Abstract
Surfactant replacement therapy (SRT) has been shown to reduce mortality and air leaks in preterm neonates from high-income countries (HICs). The safety and efficacy of SRT in low- and middle- income countries (LMICs) have not been systematically evaluated. The major objectives of this review were to assess the (1) efficacy and safety, and (2) feasibility and cost effectiveness of SRT in LMIC settings. We searched the following databases—MEDLINE, CENTRAL, CINAHL, EMBASE and WHOLIS using the search terms 'surfactant' OR 'pulmonary surfactant'. Both experimental and observational studies that enrolled preterm neonates with or at-risk of respiratory distress syndrome (RDS) and required surfactant (animal-derived or synthetic) were included. A total of 38 relevant studies were found; almost all were from level-3 neonatal units. Pooled analysis of two randomized controlled trials (RCTs) and 22 observational studies showed a significant reduction in mortality at the last available time point in neonates who received SRT (relative risk (RR) 0.67; 95% confidence interval (CI) 0.57 to 0.79). There was also a significant reduction in the risk of air leaks (five studies; RR 0.51; 0.29 to 0.90). One RCT and twelve observational studies reported the risk of bronchopulmonary dysplasia (BPD) with contrasting results; while the RCT and most before-after/cohort studies showed a significant reduction or no effect, the majority of the case-control studies demonstrated significantly higher odds of receiving SRT in neonates who developed BPD. Two studies—one RCT and one observational—found no difference in the proportion of neonates developing pulmonary hemorrhage, while another observational study reported a higher incidence in those receiving SRT. The failure rate of the intubate-surfactant-extubate (InSurE) technique requiring mechanical ventilation or referral varied from 34 to 45% in four case-series. No study reported on the cost effectiveness of SRT. Available evidence suggests that SRT is effective, safe and feasible in level-3 neonatal units and has the potential to reduce neonatal mortality and air leaks in low-resource settings as well. However, there is a need to generate more evidence on the cost effectiveness of SRT and its effect on BPD in LMIC settings.
Introduction
Preterm birth complications are the most common cause of death in children aged 5 years or less. Out of the 6.3 million children who died before age 5 in 2013, about 1 million (15.4%) died because of these conditions.1 The major morbidities that result in deaths in preterm neonates include respiratory distress syndrome (RDS), intraventricular hemorrhage and necrotizing enterocolitis. RDS, in addition to being a direct cause of mortality, also contributes indirectly by increasing the risk of intraventricular hemorrhage, bronchopulmonary dysplasia (BPD) and nosocomial infections such as ventilator-associated pneumonia.2
RDS is caused by the deficiency of pulmonary surfactant in preterm neonates. Its incidence increases with decreasing gestational age, the risk being 60% in less than 28 weeks and 30% between 28 and 34 weeks of gestation. If left untreated, it leads to high mortality; the reported case fatality is 57 to 89% in low- and middle-income countries (LMICs).3 The assessment of the burden of RDS, in general, and in LMICs, in particular, is difficult. Assuming the incidence of RDS to be 1% of all live births—a relatively conservative estimate—approximately 1.4 million neonates develop RDS every year across the globe (total live births 137 688 million per year).1
In addition to optimal respiratory support in the form of continuous positive airway pressure (CPAP) or mechanical ventilation and good supportive care, surfactant replacement therapy (SRT) forms the mainstay in the management of RDS. Since the report by Fujiwara in 1980 of the first successful use of SRT,4 numerous randomized controlled trials (RCTs) and their meta-analyses have established its efficacy in reducing mortality and air leak syndromes in RDS.5, 6 Almost all the trials evaluating the role of SRT were conducted in high-income countries (HICs). Not surprisingly, SRT is the standard of care in neonates with RDS in these countries.
Can we extrapolate the evidence from HICs to LMICs and recommend SRT as the standard of care? The answer to this question is critical because the high cost of the drug, lack of skilled personnel and a sub-optimal support system7 preclude an immediate and effective roll-out of the intervention in most neonatal units of LMICs. The paucity of evidence for efficacy and/or safety of SRT in these settings further adds to the complexity. With the lack of skilled manpower and optimal respiratory support, SRT may not be as effective; moreover, there may be safety concerns due to inadequate supervision and monitoring. The present systematic review therefore aimed to synthesize the evidence on the efficacy, safety, feasibility and cost effectiveness of implementing SRT in LMIC settings. The findings of the review should help policy makers and other stakeholders to make an informed decision regarding the introduction and/or scaling-up of the intervention in different LMIC settings.
Methods
Objectives
The two major objectives of this review were to evaluate (1) the efficacy and safety of SRT and (2) the feasibility and cost effectiveness of introducing and implementing SRT in LMIC settings.
Types of studies
For objective 1, we included all studies—both observational and experimental (RCTs and quasi-randomized trials)—from LMICs that compared the effects of SRT with no or placebo therapy in preterm neonates with RDS. For objective 2, we included all studies that reported the use of SRT in preterm neonates with or at-risk of RDS.
Interventions
All studies that compared the effect of single or multiple doses of surfactant therapy by the intratracheal route (animal-derived or synthetic surfactant preparation) with no/placebo treatment in eligible neonates were included.
Outcomes
Table 1 provides the list of critical outcomes and their definitions.
Table 1. Objectives and outcomes.
| Objectives | Outcomes | Definition |
|---|---|---|
| 1a. To evaluate the efficacy of SRT in neonates with RDS from LMIC settings | • Neonatal mortality • In-hospital mortality • Bronchopulmonary dysplasia • Air leaks | • All-cause death in the first 28 days of life • All-cause death during the initial hospital stay • Use of supplemental oxygen at 36 weeks postmenstrual age • Any air leak syndromes such as pulmonary interstitial emphysema, pneumothorax, pneumomediastinum etc. |
| 1b. To evaluate the safety of SRT in neonates with RDS from LMIC settings | • Incidence/prevalence of pulmonary hemorrhage • Incidence/prevalence of complications such as apnea, hypoxia/arrest during or immediately after SRT | • Proportion of neonates developing pulmonary hemorrhage (blood from the endotracheal tube associated with an increase in ventilator support, oxygen requirement or blood product replacement) during or immediately after SRT • Proportion of neonates with apnea (cessation of breathing for >20 s or for lesser duration but accompanied by bradycardia or cyanosis), hypoxia (SpO2 <85%) or arrest requiring cardiopulmonary resuscitation |
| 2a. To assess the feasibility of introducing and implementing SRT at both population and health facility level | • Proportion of neonates who received the entire dose of surfactant successfully • Proportion of neonates who needed referral to higher centers immediately after SRT | • Proportion of neonates who were administered one or multiple doses of surfactant without any immediate complications such as tube block, desaturations, bradycardia that warrant stoppage of SRT • Proportion of neonates who were referred to higher centers (NICU of the same hospital/other hospitals) for immediate or late complications including failure of surfactant therapy with or without CPAP |
| 2b. To evaluate the cost effectiveness of implementing SRT as compared with no surfactant therapy | Cost per -One neonatal death averted -One air leak syndrome averted -One DALY saved -One additional quality adjusted life year -One life year gained | DALY: number of years lost because of ill health, disability or early death. Quality adjusted life years: number of years of life that would be added by intervention. |
Abbreviations: CPAP, continuous positive airway pressure; DALY, disability-adjusted life year; LMIC, low- and middle-income countries; NICU, neonatal intensive care unit; RDS, respiratory distress syndrome; SRT, surfactant replacement therapy.
Search methods for identification of studies
We used two different strategies to identify the relevant studies. First, we updated two Cochrane reviews on the effects of animal-derived surfactant5 and synthetic surfactant.6 We searched the electronic bibliographic databases including MEDLINE, Cochrane CENTRAL, Embase and CINAHL from year 1998 to July 2013 using the search terms 'surfactant' OR 'pulmonary surfactant'. The search was limited by using the filters ‘human studies', ‘clinical trial' and ‘newborn'. No language restrictions were used. In addition, we also scanned the list of excluded studies in the Cochrane reviews for any observational studies on SRT.
In the second strategy, we searched PubMed, Cochrane CENTRAL and WHOLIS till July 2013. We used the following search terms for searching PubMed—(surfactant OR pulmonary surfactant) AND 'LMIC'. The search terms for LMIC were adapted from two earlier reviews.8, 9 Similar terms were used for searching the other databases. For both strategies, we updated the search to December 2014. Because of practical difficulties, we could search EMBASE and CINAHL from 2007 to 2013 only.
We scanned the title and abstract of the retrieved citations to exclude those that were obviously irrelevant. We obtained the full text of the remaining studies to identify relevant articles.
