Abstract
Substantial evidence demonstrates a link of increased plasminogen activator inhibitor-1 (PAI-1) and glomerulosclerosis and kidney fibrosis, providing a novel therapeutic option for prevention and treatment of chronic kidney diseases. Several mechanisms contributing to increased PAI-1 will be addressed, including classic key profibrotic factors such as the renin-angiotensin-system (RAS) and transforming growth factor-beta (TGF-β), and novel molecules identified by proteomic analysis, such as thymosin- β4. The fibrotic sequelae caused by increased PAI-1 in kidney depend not only on its classic inhibition of tissue-type and urokinase-type plasminogen activators (tPA and uPA), but also its influence on cell migration.
Keywords: PAI-1, kidney, EMT, angiotensin, aldosterone, thymosin β-4, Review
2. INTRODUCTION
Plasminogen activator inhibitor-1 (PAI-1) is a 50kd glycoprotein and is the primary physiological inhibitor of tissue-type and urokinase-type plasminogen activators (tPA and uPA) (1). tPA and uPA degrade both fibrin and extracellular matrix (ECM). PAI-1 thus affects both thrombosis and fibrosis. PAI-1 effects extend to cell migration through interactions with its cofactor, vitronectin (also known as protein S) and with the urokinase receptor (uPAR) and its co-receptors (2, 3). In particular, although a unique PAI-1 receptor has not been identified, PAI-1 modifies the function of the uPAR and several of its co-receptors. The normal kidney shows nearly undetectable PAI-1. Plasma PAI-1 levels increase in several chronic inflammatory states, including chronic kidney disease (CKD) (4-7). Liver and adipose tissue appear to be the primary sources of plasma PAI-1 (8, 9). PAI-1 is increased in several acute and chronic disease states, contributed to both by resident and infiltrating cells, and is linked to worsened fibrosis. Conversely, amelioration of injury is associated with less PAI-1 (10). We will review how PAI-1 actions promote renal fibrosis.
3. PAI-1 IN HUMAN CHRONIC KIDNEY DISEASE
In humans, PAI-1 is prominent in atherosclerotic lesions and in sclerotic glomeruli in e.g. arterionephrosclerosis, diabetic nephropathy and chronic allograft nephropathy (CAN). In a study of CAN, there was increased PAI-1 expression compared to transplanted kidneys followed for the same amount of time without CAN. Increased expression was present in tubules, vessels and within glomeruli, in mesangial areas, podocytes and endothelial cells in addition to infiltrating macrophages. Within this group of kidneys with CAN, there was a trend for less overall sclerotic injury to be associated with less PAI-1. These findings support the hypothesis that altered matrix metabolism and macrophage function could be involved in the development of CAN (11). Microdissected glomeruli from kidney transplants were used to study expression of PAI-1 and the thrombin receptor protease activated receptor-1, PAR-1, α2 type IV collagen and a housekeeping gene. Decreased renal function was correlated with increased mRNA levels of PAI-1 in the biopsy. PAI-1 was also increased along with α2 type IV collagen mRNA in acute rejection compared to normal kidneys (12). PAI-1 protein in diabetic nephropathy was particularly prominent in Kimmelstiel-Wilson nodules, often associated with fragmented red blood cells in regions of local injury and mesangiolysis (13).
PAI-1 levels are also influenced by PAI-1 genotype. Higher levels are seen with the 4G vs 5G polymorphism, describing the number of guanine bases (4 vs 5) in the promoter at position −675. The 5G, but not 4G, variant binds the E2F transcription repressor, and thus decreases PAI-1 transcription. The PAI-1 4G/4G genotype was linked to increased risk of vascular complications in diabetics, especially when superimposed on the ACE D/D genotype that is associated with increased RAS activity (14). Increased PAI-1 plasma levels and 4G/4G PAI-1 genotype have also been linked to increased risk of chronic allograft dysfunction (15).
Conversely, treatments that inhibit the reninangiotensin-aldosterone system (RAAS) also decrease PAI-1. Of note, combined ARB and aldosterone inhibition in humans suppressed the enhanced PAI-1 levels that occurred in response to a diuretic, whereas monotherapy with either alone had no effect (16). In a small study of hypertensive human kidney transplant recipients, elevated plasma PAI-1 levels were decreased by treatment with ARB but not by nifedipine (17). Whether this modulation affects graft outcome and tissue fibrosis has not been proven. However, plasma PAI-1 levels did correlate with rate of GFR decline in a study of renal transplant patients (18).
4. PAI-1 IN ANIMAL MODELS OF KIDNEY DISEASE
Animal models support a role for PAI-1 in the pathogenesis of diabetic kidney injury. Of note, rodent models of diabetes do not result in overt diabetic nephropathy. However, in the streptozotocin diabetic model, streptozotocin-treated PAI-1−/− mice had reduced albuminuria and fibronectin levels compared to wild-type diabetic mice (19). db/db diabetic mice with added PAI-1 deficiency had reduced albuminuria and kidney collagen levels (20). In early streptozotocin-induced nephropathy in rats, spironolactone therapy reduced PAI-1 and transforming growth factor-β (TGF-β) expression levels and matrix deposition (21). In another study, PAI-1−/− mice were protected from high fat diet-induced diabetic metabolic changes that occurred in wild-type mice (22). Furthermore, PAI-1−/− adipocytes were functionally distinct, with altered levels of key potential mediators of the dysmetabolic syndrome, including resistin, peroxisome proliferator-activated receptor-γ (PPAR-γ) and adiponectin (23). These studies suggest complex roles for PAI-1 in the pathogenesis of diabetes and its complications.
PAI-1 is also increased in many experimental models of non-diabetic glomerulosclerosis, including cyclosporin-induced glomerulosclerosis, radiation nephropathy, various models of FSGS, chronic anti-Thy-1 nephritis, aging and CAN (24, 25).
In vitro, angiotensin II directly induced PAI-1 gene expression via the AT1 receptor and aldosterone enhanced angiotensin-induced PAI-1 expression via a glucocorticoid response element in the PAI-1 promoter (26, 27). AT1 receptor blockade but not nonspecific antihypertensive treatment reduced renal PAI-1 expression induced by angiotensin II infusion (28).
Genetic PAI-1 deficiency also ameliorated sclerosis. Sclerosis-prone 129Sv mice bred to generate a PAI-1 null genotype were protected from glomerular scarring and tubulointerstitial fibrosis after 5/6 nephrectomy (Figure 1) (29). In vitro, PAI-1−/− podocytes synthesized less collagen in response to angiotensin II than wild-type cells (30). PAI-1−/− mice also had less vascular sclerosis than wild-type mice in response to angiotensin II and salt-loading or nitric oxide inhibition by L-NAME (31-33) . PAI-1−/− mice were protected from interstitial fibrosis induced by protein overload (34). Of note, these protective effects of PAI-1 deficiency occurred in several settings without changes in renal protease activity. However, macrophages and myofibroblasts were decreased, suggesting effects on cell migration independent of tPA and uPA modulation by PAI-1.
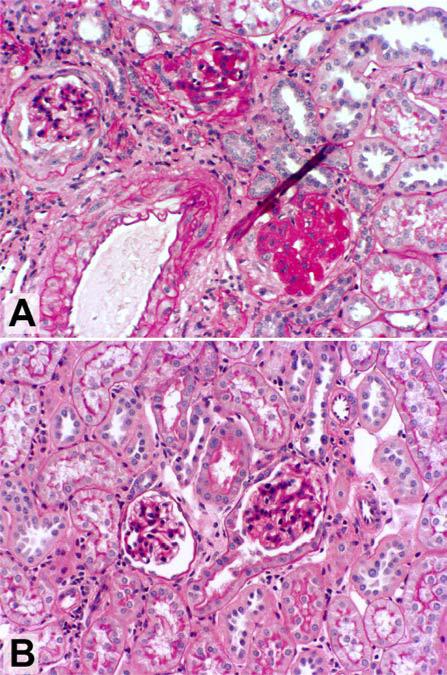
Figure 1.
Plasminogen activator inhibitor-1 (PAI-1) deficiency attenuates glomerulosclerosis in mice with remnant kidneys. Ten weeks after sclerosis-prone 129Sv mice underwent 5/6 nephrectomy, glomerulosclerosis and interstitial fibrosis were evident (A). These lesions failed to develop in 129Sv /PAI-1–/– mice (B). Magnification, ×200 (periodic acid-Schiff). Reproduced with permission from 10.
5. ANGIOTENSIN, PAI-1 AND TGFβ-1- THE TRIPLE THREATS
TGF-β is secreted as a complex, composed of three proteins derived from two genes, each encoding a procytokine. The N-terminal region, latency-associated peptide, LAP, prevents TGF-β from binding to its receptor (35). Because angiotensin, PAI-1 and TGF-β are often co-expressed in chronic renal disease and can influence expression of the other molecules, it has been challenging to unravel the independent contributions of each of these molecules to sclerosis (36). However, in the anti-Thy-1 model, combined TGF-β and angiotensin inhibition reduced PAI-1 expression and glomerular ECM accumulation and was more effective than either therapy alone (37), suggesting complex interactions and interdependent but not identical effects of sclerosis. Activation of TGF-β occurs through several mechanisms, including plasmin (38). Other mechanisms for activating TGF-β include reactive oxygen species, thrombospondin-1 (TSP-1) and αvβ6 integrin (39). This integrin is expressed in epithelia of the lung, skin and kidney, and induces activation of TGF-β1 by cleaving it from LAP. In the kidney, β6 is present in the proximal tubule, cortical thick ascending limb, inner and outer medullary collecting ducts and macula densa (40). Thus, in a bleomycin model of lung epithelial cell injury, β6−/− mice were completely protected against the fibrosis that developed in the wild type (41).
