Abstract
Background
Liver disease is a common comorbid condition in maintenance hemodialysis (MHD) patients and may be associated with poor survival. The relationship between aspartate aminotransferase (AST) and survival has not yet been addressed in these patients. We hypothesized that higher AST level is associated with higher death risk in MHD patients.
Methods
A 5-year (January 2007–December 2011) cohort of 109 718 MHD patients was studied in the USA in dialysis clinics where AST was measured in at least 50% of all outpatients in the baseline calendar quarter. Survival models were adjusted for demographic variables, and available clinical and laboratory surrogates of malnutrition-inflammation complex, and cubic survival splines were plotted.
Results
A linear association existed between baseline serum AST levels and mortality. Increasing AST of >20 IU/L was incrementally and almost linearly associated with higher death risk at all levels of adjustment. In fully adjusted models, AST levels of ≥40 IU/L were associated with the highest risk of mortality (hazard ratio: 1.46, 95% CI: 1.38–1.54). Low AST levels (<15 IU/L) were associated with increased death risk only in fully adjusted models examining hepatitis C virus-positive patients.
Conclusions
Higher AST level of >20 IU/L is incrementally associated with higher mortality in MHD patients whereas AST in the 15–20 IU/L range is associated with the greatest survival. These findings suggest that the assessment of liver function and improving liver disease may confer survival benefit to MHD patients.
Keywords: all-cause mortality, aspartate aminotransferase, end-stage renal disease, hemodialysis, liver enzymes
INTRODUCTION
End-stage renal disease (ESRD) is frequently accompanied by major chronic diseases such as coronary heart disease (13–24%), chronic heart failure (5–20%), diabetes mellitus (12–28%), hypertension (34–50%) and stroke or transient ischemic attack (6–13%) [1]. While not as common, chronic liver disease can coexist with ESRD and its presence may be associated with poor clinical outcomes. Hepatitis C virus (HCV) infection, which itself can lead to chronic kidney disease [2], is the most common cause of liver damage in maintenance hemodialysis (MHD) patients [3–8].
Some of the most useful laboratory tests to screen for liver disease are serum aspartate aminotransferase (AST) and serum alanine transaminase (ALT). Many studies report that serum AST levels are low in patients undergoing dialysis. This is true even in HCV-positive patients, and some of these studies suggest lowering the upper limits of normal AST and ALT levels in this patient population [9–14]. Previous investigations have identified vitamin B6 deficiency as a potential mechanism responsible for hypoaminotransferasemia in dialysis patients given that vitamin B6 is required as a co-enzyme for aminotransferases to express their activity [15–21]. However, subsequent investigations noted that hemodilution may also be another mechanism underlying the lower aminotransferase level in dialysis patients [22, 23].
While some studies have reported a positive association between elevated liver enzymes and increased risk of mortality in MHD patients, the relationship between AST and all-cause mortality in MHD patients has not been thoroughly examined. The present study was therefore designed to determine the relationship between serum AST level and all-cause mortality in ESRD patients maintained on hemodialysis. To this end, we examined a large national database of incident MHD patients of contemporary origin with mostly uniform practice patterns and highly standardized laboratory testing criteria that were all measured in a single laboratory. We hypothesized that higher levels of AST would be associated with increased all-cause mortality in MHD patients.
MATERIALS AND METHODS
Study population and data source
This study included MHD patients initiating treatment between 2007 and 2011, with follow-up through 31 December 2011. All data were obtained from the electronic records of one of the large dialysis organizations (LDO). The study was approved by the Institutional Review Committees of the Los Angeles Biomedical Research Institute at Harbor-UCLA, University of California, Irvine Medical Center and DaVita Clinical Research. Given the large sample size, anonymity of the patients studied and nonintrusive nature of the research, the requirement for written consent was waived.
Patients were considered to be on MHD if they were treated for at least 60 consecutive days (‘60-day rule’ as used by the United States Renal Data System). The first (baseline) studied quarter for each patient was the first 91-day period starting from the date of first dialysis treatment. Follow-up was available for 20 quarters. Patients aged <18 years at baseline, those who did not receive treatment for at least 60 days or who were missing AST at baseline were excluded from the cohort.
Clinical and demographic measures
The analytic cohort comprised of 109 718 MHD patients. Cohort construction is summarized in Supplementary data, Figure S1. Information on race/ethnicity, primary insurance and the presence of diabetes at baseline was obtained from the LDO's electronic records database. In this database, race/ethnicity is self-categorized in that dialysis patients select the race and/or ethnicity with which they were most closely identified according to US Census Bureau categorizations [24].
To minimize measurement variability, all repeated laboratory and clinical measurements for each patient during the calendar quarter of entry were averaged. Average values were obtained from up to 20 entry calendar quarters (from 1 January 2007 through 31 December 2011) for each patient. Post-hemodialysis dry weight and baseline height were used to calculate body mass index (BMI). Furthermore, the presence or absence of diabetes and pre-existing comorbid conditions were categorized into 11 baseline comorbid conditions: (i) alcohol dependence, (ii) congestive heart failure, (iii) chronic obstructive pulmonary disease, (iv) cerebrovascular disease, (v) HIV, (vi) history of cancer, (vii) history of hypertension, (viii) atherosclerotic heart disease, (ix) liver disease, (x) other cardiac disease and (xi) drug dependence.
Laboratory measures
Blood samples were drawn using uniform techniques in all dialysis clinics and were transported to a single, central laboratory typically within 24 h. All laboratory values were measured using automated and standardized methods in the central laboratory. Most laboratory parameters were measured monthly, including urea nitrogen, albumin, creatinine, total iron-binding capacity (TIBC), bicarbonate, phosphorus and calcium. Serum ferritin was measured at least quarterly. Hemoglobin was measured at least monthly in all patients and weekly to biweekly in most patients. Kt/V was used to estimate dialysis dosage, and normalized protein catabolic rate (nPCR) was measured monthly as an indicator of daily protein intake. Most blood samples were collected before dialysis, except for post-dialysis serum urea nitrogen to calculate urea kinetics.
Outcome ascertainment
The outcome of interest was all-cause death, which was ascertained from the LDO database. We evaluated the association between AST levels and all-cause mortality using Cox proportional hazards models in which patients remained at-risk until death or censoring for renal transplantation, transfer to another dialysis clinic or end of the study period (31 December 2011).
