Abstract
Background
Coxiella burnetii is a zoonotic bacterium that infects a wide range of animal species and causes the disease Q fever. Both wild and domestic ruminants may be relevant in the epidemiology of C. burnetii infection. In order to investigate the significance of the ruminant host community in the alpine and subalpine ecosystems of the Eastern Pyrenees, Northeastern Spain, in the epidemiology of Q fever, a serological survey was performed on samples from 599 wild and 353 sympatric domestic ruminants.
Results
Specific antibodies against C. burnetii were detected with a commercial enzyme-linked immunosorbent assay (ELISA). Domestic sheep showed the highest prevalence (12.7 %, CI 95 % 8.6–16.9), followed by European mouflon (Ovis orientalis musimon) with a 6.8 % prevalence (CI 95 % 1.6–12.1), red deer (Cervus elaphus) with 2.4 % (CI 95 % 0–5.6), and cattle with a prevalence of 1.1 % (CI 95 % 0–3.2). No positive domestic goats, fallow deer (Dama dama), roe deer (Capreolus capreolus) and Southern chamois (Rupicapra pyrenaica) were detected. Sheep flock prevalence was 75 % (nine of the 12 sheep flocks sampled were positive, within-flock prevalence ranging from 11.1 to 25.0 %), whereas cattle herd prevalence was 11.1 % (one out of the nine cattle herds sampled was positive, within-herd prevalence of 10.0 %.
Conclusions
Both domestic and wild ruminants from the alpine and subalpine ecosystems of the Eastern Pyrenees were exposed to C. burnetii. The higher seroprevalence in sheep and its relative abundance suggest that this species may have a major contribution to the ecology of C. burnetii. Conversely, wild ruminants do not seem to represent a relevant host community for C. burnetii maintenance in the Eastern Pyrenees.
Keywords: Coxiella burnetii, Livestock, Pyrenees, Q Fever, Spain, Wildlife
Findings
Coxiella burnetii is a zoonotic bacterium that infects a wide range of animal species and causes the disease Q fever, frequently involving several host species and ticks in natural systems [1–7]. Main sources of human infection are domestic ruminants, which mostly undergo subclinical infections [2]. Wild ruminants may also be relevant in the epidemiology of Q fever, since they can maintain and shed C. burnetii [4, 5]. However, the epidemiological role of wild ruminants is unclear and could depend on species features, density and host composition of the ecosystem, and/or the environment [2, 5, 6]. Therefore, research is needed to assess the potential role of wild ruminants in C. burnetii epidemiology.
The objectives of this study were to determine the seroprevalence against C. burnetii in wild and domestic ruminants in the Eastern Pyrenees, in order to assess the relative importance of the ruminant host species and to evaluate their potential role in the epidemiology of C. burnetii in the study area.
Blood samples from 599 wild and 353 domestic ruminants older than 1 year were collected from 2010 to 2014 in six different management units in the Catalan Eastern Pyrenees, Northeastern Spain (Table 1; Fig. 1). These areas hold most of the wild ungulate population of the Catalan Pyrenees and are managed by the regional administration, which makes them an interesting wildlife–livestock interface scenario and allows reliable sampling and data collection, respectively. These regions are mainly composed by alpine and subalpine ecosystems. Approximately 18,000 wild ungulates dwell in the study areas; 10,000 Southern chamois (Rupicapra pyrenaica), 4000 roe deer (Capreolus capreolus), 2000 red deer (Cervus elaphus), 1000 fallow deer (Dama dama) and less than 1000 European mouflon (Ovis orientalis musimon) [8, 9]. Approximately 150,000 cattle, 170,000 sheep and 13,000 goats are bred in the corresponding counties [10]. Wild ruminant species were representatively sampled in each study area, since species abundance and composition differs among the management units. Livestock (251 sheep, 11 goats, and 91 beef cattle) were sampled from 21 herds that spend the grazing period from May to November in the Alpine meadows of three of the regions (Table 1; Fig. 1). The sampled herds included 12 sheep flocks (mixed with few goats) and nine beef cattle herds, with herd sizes of 500 and 100 animals, respectively.