Studies in the β6 knockout mice provide additional insights on interactions of the RAAS, TGF-β and PAI-1. In contrast to wild-type mice, β6−/− mice did not develop fibrosis and active TGF-β and PAI-1 were not increased after UUO (41, 42). However, fibrosis could be restored in β6−/− mice by adding angiotensin II or aldosterone infusion to UUO. In these mice, PAI-1 expression and fibrosis, but not TGF-β, were increased to wild-type levels. These data support a direct link among angiotensin or aldosterone, PAI-1, and fibrosis, without dependence on TGF-β as an intermediary in this setting.
TGF-β and PAI-1 interactions have been extensively studied. TGF-β–overexpressing mice develop progressive glomerulosclerosis and also have increased PAI-1 (43). PAI-1 deficiency attenuated this TGF-β-induced kidney disease, as examined when these TGF-β1 transgenic mice were cross-bred with PAI-1−/− mice. The lack of PAI-1 resulted in less mesangial expansion and basement membrane thickening than seen in transgenic TGF-β mice with intact PAI-1, with associated decreased collagen accumulation due to decreased expression of both collagen I and collagen III. Interestingly, these results were not correlated with differences in protease activity, suggesting that other activities of PAI-1 resulted in the observed effects (43).
TGF-β1 has numerous effects beyond stimulating ECM synthesis, including effects on cell proliferation and immune modulation. Thus, TGF-β1−/− mice have multifocal inflammatory disease, resulting in early death (44, 45). Antagonism of TGF-β with a pan-antibody at low dose was protective against development of sclerosis in puromycin aminonucleoside nephropathy. However, higher doses of the anti-TGF-β antibody had no effect on glomerulosclerosis, and macrophage infiltration was increased, perhaps reflecting block of TGF-β immune modulatory effects (46).
6. EFFECTS OF PAI-1 and PAs ON GLOMERULOSCLEROSIS AND FIBROSIS
PAI-1 also impacts matrix accumulation in other organs. In the bleomycin model of pulmonary fibrosis, there was increased fibrosis in mice with over-expression of PAI-1 versus decreased fibrosis in PAI-1−/− mice (47). These results were consistent with effects on protease activity, specifically plasmin, because treatment of the PAI-1−/− mice with an inhibitor of plasmin formation, tranexamic acid, obviated the protective effect of genetic deficiency of PAI-1 (48). The impact of plasmin on injury was also shown in double tPA/uPA−/− mice that developed more severe crescentic glomerulonephritis and had correspondingly less plasmin (49). However, more recent studies point to additional mechanisms whereby PAI-1 can modulate injury (Figure 2). When the UUO model was induced in PAI-1−/− mice, there was decreased matrix accumulation, but plasmin activity was not enhanced in the PAI-1−/− mice. In addition to the decreased fibrosis, cell infiltration was also decreased, and conversely, cell infiltration was increased when PAI-1 over-expressing mice were subject to UUO (50, 51). These data suggest direct effects of PAI-1 to enhance infiltration and recruitment of profibrotic cells.
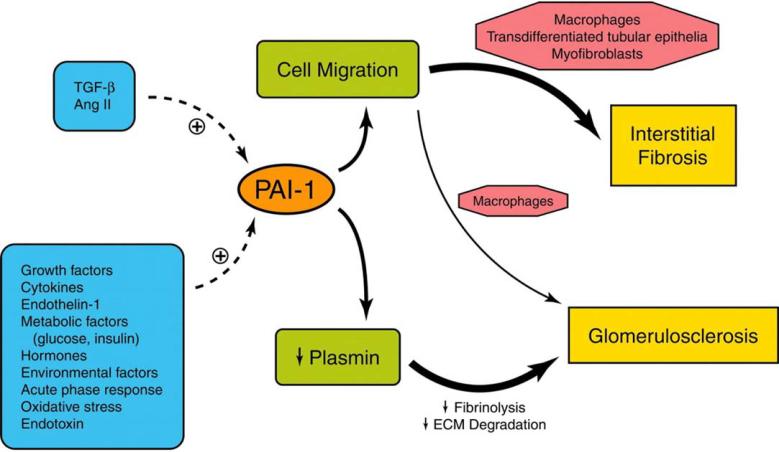
Figure 2.
Schematic summary of the primary effects of PAI-1 in chronic kidney disease (CKD). Renal PAI-1 expression can be induced by a number of factors involved in disease pathogenesis. TGF-β and angiotensin II are widely recognized inducers of PAI-1, but many other factors stimulate PAI-1 expression in CKD. In the glomerulus, sclerosis is linked to increased PAI-1 and inhibition of extracellular matrix breakdown. Within the tubulointerstitium, the profibrotic effects of PAI-1 align more closely with its ability to promote migration of monocytes/macrophages, transdifferentiated tubular epithelial cells and (myo)fibroblasts in particular. Reproduced with permission from 10.
These effects of PAI-1 on cell adhesion and migration are mediated via both protease-dependent and - independent mechanisms. Originally, PAI-1 was thought to be an inhibitor of cell migration. Smooth muscle cell migration is increased in PAI-1−/− mice, while PAI-1 overexpression in vitro inhibits this process, presumably by vitronectin effects (52, 53). These studies support that PAI-1 can have cell migration–inhibitory effects independent of its proteolytic activity. This effect on cell migration might not only modulate macrophage infiltration but also affect epithelial-mesenchymal transition (EMT), and may possibly relate to effects mediated by complex interactions with vitronectin and maybe other co-receptors for uPAR (54). PAI-1 is bound to the vitronectin in plasma, and when PAI-1 binds to tPA or uPA, this binding is reversed. PAI-1 inhibition of uPA results in inactivation of the uPA-uPAR-integrin complex. The complex is internalized by the low density lipoprotein receptor related protein, LRP. LRP modulates cell migration in several in vitro systems (55, 56). PAI-1 activates an LRP-dependent signaling pathway that contributes to PAI-1's pro-migratory effects (57). PAI-1 activates the Jak/Stat signaling pathway via binding to LRP in cultured smooth muscle cells. When PAI-1 was mutated to prevent LRP binding, this signaling pathway was not activated, nor was cell migration enhanced. Similarly, antibodies or antagonism or genetic deficiency of LRP blocked PAI-1's pro-migratory effects (57). The endocytic receptor LRP together with tPA and PAI-1 also coordinated Mac-1-dependent macrophage migration (58). Genetic inactivation of PAI-1, Mac-1 or LRP abrogated macrophage migration. The dissociation of uPAR and integrins from the matrix, induced by PAI-1 binding to uPA at the cell surface, could thus promote cell migration (59). PAI-1 also binds to vitronectin with much higher affinity than does uPAR. When PAI-1 binds to vitronectin, cell adhesion mediated by integrins is inhibited. This so-called “de-adhesion” property of PAI-1 could contribute to recruitment of macrophages. Whether this also could facilitate EMT remains to be determined. Interesting experiments have further explored these various functions of PAI-1 using a mutant PAI-1 that has normal vitronectin affinity but is not capable of inhibiting tPA or uPA. Using this compound, macrophage infiltration and markers of fibrosis were reduced, supporting a key pathophysiological role for these non-protease functions of PAI-1 (60). Increased PAI-1 is also linked to more aggressive tumor cell metastases, further indicating possible effects on cell migration and even possible direct chemotactic actions of PAI-1 (24). Additional studies in fibrotic models also observed protective effect of decreased PAI-1 independent of its regulatory function on plasmin activity.
In spontaneously hypercholesterolemic rats, treatment with a PPARγ agonist or the ARB candesartan resulted in amelioration of development of proteinuria and fibrosis, with corresponding attenuation of PAI-1 protein level, but no decrease in plasmin activity (61). Further studies on the actions of uPA in the kidney observed no protection against interstitial fibrosis induced by UUO and there was no compensatory change in renal uPAR mRNA levels, or PAI-1 protein or tPA activity levels. This study also examined activation of hepatocyte growth factor (HGF), as uPA can activate latent HGF, but this was not increased in uPA−/− mice. These studies suggest that the profibrotic actions of PAI-1 are not dependent on uPA and that uPA is not the mechanism by which HGF is activated in the kidney. The results are also in contrast to previous reports that show that increased uPA activity could attenuate pulmonary and hepatic fibrosis, suggesting organ-specific pathways of fibrogenesis (see below) (62). Additional studies in plasminogen (PLG)-deficient mice subjected to UUO showed decreased fibrosis in the PLG−/− mice and decreased EMT, associated with significant decreased phosphorylated ERK and active TGF-β. These in vivo observations were examined in further detail in vitro, where cultured murine tubular epithelial cells showed increased ERK phosphorylation when exposed to plasmin, with phenotypic transition to become more fibroblast-like, with de novo expression of fibroblast specific protein-1 and alpha-smooth muscle actin. Blocking of protease activated receptor-1 (PAR-1) genetically or with a specific anti-PAR-1 signaling peptide or an ERK kinase inhibitor blocked these in vitro EMT events. Conversely, when PAR-1 was overexpressed in these cells, ERK correlation response to plasmin was enhanced. These studies provide strong evidence for a profibrotic role for plasmin mediated through PAR-1-dependent ERK signaling, with important effects to induce EMT (63).
