Abstract
Here, we assessed the effects of long-chain fatty acids (LCFAs) and the LCFA receptor agonist GW9508 on the transcription of the gonadotropin subunit genes Cga, Lhb and Fshb because LCFA receptor GPR120 was observed in mouse gonadotropes in our recent study. A transcription assay using LβT2 cells demonstrated that LCFAs, oleic acid, α-linolenic acid, docosahexaenoic acid and palmitate, repressed the expression of Cga, Lhb, and Fshb at concentrations between 50 and 100 µM. On the other hand, treatment with 10 µM unsaturated LCFAs, oleic acid, α-linolenic acid and docosahexaenoic acid, repressed only Fshb expression, while the same dose of a saturated LCFA, palmitate, had no effect on the expression of gonadotropin subunit genes. Furthermore, GW9508 did not affect promoter activity. Next, we examined deletion mutants of the upstream region of Fshb and found that the upstream regulatory region (-2824 to -2343 bp) of Fshb was responsible for the notable repression by 10 µM unsaturated LCFAs. Our results suggest that the upstream region of Fshb is susceptible to unsaturated LCFAs. In addition, unsaturated LCFAs play a role in repressing Fshb expression through the distal -2824 to -2343 bp region, which might be independent of the LCFA receptor GPR120 pathway.
Keywords: Follicle-stimulating hormone (FSH), Free fatty acid, Gonadotropin, Luteinizing hormone (LH), Pituitary
Gonadal function is regulated mainly at the hypothalamic level of the hypothalamic-pituitary-gonadal axis. However, increasing evidence suggests that gonadal function is controlled at the pituitary level. For example, cortisol has been reported to directly suppress pulsatile luteinizing hormone (LH) secretion from the pituitary in sheep [1, 2]. Moreover, insulin, leptin, adiponectin, and other hormones are known to directly regulate LH secretion via gonadotropes [3,4,5]. These previous reports suggest that the synthesis and secretion of gonadotropic hormones can be directly regulated at the pituitary level by peripheral signals such as hormones. The pituitary gonadotropic hormones, LH and follicle-stimulating hormone (FSH), exist as heterodimers, comprised of a common glycoprotein α-subunit (Cga) and their specific β-subunits, LHβ and FSHβ, respectively. Genes encoding these three subunits are expressed in the pituitary gonadotropes, and several extracellular signals including GnRH, progesterone, estrogen, activin, and inhibin have been reported to regulate their expression via a specific upstream response element [6,7,8,9,10,11,12,13,14]. Therefore, characterization of response elements of gonadotropin subunit genes will help to determine the mechanisms underlying gonadotropin regulation at the pituitary level.
Long-chain fatty acids (LCFAs) may act as metabolic signals in the regulation of reproductive functions. This is supported by findings that peripheral or central lipoprivation suppresses pulsatile LH secretion [15, 16], and central exposure to linoleic acid increases Lhb mRNA expression levels in rats [17]. Interestingly, LCFAs are supposed to have some influence on the synthesis and secretion of gonadotropic hormones at the pituitary level, since a free fatty acid (FFA) cocktail directly increases Lhb mRNA levels and suppresses Fshb mRNA levels in the gonadotropic cell line LβT2 [18] and linoleic acid increases LH release and Lhb mRNA levels in both LβT2 cells and rat primary cultured pituitary cells [19]. Furthermore, LCFAs may induce these phenomena through LCFA receptor GPR120, which is known to be expressed in the gonadotropes of the mouse pituitary [20] and LβT2 cells [19]. However, in previous studies with LβT2 cells, the role of LCFAs in the gene expression and secretion of gonadotropic hormones has been examined using concentrations of LCFAs of several hundred micromolar. Therefore, the effect of lower than 100 μM LCFAs on the gene expression and secretion of gonadotropic hormones is still unclear.
In the present study, we investigated the effects of concentrations of LCFAs below 100 μM and a LCFA receptor agonist, GW9508, on the transcription of gonadotropin subunit genes, Cga, Lhb and Fshb, in LβT2 cells and identified the gene regulatory region responsive to LCFAs.
Materials and Methods
Cell culture
The gonadotropic cell line LβT2 (kindly provided by Dr PL Mellon) was maintained in monolayer cultures in Dulbecco’s modified Eagle’s medium (Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum and 0.5% penicillin/streptomycin solution (Sigma-Aldrich, St. Louis, MO, USA) in a humidified 5% CO2/95% air incubator at 37°C.
At the time of the reporter assay, cells (2 × 104) were seeded in 100 µl Opti-MEM (Life Technologies) per well of a 96-well plate, 24 h prior to transfection and maintained in a humidified 5% CO2/95% air incubator at 37°C.
