Abstract
We conducted a systematic review to evaluate the (1) feasibility and efficacy and (2) safety and cost effectiveness of continuous positive airway pressure (CPAP) therapy in low- and middle-income countries (LMIC). We searched the following electronic bibliographic databases—MEDLINE, Cochrane CENTRAL, CINAHL, EMBASE and WHOLIS—up to December 2014 and included all studies that enrolled neonates requiring CPAP therapy for any indication. We did not find any randomized trials from LMICs that have evaluated the efficacy of CPAP therapy. Pooled analysis of four observational studies showed 66% reduction in in-hospital mortality following CPAP in preterm neonates (odds ratio 0.34, 95% confidence interval (CI) 0.14 to 0.82). One study reported 50% reduction in the need for mechanical ventilation following the introduction of bubble CPAP (relative risk 0.5, 95% CI 0.37 to 0.66). The proportion of neonates who failed CPAP and required mechanical ventilation varied from 20 to 40% (eight studies). The incidence of air leaks varied from 0 to 7.2% (nine studies). One study reported a significant reduction in the cost of surfactant usage with the introduction of CPAP. Available evidence suggests that CPAP is a safe and effective mode of therapy in preterm neonates with respiratory distress in LMICs. It reduces the in-hospital mortality and the need for ventilation thereby minimizing the need for up-transfer to a referral hospital. But given the overall paucity of studies and the low quality evidence underscores the need for large high-quality studies on the safety, efficacy and cost effectiveness of CPAP therapy in these settings.
Introduction
Of the total 2.9 million neonates who die every year, nearly 1 million (35%) die of preterm birth complications.1 Preterm neonates are not only at high risk of mortality but also are at risk for developing serious morbidities like respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis and infections.
Despite advances in neonatal intensive care, respiratory distress syndrome remains the single, most important cause of mortality among preterm neonates.2 More than 50% of neonates born before 31 weeks of gestation develop respiratory distress syndrome, which is due to deficiency of pulmonary surfactant production.3, 4 Despite the advent of effective prevention and management strategies such as antenatal steroids, exogenous surfactant therapy and newer ventilatory techniques, nearly 40% very-preterm neonates either die or develop bronchopulmonary dysplasia by 36 weeks postconceptional age.5, 6, 7
Continuous positive airway pressure (CPAP), which refers to the application of continuous distending pressure in a spontaneously breathing neonate, increases the functional residual capacity of the lung resulting in better gas exchange.8 CPAP has been shown to reduce the risk of mortality by 48%6 and the need for surfactant and mechanical ventilation by about 50%.9 In addition, the use of CPAP has been found to decrease hospital stay10 and need for referrals and up-transfers to tertiary units, saving nearly $10 000 for every six neonates treated.11 Altogether, these benefits have made CPAP the standard of care in managing sick preterm neonates with respiratory distress in high-income countries.
Yet, it still remains unclear whether the same benefits pertain to low- and middle-income countries (LMIC) where the availability of trained doctors and nursing staff, round the clock monitoring facilities, and optimal CPAP devices and interfaces is still sub-optimal. The problem is compounded by difficulty in delivering CPAP in the delivery room/during transport, lack of blenders and pulse-oximeters, lack of blood gas analyzers, chest x-ray equipment and finally the cost issues.12 Without optimal equipment and skilled manpower, it is likely that CPAP may not be as effective and possibly less safe in LMIC settings. On the other hand, the higher costs of exogenous surfactant therapy and newer sophisticated ventilators may make CPAP the way forward for managing respiratory distress in these settings.
The question can be answered only by examining available evidence on the use of CPAP from LMIC. Therefore, we planned a systematic review to evaluate the efficacy, safety, feasibility and cost effectiveness of introducing and implementing CPAP at both population and health facility level in LMIC. The findings of the review would provide the policy makers available information to guide the upscale of the intervention in different settings from LMIC.
Methods
Objectives
The major objectives of this review were to assess the1 feasibility and efficacy and2 safety and cost effectiveness of CPAP therapy in preterm neonates from LMICs (Table 1).
Table 1. Objectives, outcomes and definitions.
| Objectives | Outcomes | Definitions |
|---|---|---|
| Is it feasible and effective to introduce and implement CPAP therapy in LMIC settings? | In-hospital/neonatal mortality after introduction of CPAPProportion of neonates who require intubation and mechanical ventilationProportion of neonates who needed referral to higher centers immediately after institution of CPAP therapy | Mortality before discharge or in the first 28 days of lifeProportion of neonates who develop CPAP failure/require re-intubation as per pre-defined criteria used in each studyProportion of neonates who were referred to higher centers (NICU of the same hospital/other hospitals) for immediate or late complications including failure of CPAP therapy |
| Is it safe to implement CPAP therapy in LMIC settings? | Proportion of neonates developing pulmonary air-leak, sepsis, local trauma, shock during CPAP therapy | Proportion of neonates developing pneumothorax or pulmonary interstitial air collection, local nasal trauma or shock, defined as need for vasopressors to maintain blood pressure and peripheral perfusion |
| Is it cost-effective to implement CPAP therapy in LMIC settings? | Cost per one neonatal death avertedCost per one ventilation avertedCost to the health facility and family | The actual cost to the health system for every neonatal death avertedCost per one ventilation avertedCost to health facility and family |
Abbreviations: CPAP, continuous positive airway pressure; LMIC, low- and middle-income countries; NICU, neonatal intensive care unit.