Statistical analysis
Data were summarized using proportions, means (±standard deviation, SD) or median [interquartile range (IQR)] as appropriate, and multiple linear regression models were fitted to construct Pearson's and partial correlations. Plots of log [−log (survival rate)] against log (survival time) were performed to test the proportionality assumption. We divided serum AST levels into eight categories (<10, 10–<15, 15–<20, 20–<25, 25–<30, 30–<35, 35–<40 and ≥40 IU/L), and using Cox proportional hazards regression models, we evaluated the association between all-cause mortality and AST within the aforementioned eight AST groups using 15–<20 IU/L as reference.
For each analysis, three models were examined based on the level of multivariate adjustment: (i) a minimally adjusted model that included mortality as the outcome, AST groups (<10, 10–<15, 15–<20, 20–<25, 25–<30, 30–<35, 35–<40 and ≥40 IU/L) and entry calendar quarter (q1 through q20) as covariates; (ii) case-mix-adjusted models that included all of the above plus age, gender, ethnicity (African American and other self-categorized black, non-Hispanic white, Asian, Hispanic and other), diabetes and 11 pre-existing comorbid states, baseline HCV status, primary insurance (Medicare, Medicaid and other), dialysis dosage as indicated by Kt/V and residual renal function during the entry quarter (i.e. urinary urea clearance); and (iii) malnutrition-inflammation complex syndrome (MICS)-adjusted models, which included all of the covariates in the case-mix model as well as 13 surrogates of nutritional status and inflammation including BMI and 12 laboratory variables, together also known as MICS, with known association with clinical outcomes in MHD patients: (a) serum albumin, (b) serum creatinine, (c) serum TIBC, (d) serum ferritin, (e) serum phosphorus, (f) serum calcium, (g) serum bicarbonate, (h) peripheral white blood cell (WBC) count, (i) lymphocyte percentage, (j) hemoglobin, (k) iron saturation and (l) nPCR as an indicator of daily protein intake, also known as the normalized protein nitrogen appearance (nPNA).
Associations between continuous AST and mortality across the three levels of multivariable adjustment were also modeled using restricted cubic splines with knots at AST of 10, 25 and 40 IU/L. In order to verify the validity of the AST–mortality association, we used Cox models to examine eight-category AST and mortality within strata of baseline HCV status using the above-mentioned three levels of multivariate adjustment. We also performed sensitivity analyses with ALT as the exposure, modeling the ALT-mortality association with Cox regression and splines.
Finally, subgroup analyses were conducted to examine the associations of mortality with higher AST (≥20 IU/L) versus reference lower AST (<20 IU/L) across strata of a priori selected subgroups including: gender (male, female), age (<65, ≥65 years), race/ethnicity (Caucasian, African American, Hispanic, Asian), diabetes (diabetic, non-diabetic), albumin (≥3.8, <3.8 g/dL), baseline HCV status and ever HCV status. Missing covariate data (<1% for most laboratory and demographic variables) were imputed by means or medians of recorded values. Data on baseline HCV status and ever HCV status were missing in 31 and 12%, respectively, and were imputed by means when used as a covariate but not imputed when used in the subgroup analyses. Most analyses were carried out with SAS version 9.4, SAS Institute, Inc., Cary, NC. Splines were carried out using Stata version 13.1 (Stata Corporation, College Station, TX).
RESULTS
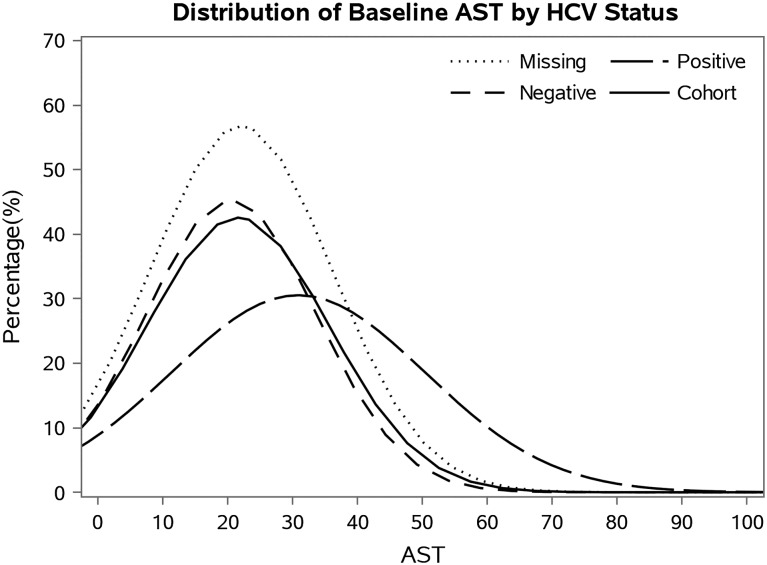
The original 5-year national database included 112 017 MHD patients. After excluding patients with no information on baseline AST, the final study population consisted of 109 718 patients, of which 5896 (5.4%) were HCV positive at baseline. These patients had a median follow-up time of 493 days (IQR = 230–921) and 29 000 deaths (26%). The patient's average age was 63 ± 15 years old with 44% female, 33% African Americans and 58% diabetics. Table 1 shows baseline demographic, clinical and laboratory characteristics of all patients with baseline AST serum measurements as well as those in each AST subset (<10, 10–<15, 15–<20, 20–<25, 25–<30, 30–<35, 35–<40, ≥40 IU/L). Approximately 2% of patients had plasma AST levels of <10 IU/L and 5% had AST levels of >40 IU/L. Patients with AST of <10 IU/L were younger, included more men and tended to have a lower prevalence of diabetes when compared with patients in the highest AST levels. Mean (±SD) baseline AST concentration was 30.9 ± 19.6 IU/L in HCV-positive patients and 20.6 ± 13.2 IU/L in HCV-negative patients (Figure 1).
Table 1.