LCFA stock and working solutions
The LCFAs, oleic acid, α-linolenic acid, docosahexaenoic acid (DHA) and palmitate, were dissolved in 50% ethanol at 65°C to prepare 500 mM stock solutions. Stock solutions and fatty acid-free bovine serum albumin (Sigma-Aldrich) in Opti-MEM were vortexed for 40 min and filtered to obtain a 10 mM homogenous mixture in Opti-MEM containing 2% bovine serum albumin.
Reporter assay
Upstream regions of the rat Cga (NC_005104.4), Lhb (NC_005100.4) and Fshb (NC_005102.4) genes were amplified using specific primer sets. The fragments were ligated into the secreted alkaline phosphatase (SEAP) plasmid vector pSEAP2-Basic (Clontech Laboratories, Palo Alto, CA, USA) as described previously [21,22,23]. The resulting reporter vectors contained the following gonadotropin subunit upstream regions: -3793/+37 (Cga); -2930/+17 (Lhb); and -2824/+28, -2342/+28, -1718/+28, -1499/+28, -1219/+28, -970/+28, -650/+28, -346/+28, -43/+28 (Fshb).
Transfection was performed using 200 ng DNA and 0.3 µl FuGENE HD (Roche Diagnostics, Basel, Switzerland) per well according to the protocol described in a previous study [24]. The cells were treated with an LCFA solution (10 µl per well) or the LCFA receptor agonist GW9508 at 7–8 h after transfection. After another 72 h of incubation, 5 µl of culture medium from each well was assayed for SEAP activity using the Phospha-Light Reporter Gene Assay System (Applied Biosystems, Foster City, CA, USA) and Powerscan H1 microplate luminometer (DS Pharma Biomedical, Osaka, Japan) according to the manufacturers’ protocols.
Statistical analysis
All values were expressed as the mean ± SEM. Dunnett’s method was used to analyze the effect of LCFAs or GW9508 on SEAP activity. P-values of < 0.05 were considered significant.
Results
Effect of LCFAs or GW9508 on transcriptional activation of gonadotropin subunit genes
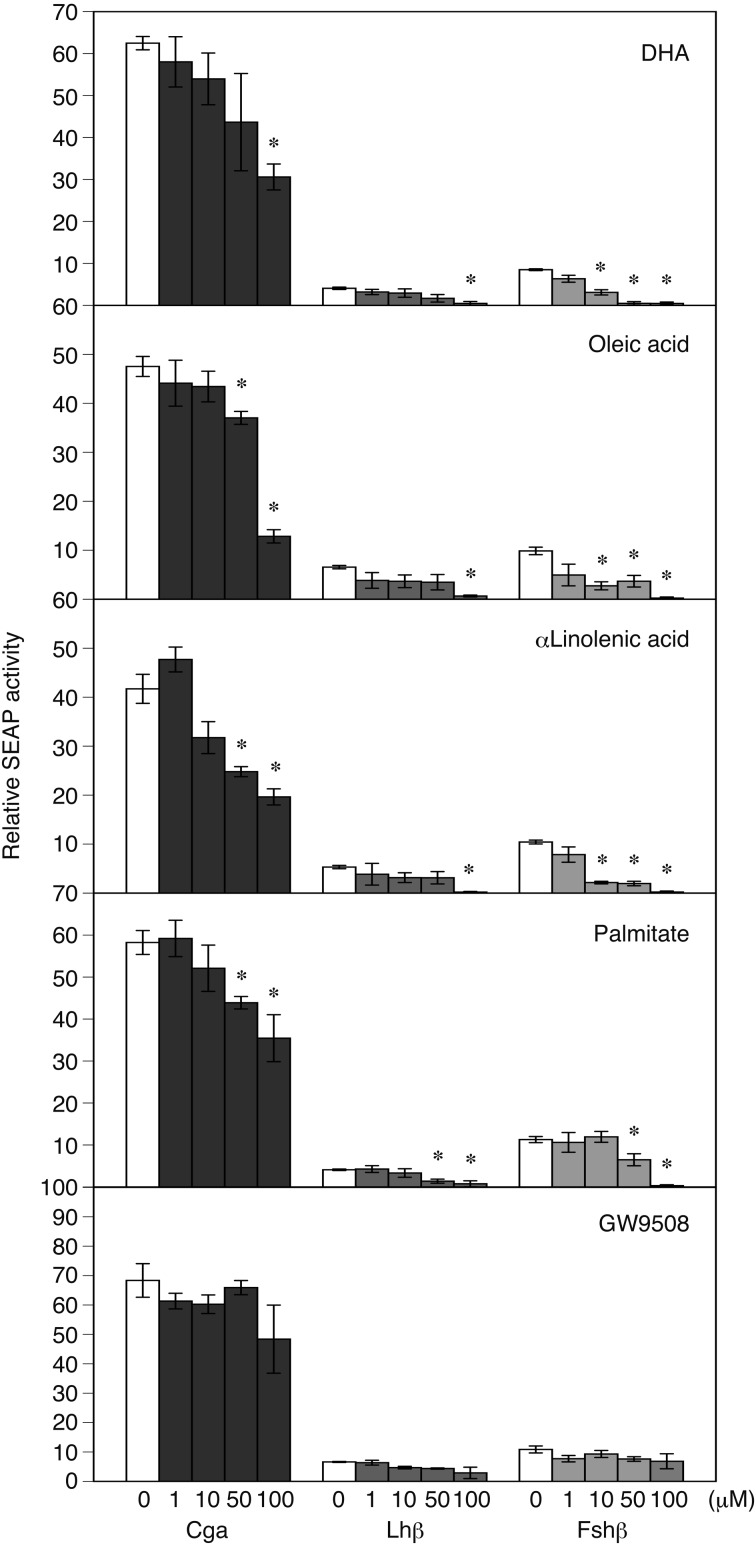