Data extraction and management
Two review authors (MJS and NG) independently identified and assessed the studies for inclusion in this review. Each reviewer separately extracted data using the standardized data extraction forms. Any disagreements were resolved through discussion with the third author (RA).
Methodological quality
We independently evaluated the methodological quality of the included studies using the standard methods of the Cochrane Review Group including allocation concealment, blinding and completeness of follow up (classified as yes, no or unclear). For objective 2, we did not carry out any quality assessment (because of the nature of the studies included).
Statistical analysis
Meta-analysis was performed using Stata 11.2 software (StataCorp, College Station, TX, USA). Pooled estimates were calculated from the relative risks (RRs) and 95% CIs of the individual studies by generic inverse variance method with the user-written ‘metan' command in Stata. We examined heterogeneity among the included studies by eyeballing the forest plots and quantified it using the I2 statistic. If significant heterogeneity existed in both direction and magnitude of the effect sizes of individual studies, we did not pool them. If heterogeneity was essentially due to a difference in the magnitude and not in the direction of effect sizes, we pooled the studies by the random effects model.
Results
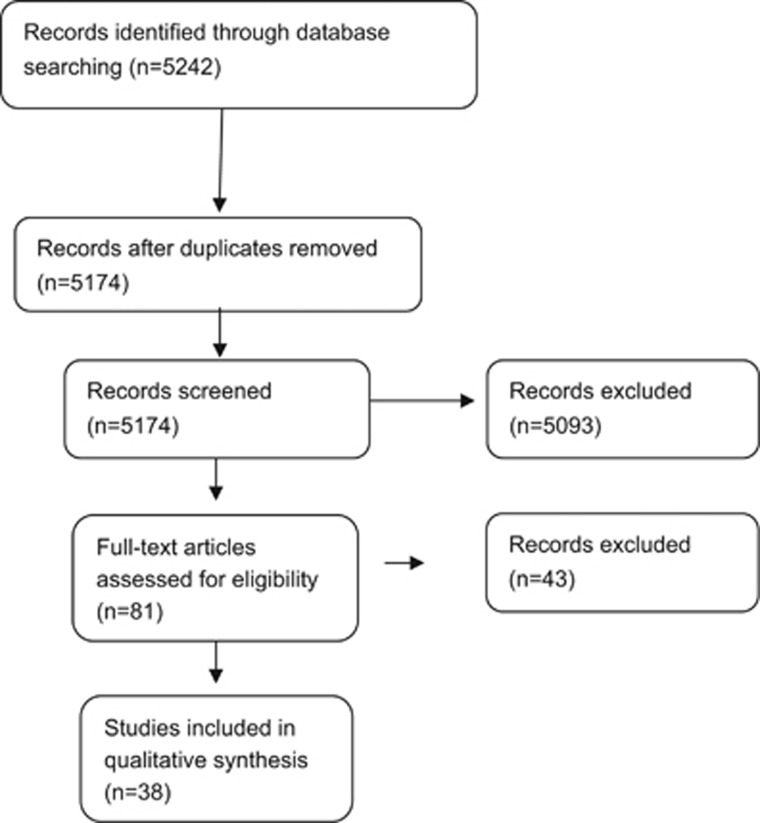
Figure 1 depicts the number of studies identified by the two search strategies.
Figure 1.
Flow chart depicting the selection of studies included in the meta-analysis.
The Cochrane review on animal-derived surfactant treatment included 13 RCTs conducted in level-3 neonatal intensive care units (NICUs) of HICs.5 The other review on synthetic surfactant treatment in established RDS included six RCTs and was last updated in 1998.6 Upon updating the two reviews, we did not identify any additional eligible studies for inclusion in our review.
After screening the title/abstract or assessing the full text of potentially eligible articles identified by the second search strategy, we included a total of 38 studies: two RCTs, 12 before-and-after studies, eight each of concurrent control and case-control studies, and eight case-series from LMICs. Descriptions of the observational studies have been provided as and when applicable in the subsequent sections.
Effect on mortality
We identified two RCTs from LMICs that examined the effect of surfactant therapy on mortality in preterm neonates with RDS. One RCT compared the effect of synthetic surfactant with no surfactant and found no difference in the risk of mortality (RR 0.73; 95% CI 0.37 to 1.42).10 In another RCT, preterm neonates (24–31 weeks gestation) with signs of respiratory distress within 60 min of birth were randomized into nasal intermittent positive pressure ventilation (NIPPV)+surfactant or NIPPV alone. Both groups were initially stabilized with CPAP and were switched to NIPPV within the first hour. Surfactant therapy was administered to either group (repeat dose for NIPPV+surfactant group and first dose for NIPPV group) if the infants needed a FiO2 of >0.45 to maintain the targeted saturation. There was no statistically significant difference in mortality between the two groups (RR 0.52; 95% CI 0.05 to 5.40).11
A total of 22 observational studies reported the effect of SRT on in-hospital or neonatal mortality in preterm neonates with RDS (Table 2). These studies can be broadly categorized into three groups: before and after time-series/comparison with historical controls, comparison with concurrent controls—those who received and those who did not receive surfactant therapy—and case-control studies (‘typical' case-control or analysis of prospective data as though it were a case-control study).
Table 2. Studies on effectiveness and feasibility of SRT in LMIC settings.
|
Studies with a control group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author, year | Country | Setting (description, if available) | Study design | Exposed group | Control group | Results | Effect size RR/mean difference (95% CI) | Comments | ||
| Flores-Nava et al.10 | Mexico | NICU | RCT | Preterm neonates (<34 weeks) with RDS and received four doses of synthetic surfactant (n=20) | Preterm neonates (<34 weeks) with RDS and received air as placebo (n=20) | Mortality Pneumothx Pulmonary hemorrhage | Exp 8 (40) 2 (10) 3 (15) | Unexp 11 (55) 4 (20) 3 (15) | 0.73 (0.37–1.42) 0.5 (0.10–2.43) 1.0 (0.23–4.37) | Full text in Spanish; information obtained from abstract which is available in English |
| Duman et al.11 | Turkey | NICU; university hospital | RCT | Preterm neonates (24–31 weeks) with signs of respiratory distress within 60 min of life who were initially stabilized on NCPAP and were switched to NIPPV within the first hour and received very early surfactant. Repeat dose was administered if the infants needed a FiO2 of >0.45 to maintain the targeted saturation; then they continued with invasive mechanical ventilation (n=29) | Preterm neonates (24–316/7 weeks) with signs of respiratory distress within 60 min of life who were initially stabilized on NCPAP and were switched to NIPPV within the first hour and did not receive very early surfactant. However, this group received surfactant if the infants needed a FiO2 of >0.45 to maintain the targeted saturation; then they continued with invasive mechanical ventilation (n=30) | Mortality BPD Pneumothx Severe IVH | Exp 1 (3.4) 11 (35.7) 0 (0) 2 (6.9) | Unexp 2 (6.6) 12 (44.4) 1 (3.6) 3 (10) | 0.51 (0.49–5.40) 0.95 (0.50–1.80) — 0.69 (0.12–3.83) | Surfactant therapy was administered to either group (repeat dose for NIPPV+surfactant and first dose for NIPPV) if the infants needed a FiO2 of >0.45 to maintain the targeted saturation; then they continued with invasive mechanical ventilation |
| Ballot et al.12 | South Africa | NICU; university hospital | Comparison of two time periods—before and after introduction of SRT | Neonates admitted between Nov 1991 and Nov 1992 and received surfactant as part of RCT protocol that involved four groups of surfactant therapy on the basis of FiO2 requirement (n=78) | Neonates admitted between 1989 and June 1991 before introduction of SRT (n=156) | Mortality BPD 36 weeks Pneumothx Severe IVH | Exp 9 (11.5) 2 (2.6) 5 (6.4) 10 (12.8) | Unexp 21 (13) 10 (6.4) 27 (17) 16 (10) | Unadjusted RR0 86 (0.41–1.78) 0.4 (0.09–1.78) 0.37 (0.15–0.92) 1.25 (0.6–2.62) | 32 (41%) Neonates did not receive surfactant in the exposed group; exposed group had two sub-groups—moderate and severe RDS. We combined the data of the two groups |
| Cooper et al.13 | South Africa | NICU; university hospital | Time-series: from comparison of outcomes of low BW neonates from1950 to 1996 | — | — | For each 100 g category between 800 and 1300 g, there was an increase in survival of 10–15% between 1990/91 and 1995/96 | — | Authors attributed this increased survival between these two periods to use of antenatal steroids and surfactant therapy | ||
| Rossello et al.14 | Five Latin American countries | 19 NICUs;?referral hospitals | Comparison with historical controls (born in the previous 2 years) | Preterm neonates with RDS and received surfactant (n=348) | Preterm neonates with RDS who did not receive surfactant (n=348) | Neonatal mortality In-hospital mortality BPD | Exp NA NA NA | Unexp NA NA NA | BW-stratified RR 0.79 (0.68–0.92) 0.82 (0.71–0.94) 1.8 (1.34–2.42) | |
| Lim et al.15 | Malaysia | ?NICU of university hospital | Comparison with historical controls | Preterm neonates weighing 800 g or more with features of RDS and received surfactant (Survanta) (n=30) | Preterm neonates with RDS who did not receive surfactant (n=30) | Mortality Vent. durn (days) | Exp 3 (10) 7.5 | Unexp 10 (33) 18.9 | Unadjusted RR 0.3 (0.09 to 0.98) Mean difference; ? P=0.02 | Full text not available |
