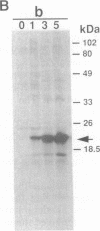
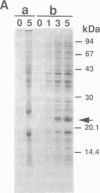
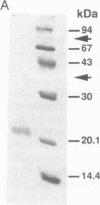
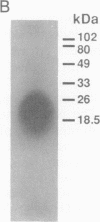
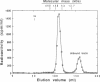
Abstract
We have designed a streptavidin-metallothionein chimeric protein in which the streptavidin moiety provides a means of binding the metallothionein moiety tightly to specific biological targets. A gene fusion of streptavidin with mouse metallothionein I was efficiently expressed in Escherichia coli, and the expressed chimeric protein was purified to homogeneity by a simple procedure. The purified chimera, consisting of four identical subunits, bound one biotin and approximately seven Cd2+ ions per subunit (19.5 kDa). This indicates that both the streptavidin and the metallothionein moieties are fully functional. The high binding affinity of the chimera both for biotin and for heavy metal ions allows the specific labeling or conjugation of any biological material containing unhindered biotin with a variety of different heavy metal ions and their isotopes, thereby opening the way for simultaneous assay systems for a large number of biological targets.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer E. A., Ben-Hur H., Gitlin G., Wilchek M. An improved method for the single-step purification of streptavidin. J Biochem Biophys Methods. 1986 Sep;13(2):103–112. doi: 10.1016/0165-022x(86)90022-9. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Ben-Hur H., Hiller Y., Wilchek M. Postsecretory modifications of streptavidin. Biochem J. 1989 Apr 15;259(2):369–376. doi: 10.1042/bj2590369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer E. A., Ben-Hur H., Wilchek M. Isolation and properties of streptavidin. Methods Enzymol. 1990;184:80–89. doi: 10.1016/0076-6879(90)84262-f. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Wilchek M. The use of the avidin-biotin complex as a tool in molecular biology. Methods Biochem Anal. 1980;26:1–45. doi: 10.1002/9780470110461.ch1. [DOI] [PubMed] [Google Scholar]
- CHAIET L., MILLER T. W., TAUSIG F., WOLF F. J. ANTIBIOTIC MSD-235. II. SEPARATION AND PURIFICATION OF SYNERGISTIC COMPONENTS. Antimicrob Agents Chemother (Bethesda) 1963;161:28–32. [PubMed] [Google Scholar]
- CHAIET L., WOLF F. J. THE PROPERTIES OF STREPTAVIDIN, A BIOTIN-BINDING PROTEIN PRODUCED BY STREPTOMYCETES. Arch Biochem Biophys. 1964 Jul 20;106:1–5. doi: 10.1016/0003-9861(64)90150-x. [DOI] [PubMed] [Google Scholar]
- Glanville N., Durnam D. M., Palmiter R. D. Structure of mouse metallothionein-I gene and its mRNA. Nature. 1981 Jul 16;292(5820):267–269. doi: 10.1038/292267a0. [DOI] [PubMed] [Google Scholar]
- Green N. M. Avidin and streptavidin. Methods Enzymol. 1990;184:51–67. doi: 10.1016/0076-6879(90)84259-j. [DOI] [PubMed] [Google Scholar]
- Green N. M. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Harnly J. M., Garland D. L. Multielement atomic absorption methods of analysis. Methods Enzymol. 1988;158:145–156. doi: 10.1016/0076-6879(88)58053-9. [DOI] [PubMed] [Google Scholar]
- Hiller Y., Gershoni J. M., Bayer E. A., Wilchek M. Biotin binding to avidin. Oligosaccharide side chain not required for ligand association. Biochem J. 1987 Nov 15;248(1):167–171. doi: 10.1042/bj2480167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K., Wood S. W., Brinton C. C., Montibeller J. A., Finn F. M. Iminobiotin affinity columns and their application to retrieval of streptavidin. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4666–4668. doi: 10.1073/pnas.77.8.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. B., Arlinghaus H. F., Schmitt H. W., Sachleben R. A., Brown G. M., Thonnard N., Sloop F. V., Foote R. S., Larimer F. W., Woychik R. P. An approach to the use of stable isotopes for DNA sequencing. Genomics. 1991 Jan;9(1):51–59. doi: 10.1016/0888-7543(91)90220-9. [DOI] [PubMed] [Google Scholar]
