Abstract
The endoplasmic reticulum (ER) stress response has been implicated in the development, atresia and luteinization of ovarian follicles. However, there have been few reports concerning the role of Herp, an ER stress-induced protein, in follicular development. The present study aims to detect the distribution and cyclic variations of Herp during the estrous cycle and to reveal the roles of Herp in regulating the cell cycle, apoptosis and steroid hormone biosynthesis in mouse granulosa cells. In this study, immunohistochemistry staining showed that Herp expression was primarily in the granulosa cells and oocytes. Furthermore, we constructed recombinant lentiviral vectors for Herp short hairpin interfering RNA (shRNA) expression; immunofluorescence staining, real-time quantitative PCR (RT-qPCR) and western blot analysis revealed that Herp was successfully knocked down. Flow cytometry showed that knockdown of Herp arrested granulosa cells at the S phase of the cell cycle. More importantly, ELISA analysis revealed that Herp knockdown significantly upregulated the concentration of estradiol (E2) in the culture supernatants. RT-qPCR was performed to determine the regulatory mechanism of Herp knockdown in the cell cycle, and in steroid synthesis, RT-qPCR analysis revealed that Herp knockdown upregulated the mRNA expression of steroidogenic enzymes (Cyp19a1) and downregulated metabolic enzymes (Cyp1b1) and cell cycle factors (cyclin A1, cyclin B1 and cyclin D2). These results suggest that Herp may regulate the cell cycle and hormone secretions in mouse granulosa cells. The present study helps to elucidate the physiological functions of Herp as they relate to reproduction.
Keywords: Cell cycle, Granulosa cells, Herp, Short hairpin interfering RNA, Steroidogenesis
In mammals, the ovary is an extremely dynamic organ that includes follicles in various stages of development. Ovarian follicles develop and progress through the primordial, primary, secondary and antral stages [1]. However, only very few follicles reach the ovulatory stage and subsequently form a corpus luteum (CL), with most undergoing atresia [2]. During folliculogenesis, gonadotropins, such as follicle-stimulating hormone (FSH) and luteinizing hormone (LH), play an important role. The pituitary surge of LH/FSH has been thoroughly demonstrated to initiate a complex series of cellular and molecular events in periovulatory follicles leading to the resumption of oocyte meiosis, breakdown of the follicle wall and oocyte release, followed by the subsequent luteinization of the postovulatory follicle [2]. As found in numerous studies, not only endocrine but also paracrine and autocrine factors, which include gonadal steroids, growth factors, cytokines and intracellular proteins, play important roles in ovarian follicular development [3]; for example, an increasing body of evidence indicates that members of the mammalian endoplasmic reticulum (ER) stress response-related molecular chaperones regulate key genes that are crucial for follicular development, atresia and luteinization [4,5,6,7]. To date, the exact molecular mechanisms and interactions between the ER stress response and follicular development remain unclear.
The ER not only plays an important role in protein and lipid biogenesis, folding and trafficking but is also involved in the maintenance of Ca2+ homeostasis [8]. The ER serves to control the quality of proteins by facilitating protein folding and recognizing and retaining misfolded proteins [9, 10]. In granulosa cells, the ER can regulate expression of the luteinizing hormone receptor (LHR) [11]. However, ER functional overload triggers a protective mechanism known as the ER stress response [12]. Previous studies have demonstrated that the ER stress response regulates the development, maintenance and regression of the corpus luteum [13, 14]. The ER stress response reduces steroidogenic enzyme expression by modulating the ATF6 pathway in hCG-stimulated Leydig cells [15]. Our previous studies demonstrated that the ER stress response is involved in granulosa cell apoptosis during follicular atresia in goat and mouse ovaries [4, 5]. The homocysteine-responsive ER-resident protein (Herp) localized in the ER membrane is induced in the ER stress response [16, 17]. Herp degrades unfolded and misfolded proteins in the ER via the ER-associated protein degradation (ERAD) pathway [18,19,20], and it stabilizes ER Ca2+ homeostasis and maintains mitochondrial function in neuronal cells [21]. Herp knockout is more susceptible to ER stress-induced apoptosis in muscle cells [22], and Herp is ubiquitously expressed in various organs, such as the heart, liver, skeletal muscle, kidneys and pancreas [16]. These characteristics suggest that Herp may play important roles in these organs. Previous studies also showed that Herp may be implicated in the pathogenesis of type 2 diabetes, neurodegeneration and sarcopenia [22,23,24,25,26].
Although Herp is a stress-response protein, there is evidence showing that it has varying roles in different tissues. Although ubiquitously expressed in various organs, we do not know whether Herp is expressed in the ovary, and the role of this gene in follicular development remains to be elucidated. In the present study, we aimed to detect the distribution and cyclic variations of Herp in the development of the mouse ovary in vivo and further reveal the roles of Herp in the regulation of the cell cycle, apoptosis and steroid hormone biosynthesis in mouse granulosa cells in vitro.
Materials and Methods
Ovary collection
Female Kunming White outbred strain mice were obtained from the Laboratory Animal Center of the Fourth Military Medical University. The mice were housed under a 14 h light and 10 h dark cycle at a room temperature of 23 ± 2ºC. The mice were provided food and water ad libitum. All procedures were approved by the Committee for the Ethics on Animal Care and Experiments of Northwest A&F University.
Mature female mice (8 weeks old) were used in determining the stages of the estrous cycle by collecting vaginal smears and observing the pudendum [27]. Vaginal smears were collected on glass slides daily (0900 h). Only mice exhibiting regular 4- or 5-day normal estrous cycles (n = 10, at least three consecutive cycles) were used for the subsequent experiments. Ovaries were collected and quickly embedded in OCT compound (Sakura Finetek, Torrance, CA, USA) for immunohistochemical studies or stored at –80ºC until use in western blot analysis [28]. Pregnant mare serum gonadotropin (PMSG) were used to synchronize the estrus cycle in immature 21-day-old female mice. Each mouse was intraperitoneally injected with 5 IU PMSG to stimulate follicular growth. After 48 h, the ovaries were used for culturing granulosa cells in vitro.