Plasmin also acts as a potent proinflammatory activator of monocyte-derived macrophage and triggers cytokine induction (64). Annexin A2 is a calcium and phospholipid-binding protein. The annexin A2 heterotetramer, composed of annexin A2 and S100A10, is a co-receptor for plasmin and regulates plasmin activity (65-67). Annexin A2 mediates plasmin induced-activation of macrophages via intracellular Janus kinase JAK1/Stat3 signaling (64, 66). Annexin A2 is also present on renal tubular epithelial cells (68). In an acute renal failure model in mice, expression of annexin A2 parallels the time course of tubular injury and recovery (69). Whether annexin II modulates plasmin's effects on EMT and/or fibrosis remains to be determined.
A possible impact of EMT on injury has been shown in elegant studies by Neilson and colleagues in vivo in the UUO model (70). Interestingly, tPA−/− mice showed a decrease in interstitial collagen after UUO, linked to decreased matrix metalloproteinase-9 (MMP-9) induction and preserved tubular basement membrane integrity (71, 72), events that could prevent EMT. Modulation of the EMT process by tPA is further supported by a recent finding that tPA directly promotes murine myofibroblast activation through LRP-mediated integrin signaling (73). These findings contrast with the protective effects of increased tPA to protect against glomerular matrix accumulation.
These data suggest complex effects of PAI-1 beyond plasmin inactivation that could particularly enhance infiltration of cells into the interstitium, including EMT or macrophage influx. Whether similar effects of PAI-1 on cell migration in the glomerulus occur has not yet been established.
7. POSSIBLE ORGAN-SPECIFIC PAI-1 EFFECTS
The plasmin/PA system has varying effects in different organs. Renal fibrosis after UUO was less severe in plasminogen-deficient mice, associated with lower levels of active TGF-β (63, 74). These findings contrast with outcomes in the bleomycin-induced lung injury model, where fibrosis was worse in plasminogen-deficient mice, suggesting that the fibrogenic effects of plasminogen might be organ-specific (75). The contribution of PAI-1 to aldosterone-induced injury also showed organ specific effects. Infusion of aldosterone with salt in the drinking water in wild-type mice induced increased albuminuria and glomerulomegaly due primarily to mesangial expansion, with increased renal collagen content, with corresponding increases in mRNA for collagen I, collagen III, osteopontin, fibronectin and the macrophage marker F4/80 and monocyte chemotactic protein. In contrast, PAI-1−/− mice were partially protected from these injuries, with less mesangial matrix expansion and without significant increase in collagen III and with less osteopontin expression. Of interest, TGF-β was not increased in this model. A differential effect on kidney versus heart injury was observed in that aldosterone induced similar cardiac hypertrophy in both wild-type and PAI-1−/− mice, perhaps related to diverse effects on infiltrating cells in these two organs (76).
Effects of PAI-1 on cardiac injury were examined in a model of uninephrectomy with angiotensin infusion. This infusion caused aortic remodeling with increased medial, adventitial and overall wall thickness, which was attenuated by the novel orally active small molecule PAI-1 inhibitor, PAI-039. The angiotensin II-induced increased aortic osteopontin was significantly decreased by the PAI-1 inhibitor. However, overall cardiac hypertrophy was not affected, and the PAI-1 inhibitor actually increased angiotensin II-induced cardiac fibrosis. Whether these varying effects are species specific or organ specific remains to be determined (31).
8. NOVEL MOLECULES AND RAS-PAI-1 INTERACTIONS: THYMOSIN β-4 AND Ac-SDKP
Recent studies suggest that thymosin β-4 may be one intermediary linking actions of angiotensin II and PAI-1. Thymosin β-4 is a ubiquitously expressed G-actin binding protein with both nuclear and cytoplasmic locations (77, 78). Thymosin β-4 accelerates dermal wound healing, related to anti-inflammatory activity, increased tissue remodeling and angiogenesis and increased collagen deposition and wound contraction (79). In cultured corneal cells, thymosin β-4 increases TGF-β and laminin-5 (80). Thymosin β-4 had direct effects on corneal cell migration, and additional effects that may be in part mediated by induction of TGF-β. Interestingly, thymosin β-4 promotes cardiac myocyte survival after ischemia, linked to increased vascular endothelial growth factor (VEGF) expression and activation of integrin-linked kinase (ILK) (81). Thymosin β-4 plays a key role in cardiac development, stimulates coronary vasculogenesis and angiogenesis, and induces adult epicardial progenitor mobilization and neovascularization (82).
In contrast to these potentially beneficial effects after dermal, corneal and cardiac injury, thymosin β-4 appears to potentiate glomerulosclerosis. We performed proteomic analysis of isolated laser-capture microdissected glomeruli from frozen sections. Thymosin β-4 was increased in 5/6 nephrectomy rats, markedly in sclerotic glomeruli, but also in nonsclerotic glomeruli, compared to normal (83). Immunohistochemistry showed that increased glomerular thymosin β-4 was contributed to by glomerular endothelial cell (GEN) staining. We further investigated the functional significance of up-regulated thymosin β-4, by manipulating its expression in vitro. Knock-down of thymosin β-4 by siRNA in cultured GEN prevented the increased PAI-1 expression seen in response to angiotensin II in normal GEN with intact thymosin β-4 (83). This data supports that thymosin β-4 is not merely a marker of sclerosis, but promotes matrix accumulation within glomeruli.
Our new preliminary data show that thymosin β-4 is also up-regulated in the fibrotic interstitium in the UUO model in a variety of cells, including myofibroblasts, distal tubules, macrophages and also occasional peritubular capillary and proximal tubular cells (84). We also found that integrin β6−/− mice with UUO that were protected from fibrosis had little thymosin β-4 expression. In contrast, when addition of AngII infusion in these mice restored fibrosis, thymosin β-4 was markedly upregulated to levels similar to that seen in wild-type mice after UUO with consequent fibrosis (85).
Thymosin β-4 is degraded by endoproteinases or prolyloligopeptidase (POP) to Ac-SDKP which also has manifold actions. Ac-SDKP is further degraded to an inactive metabolite by ACE. Thus, ACEI increases Ac-SDKP plasma levels four- to five-fold (86, 87). Conversely, administration of a monoclonal antibody to Ac-SDKP blocked beneficial effects of ACEI or infused Ac-SDKP, resulting in worse cardiac fibrosis and hypertension in response to AngII (88). Ac-SDKP inhibited TGF-β1 induction of increased PAI-1 and α2 type I collagen in vitro in mesangial cells, linked to inhibition of Smad2 phosphorylation (89). Ac-SDKP enhanced corneal endothelial cell proliferation both in vitro and in vivo (90), and exogenous Ac-SDKP infusion improved fibrosis in a rat anti-GBM nephritis model (91) and ameliorated both renal insufficiency and mesangial expansion in the db/db mouse model of diabetes (92). Further, Ac-SDKP blocked collagen synthesis in a cardiac fibrosis model (93, 94). Our preliminary data suggest synergistic beneficial effects of ARB and exogenous Ac-SDKP on injury in UUO, with decreased thymosin β-4 and PAI-1 (95).
These data support that the balance of thymosin β-4 and Ac-SDKP is important in modulating fibrotic vs antifibrotic responses, and that beneficial actions of ACEI are at least in part contributed to by enhanced Ac-SDKP.
9. REGRESSION OF RENAL FIBROSIS
The RAS is a central target for treatment of progressive renal diseases. ACEI or ARB are effective in treatment of CKD, perhaps even better than other antihypertensives. The apparent superior efficacy of ACEI was first linked to decreased intraglomerular hypertension and hyperfiltration, due to preferential decrease of efferent arteriolar resistance. Evidence then emerged that additional non-hemodynamic effects of angiotensin II are also pivotal for progressive renal injury, including induction of aldosterone, increased ECM synthesis, immune modulation, induction of other growth factors such as platelet-derived growth factor B (PDGF-B), TGF-β, connective tissue growth factor (a downstream mediator of TGF-β actions), and increased PAI-1 (96). Angiotensin can directly induce PAI-1. The RAS also modulates the inflammatory response, at least in part by affecting both lymphocytes and macrophages, which express RAS components, including angiotensinogen and angiotensin receptors.
Recent experimental and clinical data have explored the potential reversibility of glomerulosclerosis, and examined possible mechanisms of this glomerular remodeling (96, 97). In studies in the rat 5/6 nephrectomy model induced by renal artery branch ligation, we started treatment with high or normal dose ACEI at 8 weeks after injury, a point of established sclerosis as verified by renal biopsy. Higher doses of ACEI were more effective than normal antihypertensive doses of ACEI in ameliorating development of glomerulosclerosis over the next 4 weeks, although both glomerular and systemic hemodynamic effects were similar with both doses (96). In some rats, regression of glomerulosclerosis occurred in response to high dose ACEI, as shown by less severe sclerosis at autopsy than at biopsy 4 weeks earlier at initiation of treatment (96). Similar results are seen in aging rats, where existing aortic and glomerular sclerosis are reversed by high dose ARB and are associated with decreased PAI-1 levels (98).
