm was blocked only after addition of the ETB receptor antagonist. These results suggest that EDN-1 suppresses PMN phagocytosis for sperm mainly via the ETB receptor. In fact, a 2 h exposure of PMNs to EDN-1 induced a downregulation of the mRNA expression of ETB receptor without any effect on the ETA receptor, supporting the hypothesis of an ETB receptor-mediated pathway. The downregulation of mRNA expression of the ETB receptor may be due to the decrease in the stability of mRNA as a result of rapid internalization of ligand-bound complexes after binding of EDN-1 to the ETB receptor [29, 30]. Previously, it has been shown that the effect of EDN-1 is dependent mainly on the type of receptor mediated [10]. In vascular tissue, the ETB receptor is mainly situated on endothelial cells and mediates release of relaxing factors such as prostacyclin and nitric oxide. Meanwhile, the ETA receptor is present on vascular smooth muscle cells and mediates contraction [31].
Previously, it has been shown that EDN-1 impairs respiratory burst and bacterial clearance from the blood and tissue in rabbits [15, 16]. On the other hand, the study by Ishida et al. [32] revealed that EDN-1 by itself was not an effective stimulus for inducing superoxide generation in human neutrophils but that it primed neutrophils for enhanced superoxide production when they were stimulated by the chemotactic peptide FMLP. Also, EDN-1 and EDN-2 induced enhancement of the concentration of cytosolic free Ca2q [33,34,35], and EDN-1 has been reported to stimulate neutrophils migration through activation of protein kinase G [14].
PMNs either directly phagocytize sperm or trap and immobilize them through formation of NETs, webs of DNA normally extruded outside the cell in order to physically fix sperm and restrict their motility until they are phagocytized by another PMN [36]. Recently, we demonstrated that stimulation of PMNs with an LH-stimulated BOEC supernatant prior to phagocytosis prevented the formation of NETs for sperm entanglement [6]. The present results showed that incubation of PMNs with EDN-1 prior to phagocytosis assay completely suppressed the NETs formation. Moreover, our results showed that incubation of PMNs with EDN-1 resulted in decreased superoxide generation by phagocytizing PMNs for sperm. It has been shown that neutrophils exert an oxidative killing mechanism via the non-mitochondrial generation of superoxide anions (O2–) and ROS through NADPH oxidase in a process known as the respiratory burst [37]. On the other hand, NET production is dependent on ROS and H2O2 production via NADPH oxidase [38, 39]. Therefore, we speculate that EDN-1 decreases superoxide production leading to suppression of NETs formation, decreasing the possibility of direct exposure of sperm to PMNs and immobilization of sperm and in turn contributing to protection of sperm from phagocytosis by PMNs.
Additionally, our results showed that the combinations of oviductal concentrations of EDN-1 and PGE2 resulted in suppression of the phagocytic activity for sperm and superoxide generation by PMNs in response to sperm without any additive effect compared with PGE2 only. Therefore, it is suggested that PGE2 and EDN-1 independently exert their suppressive effect on PMN phagocytosis for sperm. Additionally, the suppressive effects of PGE2 on PMN phagocytosis for sperm was significantly stronger than that for EDN-1. Thus, it seems that PGE2 is the main suppressive factor for PMNs phagocytosis in response to sperm, and that EDN-1 independently contributes to suppression of PMNs phagocytosis for sperm. Furthermore, EDN-1 has previously been shown to enhance the oviductal secretions of PGE2 [11, 12]. Thus, these findings suggest that EDN-1 either directly suppresses PMNs phagocytosis for sperm or indirectly suppresses it through stimulation of the oviductal release of PGE2.
Taken together, the results of this study provides novel evidence indicating that EDN-1 may play an immunological role inside the oviduct milieu. Our findings suggest that EDN-1 helps PGE2 action in maintaining sperm survival in the bovine oviduct through downregulation of the phagocytic activity of PMNs for sperm.