Immunohistochemistry
Immunohistochemistry was used to localize Herp protein in the ovarian tissues of the mice. The ovaries were fixed in 4% paraformaldehyde in PBS (pH = 7.4) for 24 h, dehydrated through a graded ethanol series and embedded in paraffin. Five-millimeter-thick sections were mounted onto glass slides precoated with Poly-L-Lysine Solution (Sigma, St. Louis, MO, USA) and incubated overnight at 37ºC. After dehydrating, the samples were placed in citrate buffer (pH = 6.0). Antigen retrieval was performed by treating samples in a microwave oven at 92ºC for 15 min; slides were then cooled and then washed in PBS. The sections were pretreated with 3% (vol/vol) H2O2 in methanol to quench endogenous peroxidase activity. After washing with PBS, the sections were incubated with 10% goat serum for 30 min at 37ºC. After blocking, the sections were incubated overnight at 4ºC with rabbit polyclonal antibody against Herp (Santa Cruz, sc-98669; 1:50 dilutions) in a humidified chamber. After washing followed by incubation with biotinylated anti-rabbit IgG antibody (Beijing 4A Biotech, Beijing, China) at 37ºC for 1 h, the sections were incubated with HRP-labeled streptavidin (SA-HRP) at 37ºC for 30 min. Thereafter, positive reactions were visualized with a diaminobenzidine (DAB) (Sigma, St. Louis, MO, USA)-peroxidase substrate and 30 sec counterstaining with hematoxylin. Finally, the sections were counterstained, dehydrated and mounted. Negative control slides were incubated with preimmune serum instead of the primary antibody. The slides were imaged using a digital microscope (BA400, Motic, Wetzlar, Germany).
Primary mouse granulosa cells culture and Herp short hairpin interfering RNA (shRNA) lentivirus transduction
After superovulation, the ovaries were excised, and the granulosa cells were released by puncturing the follicles with 26-gage needles. The granulosa cells were washed and collected via brief centrifugation, and cell viability was determined via trypan blue exclusion. The cells were divided into 24-well culture plates (1 × 105 per well) and cultured in DMEM/F-12 medium (1:1, HyClone) supplemented with 10% fetal bovine serum (FBS) (Life Technologies), 100 units/ml penicillin and 100 μg/ml streptomycin solutions at 37ºC under a 5% CO2 atmosphere.
The U6 RNAi cassette fragment from pSilencer 2.1-U6 hygro (Cat. No. AM5760, Life Technologies, Carlsbad, CA, USA) was amplified and cloned into pCD513B-1 (SBI, Mountain View, CA, USA), which contains a GFP expression construct, to generate a pCD513B-U6 lentiviral vector [29]. Lentivirus vectors encoding the Herp shRNA (shHerp) and non-silencing negative control (shNC) were constructed by our group. The sequence of the shNC was 5′-GATCCGATGAAATGGGTAAGTACATTCAAGAGATGTACTTACCCATTTCATCTTTTTTG-3′. The sequence of the shHerp was 5′-GATCCGAGCAGCCGGACAACTCTAATCTCGAGATTAGAGTTGTCCGGCTGCTCTTTTTG-3′. The recombinant lentivirus vector was packaged and transduced into HEK 293T cells. The medium was harvested 48 h after transfection, purified via low-speed centrifugation, and filtered through a 0.45-µm PVDF filter. The viral titers (IU/ml) were calculated according to the following formula: number of GFP-positive cells × dilution multiple/the amount of virus solution (ml). An appropriate number of lentiviral particles (MOI = 20) were transduced into primary granulosa cells using 8 µg/ml polybrene. After 12 h of incubation, the medium containing the virus was removed and replaced with fresh culture medium. The cells were harvested after an additional 48 h.
RNA extraction and real-time quantitative PCR analysis
Total RNA was extracted from frozen ovaries and granulosa cells using TRIzol (TaKaRa, Dalian, China) according to the manufacturer’s instructions. The cDNAs were synthesized using a PrimeScriptTM RT Reagent Kit (TaKaRa). Real-time quantitative PCR (RT-qPCR) was performed using a Bio-Rad iQ5 and the Bio-Rad iQ5 Optical System Software (Bio-Rad Laboratories, Hercules, CA, USA) along with a the SYBR Premix Ex Taq II Kit (TaKaRa) according to the manufacturer’s protocol. The sequences of the specific primers used are listed in Table 1. These reactions were repeated three times for each sample as technical replicates. Gene mRNA quantifications were performed using the 2–∆∆Ct method, and the amount of transcript in each sample was normalized using β-actin as the internal control gene to correct for differences in the cDNA used.
Table 1. Primer sequences used for real-time quantitative PCR (RT-qPCR).
| Gene | GenBank Accession | Forward (5′–3′) | Reverse (5′–3′) | Product (bp) |
| β-actin | NM_007393 | GCAAGCAGGAGTACGATGAG | CCATGCCAATGTT GTCTCTT | 148 |
| Herp | NM_022331.1 | GCAGTTGGAGTGTGAGTCG | TCTGTGGATTCAGCACCCTTT | 229 |
| Cyplbl | NM_009994.1 | CACTATTACGGACATCTTCGG | AGGTTGGGCTGGTCACTC | 168 |
| Star | NM_011485.4 | CTTGGCTGCTCAGTATTGAC | TGGTGGACAGTCCTTAACAC | 153 |
| Cyp11a1 | NM_019779.3 | CGATACTCTTCTCATGCGAG | CTTTCTTCCAGGCATCTGAAC | 126 |
| Cyp19a1 | NM_007810.3 | GACACATCATGCTGGACACC | CAAGTCCTTGACGGATCGTT | 179 |
| Ptgs2 | NM_011198.3 | CTCTATCACTGGCACCCCCTG | GAAGCGTTTGCGGTACTCATT | 261 |
| Has2 | NM_008216.3 | ACCCTGCCTCATCTGTGGAGA | TGTTGGTAAGGTGCCTGTCGT | 306 |
| Cyclin A1 | Z26580.1 | GCCTTCACCATTCATGTGGAT | TTGCTGCGGGTAAAGAGACAG | 118 |
| Cyclin B1 | NM_172301.3 | AAGGTGCCTGTGTGTGAACC | GTCAGCCCCATCATCTGCG | 228 |
| Cyclin D2 | NM_009829.3 | ACACCGACAACTCTGTGAAGC | GCCAGGTTCCACTTCAGCTTA | 79 |
| p53 | AB020317.1 | TACAAGAAGTCACAGCACAT | GATAGGTCGGCGGTTCAT | 267 |
| Bcl-2 | NM_009741.4 | CGAGAAGAAGAGGGAATCACAGG | AATCCGTAGGAATCCCAACC | 133 |
| Bax | NM_007527.3 | AGGATGCGTCCACCAAGAA | CAAAGTAGAAGAGGGCAACCAC | 195 |
| Caspase-3 | NM_001284409.1 | TGACTGGAAAGCCGAAACTC | GCAAGCCATCTCCTCATCAG | 101 |
Immunofluorescent staining
After transduction with the shHerp lentivirus for 48 h, granulosa cells were first fixed in 4% paraformaldehyde for 20 min, permeabilized with 0.1% Triton X-100 in PBS for 20 min, blocked with 5% BSA in PBS for 1 h at room temperature and then co-incubated with anti-Herp antibody (Santa Cruz, sc-98669; 1:50 dilutions) overnight at 4ºC. After washing followed by incubation with anti-rabbit secondary antibody (Invitrogen, A31572; 1:500 dilutions) for 1 h at 37ºC, the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) for 5 min. The fluorescent signals were examined under a Nikon epifluorescence microscope (Eclipse 80i; Nikon, Tokyo, Japan).