The promoter activity of Fshb (-2824/+28) was significantly repressed by not only treatment with both 100 µM and 50 µM DHA, oleic acid, α-linolenic acid or palmitate but was also significantly repressed by 10 µM DHA, oleic acid or α-linolenic acid (P < 0.05; Fig. 1). On the other hand, the promoter activity of Cga (-3793/+37) was significantly repressed by treatment with 100 µM DHA and by both 100 µM and 50 µM of oleic acid, α-linolenic acid or palmitate, but it was not significantly repressed by 10 µM LCFAs. Similar to the results for Cga, the promoter activity of Lhb (-2930/+17) was significantly repressed by treatment with 100 µM DHA, oleic acid, α-linolenic acid or palmitate and by treatment with 50 µM palmitate.
Fig. 1.
Transient transfection assay of the rat gonadotropin subunit gene promoters in LβT2 cells with or without treatment with oleic acid, α-linolenic acid, docosahexaenoic acid (DHA), palmitic acid or the long-chain fatty acid (LCFA) receptor agonist GW9508. Reporter constructs containing Cga (-3793/+37 b), Lhb (-2930/+17 b) or Fshb (-2824 to +28 b) promoters fused with the secreted alkaline phosphatase (SEAP) gene in the pSEAP-Basic vector were transfected into LβT2 cells. The cells were exposed to oleic acid, α-linolenic acid, DHA, palmitic acid, or GW9508 (1, 10, 50 or 100 µM) 7–8 h after transfection. An aliquot of the culture medium was used for the SEAP assay. SEAP activities are presented as the activity relative to that of the basic vector. Values are the mean ± SEM of four independent experiments. * P < 0.05 vs. pSEAP2-Basic (Dunnett’s method).
Next, we determined whether LCFAs regulate the synthesis of gonadotropes through GPR120 at the pituitary level and found that the LCFA receptor agonist GW9508 did not affect promoter activity at any concentration of LCFA used in this study.
Deletion analysis for the Fshb upstream region
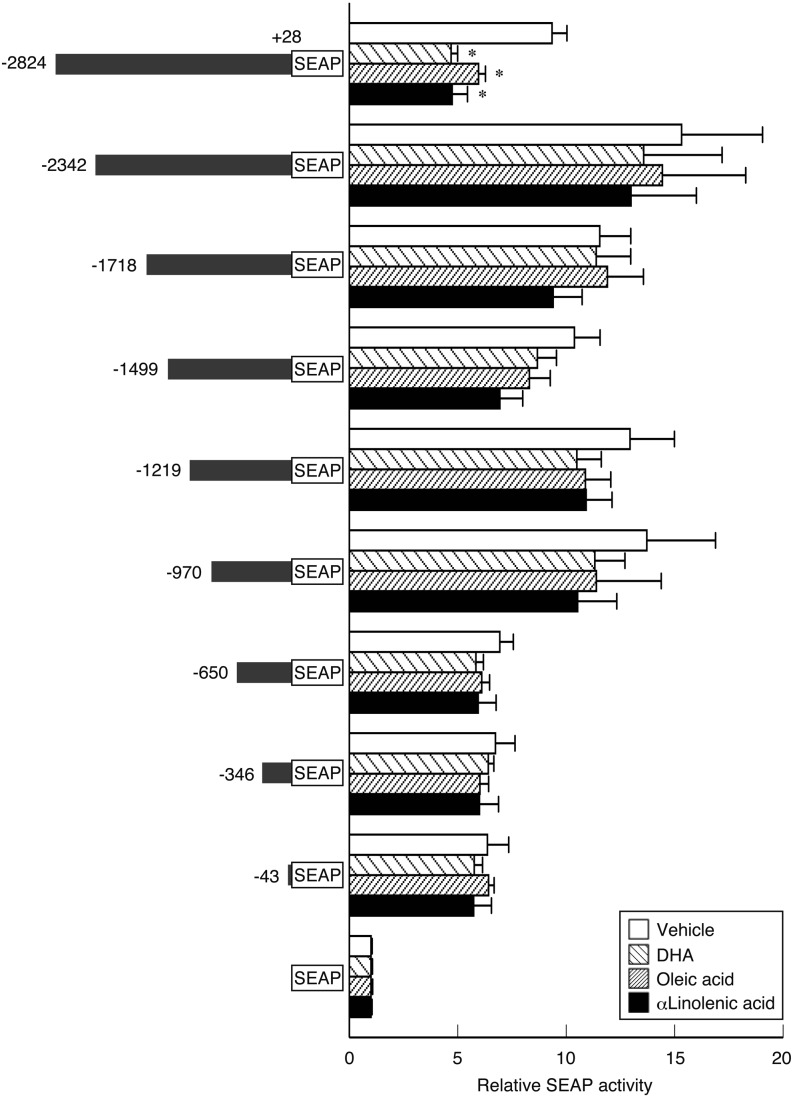