- Kägi J. H., Schäffer A. Biochemistry of metallothionein. Biochemistry. 1988 Nov 15;27(23):8509–8515. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Michel R. G. Atomic fluorescence spectrometry. Methods Enzymol. 1988;158:222–243. doi: 10.1016/0076-6879(88)58058-8. [DOI] [PubMed] [Google Scholar]
- Olivares J. A. Inductively coupled plasma-mass spectrometry. Methods Enzymol. 1988;158:205–222. doi: 10.1016/0076-6879(88)58057-6. [DOI] [PubMed] [Google Scholar]
- Osteryoung J. Electrochemical methods of analysis. Methods Enzymol. 1988;158:243–267. doi: 10.1016/0076-6879(88)58059-x. [DOI] [PubMed] [Google Scholar]
- Otvos J. D., Armitage I. M. Structure of the metal clusters in rabbit liver metallothionein. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7094–7098. doi: 10.1073/pnas.77.12.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risby T. H. Flame atomic emission spectrometry. Methods Enzymol. 1988;158:180–190. doi: 10.1016/0076-6879(88)58055-2. [DOI] [PubMed] [Google Scholar]
- Sachleben R. A., Brown G. M., Sloop F. V., Arlinghaus H. F., England M. W., Foote R. S., Larimer F. W., Woychik R. P., Thonnard N., Jacobson K. B. Resonance ionization spectroscopy for multiplex sequencing of tin-labeled DNA. Genet Anal Tech Appl. 1991 Sep;8(6):167–170. doi: 10.1016/1050-3862(91)90057-x. [DOI] [PubMed] [Google Scholar]
- Sano T., Cantor C. R. A streptavidin-protein A chimera that allows one-step production of a variety of specific antibody conjugates. Biotechnology (N Y) 1991 Dec;9(12):1378–1381. doi: 10.1038/nbt1291-1378. [DOI] [PubMed] [Google Scholar]
- Sano T., Cantor C. R. Expression of a cloned streptavidin gene in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jan;87(1):142–146. doi: 10.1073/pnas.87.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T., Cantor C. R. Expression vectors for streptavidin-containing chimeric proteins. Biochem Biophys Res Commun. 1991 Apr 30;176(2):571–577. doi: 10.1016/s0006-291x(05)80222-0. [DOI] [PubMed] [Google Scholar]
- Slavin W. Atomic absorption spectrometry. Methods Enzymol. 1988;158:117–145. doi: 10.1016/0076-6879(88)58052-7. [DOI] [PubMed] [Google Scholar]
- Stillman M. J., Cai W., Zelazowski A. J. Cadmium binding to metallothioneins. Domain specificity in reactions of alpha and beta fragments, apometallothionein, and zinc metallothionein with Cd2+. J Biol Chem. 1987 Apr 5;262(10):4538–4548. [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Wilchek M., Bayer E. A. Applications of avidin-biotin technology: literature survey. Methods Enzymol. 1990;184:14–45. doi: 10.1016/0076-6879(90)84257-h. [DOI] [PubMed] [Google Scholar]
- Wilchek M., Bayer E. A. Introduction to avidin-biotin technology. Methods Enzymol. 1990;184:5–13. doi: 10.1016/0076-6879(90)84256-g. [DOI] [PubMed] [Google Scholar]
- Wilchek M., Bayer E. A. The avidin-biotin complex in bioanalytical applications. Anal Biochem. 1988 May 15;171(1):1–32. doi: 10.1016/0003-2697(88)90120-0. [DOI] [PubMed] [Google Scholar]
- Willner H., Vasák M., Kägi J. H. Cadmium-thiolate clusters in metallothionein: spectrophotometric and spectropolarimetric features. Biochemistry. 1987 Sep 22;26(19):6287–6292. doi: 10.1021/bi00393a049. [DOI] [PubMed] [Google Scholar]
- Wolnik K. A. Inductively coupled plasma-emission spectrometry. Methods Enzymol. 1988;158:190–205. doi: 10.1016/0076-6879(88)58056-4. [DOI] [PubMed] [Google Scholar]