Types of studies
For the first objective, we included both observational and interventional studies (randomized controlled trials and quasi-randomized trials) from LMICs that had evaluated the use of CPAP in preterm neonates. For the second objective, we included all studies from LMICs that had reported the use of CPAP in neonates (including case series that reported complications following CPAP therapy).
Interventions
We included all studies that evaluated the use of CPAP therapy in eligible neonates.
Outcome measures and their definitions
Table 1 provides the list of critical outcomes and their definitions.
Search methods for identification of studies
We searched the following electronic bibliographic databases—MEDLINE, Cochrane CENTRAL, CINAHL, EMBASE and WHOLIS—up to December 2014. We used the following search terms for searching Medline—‘continuous positive airway pressure', ‘respiration, artificial', ‘positive-pressure respiration' or the text words: ‘continuous distending pressure', ‘CPAP', ‘CDAP', ‘distending pressure', ‘continuous positive transpulmonary pressure', ‘continuous transpulmonary pressure', ‘continuous inflating pressure', ‘positive pressure', ‘positive expiratory pressure', ‘positive end expiratory pressure', ‘PEEP') AND LMIC. Similar terms were used for searching the other databases—LILACS, Popline and BiblioMap.
The search terms for LMIC were adapted from the two systematic reviews on cost effective interventions in LMIC.13, 14 No language restrictions were used and no studies were excluded on the basis of study design. All causes of respiratory distress were considered. We scanned the title and abstract of the retrieved citations to exclude those that were obviously irrelevant. We retrieved the full text of the remaining studies to identify the relevant articles. The reference lists of included articles were also searched.
Data extraction and management
Data extraction was done using a data extraction form pre-designed and tested by the authors. Two reviewers (AT and MJS) independently extracted the relevant information including the number of participants, the number of events, adjusted odds ratio (OR) and its 95% confidence interval (CI).
Given the types of studies expected to be included and the broad objectives of the review, we did not intend to do any quality assessment of the included studies or meta-analysis of the results.
Statistical analysis
Meta-analysis was performed with user written programs on Stata 11.2 software (StataCorp, College Station, TX, USA). Pooled estimates of the outcome measures were calculated from the relative risks or OR and 95% CI of the individual studies by generic inverse variance method. We examined for heterogeneity among the included studies by inspecting the forest plots and quantifying the impact of heterogeneity using a measure of the degree of inconsistency in the studies' results (I2 statistic). We intended to use the fixed-effect model if the I2 statistic was <60% if the I2 statistic was 60% or more, we planned to use the random effects model provided no major causes for heterogeneity could be identified.
Results
Figure 1 depicts the number of studies identified by the literature search and the studies included after reviewing the title/abstract or the full text. The search strategy identified 645 records, of which 597 were excluded after scanning title and abstract. Among the remaining 48 studies, 22 were eligible for inclusion in the systematic review. Description of the included studies has been provided as and when applicable in the following sections.
Figure 1.
Flow chart depicting the selection of studies included in the review.
Feasibility and efficacy
In-hospital/neonatal mortality
We did not identify any randomized trial that had compared the effect of CPAP with other methods such as oxygen by hood on neonatal mortality. We identified a total of 13 observational studies had reported the effect of CPAP therapy on in-hospital or neonatal mortality in preterm neonates (Table 2). These studies can be broadly categorized into three groups: time-series/comparison with historical controls, case-control studies (‘typical' case-control or analysis of prospective data like a case-control study) and prospective observational studies of CPAP therapy.
Table 2. Studies on feasibility and/or effectiveness of CPAP therapy in LMIC settings.
| Author, year | Country | Setting | Study design | Study population | CPAP Strategy | Results | Comments |
|---|---|---|---|---|---|---|---|
| Studies with a control group | |||||||
| Koyamaibole, 200515 | Fiji | Referral hospital (only hospital providing NICU services in Fiji) | Comparison of two time periods—before and after introduction of bCPAP | Median weight 2765 g (1785–3300); 70 (12.6%) were 1000–1500 g | CPAP was considered for neonates with grunting, severe chest indrawing, severe respiratory distress and hemoglobin oxygen saturation <90% despite oxygen | Among the 105 neonates who received CPAP, 24 (22.8%) failed and required mechanical ventilationTrend towards lower mortality in the period when bCPAP was used (OR 0.74; 95% CI 0.52 to1.03; P=0.06) | Not a randomized trial; ventilator assistance was available as backup if required so the findings may not be translated to a scenario where it is used in isolation |
| Ballot, 201016 | South Africa | Tertiary care neonatal unit | Retrospective chart review | All very low birth weight neonates admitted over a one-year period (n=474) | CPAP commenced when the infant showed signs of respiratory failure; exact strategy not given; CPAP use between survivors vs non-survivors was evaluated | Nasal CPAP use was associated with decrease in mortality from 32.8% to 16.7%Use of nasal CPAP resulted in improved survival among very low birth weight infants (OR 4.58; 95% CI 1.58 to 13.31) | No information regarding respiratory support in the control group or other confounding variables |
| Peiper, 200317 | South Africa | Tertiary care neonatal unit | Prospective data collection | All admissions with birth weight<1200 g who were refused admission to the unit | CPAP protocol was initiated with a pressure of 5 cmH2O and then increased to stabilize respiratory movements and achieve target pressure of arterial oxygen (PaO2) | 21 neonates total; 11 received CPAPSurvival of neonates with respiratory distress managed with CPAP was 81.8% (9/11) vs 20% (2/10) with head box oxygenSurvival to discharge 45.4% (5/11) vs 20%(2/10) | Skewed gender distribution, intention to treat analysis was not done; small sample size |