Demographic and clinical characteristics of 109 718 MHD patients, including patients in eight groups of AST
| Characteristics | All MHD patients | AST (IU/L) |
P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AST < 10 | 10 ≤ AST<15 | 15 ≤ AST < 20 | 20 ≤ AST < 25 | 25 ≤ AST < 30 | 30 ≤ AST < 35 | 35 ≤ AST < 40 | AST ≥ 40 | |||
| n = 109 718 | n = 2399 | n = 22 997 | n = 36 585 | n = 22 766 | n = 11 071 | n = 5415 | n = 2965 | n = 5520 | ||
| Age (years) | 63 ± 15 | 56 ± 17 | 61 ± 15 | 63 ± 15 | 65 ± 15 | 64 ± 15 | 63 ± 15 | 62 ± 14 | 60 ± 14 | <0.001 |
| Gender (% women) | 44 | 29 | 42 | 45 | 45 | 50 | 43 | 41 | 41 | 0.35 |
| Diabetes mellitus (%) | 58 | 46 | 54 | 59 | 61 | 49 | 61 | 61 | 58 | <0.001 |
| Race (%) | ||||||||||
| White | 47 | 42 | 46 | 48 | 48 | 47 | 45 | 41 | 40 | <0.001 |
| African American | 31 | 35 | 33 | 30 | 29 | 31 | 33 | 36 | 39 | 0.008 |
| Hispanic | 15 | 18 | 15 | 15 | 15 | 14 | 14 | 15 | 13 | <0.001 |
| Asian | 3 | 2 | 2 | 3 | 4 | 4 | 4 | 4 | 4 | <0.001 |
| Other | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 3 | 4 | <0.001 |
| Primary insurance (%) | ||||||||||
| Medicare | 54 | 46 | 52 | 54 | 55 | 55 | 55 | 53 | 51 | <0.001 |
| Medicaid | 7 | 9 | 7 | 6 | 7 | 7 | 8 | 9 | 9 | 0.01 |
| Other | 39 | 45 | 41 | 40 | 38 | 38 | 37 | 38 | 40 | 0.006 |
| Dialysis access type (%) | ||||||||||
| CVC | 78 | 61 | 72 | 77 | 80 | 81 | 82 | 82 | 83 | <0.001 |
| AV fistula | 15 | 30 | 19 | 15 | 13 | 12 | 11 | 11 | 10 | <0.001 |
| AV graft | 4 | 5 | 5 | 4 | 4 | 4 | 3 | 4 | 3 | <0.001 |
| AV other | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <0.001 |
| Unknown | 3 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | <0.001 |
| Kt/V (dialysis dose) | 1.47 ± 0.33 | 1.42 ± 0.31 | 1.45 ± 0.31 | 1.47 ± 0.32 | 1.49 ± 0.34 | 1.48 ± 0.33 | 1.46 ± 0.32 | 1.47 ± 0.34 | 1.45 ± 0.33 | <0.001 |
| KRU (residual renal function) (mL/min) | 4.06 ± 3.55 | 3.63 ± 3.30 | 3.96 ± 3.28 | 4.07 ± 3.41 | 4.13 ± 3.79 | 3.45 ± 2.72 | 4.05 ± 3.51 | 4.44 ± 4.68 | 4.04 ± 4.14 | <0.001 |
| PT/INR | 2.00 ± 1.03 | 1.57 ± 0.70 | 1.86 ± 1.05 | 1.99 ± 1.04 | 2.00 ± 0.94 | 2.12 ± 1.07 | 2.16 ± 1.01 | 2.28 ± 1.24 | 2.07 ± 1.11 | <0.001 |
| Comorbidities (%) | ||||||||||
| Alcohol dependence | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <0.001 |
| Congestive heart failure | 37 | 33 | 34 | 37 | 38 | 39 | 40 | 38 | 38 | <0.001 |
| Chronic obstructive pulmonary disease | 5 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | <0.001 |
| Cerebrovascular disease | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | <0.001 |
| HIV-positive status | <1 | <1 | <1 | <1 | <1 | <1 | 1 | 1 | 2 | 0.69 |
| History of cancer | 2 | 2 | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 0.69 |
| History of hypertension | 51 | 54 | 51 | 51 | 51 | 52 | 51 | 51 | 49 | <0.001 |
| Atherosclerotic heart disease | 14 | 12 | 13 | 15 | 15 | 16 | 15 | 15 | 14 | 0.647 |
| Liver disease | 1 | <1 | <1 | 1 | 1 | 2 | 2 | 4 | 5 | 0.002 |
| Other cardiovascular disease | 15 | 12 | 15 | 15 | 16 | 17 | 16 | 16 | 15 | <0.001 |
| Drug dependence | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <0.001 |
| Ever HCV positive | 8 | 3 | 2 | 4 | 7 | 13 | 19 | 24 | 31 | <0.001 |
| Serum levels | ||||||||||
| ALT (IU/L) | 16.1 ± 14.2 | 8.16 ± 3.50 | 10.2 ± 4.08 | 13.2 ± 5.34 | 17.3 ± 7.93 | 21.7 ± 11.8 | 26.2 ± 14.7 | 29.3 ± 15.1 | 47.9 ± 43.5 | <0.001 |
| Albumin (g/dL) | 3.51 ± 0.48 | 3.69 ± 0.48 | 3.58 ± 0.45 | 3.54 ± 0.46 | 3.50 ± 0.46 | 3.45 ± 0.48 | 3.39 ± 0.51 | 3.35 ± 0.52 | 3.22 ± 0.55 | <0.001 |