| Ho and Chang16 | Malaysia | NICU; referral hospital; six ventilator beds | Comparison of two time periods—before and after introduction of evidence-based practices including SRT | VLBW neonates admitted between Jan and June 2003 (n=60); 45% of them received surfactant | VLBW neonates between Jan and Jun 1993 (n=69); 10% of them received surfactant | Mortality BPD 28d Pneumothx | Exp 11 (18.3) 2 (3.3) 1 (1.7) | Unexp 26 (37.7) 2 (2.9) 6 (8.7) | Unadjusted RR 0.49 (0.26–0.90) 1.15 (0.17–7.91) 0.19 (0.02–1.55) | Outcome data of only those babies with RDS or those who received surfactant not available |
| Kopelman et al.17 | Brazil | NICU; ?referral hospital | Comparison of two time periods—before and after introduction of SRT | Neonates admitted between Jan and Dec 1992 (n=30) | Neonates admitted between Jan and Dec 1991 before introduction of SRT (n=36) | Mortality Pneumothx | Exp NA NA | Unexp NA NA | P<0.05 P<0.05 | Full text not available |
| Tapia et al.18 | Chile | NICU; ?referral hospital | Comparison of two time periods—before and after introduction of SRT | Neonates admitted between Dec 1990 and Nov 1991 and received surfactant (n=73) | Neonates admitted between Dec 1989 and Nov 1990 before introduction of SRT (n=77) | Mortality Survival without BPD Air leaks Apnea | Exp 22 (30.1) 39 (53.4) NA 12 (21.9%) | Unexp 44 (57.1) 27 (35.1) NA 3 (3.9%) | Unadjusted RR 0.53 (0.35–0.79) 1.52 (1.05–2.21) P>0.05 4.22 (1.24–14.3) | Full text in Spanish; information obtained by using Google Translator |
| Verhagen et al.19 | Curaçao | NICU; ?university hospital | Comparison of two time periods—before and after introduction of SRT | VLBW neonates between 1994 and 1998 (n=32) | VLBW neonates admitted between 1991 and 1994 (n=54) | Mortality BPD ROP | Exp 8 (25) 9 (28) 3 (9) | Unexp 17 (31.5) 16 (30) 0 (0) | Unadjusted RR 0.79 (0.39–1.63) 0.95 (0.48–1.89) | Full text not available |
| Barria et al.20 | Chile | NICUs; public referral hospitals | Time-series; prospective data from database of Surfactant National Program for the period between 1998 and 2005 | Surfactant use in neonates born before 32 weeks increased from 24% in 1998 to 51.1% in 2005 | — | — | There was a 15.3% relative reduction in mortality during the period from 1998 to 2005 | Full text in Spanish—data extracted with the help of Google Translator | ||
| Prigenzi et al.21 | Brazil | NICU; level-3 hospital | Comparison of two time periods | VLBW neonates admitted between 1995 and 1997;14% of them received surfactant | VLBW neonates between 1998 and 2000; 28% of them received surfactant | — | Mortality decreased from 36.2% to 29.5% | Full text in Portuguese;There was a concomitant increase in use of antenatal steroids also (from 25 to 42%) | ||
| Fustinana et al.22 | Argentina | NICU; ?referral hospital | Comparison of two time periods—before and after introduction of SRT | VLBW neonates admitted between 1989 and 1992 (n=145) | VLBW neonates between 1993 and 2002 (n=342) | Mortality in different BW groups: 500–749 750–999 1000–1299 1250–1500 | Exp 1/20 11/33 24/36 41/55 | Unexp 18/45 49/72 76/95 107/119 | RR 0.12 (0.02–0.87) 0.49 (0.29–0.81) 0.83 (0.65–1.07) 0.83 (0.70–0.98) | Overall, the survival rate improved significantly between the two periods by 23% from 52 to 75% |
| Chye and Lim23 | Malaysia | NICU; university hospital | Retrospective cum prospective observational study | Neonates with RDS who received IMV and surfactant (n=?) | Neonates with RDS who received IMV but did not receive surfactant (n=?) | NA | Adjusted OR for survival 3.02 (1.49–6.1) | — | ||
| Narang et al.24 | India | NICU; tertiary referral hospital | ?prospective study | Neonates with RDS who received surfactant (n=88) | Neonates with RDS who did not receive surfactant (n=119) | Mortality Air leak BPD 28d PDA Sepsis | Exp 33 (37.5) 7 (7.9) 6/65 41 (46.6) 28 (31.8) | Unexp 67 (56.3) 11 (9.9) 12/48 70 (58.8) 60 (50.4) | Unadjusted RR 0.67 (0.49–0.91) 0.86 (0.35–2.13) 0.37 (0.15–0.91) 0.79 (0.6–1.04) 0.63 (0.44–0.90) | Surfactant treatment was based on the affordability of the family |
| Qian et al.25 | China | NICU; tertiary maternity and child hospitals | Secondary analysis of prospectively collected data form a network of NICUs | Neonates born before 35 weeks and received surfactant (mostly for RDS) (n=238) | Neonates born before 35 weeks but did not receive surfactant (n=639) | Mortality | Exp 60 (25) | Unexp 211 (33) | 0.76 (0.6–0.98) | The objective of the study was to evaluate the outcome of neonatal respiratory failure |
| Qian et al.26 | China | NICUs; level-3 hospitals | Prospective data collected from a network of 23 NICUs | Neonates with RDS and received surfactant (n=241) | Neonates with RDS but did not receive surfactant (n=470) | Mortality | Exp 51 (21.1) | Unexp 172 (36.6) | 0.58 (0.44–0.76) | — |
| Wang et al.27 | China | NICU; referral hospitals | Retrospective chart review | Neonates with RDS who received surfactant (n=1624) | Neonates with RDS who did not receive surfactant (n=1343) | Mortality | Exp 327(20.1) | Unexp 376(28) | Unadjusted RR 0.72 (0.63–0.82) Adjusted OR (multivariate) 0.43 (0.36–0.51) | The objective of the study was to evaluate the outcome of neonatal respiratory failure |
| Cai et al.28 | China | ?NICU of referral hospital | ?RCT | Neonates with RDS who received surfactant (n=29) along with NIPPV | Neonates with RDS who received only NIPPV (n=17) | Mortality | Exp 28 (96.4) | Unexp 12 (70.1) | Unadjusted RR 1.37 (1.0–1.87) | Full text not available |
| Li et al.29 | China | ?NICU of referral hospital | Retrospective chart review | Neonates with RDS who received surfactant (n=14) | Neonates with RDS who received only CPAP and not surfactant (n=8) | Mortality | Exp 2 (20.1) | Unexp 1 (28) | Unadjusted RR 1.14 (0.12–10.7) | The study had one more group of neonates who were treated with intravenous mucosolva—a surface active agent. Data from this group was not included |
| Sun et al.30 | China | NICU; ?level-3 in Hebei province | ?review that includes data from two prospective multicenter studies | Preterm neonates who received surfactant for RDS (n=514) | Preterm neonates with RDS who did not receive surfactant (n=367) | Mortality | Exp 136 (26.4) | Unexp 152 (41.4) | Unadjusted RR 0.64 (0.53–0.77) | Data from network in Hebei province provided here; data from the other network already included25 |
| Malaysian VLBW study Group31 | Malaysia | 23 NICUs; district as well as university hospitalsAll NICUs had ventilator facilities | Prospective data collection; analyzed like a case–control study | Cases: VLBW neonates who died during initial hospital stay (n=543) | Controls: VLBW neonates who survived until discharge (n=325) | Surfactant | Cases 8 (1.5) | Controls 8 (2.5) | Adjusted OR 0.1 (0.0–0.2) | Proportion of neonates who received surfactant was very low in both groups |
| Boo et al.32 | Malaysia | NICU referral maternity hospital | ?Prospective study—analyzed like case–control | Cases: ELBW neonates who died during initial hospital stay (n=103) | Controls: ELBW neonates who survived until discharge (n=49) | Surfactant | Cases 17 (16.5) | Controls 12 (24.4) | Unadjusted OR 1.6 (0.7–4.1) Adjusted OR ?; P>0.05 | Proportion of neonates who received surfactant was low in both groups |
| Grupo Colaborativo Neocosur33 | Four South American countries (Argentina, Chile, Peru and Uruguay) | NICU;?referral hospitals | Prospective observational study; analyzed like a case–control study | Cases: VLBW neonates who died during initial hospital stay (n=104) | Controls: VLBW neonates who survived till discharge (n=281) | Surfactant | Cases NA | Controls NA | Adjusted OR ? but P>0.05 | Surfactant therapy was not a risk factor for death but was a significant factor for BPD |