To confirm the marked repression of Fshb by 10 µM oleic acid, α-linolenic acid and DHA, deletion mutants of the upstream region of Fshb were examined using transfection and reporter gene assays. Oleic acid, α-linolenic acid and DHA significantly repressed promoter activity of the −2824 to +28 b region (Fig. 2). However, the -2342/+28, -1718/+28, -1499/+28, -1219/+28, -970/+28, -650/+28, -346/+28, and -43/+28 b regions lost the repression by oleic acid, α-linolenic acid and DHA.
Fig. 2.
Deletion analysis of the rat Fshb promoter region (-2824 to +28 b regions) in LβT2 cells with or without treatment with 10 µM unsaturated LCFAs oleic acid, α-linolenic acid, or docosahexaenoic acid (DHA). On the left, reporter constructs are shown containing serial deletion mutants of the Fshb promoter fused with the secreted alkaline phosphatase (SEAP) gene in the pSEAP2-Basic vector that were transfected into LβT2 cells. On the right, SEAP activities are represented as the activity relative to that of the basic vector. Values are the mean ± SEM for four independent experiments. * P < 0.05 vs. pSEAP2-Basic (Dunnett’s method).
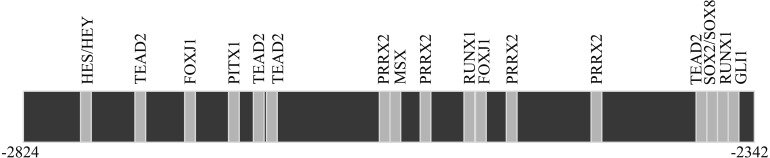
Figure 3 shows the locations of putative binding sites for transcription factors between the -2824 to -2343 b region of the rat Fshb upstream promoter, which responded to unsaturated LCFAs. Among the transcription factors binding to these sites, FOXJ1, GLU1, MSX, PITX1, PRRX2, RUNX1 and SOX2 were identified as those with expression in the murine pituitary gland.
Fig. 3.
Locations of gene transcription factor binding sites in the -2824 to -2343 b region upstream of the Fshb promoter that was responsive to unsaturated LCFAs. Abbreviations of transcription factors: Forkhead box protein J1 (FOXJ1), glioma-associated oncogene homolog 1 (GLI1), hairy and enhancer of split (HES)/split-related with YRPW motif (HEY), Msh homeobox 1-like protein (MSX), paired-like homeodomain 1 (PITX1), paired related homeobox 2 (PRRX2), runt-related transcription factor 1 (RUNX1), sex-determining region Y-box 2 (SOX2)/sex-determining region Y-box 8 (SOX8), and TEA domain family member 2 (TEAD2).