| Kawaza, 201418 | Malawi | Referral hospital | Prospective observational study with two groups – CPAP with Hudson prongs vs standard care (oxygen with nasal cannulae) | Neonates weighing 1000 g and presenting with severe respiratory distress | Low-cost bCPAP system delivered by Hudson nasal prongs | Survival rate for neonates receiving bCPAP was 71.0% (44/62) compared with 44.0% (11/25) for controls64.6% (31/48) of neonates with RDS receiving bCPAP survived to discharge, compared to 23.5% (4/17) of controls | Control group received standard care (oxygen by cannula); they were shifted to CPAP group if CPAP device was available |
| Jeena, 200219 | South Africa | NICU, teaching hospital | Retrospective review of cases seen at King Edward VIII Hospital | Nasal CPAP was required by 85 neonatesMedian weight 1659 g and gestation 34 weeks. | CPAP was considered for neonates with respiratory failure Maximum CPAP 6 cm and maximum FiO2 60% | 63 neonates (74%) were initially successfully supported with nasal CPAP aloneOf these, 50 (79%) required no further respiratory support until discharge and seven received IPPV subsequentlyMortality rate of 25% in the 85 neonates who received CPAPMortality in neonates successfully managed with CPAP 18%Mortality on CPAP was only 9% for those infants who were not offered ventilation | — |
| Studies with no control group | |||||||
| Saxena, 201220 | India | NICU, teaching hospital | Prospective observational study | All preterm neonates diagnosed with RDS Nasal CPAP alone was given to all spontaneously breathing neonates (n=50)Gestational age 31 (25–35) weeks, birth weight 1543 (710–2700) g | Trial of nasal CPAP was given to all spontaneously breathing newborns | Among the neonates who received CPAP alone 46/50 survived (92%)9/24 (37.5%) neonates <28 weeks, 24/35 (68.5%) 29–32 weeks and 17/24 (70.8%) of 33–36 weeks gestational age neonates could be managed successfully with CPAP and did not require mechanical ventilation | No details of CPAP delivery devices, pressures at the time of initiation, whether breastfeeding |
| Singh, 199321 | India | Tertiary care teaching hospital | Uncontrolled observational study | Not available | Clearly delineated CPAP protocol | 25/33 (75.8%) neonates who received CPAP and 25/57 (44%) neonates who received ventilation survived | No control group, details of patient population and illness not available |
| Rojas, 200922 | Colombia Multicenter trial | Tertiary care center | Randomized trial of INSURE vs CPAP only arm | Preterm infants 27–32 weeks, with O2 requirement or respiratory distress at 15–60 min of age were randomized into INSURE or early CPAP | — | Of the 137 babies treated with CPAP, mortality was 13/137 (9%), mechanical ventilation was needed in 53 (39%),12 (9%) babies had pneumothorax | Case series of only CPAP arm included |
| Hendrik, 201023 | South Africa | Secondary level unit | No comparison | Mean birth weight: 1166 g Mean gestational age: 31 weeks Male: 22/34 (65%) | CPAP protocol clearly delineated, case series | >1800 g: 4/17 neonates had failure<1800 g: 11/34 neonates had failureSurvival 80% | Case series |
| Shrestha, 201024 | Nepal | Secondary level unit | Uncontrolled observational study | All babies with respiratory distress Gestational age 28- 37 weeks Weight 800-2700 g | — | 15 babies; mortality was 33% (4/15) | Case series |
| Heuvel, 201125 | Malawi | Secondary care unit | Case series of 11 babies | Weight 1000-2500 g | CPAP considered for babies with respiratory distress | 5 babies received CPAP and 3 survived (60%) | Case series |
| Kirsten, 201226 | South Africa | Tertiary care NICU | Observational study | Preterm neonates 500 -1000 g⩾25 weeks were included | Clear protocol; started at 4–6 cm of water. FiO2 titrated based on oxygen concentration | 80% survival until discharge for neonates who received only CPAPNasal CPAP was associated with an improved survival at day 3, day 7 and at dischargeBirth weight less than 750 g identified as independent risk factor for failed CPAP | Observational study |
| Boo, et al. 200027 | Malaysia | NICU | Case–control study | 97 preterm infants<37 weeks | CPAP started for infants with respiratory distress; given with either bubble or ventilator CPAP | 37 infants (38.1%) failed CPAPOverall mortality rate not reported | |
| Urs, 200929 | India | NICU, teaching hospital | Prospective observational study | All neonates diagnosed with RDS (n=50) CPAP failure (n=10) CPAP success (n=40) Overall 33 neonates (1000-1500 g), 4 (⩽999 g), 13 (1501-2000 g) | CPAP considered for neonates with FiO2 requirement >0.40 to maintain PaO2 >60 mm Hg with pH <7.25, PaCO2 >50 mm and Downe's score >4 | BCPAP proved to be effective in 40/50 (80%) neonates.Success rate of bCPAP in mild, moderate and severe RDS was 100%, 93.1 and 46.6% respectivelyMortality rate not reported | No details on safety of CPAP delivery, outcome of neonates enrolled in study |
| Pillai, 201129 | India | NICU, teaching hospital | Prospective observational study | Very low birth weight infants <36 weeks eligible for enrollment (n=62) | CPAP considered for neonates with any respiratory distress Initiating pressure: 4-5 cm water, FiO2: 0.4 to 0.5; Target saturations 88-93% | 16 neonates (25.8%) failed CPAPMortality not reported | No details on safety of CPAP delivery, outcome of neonates enrolled in study |
| Koti, 200930 | India | Level III NICU | Prospective observational study | 56 Inborn preterm infants (gestation 28 to 34 weeks) with respiratory distress and chest x-ray suggestive of RDS | BCPAP with bi-nasal prongs started at 5 cm of water and adjusted to minimize chest retractions. FiO2 was adjusted to maintain SpO2between 87 and 95% | Fourteen (25%) babies failed CPAP.Of the 14 infants who failed CPAP, in 4 (28.5%) ventilation was started after an initial recovery from CPAP.Six (6/64; 9.4%) babies died during hospital stay | |
| Bassiouny, 199431 | Oman | NICU | Prospective observational study | 44 preterm infants with RDS enrolled | CPAP delivered using Beneveniste's valve and silastic nasal prongs | 27/44 cases successfully treated with CPAP (61%) and 17 cases (39%) failed to respond | Full text could not be retrieved |
Abbreviations: CPAP, continuous positive airway pressure; INSURE, intubate-surfactant-and-extubate; IPPV, intermittent positive pressure ventilation; LMIC, low- and middle-income countries; NICU, neonatal intensive care unit; RDS, respiratory distress syndrome.