| Calcium (mg/dL) | 8.68 ± 0.63 | 8.67 ± 0.66 | 8.65 ± 0.63 | 8.69 ± 0.62 | 8.72 ± 0.62 | 8.70 ± 0.64 | 8.66 ± 0.65 | 8.62 ± 0.66 | 8.54 ± 0.66 | <0.001 |
| Bicarbonate (mg/dL) | 23.6 ± 2.70 | 22.7 ± 2.75 | 23.2 ± 2.69 | 23.6 ± 266 | 23.8 ± 2.68 | 23.9 ± 2.72 | 23.8 ± 2.73 | 23.7 ± 2.76 | 23.6 ± 2.76 | <0.001 |
| Creatinine (mg/dL) | 5.86 ± 2.36 | 7.59 ± 3.01 | 6.39 ± 2.49 | 5.86 ± 2.28 | 5.59 ± 2.22 | 5.48 ± 2.23 | 5.48 ± 2.33 | 5.46 ± 2.24 | 5.44 ± 2.24 | 0.001 |
| Ferritin (ng/mL) | 283 (164, 486) | 231 (133, 400) | 253 (148, 428) | 273 (160, 459) | 292 (170, 501) | 305 (177, 526) | 332 (188, 573) | 241 (193, 599) | 400 (217, 719) | <0.001 |
| Blood hemoglobin (g/dL) | 11.1 ± 1.18 | 10.9 ± 1.23 | 11.0 ± 1.15 | 11.1 ± 1.15 | 11.2 ± 1.17 | 11.2 ± 1.22 | 11.2 ± 1.26 | 11.1 ± 1.25 | 11.0 ± 1.30 | <0.001 |
| Iron saturation ratio | 23.1 ± 9.13 | 22.7 ± 8.71 | 22.2 ± 8.11 | 22.5 ± 1.19 | 23.0 ± 8.62 | 23.7 ± 9.37 | 24.5 ± 10.8 | 25.4 ± 11.6 | 27.8 ± 14.3 | <0.001 |
| Lymphocyte (% of total WBC) | 20.7 ± 7.54 | 21.1 ± 7.56 | 20.6 ± 7.17 | 20.6 ± 7.33 | 20.6 ± 7.53 | 20.7 ± 7.91 | 20.9 ± 8.08 | 21.3 ± 8.25 | 21.6 ± 8.53 | <0.001 |
| Protein catabolic rate (g/kg/day) | 0.79 ± 0.22 | 0.82 ± 0.22 | 0.80 ± 0.21 | 0.79 ± 0.22 | 0.80 ± 0.22 | 0.79 ± 0.22 | 0.77 ± 0.22 | 0.77 ± 0.23 | 0.76 ± 0.23 | <0.001 |
| Phosphorus (mg/dL) | 4.92 ± 1.15 | 5.38 ± 1.27 | 5.10 ± 1.18 | 4.93 ± 1.13 | 4.83 ± 1.12 | 4.78 ± 1.13 | 4.77 ± 1.13 | 4.79 ± 1.16 | 4.79 ± 1.16 | <0.001 |
| TIBC (mg/dL) | 225 ± 49.1 | 225 ± 44.2 | 223 ± 45.4 | 224 ± 46.6 | 226 ± 50.0 | 227 ± 51.9 | 227 ± 54.9 | 229 ± 57.0 | 226 ± 61.0 | <0.001 |
| White blood cell (×103/µL) | 7.82 ± 2.68 | 7.69 ± 2.50 | 7.86 ± 2.49 | 7.87 ± 2.58 | 7.82 ± 2.60 | 7.78 ± 3.02 | 7.75 ± 3.03 | 7.67 ± 3.07 | 7.62 ± 3.05 | <0.001 |
| Body mass index (kg/m2) | 28.1 ± 7.33 | 28.6 ± 7.57 | 29.0 ± 7.65 | 28.3 ± 7.34 | 27.8 ± 7.19 | 27.5 ± 7.01 | 27.2 ± 7.04 | 27.1 ± 6.81 | 26.6 ± 6.96 | <0.001 |
Dichotomous/dummy variables are presented as percentage; continuous variables are presented as mean ± SD or median (IQR).
ALT, alanine transaminase; AV, arteriovenous; CVC, central venous catheter; HCV, hepatitis C virus; IQR, interquartile range; KRU, kidney residual time; PR/INR, prothrombin time/international normalized ratio; SD, standard deviation; TIBC, total iron-binding capacity; WBC, white blood cell.
FIGURE 1:
Distribution of baseline AST by baseline HCV status.
Comparisons of the bivariate (unadjusted) and multivariate adjusted correlation coefficients between AST and some clinically relevant variables in the baseline quarter are shown in Supplementary data, Table S1. Serum albumin, ferritin and TIBC concentrations displayed the strongest correlations with AST.
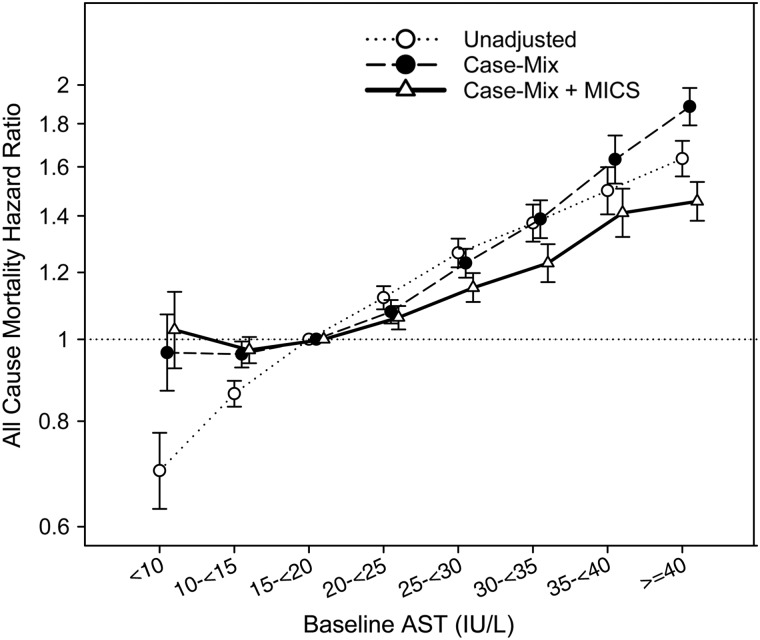
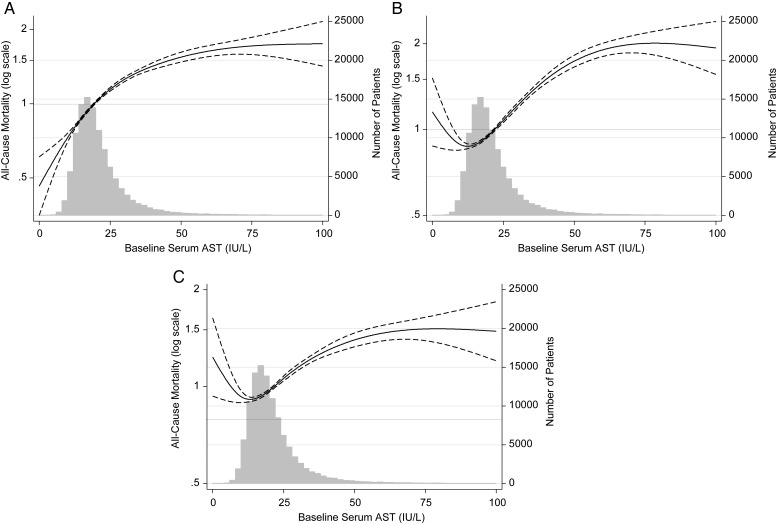
Figure 2 displays 5-year death hazard ratios (HR) for eight categories of AST in all 109 718 MHD patients studied. Using AST 15–<20 IU/L as reference, there was an incremental linear association between AST levels and all-cause mortality in all levels of adjustment. In case-mix and fully adjusted models, the higher survival for patients with AST of <10 was attenuated and no longer significant. In fully adjusted models, AST levels of ≥40 IU/L were associated with the highest risk of mortality (HR: 1.46, 95% CI: 1.38–1.54). Similar results were seen in Figure 3, which displays continuous AST and all-cause mortality using splines. Harrell's c-index was 0.74. In sensitivity analyses using ALT, we also found an incremental, almost linear relationship between this liver enzyme and mortality (Supplementary data, Figures S2 and S3).