More recent studies by the groups of Ritz, Remuzzi, Zatz, Chatziantoniou, and further studies by our group, have further explored the mechanisms and potential for regression of glomerulosclerosis (99-103). Although glomerular pressure again was lowered to a similar extent by an extremely high dose and a lower dose of the ARB losartan, the higher dose was more effective and decreased renal inflammation and restored glomerular and interstitial injury to pretreatment levels (104). We also showed that high dose ARB was particularly effective in achieving regression, numerically even better than ACEI (103). Of interest, inhibition of aldosterone by spironolactone also decreased PAI-1 levels and induced regression of sclerosis in some animals (105, 106). The group of Ritz investigated regression, comparing injury in subgroups of rats sacrificed at different times after subtotal nephrectomy induced by cautery without ligation, a model with less severe hypertension and sclerosis than the ligation model. Glomerulosclerosis, vascular lesions and tubulointerstitial fibrosis were less in rats sacrificed at 12 weeks after 5/6 nephrectomy after 4 weeks of ACEI than in those sacrificed at 8 weeks (99). The efficacy of combination therapy with an ACEI, ARB and statin on sclerosis and regression beyond monotherapy with any one of these drugs was shown by Remuzzi's group (101), supporting that a multipronged therapeutic approach can achieve better results than targeting of a single pathway.
The molecular composition of sclerotic glomeruli differs from that of the normal mesangial matrix and appears to be more resilient to proteolytic degradation, perhaps explaining why glomerulosclerosis regression is not achieved in all 5/6 nephrectomized rats treated with high dose RAS blockers. Nonetheless, a recent mouse UUO study reported convincing evidence of interstitial matrix remodeling when UUO was released after 7 days (54).
Although regression of sclerosis was achieved in the above studies using angiotensin inhibition, regression did not occur in all animals. Further, glomerular structure did not completely return to normal, suggesting that additional mechanisms such as reactive oxygen species, abnormal cell growth, etc. promoting sclerosis were still active, or that inherent limits of regenerative capacity of remaining glomerular components exist.
9.1. ECM modulation by RAS and aldosterone
The mechanisms of the observed regression in the above studies have been partially elucidated. The balance of ECM synthesis and proteases and their inhibitors is key in determining whether ECM accumulation occurs. Key modulators of ECM turnover in the glomerulus include MMPs 2 and 9 and tissue inhibitors of matrix metalloproteases (TIMPs). Key factors that promote ECM synthesis include TGF-β1. In our studies of regression, high-dose angiotensin inhibition did not increase MMP-2 or −9 messenger RNA or activity; these were actually reduced. TGF-β1 messenger RNA was also not changed (103). Regression of sclerosis induced by angiotensin inhibition was, however, tightly linked to decreases in PAI-1 and TIMP-1 (103, 106). Kidney plasmin activity was markedly decreased in untreated 5/6 nephrectomy rats, but was restored towards normal when regression was achieved by high dose ARB. Thus, ARB decreased TIMP-1 and PAI-1, and increased plasmin, with net effect to induce regression of sclerosis. The decreased PAI-1 may also have contributed to less inflammatory cell infiltrate. Inhibition of aldosterone also resulted in regression in some rats, an effect that was enhanced by blood pressure control with nonspecific antihypertensives (106). This effect was again linked to decreased PAI-1.
9.2. Cell responses mediated by RAS and aldosterone
The complex interactions of cells within the glomerulus determine the potential for regression of sclerosis. Mesangial cells readily proliferate and can be replenished after injury. However, the interaction between podocytes and endothelium is quite complex. The podocyte normally secretes growth factors, including VEGF-A and angiopoietin-1, that are necessary for normal glomerular endothelial cell survival and function (107, 108). The mechanism whereby such mediators from the podocytes can flow in a countercurrent fashion across the GBM to the endothelium could relate to the recently described subpodocyte space, cave-like domains under podocytes overlying the GBM with limited egress, which could locally alter transcapillary pressures and flow (109).
Glomerular VEGF and capillary density were decreased in the rat remnant kidney model of glomerulosclerosis and, conversely, treatment with exogenous VEGF ameliorated development of glomerulosclerosis and tubulointerstitial fibrosis (110). In studies of regression induced by ACEI, podocyte number was not changed but the volume of the podocytes was increased. Interestingly, this regression induced by ACEI also decreased mesangial and endothelial cell proliferation in this model with a corresponding reduction in glomerular volume and capillary number (100). In our recent preliminary confocal Z-section studies in the remnant kidney ligation model, we observed decreased capillary branching in untreated sclerotic rats, with increased capillary branching and length occurring when regression of sclerosis was induced by angiotensin inhibition. This observation indicates that increased capillary growth occurs and contributes to induction of regression, i.e. more open capillary loops and less sclerosis (111). In vitro, we showed that ARBs may affect injured podocytes, restoring their synthesis of the key angiogenic factors VEGF and angiopoietin-1, and thus promoting capillary growth (112). The endothelial cell growth in response to podocyte-conditioned media was blocked by antibodies inhibiting these angiogenic proteins. These data imply that angiotensin inhibition could contribute to regression by restoring the injured podocyte's ability to induce capillary growth (112).
9.3. Angiotensin receptors: AT1 vs AT2
Angiotensin transduces effects via two major angiotensin receptors: the angiotensin type 1 (AT1) and angiotensin type 2 (AT2) receptors. The AT1 receptor mediates classic angiotensin actions, including vasoconstriction, growth and matrix synthesis, whereas the AT2 receptor counteracts these effects, mediating vasodilation and apoptosis (113). Additional novel RAS actions are contributed to by ACE2, angiotensin metabolites and the renin receptor (114-116).
AT2 receptor expression can be altered after injury. Thus, the AT2 receptor was upregulated after angiotensin infusion (117), and in response to acute injury (118). AT2-induced apoptosis could minimize injury by removal of injured cells before activation of profibrotic chemokines and cytokines occurs. AT2 receptor-mediated vasodilation occurs due to nitric oxide, or indirectly via the bradykinin B2 receptor (119). Not all AT2 actions appear to be beneficial. The AT2 receptor also increases the chemokine RANTES in response to angiotensin II, a potential profibrotic mechanism (120, 121).
The net effect of the AT2 receptor in interstitial fibrosis was examined in AT2−/− mice with UUO. AT2−/− mice developed worse fibrosis than wild-type counterparts after UUO, linked to marked decrease in apoptosis of interstitial and tubular epithelial cells (122). Similar observations have been made in the hamster cardiomyopathic model of spontaneous cardiac fibrosis, where blockade of the AT1 receptor resulted in less cardiac fibrosis. In contrast, antagonism of the AT2 receptor in these hamsters resulted in worse injury, linked to inhibition of apoptosis in cardiac neomyocytes in parallel in vitro experiments (123). Further, compared to wild-type, mice with overexpression of the AT2 receptor (AT2 Tg) developed less glomerular injury, less proteinuria, less PDGF-B and TGF-β expression and more nitric oxide after subtotal nephrectomy. The protective effect of AT2 overexpression was blocked by treatment with an AT2 receptor antagonist, supporting that the decreased injury was AT2-mediated (124). Our recent study investigated the effects of the AT2 receptor in existing glomerulosclerosis. AT2 receptor antagonism was started at 8 weeks after subtotal nephrectomy, a time point by which moderate glomerulosclerosis had developed. AT2 receptor antagonist treatment resulted in worsened glomerulosclerosis over the next 4 weeks compared to AT1 receptor blocker treatment, where regression occurred. Importantly, adding AT2 receptor blocker to concomitant AT1 receptor blocker prevented the beneficial effects of the ARB and worsened sclerosis (125). The protective mechanisms of AT2 receptor in this model were linked to shifts in apoptosis/proliferation and change in fibrinolytic/proteolytic effects, with increased PAI-1 in rats receiving the AT2 receptor antagonist. In contrast, sclerosis was decreased when AT2 was blocked before sclerosis occurred, i.e. at the onset of injury in the rat radiation nephropathy model or the time of renal ablation in the subtotal nephrectomy model (126). These results suggest that mechanisms activated by AT2 may have varying effects depending on stage and type of injury. The results further suggest that efficacy of AT1 receptor blockers could in part be due to increased available angiotensin II to bind to and activate the AT2 receptor.
10. RAS, PAI-1 AND DYSMETABOLIC SYNDROME
The RAS and PAI-1 also have broad-ranging effects on risk for cardiovascular and renal disease by modulating obesity and dysmetabolic syndrome. Data from our group and others have recently shown that pharmacological blockage or genetic deletion of AT1 attenuates development of high fat diet-induced obesity, and decreases expression of key markers of dysmetabolic syndrome, associated with decreased PAI-1 (22, 127). Protection against adverse effects of high fat diet, such as increased adiposity, hyperglycemia, hyperinsulinemia and altered balance of key-adipogenic molecules, was also seen in PAI-1 deficient mice (22). Interestingly, weight loss from either gastric restrictive surgery or exercise and diet is associated with a significant decrease in adipose tissue macrophage number, reduction of inflammatory state and normalization of inflammatory mediators within the adipose tissue (including macrophage inhibitory factor, PAI-1, and other acute phase proteins) (128, 129).
11. SUMMARY AND PERSPECTIVE
PAI-1 is a multifunctional glycoprotein with complex actions in the kidney with net effect to increase ECM accumulation. Increased local PAI-1 is tightly linked to fibrosis and glomerulosclerosis. Conversely, inhibition of PAI-1 prevents CKD progression and may even facilitate regression of glomerulosclerosis, likely due to effects on proteolysis. In contrast, in the renal interstitium, PAI-1 interactions with vitronectin appear key and facilitate cell migration and potentially EMT. Additional complex interactions with e.g. angiotensin, aldosterone, TGF-β and thymosin β-4 are emerging, which may vary in heart, aorta and kidney. Further understanding of local actions of PAI-1 and its interactins with other modulators of fibrosis may direct specific effective approaches for treatment of progressive kidney disease.