Time series/comparison with historical controls: one study from Fiji evaluated mortality data from two time periods–18 months before and 18 months after the introduction of bubble CPAP (bCPAP). 15 In the former period, there were 79/1106 deaths (7.1%) while in the latter there were 74/1382 deaths (5.4%), suggesting a trend toward lower mortality (OR 0.74, 95% CI 0.52 to 1.03; P=0.06).
Case–control studies: a retrospective chart review from South Africa found that the use of nasal CPAP was associated with lower mortality among very low birth weight neonates (16.7 vs 32.8% OR 0.22, 0.08 to 0.63). 16 The confounding effect of other variables was, however, unclear. Another small study from South Africa reported a mortality of 18.2% (2/11) with CPAP as against 80% (8/10; OR 0.06, 0.004 to 0.66) for initial treatment of respiratory distress with head box oxygen, with no backup of mechanical ventilation in the unit. 17 One non-randomized study from Malawi compared the effects of nasal CPAP with oxygen therapy by nasal cannulae. The study reported a significantly lower mortality in the CPAP group as compared with the control group (29.0 vs 56.0% OR 0.32, 95% CI 0.12 to 0.83). 18 Another retrospective study from South Africa compared outcomes of neonates manages with CPAP as against invasive ventilation. 19 The reported mortality in the CPAP group (25%) was comparable to the group that received ventilation (39%). The authors remarked that mortality in neonates successfully managed with CPAP was 18% and this dropped to 9% after correcting for neonates who were not offered ventilation.
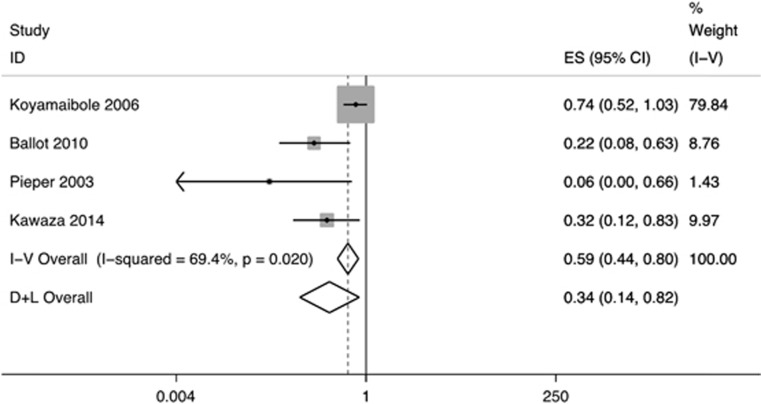
Uncontrolled observational studies: the reported mortality rates range from 8 to 26.6% in neonates who received CPAP. 20, 21, 22, 23, 24, 25, 26 Pooled analysis of the four studies that provided complete data showed 66% reduction in in-hospital mortality following CPAP therapy in preterm neonates (OR 0.34, 95% CI 0.14 to 0.82; random-effects model; Figure 2).
Figure 2.
Effect of CPAP therapy on in-hospital mortality. ‘I-V Overall' refers to the estimate by fixed effects model while ‘D+L Overall' refers to the pooled estimate. CPAP, continuous positive airway pressure; ES, effect size; ID, identification.
Proportion of neonates who failed CPAP and required mechanical ventilation
Eight studies from LMIC settings had reported this outcome (Table 2).20, 22, 23, 27, 28, 29, 30, 31 Except two studies that reported a higher failure rate of 38%27 and 40%,31 other studies reported a failure of 20 to 25%. One study from India reported that the Institution of CPAP alone in all spontaneously breathing preterm neonates with respiratory distress syndrome and administration of surfactant to only those needing mechanical ventilation reduced the need for intubations and surfactant administration without affecting the outcome adversely.20
One study had reported the need of referral of one neonate due to non availability of ventilator in the unit.20 The other neonates who required mechanical ventilation as a primary mode (33/83; 39.7%) were managed in the same unit. A before–after study from a referral hospital in Fiji suggested a reduction in the need of mechanical ventilation with the use of CPAP. The introduction of bCPAP was associated with a 50 per cent reduction in the need for mechanical ventilation—from 113/1106 (10.2%) prior to bCPAP to 70/1382 (5.1%) after introduction of CPAP (relative risk 0.5, 95% CI 0.37 to 0.66).15
Safety of implementation of CPAP therapy
Nine studies (India 2, Brazil 1, Oman 1 and Malaysia 1, South Africa 2, Malawi 2) had commented on the incidence of air leaks (Table 3). Of these, seven reported no pneumothorax in neonates receiving CPAP therapy.18, 23, 25, 26, 28, 31, 32 One study reported the development of pneumothorax in two neonates (2/56; 3.5%). Both the neonates did not require mechanical ventilation and were stabilised on CPAP.30 In contrast, a study from Malaysia reported a relatively higher incidence of pneumothorax after the implementation of CPAP therapy (7/97; 7.2%).27
Table 3. Studies on safety of CPAP therapy in LMIC settings.