Discussion
In this study, we determined the effect of LCFAs and the LCFA receptor agonist GW9508 on the transcription of gonadotropin hormone subunit genes, including Cga, Lhb and Fshb, and identified the gene regulatory regions that were responsive to LCFAs. As shown in Fig. 1, unsaturated LCFAs, DHA, oleic acid and α-linolenic acid, markedly repressed the expression of Fshb at a low concentration (10 µM), while the saturated LCFA, palmitate, caused no apparent change in gonadotropin subunit gene expression at the same dose. Further, treatment with 50–100 µM LCFAs repressed expression of the gonadotropin subunit genes Cga, Lhb and Fshb (Fig. 1). In previous studies, the role of LCFAs on the transcription of gonadotropin hormone subunit genes has been examined using concentrations in the range of several hundred micromolars of LCFAs [17,18,19]. In this study, however, the low concentration of 10 µM unsaturated LCFAs repressed the expression of Fshb. The concentration of nonesterified fatty acids in the rodent peripheral blood is in the hundreds of micromolar range [25, 26]; therefore, the concentration of LCFAs used in this study is similar to the physiological level or even slightly lower than that recorded in rodent peripheral blood. These findings suggest that the transcription of Fshb is more sensitive or susceptible to unsaturated LCFAs compared with the previously reported concentration and that unsaturated LCFAs may be involved in the repression of Fshb transcription. Reproductive aging in women is characterized by shortening of the estrous cycle length [27], increased incidences of oocyte spindle aberrations or aneuploidy [28] and declining fertility [29]. Similar to humans, female rodents also show similar age-related physiological changes in reproductive function [30, 31]. These changes are associated with elevated baseline levels of serum FSH in humans [32] and mice [33]. Interestingly, dietary administration of omega-3 polyunsaturated fatty acids such as DHA decreased serum baseline FSH levels and FSH response to GnRH without changing serum LH levels in reproductive-age women with normal body weights [34]. Furthermore, Nehra et al. [35] reported that dietary treatment with a diet rich in DHA prolonged reproductive lifespan and improved oocyte quality in mice. Thus, the effect of a low concentration of unsaturated LCFAs on the transcription of Fshb in this study may have identified a part of the mechanism of unsaturated LCFAs in the delay of female reproductive aging and improvement of oocyte quality.
A transfection assay using serial deletion mutants of the -2824 to +28 b upstream region of Fshb revealed that the 10 µM unsaturated LCFA-responsive region for reducing Fshb transcription is located in the -2824 to -2343 b region (Fig. 2). Previous studies have shown that an FFA cocktail or linoleic acid reduces basal Fshb mRNA levels by disrupting the effects of activin on transcription of Fshb in LβT2 cells [17, 18]. LβT2 cells have been reported to produce activin endogenously to maintain basal Fshb expression [36]. These previous results imply that the reduction of Fshb transcription caused by unsaturated LCFAs via the -2824 to -2343 b region in the present study may also be mediated by the disruption of activin effects on Fshb expression. However, Suszko et al. [9] reported that the most activin-responsive domain of the rat Fshb promoter within the proximal promoter region was between -230 and -199 b. Therefore, the -2824 to -2343 b region is a novel region for the transcriptional control with unsaturated LCFAs. It is not clear whether this region is an activin-responsive domain leading to the disruption of Fshb transcription by unsaturated LCFAs. Interestingly, the -2824 to -2343 b region may contain plural putative regulatory elements for diverse transcription factors, including Forkhead box protein J1 (FOXJ1), glioma-associated oncogene homolog 1 (GLI1), hairy and enhancer of split/split-related with YRPW motif, Msh homeobox 1-like protein (MSX), paired-like homeodomain 1 (PITX1), paired related homeobox 2 (PRRX2), runt-related transcription factor 1 (RUNX1), sex-determining region Y-box 2 (SOX2)/sex-determining region Y-box 8, and TEA domain family member 2 (Fig. 3). In particular, FOXJ1, GLI1, MSX, PITX1, PRRX2, RUNX1, and SOX2 are expressed in the murine pituitary gland [37,38,39,40,41,42]. Therefore, unsaturated LCFAs may directly and/or indirectly modulate the binding of any of these factors. Indeed, in a previous study [43], PITX1 was observed to regulate murine and human Fshb transcription. Nevertheless, the role of the -2824 to -2343 b upstream region of rat Fshb and its molecular mechanism for the suppression by unsaturated LCFAs will need to be clarified by further investigations.
We hypothesized that LCFAs directly regulate the synthesis of gonadotropes through GPR120 at the pituitary level, because GPR120 expression has been observed in the gonadotropes of the mouse pituitary [20]. To test our hypothesis, the LCFA receptor agonist GW9508 was used. However, treatment with GW9508 did not affect gonadotropin subunit gene expression at the LCFA doses used in our study, although LCFAs repressed the promoter activity of gonadotropin subunit genes in LβT2 pituitary gonadotropic cells (Fig. 1). The dose of GW9508 used in this study was similar to that used in a previous study in which LβT2 cells were exposed to GW9508 [19] and induced an agonistic effect on GRP120 [44]. These results indicate that the reduction of transcription of Fshb is likely mediated by the β-oxidation products of unsaturated LCFAs and/or by the direct or indirect action of unsaturated LCFAs on the Fshb transcriptional regulatory mechanisms.