ACKNOWLEDGEMENT
The authors acknowledge research grant support from the National Institutes of Health, DK56942 (ABF) and DK44757 (ABF).
REFERENCES
- 1.Loskutoff DJ, Edgington TS. An inhibitor of plasminogen activator in rabbit endothelial cells. J Biol Chem. 1981;256:4142–4145. [PubMed] [Google Scholar]
- 2.Lijnen HR. Pleiotropic functions of plasminogen activator inhibitor-1. J Thromb Haemost. 2005;3:35–45. doi: 10.1111/j.1538-7836.2004.00827.x. [DOI] [PubMed] [Google Scholar]
- 3.Dellas C, Loskutoff DJ. Historical analysis of PAI-1 from its discovery to its potential role in cell motility and disease. Thromb Haemost. 2005;93:631–640. doi: 10.1160/TH05-01-0033. [DOI] [PubMed] [Google Scholar]
- 4.Shik J, Parfrey PS. The clinical epidemiology of cardiovascular disease in chronic kidney disease. Curr Opin Nephrol Hypertens. 2005;14:550–557. doi: 10.1097/01.mnh.0000170752.64150.88. [DOI] [PubMed] [Google Scholar]
- 5.Menon V, Gul A, Sarnak MJ. Cardiovascular risk factors in chronic kidney disease. Kidney Int. 2005;68:1413–1418. doi: 10.1111/j.1523-1755.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 6.Wali RK, Henrich WL. Chronic kidney disease: a risk factor for cardiovascular disease. Cardiol Clin. 2005;23:343–362. doi: 10.1016/j.ccl.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Vaughan DE. PAI-1 and atherothrombosis. J Thromb Haemost. 2005;3:1879–1883. doi: 10.1111/j.1538-7836.2005.01420.x. [DOI] [PubMed] [Google Scholar]
- 8.de Boer JP, Abbink JJ, Brouwer MC, Meijer C, Roem D, Voorn GP, Lambers JW, van Mourik JA, Hack CE. PAI-1 synthesis in the human hepatoma cell line HepG2 is increased by cytokines--evidence that the liver contributes to acute phase behaviour of PAI-1. Thromb Haemost. 1991;65:181–185. [PubMed] [Google Scholar]
- 9.Pandey M, Loskutoff DJ, Samad F. Molecular mechanisms of tumor necrosis factor-alpha-mediated plasminogen activator inhibitor-1 expression in adipocytes. Faseb J. 2005;19:1317–1319. doi: 10.1096/fj.04-3459fje. [DOI] [PubMed] [Google Scholar]
- 10.Eddy AA, Fogo AB. Plasminogen activator inhibitor-1 in chronic kidney disease: evidence and mechanisms of action. J Am Soc Nephrol. 2006;17:2999–3012. doi: 10.1681/ASN.2006050503. [DOI] [PubMed] [Google Scholar]
- 11.Revelo MP, Federspiel C, Helderman H, Fogo AB. Chronic allograft nephropathy: expression and localization of PAI-1 and PPAR-gamma. Nephrol Dial Transplant. 2005;20:2812–2819. doi: 10.1093/ndt/gfi172. [DOI] [PubMed] [Google Scholar]
- 12.Delarue F, Hertig A, Alberti C, Vigneau C, Ammor M, Berrou J, Akposso K, Peraldi MN, Rondeau E, Sraer JD. Prognostic value of plasminogen activator inhibitor type 1 mRNA in microdissected glomeruli from transplanted kidneys. Transplantation. 2001;72:1256–1261. doi: 10.1097/00007890-200110150-00013. [DOI] [PubMed] [Google Scholar]
- 13.Paueksakon P, Revelo MP, Ma LJ, Marcantoni C, Fogo AB. Microangiopathic injury and augmented PAI-1 in human diabetic nephropathy. Kidney Int. 2002;61:2142–2148. doi: 10.1046/j.1523-1755.2002.00384.x. [DOI] [PubMed] [Google Scholar]
- 14.Kimura H, Gejyo F, Suzuki Y, Suzuki S, Miyazaki R, Arakawa M. Effects of polymorphisms in angiotensin converting enzyme and plasminogen activator-1 genes on the development of diabetic nephropathy and macroangiopathy. Kidney Int. 1998;54:1659–1669. doi: 10.1046/j.1523-1755.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 15.Reis K, Arinsoy T, Derici U, Gonen S, Bicik Z, Soylemezoglu O, Yasavul U, Hasanoglu E, Sindel S. Angiotensinogen and plasminogen activator inhibitor-1 gene polymorphism in relation to chronic allograft dysfunction. Clin Transplant. 2005;19:10–14. doi: 10.1111/j.1399-0012.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 16.Sawathiparnich P, Murphey LJ, Kumar S, Vaughan DE, Brown NJ. Effect of combined AT1 receptor and aldosterone receptor antagonism on plasminogen activator inhibitor-1. J Clin Endocrinol Metab. 2003;88:3867–3873. doi: 10.1210/jc.2003-030374. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa A, Ohta N, Ozono S, Kawabe K, Kitamura T. Inhibition of plasminogen activator inhibitor-1 by angiotensin II receptor blockers on cyclosporine-treated renal allograft recipients. Transplant Proc. 2005;37:994–996. doi: 10.1016/j.transproceed.2004.12.226. [DOI] [PubMed] [Google Scholar]
- 18.Lahlou A, Peraldi MN, Thervet E, Flahault A, Delarue F, Soubrier F, Rossert J, Hertig A, Rondeau E. Chronic graft dysfunction in renal transplant patients: potential role of plasminogen activator inhibitor type 1. Transplantation. 2002;73:1290–1295. doi: 10.1097/00007890-200204270-00018. [DOI] [PubMed] [Google Scholar]
- 19.Nicholas SB, Aguiniga E, Ren Y, Kim J, Wong J, Govindarajan N, Noda M, Wang W, Kawano Y, Collins A, Hsueh WA. Plasminogen activator inhibitor-1 deficiency retards diabetic nephropathy. Kidney Int. 2005;67:1297–1307. doi: 10.1111/j.1523-1755.2005.00207.x. [DOI] [PubMed] [Google Scholar]
- 20.Collins SJ, Alexander SL, Lopez-Guisa JM, Cai X, Maruvada R, Chua SC, Zhang G, Okamura DM, Matsuo S, Eddy AA. Plasminogen activator inhibitor-1 deficiency has renal benefits but some adverse systemic consequences in diabetic mice. Nephron Exp Nephrol. 2006;104:e23–34. doi: 10.1159/000093673. [DOI] [PubMed] [Google Scholar]
- 21.Fujisawa G, Okada K, Muto S, Fujita N, Itabashi N, Kusano E, Ishibashi S. Spironolactone prevents early renal injury in streptozotocin-induced diabetic rats. Kidney Int. 2004;66:1493–1502. doi: 10.1111/j.1523-1755.2004.00913.x. [DOI] [PubMed] [Google Scholar]
- 22.Ma LJ, Mao SL, Taylor KL, Kanjanabuch T, Guan Y, Zhang Y, Brown NJ, Swift LL, McGuinness OP, Wasserman DH, Vaughan DE, Fogo AB. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes. 2004;53:336–346. doi: 10.2337/diabetes.53.2.336. [DOI] [PubMed] [Google Scholar]
- 23.Liang X, Kanjanabuch T, Mao SL, Hao CM, Tang YW, Declerck PJ, Hasty AH, Wasserman DH, Fogo AB, Ma LJ. Plasminogen activator inhibitor-1 modulates adipocyte differentiation. Am J Physiol Endocrinol Metab. 2006;290:E103–E113. doi: 10.1152/ajpendo.00605.2004. [DOI] [PubMed] [Google Scholar]
- 24.Eddy AA. Plasminogen activator inhibitor-1 and the kidney. Am J Physiol Renal Physiol. 2002;283:F209–220. doi: 10.1152/ajprenal.00032.2002. [DOI] [PubMed] [Google Scholar]
- 25.Takeshita K, Yamamoto K, Ito M, Kondo T, Matsushita T, Hirai M, Kojima T, Nishimura M, Nabeshima Y, Loskutoff DJ, Saito H, Murohara T. Increased expression of plasminogen activator inhibitor-1 with fibrin deposition in a murine model of aging, “Klotho” mouse. Semin Thromb Hemost. 2002;28:545–554. doi: 10.1055/s-2002-36699. [DOI] [PubMed] [Google Scholar]
- 26.Brown NJ, Vaughan DE, Fogo AB. The reninangiotensin-aldosterone system and fibrinolysis in progressive renal disease. Semin Nephrol. 2002;22:399–406. doi: 10.1053/snep.2002.34725. [DOI] [PubMed] [Google Scholar]
- 27.Brown NJ, Kim KS, Chen YQ, Blevins LS, Nadeau JH, Meranze SG, Vaughan DE. Synergistic effect of adrenal steroids and angiotensin II on plasminogen activator inhibitor-1 production. J Clin Endocrinol Metab. 2000;85:336–344. doi: 10.1210/jcem.85.1.6305. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura S, Nakamura I, Ma L-J, Vaughan DE, Fogo AB. Plasminogen activator inhibitor-1 expression is regulated by the angiotensin type 1 receptor in vivo. Kidney Int. 2000;58:251–259. doi: 10.1046/j.1523-1755.2000.00160.x. [DOI] [PubMed] [Google Scholar]
- 29.Ma LJ, Naito T, Han JY, Fogo AB. Plasminogen activator inhibitor-1 (PAI-1) deficiency prevents the development of glomerulosclerosis in the subtotal nephrectomy (5/6 Nx) in the mouse. J Am Soc Nephrol. 2005;16:653A. [Google Scholar]
- 30.Naito T, Rodriguez G, Borza D-B, Ma LJ, Pozzi A, Fogo AB. Podocyte PAI-1 affects angiotensin II (AngII)-induced ECM accumulation. Lab Invest. 2006;86:264A. [Google Scholar]