| Author, year | Country | Setting | Study design | Study population | CPAP strategy | Results | Comments |
|---|---|---|---|---|---|---|---|
| CPAP and pneumothorax | |||||||
| Kawaza, 201418 | Malawi | Referral hospital | Prospective observational study with two groups – CPAP with Hudson prongs vs standard care (oxygen with nasal cannulae) | Neonates weighing 1000 g and presenting with severe respiratory distress | Low-cost bCPAP system delivered by Hudson nasal prongs | No pneumothorax reported | |
| Hendrik, 201023 | South Africa | Secondary level unit | No comparison | Mean birth weight: 1166 g Mean gestational age: 31 weeks Male: 22/34 (65%) | CPAP protocol clearly delineated, case series | No pneumothorax reported | |
| Heuvel, 201125 | Malawi | Secondary care unit | Case series of 11 infants | Weight 1000–2500 g | CPAP considered for infants with respiratory distress | No pneumothorax reported | |
| Kirsten, 201226 | South Africa | Tertiary care NICU | Observational study | Preterm neonates 500 –1000 g ⩾25 weeks | Clear protocol; started at 4-6 cm of water. FiO2 titrated based on oxygen concentration | No pneumothorax reported | |
| Boo, 200027 | Malaysia | NICU | Case–control study | 97 preterm infants <37 weeks | CPAP started for infants with respiratory distress; and given with either bCPAP or ventilator CPAP | Pneumothorax reported for 7/97 babies: 7.2%(5/37 in CPAP failure group and 2/60 in CPAP success group) | |
| Urs, 200928 | India | NICU, teaching hospital | Prospective observational Study | All neonates diagnosed with RDS (n=50) CPAP failure (n=10) CPAP success (n=40) Overall 33 neonates (1000–1500 g), 4 (⩽999 g), 13 (1501–2000 g) | CPAP considered for neonates with FiO2 requirement >0.40 to maintain PaO2>60 mmHg with pH <7.25, PaCO2 >50 mm and Downes' score >4 | No baby developed pneumothorax | |
| Koti, 200930 | India | Level III NICU | Prospective observational study | 56 inborn preterm infants (gestation 28 to 34 weeks) with respiratory distress and chest x- ray suggestive of RDS | BCPAP with bi-nasal prongs (Fisher and Paykel Healthcare, New Zealand) started at 5 cm of water and adjusted to minimize chest retractions. FiO2 adjusted to maintain SpO2between 87% and 95% | Two babies had pneumothorax but both stabilized on bCPAP and required neither ventilation nor chest tube drainage | |
| Bassiouny, 199431 | Oman | NICU | Prospective observational study | 44 preterm infants with RDS enrolled | CPAP delivered using Beneveniste's valve and silastic nasal prongs | No pneumothorax reported | |
| Rego, 200232 | Brazil | NICU level 3 | Randomized controlled clinical trial | 99 neonates ⩽2500 g | Randomized to Hudson or Argyle nasal prongs | No pneumothorax reported | |
| CPAP and nasal trauma | |||||||
| Rego, 200232 | Brazil | NICU level 3 | Randomized controlled clinical trial | 99 neonates ⩽2500 g | Randomized to Hudson or Argyle nasal prongs | Argyle nasal prongs associated with more hyperemia No cases of pneumothorax reported in either group | Comparison of two CPAP delivery methods |
| Yong, 200533 | Malaysia | NICU level 3 | Randomized controlled clinical trial | 89 neonates <1500 g | 41 randomized to mask group and 48 to prong group | No significant trauma difference in the two groups Almost all neonates developed trauma | Comparison of two CPAP delivery methods |
| Do Nascimento, 200934 | Brazil | Neonatal unit maternity hospital | Quantitative, descriptive, cross sectional | 147 neonates of which 123 (83.7%) <37 weeks | No mention of duration, CPAP settings or other details Nasal protection observed in 142 (96.6%). 100% received humidification while 127 (86.4%) were heated | 117 neonates had mild (hyperemia), 29 had moderate (bleeding with erosion) and 1 had necrosis in addition to bleeding and erosion | No mention of duration, CPAP settings, or other details |
| CPAP and ROP | |||||||
| Hakeem Abdel, 201236 | Egypt | NICU | Prospective observational study | Preterm neonates <32 weeks and <1500 g Infants whose gestational age >32 weeks or birth weight >1500 g included if exposed to oxygen therapy for> 7 days | Perinatal risk factors for ROP assessed using univariate and multivariate analyses | No association of CPAP therapy with ROP (P>0.05) | No mention of duration, CPAP settings, or other details |
| Kumar, 201137 | India | NICU level 3 | Retrospective evaluation of prospectively collected data | Neonates with gestation ⩽32 weeks or birth weight ⩽1500 g screened. Infants with birth weight of 1501-1800 g or gestation of 33-34 weeks also screened in the presence of additional risk factors | Perinatal risk factors for ROP assessed using univariate and multivariate analyses | CPAP associated with severe ROP on univariate analysis but not on multivariate analysis | No mention of duration, CPAP settings, or other details |
Abbreviations: CPAP, continuous positive airway pressure; LMIC, low- and middle-income countries; NICU, neonatal intensive care unit; ROP, retinopathy of prematurity.