The present results showing that LCFAs repressed the transcription of Cga and Lhb (Fig. 1) contradict the results of previous studies. Sharma et al. [18] reported that an FFA cocktail induced an increase in Lhb mRNA levels in LβT2 cells. Garrel et al. [17, 19] reported that linoleic acid treatment induced an increase in the Lhb mRNA levels, while it had no effect on Cga mRNA levels in LβT2 cells or rat anterior pituitary cells. The inconsistency may depend on the difference in experimental methods used. Previous studies have demonstrated the effect of LCFAs on gonadotropin hormone subunit mRNA levels using real-time PCR, whereas we investigated the function of the enhancer/promoter elements of the gonadotropin genes using the SEAP reporter gene assay system. We only examined the region ~3.8 kb upstream of the rat gonadotropic subunit genes. In the SEAP reporter gene assay system, it may be necessary to analyze additional upstream regions to determine the gonadotropin transcription control mechanism by LCFAs. The upstream regions of Cga and Lhb examined in this study may lack the specific response regions for LCFAs, in contrast to the results measured for Cga and Lhb mRNA in the previous studies. The evidence concerning the LCFA response in the transcription of Cga and Lhb is not conclusive.
In conclusion, the promoter assay using reporter vectors revealed that the transcription of Fshb was susceptible to unsaturated LCFAs compared with the transcription of Cga and Lhb. Furthermore, the unsaturated LCFAs, DHA, oleic acid and α-linolenic acid, exhibited repressive regulation of rat Fshb expression at -2824 and -2343 b of the 5′-flanking region and might be independent of the GPR120 pathway.
Acknowledgments
We thank Dr PL Mellon for providing us with LβT2 cells. We also thank Dr Takao Susa for technical assistance and advice. This work was supported in part by a Japan Society for the Promotion of Science KAKENHI Grant (23780282 and 26450452 awarded to RM and 26292166 to YK) and Kinki University (RK-058 awarded to RM). This study was also supported by the Meiji University International Institute for BioResource Research (MUIIR) and by Research Funding for the Computational Software Support Program from Meiji University.
References
- 1.Pierce BN, Stackpole CA, Breen KM, Clarke IJ, Karsch FJ, Rivalland ET, Turner AI, Caddy DJ, Wagenmaker ER, Oakley AE, Tilbrook AJ. Estradiol enables cortisol to act directly upon the pituitary to suppress pituitary responsiveness to GnRH in sheep. Neuroendocrinology 2009; 89: 86–97. [DOI] [PubMed] [Google Scholar]
- 2.Breen KM, Davis TL, Doro LC, Nett TM, Oakley AE, Padmanabhan V, Rispoli LA, Wagenmaker ER, Karsch FJ. Insight into the neuroendocrine site and cellular mechanism by which cortisol suppresses pituitary responsiveness to gonadotropin-releasing hormone. Endocrinology 2008; 149: 767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avelino-Cruz JE, Flores A, Cebada J, Mellon PL, Felix R, Monjaraz E. Leptin increases L-type Ca2+ channel expression and GnRH-stimulated LH release in LbetaT2 gonadotropes. Mol Cell Endocrinol 2009; 298: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu M, Tang Q, Olefsky JM, Mellon PL, Webster NJ. Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in LbetaT2 gonadotropes. Mol Endocrinol 2008; 22: 760–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, Pinilla L, Tena-Sempere M, Dieguez C, Castaño JP, Malagon MM. Regulation of pituitary cell function by adiponectin. Endocrinology 2007; 148: 401–410. [DOI] [PubMed] [Google Scholar]
- 6.Fortin J, Ongaro L, Li Y, Tran S, Lamba P, Wang Y, Zhou X, Bernard DJ. Activin signaling in gonadotropes: What does the FOX say to the SMAD? Mol Endocrinol 2015; 29: 963–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran S, Lamba P, Wang Y, Bernard DJ. SMADs and FOXL2 synergistically regulate murine FSHbeta transcription via a conserved proximal promoter element. Mol Endocrinol 2011; 25: 1170–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciccone NA, Lacza CT, Hou MY, Gregory SJ, Kam KY, Xu S, Kaiser UB. A composite element that binds basic helix loop helix and basic leucine zipper transcription factors is important for gonadotropin-releasing hormone regulation of the follicle-stimulating hormone beta gene. Mol Endocrinol 2008; 22: 1908–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suszko MI, Balkin DM, Chen Y, Woodruff TK. Smad3 mediates activin-induced transcription of follicle-stimulating hormone beta-subunit gene. Mol Endocrinol 2005; 19: 1849–1858. [DOI] [PubMed] [Google Scholar]