| Cases: VLBW neonates with BPD (n=88) | Controls: VLBW neonates without BPD (n=297) | Surfactant | Cases NA | Controls NA | Adjusted OR 4.7 (1.18–18.7) | |||||
| Cunha et al.34 | Brazil | NICU; university hospital | Prospective study—analyzed like case–control | Cases: VLBW neonates who required ventilation in first week and developed BPD (n=45) | Controls: VLBW neonates who required ventilation in first week but did not develop BPD (n=41) | Surfactant | Cases 23 (51.1) | Controls 10 (24.4) | Unadjusted RR 1.68 (1.14–2.48) Adjusted OR ?; P>0.05 | On multivariate analysis, surfactant therapy was not found to be a risk factor of BPD |
| Tapia et al.35 | South American countries (Argentina and Chile) | NICU; ?referral hospitals | Prospective observational study; analyzed like a case–control study | Cases: VLBW neonates with BPD (n=445) | Controls: VLBW neonates without BPD (n=1380) | Surfactant | Cases NA | Controls NA | Adjusted OR 1.40 (1.08–1.82) | — |
| Demirel et al.36 | Turkey | NICU; ?referral hospital | Case–control study | Cases: Extramural (outborn) VLBW neonates with BPD any stage (n=56) | Controls: Outborn VLBW neonates without BPD (n=50) | Surfactant | Cases 20 (35.7) | Controls 6 (12) | Adjusted OR for moderate/severe BPD 7.54 (2.15–26.4) | For logistic regression, authors combined the mild BPD group (~50% of total BPD) with no BPD group, and then compared them with moderate /severe BPD |
| Goncalves et al.37 | Brazil | NICU; ?level-3 referral hospital | Retrospective chart review | Cases: Preterm (<34 weeks) neonates with BPD (n=13) | Controls: Preterm (<34 week) neonates without BPD (n=200) | Surfactant | Cases 10 (76.9) | Controls 29(14.5) | Adjusted OR 5.3 (1.1–26.5) | Article in Portuguese |
| Lima et al.38 | Brazil | NICU; level-3 hospital | Cross-sectional study; analyzed like case–control | Cases: VLBW neonates with BPD (n=57) | Controls: VLBW neonates without BPD (n=266) | Surfactant | Cases 46 (80.7) | Controls 114 (42.9) | Unadjusted OR 5.58 (2.68–12.4) | Infants who received surfactant had significantly lower birth weight and gestational age |
|
Studies with no control group | |||||||
|---|---|---|---|---|---|---|---|
| Author, year | Country | Setting | Study design | Study population | Surfactant – type, strategy | Results | Comments |
| Kirsten et al.42 | South Africa | Neonatal high-care ward with no backup ventilation; referral hospital | Case series | InSurE method applied to extremely low BW neonates born at or after 25 weeks AND failed CPAP (n=97) | InSurE for those who failed CPAP (FiO2 ⩾0.4 after 1 h of CPAP, severe chest retractions, apnea); natural surfactant (Poractant) | Failure of InSurE: 33 (34.0%) Mortality in first week: 25 (25.8%) Mortality till discharge: 36 (37.1%) | Out of the 33 neonates who failed InSurE, 15 were admitted to NICU and required mechanical ventilation; CPAP failure (requirement of InSurE): 31.4% |
| Afjeh and Sabzehei43 | Iran | NICU; ?university hospital | Case series | Preterm neonates born at 25–32 weeks with BW 600–1500 g and having RDS (n=66) | InSurE method (extubation within 2–3 min following surfactant therapy) | Failure of InSurE: 39.4% success was dependent upon gestation | About 40% of preterm neonates (25–32 weeks) fail InSurE therapy and would require mechanical ventilation (i.e., referral if adequate facilities are not available) |
| Kanmaz et al.41 | Turkey | NICU; teaching hospital | RCT comparing surfactant administration via thin catheter with tracheal instillation; only data from the second group presented | Preterm neonates born before 32 weeks and having RDS with FiO2 requirement of ⩾0.4 in first 2 h of life and received surfactant by InSurE method (n=100) | InSurE method (extubation immediately following surfactant therapy);natural surfactant (Poractant) | Requirement of mechanical ventilation in first 72 h—45% | About 45% of preterm neonates (<32 wk) fail InSurE therapy and require mechanical ventilation;need for ventilation was significantly lower in the thin catheter group (30% RR 0.52; CI 0.29–0.94) |
| Tagare et al.44 | India | NICU; level-3 referral hospital | Case series | Preterm neonates with RDS on CPAP for at least 30 min with FiO2 ⩾0.4 in first 6 h and received InSurE (n=28) | InSurE method (extubation immediately following surfactant therapy);natural surfactant (Beractant) | Failure of InSurE: 43% | 43% Failed InSurELater age at InSurE was the only factor which emerged significant after multivariate analysis (4.9 vs 2.5 h) |
Abbreviations: BPD, bronchopulmonary dysplasia; BW, birth weight; CI, confidence interval; ELBW, extremely low birth weight; Exp, exposed; IMV, intermittent mandatory ventilation; InSurE, intubate-surfactant-extubate; IVH, intraventricular hemorrhage; LMIC, low- and middle- income countries; NA, information not available; NCPAP, nasal continuous positive airway pressure; NICU, neonatal intensive care unit; NIPPV, nasal intermittent positive pressure ventilation; OR, odds ratio; PDA, patent ductus arteriosus; Pneumothx: pneumothorax; RCT, randomized controlled trial; RDS, respiratory distress syndrome; ROP, retinopathy of prematurity; RR, relative risk; SRT, surfactant replacement therapy; Unexp, unexposed; Vent. durn, ventilation duration; VLBW, very low birth weight.
Before-and-after studies/comparison with historical controls
Eleven studies compared the in-hospital/neonatal mortality of neonates with RDS admitted in two time periods—before and after introduction of SRT.12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 Of these studies, nine reported a significant reduction in mortality between the two time periods.13, 14, 15, 16, 17, 18, 20, 21, 22 One study from South Africa, which compared the mortality of neonates born between 1991 and 1992 with those born between 1989 and June 1991, did not report any difference (RR 0.86; 95% CI 0.41 to 1.78).12 However, surfactant was administered only in severe RDS with a high FiO2 requirement in the former period; about 41% of neonates with RDS did not receive surfactant. Another study from Curaçao compared the outcomes of very low birth weight (VLBW) neonates admitted between 1994 and 1998 with those admitted between 1991 and 1994 and found no significant difference in mortality.19
Comparison of concurrent controls
Eight studies compared the risk of mortality between neonates who received SRT with those who did not receive it (Table 2).23, 24, 25, 26, 27, 28, 29, 30 In most studies, the decision to administer surfactant was based on financial considerations. Six studies reported a significant reduction in mortality in the surfactant treated group, the RRs varying from 0.43 to 0.76.23, 24, 25, 26, 27, 30 Two studies from China that compared surfactant therapy with NIPPV/CPAP with only NIPPV/CPAP reported no significant reduction in mortality.28, 29
Case-control studies
Three studies compared the odds of receiving surfactant therapy in those who died with those who survived to discharge.31, 32, 33 The proportion of neonates who received surfactant was low (<25%) in two studies;31, 32 it was not reported in the third.33 Although one study reported significantly lower odds of mortality in neonates who received SRT(32),31 the other two did not report any reduction.32, 33
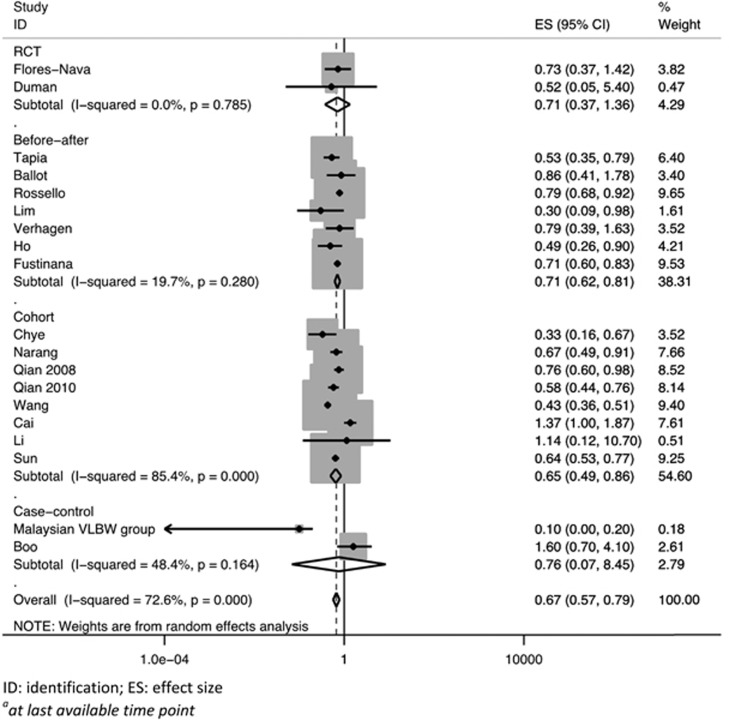
Pooled analysis of all the studies including the two randomized trials showed a significant reduction in the risk of mortality at the last available time point in neonates who received surfactant therapy (pooled RR 0.67; 95% CI 0.57 to 0.79; Figure 2).