- 31.Weisberg AD, Albornoz F, Griffin JP, Crandall DL, Elokdah H, Fogo AB, Vaughan DE, Brown NJ. Pharmacological inhibition and genetic deficiency of plasminogen activator inhibitor-1 attenuates angiotensin II/salt-induced aortic remodeling. Arterioscler Thromb Vasc Biol. 2005;25:365–371. doi: 10.1161/01.ATV.0000152356.85791.52. [DOI] [PubMed] [Google Scholar]
- 32.Kaikita K, Fogo AB, Ma LJ, Schoenhard JA, Brown NJ, Vaughan DE. Plasminogen activator inhibitor-1 deficiency prevents hypertension and vascular fibrosis in response to long-term nitric oxide synthase inhibition. Circulation. 2001;104:839–844. doi: 10.1161/hc3301.092803. [DOI] [PubMed] [Google Scholar]
- 33.Kaikita K, Schoenhard JA, Painter CA, Ripley RT, Brown NJ, Fogo AB, Vaughan DE. Potential roles of plasminogen activator system in coronary vascular remodeling induced by long-term nitric oxide synthase inhibition. J Mol Cell Cardiol. 2002;34:617–627. doi: 10.1006/jmcc.2002.2001. [DOI] [PubMed] [Google Scholar]
- 34.Oda T, Kim HG, Wing D, Lopez-Guisa J, Jernigan S, Eddy AA. Effects of genetic PAI-1 deficiency in mice with protein-overload proteinuria. J Am Soc Nephrol. 1999;10:578A. [Google Scholar]
- 35.Sharma K, Ziyadeh FN. The emerging role of transforming growth factor-beta in kidney diseases. Am J Physiol. 1994;266:F829–F842. doi: 10.1152/ajprenal.1994.266.6.F829. [DOI] [PubMed] [Google Scholar]
- 36.Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, Noble NA, Border W. Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int. 2006;69:105–113. doi: 10.1038/sj.ki.5000011. [DOI] [PubMed] [Google Scholar]
- 37.Yu L, Border WA, Anderson I, McCourt M, Huang Y, Noble NA. Combining TGF-beta inhibition and angiotensin II blockade results in enhanced antifibrotic effect. Kidney Int. 2004;66:1774–1784. doi: 10.1111/j.1523-1755.2004.00901.x. [DOI] [PubMed] [Google Scholar]
- 38.Lyons RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent recombinant transforming growth factor-beta 1 by plasmin. J Cell Biol. 1990;110:1361–1367. doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, Murphy-Ullrich JE. Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J Biol Chem. 1995;270:7304–7310. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- 40.Arend LJ, Smart AM, Briggs JP. Mouse beta (6) integrin sequence, pattern of expression, and role in kidney development. J Am Soc Nephrol. 2000;11:2297–2305. doi: 10.1681/ASN.V11122297. [DOI] [PubMed] [Google Scholar]
- 41.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 42.Ma LJ, Yang HC, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB. Transforming growth factor β (TGF-β) dependent and independent pathways of induction of tubulointerstitial fibrosis in αvβ6−/− mice. Am J Pathol. 2003;163:1261–1273. doi: 10.1016/s0002-9440(10)63486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krag S, Danielsen CC, Carmeliet P, Nyengaard J, Wogensen L. Plasminogen activator inhibitor-1 gene deficiency attenuates TGF-beta1-induced kidney disease. Kidney Int. 2005;68:2651–2666. doi: 10.1111/j.1523-1755.2005.00737.x. [DOI] [PubMed] [Google Scholar]
- 44.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aoki CA, Borchers AT, Li M, Flavell RA, Bowlus CL, Ansari AA, Gershwin ME. Transforming growth factor beta (TGF-beta) and autoimmunity. Autoimmun Rev. 2005;4:450–459. doi: 10.1016/j.autrev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Ma LJ, Jha S, Ling H, Pozzi A, Ledbetter S, Fogo AB. Divergent effects of low versus high dose anti-TGF-beta antibody in puromycin aminonucleoside nephropathy in rats. Kidney Int. 2004;65:106–115. doi: 10.1111/j.1523-1755.2004.00381.x. [DOI] [PubMed] [Google Scholar]
- 47.Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest. 1996;97:232–237. doi: 10.1172/JCI118396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest. 2000;106:1341–1350. doi: 10.1172/JCI10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitching AR, Holdsworth SR, Ploplis VA, Plow EF, Collen D, Carmeliet P, Tipping PG. Plasminogen and plasminogen activators protect against renal injury in crescentic glomerulonephritis. J Exp Med. 1997;185:963–968. doi: 10.1084/jem.185.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oda T, Jung YO, Kim HS, Cai X, Lopez-Guisa JM, Ikeda Y, Eddy AA. PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int. 2001;60:587–596. doi: 10.1046/j.1523-1755.2001.030002587.x. [DOI] [PubMed] [Google Scholar]
- 51.Matsuo S, Lopez-Guisa JM, Cai X, Okamura DM, Alpers CE, Bumgarner RE, Peters MA, Zhang G, Eddy AA. Multifunctionality of PAI-1 in fibrogenesis: evidence from obstructive nephropathy in PAI-1-overexpressing mice. Kidney Int. 2005;67:2221–2238. doi: 10.1111/j.1523-1755.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- 52.Redmond EM, Cullen JP, Cahill PA, Sitzmann JV, Stefansson S, Lawrence DA, Okada SS. Endothelial cells inhibit flow-induced smooth muscle cell migration: role of plasminogen activator inhibitor-1. Circulation. 2001;103:597–603. doi: 10.1161/01.cir.103.4.597. [DOI] [PubMed] [Google Scholar]
- 53.Proia RR, Nelson PR, Mulligan-Kehoe MJ, Wagner RJ, Kehas AJ, Powell RJ. The effect of endothelial cell overexpression of plasminogen activator inhibitor-1 on smooth muscle cell migration. J Vasc Surg. 2002;36:164–171. doi: 10.1067/mva.2002.123687. [DOI] [PubMed] [Google Scholar]
- 54.Fogo AB. Renal fibrosis: not just PAI-1 in the sky. J Clin Invest. 2003;112:326–328. doi: 10.1172/JCI19375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Czekay RP, Aertgeerts K, Curriden SA, Loskutoff DJ. Plasminogen activator inhibitor-1 detaches cells from extracellular matrices by inactivating integrins. J Cell Biol. 2003;160:781–791. doi: 10.1083/jcb.200208117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Degryse B, Neels JG, Czekay RP, Aertgeerts K, Kamikubo Y, Loskutoff DJ. The low density lipoprotein receptor-related protein is a motogenic receptor for plasminogen activator inhibitor-1. J Biol Chem. 2004;279:22595–22604. doi: 10.1074/jbc.M313004200. [DOI] [PubMed] [Google Scholar]
- 58.Cao C, Lawrence DA, Li Y, Von Arnim CA, Herz J, Su EJ, Makarova A, Hyman BT, Strickland DK, Zhang L. Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. Embo J. 2006;25:1860–1870. doi: 10.1038/sj.emboj.7601082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Czekay RP, Loskutoff DJ. Unexpected role of plasminogen activator inhibitor 1 in cell adhesion and detachment. Exp Biol Med (Maywood) 2004;229:1090–1096. doi: 10.1177/153537020422901102. [DOI] [PubMed] [Google Scholar]
- 60.Huang Y, Haraguchi M, Lawrence DA, Border WA, Yu L, Noble NA, mutant A. noninhibitory plasminogen activator inhibitor type 1 decreases matrix accumulation in experimental glomerulonephritis. J Clin Invest. 2003;112:379–388. doi: 10.1172/JCI18038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Omasu F, Oda T, Yamada M, Yoshizawa N, Yamakami K, Sakurai Y, Miura S. Effects of pioglitazone and candesartan on renal fibrosis and the intrarenal plasmin cascade in spontaneously hypercholesterolemic rats. Am J Physiol Renal Physiol. 2007;293:F1292–F1298. doi: 10.1152/ajprenal.00232.2007. [DOI] [PubMed] [Google Scholar]
- 62.Yamaguchi I, Lopez-Guisa JM, Cai X, Collins SJ, Okamura DM, Eddy AA. Endogenous urokinase lacks antifibrotic activity during progressive renal injury. Am J Physiol Renal Physiol. 2007;293:F12–19. doi: 10.1152/ajprenal.00380.2006. [DOI] [PubMed] [Google Scholar]
- 63.Zhang G, Kernan KA, Collins SJ, Cai X, Lopez-Guisa JM, Degen JL, Shvil Y, Eddy AA. Plasmin (ogen) promotes renal interstitial fibrosis by promoting epithelialto-mesenchymal transition: role of plasmin-activated signals. J Am Soc Nephrol. 2007;18:846–859. doi: 10.1681/ASN.2006080886. [DOI] [PubMed] [Google Scholar]
- 64.Li Q, Laumonnier Y, Syrovets T, Simmet T. Plasmin triggers cytokine induction in human monocyte-derived macrophages. Arterioscler Thromb Vasc Biol. 2007;27:1383–1389. doi: 10.1161/ATVBAHA.107.142901. [DOI] [PubMed] [Google Scholar]