Acknowledgments
This study was supported in part by Global COE Program of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Ellington JE. The bovine oviduct and its role in reproduction: a review of the literature. Cornell Vet 1991; 81: 313–328. [PubMed] [Google Scholar]
- 2.Kölle S, Reese S, Kummer W. New aspects of gamete transport, fertilization, and embryonic development in the oviduct gained by means of live cell imaging. Theriogenology 2010; 73: 786–795. [DOI] [PubMed] [Google Scholar]
- 3.Kowsar R, Hambruch N, Liu J, Shimizu T, Pfarrer C, Miyamoto A. Regulation of innate immune function in bovine oviduct epithelial cells in culture: the homeostatic role of epithelial cells in balancing Th1/Th2 response. J Reprod Dev 2013; 59: 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol 2007; 120: 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulek K, Swaidani S, Aronica M, Li X. Epithelium: the interplay between innate and Th2 immunity. Immunol Cell Biol 2010; 88: 257–268. [DOI] [PubMed] [Google Scholar]
- 6.Marey MA, Liu J, Kowsar R, Haneda S, Matsui M, Sasaki M, Takashi S, Hayakawa H, Wijayagunawardane MP, Hussein FM, Miyamoto A. Bovine oviduct epithelial cells downregulate phagocytosis of sperm by neutrophils: prostaglandin E2 as a major physiological regulator. Reproduction 2014; 147: 211–219. [DOI] [PubMed] [Google Scholar]
- 7.Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci USA 1989; 86: 2863–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanagisawa M, Inoue A, Takuwa Y, Mitsui Y, Kobayashi M, Masaki T. The human preproendothelin-1 gene: possible regulation by endothelial phosphoinositide turnover signaling. J Cardiovasc Pharmacol 1989; 13(Suppl 5): S13–S17, discussion :S18. [PubMed] [Google Scholar]
- 9.Kenigsberg D. Endothelin and reproduction? J Clin Endocrinol Metab 1992; 74: 12–13. [DOI] [PubMed] [Google Scholar]
- 10.Masaki T, Ninomiya H, Sakamoto A, Okamoto Y. Structural basis of the function of endothelin receptor. Mol Cell Biochem 1999; 190: 153–156. [PubMed] [Google Scholar]
- 11.Wijayagunawardane MP, Miyamoto A, Sato K. Prostaglandin E2, prostaglandin F2 alpha and endothelin-1 production by cow oviductal epithelial cell monolayers: effect of progesterone, estradiol 17 beta, oxytocin and luteinizing hormone. Theriogenology 1999; 52: 791–801. [DOI] [PubMed] [Google Scholar]
- 12.Wijayagunawardane MP, Miyamoto A, Taquahashi Y, Acosta TJ, Nishimura M, Sato K. Angiotensin II and atrial natriuretic peptide in the cow oviductal contraction in vitro: direct effect and local secretion of prostaglandins, endothelin-1, and angiotensin II. Biol Reprod 2001. a; 65: 799–804. [DOI] [PubMed] [Google Scholar]
- 13.Wijayagunawardane MP, Miyamoto A, Taquahashi Y, Gabler C, Acosta TJ, Nishimura M, Killian G, Sato K. In vitro regulation of local secretion and contraction of the bovine oviduct: stimulation by luteinizing hormone, endothelin-1 and prostaglandins, and inhibition by oxytocin. J Endocrinol 2001. b; 168: 117–130. [DOI] [PubMed] [Google Scholar]
- 14.Elferink JG, De Koster BM. The involvement of protein kinase G in stimulation of neutrophil migration by endothelins. Eur J Pharmacol 1998; 350: 285–291. [DOI] [PubMed] [Google Scholar]
- 15.Schmeck J, Heller A, Phan TL, Urbschek R, Koch T. Effects of endothelin-1 on bacterial clearance in rabbits. Eur J Anaesthesiol 1999; 16: 169–175. [DOI] [PubMed] [Google Scholar]
- 16.Heller A, Schmeck J, Heller S, Phan H, Nebe T, Urbaschek R, Koch T. Endothelin-1 impairs neutrophil respiratory burst and elimination of Escherichia coli in rabbits. Crit Care Med 2000; 28: 1515–1521. [DOI] [PubMed] [Google Scholar]
- 17.Wijayagunawardane MPB, Miyamoto A, Cerbito WA, Acosta TJ, Takagi M, Sato K. Local distributions of oviductal estradiol, progesterone, prostaglandins, oxytocin and endothelin-1 in the cyclic cow. Theriogenology 1998; 49: 607–618. [DOI] [PubMed] [Google Scholar]