Table 1.
Prevalence of Coxiella burnetii antibody positive wild and domestic ruminants in National Game Reserves (NGR) and Controlled Hunting Areas (CHA) in the Eastern Pyrenees, Spain
| NGR Freser-Setcases |
NGR Alt Pallars |
CHA Vall d’Aran |
NGR Cadí |
NGR Boumort |
NGR Cerdanya-Alt Urgell |
Total | |
|---|---|---|---|---|---|---|---|
| Southern chamois (Rupicapra pyrenaica) |
0/150 0 % |
0/36 0 % |
0/44 0 % |
0/76 0 % |
– | 0/17 0 % |
0/323 0 % |
| European mouflon (Ovis orientalis musimon) |
6/82 7.3 % (1.7–13.0) |
0/6 0 % |
– | – | – | – | 6/88 6.8 % (1.6–12.1) |
| Roe deer (Capreolus capreolus) |
0/35 0 % |
0/16 0 % |
0/18 0 % |
0/11 0 % |
0/1 0 % |
0/11 0 % |
0/92 0 % |
| Red deer (Cervus elaphus) |
– | 1/15 6.7 % (0–19.3) |
0/6 0 % |
1/25 4.0 % (0–11.7) |
0/36 0 % |
0/3 0 % |
2/85 2.4 % (0–5.6) |
| Fallow deer (Dama dama) |
– | 0/11 0 % |
– | – | – | – | 0/11 0 % |
| TOTAL WILD RUMINANTS | 6/267 2.2 % (0.5–4.0) |
1/84 1.2 % (0–3.5) |
0/68 0 % |
1/112 0.9 % (0–2.6) |
0/37 0 % |
0/31 0 % |
8/599 1.3 % (0.4–2.3) |
| Sheep (Ovis aries) |
4/81 4.9 % (0.2–9.7) |
21/140 15.0 % (9.1–20.9) |
7/30 23.3 % (8.2–38.5) |
– | – | – | 32/251 12.7 % (8.6–16.9) |
| Cattle (Bos taurus) |
0/70 0 % |
1/21 4.8 % (0–13.9) |
– | – | – | – | 1/91 1.1 % (0–3.2) |
| Goat (Capra hircus) |
0/4 0 % |
0/7 0 % |
– | – | – | – | 0/11 0 % |
| TOTAL DOMESTIC RUMINANTS | 4/155 2.6 % (0.1–5.1) |
22/168 13.1 % (8.0–18.2) |
7/30 23.3 % (8.2–38.5) |
– | – | – | 33/353 9.3 % (6.3–12.4) |
| TOTAL | 10/422 2.4 % (0.9–3.8) |
23/252 9.1 % (5.6–12.7) |
7/98 7.1 % (2.0–12.2) |
1/112 0.9 % (0–2.6) |
0/37 0 % |
0/31 0 % |
41/952 4.3 % (3.0–5.6) |
Positive individuals over the sampled animals are shown, followed by prevalence as a percentage and the CI 95 % between parentheses
Fig. 1.