Western blot analysis
Frozen ovaries and granulosa cells were lysed with RIPA lysis buffer (Nanjing KeyGen Biotech, Nanjing, China). The protein concentration was determined using a BCA Protein Assay Kit (Nanjing KeyGen Biotech). Equal total proteins were separated via 12% SDS-PAGE gel and electrotransferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were then blocked with 10% fatty acid-free milk in TBST for 1 h at room temperature and incubated overnight at 4ºC in blocking solution containing rabbit polyclonal antibody against Herp (Santa Cruz, 1:100 dilutions) and mouse monoclonal antibody against β-actin (Tianjin Sanjian Biotech, Tianjin, China; 1:1000 dilutions). The following day, the membranes were incubated with a secondary antibody conjugated to horseradish peroxidase (Zhongshan Golden Bridge Biotechnology, Nanjing, China; 1:5000 dilutions) at room temperature for 1 h. Finally, immunoreactive bands were visualized using a Gel Imaging System (Tannon Science & Technology, Shanghai, China) and then digitized with the Quantity One software (Bio-Rad Laboratories).
ELISA for measurements of steroid hormones
After transduction with the shHerp lentivirus for 48 h, the cell numbers were counted, and the concentrations of estradiol (E2) and progesterone (P4) in the culture supernatants were measured with ELISA kits (Beijing North Institute of Biological Technology, Beijing, China) according to the manufacturer’s instructions. The sensitivity and inter- and intra-assay CVs of the E2 and P4 ELISA kits are as follows: 25 pg/ml, < 15% and < 10% for E2 and 0.2 ng/ml, < 15% and < 10% for P4, respectively.
Cell cycle analysis
After transduction with the shHerp lentivirus for 48 h, cultured granulosa cells were washed twice with PBS, trypsinized, harvested via centrifugation at 1000 rpm for 3 min and then suspended in PBS. The cells were then centrifuged at 1000 rpm for 3 min and fixed in 70% ice-cold ethanol overnight at 4ºC. The fixed cells were washed again in PBS and stained using propidium iodide (PI)/RNase A solution at 37ºC in a dark chamber for 30 min. Detection was performed via flow cytometry (EPICS Altra, Beckman Coulter, Brea, CA, USA). For each determination, a minimum of 2 × 104 cells was analyzed. All experiments were independently repeated five times.
Apoptosis analysis
After transduction, apoptotic cells were quantified with an Annexin V-PE and PI apoptosis detection kit (Nanjing KeyGen Biotech). The cells were washed with PBS, trypsinized and harvested via centrifugation at 1000 rpm for 3 min. The cells were resuspended in 50 μl binding buffer, 5 μl PI was added, and the mixture was incubated. Then, 450 μl binding buffer was added, followed by the addition of 1 μl Annexin V-PE; the mixture was then incubated for 15 min. Apoptosis was detected using flow cytometry (EPICS Altra, Beckman Coulter, Brea, CA, USA) within 1 h. The number of early apoptotic cells was determined by counting the percentage of annexin V-PE+/PI– cells. Late apoptotic cells were obtained by counting the percentage of annexin V-PE+/PI+ cells. Annexin V-PE–/PI– cells were considered to be surviving cells. The experiments were independently repeated three times.
Statistical analyses
The experimental results are presented as the means ± SEM of triplicate experiments. Data were analyzed with one-way ANOVA, followed by Fisher’s least significant different test (Fisher LSD) and an Independent-Samples T test with the SPSS (Statistical Package for the Social Sciences) software (Version 13.0; SPSS, Chicago, IL, USA). Differences were considered significant when P < 0.05.
Results
Immunohistochemical localization of Herp protein in the estrous ovary
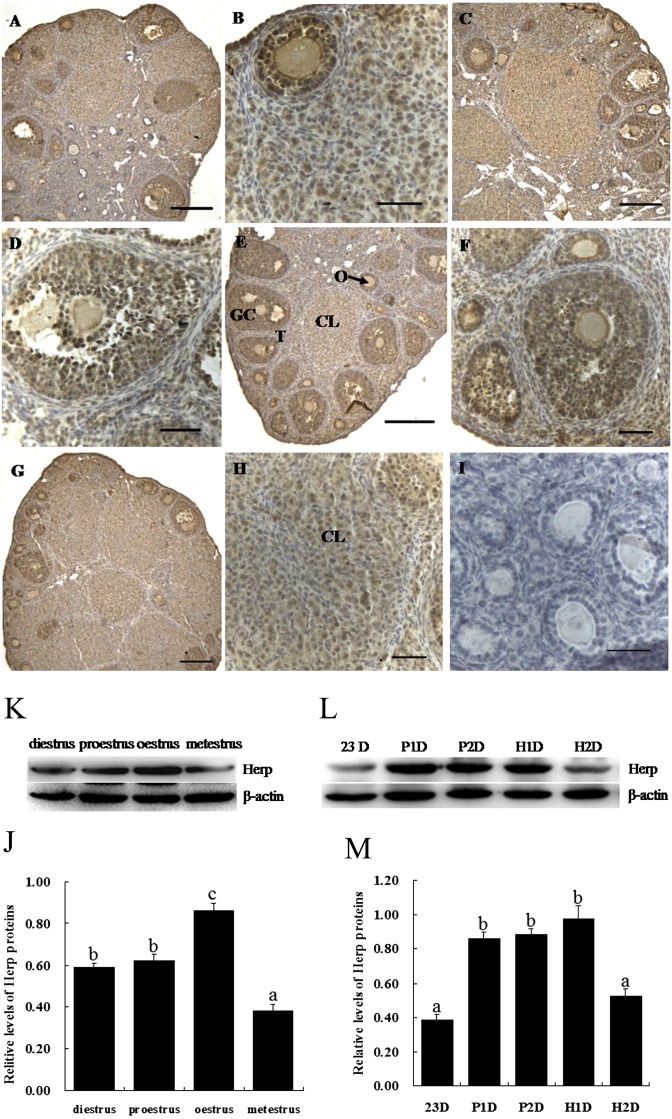
To elucidate whether Herp was expressed in ovaries during the estrous cycle, we collected ovaries during four phases of the estrous cycle. We found that the expression of Herp was ubiquitous throughout the ovary during the estrous cycle. High expression of Herp protein was found in the oocytes and granulosa cells at various stages of the estrous cycle (Fig. 1A–H). Herp immunoreactivity was stronger in the perinuclear space compared with oocyte nuclei (Fig. 1B, D and F). In follicles at different stages of follicular development, the expression of Herp was high in the granulosa cells; however, the corpus luteum exhibited a low Herp expression. The western blot results showed that the expression of Herp was higher at estrous and lower at metestrous during the estrous cycle (P < 0.05, Fig. 1I and J). After PMSG and hCG treatment, the levels of Herp in the ovary were higher after treatment with PMSG for 1 day and 2 day and treatment with hCG for 1 day than 23 day and treatment with hCG for 2 day (P < 0.05, Fig. 1K and L).