Figure 2.
Pooled effect of surfactant replacement therapy on mortalitya in studies from low- and middle-income countries settings.
Effect on BPD
The randomized trial on NIPPV+surfactant vs NIPPV alone found no difference in the incidence of BPD (RR 0.95; 95% CI 0.50 to 1.80).11 A total of six before-and-after/cohort studies reported the effect of SRT on the risk of BPD (Table 2).12, 14, 16, 18, 19, 24 Of these, three did not show any significant benefit.12, 16, 19 One prospective study from India reported a significantly lower incidence of BPD in the surfactant group.24 Another study that compared the outcomes of neonates at two different time points—before and after introduction of surfactant—also reported a significant improvement in the incidence of survival without BPD.18 In contrast, the study from a network of NICUs in Latin America reported a significantly higher risk of BPD in neonates who received surfactant therapy.14
Six case-control studies compared the odds of receiving surfactant therapy in VLBW neonates who developed BPD with those who did not (Table 2).33, 34, 35, 36, 37, 38 One study found no association between surfactant use and BPD.34 Another study reported significantly higher odds of BPD in those who received surfactant therapy, but the effect was not adjusted for key confounders such as gestation and birth weight.32 The other four studies reported significantly higher odds of BPD in those who received SRT even after adjusting for potential confounders by multivariate regression analysis. The adjusted odds ratios varied from 1.4 to 7.5.
Given the huge heterogeneity, we did not attempt to pool the results of individual studies.
Effect on air leaks
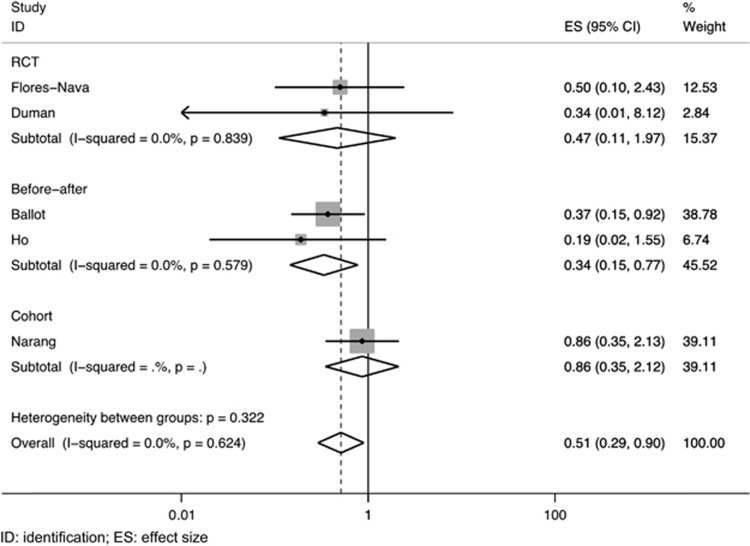
A total of six studies—four observational and two randomized trials—compared the risk of air leaks in neonates who received surfactant therapy with those who did not receive it (Table 2).10, 11, 12, 16, 18, 24 Of these, four did not show any significant difference.10, 16, 18, 24 One study from South Africa reported a significantly lower incidence of pneumothorax in the surfactant group.12 Pooled analysis of the five studies with complete data showed a significant reduction in the risk of air leaks following SRT (pooled RR 0.51; 95% CI 0.29 to 0.90; Figure 3).
Figure 3.
Pooled effect of surfactant replacement therapy on air leaks in studies from low- and middle-income countries settings.
Safety of SRT
One RCT reported the incidence of pulmonary hemorrhage and found no difference between SRT and control groups.10 We identified one observational study from Uruguay that compared the outcomes of neonates who received SRT with those of historical controls in 19 NICUs across five Latin American countries.14 The proportion of neonates with pulmonary hemorrhage (and patent ductus arteriosus) was significantly higher in neonates who received surfactant. In contrast, another study from Chile that reported data from the network of NICUs participating in the national program on surfactant use found no difference in the proportion of neonates developing pulmonary hemorrhage before and after introduction of SRT.20 However, the proportion with pulmonary hemorrhage was very high—about 30% in both groups (Table 3). One study from Thailand compared the proportion of neonates with pulmonary hemorrhage in two time periods—immediately after introduction of surfactant (1999 to 2002) with 1 to 3 years after (2003 to 05).39 The authors reported a significant reduction in pulmonary hemorrhage in the latter period (RR 0.36; 95% CI 0.17 to 0.76). Five case-series from Brazil, India, South Africa and Turkey reported the proportion of neonates developing pulmonary hemorrhage during/after SRT (Table 3)40, 41 which varied from 5 to 12%. All of them were conducted in level-3 NICUs. None of the included studies reported the incidence of apnea or cardiac arrest during or immediately after SRT.
Table 3. Studies on safety of SRT in LMIC settings.
| Studies with no control group | |||||||
|---|---|---|---|---|---|---|---|
| Author, year | Country | Setting | Study design | Study population | Surfactant—type, strategy | Results | Comments |
| Davies and Rothberg40 | South Africa | NICU; state academic and private referral hospitals | Case series | Neonates who received surfactant therapy (n=155) | Rescue therapy followed by mechanical ventilation; natural surfactant (Beractant) | Pulm. hge: 18 (11.6%) PDA: 58 (37.4%) Severe IVH: 28 (18.1%) | |
| Sanghvi and Merchant45 | India | NICU; referral hospital | Case series | Preterm neonates with RDS, requiring mechanical ventilation, and arterial to alveolar PO2 ratio <0.22 (n=22) Mean BW: 1179 (600–1720) g Mean gest: 29.8 (26–33) weeks | Beractant and synthetic (Exosurf); rescue therapy | Pulm. hge: 2 (9%) Air leak: 0 Sepsis: 10 (45%) PDA: 5 (23%) | Sepsis was responsible for 54% of mortality |
| Lefort et al.46 | Brazil | NICU | RCT comparing early vs late surfactant administration; data from both groups combined | 34 Weeks or less (n=75); gestational age: 29.6±2.5 (early group) BW 1275.29±333.7 (early group) | Rescue therapy followed by mechanical ventilation; natural surfactant | Pulm. hge: 7 (9.3%) | — |
| Surmeli-Onay et al.47 | Turkey | NICU; university hospital Level-3 NICU | Case series | Late preterm neonates (34–36 weeks) who received surfactant therapy (n=77) for any indication | Beractant and Poractant in 2:1 ratio | Air leaks: 8 (10.4%) Pulm. hge: 4 (5.2%) | Out of 77, only 51 had RDS; others had ARDS, CDH, pneumonia, etc. |
| Kanmaz et al.41 | Turkey | NICU; teaching hospital | RCT comparing surfactant administration via thin catheter with tracheal instillation; only data from second group presented | Preterm neonates born before 32 weeks and having RDS with FiO2 requirement of ⩾0.4 in first 2 h of life (n=100) | InSurE method (extubation immediately following surfactant therapy) | Pulm hge: 7 (7%) | — |
|
Studies with a control group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author, year | Country | Setting (description, if available) | Study design | Exposed group | Control group | Results | Effect size RR/mean difference (95% CI) | Comments | ||
| Rossello et al.14 | Five Latin American countries | 19 NICUs; ?referral hospitals | Comparison with historical controls (born in the previous 2 years) | Preterm neonates with RDS and received surfactant (n=348) | Preterm neonates with RDS who did not receive surfactant (n=348) | Pulm. hge PDA | Exp NA NA | Unexp NA NA | BW stratified RR 1.53 (1.11–2.1) 1.35 (1.17–1.56) | — |
| Chotigeat et al.41 | Thailand | NICU; referral hospital | Comparison of two time periods –first 3 years vs next 3 years after introduction of surfactant | Neonates admitted between 2003 and 2005 with RDS and received surfactant (n=91) | Neonates admitted between 1999 and 2002 with RDS and received surfactant (n=33) | Pulm hge Sepsis | Exp 11 (12) 23 (25) | Unexp 11 (33.3) 15 (45.4) | Unadjusted RR 0.36 (0.17–0.76) 0.56 (0.33–0.93) | Exposed group (2003–05) received surfactant much earlier than the unexposed group |
| Barria et al.20 | Chile | NICUs; public referral hospitals | Prospective data from database of Surfactant National Program—data compared with historical controls | Preterm neonates (<32 weeks) who received Exosurf or Survanta (n=2868) Mean BW and gestational age (mean±s.d.) were 1174 g (± 385) and 28.6 weeks (± 2.3), respectively | Preterm neonates who were born prior to introduction of surfactant (n=?) | Pulm hge | Exp 30.1% | Unexp 29.8% | P>0.05 | Full text in Spanish—data extracted with the help of Google Translator |
Abbreviations: ARDS, acute respiratory distress syndrome; BW, birth weight; CDH, congenital diaphragmatic hernia; CI, confidence interval; Exp, exposed; InSurE, intubate-surfactant-extubate; IVH, intraventricular hemorrhage; LMIC, low- and middle-income countries; NICU, neonatal intensive care unit; Pulm hge: pulmonary hemorrhage; PDA, patent ductus arteriosus; RCT, randomized controlled trial; RDS, respiratory distress syndrome; RR, relative risk; SRT, surfactant replacement therapy; Unexp, unexposed.