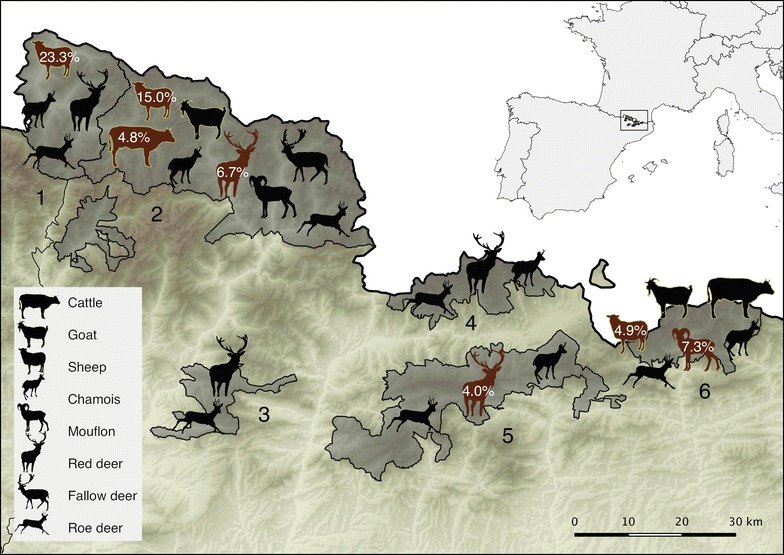
Prevalence of Coxiella burnetii specific antibodies assayed by ELISA in the Eastern Pyrenees. Six different management units were sampled: 1 Controlled Hunting Area of Vall d’Aran; 2 National Game Reserve of Alt Pallars; 3 National Game Reserve of Boumort; 4 National Game Reserve of Cerdanya-Alt Urgell; 5 National Game Reserve of Cadí; 6 National Game Reserve of Freser-Setcases
The wild ruminant blood samples were obtained directly from the heart from animals hunted during the regular hunting season, mainly from summer to early spring. The livestock samples were obtained from the jugular vein in sheep and goats, and from the medial coccygeal vein in cattle, within the yearly livestock health campaigns. Blood samples were allowed to clot at environmental temperature and transported to the laboratory, where they were centrifuged at 1500×g for 10 min. Sera were frozen at −20 °C within 24 h from sample collection and until analysis. Specific antibodies against C. burnetii phase I and phase II antigens were tested by a commercial indirect enzyme-linked immunosorbent assay (ELISA) that detects IgG from ruminant species (Q-Fever Antibody Test Kit; IDEXX, Westbrook, Maine, USA). The analyses were performed following the manufacturer’s instructions and results were read at optical density of 450 nm. Although the ELISA used has not been specifically validated for wild species, and both sensitivity and specificity could be lower than those described for domestic ruminants, phylogenetic differences between wild and domestic ruminant species are not higher than among livestock. Moreover, C. burnetii ELISA test for livestock have been previously used to study Q fever in wild ruminants [11, 12].
Binomial tests were performed to determine differences between species prevalence, and significance was set at 0.05. All statistical analyses were performed with R software [13]. EpiR package was used to calculate the prevalence estimates [14].
Table 1 shows the seroprevalence estimates for the domestic and wild ruminants. C. burnetti ELISA positive individuals were found in European mouflon and red deer and in sheep and cattle. C. burnetii antibodies were not detected in domestic goats, Southern chamois, roe deer, and fallow deer. Among the positive species, domestic sheep prevalence was statistically higher than in cattle (p = 0.00255) and red deer (p = 0.01112), but not as compared to mouflon (p = 0.1865). Nine out of the 12 sheep flocks sampled were positive (75 %) and within-flock prevalence ranged from 11.1 to 25.0 %, whereas only one out of the nine cattle herds sampled was positive with a within-herd prevalence of 10.0 %. No sex and age differences were found for C. burnetii seroprevalence in any species.
Although previous reports demonstrate that cattle is overall the domestic ruminant species showing a higher overall mean apparent prevalence (20 %) as compared to small ruminants (15 %), sheep and goats may show the highest seroprevalence in certain epidemiological scenarios [5, 6, 15–17]. The higher seroprevalence consistently found in sheep as compared to cattle in the Eastern Pyrenees are in accordance with previous reports in semi-extensive and extensive grazing systems [17]. High C. burnetii seroprevalence with zoonotic risk has been reported in goats [12, 13, 18, 19], but the low sample size of our study does not allow to draw strong conclusions. However, the absence of seropositive goats, altogether with the low goat population and the few goats per herd in the study area [10], do not allow to point to this species as important for C. burnetti spread in the Eastern Pyrenees.
The seroprevalence against C. burnetii found in European mouflon is in accordance with previous reports for this species [20, 21]. Mouflon is taxonomically considered a sheep, and in the Alpine meadows of the National Game Reserve of Freser-Setcases, where C. burnetii-seropositive mouflons have been detected, mouflon and sheep commingle and even interbreed. This could explain the high seroprevalence in mouflon being not statistically different from sheep as observed in this study. Red deer has previously been demonstrated as competent hosts for C. burnetii, with higher seroprevalences than found in this study. Although red deer has been recognized as an important reservoir host for C. burnetii in the Iberian Peninsula, [2, 4, 6], red and roe deer do not seem to play an important role in the ecology of C. burnetii in the alpine and subalpine ecosystems of North-Eastern Iberian peninsula. Animal host density and community composition as well as landscape distribution of resources can also influence the dynamics of infectious diseases in wild animal populations [22]. This could explain the different seroprevalences for C. burnetii found in studies carried out in different ecosystems with different host communities.