Fig. 1.
Localization and expression of Herp protein in mouse ovaries during the estrous cycle. A and B: diestrus, C and D: proestrus, E and F: oestrus, G and H: metestrus. I: negative control. Positive immunostaining for Herp is indicated by a brown reaction product. GC, granulosa cells; O, oocyte; T, theca cells; CL, corpus luteum. Scale bars, 50 μm (A, C, E, G and I) and 100 μm (B, D, F, and H). J and K: The protein level of Herp in the mouse ovary during the estrous cycle. L and M: The protein level of Herp in the mouse ovary after treatment with PMSG or hCG. P1D and P2D, treated with PMSG for 1 day and treated with PMSG for 2 day, respectively; H1D and H2D, treated with hCG for 1 day after treatment with PMSG for 2 day and treated with hCG for 2 day after treatment with PMSG for 2 day, respectively. Analyses of band intensity on films are presented as the relative ratio of Herp to β-actin. Statistical analysis is shown in the bar graphs. Data are presented as the mean ± SEM. Bars with different letters are significantly different (P < 0.05).
Effect of Herp knockdown on the expression of Herp in primary mouse granulosa cells
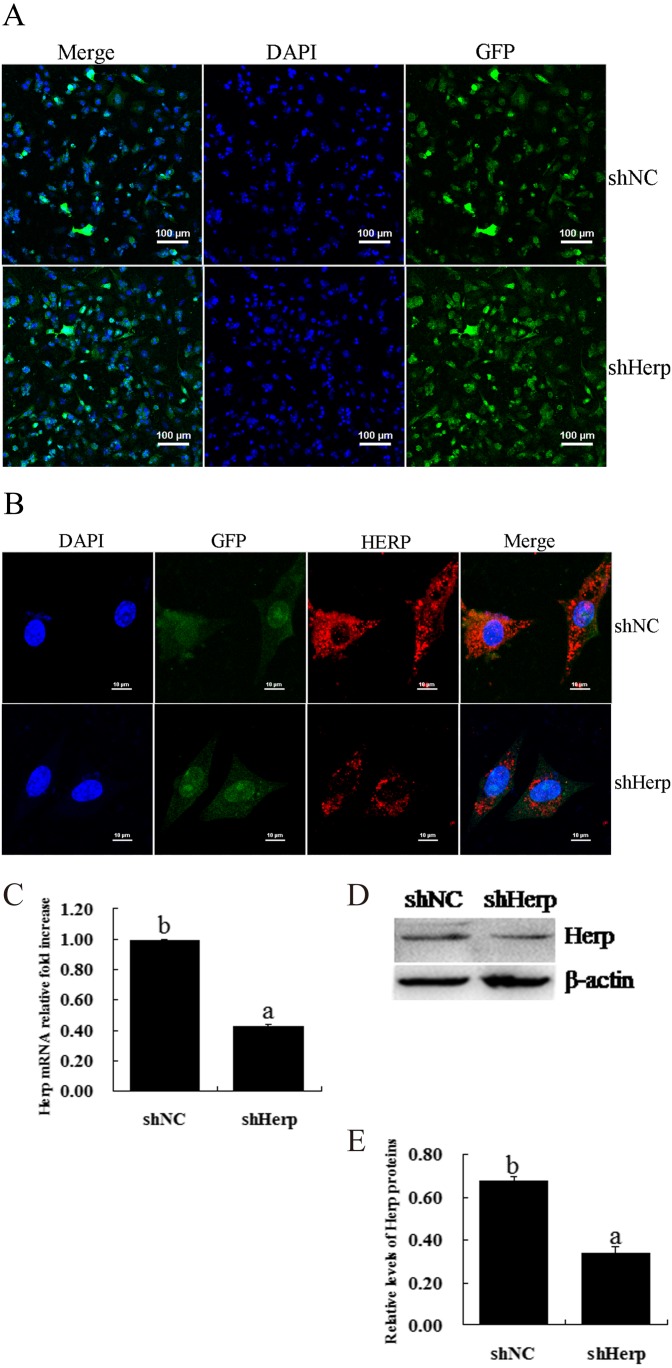
To identify the effect of shHerp, granulosa cells were transduced with the pCD513B-U6-shHerp lentivirus (Fig. 2A). The transduction efficiency was more than 80% (the results are not shown). Immunofluorescence staining showed that Herp was primarily located around the nucleus and that Herp knockdown markedly reduced the expression of Herp in mouse granulosa cells (Fig. 2B). The results of RT-qPCR showed that shHerp significantly decreased expression compared with the control group and downregulated the expression of the Herp gene by more than 60% (P < 0.05, Fig. 2C). Western blot showed that shHerp prominently downregulated the expression of Herp protein (P < 0.05, Fig. 2D and E).
Fig. 2.
Effective inhibition of Herp expression in primary mouse granulosa cells via transduction of the shHerp lentiviruses. A: Fluorescence images of granulosa cells transduced by lentivirus for 48 h. Scale bars, 100 μm. B: Immunofluorescent analysis of Herp protein expression levels in granulosa cells transduced with the shHerp lentivirus for 48 h. C: Relative mRNA expression of the Herp gene in granulosa cells transduced with the shHerp lentivirus for 48 h. The amounts of mRNA were normalized to that of β-actin. D and E: Western blot analysis of Herp protein expression levels in granulosa cells transduced with shHerp lentiviruses for 48 h. The statistical analysis is shown in the bar graphs. Data are presented as the mean ± SEM. Bars with different letters are significantly different (P < 0.05).