Feasibility of introducing SRT
None of the included studies specifically reported the proportion of neonates receiving surfactant dose(s) successfully. One study from a neonatal unit in South Africa with no backup ventilation facilities (Table 2) reported the proportion of neonates who needed referral following SRT.42 The study included extremely low birth weight neonates born at or after 25 weeks' gestation who were intubated, administered surfactant and then immediately extubated (InSurE strategy) to CPAP. The proportion of neonates who failed InSurE and required mechanical ventilation/referral was 34% (95% CI 24 to 45% n=33). Another case-series from Iran employed the InSurE technique in VLBW neonates (25 to 32 weeks' gestation).43 About 40% failed to improve and required mechanical ventilation (95% CI 28 to 52%). One case-series from Turkey employed the InSurE method in neonates born before 32 weeks' gestation and having an FiO2 requirement of >0.4 in the first 2 h of life.41 About 45% of the enrolled neonates required mechanical ventilation in the first 72 h (95% CI 35 to 55%). A recently published case-series from India reported almost the same figures for failure of InSurE (43% 95% CI 24 to 63%).44
Cost-effectiveness
None of the studies from LMICs reported this outcome.
Discussion
Introduction of SRT in the management of RDS is one of the most important advancements in the field of neonatology. However, in the absence of an evidence base from LMICs, completely confidence about the efficacy and safety of SRT in LMIC settings is not possible. We found two RCTs that did not find any difference in mortality following therapy with synthetic surfactant.10 Pooled analysis of all studies—observational as well as randomized—showed a significant reduction in the risk of mortality at the last available time point (RR 0.67; 95% CI 0.57 to 0.79) and air leaks (RR 0.51; 95% CI 0.29 to 0.90) in neonates who received SRT. These results are quite consistent with the data from HICs where there is moderate quality evidence from randomized trials that SRT reduces the risk of neonatal mortality (RR 0.68; 95% CI 0.57 to 0.82) and air leaks (RR 0.47; 95% CI 0.39 to 0.58) in preterm neonates with RDS.5
There is low-quality evidence from HICs that SRT does not reduce the risk of BPD.5 However, the studies from LMICs that compared the risk of BPD in neonates who received SRT with those who did not receive surfactant produced contrasting results. Although some cohort studies reported significantly lower risk of BPD, majority of the case-control studies demonstrated significantly higher odds of exposure to SRT in VLBW neonates with BPD. In contrast, a randomized trial did not find any significant difference in the incidence of BPD.11 One possible reason for this discrepancy is that more immature neonates, who would have otherwise died, survived following SRT and developed BPD. The uncertain risk of BPD following SRT has practical implications in LMIC settings because of the long duration of hospital stay with its associated cost implications.
The proportion of neonates developing pulmonary hemorrhage varied from 5 to 12% in LMIC settings.40, 41, 45, 46, 47 In contrast, the two studies from HICs that compared early vs late surfactant therapy reported an incidence of 5.9%[ref. 48] and 6.3%,49 respectively. However, the reduction in the proportion of neonates developing pulmonary hemorrhage by almost two-thirds after 1 to 3 years of implementing SRT in Thailand39 highlights the fact that the incidence is likely to be higher in LMIC settings that are just beginning to implement SRT but it might decrease with increasing experience. There is no information about the development of apnea or cardiac arrest following SRT from either LMIC or HIC settings. Transient desaturations invariably occur following SRT. Non-availability of this data may reflect its genuine non-existence, but there is need for vigilance about these potential complications, especially during initial implementation of SRT in LMIC settings.
There are no data on the proportion of neonates who received surfactant dose(s) successfully. This could possibly indicate that all neonates who were to receive surfactant therapy received it successfully without the need to stop because of immediate complications. Given that most studies were conducted in level-3 NICUs, this possibility seems more likely. About 40% of preterm neonates with RDS across different gestation categories receiving surfactant by InSurE failed and required mechanical ventilation. The possible reasons of such high failure rates are (1) inclusion of more immature neonates; (2) inadequate manpower resulting in sub-optimal monitoring; and (3) limited experience and expertize of doctors and nursing staff in most LMIC settings. But the number of studies reporting the failure rates is small (n=4)—it is possible that the failure rates are lower in other settings from LMICs. Nevertheless, the high risk of failure highlights the importance of provision of mechanical ventilation or referral, if ventilation facilities are not available. The paucity of studies from level-2 facilities without ventilation backup precludes any comment about the feasibility of SRT in these settings. None of the studies from LMICs reported on the cost effectiveness of SRT.
Implications for policy makers
With significant benefits observed in terms of reduction of mortality and air leaks without major harm, policy makers and other stakeholders are likely to give a high value to the use of SRT in the management of RDS in all resource-restricted countries. This is reflected by the inclusion of surfactant in the World Health Organization's Essential drug list.50 However, the most important concern lies in the cost of SRT, which varies from US$ 100 to 500 in different countries. Surfactant therapy could also increase the total cost of care of preterm neonates in a health facility because of increased survival of more immature infants who are likely to have a prolonged hospital stay. Still, the incremental cost effectiveness ratio is likely to be favorable if the quality adjusted life years for survivors of preterm births are taken into account.51
Various other factors, including availability of skilled personnel and an optimal support system, need to be ensured for an effective roll-out of the intervention. SRT should be reserved for symptomatic neonates with RDS who do not improve with CPAP or require intubation and assisted ventilation due to worsening disease. Even the recent trials on SRT52, 53, 54, 55 do not support the use of prophylactic SRT in extremely preterm neonates. However, all these trials were conducted in HICs in very preterm neonates (<28 weeks) where the use of antenatal steroid coverage was >90%.52, 53, 56, 57
Implications for researchers
The results of this review are primarily on the basis of observational studies with a dearth of high-quality evidence, including RCTs, from LMIC settings. Given the nature of studies included in the present review, the quality of evidence is considered to be low. There is a need to generate more evidence on the cost effectiveness of SRT and its effect on BPD in LMIC settings. Although it may not be ethical to carry out RCTs, large, multicenter, high-quality observational studies are quite feasible in these settings.
There is definitely a need to search for the exact timing of administering rescue surfactant in symptomatic infants with RDS especially among moderately preterm infants (28 to 33 weeks) who constitute the major portion of the beneficiaries in LMIC settings. Administering rescue surfactant early in the course of disease will result in its overuse when the majority of symptomatic infants can be managed with CPAP alone. However, late administration may result in the reduction or complete loss of its beneficial effect. Some data support early addition of surfactant as compared with CPAP alone by reducing the need for subsequent mechanical ventilation.58, 59
There is also a need to explore alternate simpler methods of administering surfactant which do not involve intubation. These minimally invasive techniques will be very useful for LMIC settings where inadequately trained health-care professionals are one of the major challenges for the successful administration of SRT. Various methods such as the ‘minimally invasive surfactant therapy',60, 61 ‘aerosol technique',62 surfactant administration via laryngeal mask airway63 or nasopharyngeal installation64 have been tried. Preliminary data on these ‘minimally invasive techniques' are promising; more evidence is required before they can be adopted in LMIC settings.