- 10.Coss D, Jacobs SB, Bender CE, Mellon PL. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone beta gene by gonadotropin-releasing hormone. J Biol Chem 2004; 279: 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Conner JL, Wade MF, Prendergast P, Edwards DP, Boonyaratanakornkit V, Mahesh VB. A 361 base pair region of the rat FSH-beta promoter contains multiple progesterone receptor-binding sequences and confers progesterone responsiveness. Mol Cell Endocrinol 1997; 136: 67–78. [DOI] [PubMed] [Google Scholar]
- 12.Bailey JS, Rave-Harel N, McGillivray SM, Coss D, Mellon PL. Activin regulation of the follicle-stimulating hormone beta-subunit gene involves Smads and the TALE homeodomain proteins Pbx1 and Prep1. Mol Endocrinol 2004; 18: 1158–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller CD, Miller WL. Transcriptional repression of the ovine follicle-stimulating hormone-beta gene by 17 beta-estradiol. Endocrinology 1996; 137: 3437–3446. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida S, Kato T, Nishimura N, Kanno N, Chen M, Ueharu H, Nishihara H, Kato Y. Transcription of follicle-stimulating hormone subunit genes is modulated by porcine LIM homeobox transcription factors, LHX2 and LHX3. J Reprod Dev 2016(in press) http://doi.org/10.1262/jrd.2015-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sajapitak S, Iwata K, Shahab M, Uenoyama Y, Yamada S, Kinoshita M, Bari FY, I’Anson H, Tsukamura H, Maeda K. Central lipoprivation-induced suppression of luteinizing hormone pulses is mediated by paraventricular catecholaminergic inputs in female rats. Endocrinology 2008; 149: 3016–3024. [DOI] [PubMed] [Google Scholar]
- 16.Shahab M, Sajapitak S, Tsukamura H, Kinoshita M, Matsuyama S, Ohkura S, Yamada S, Uenoyama Y, I’Anson H, Maeda K. Acute lipoprivation suppresses pulsatile luteinizing hormone secretion without affecting food intake in female rats. J Reprod Dev 2006; 52: 763–772. [DOI] [PubMed] [Google Scholar]
- 17.Garrel G, Simon V, Denoyelle C, Ishaq M, Rouch C, Dairou J, Magnan C, Migrenne S, Cruciani-Guglielmacci C, Cohen-Tannoudji J. Unsaturated fatty acids disrupt Smad signaling in gonadotrope cells leading to inhibition of FSHβ gene expression. Endocrinology 2014; 155: 592–604. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, Morinaga H, Hwang V, Fan W, Fernandez MO, Varki N, Olefsky JM, Webster NJ. Free fatty acids induce Lhb mRNA but suppress Fshb mRNA in pituitary LβT2 gonadotropes and diet-induced obesity reduces FSH levels in male mice and disrupts the proestrous LH/FSH surge in female mice. Endocrinology 2013; 154: 2188–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrel G, Simon V, Denoyelle C, Cruciani-Guglielmacci C, Migrenne S, Counis R, Magnan C, Cohen-Tannoudji J. Unsaturated fatty acids stimulate LH secretion via novel PKCepsilon and -theta in gonadotrope cells and inhibit GnRH-induced LH release. Endocrinology 2011; 152: 3905–3916. [DOI] [PubMed] [Google Scholar]
- 20.Moriyama R, Deura C, Imoto S, Nose K, Fukushima N. Expression of the long-chain fatty acid receptor GPR120 in the gonadotropes of the mouse anterior pituitary gland. Histochem Cell Biol 2015; 143: 21–27. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa A, Kato T, Susa T, Sano A, Kato Y. Molecular cloning and characterization of porcine homeodomain transcription factor Msx1. J Reprod Dev 2009; 55: 278–282. [DOI] [PubMed] [Google Scholar]
- 22.Aikawa S, Susa T, Sato T, Kitahara K, Kato T, Kato Y. Transcriptional activity of the 5′ upstream region of the porcine glycoprotein hormone alpha subunit gene. J Reprod Dev 2005; 51: 117–121. [DOI] [PubMed] [Google Scholar]
- 23.Kato T, Ishikawa A, Yoshida S, Sano Y, Kitahara K, Nakayama M, Susa T, Kato Y. Molecular cloning of LIM homeodomain transcription factor Lhx2 as a transcription factor of porcine follicle-stimulating hormone beta subunit (FSHβ) gene. J Reprod Dev 2012; 58: 147–155. [DOI] [PubMed] [Google Scholar]
- 24.Susa T, Kato T, Kato Y. Reproducible transfection in the presence of carrier DNA using FuGENE6 and Lipofectamine2000. Mol Biol Rep 2008; 35: 313–319. [DOI] [PubMed] [Google Scholar]