Effect of Herp knockdown on E2 and P4 production in granulosa cells
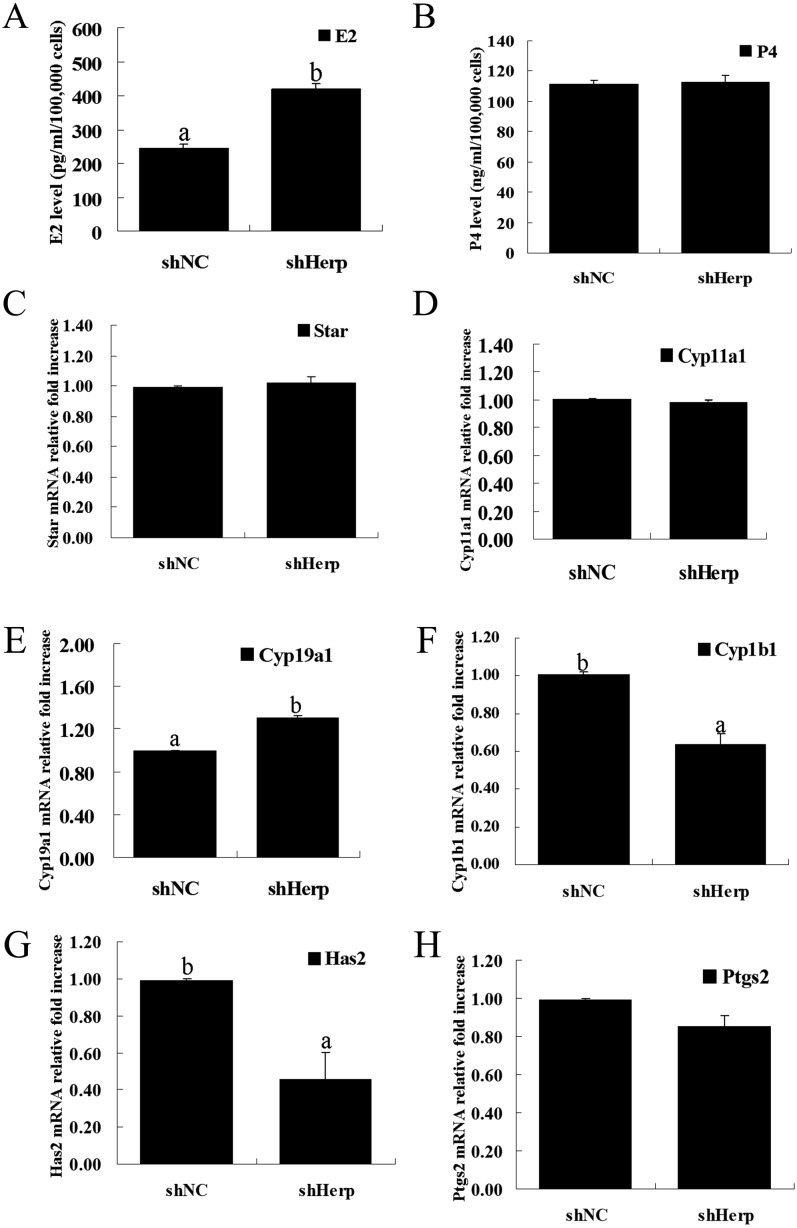
To assess the effect of Herp knockdown on steroid hormones, we measured the concentration of E2 and P4 in the culture medium at 48 h post transduction. After transduction, the concentration of E2 in the granulosa cell culture medium was significantly increased in the shHerp group compared with the shNC group (P < 0.05, Fig. 3A). However, the concentration of P4 showed no significant differences between the shHerp and shNC groups (Fig. 3B).
Fig. 3.
Effect of Herp knockdown on the secretion of E2 and P4 in primary mouse granulosa cells caused by transduction with the shHerp lentivirus. A and B: Concentration of E2 and P4 in the culture medium of granulosa cells transduced with the shHerp lentivirus for 48 h. C–H: Relative mRNA expression of genes (Star, Cyp11a1, Cyp19a1, Cyp1b1, Has2 and Ptgs2) in granulosa cells transduced with the shHerp lentiviruses for 48 h. The amounts of mRNA were normalized to that of β-actin. The statistical analysis is shown in the bar graphs. Data are presented as the means ± SEM. Bars with different letters are significantly different (P < 0.05).
To further confirm the higher release of E2 via Herp knockdown, we analyzed the mRNA expression of steroidogenic enzymes (Cyp11a1, Star and Cyp19a1) and metabolic enzymes (Cyp1b1). RT-qPCR showed that Herp knockdown did not affect the mRNA expression of Cyp11a1 and Star (Fig. 3C and D), which are important for P4 synthesis as rate-limiting enzymes. Herp knockdown significantly increased the mRNA expression of Cyp19a1 (P < 0.05, Fig. 3E), which is important for E2 synthesis. However, Herp knockdown significantly decreased the mRNA expression of Cyp1b1 (P < 0.05, Fig. 3F), which is important for E2 metabolism. Furthermore, Herp knockdown significantly increased the mRNA expression of Has2 (P < 0.05, Fig. 3G) but had no effect on Ptgs2 (Fig. 3H), which are important for the cumulus expansion and luteinization of primary granulosa cells.
Effect of Herp knockdown on the cell cycle of granulosa cells
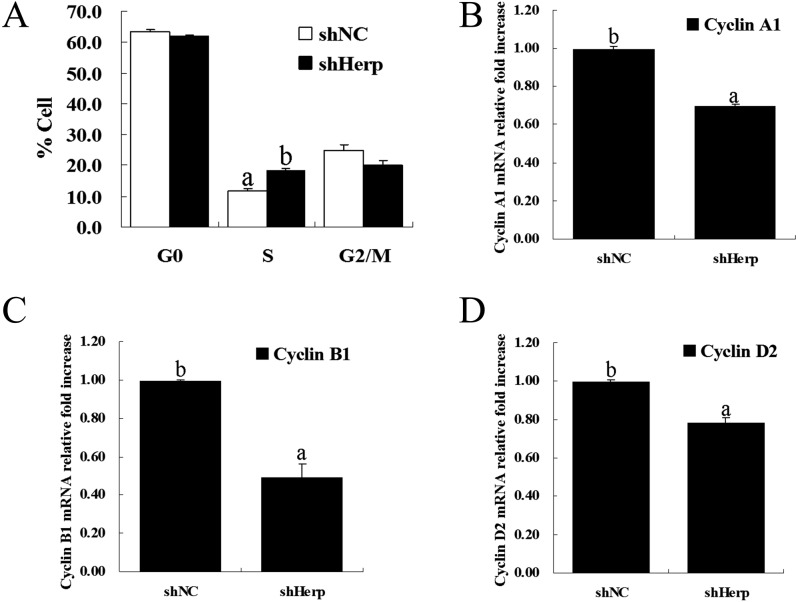
To determine whether Herp is involved in the regulation of cell cycle progression, we measured the cell cycle progression of transduced granulosa cells via flow cytometry following staining with PI. A significant number of GCs were arrested at the S phase in the shHerp group compared with the shNC group (P < 0.05, Fig. 4A and Supplementary Fig. 1: on-line only).
Fig. 4.
Effects of Herp knockdown on the cell cycle. A: Analysis of the cell cycle via flow cytometry in granulosa cells tranduced with the shHerp lentivirus for 48 h. B–D: Relative mRNA expression of cell cycle-related genes (cyclin A1, cyclin B1 and cyclin D2) in granulosa cells transduced with the shHerp lentivirus for 48 h. The amounts of mRNA were normalized to that of β-actin. The statistical analysis is shown in the bar graphs. Data are presented as the mean ± SEM. Bars with different letters are significantly different (P < 0.05).