- 65.Fitzpatrick SL, Kassam G, Choi KS, Kang HM, Fogg DK, Waisman DM. Regulation of plasmin activity by annexin II tetramer. Biochemistry. 2000;39:1021–1028. doi: 10.1021/bi991411z. [DOI] [PubMed] [Google Scholar]
- 66.Laumonnier Y, Syrovets T, Burysek L, Simmet T. Identification of the annexin A2 heterotetramer as a receptor for the plasmin-induced signaling in human peripheral monocytes. Blood. 2006;107:3342–3349. doi: 10.1182/blood-2005-07-2840. [DOI] [PubMed] [Google Scholar]
- 67.Kim J, Hajjar KA. Annexin II: a plasminogenplasminogen activator co-receptor. Front Biosci. 2002;7:d341–348. doi: 10.2741/kim. [DOI] [PubMed] [Google Scholar]
- 68.Kumar V, Farell G, Deganello S, Lieske JC. Annexin II is present on renal epithelial cells and binds calcium oxalate monohydrate crystals. J Am Soc Nephrol. 2003;14:289–297. doi: 10.1097/01.asn.0000046030.24938.0a. [DOI] [PubMed] [Google Scholar]
- 69.Cheng CW, Rifai A, Ka SM, Shui HA, Lin YF, Lee WH. A Chen: Calcium-binding proteins annexin A2 and S100A6 are sensors of tubular injury and recovery in acute renal failure. Kidney Int. 2005;68:2694–2703. doi: 10.1111/j.1523-1755.2005.00740.x. [DOI] [PubMed] [Google Scholar]
- 70.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang J, Shultz RW, Mars WM, Wegner RE, Li Y, Dai C, Nejak K, Liu Y. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J Clin Invest. 2002;110:1525–1538. doi: 10.1172/JCI16219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006;281:2120–2127. doi: 10.1074/jbc.M504988200. [DOI] [PubMed] [Google Scholar]
- 73.Hu K, Wu C, Mars WM, Liu Y. Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor-related protein 1-mediated integrin signaling. J Clin Invest. 2007;117:3821–3832. doi: 10.1172/JCI32301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edgtton KL, Gow RM, Kelly DJ, Carmeliet P, Kitching AR. Plasmin is not protective in experimental renal interstitial fibrosis. Kidney Int. 2004;66:68–76. doi: 10.1111/j.1523-1755.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- 75.Swaisgood CM, French EL, Noga C, Simon RH, Ploplis VA. The development of bleomycin-induced pulmonary fibrosis in mice deficient for components of the fibrinolytic system. Am J Pathol. 2000;157:177–187. doi: 10.1016/S0002-9440(10)64529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma J, Weisberg A, Griffin JP, Vaughan DE, Fogo AB, Brown NJ. Plasminogen activator inhibitor-1 deficiency protects against aldosterone-induced glomerular injury. Kidney Int. 2006;69:1064–1072. doi: 10.1038/sj.ki.5000201. [DOI] [PubMed] [Google Scholar]
- 77.Huff T, Rosorius O, Otto AM, Muller CS, Ballweber E, Hannappel E, Mannherz HG. Nuclear localisation of the G-actin sequestering peptide thymosin beta4. J Cell Sci. 2004;117:5333–5341. doi: 10.1242/jcs.01404. [DOI] [PubMed] [Google Scholar]
- 78.Mora CA, Baumann CA, Paino JE, Goldstein AL, Badamchian M. Biodistribution of synthetic thymosin beta 4 in the serum, urine, and major organs of mice. Int J Immunopharmacol. 1997;19:1–8. doi: 10.1016/s0192-0561(97)00005-2. [DOI] [PubMed] [Google Scholar]
- 79.Huff T, Otto AM, Muller CS, Meier M, Hannappel E. Thymosin beta4 is released from human blood platelets and attached by factor XIIIa (transglutaminase) to fibrin and collagen. Faseb J. 2002;16:691–696. doi: 10.1096/fj.01-0713com. [DOI] [PubMed] [Google Scholar]
- 80.Sosne G, Xu L, Prach L, Mrock LK, Kleinman HK, Letterio JJ, Hazlett LD, Kurpakus-Wheater M. Thymosin beta 4 stimulates laminin-5 production independent of TGF-beta. Exp Cell Res. 2004;293:175–183. doi: 10.1016/j.yexcr.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 81.Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 82.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 83.Xu BJ, Shyr Y, Liang X, Ma LJ, Donnert EM, Roberts JD, Zhang X, Kon V, Brown NJ, Caprioli RM, Fogo AB. Proteomic patterns and prediction of glomerulosclerosis and its mechanisms. J Am Soc Nephrol. 2005;16:2967–2975. doi: 10.1681/ASN.2005030262. [DOI] [PubMed] [Google Scholar]
- 84.Orhan D, Donnert EM, Xu BJ, Gaspert A, Ma J, Fogo AB. Thymosin β-4 is increased in renal interstitial fibrosis. Lab Invest. 2007;87:274A. [Google Scholar]
- 85.Potthoff SA, Zuo Y, Ma L-J, Fogo AB. Angiotensin II-induced Restored Fibrosis in β6 −/− mice is Linked to Thymosin β-4. J Am Soc Nephrol. 2007;18:419A. [Google Scholar]
- 86.Azizi M, Ezan E, Nicolet L, Grognet JM, Menard J. High plasma level of N-acetyl-seryl-aspartyl-lysyl-proline: a new marker of chronic angiotensin-converting enzyme inhibition. Hypertension. 1997;30:1015–1019. doi: 10.1161/01.hyp.30.5.1015. [DOI] [PubMed] [Google Scholar]
- 87.Azizi M, Rousseau A, Ezan E, Guyene TT, Michelet S, Grognet JM, Lenfant M, Corvol P, Menard J. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetylseryl-aspartyl-lysyl-proline. J Clin Invest. 1996;97:839–844. doi: 10.1172/JCI118484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peng H, Carretero OA, Vuljaj N, Liao TD, Motivala A, Peterson EL, Rhaleb NE. Angiotensin-converting enzyme inhibitors: a new mechanism of action. Circulation. 2005;112:2436–2445. doi: 10.1161/CIRCULATIONAHA.104.528695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kanasaki K, Koya D, Sugimoto T, Isono M, Kashiwagi A, Haneda M. N-Acetyl-seryl-aspartyl-lysylproline inhibits TGF-beta-mediated plasminogen activator inhibitor-1 expression via inhibition of Smad pathway in human mesangial cells. J Am Soc Nephrol. 2003;14:863–872. doi: 10.1097/01.asn.0000057544.95569.ec. [DOI] [PubMed] [Google Scholar]
- 90.Wang D, Carretero OA, Yang XY, Rhaleb NE, Liu YH, Liao TD, Yang XP. N-acetyl-seryl-aspartyl-lysyl-proline stimulates angiogenesis in vitro and in vivo. Am J Physiol Heart Circ Physiol. 2004;287:H2099–2105. doi: 10.1152/ajpheart.00592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Omata M, Taniguchi H, Koya D, Kanasaki K, Sho R, Kato Y, Kojima R, Haneda M, Inomata N. N-acetyl-serylaspartyl-lysyl-proline ameliorates the progression of renal dysfunction and fibrosis in WKY rats with established anti-glomerular basement membrane nephritis. J Am Soc Nephrol. 2006;17:674–685. doi: 10.1681/ASN.2005040385. [DOI] [PubMed] [Google Scholar]
- 92.Shibuya K, Kanasaki K, Isono M, Sato H, Omata M, Sugimoto T, Araki S, Isshiki K, Kashiwagi A, Haneda M, Koya D. N-acetyl-seryl-aspartyl-lysyl-proline prevents renal insufficiency and mesangial matrix expansion in diabetic db/db mice. Diabetes. 2005;54:838–845. doi: 10.2337/diabetes.54.3.838. [DOI] [PubMed] [Google Scholar]
- 93.Rasoul S, Carretero OA, Peng H, Cavasin MA, Zhuo J, Sanchez-Mendoza A, Brigstock DR, Rhaleb NE. Antifibrotic effect of Ac-SDKP and angiotensin-converting enzyme inhibition in hypertension. J Hypertens. 2004;22:593–603. doi: 10.1097/00004872-200403000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng H, Carretero OA, Brigstock DR, Oja-Tebbe N, Rhaleb NE. Ac-SDKP reverses cardiac fibrosis in rats with renovascular hypertension. Hypertension. 2003;42:1164–1170. doi: 10.1161/01.HYP.0000100423.24330.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zuo YQ, Potthoff SA, Tennant PR, Yang HC, Ma LJ, Fogo AB. Synergistic Effects of AcSDKP and Angiotensin Receptor Blocker on Repressing Thymosin β4 (Thyβ4) Expression in Early Renal Interstitial Fibrosis. J Am Soc Nephrol. 2007;18:202A. [Google Scholar]
- 96.Fogo AB. Progression and potential regression of glomerulosclerosis (Nephrology Forum). Kidney Int. 2001;59:804–819. doi: 10.1046/j.1523-1755.2001.059002804.x. [DOI] [PubMed] [Google Scholar]
- 97.Fogo AB. Can glomerulosclerosis be reversed? Nat Clin Pract Nephrol. 2006;2:290–291. doi: 10.1038/ncpneph0200. [DOI] [PubMed] [Google Scholar]