Strengths and weaknesses
Ours is possibly the first attempt to systematically review and synthesize the available evidence on the efficacy, safety, feasibility and cost effectiveness of SRT in low-resource settings. The studies in this review are limited by their study design and quality and are predominantly from single centers confined to level-3 neonatal units. Some of the observational studies had the limitation of using surfactant therapy based on the financial status of the parents, which could introduce serious bias in the results. Lastly, the small number of studies included in the review belie the fact that use of SRT is quite common in most resource-constricted settings.
Conclusion
Available evidence suggests that SRT is an effective, safe and feasible intervention in level-3 neonatal units and has the potential to reduce neonatal mortality and air leaks in low-resource settings also. SRT should be provided in settings where there is adequate manpower, professional skills and desired infrastructure to administer surfactant. To achieve maximum gains, it should be combined with more cost-effective therapies such as antenatal steroids and use of early/delivery room CPAP.
Acknowledgments
We acknowledge Dr Sushil Kumar, University of Minnesota, Minneapolis, USA for helping us in searching the various databases. This review was funded by the Department of Maternal, Newborn, Child and Adolescent Health and Development, World Health Organization, Geneva, Switzerland.
Author contributions
MJS prepared the study protocol, applied the search strategy, extracted data, did the statistical analysis and modified the manuscript. NG retrieved the articles, extracted data, helped in analysis and wrote the first draft of the manuscript. KJ retrieved the articles, extracted data and helped in analysis. RA and VKP modified the study protocol, supervised data extraction, helped in statistical analysis and finalized the manuscript. MJS and VKP acted as the guarantors of the paper.
The authors declare no conflict of interest.
References
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015; 385(9966): 430–440. [DOI] [PubMed] [Google Scholar]
- Fehlmann E, Tapia JL, Fernandez R, Bancalari A, Fabres J, D'Apremont I et al. [Impact of respiratory distress syndrome in very low birth weight infants: a multicenter South-American study]. Arch Argent Pediatr 2010; 108(5): 393–400. [DOI] [PubMed] [Google Scholar]
- Kumar A, Bhat BV. Epidemiology of respiratory distress of newborns. Indian J Pediatr 1996; 63(1): 93–98. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Maeta H, Chida S, Morita T, Watabe Y, Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet 1980; 1(8159): 55–59. [DOI] [PubMed] [Google Scholar]
- Seger N, Soll R. Animal derived surfactant extract for treatment of respiratory distress syndrome. Cochrane Database Syst Rev 2009; (2): CD007836. [DOI] [PMC free article] [PubMed]
- Soll RF. Synthetic surfactant for respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev 2000; (2): CD001149. [DOI] [PMC free article] [PubMed]
- Vidyasagar D, Velaphi S, Bhat VB. Surfactant replacement therapy in developing countries. Neonatology 2011; 99(4): 355–366. [DOI] [PubMed] [Google Scholar]
- Pande S, Hiller JE, Nkansah N, Bero L. The effect of pharmacist-provided non-dispensing services on patient outcomes, health service utilisation and costs in low- and middle-income countries. Cochrane Database Syst Rev 2013; (2): CD010398. [DOI] [PMC free article] [PubMed]
- Shroufi A, Chowdhury R, Anchala R, Stevens S, Blanco P, Han T et al. Cost effective interventions for the prevention of cardiovascular disease in low and middle income countries: a systematic review. BMC Public Health 2013; 13: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Nava G, López-Padilla M, Barrera-Millán E, Escobedo-Chávez E, Thompson-Chagoyan O, Humberto JR. Ensayo clínico con un surfactante artificial para el tratamiento del síndrome de dificultad respiratoria neonatal. Perinatol Reprod Hum 1995; 9(3): 149–155. [Google Scholar]
- Duman N, Tuzun F, Sever AH, Arslan MK, Iscan B, Dilek M et al. Nasal intermittent positive pressure ventilation with or without very early surfactant therapy for the primary treatment of respiratory distress syndrome. J Matern Fetal Neonatal Med 2016; 29(2): 252–257. [DOI] [PubMed] [Google Scholar]
- Ballot DE, Rothberg AD, Davies VA. The selection of infants for surfactant replacement therapy under conditions of limited financial resources. S Afr Med J 1995; 85(7): 640–643. [PubMed] [Google Scholar]
- Cooper PA, Saloojee H, Bolton KD, Mokhachane M. Survival of low-birth-weight infants at Baragwanath Hospital—1950-1996. S Afr Med J 1999; 89(11): 1179–1181. [PubMed] [Google Scholar]
- Rossello JD, Hayward PE, Martell M, Del Barco M, Margotto P, Grandzoto J et al. Hyaline membrane disease (HMD) therapy in Latin America: impact of exogenous surfactant administration on newborn survival, morbidity and use of resources. J Perinat Med 1997; 25(3): 280–287. [PubMed] [Google Scholar]
- Lim WL, Lim CT, Chye JK. The effectiveness of surfactant replacement therapy for preterm infants with respiratory distress syndrome. Med J Malaysia 1998; 53(4): 376–384. [PubMed] [Google Scholar]
- Ho JJ, Chang AS. Changes in the process of care and outcome over a 10-year period in a neonatal nursery in a developing country. J Trop Pediatr 2007; 53(4): 232–237. [DOI] [PubMed] [Google Scholar]
- Kopelman BI, Miyoshi MH, Guinsburg R, Schiavon AV. Impacto da reposiçäo com surfactante exógeno nos custos hospitalares de recém-nascidos prematuros com síndrome do desconforto respiratório. Pediatr Modern 1993; 29(3): 377–381. [Google Scholar]
- Tapia JL, Oto MA, Ramírez R, Henríquez MT, Pilar Fernández F, Álvarez J. Terapia con surfactante exógeno en recién nacidos con enfermedad de membrana hialina. Revista chilena de pediatría 1994; 65: 137–142. [Google Scholar]
- Verhagen AA, van der Meulen GN, Wiersma HE, Keli SO, Angelista IR, Muskiet FD et al. Respiratory distress syndrome in Curaçao. Conventional versus surfactant treatment. West Indian Med J 2002; 51(2): 68–73. [PubMed] [Google Scholar]
- Barria RM, Pino PZ, Becerra CF. Mortalidad en prematuros tratados con surfactante exógeno. Rev Chil Pediatr 2008; 79(1): 36–44. [Google Scholar]
- Prigenzi MLH, Trindade CEP, Rugolo LMSS, Silveira LVA. Fatores de risco associados à mortalidade de recém-nascidos de muito baixo peso na cidade de Botucatu, São Paulo, no período 1995-2000. Revista Brasileira de Saúde Materno Infantil 2008; 8: 93–101. [Google Scholar]
- Fustinana CA, Izbizky G, Rodriguez D, Mariani G, Ceriani Cernadas JM. Effectiveness assessment of a neonatal intensive care program for very low birth weight infants. Impact of surfactant administration. Arch Argent Pediatr 2009; 107(1): 9–15. [DOI] [PubMed] [Google Scholar]
- Chye JK, Lim CT. Very low birth weight infants—mortality and predictive risk factors. Singapore Med J 1999; 40(9): 565–570. [PubMed] [Google Scholar]
- Narang A, Kumar P, Dutta S, Kumar R. Surfactant therapy for hyaline membrane disease: the Chandigarh experience. Indian Pediatr 2001; 38(6): 640–646. [PubMed] [Google Scholar]
- Qian L, Liu C, Zhuang W, Guo Y, Yu J, Chen H et al. Neonatal respiratory failure: a 12-month clinical epidemiologic study from 2004 to 2005 in China. Pediatrics 2008; 121(5): e1115–e1124. [DOI] [PubMed] [Google Scholar]
- Qian LL, Liu CQ, Guo YX, Jiang YJ, Ni LM, Xia SW et al. Current status of neonatal acute respiratory disorders: a one-year prospective survey from a Chinese neonatal network. Chin Med J (Engl) 2010; 123(20): 2769–2775. [PubMed] [Google Scholar]
- Wang H, Gao X, Liu C, Yan C, Lin X, Yang C et al. Morbidity and mortality of neonatal respiratory failure in China: surfactant treatment in very immature infants. Pediatrics 2012; 129(3): e731–e740. [DOI] [PubMed] [Google Scholar]