Previous studies have failed to demonstrate antibodies against C. burnetti in mountain ungulates such as Alpine ibex (Capra ibex) [12] and Southern chamois [23], or has revealed low prevalences in e.g. Alpine chamois (Rupicapra rupicapra) [24]. This is in agreement with the findings of our study, where antibodies against C. burnetii were not detected in chamois although chamois was the most abundant and sampled wild species in the study areas. Overall, wild ruminant seroprevalence was not related to the species abundance or density. This suggests that other ecological, behavioural and/or environmental factors, and the resulting interspecific contact and transmission rates, may enhance or reduce C. burnetii exposure [5, 6].
Inter-species contact between wild and domestic ruminants takes place in mountain habitats [25], which may favour transmission of pathogens such as C. burnetii. Wild ruminants can become infected and shed C. burnetii as demonstrated for red deer [2, 4, 6] and roe deer [26], and therefore can potentially contribute to the spread of C. burnetii. However, the lower seroprevalence found in wild ruminants as compared to livestock suggests that no single species contribute significantly in the maintenance of C. burnetii transmission in the alpine and subalpine habitats of the Eastern Pyrenees, but are rather susceptible hosts as a part of the maintenance community in a complex multi-host system [27]. On the other hand, the relatively high overall and within-flock prevalence found in domestic sheep and the consistent detection of high prevalences in sheep in all the studied areas suggest a relative importance of this species as a source of C. burnetii spillover events for other susceptible hosts. This is further supported by the fact that sheep is the most abundant species in the study area [10]. This differs from epidemiological scenarios reported elsewhere, where wild ruminant species are recognized as maintenance hosts [2, 4, 6, 7, 26]. Further molecular analyses to characterize the strains infecting sheep and wild ruminants from the Eastern Pyrenees should be performed to ascertain the existence of independent sylvatic and domestic cycles.
To the authors’ knowledge, exposure to C. burnetii has been confirmed at the wild-domestic ruminant interface in the Eastern Pyrenees for the first time. This study demonstrates that both domestic and wild ruminants from the alpine and subalpine ecosystems of the Eastern Pyrenees are exposed to C. burnetii. The higher seroprevalence in sheep suggests that this species may be of major importance in the ecology of C. burnetii. Conversely, wild ruminants do not seem to represent a relevant community of hosts for the maintenance of C. burnetii. Identification and characterization of the C. burnetii strains infecting domestic and wild ruminants in the Eastern Pyrenees is needed in order to determine whether there is a domestic cycle with spillovers to wild ruminants or independent domestic and sylvatic cycles. Although red deer do not seem to play an important role in the ecology of C. burnetii, the finding of positive red deer and European mouflon individuals makes it advisable for hunters and game rangers to use prophylactic measures in order to prevent exposure to C. burnetii during post-mortem management of these species.
Authors’ contributions
OC, SL and JRLO planned the study; XFA, OC, ACC and JRLO participated in sample collection; XFA, OC and ACC performed the laboratory analyses; SL and JRLO obtained the funding; XFA, OC and JRLO redacted the initial draft. All authors read and approved the final the manuscript.
Acknowledgements
The authors are grateful to the gamekeepers and directors of all the National Game Reserves and the Controlled Hunting Area from Catalonia for their help and collaboration in wild ruminant sample collection. The authors are also indebted to the owners of the livestock herds and flocks, who kindly agreed to collaborate with the study, as well as with the local veterinary practitioners, in particular Joan Planas, Josep Gusard and Rogeli Feixa, for their assistance with livestock sample collection. X. Fernández-Aguilar was supported by the FI-DGR program from the Government of Catalonia. This study benefited from the research Project CGL2009-11631 of the Spanish MICINN.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Xavier Fernández-Aguilar, Email: xfdezaguilar@gmail.com.