- 25.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 2011; 60: 2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goncalves de Albuquerque CF, Burth P, Younes Ibrahim M, Garcia DG, Bozza PT, Castro Faria Neto HC, Castro Faria MV. Reduced plasma nonesterified fatty acid levels and the advent of an acute lung injury in mice after intravenous or enteral oleic acid administration. Mediators Inflamm 2012; 2012: 601032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenton EA, Landgren BM, Sexton L, Harper R. Normal variation in the length of the follicular phase of the menstrual cycle: effect of chronological age. Br J Obstet Gynaecol 1984; 91: 681–684. [DOI] [PubMed] [Google Scholar]
- 28.Jones KT. Meiosis in oocytes: predisposition to aneuploidy and its increased incidence with age. Hum Reprod Update 2008; 14: 143–158. [DOI] [PubMed] [Google Scholar]
- 29.Heffner LJ. Advanced maternal age—how old is too old? N Engl J Med 2004; 351: 1927–1929. [DOI] [PubMed] [Google Scholar]
- 30.Gosden RG, Laing SC, Felicio LS, Nelson JF, Finch CE. Imminent oocyte exhaustion and reduced follicular recruitment mark the transition to acyclicity in aging C57BL/6J mice. Biol Reprod 1983; 28: 255–260. [DOI] [PubMed] [Google Scholar]
- 31.Wu JM, Zelinski MB, Ingram DK, Ottinger MA. Ovarian aging and menopause: current theories, hypotheses, and research models. Exp Biol Med (Maywood) 2005; 230: 818–828. [DOI] [PubMed] [Google Scholar]
- 32.Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab 1996; 81: 1038–1045. [DOI] [PubMed] [Google Scholar]
- 33.Bernstein LR, Mackenzie AC, Kraemer DC, Morley JE, Farr S, Chaffin CL, Merchenthaler I. Shortened estrous cycle length, increased FSH levels, FSH variance, oocyte spindle aberrations, and early declining fertility in aging senescence-accelerated mouse prone-8 (SAMP8) mice: concomitant characteristics of human midlife female reproductive aging. Endocrinology 2014; 155: 2287–2300. [DOI] [PubMed] [Google Scholar]
- 34.Al-Safi ZA, Liu H, Carlson NE, Chosich J, Harris M, Bradford AP, Robledo C, Eckel RE, Polotsky AJ. Omega-3 fatty acid supplementation lowers serum FSH in normal weight but not obese women. J Clin Endocrinol Metab 2016; 101: 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nehra D, Le HD, Fallon EM, Carlson SJ, Woods D, White YA, Pan AH, Guo L, Rodig SJ, Tilly JL, Rueda BR, Puder M. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell 2012; 11: 1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang HJ, Miller WL, Mellon PL. Cell-specific transcriptional regulation of follicle-stimulating hormone-beta by activin and gonadotropin-releasing hormone in the LbetaT2 pituitary gonadotrope cell model. Endocrinology 2001; 142: 2284–2295. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura N, Ueharu H, Nishihara H, Shibuya S, Yoshida S, Higuchi M, Kanno N, Horiguchi K, Kato T, Kato Y. Search for regulatory factors of the pituitary-specific transcription factor PROP1 gene. J Reprod Dev 2015; 62: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lampichler K, Ferrer P, Vila G, Lutz MI, Wolf F, Knosp E, Wagner L, Luger A, Baumgartner-Parzer S. The role of proto-oncogene GLI1 in pituitary adenoma formation and cell survival regulation. Endocr Relat Cancer 2015; 22: 793–803. [DOI] [PubMed] [Google Scholar]
- 39.MacKenzie A, Ferguson MW, Sharpe PT. Hox-7 expression during murine craniofacial development. Development 1991; 113: 601–611. [DOI] [PubMed] [Google Scholar]
- 40.Szeto DP, Ryan AK, O’Connell SM, Rosenfeld MG. P-OTX: a PIT-1-interacting homeodomain factor expressed during anterior pituitary gland development. Proc Natl Acad Sci USA 1996; 93: 7706–7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Susa T, Kato T, Yoshida S, Yako H, Higuchi M, Kato Y. Paired-related homeodomain proteins Prx1 and Prx2 are expressed in embryonic pituitary stem/progenitor cells and may be involved in the early stage of pituitary differentiation. J Neuroendocrinol 2012; 24: 1201–1212. [DOI] [PubMed] [Google Scholar]
- 42.Breen KM, Thackray VG, Coss D, Mellon PL. Runt-related transcription factors impair activin induction of the follicle-stimulating hormone β-subunit gene. Endocrinology 2010; 151: 2669–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamba P, Khivansara V, D’Alessio AC, Santos MM, Bernard DJ. Paired-like homeodomain transcription factors 1 and 2 regulate follicle-stimulating hormone beta-subunit transcription through a conserved cis-element. Endocrinology 2008; 149: 3095–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Briscoe CP, Peat AJ, McKeown SC, Corbett DF, Goetz AS, Littleton TR, McCoy DC, Kenakin TP, Andrews JL, Ammala C, Fornwald JA, Ignar DM, Jenkinson S. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol 2006; 148: 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]