Three studies from LMIC settings had reported the occurrence of nasal trauma after the institution of CPAP therapy.32, 33, 34 The study by Rego suggested increased occurrence of hyperemia with one specific type nasal prongs.32 The studies by Yong33 and Nascimento34 suggested that nasal injury was observed in nearly all neonates instituted on CPAP and the risk was related to the duration of CPAP therapy (Table 3). In the study by Nascimento, mild hyperemia was observed in 79.6% (117/147) neonates and bleeding in 19.7% (29/147) neonates. The study suggested that training and educational programs can improve the care of newborns who are on CPAP and can help prevent complications related to CPAP use.34 Another single center study suggested the utility of silicone gel sheeting to reduce the incidence of nasal injury.35
No study from LMIC had reported the proportion of neonates who developed shock after institution of CPAP therapy. Two studies from LMIC reported no association of retinopathy of prematurity and institution of CPAP therapy.36, 37 Both these studies were retrospective single center studies (Table 3).
Cost-effectiveness of CPAP therapy
Cost per one neonatal death or ventilation averted
No study from LMIC had reported this outcome.
Cost to the health facility and family
One study from Fiji—a retrospective evaluation of prospectively collected data—had reported the cost to the health facility.15 The study included only the costs of the machines. For 6 years before the introduction of bCPAP, the NICU had five ventilators: three Bear Cub, cost of $40 000 each; and two Servo3000, cost of $65 000 each. In May 2003, bCPAP was introduced. Equipment was purchased to provide bCPAP to two neonates at any one time. The costs were $6000 for each CPAP machine and $300 for circuitry.15
We identified one study by Levesque on the impact of implementing five potentially better respiratory practices on neonatal outcomes and costs.38 The implemented practices included the exclusive use of bCPAP, provision of bCPAP in the delivery room, strict intubation criteria and strict extubation criteria, and prolonged CPAP to avoid supplemental oxygen. The study reported that the non-personnel cost of care for neonates <33 weeks' gestation was similar during the first 12 weeks of hospitalization before and after the guideline was implemented. The percentage of hospitalization days spent with a 1:1 staffing ratio was also similar before and after implementation of the guideline. However, the specific cost for surfactant replacement therapy was significantly lower in the latter period.38 The cost of the nine stationary and three portable bCPAP units was much lower than the estimated 2007 cost of replacing the nine out-of-warranty ventilators with new basic model conventional ventilators ($19 500 for bCPAP vs $135 000 for ventilators).
Discussion
CPAP has now become a standard of care for all preterm neonates with respiratory distress. Evidence from high-quality studies suggests significant survival advantage in preterm neonates with severe respiratory distress and managed with CPAP as compared with those managed with only oxygen.9 But the evidence is based on studies from only high-income countries. In the absence of such evidence base from LMICs, one cannot be really sure about the efficacy and safety of CPAP therapy in LMIC settings.
We found a significant reduction in the risk of in-hospital mortality following introduction of CPAP therapy (Figure 2). The pooled effect size (OR 0.34, 95% CI 0.14 to 0.82) suggested similar, if not better, beneficial effect when compared with that reported from high-income countries (relative risk 0.52, 95% CI 0.32 to 0.87). 9Given the nature of studies—before and after and case–control—included in the present review, the quality of evidence is likely to be low. There is a need to generate more evidence on the efficacy of CPAP in preterm neonates from LMICs. It may not be ethical to do randomized studies on the effects of CPAP now but it is definitely possible to have large high-quality observational studies from these settings.
The current review suggested that implementation of CPAP therapy is feasible in level 2 to 3 NICUs of LMICs. Only 25 to 40% of preterm neonates receiving CPAP therapy required mechanical ventilation (and referral, if ventilation facilities not available). A recent systematic review, which suggested a reduction in mechanical ventilation by 30 to 50%, had included studies of neonates managed with only bCPAP; it did not include other potential studies that had evaluated the effect of CPAP on reduction in the need of mechanical ventilation.19, 27 Nurses can institute CPAP easily after 1 to 2 months of training and institution of CPAP has the potential to bring down the requirement and the cost of surfactant therapy.15 This reduction has huge financial implications for LMIC.
The studies on safety of CPAP therapy suggested a very-low risk of pneumothorax (0 to 7.2%). When considering the lack of skilled manpower and the sub-optimal equipments available in most LMIC settings, the low risk is definitely reassuring. The recent systematic review on the efficacy and safety of bCPAP in LMIC settings also reported similar results.39 We found a high risk of nasal trauma in neonates managed with CPAP. Up to 20% neonates developed nasal bleeding in one study.34 This reinforces the need for good nursing care and monitoring.15, 23, 25 With improving survival of very preterm neonates and need for longer duration of CPAP administration, nasal mucosal injury attains importance, given that it predisposes to immediate as well as long-term functional and cosmetic sequelae.40
Implications for policy makers
CPAP appears to be a promising and a safe technology for respiratory support in neonates with respiratory distress. In addition, due to lower initial costs, it has the potential for being up-scaled for management of respiratory distress in developing countries. But factors like cost and availability of consumables and additional equipment like humidifier and availability of skilled staff can limit the up-scaling of CPAP therapy. In addition, the use of CPAP also requires regular training of staff for optimal delivery of CPAP.