Óscar Cabezón, Email: ocabezon@yahoo.com.
Andreu Colom-Cadena, Email: andreuccadena@gmail.com.
Santiago Lavín, Email: Santiago.Lavin@uab.cat.
Jorge Ramón López-Olvera, Email: Jordi.Lopez.Olvera@uab.cat.
References
- 1.Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruiz-Fons F, Rodríguez Ó, Torina A, Naranjo V, Gortázar C, de la Fuente J. Prevalence of Coxiella burnetti infection in wild and farmed ungulates. Vet Microbiol. 2008;126:282–286. doi: 10.1016/j.vetmic.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Angelakis E, Raoult D. Q fever. Vet Microbiol. 2010;140:297–309. doi: 10.1016/j.vetmic.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 4.González-Barrio D, Almería S, Caro MR, Salinas J, Ortiz JA, Gortázar C, Ruiz-Fons F. Coxiella burnetii shedding by farmed red deer (Cervus elaphus) Transbound Emerg Dis. 2015;62:572–574. doi: 10.1111/tbed.12179. [DOI] [PubMed] [Google Scholar]
- 5.Ohlson A, Malmsten J, Frössling J, Bölske G, Aspán A, Dalin A-M, Lindberg A. Surveys on Coxiella burnetii infections in Swedish cattle, sheep, goats and moose. Acta Vet Scand. 2014;56:39. doi: 10.1186/1751-0147-56-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.González-Barrio D, Velasco Ávila AL, Boadella M, Beltrán-Beck B, Barasona JÁ, Santos JPV, Queirós J, García-Pérez AL, Barral M, Ruiz-Fons F. Host and environmental factors modulate the exposure of free-ranging and farmed red deer (Cervus elaphus) to Coxiella burnetii. Appl Environ Microbiol. 2015;81:6223–6231. doi: 10.1128/AEM.01433-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Astobiza I, Barral M, Ruiz-Fons F, Barandika JF, Gerrikagoitia X, Hurtado A, García-Pérez AL. Molecular investigation of the occurrence of Coxiella burnetii in wildlife and ticks in an endemic area. Vet Microbiol. 2011;147:190–194. doi: 10.1016/j.vetmic.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 8.Casanovas R, Leal R, Roldán J. Reserves nacionals de caça: 40è aniversari 1966–2006. Barcelona: Generalitat de Catalunya, Departament de Medi Ambient i Habitatge; 2007. p. 224. http://www.gencat.cat/mediamb/publicacions/monografies/40anys_rnc.pdf. Assessed 21 Mar 2016.
- 9.Departament de Medi Ambient. Pla estratègic de la caça a Catalunya. Barcelona, Spain: Generalitat de Catalunya, Departament de Medi Ambient; 2009. p. 233 http://agricultura.gencat.cat/ca/ambits/medi-natural/casa/dar_estudis_informes/dar_pla_estrategic_caca_catalunya/. Accessed 21 Mar 2016.
- 10.Institut d’Estadística de Catalunya. Anuari estadístic de Catalunya. Agricultura, ramaderia i pesca; Ramaderia; Caps de bestiar. Per espècies; Comarques, àmbits i províncies; 2009. http://www.idescat.cat/pub/?id=aec&n=449. Accessed 21 Mar 2016.
- 11.López-Olvera JR, Vidal D, Vicente J, Pérez M, Luján L, Gortázar C. Serological survey of selected infectious diseases in mouflon (Ovis aries musimon) from south-central Spain. Eur J Wildl Res. 2009;55:75–79. doi: 10.1007/s10344-008-0215-6. [DOI] [Google Scholar]
- 12.Marreros N, Hüssy D, Albini S, Frey CF, Abril C, Vogt HR, et al. Epizootiologic investigations of selected abortive agents in free-ranging Alpine ibex (Capra ibex ibex) in Switzerland. J Wildl Dis. 2011;47:530–543. doi: 10.7589/0090-3558-47.3.530. [DOI] [PubMed] [Google Scholar]
- 13.A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. http://www.R-project.org. Accessed 4 Jan 2016.