Additionally, to further confirm the results of the cell cycle analysis, the mRNA expression of cell cycle factors (cyclin A1, cyclin B1 and cyclin D2) was determined using RT-qPCR. The results showed that Herp knockdown significantly decreased the mRNA expression of cyclin A1, cyclin B1 and cyclin D2 (P < 0.05, Fig. 4B and D).
Effect of Herp knockdown on granulosa cells apoptosis
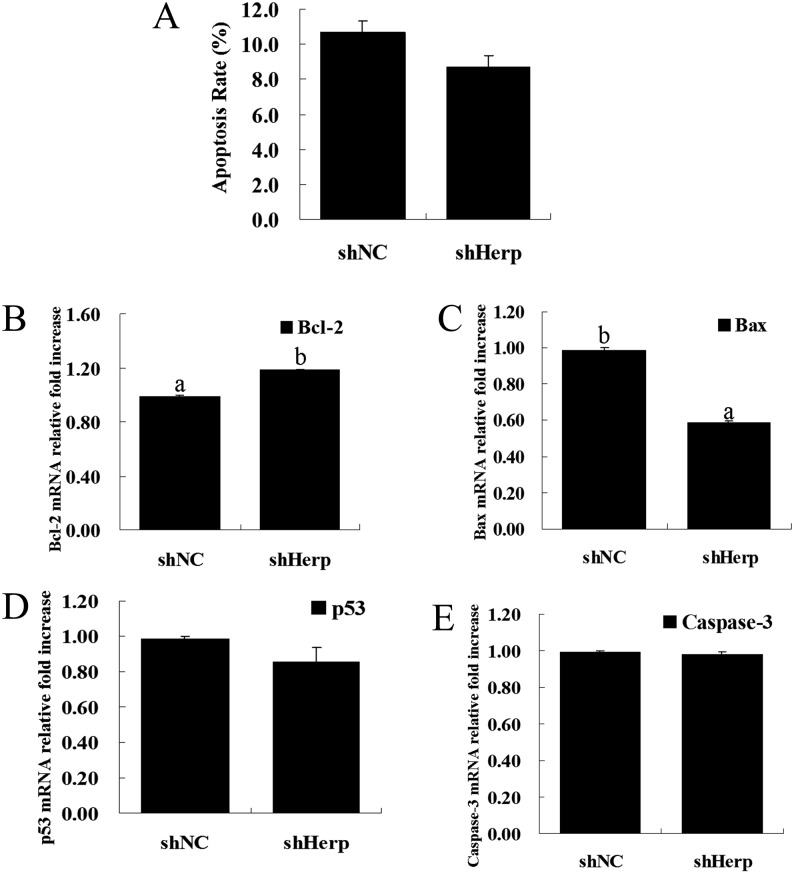
To elucidate the roles of Herp in the regulation of granulosa cell apoptosis, we determined the apoptotic rate of transduced granulosa cells with Annexin V-PE/PI double staining using flow cytometry. Herp knockdown did not alter apoptosis in the shHerp group compared with the shNC group (Fig. 5A and Supplementary Fig. 2: on-line only).
Fig. 5.
Effects of Herp knockdown on cell apoptosis. A: Measurement of cell apoptosis via flow cytometry in granulosa cells tranduced with the shHerp lentivirus for 48 h. B–E: Relative mRNA expression of cell apoptosis-related genes (Bcl-2, Bax, p53 and Caspase-3) in granulosa cells transduced with the shHerp lentivirus for 48 h. The amounts of mRNA were normalized to that of β-actin. The statistical analysis is shown in the bar graphs. Data are presented as the mean ± SEM. Bars with different letters are significantly different (P < 0.05).
To further demonstrate the effects of Herp knockdown on cell apoptosis, we measured the mRNA expression of p53, Bcl-2, Bax and Caspase-3 in transduced granulosa cells. RT-qPCR showed that Herp knockdown significantly increased the mRNA expression of Bcl-2 (P < 0.05, Fig. 5B) and significantly decreased the mRNA expression of Bax (P < 0.05, Fig. 5C). There were no significant differences in mRNA expression of p53 and Caspase-3 (Fig. 5D and E).
Discussion
Previous studies have reported that Herp, which is localized in the ER, is ubiquitously expressed in various organs, such as the heart, liver, skeletal muscle, kidneys and pancreas [16]. Herp degrades unfolded and misfolded proteins in the ER, stabilizes ER Ca2+ homeostasis and maintains mitochondrial function in neuronal cells [18, 19, 21]. In the present study, we attempted to determine for the first time the in vivo localization of Herp in mouse ovaries during the estrous cycle (Fig. 1). The expression of Herp was primarily in the oocytes and granulosa cells in various stages of follicular development. The higher expression of Herp at the estrous stages suggested its involvement in ovulation (Fig. 1J and K). Significantly increased induction of Herp expression by PMSG and hCG also indicated that Herp expression may be affected by ovulation and hormonal regulation (Fig. 1L and M). The in vitro results showed that Herp was involved in cell cycle control, steroid synthesis and regulation of the expression of related genes in mouse granulosa cells, indicating that Herp may play an important role in regulating the function of granulosa cells and may further influence follicular development.
Follicular development and ovulation are primarily dependent on the proliferation and luteinization of granulosa cells, which synthesize steroid hormones (such as E2 and P4) that have important roles [30,31,32]. Granulosa cells secrete sex steroid, as well as a series of growth factors involved in interactions with oocytes and theca cells during folliculogenesis. In the present study, we observed that knockdown of Herp significantly increased E2 production without affecting the P4 concentration in the primary cultured granulosa cells compared with the control group after transduction with the shHerp lentivirus for 48 h. Higher level of E2 level may have been due to an increase in the expression of Cyp19a1 (Fig. 3E), which is the rate-limiting enzyme in controlling androgen aromatization to estrogen [33]; higher E2 may also have been due to a decrease in the mRNA of Cyp1b1 (Fig. 3F), which is a crucial enzyme in regulating estrogen metabolism [34]. We also detected Has2 and Ptgs2, which are important for cumulus expansion and luteinization [35, 36]. We found that the knockdown of Herp could decrease the expression of Has2 mRNA (Fig. 3H), implying that Herp has a positive effect on Has2 regulation. We infer that Herp may be involved in folliculogenesis, cumulus expansion and ovulation through regulation of the expression of these genes in the mouse ovary.