- 98.Ma LJ, Nakamura S, Whitsitt JS, Marcantoni C, Davidson JM, Fogo AB. Regression of sclerosis in aging by an angiotensin inhibition-induced decrease in PAI-1. Kidney Int. 2000;58:2425–2436. doi: 10.1046/j.1523-1755.2000.00426.x. [DOI] [PubMed] [Google Scholar]
- 99.Adamczak M, Gross ML, Krtil J, Koch A, Tyralla K, Amann K, Ritz E. Reversal of glomerulosclerosis after high-dose enalapril treatment in subtotally nephrectomized rats. J Am Soc Nephrol. 2003;14:2833–2842. doi: 10.1097/01.asn.0000095248.91994.d3. [DOI] [PubMed] [Google Scholar]
- 100.Adamczak M, Gross ML, Amann K, Ritz E. Reversal of glomerular lesions involves coordinated restructuring of glomerular microvasculature. J Am Soc Nephrol. 2004;15:3063–3072. doi: 10.1097/01.ASN.0000146121.72699.86. [DOI] [PubMed] [Google Scholar]
- 101.Zoja C, Corna D, Camozzi D, Cattaneo D, Rottoli D, Batani C, Zanchi C, Abbate M, Remuzzi G. How to fully protect the kidney in a severe model of progressive nephropathy: a multidrug approach. J Am Soc Nephrol. 2002;13:2898–2908. doi: 10.1097/01.asn.0000034912.55186.ec. [DOI] [PubMed] [Google Scholar]
- 102.Boffa JJ, Lu Y, Placier S, Stefanski A, Dussaule JC, Chatziantoniou C. Regression of renal vascular and glomerular fibrosis: role of angiotensin II receptor antagonism and matrix metalloproteinases. J Am Soc Nephrol. 2003;14:1132–1144. doi: 10.1097/01.asn.0000060574.38107.3b. [DOI] [PubMed] [Google Scholar]
- 103.Ma LJ, Nakamura S, Aldigier JC, Rossini M, Yang H, Liang X, Nakamura I, Marcantoni C, Fogo AB. Regression of glomerulosclerosis with high-dose angiotensin inhibition is linked to decreased plasminogen activator inhibitor-1. J Am Soc Nephrol. 2005;16:966–976. doi: 10.1681/ASN.2004060492. [DOI] [PubMed] [Google Scholar]
- 104.Fujihara CK, Velho M, Malheiros DM, Zatz R. An extremely high dose of losartan affords superior renoprotection in the remnant model. Kidney Int. 2005;67:1913–1924. doi: 10.1111/j.1523-1755.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 105.Brown NJ, Nakamura S, Ma LJ, Nakamura I, Donnert E, Freeman M, Vaughan DE, Fogo AB. Aldosterone modulates plasminogen activator inhibitor-1 and glomerulosclerosis in vivo. Kidney Int. 2000;58:1219–1227. doi: 10.1046/j.1523-1755.2000.00277.x. [DOI] [PubMed] [Google Scholar]
- 106.Aldigier JC, Kanjanbuch T, Ma LJ, Brown NJ, Fogo AB. Regression of existing glomerulosclerosis by inhibition of aldosterone. J Am Soc Nephrol. 2005;16:3306–3314. doi: 10.1681/ASN.2004090804. [DOI] [PubMed] [Google Scholar]
- 107.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Satchell SC, Mathieson PW. Angiopoietins: microvascular modulators with potential roles in glomerular pathophysiology. J Nephrol. 2003;16:168–178. [PubMed] [Google Scholar]
- 109.Salmon AH, Toma I, Sipos A, Muston PR, Harper SJ, Bates DO, Neal CR, Peti-Peterdi J. Evidence for restriction of fluid and solute movement across the glomerular capillary wall by the subpodocyte space. Am J Physiol Renal Physiol. 2007;293:F1777–F1786. doi: 10.1152/ajprenal.00187.2007. [DOI] [PubMed] [Google Scholar]
- 110.Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol. 2001;12:1448–1457. doi: 10.1681/ASN.V1271448. [DOI] [PubMed] [Google Scholar]
- 111.Scruggs B, Donnert E, Ma L-J, Bertram J, Fogo AB. Capillary branching contributes to regression of sclerosis by angiotensin receptor blocker (ARB). J Am Soc Nephrol. 2005;16:674A. doi: 10.1016/j.ajpath.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liang XB, Ma LJ, Naito T, Wang Y, Madaio M, Zent R, Pozzi A, Fogo AB. Angiotensin type 1 receptor blocker restores podocyte potential to promote glomerular endothelial cell growth. J Am Soc Nephrol. 2006;17:1886–1895. doi: 10.1681/ASN.2005020205. [DOI] [PubMed] [Google Scholar]
- 113.Ma LJ, Fogo AB. Role of angiotensin II in glomerular injury. Semin Nephrol. 2001;21:544–553. doi: 10.1053/snep.2001.26793. [DOI] [PubMed] [Google Scholar]
- 114.Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25:291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ferrario CM, Chappell MC. Novel angiotensin peptides. Cell Mol Life Sci. 2004;61:2720–2727. doi: 10.1007/s00018-004-4243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nguyen G. The (pro)renin receptor: pathophysiological roles in cardiovascular and renal pathology. Curr Opin Nephrol Hypertens. 2007;16:129–133. doi: 10.1097/MNH.0b013e328040bfab. [DOI] [PubMed] [Google Scholar]
- 117.Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press. 2003;12:70–88. doi: 10.1080/08037050310001057. [DOI] [PubMed] [Google Scholar]
- 118.Ruiz-Ortega M, Esteban V, Suzuki Y, Ruperez M, Mezzano S, Ardiles L, Justo P, Ortiz A, Egido J. Renal expression of angiotensin type 2 (AT2) receptors during kidney damage. Kidney Int Suppl. 2003;64:S21–S26. doi: 10.1046/j.1523-1755.64.s86.5.x. [DOI] [PubMed] [Google Scholar]
- 119.Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension. 2003;42:600–604. doi: 10.1161/01.HYP.0000090323.58122.5C. [DOI] [PubMed] [Google Scholar]
- 120.Wolf G. “The road not taken”: role of angiotensin II type 2 receptor in pathophysiology. Nephrol Dial Transplant. 2002;17:195–198. doi: 10.1093/ndt/17.2.195. [DOI] [PubMed] [Google Scholar]
- 121.Wolf G, Ziyadeh FN, Thaiss F, Tomaszewski J, Caron RJ, Wenzel U, Zahner G, Helmchen U, Stahl RA. Angiotensin II stimulates expression of the chemokine RANTES in rat glomerular endothelial cells. Role of the angiotensin type 2 receptor. J Clin Invest. 1997;100:1047–1058. doi: 10.1172/JCI119615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ma J, Nishimura H, Fogo AB, Kon V, Inagami T, Ichikawa I. Accelerated fibrosis and collagen deposition develop in the renal interstitium of angiotensin type 2 receptor null mutant mice during ureteral obstruction. Kidney Int. 1998;53:937–944. doi: 10.1111/j.1523-1755.1998.00893.x. [DOI] [PubMed] [Google Scholar]
- 123.Ohkubo N, Matsubara H, Nozawa Y. Angiotensin type 2 receptors are reexpressed by cardiac fibroblasts from failing myopathic hamster hearts and inhibit cell growth and fibrillar collagen metabolism. Circulation. 1997;96:3954–3962. doi: 10.1161/01.cir.96.11.3954. [DOI] [PubMed] [Google Scholar]
- 124.Hashimoto N, Maeshima Y, Satoh M, Odawara M, Sugiyama H, Kashihara N, Matsubara H, Yamasaki Y, Makino H. Overexpression of angiotensin type 2 receptor ameliorates glomerular injury in a mouse remnant kidney model. Am J Physiol Renal Physiol. 2004;286:F516–525. doi: 10.1152/ajprenal.00294.2003. [DOI] [PubMed] [Google Scholar]
- 125.Naito T, Ma LJ, Donnert E, Fogo AB. Angiotensin type 2 receptor antagonist (AT2RA) worsens glomerulosclerosis in the rat remnant kidney model. J Am Soc Nephrol. 2005;16:654A. [Google Scholar]
- 126.Cao Z, Bonnet F, Candido R, Nesteroff SP, Burns WC, Kawachi H, Shimizu F, Carey RM, De Gasparo M, Cooper ME. Angiotensin type 2 receptor antagonism confers renal protection in a rat model of progressive renal injury. J Am Soc Nephrol. 2002;13:1773–1787. doi: 10.1097/01.asn.0000019409.17099.33. [DOI] [PubMed] [Google Scholar]
- 127.Kouyama R, Suganami T, Nishida J, Tanaka M, Toyoda T, Kiso M, Chiwata T, Miyamoto Y, Yoshimasa Y, Fukamizu A, Horiuchi M, Hirata Y, Ogawa Y. Attenuation of diet-induced weight gain and adiposity through increased energy expenditure in mice lacking angiotensin II type 1a receptor. Endocrinology. 2005;146:3481–3489. doi: 10.1210/en.2005-0003. [DOI] [PubMed] [Google Scholar]
- 128.van Dielen FM, Buurman WA, Hadfoune M, Nijhuis J, Greve JW. Macrophage inhibitory factor, plasminogen activator inhibitor-1, other acute phase proteins, and inflammatory mediators normalize as a result of weight loss in morbidly obese subjects treated with gastric restrictive surgery. J Clin Endocrinol Metab. 2004;89:4062–4068. doi: 10.1210/jc.2003-032125. [DOI] [PubMed] [Google Scholar]
- 129.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290:E961–967. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]