Strengths and weaknesses
Ours is possibly the first attempt to review and synthesize the available evidence on the effect of CPAP therapy on major outcomes including mortality and air leaks in preterm neonates. Given the paucity of randomized trials, we included observational studies so as to inform policy making. The studies in this review are limited by their study design and quality. We believe that CPAP is being widely used in LMICs than what is evident from the present review. Given the detailed search, the discrepancy is more to do with the ‘real' paucity of studies from these settings. Possibly, the lack of resources, particularly the manpower, limits the capacity of health care providers from LMICs to publish their experiences in peer-reviewed journals.
Conclusion
Available evidence from observational studies suggests that CPAP is a safe and effective mode of therapy in preterm neonates with respiratory distress in LMIC. It reduces the in-hospital mortality and the need for ventilation thereby minimizing the need for up-transfer to a referral hospital. But given the overall paucity of studies and the low-quality evidence, there is an urgent need for high-quality studies on not only the safety and efficacy but also on the cost effectiveness of CPAP therapy in these settings.
Acknowledgments
We acknowledge Dr Sushil Kumar, University of Minnesota, Minneapolis, MN, USA, for helping us in searching the various databases. The Department of Maternal, Newborn, Child and Adolescent Health and Development (MCA), World Health Organization, Geneva, Switzerland funded this review.
Author contributions
AT applied the search strategy, retrieved articles, extracted data and wrote the manuscript. MJS developed the study protocol, extracted data, did the statistical analysis, and modified the manuscript. AC retrieved articles, extracted data, and modified the manuscript. RA and VKP guided development of the protocol, supervised data extraction, and modified the manuscript. MJS and RA act as the guarantors of the paper.
The authors declare no conflict of interest.
References
- Lawn JE, Blencowe H, Oza S, You D, Lee ACC, Waiswa P et al. Every Newborn: progress, priorities, and potential beyond survival. Lancet 2014; 384(9938): 189–205. [DOI] [PubMed] [Google Scholar]
- Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev 2007; (4): CD003063. [DOI] [PMC free article] [PubMed]
- St Clair C, Norwitz ER, Woensdregt K, Cackovic M, Shaw JA, Malkus H et al. The probability of neonatal respiratory distress syndrome as a function of gestational age and lecithin/sphingomyelin ratio. Am J Perinatol 2008; 25(8): 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanaratnem J, Finer NN. Neonatal acute respiratory failure. Curr Opin Pediatr 2000; 12(3): 227–232. [DOI] [PubMed] [Google Scholar]
- Van Marter LJ, Allred EN, Leviton A, Pagano M, Parad R, Moore M et al. Antenatal glucocorticoid treatment does not reduce chronic lung disease among surviving preterm infants. J Pediatr 2001; 138(2): 198–204. [DOI] [PubMed] [Google Scholar]
- Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet J-M, Carlin JB et al. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med 2008; 358(7): 700–708. [DOI] [PubMed] [Google Scholar]
- Kirpalani H, Millar D, Lemyre B, Yoder BA, Chiu A, Roberts RS et al. A trial comparing noninvasive ventilation strategies in preterm infants. N Engl J Med 2013; 369(7): 611–620. [DOI] [PubMed] [Google Scholar]
- Gregory GA, Kitterman JA, Phibbs RH, Tooley WH, Hamilton WK. Treatment of the idiopathic respiratory-distress syndrome with continuous positive airway pressure. N Engl J Med 1971; 284(24): 1333–1340. [DOI] [PubMed] [Google Scholar]
- Ho JJ, Subramaniam P, Henderson-Smart DJ, Davis PG. Continuous distending pressure for respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev 2002; (2): CD002271. [DOI] [PubMed]
- Diblasi RM. Nasal continuous positive airway pressure (CPAP) for the respiratory care of the newborn infant. Respir Care 2009; 54(9): 1209–1235. [PubMed] [Google Scholar]
- Buckmaster AG, Arnolda G, Wright IMR, Foster JP, Henderson-Smart DJ. Continuous positive airway pressure therapy for infants with respiratory distress in non tertiary care centers: a randomized, controlled trial. Pediatrics 2007; 120(3): 509–518. [DOI] [PubMed] [Google Scholar]
- Sundaram V, Chirla D, Panigrahy N, Kumar P. Current status of NICUs in India: a nationwide survey and the way forward. Indian J Pediatr 2014; 81(11): 1198–1204. [DOI] [PubMed] [Google Scholar]
- Pande S, Hiller JE, Nkansah N, Bero L. The effect of pharmacist-provided non-dispensing services on patient outcomes, health service utilisation and costs in low- and middle-income countries. Cochrane Database Syst Rev 2013; 2: CD010398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroufi A, Chowdhury R, Anchala R, Stevens S, Blanco P, Han T et al. Cost effective interventions for the prevention of cardiovascular disease in low and middle income countries: a systematic review. BMC Public Health 2013; 13: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyamaibole L, Kado J, Qovu JD, Colquhoun S, Duke T. An evaluation of bubble-CPAP in a neonatal unit in a developing country: effective respiratory support that can be applied by nurses. J Trop Pediatr 2006; 52(4): 249–253. [DOI] [PubMed] [Google Scholar]
- Ballot DE, Chirwa TF, Cooper PA. Determinants of survival in very low birth weight neonates in a public sector hospital in Johannesburg. BMC Pediatr 2010; 10: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper CH, Smith J, Maree D, Pohl FC. Is nCPAP of value in extreme preterms with no access to neonatal intensive care? J Trop Pediatr 2003; 49(3): 148–152. [DOI] [PubMed] [Google Scholar]
- Kawaza K, Machen HE, Brown J, Mwanza Z, Iniguez S, Gest A et al. Efficacy of a low-cost bubble CPAP system in treatment of respiratory distress in a neonatal ward in Malawi. PLoS One 2014; 9(1): e86327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeena P, Pillay P, Adhikari M. Nasal CPAP in newborns with acute respiratory failure. Ann Trop Paediatr 2002; 22(3): 201–207. [DOI] [PubMed] [Google Scholar]
- Saxena A, Thapar RK, Sondhi V, Chandra P. Continuous positive airway pressure for spontaneously breathing premature infants with respiratory distress syndrome. Indian J Pediatr 2012; 79(9): 1185–1191. [DOI] [PubMed] [Google Scholar]
- Singh M, Deorari AK, Paul VK, Mittal M, Shanker S, Munshi U et al. Three-year experience with neonatal ventilation from a tertiary care hospital in Delhi. Indian Pediatr 1993; 30(6): 783–789. [PubMed] [Google Scholar]
- Rojas MA, Lozano JM, Rojas MX, Laughon M, Bose CL, Rondon MA et al. Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: a randomized, controlled trial. Pediatrics 2009; 123(1): 137–142. [DOI] [PubMed] [Google Scholar]
- Hendriks HJ Is CPAP a feasible treatment modality in a rural district hospital for neonates with respiratory distress syndrome?. MSc thesis, University of Stellenbosch, South Africa, 2010.