FIGURE 2:
Association of AST and all-cause mortality using Cox proportional hazards models in the entire cohort of 109 718 MHD patients.
FIGURE 3:
Cubic spline models of Cox proportional regression analyses reflecting adjusted mortality predictability (with 95% CI) according to increasing serum ALT concentrations in the entire cohort of 109 718 MHD patients (A: unadjusted; B: case-mix adjusted; C: case-mix and MICS adjusted).
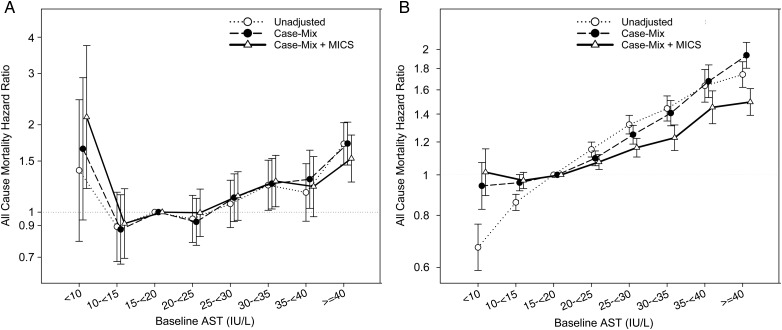
Figure 4 shows the association between AST and all-cause mortality within strata of HCV status. In the fully adjusted model, the lowest AST is associated with higher death risk only in HCV-positive patients (Figure 4A, HR: 2.13, 95% CI: 1.21–3.74). The incremental, almost linear association between AST and mortality among HCV-negative MHD patients (Figure 4B) is almost identical to that seen in the entire cohort (Figure 2).
FIGURE 4:
(A) Association of AST and all-cause mortality using Cox proportional hazards models in 5896 HCV-positive MHD patients. (B) Association of AST and all-cause mortality using Cox proportional hazards models in 69 849 HCV-negative MHD patients.
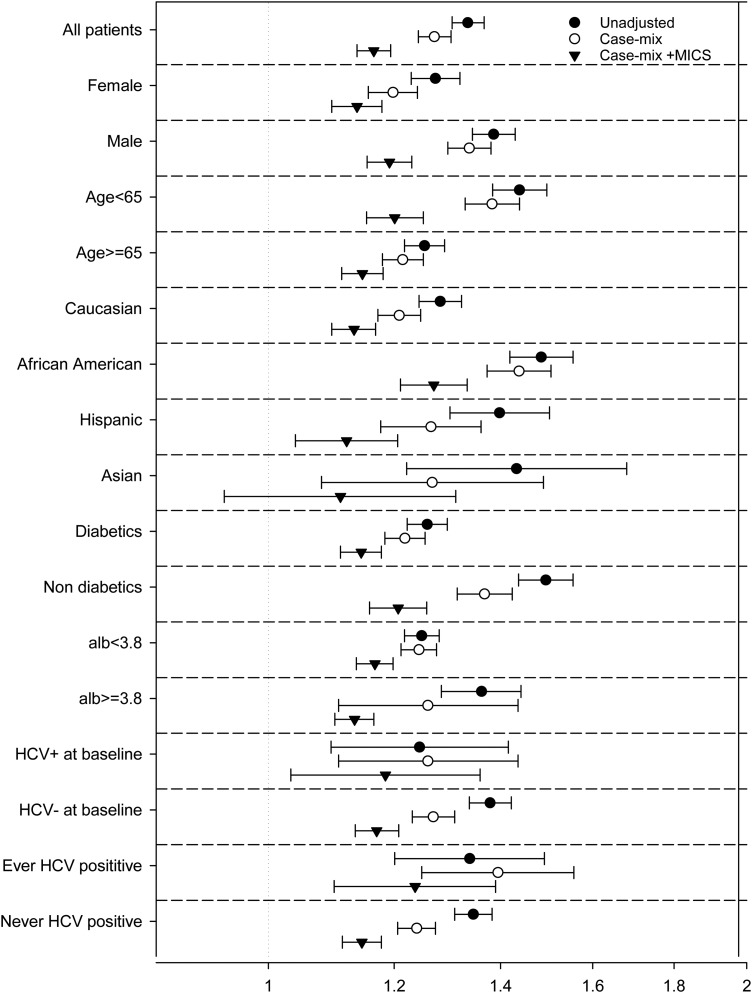
In subgroup analyses, the unadjusted all-cause mortality HRs were above unity in almost all examined subgroups, indicating a higher risk of death with higher AST (≥20 IU/L) compared with low AST (<20 IU/L). These associations persisted after full adjustment in all subgroups but Asian (Figure 5).
FIGURE 5:
Association of AST and all-cause mortality in patients with AST of ≥20 IU/L in selected subgroups of MHD patients.
In logistic regressions adjusted for case-mix and MICS covariates, main predictors of high AST (AST ≥20 IU/L) included Asian race, HIV positivity, baseline HCV-positive status and serum TIBC. After adjustment, we found that the odds of experiencing high AST levels were 59% higher for Asians compared with all other race/ethnicities, 164% higher for HIV-positive patients and 277% higher for HCV-positive patients. In addition, a 100 mg/dL increase in serum TIBC increased the odds of finding an elevated AST level by 171%. Other significant predictors of high AST included central venous catheter access, liver disease, alcohol dependence and iron saturation ratio (Table 2).
Table 2.