- 18.Jiemtaweeboon S, Shirasuna K, Nitta A, Kobayashi A, Schuberth HJ, Shimizu T, Miyamoto A. Evidence that polymorphonuclear neutrophils infiltrate into the developing corpus luteum and promote angiogenesis with interleukin-8 in the cow. Reprod Biol Endocrinol 2011; 9: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parrish JJ, Susko-Parrish J, Winer MA, First NL. Capacitation of bovine sperm by heparin. Biol Reprod 1988; 38: 1171–1180. [DOI] [PubMed] [Google Scholar]
- 20.Parrish JJ, Susko-Parrish JL, Handrow RR, Sims MM, First NL. Capacitation of bovine spermatozoa by oviduct fluid. Biol Reprod 1989; 40: 1020–1025. [DOI] [PubMed] [Google Scholar]
- 21.Kovács A, Foote RH. Viability and acrosome staining of bull, boar and rabbit spermatozoa. Biotech Histochem 1992; 67: 119–124. [DOI] [PubMed] [Google Scholar]
- 22.Bavister BD, Leibfried ML, Lieberman G. Development of preimplantation embryos of the golden hamster in a defined culture medium. Biol Reprod 1983; 28: 235–247. [DOI] [PubMed] [Google Scholar]
- 23.Guthrie HD, Liu J, Critser JK. Osmotic tolerance limits and effects of cryoprotectants on motility of bovine spermatozoa. Biol Reprod 2002; 67: 1811–1816. [DOI] [PubMed] [Google Scholar]
- 24.Matthijs A, Harkema W, Engel B, Woelders H. In vitro phagocytosis of boar spermatozoa by neutrophils from peripheral blood of sows. J Reprod Fertil 2000; 120: 265–273. [PubMed] [Google Scholar]
- 25.Liu J, Marey MA, Kowsar R, Hambruch N, Shimizu T, Haneda S, Matsui M, Sasaki M, Hayakawa H, Pfarrer C, Miyamoto A. An acute-phase protein as a regulator of sperm survival in the bovine oviduct: alpha 1-acid-glycoprotein impairs neutrophil phagocytosis of sperm in vitro. J Reprod Dev 2014; 60: 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987; 162: 156–159. [DOI] [PubMed] [Google Scholar]
- 27.Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature 1990; 348: 732–735. [DOI] [PubMed] [Google Scholar]
- 28.Alghamdi AS, Foster DN. Seminal DNase frees spermatozoa entangled in neutrophil extracellular traps. Biol Reprod 2005; 73: 1174–1181. [DOI] [PubMed] [Google Scholar]
- 29.Resink TJ, Scott-Burden T, Bühler FR. Activation of multiple signal transduction pathways by endothelin in cultured human vascular smooth muscle cells. Eur J Biochem 1990; 189: 415–421. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai T, Morimoto H, Kasuya Y, Takuwa Y, Nakauchi H, Masaki T, Goto K. Level of ETB receptor mRNA is down-regulated by endothelins through decreasing the intracellular stability of mRNA molecules. Biochem Biophys Res Commun 1992; 186: 342–347. [DOI] [PubMed] [Google Scholar]
- 31.Masaki T. Possible role of endothelin in endothelial regulation of vascular tone. Annu Rev Pharmacol Toxicol 1995; 35: 235–255. [DOI] [PubMed] [Google Scholar]
- 32.Ishida K, Takeshige K, Minakami S. Endothelin-1 enhances superoxide generation of human neutrophils stimulated by the chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine. Biochem Biophys Res Commun 1990; 173: 496–500. [DOI] [PubMed] [Google Scholar]
- 33.Lopez Farre A, Riesco A, Moliz M, Egido J, Casado S, Hernando L, Caramelo C. Inhibition by L-arginine of the endothelin-mediated increase in cytosolic calcium in human neutrophils. Biochem Biophys Res Commun 1991; 178: 884–891. [DOI] [PubMed] [Google Scholar]
- 34.Elferink JG, de Koster BM. Endothelin-induced activation of neutrophil migration. Biochem Pharmacol 1994; 48: 865–871. [DOI] [PubMed] [Google Scholar]
- 35.Elferink JGR, de Koster BM. The effect of endothelin-2 (ET-2) on migration and changes in cytosolic free calcium of neutrophils. Naunyn Schmiedebergs Arch Pharmacol 1996; 353: 130–135. [DOI] [PubMed] [Google Scholar]
- 36.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 2004; 303: 1532–1535. [DOI] [PubMed] [Google Scholar]
- 37.Robinson JM. Reactive oxygen species in phagocytic leukocytes. Histochem Cell Biol 2008; 130: 281–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007; 176: 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neeli I, Dwivedi N, Khan S, Radic M. Regulation of extracellular chromatin release from neutrophils. J Innate Immun 2009; 1: 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]