Folliculogenesis includes follicular growth and atresia. This process is involved in numerous ovarian factors that regulate cell proliferation, differentiation and apoptosis. Follicular atresia may be initiated by the apoptosis of granulosa cells. However, we do not know whether Herp regulates the cell cycle and apoptosis of granulosa cells. In the present study, we detected various phases of the cell cycle of granulosa cells after Herp knockdown. The S phase of the shHerp group was significantly arrested compared with that in the shNC group (18.18 ± 1.02% and 11.55 ± 0.91%, respectively) (Fig. 4A). We speculated that Herp may play an important role in regulating granulosa cell proliferation by affecting cell cycle progression to further modulate ovarian development. We further detected the expression of genes related to the cell cycle. Knockdown of Herp decreased the mRNA level of cyclin A1, cyclin B1 and cyclin D2 (Fig. 4B–D). Cyclin A1 is a key regulator of cell cycle progression from the S phase to the G2/M phase; this knockdown therefore resulted in S phase arrest [37]. The physiological function of cyclin D2 is to promote the G1 to S phase transition in a coordinated manner together with its antagonist, p27Kip1 [38]. Similarly, cyclin B1 is involved in normal cell cycle progression, and cyclin B1-Cdk1 is involved in mitotic exit and the start of a new cell division [39]. These results of cell cycle analysis and the expression of cyclin A1, cyclin B1 and cyclin D2 indicated that Herp may promote normal granulosa cell growth and proliferation.
Previous studies demonstrated that Herp depletion prevents cell death during glucose starvation [40]. Similarly, our recent study also showed that knockdown of Herp inhibits zearalenone-induced cell death in RAW 264.7 macrophages. Based on these studies, we predicted that knockdown of Herp could promote cell survival by inhibiting apoptosis in mouse granulosa cells. Although our results showed that knockdown of Herp could not inhibit apoptosis compared with the control in mouse granulosa cells (Fig. 5A), we found an increase in the mRNA level of Bcl-2 and a decrease in the mRNA expression of Bax after Herp knockdown (Fig. 5B and C). Bcl-2 is an anti-apoptotic molecule that is associated with cell survival [41]. We did not detect significant alterations in the mRNA levels of p53 and Caspase-3, and it may not be possible to further decrease the percentage of apoptosis (10.7 ± 0.20% and 8.7 ± 0.77% in the control group and Herp knockdown group, respectively) in this normal culture model of granulosa cells. An induction model of apoptosis should be established to determine whether knockdown of Herp could promote cell survival or inhibit apoptosis in granulosa cells.
In summary, this study documented for the first time that the expression of Herp in granulosa cells is stage specific in the estrous cycle and is regulated by gonadotropin. Herp activation downregulates estrogen production in granulosa cells, possibly by controlling the expression of steroidogenic genes, which could regulate folliculogenesis and ovulation. The present study provided new insights into the function of Herp and the molecular mechanisms involved in the regulation of folliculogenesis and ovulation.
Supplementary
Acknowledgments
This research study was funded by the National Natural Science Foundation of China (No. 31201966; No. 31372499). Fenglei Chen designed the study, performed the experiments and wrote the manuscript. Nan Wang, Diqi Yang and Xin Wen were involved in performing the experiments and discussing the results. Dong Zhou, Keqiong Tang and Pengfei Lin provided advice on all aspects of the study. Tagwa Norain Mahmoud edited the language of the manuscript. Anhua Wang and Yaping Jin conducted the experiments, interpreted the data and supervised the research project.
References
- 1.Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev 2012; 58: 44–50. [DOI] [PubMed] [Google Scholar]
- 2.Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction 2006; 132: 191–206. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson E, Skinner MK. Cellular interactions that control primordial follicle development and folliculogenesis. J Soc Gynecol Investig 2001; 8(Suppl Proceedings): S17–S20. [DOI] [PubMed] [Google Scholar]
- 4.Lin P, Yang Y, Li X, Chen F, Cui C, Hu L, Li Q, Liu W, Jin Y. Endoplasmic reticulum stress is involved in granulosa cell apoptosis during follicular atresia in goat ovaries. Mol Reprod Dev 2012; 79: 423–432. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Lin P, Chen F, Wang A, Lan X, Song Y, Jin Y. Luman recruiting factor regulates endoplasmic reticulum stress in mouse ovarian granulosa cell apoptosis. Theriogenology 2013; 79: 633–639.e3. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Pei X, Jin Y, Wang Y, Zhang C. The roles of endoplasmic reticulum stress response in female mammalian reproduction. Cell Tissue Res 2015. (in press). [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Sun M, Shan Y, Zheng X, Ma H, Ma W, Wang Z, Pei X, Wang Y. Endoplasmic reticulum stress-mediated apoptotic pathway is involved in corpus luteum regression in rats. Reprod Sci 2015; 22: 572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai E, Teodoro T, Volchuk A. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology (Bethesda) 2007; 22: 193–201. [DOI] [PubMed] [Google Scholar]
- 9.Bergeron JJ, Brenner MB, Thomas DY, Williams DB. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci 1994; 19: 124–128. [DOI] [PubMed] [Google Scholar]
- 10.Ruddon RW, Bedows E. Assisted protein folding. J Biol Chem 1997; 272: 3125–3128. [DOI] [PubMed] [Google Scholar]
- 11.Kogure K, Nakamura K, Ikeda S, Kitahara Y, Nishimura T, Iwamune M, Minegishi T. Glucose-regulated protein, 78-kilodalton is a modulator of luteinizing hormone receptor expression in luteinizing granulosa cells in rats. Biol Reprod 2013; 88: 8. [DOI] [PubMed] [Google Scholar]
- 12.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 2011; 334: 1081–1086. [DOI] [PubMed] [Google Scholar]
- 13.Park HJ, Park SJ, Koo DB, Kong IK, Kim MK, Kim JM, Choi MS, Park YH, Kim SU, Chang KT, Park CK, Chae JI, Lee DS. Unfolding protein response signaling is involved in development, maintenance, and regression of the corpus luteum during the bovine estrous cycle. Biochem Biophys Res Commun 2013; 441: 344–350. [DOI] [PubMed] [Google Scholar]
- 14.Park HJ, Park SJ, Koo DB, Lee SR, Kong IK, Ryoo JW, Park YI, Chang KT, Lee DS. Progesterone production is affected by unfolded protein response (UPR) signaling during the luteal phase in mice. Life Sci 2014; 113: 60–67. [DOI] [PubMed] [Google Scholar]