- Shrestha M, Basnet S, Shrestha P. Bubble CPAP in Neonatal Unit of TUTH. J Nepal Paediatr Soc 2010; 30: 64–68. [Google Scholar]
- Van den Heuvel M, Blencowe H, Mittermayer K, Rylance S, Couperus A, Heikens GT et al. Introduction of bubble CPAP in a teaching hospital in Malawi. Ann Trop Paediatr 2011; 31(1): 59–65. [DOI] [PubMed] [Google Scholar]
- Kirsten GF, Kirsten CL, Henning PA, Smith J, Holgate SL, Bekker A et al. The outcome of ELBW infants treated with NCPAP and InSurE in a resource-limited institution. Pediatrics 2012; 129(4): e952–e959. [DOI] [PubMed] [Google Scholar]
- Boo NY, Zuraidah AL, Lim NL, Zulfiqar MA. Predictors of failure of nasal continuous positive airway pressure in treatment of preterm infants with respiratory distress syndrome. J Trop Pediatr 2000; 46(3): 172–175. [DOI] [PubMed] [Google Scholar]
- Urs PS, Khan F, Maiya PP. Bubble CPAP - a primary respiratory support for respiratory distress syndrome in newborns. Indian Pediatr 2009; 46(5): 409–411. [PubMed] [Google Scholar]
- Pillai MS, Sankar MJ, Mani K, Agarwal R, Paul VK, Deorari AK. Clinical prediction score for nasal CPAP failure in pre-term VLBW neonates with early onset respiratory distress. J Trop Pediatr 2011; 57(4): 274–279. [DOI] [PubMed] [Google Scholar]
- Koti J, Murki S, Gaddam P, Reddy A, Reddy MDR. Bubble CPAP for respiratory distress syndrome in preterm infants. Indian Pediatr 2010; 47(2): 139–143. [DOI] [PubMed] [Google Scholar]
- Bassiouny MR, Gupta A, el Bualy M. Nasal continuous positive airway pressure in the treatment of respiratory distress syndrome: an experience from a developing country. J Trop Pediatr 1994; 40(6): 341–344. [DOI] [PubMed] [Google Scholar]
- Rego MAC, Martinez FE. Comparison of two nasal prongs for application of continuous positive airway pressure in neonates. Pediatr Crit Care Med 2002; 3(3): 239–243. [DOI] [PubMed] [Google Scholar]
- Yong S-C, Chen S-J, Boo N-Y. Incidence of nasal trauma associated with nasal prong versus nasal mask during continuous positive airway pressure treatment in very low birthweight infants: a randomised control study. Arch Dis Child Fetal Neonatal Ed 2005; 90(6): F480–F483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Nascimento RM, Ferreira ALC, Coutinho ACFP, Santos Veríssimo RCS. The frequency of nasal injury in newborns due to the use of continuous positive airway pressure with prongs. Rev Lat Am Enfermagem 2009; 17(4): 489–494. [DOI] [PubMed] [Google Scholar]
- Günlemez A, Isken T, Gökalp AS, Türker G, Arisoy EA. Effect of silicon gel sheeting in nasal injury associated with nasal CPAP in preterm infants. Indian Pediatr 2010; 47(3): 265–267. [DOI] [PubMed] [Google Scholar]
- Hakeem AHAA, Mohamed GB, Othman MF. Retinopathy of prematurity: a study of prevalence and risk factors. Middle East Afr J Ophthalmol 2012; 19(3): 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Sankar MJ, Deorari A, Azad R, Chandra P, Agarwal R et al. Risk factors for severe retinopathy of prematurity in preterm low birth weight neonates. Indian J Pediatr 2011; 78(7): 812–816. [DOI] [PubMed] [Google Scholar]
- Levesque BM, Kalish LA, LaPierre J, Welch M, Porter V. Impact of implementing 5 potentially better respiratory practices on neonatal outcomes and costs. Pediatrics 2011; 128(1): e218–e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Duke T, Davis P. Efficacy and safety of bubble CPAP in neonatal care in low and middle income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed 2014; 99(6): F495–F504. [DOI] [PubMed] [Google Scholar]
- Smith LP, Roy S. Treatment strategy for iatrogenic nasal vestibular stenosis in young children. Int J Pediatr Otorhinolaryngol 2006; 70(8): 1369–1373. [DOI] [PubMed] [Google Scholar]