- Cai C, Lv T, Wang X, Zhao G, Du G. Effect of calsurf combined with nasal intermittent positive pressure ventilation (NIPPV) in 46 neonates with respiratory distress syndrome (NRDS): clinical analysis. Prog Modern Biomed 2012; 12: 5874–5877. [Google Scholar]
- Li Y. Clinical analysis and discussion on treatment of 36 cases with hyaline membrane disease. Modern Preventive Med 2010; 37: 1990–1991. [Google Scholar]
- Sun B, Ma L, Liu X, Gao X, Ni L. Development of neonatal respiratory and intensive care: Chinese perspectives. Neonatology 2012; 101(2): 77–82. [DOI] [PubMed] [Google Scholar]
- Malaysian Very Low Birth Weight Study Group. A national study of risk factors associated with mortality in very low birthweight infants in the Malaysian neonatal intensive care units. J Paediatr Child Health 1997; 33(1):18–25. [DOI] [PubMed] [Google Scholar]
- Boo NY, Puah CH, Lye MS. The role of expressed breastmilk and continuous positive airway pressure as predictors of survival in extremely low birthweight infants. J Trop Pediatr 2000; 46(1): 15–20. [DOI] [PubMed] [Google Scholar]
- Grupo Colaborativo Neocosur. Very-low-birth-weight infant outcomes in 11 South American NICUs. J Perinatol 2002; 22(1): 2–7. [DOI] [PubMed] [Google Scholar]
- Cunha GS, Mezzacappa-Filho F, Ribeiro JD. Risk factors for bronchopulmonary dysplasia in very low birth weight newborns treated with mechanical ventilation in the first week of life. J Trop Pediatr 2005; 51(6): 334–340. [DOI] [PubMed] [Google Scholar]
- Tapia JL, Agost D, Alegria A, Standen J, Escobar M, Grandi C et al. Bronchopulmonary dysplasia: incidence, risk factors and resource utilization in a population of South American very low birth weight infants. J Pediatr (Rio J) 2006; 82(1): 15–20. [DOI] [PubMed] [Google Scholar]
- Demirel N, Bas AY, Zenciroglu A. Bronchopulmonary dysplasia in very low birth weight infants. Indian J Pediatr 2009; 76(7): 695–698. [DOI] [PubMed] [Google Scholar]
- Goncalves DD, da Silva LG, de M Paula G, Bonfim O, Moreira ME, Assumpcao AM et al. Preterm premature rupture of the fetal membranes: factors associated with bronchopulmonary dysplasia. Rev Bras Ginecol Obstet 2010; 32(10): 497–503. [PubMed] [Google Scholar]
- de Oliveira Lima MR, do A Andrade M, Guimarães do Araújo AP, Figueroa JN, Barboza de Andrade L. Influéncia de fatores maternos e neonatais no desenvolvimento da displasia broncopulmonarien. Rev Assoc Med Bras 2011; 57(4):398–403. [DOI] [PubMed] [Google Scholar]
- Chotigeat U, Promwong N, Kanjanapattanakul W, Khorana M, Sangtawesin V, Horpaopan S. Comparison outcomes of surfactant therapy in respiratory distress syndrome in two periods. J Med Assoc Thai 2008; 91(Suppl 3): S109–S114. [PubMed] [Google Scholar]
- Davies VA, Rothberg AD, Ballot DE. The introduction of surfactant replacement therapy into South Africa. S Afr Med J 1995; 85(7):637–640. [PubMed] [Google Scholar]
- Kanmaz HG, Erdeve O, Canpolat FE, Mutlu B, Dilmen U. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics 2013; 131(2): e502–e509. [DOI] [PubMed] [Google Scholar]
- Kirsten GF, Kirsten CL, Henning PA, Smith J, Holgate SL, Bekker A et al. The outcome of ELBW infants treated with NCPAP and InSurE in a resource-limited institution. Pediatrics 2012; 129(4): e952–e959. [DOI] [PubMed] [Google Scholar]
- Afjeh SA, Sabzehei MK. The INSURE method in VLBW preterm infant with RDS. Pajoohandeh J 2010; 15(5): 199–203. [Google Scholar]
- Tagare A, Kadam S, Vaidya U, Pandit A. Outcome of intubate surfactant rapidly extubate (InSuRE): an Indian experience. Indian J Pediatr 2014; 81(1): 20–23. [DOI] [PubMed] [Google Scholar]
- Sanghvi KP, Merchant RH. Single dose surfactant rescue therapy in neonatal respiratory distress syndrome. Indian Pediatrics 1998; 35(6): 533–536. [PubMed] [Google Scholar]
- Lefort S, Diniz EM, Vaz FA. Clinical course of premature infants intubated in the delivery room, submitted or not to porcine-derived lung surfactant therapy within the first hour of life. J Matern Fetal Neonatal Med 2003; 14(3): 187–196. [DOI] [PubMed] [Google Scholar]
- Surmeli-Onay O, Korkmaz A, Yigit S, Yurdakok M. Surfactant therapy in late preterm infants: respiratory distress syndrome and beyond. Turk J Pediatr 2012; 54(3): 239–246. [PubMed] [Google Scholar]
- The OSIRIS Collaborative Group (open study of infants at high risk of or with respiratory insufficiency—the role of surfactant. Early versus delayed neonatal administration of a synthetic surfactant—the judgment of OSIRIS. Lancet 1992; 340(8832): 1363–1369. [PubMed] [Google Scholar]
- Gortner L, Wauer RR, Hammer H, Stock GJ, Heitmann F, Reiter HL et al. Early versus late surfactant treatment in preterm infants of 27 to 32 weeks' gestational age: a multicenter controlled clinical trial. Pediatrics 1998; 102(5): 1153–1160. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Model List of Essential Medicines. 18th list. April 2013. Available at: http://www.who.int/medicines/publications/essentialmedicines/4th_EMLc_FINAL_web_8Jul13.pdf. (accessed on 31 May, 2015).
- Salinas-Escudero G, Reyes-Lopez A, Garduno-Espinosa J, Villasis-Keever MA, Martinez-Valverde S, Munoz-Hernandez O. Economic evaluation of the use of exogenous pulmonary surfactants in preterm newborns in a Mexican population. Salud Publica Mex 2012; 54(Suppl 1): S73–S81. [DOI] [PubMed] [Google Scholar]
- Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med 2010; 362(21): 1970–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn MS, Kaempf J, de Klerk A, de Klerk R, Reilly M, Howard D et al. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics 2011; 128(5): e1069–e1076. [DOI] [PubMed] [Google Scholar]
- Rojas-Reyes MX, Morley CJ, Soll R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2012; 3: CD000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polin RA, Carlo WA. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics 2014; 133(1): 156–163. [DOI] [PubMed] [Google Scholar]
- Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med 2008; 358(7): 700–708. [DOI] [PubMed] [Google Scholar]
- Sandri F, Plavka R, Ancora G, Simeoni U, Stranak Z, Martinelli S et al. Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics 2010; 125(6): e1402–e1409. [DOI] [PubMed] [Google Scholar]
- Rojas MA, Lozano JM, Rojas MX, Laughon M, Bose CL, Rondon MA et al. Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: a randomized, controlled trial. Pediatrics 2009; 123(1): 137–142. [DOI] [PubMed] [Google Scholar]
- Kandraju H, Murki S, Subramanian S, Gaddam P, Deorari A, Kumar P. Early routine versus late selective surfactant in preterm neonates with respiratory distress syndrome on nasal continuous positive airway pressure: a randomized controlled trial. Neonatology 2013; 103(2): 148–154. [DOI] [PubMed] [Google Scholar]
- Dargaville PA, Aiyappan A, De Paoli AG, Kuschel CA, Kamlin CO, Carlin JB et al. Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed 2013; 98(2): F122–F126. [DOI] [PubMed] [Google Scholar]
- Aguar M, Cernada M, Brugada M, Gimeno A, Gutierrez A, Vento M. Minimally invasive surfactant therapy with a gastric tube is as effective as the intubation, surfactant, and extubation technique in preterm babies. Acta Paediatr 2014; 103(6): e229–e233. [DOI] [PubMed] [Google Scholar]
- Berggren E, Liljedahl M, Winbladh B, Andreasson B, Curstedt T, Robertson B et al. Pilot study of nebulized surfactant therapy for neonatal respiratory distress syndrome. Acta Paediatr 2000; 89(4): 460–464. [DOI] [PubMed] [Google Scholar]
- Attridge JT, Stewart C, Stukenborg GJ, Kattwinkel J. Administration of rescue surfactant by laryngeal mask airway: lessons from a pilot trial. Am J Perinatol 2013; 30(3): 201–206. [DOI] [PubMed] [Google Scholar]
- Ten Centre Study Group. Ten centre trial of artificial surfactant (artificial lung expanding compound) in very premature babies. Br Med J (Clin Res Ed) 1987; 294(6578):991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]