- 15.Park SJ, Kim TS, Park CK, Lee SH, Kim JM, Lee KS, Lee IK, Park JW, Lawson MA, Lee DS. hCG-induced endoplasmic reticulum stress triggers apoptosis and reduces steroidogenic enzyme expression through activating transcription factor 6 in Leydig cells of the testis. J Mol Endocrinol 2013; 50: 151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kokame K, Agarwala KL, Kato H, Miyata T. Herp, a new ubiquitin-like membrane protein induced by endoplasmic reticulum stress. J Biol Chem 2000; 275: 32846–32853. [DOI] [PubMed] [Google Scholar]
- 17.van Laar T, Schouten T, Hoogervorst E, van Eck M, van der Eb AJ, Terleth C. The novel MMS-inducible gene Mif1/KIAA0025 is a target of the unfolded protein response pathway. FEBS Lett 2000; 469: 123–131. [DOI] [PubMed] [Google Scholar]
- 18.Kim TY, Kim E, Yoon SK, Yoon JB. Herp enhances ER-associated protein degradation by recruiting ubiquilins. Biochem Biophys Res Commun 2008; 369: 741–746. [DOI] [PubMed] [Google Scholar]
- 19.Kny M, Standera S, Hartmann-Petersen R, Kloetzel PM, Seeger M. Herp regulates Hrd1-mediated ubiquitylation in a ubiquitin-like domain-dependent manner. J Biol Chem 2011; 286: 5151–5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okuda-Shimizu Y, Hendershot LM. Characterization of an ERAD pathway for nonglycosylated BiP substrates, which require Herp. Mol Cell 2007; 28: 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan SL, Fu W, Zhang P, Cheng A, Lee J, Kokame K, Mattson MP. Herp stabilizes neuronal Ca2+ homeostasis and mitochondrial function during endoplasmic reticulum stress. J Biol Chem 2004; 279: 28733–28743. [DOI] [PubMed] [Google Scholar]
- 22.Nogalska A, Engel WK, McFerrin J, Kokame K, Komano H, Askanas V. Homocysteine-induced endoplasmic reticulum protein (Herp) is up-regulated in sporadic inclusion-body myositis and in endoplasmic reticulum stress-induced cultured human muscle fibers. J Neurochem 2006; 96: 1491–1499. [DOI] [PubMed] [Google Scholar]
- 23.Chigurupati S, Wei Z, Belal C, Vandermey M, Kyriazis GA, Arumugam TV, Chan SL. The homocysteine-inducible endoplasmic reticulum stress protein counteracts calcium store depletion and induction of CCAAT enhancer-binding protein homologous protein in a neurotoxin model of Parkinson disease. J Biol Chem 2009; 284: 18323–18333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sai X, Kokame K, Shiraishi H, Kawamura Y, Miyata T, Yanagisawa K, Komano H. The ubiquitin-like domain of Herp is involved in Herp degradation, but not necessary for its enhancement of amyloid beta-protein generation. FEBS Lett 2003; 553: 151–156. [DOI] [PubMed] [Google Scholar]
- 25.Slodzinski H, Moran LB, Michael GJ, Wang B, Novoselov S, Cheetham ME, Pearce RK, Graeber MB. Homocysteine-induced endoplasmic reticulum protein (herp) is up-regulated in parkinsonian substantia nigra and present in the core of Lewy bodies. Clin Neuropathol 2009; 28: 333–343. [PubMed] [Google Scholar]
- 26.Yan S, Zheng C, Chen ZQ, Liu R, Li GG, Hu WK, Pei H, Li B. Expression of endoplasmic reticulum stress-related factors in the retinas of diabetic rats. Exp Diabetes Res 2012; doi: 10.1155/2012/743780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun M, Sui Y, Li L, Su W, Hao F, Zhu Q, Di W, Gao H, Ma T. Anoctamin 1 calcium-activated chloride channel downregulates estrogen production in mouse ovarian granulosa cells. Endocrinology 2014; 155: 2787–2796. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Jin Y, Lin P, Hu L, Cui C, Li X, Li Q, Wang A. The expression and localization of LRF in the female reproductive tract of cycling mice throughout the estrous cycle. J Immunoassay Immunochem 2013; 34: 313–322. [DOI] [PubMed] [Google Scholar]
- 29.An J, Zhang X, Qin J, Wan Y, Hu Y, Liu T, Li J, Dong W, Du E, Pan C, Zeng W. The histone methyltransferase ESET is required for the survival of spermatogonial stem/progenitor cells in mice. Cell Death Dis 2014; 5: e1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillio-Meina C, Hui YY, LaVoie HA. Expression of CCAAT/enhancer binding proteins alpha and beta in the porcine ovary and regulation in primary cultures of granulosa cells. Biol Reprod 2005; 72: 1194–1204. [DOI] [PubMed] [Google Scholar]
- 31.Guthrie HD, Cooper BS, Welch GR, Zakaria AD, Johnson LA. Atresia in follicles grown after ovulation in the pig: measurement of increased apoptosis in granulosa cells and reduced follicular fluid estradiol-17 beta. Biol Reprod 1995; 52: 920–927. [DOI] [PubMed] [Google Scholar]
- 32.Riaz H, Dong P, Shahzad M, Yang L. Constitutive and follicle-stimulating hormone-induced action of somatostatin receptor-2 on regulation of apoptosis and steroidogenesis in bovine granulosa cells. J Steroid Biochem Mol Biol 2014; 141: 150–159. [DOI] [PubMed] [Google Scholar]
- 33.Belani M, Purohit N, Pillai P, Gupta S, Gupta S. Modulation of steroidogenic pathway in rat granulosa cells with subclinical Cd exposure and insulin resistance: an impact on female fertility. Biomed Res Int 2014; doi: 10.1155/2014/460251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci USA 1996; 93: 9776–9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park ES, Lind AK, Dahm-Kähler P, Brännström M, Carletti MZ, Christenson LK, Curry TE, Jr, Jo M. RUNX2 transcription factor regulates gene expression in luteinizing granulosa cells of rat ovaries. Mol Endocrinol 2010; 24: 846–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards JS. Genetics of ovulation. Semin Reprod Med 2007; 25: 235–242. [DOI] [PubMed] [Google Scholar]
- 37.Xu X, Zhang H, Zhang Q, Huang Y, Dong J, Liang Y, Liu HJ, Tong D. Porcine epidemic diarrhea virus N protein prolongs S-phase cell cycle, induces endoplasmic reticulum stress, and up-regulates interleukin-8 expression. Vet Microbiol 2013; 164: 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherr CJ. D-type cyclins. Trends Biochem Sci 1995; 20: 187–190. [DOI] [PubMed] [Google Scholar]
- 39.Lindqvist A, van Zon W, Karlsson Rosenthal C, Wolthuis RM. Cyclin B1-Cdk1 activation continues after centrosome separation to control mitotic progression. PLoS Biol 2007; 5: e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quiroga C, Gatica D, Paredes F, Bravo R, Troncoso R, Pedrozo Z, Rodriguez AE, Toro B, Chiong M, Vicencio JM, Hetz C, Lavandero S. Herp depletion protects from protein aggregation by up-regulating autophagy. Biochim Biophys Acta 2013; 1833: 3295–3305. [DOI] [PubMed] [Google Scholar]
- 41.Borner C. The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol 2003; 39: 615–647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.