Multivariate logistic regression model predicting AST ≥20 IU/L in 109 718 MHD patients
| Variable | Unadjusted |
Case-mix |
Case-mix + MICS |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age (Δ10 years) | 1.07 (1.06, 1.08) | <0.001 | 1.12 (1.11, 1.13) | <0.001 | 1.03 (1.01, 1.04) | <0.001 |
| Female | 1.01 (0.99, 1.04) | 0.28 | 1.04 (1.00, 1.07) | 0.03 | 0.96 (0.93, 0.99) | 0.03 |
| Diabetes mellitus | 1.06 (1.03, 1.09) | <0.001 | 1.04 (1.01, 1.08) | 0.02 | 0.97 (0.94, 1.01) | 0.14 |
| Race | ||||||
| White | 0.96 (0.94, 0.98) | 0.001 | 0.86 (0.79, 0.93) | <0.001 | 0.84 (0.77, 0.91) | <0.001 |
| Black | 0.99 (0.97, 1.02) | 0.67 | 0.98 (0.94, 1.01) | 0.20 | 1.09 (1.04, 1.13) | <0.001 |
| Asian | 1.51 (1.41, 1.61) | <0.001 | 1.63 (1.50, 1.77) | <0.001 | 1.59 (1.46, 1.73) | <0.001 |
| Hispanic | 0.96 (0.93, 1.00) | 0.03 | 1.03 (0.98, 1.08) | 0.27 | 1.07 (1.02, 1.13) | 0.005 |
| Other | 1.11 (1.04, 1.18) | 0.002 | 1.17 (1.07, 1.27) | <0.001 | 1.20 (1.10, 1.31) | <0.001 |
| Primary insurance | ||||||
| Medicare | 1.05 (1.02, 1.07) | <0.001 | 1.01 (0.98, 1.04) | 0.65 | 0.98 (0.94, 1.01) | 0.15 |
| Medicaid | 1.06 (1.01, 1.11) | 0.02 | 0.97 (0.91, 1.04) | 0.38 | 0.98 (0.92, 1.05) | 0.64 |
| Other | 0.94 (0.92, 0.96) | <0.001 | 0.99 (0.96, 1.03) | 0.65 | 1.03 (0.99, 1.06) | 0.15 |
| Access type | ||||||
| CVC | 1.44 (1.40, 1.49) | <0.001 | 1.13 (1.03, 1.23) | 0.01 | 1.15 (1.05, 1.26) | 0.003 |
| AV fistula | 0.65 (0.63, 0.68) | <0.001 | 0.65 (0.62, 0.68) | <0.001 | 0.66 (0.63, 0.70) | <0.001 |
| AV graft | 0.80 (0.75, 0.85) | <0.001 | 0.71 (0.66, 0.77) | <0.001 | 0.72 (0.66, 0.78) | <0.001 |
| AV other | 0.74 (0.50, 1.11) | 0.15 | 0.68 (0.42, 1.11) | 0.12 | 0.70 (0.43, 1.15) | 0.16 |
| Unknown | 0.97 (0.90, 1.03) | 0.31 | 0.89 (0.81, 0.97) | 0.01 | 0.87 (0.79, 0.95) | 0.003 |
| Kt/V (dialysis dose) (Δ1) | 1.18 (1.13, 1.22) | <0.001 | 1.11 (1.05, 1.16) | <0.001 | 0.90 (0.85, 0.96) | <0.001 |
| Residual renal function | 1.02 (1.01, 1.03) | <0.001 | 1.02 (1.01, 1.03) | <0.001 | 1.00 (0.99, 1.01) | 0.90 |
| Comorbid States | ||||||
| Alcohol | 1.87 (1.45, 2.39) | <0.001 | 1.55 (1.12, 2.15) | 0.009 | 1.47 (1.05, 2.08) | 0.03 |
| Congestive heart failure | 1.05 (1.02, 1.08) | <0.001 | 1.07 (1.03, 1.10) | <0.001 | 1.07 (1.04, 1.11) | <0.001 |
| COPD | 0.99 (0.93, 1.04) | 0.61 | 0.81 (0.75, 0.87) | <0.001 | 0.95 (0.84, 1.07) | 0.41 |
| Cerebrovascular disease | 1.03 (0.94, 1.13) | 0.52 | 0.95 (0.85, 1.07) | 0.43 | 0.81 (0.75, 0.87) | <0.001 |
| HIV | 3.48 (2.88, 4.22) | <0.001 | 3.24 (2.55, 4.11) | <0.001 | 2.64 (2.05, 3.39) | <0.001 |
| History of cancer | 0.99 (0.91, 1.07) | 0.77 | 0.96 (0.87, 1.07) | 0.47 | 0.89 (0.80, 0.99) | 0.03 |
| History of hypertension | 0.95 (0.93, 0.98) | <0.001 | 0.92 (0.89, 0.95) | <0.001 | 1.01 (0.98, 1.04) | 0.64 |
| Atherosclerotic heart disease | 0.99 (0.96, 1.03) | 0.82 | 0.97 (0.93, 1.02) | 0.29 | 0.97 (0.92, 1.02) | 0.19 |
| Liver disease | 2.20 (1.99, 2.44) | <0.001 | 2.14 (1.87, 2.45) | <0.001 | 1.91 (1.66, 2.20) | <0.001 |
| Other cardiovascular disease | 1.06 (1.03, 1.10) | <0.001 | 1.08 (1.03, 1.13) | 0.004 | 1.04 (0.99, 1.10) | 0.11 |
| Drug dependence | 1.76 (1.39, 2.27) | <0.001 | 0.98 (0.71, 1.37) | 0.91 | 0.93 (0.66, 1.32) | 0.70 |
| HCV-positive status | 4.78 (4.31, 4.87) | <0.001 | 4.95 (4.65, 5.27) | <0.001 | 3.77 (3.53, 4.03) | <0.001 |
| Serum levels | ||||||
| Albumin (g/dL) (Δ0.5 g/dL) | 0.76 (0.75, 1.77) | <0.001 | 0.81 (0.80, 0.82) | <0.001 | 0.65 (0.64, 0.67) | <0.001 |
| Calcium (mg/dL) | 0.90 (0.88, 0.92) | <0.001 | 0.96 (0.94, 0.99) | 0.003 | 1.07 (1.04, 1.10) | <0.001 |
| Bicarbonate (mg/dL) | 1.05 (1.04, 1.06) | <0.001 | 1.04 (1.04, 1.05) | <0.001 | 1.02 (1.02, 1.03) | <0.001 |
| Creatinine (mg/dL) | 0.88 (0.88, 0.89) | <0.001 | 0.87 (0.86, 0.87) | <0.001 | 0.92 (0.91, 0.92) | <0.001 |
| Ferritin (Δ100 ng/mL) | 1.08 (1.07, 1.08) | <0.001 | 1.07 (1.07, 1.08) | <0.001 | 1.09 (1.08, 1.10) | <0.001 |
| Blood hemoglobin (g/dL) | 1.03 (1.02, 1.04) | <0.001 | 1.04 (1.02, 1.05) | <0.001 | 1.08 (1.06, 1.10) | <0.001 |
| Iron saturation ratio (Δ10%) | 1.23 (1.21, 1.24) | <0.001 | 1.22 (1.20, 1.24) | <0.001 | 1.20 (1.17, 1.22) | <0.001 |
| Lymphocyte | ||||||
| (% of total WBC) (Δ10%) | 1.04 (1.03, 1.06) | <0.001 | 1.03 (1.01, 1.05) | 0.01 | 1.03 (1.00, 1.05) | 0.02 |
| Protein catabolic rate (g/kg/day) | 0.74 (0.70, 0.78) | <0.001 | 0.75 (0.70, 0.81) | <0.001 | 1.25 (1.14, 1.36) | <0.001 |
| Phosphorus (mg/dL) | 0.82 (0.81, 0.83) | <0.001 | 0.82 (0.81, 0.84) | <0.001 | 0.89 (0.88, 0.91) | <0.001 |
| TIBC (Δ100 mg/dL) | 1.20 (1.18, 1.24) | <0.001 | 1.26 (1.22, 1.30) | <0.001 | 2.71 (2.60, 2.84) | <0.001 |
| White blood cell (×103/µL) | 0.99 (0.98, 0.99) | <0.001 | 0.99 (0.98, 0.99) | <0.001 | 0.99 (0.98, 1.00) | <0.001 |
| BMI (kg/m2) | 0.91 (0.90, 0.92) | <0.001 | 0.93 (0.92, 0.94) | <0.001 | 0.93 (0.92, 0.94) | <0.001 |
AV, arteriovenous; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CVC, central venous catheter; HCV, hepatitis C virus; TIBC, total iron-binding capacity; WBC, white blood cell.