- 14.epiR: An R Package for the Analysis of Epidemiological Data. R package version 0.9-62.
- 15.Guatteo R, Seegers H, Taurel AF, Joly A, Beaudeau F. Prevalence of Coxiella burnetii infection in domestic ruminants: a critical review. Vet Microbiol. 2011;149:1–16. doi: 10.1016/j.vetmic.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Hogerwerf L, Courcoul A, Klinkenberg D, Beaudeau F, Vergu E, Nielen M. Dairy goat demography and Q fever infection dynamics. Vet Res. 2013;44:28. doi: 10.1186/1297-9716-44-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz-Fons F, Astobiza I, Barandika JF, Hurtado A, Atxaerandio R, Juste RA, García-Pérez AL. Seroepidemiological study of Q fever in domestic ruminants in semi-extensive grazing systems. BMC Vet Res. 2010;6:3. doi: 10.1186/1746-6148-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harchette TF, Hudson RC, Schlech WF, Campbell NA, Hatchette JE, Ratnam S, Raoult D, Donovan C, Marrie TJ. Goat-associated Q Fever: a new disease in Newfoundland. Emerg Infect Dis. 2001;7:413–419. doi: 10.3201/eid0703.017308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meadows S, Jones-Bitton A, McEwen S, Jansen J, Menxies P. Coxiella burnetii seropositivity and associated risk factors in goats in Ontario, Canada. Prev Vet Med. 2015;121:199–205. doi: 10.1016/j.prevetmed.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Ioannou I, Sandalakis V, Kassinis N, Chochlakis D, Papadopoulos B, Loukaides F, Tselentis Y, Psaroulaki A. Tick-borne bacteria in mouflons and their ectoparasites in Cyprus. J Wildl Dis. 2011;47:300–306. doi: 10.7589/0090-3558-47.2.300. [DOI] [PubMed] [Google Scholar]
- 21.Psaroulaki A, Chochlakis D, Angelakis E, Ioannou I, Tselentis Y. Coxiella burnetii in wildlife and ticks in an endemic area. Trans R Soc Trop Med Hyg. 2014;108:625–631. doi: 10.1093/trstmh/tru134. [DOI] [PubMed] [Google Scholar]
- 22.Barasona JA, Latham M, Acevedo P, Armenteros JA, Latham A, Gortázar C, Carro F, Soriguer RC, Vicente J. Spatiotemporal interactions between wild boar and cattle: implications for cross-species disease transmission. Vet Res. 2014;45:122. doi: 10.1186/s13567-014-0122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Blasio A, Marenzoni ML, Di Sabatino D, Giovannini A, Latini R, Gentile L. Retrospective serological study to monitor the health status of Apennine chamois (Rupicapra pyrenaica ornata) Eur J Wildl Res. 2015;61:479–482. doi: 10.1007/s10344-015-0906-8. [DOI] [Google Scholar]
- 24.Pioz M, Loison A, Gibert P, Jullien JM, Artois M, Gilot-Fromont E. Antibodies against Salmonella is associated with reduced reproductive success in female alpine chamois (Rupicapra rupicapra) Can J Zool. 2008;86:1111–1120. doi: 10.1139/Z08-089. [DOI] [Google Scholar]
- 25.Richomme C, Gauthier D, Fromont E. Contact rates and exposure to inter-species disease transmission in mountain ungulates. Epidemiol Infect. 2006;134:21–30. doi: 10.1017/S0950268805004693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rijks JM, Roest HIJ, van Tulden PW, Kik MJL, Gröne A. Infection in roe deer during Q Fever epidemic, the Netherlands. Emerg Infect Dis. 2011;17:2369–2371. doi: 10.3201/eid1712.110580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haydon DT, Cleaveland S, Taylor LH, Laurenson MK. Identifying reservoirs of infection: a conceptual and practical challenge. Emerg Infect Dis. 2002;8:1468–1473. doi: 10.3201/eid0812.010317. [DOI] [PMC free article] [PubMed] [Google Scholar]