DISCUSSION
We examined the association between AST level and all-cause mortality in a nationally representative cohort of 109 718 incident MHD patients. As expected, higher AST levels were associated with increased all-cause mortality. Patients with AST levels of 15–20 IU/L showed the best survival whereas AST levels of >20 IU/L were associated with a significantly higher risk of mortality. This association was consistent across various subgroups of MHD patients. However, when we examined the association within strata of baseline HCV status, lower AST levels were also associated with increased mortality in HCV-positive patients.
There are several mechanisms that can explain the findings from this investigation. One obvious possibility is that higher AST levels are indicative of underlying liver disease, which can be associated with increased morbidity and mortality. The similar results observed from the sensitivity analysis using ALT as an exposure further support this mechanism. Nevertheless, the data from our study clearly indicate a statistically significant trend for increased all-cause mortality for small incremental increases in AST levels even within the ‘normal’ range in the MHD patient population. Therefore, even patients with presumed normal liver function based on liver enzyme levels can be at risk for increased mortality. Hence, additional underlying mechanisms may be at play that can contribute to these observations. This is especially true given the complexity of factors that contribute to all-cause mortality in patients with ESRD. For example, there may be unique underlying biologic factors in MHD patients that impact AST levels and all-cause mortality. This is supported by a study conducted by Skaaby et al. that found a statistically significant inverse association between vitamin D level and incident liver disease. These investigators found that the risk of having a higher plasma level of ALT, AST or GGT (gamma-glutamyl transferase) tended to be increased with lower nutritional vitamin D levels [25, 26]. Given the abundance of evidence connecting nutritional vitamin D deficiency to mortality, low vitamin D levels can be a potential mechanism by which elevated AST levels may be linked with increased risk of mortality [27–29].
In addition, a recent report by Van Beek et al. found that variations in liver enzyme levels can be explained by genetic variations that are then modified by each patient's environmental factors [30]. In the case of MHD patients, the observed variations in serum AST levels may be partly due to hereditary factors that are impacted by the biological effects of ESRD and the patient's environment. The latter would include metabolic risk factors such as dyslipidemia, inflammation, alcohol use, smoking, vitamin D levels and coffee consumption [30]. Hence, future studies will need to assess the potential genetic, biologic and environmental factors that may be responsible for the association of plasma AST levels with all-cause mortality in MHD patients.
Contrary to HCV-negative patients, low AST level was associated with higher risk of mortality in patients with chronic HCV infection. The most reasonable explanation for this is that HCV patients with low AST levels may have minimal parenchymal reserve and low AST level is just a surrogate marker indicating the severity of their end-stage liver disease. Unfortunately, we could not test this hypothesis as we did not have reliable markers of liver parenchymal function in our database such as spontaneous prothrombin level or serum cholinesterase. Further studies are needed to explore this potential explanation.
Several limitations of this study should be mentioned. First, the current findings should be qualified given the observational nature of our study design. Another limitation is the potential confounding of therapy with pharmacologic agents, which may have led to increased AST levels. This was not examined because home medication data were not available systematically in this national cohort. It should be mentioned that some patients needed to be excluded given the fact that baseline AST measurements were not available for them. However, we do not believe confounding by indication was present given that the decision to measure AST levels was made uniformly at the clinic level and was not individualized. In addition, we believe the risk of selection bias was not high given that all of the LDO facilities are under uniform administrative care, and all laboratory tests are performed in one single laboratory with optimal quality-assurance monitoring.
In summary, little is known about the association between AST and mortality in dialysis patients, and in this study, we sought to clarify this relationship. Higher AST levels were incrementally associated with increased all-cause mortality even in fully adjusted models. Moreover, we found an association between low levels of AST and increased mortality in HCV-positive patients. While significantly elevated levels of AST are noted and addressed by most clinicians, moderate increases within the normal range and lower than expected levels may also have clinical relevance and significance. Therefore, future studies are needed to further delineate the link between alterations in serum AST levels and outcomes and describe the mechanisms that can explain these associations.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
FUNDING
This study was supported by KKZ's research grants from the NIH/NIDDK (K24-DK091419), and philanthropist grants from Mr Louis Chang, Mr Harold Simmons and AVEO. H.M. is supported by Award Number 1IK2CX001043 from the Clinical Research and Development Service of the VA Office of Research and Development.
CONFLICT OF INTEREST STATEMENT
K.K.-Z. has received honoraria from Genzyme/Sanofi and Shire and was the medical director of DaVita Harbor-UCLA/MFI in Long Beach, CA, USA, during 2007–12. Other authors have not declared any conflicts of interest.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank DaVita Clinical Research® (DCR) for providing the clinical data for this study.
REFERENCES
- 1.Go AS, Chertow GM, Fan D et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 2.Molnar MZ, Alhourani HM, Wall BM. et al. Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology 2015; 61: 1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ et al. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol 2007; 18: 1584–1593 [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, McAllister CJ, Miller LG. Clinical characteristics and mortality in hepatitis C-positive haemodialysis patients: a population based study. Nephrol Dial Transplant 2005; 20: 1662–1669 [DOI] [PubMed] [Google Scholar]
- 5.Meyers CM, Seeff LB, Stehman-Breen CO et al. Hepatitis C and renal disease: an update. Am J Kidney Dis 2003; 42: 631–657 [DOI] [PubMed] [Google Scholar]
- 6.Batty DS Jr, Swanson SJ, Kirk AD et al. Hepatitis C virus seropositivity at the time of renal transplantation in the United States: associated factors and patient survival. Am J Transplant 2001; 1: 179–184 [PubMed] [Google Scholar]
- 7.Schneeberger PM, Keur I, van Loon AM et al. The prevalence and incidence of hepatitis C virus infections among dialysis patients in the Netherlands: a nationwide prospective study. J Infect Dis 2000; 182: 1291–1299 [DOI] [PubMed] [Google Scholar]
- 8.Dussol B, Berthezene P, Brunet P et al. Hepatitis C virus infection among chronic dialysis patients in the south of France: a collaborative study. Am J Kidney Dis 1995; 25: 399–404 [DOI] [PubMed] [Google Scholar]
- 9.Wolf PL, Williams D, Coplon N et al. Low aspartate transaminase activity in serum of patients undergoing chronic hemodialysis. Clin Chem 1972; 18: 567–568 [PubMed] [Google Scholar]
- 10.Warnock LG, Stone WJ, Wagner C. Decreased aspartate aminotransferase (‘SGOT’) activity in serum of uremic patients. Clin Chem 1974; 20: 1213–1216 [PubMed] [Google Scholar]
- 11.Dobbelstein H, Korner WF, Mempel W et al. Vitamin B6 deficiency in uremia and its implications for the depression of immune responses. Kidney Int 1974; 5: 233–239 [DOI] [PubMed] [Google Scholar]
- 12.Cohen GA, Goffinet JA, Donabedian RK et al. Observations on decreased serum glutamic oxalacetic transaminase (SGOT) activity in azotemic patients. Ann Intern Med 1976; 84: 275–280 [DOI] [PubMed] [Google Scholar]
- 13.Hung KY, Lee KC, Yen CJ et al. Revised cutoff values of serum aminotransferase in detecting viral hepatitis among CAPD patients: experience from Taiwan, an endemic area for hepatitis B. Nephrol Dial Transplant 1997; 12: 180–183 [DOI] [PubMed] [Google Scholar]
- 14.Hung KY, Shyu RS, Huang CH et al. Viral hepatitis in continuous ambulatory peritoneal dialysis patients in an endemic area for hepatitis B and C infection: the Taiwan experience. Blood Purif 1997; 15: 195–199 [DOI] [PubMed] [Google Scholar]
- 15.Stone WJ, Warnock LG, Wagner C. Vitamin B6 deficiency in uremia. Am J Clin Nutr 1975; 28: 950–957 [DOI] [PubMed] [Google Scholar]
- 16.Teehan BP, Smith LJ, Sigler MH et al. Plasma pyridoxal-5′-phosphate levels and clinical correlations in chronic hemodialysis patients. Am J Clin Nutr 1978; 31: 1932–1936 [DOI] [PubMed] [Google Scholar]
- 17.Kleiner MJ, Tate SS, Sullivan JF et al. Vitamin B6 deficiency in maintenance dialysis patients: metabolic effects of repletion. Am J Clin Nutr 1980; 33: 1612–1619 [DOI] [PubMed] [Google Scholar]
- 18.Kopple JD, Mercurio K, Blumenkrantz MJ et al. Daily requirement for pyridoxine supplements in chronic renal failure. Kidney Int 1981; 19: 694–704 [DOI] [PubMed] [Google Scholar]
- 19.Ross EA, Shah GM, Reynolds RD et al. Vitamin B6 requirements of patients on chronic peritoneal dialysis. Kidney Int 1989; 36: 702–706 [DOI] [PubMed] [Google Scholar]
- 20.Spannuth CL Jr, Warnock LG, Wagner C et al. Increased plasma clearance of pyridoxal 5′-phosphate in vitamin B6-deficient uremic man. J Lab Clin Med 1977; 90: 632–637 [PubMed] [Google Scholar]
- 21.Ono K, Ono T, Matsumata T. The pathogenesis of decreased aspartate aminotransferase and alanine aminotransferase activity in the plasma of hemodialysis patients: the role of vitamin B6 deficiency. Clin Nephrol 1995; 43: 405–408 [PubMed] [Google Scholar]
- 22.Yasuda K, Okuda K, Endo N et al. Hypoaminotransferasemia in patients undergoing long-term hemodialysis: clinical and biochemical appraisal. Gastroenterology 1995; 109: 1295–1300 [DOI] [PubMed] [Google Scholar]
- 23.Liberato IR, Lopes EP, Cavalcante MA et al. Liver enzymes in patients with chronic kidney disease undergoing peritoneal dialysis and hemodialysis. Clinics 2012; 67: 131–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NIDDK USRDSUADRAoE-SRDitUSINIoH. 2008 http://www.usrds.org/atlas_2006.htm (6 August 2010, date last accessed)
- 25.Skaaby T, Husemoen LL, Borglykke A et al. Vitamin D status, liver enzymes, and incident liver disease and mortality: a general population study. Endocrine 2014; 47: 213–220 [DOI] [PubMed] [Google Scholar]
- 26.Skaaby T, Husemoen LL, Linneberg A. Does liver damage explain the inverse association between vitamin D status and mortality? Ann Epidemiol 2013; 23: 812–814 [DOI] [PubMed] [Google Scholar]
- 27.Schottker B, Jorde R, Peasey A et al. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ 2014; 348: g3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalantar-Zadeh K, Kovesdy CP. Clinical outcomes with active versus nutritional vitamin D compounds in chronic kidney disease. Clin J Am Soc Nephrol 2009; 4: 1529–1539 [DOI] [PubMed] [Google Scholar]
- 29.Kalantar-Zadeh K, Shah A, Duong U. Kidney bone disease and mortality in CKD: revisiting the role of vitamin D, calcimimetics, alkaline phosphatase, and minerals. Kidney Int Suppl 2010; 117: S10–S21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Beek JH, de Moor MH, de Geus EJ et al. The genetic architecture of liver enzyme levels: GGT, ALT and AST. Behav Genet 2013; 43: 329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.