Abstract
Despite decades of significant progress in understanding the molecular mechanisms of malignant tumorigenic cells, it remains elusive what these tumorigenic cells are and what controls the growth of these malignant cells. Recently, we have mechanically selected and grown highly malignant and tumorigenic tumor-repopulating cells (TRCs), a small sub-population of cancer cells, by culturing single cancer cells in soft fibrin matrices. However, it is unclear what regulates TRC growth besides Sox2. Here we show that nuclear protein 1 (Nupr1), a protein independent of Sox2, is downregulated in TRCs of melanoma, ovarian cancer and breast cancer cultured in soft fibrin matrices. Nupr1 expression depends on nuclear translocation of YAP that is enriched at the Nupr1 promoter sites; YAP is controlled by Cdc42-mediated F-actin and Lats1 interactions. Nupr1 regulates tumor-suppressor p53 and negatively regulates Nestin and Tert that are independent of Sox2 and promote TRC growth. Silencing Nupr1 increases TRC growth and Nupr1 overexpression inhibits TRC growth in culture and in immune-competent mice. Our results suggest that Nupr1 is a suppressor of growth of highly tumorigenic TRCs and may have a critical role in cancer progression.
Introduction
Despite decades of continuous efforts and significant progress in understanding the molecular mechanisms of malignant tumorigenic cells,1 it remains unresolved what these tumorigenic cells are and what controls the growth and proliferation of these tumor cells. Recently, we mechanically selected tumorigenic tumor-repopulating cells (TRCs) independent of surface stem cell markers, a small sub-population of cancer cells, by culturing single cancer cells from cancer cell lines or primary tumors in three-dimensional (3D) soft fibrin matrices.2 These TRCs exhibit highly tumorigenic and proliferative capacity and they efficiently repopulate tumors at distant organs in wild-type syngeneic and non-syngeneic mice.2 The soft fibrin matrices promote TRC growth by facilitating Sox2 expression.3 However, the melanoma cells can significantly increase their proliferation when Sox2 expression is only modestly increased,3 suggesting that other factors that are regulated by matrix softness may contribute to TRC proliferation independent of Sox2. Using microarrays to screen for potential factors, we have identified nuclear protein 1 (Nupr1) that is markedly reduced in the TRCs in 3D soft fibrin matrices when compared with melanoma cells on 2D rigid plastic. Nupr1 is a DNA-binding protein implicated in regulation of cell cycle and apoptosis.4 Nupr1 is also called COM1 (candidate of metastasis 1) because it is expressed in metastatic cancer cells.5 The exact role of Nupr1 in cancer is poorly understood as several studies have associated Nupr1 with cancer cells' ability to survive6, 7, 8, 9 while another shows its tumor-suppressing capabilities.10 Here we reveal that Nupr1 is downregulated in TRCs to facilitate proliferation of the tumorigenic cells and its expression depends on nuclear Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ).
Results
Substrate stiffness regulates Sox2-independent Nupr1
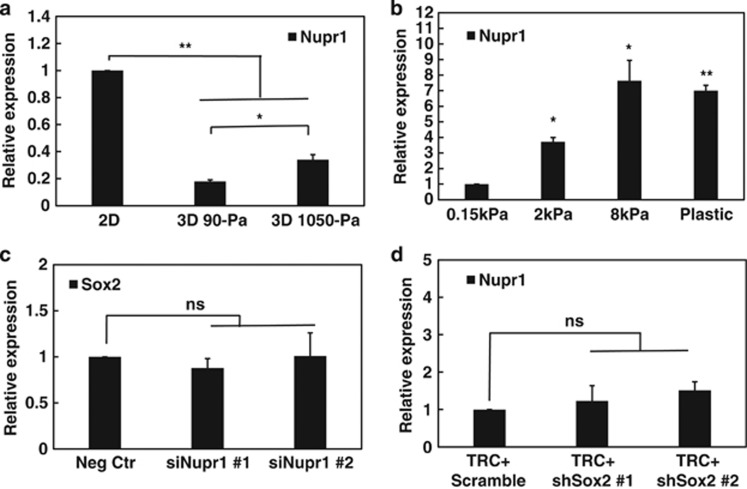
To determine the impact of matrix rigidity on Nupr1, we compared its expression in cells that are cultured on 2D rigid plastic with that in cells in 3D soft fibrin matrices. Nupr1 mRNA expression was decreased by ~70% in 3D soft (90-Pa) fibrin matrices (Figure 1a) and Nupr1 protein levels were decreased considerably (Supplementary Figure 1a) when compared with that on 2D rigid plastic. Nupr1 expression was also much lower in human ovarian cancer cells (A2780) and human breast cancer cells (MCF-7) cultured in 3D soft fibrin gels (Supplementary Figure 1b), suggesting that low mRNA expression of Nupr1 is not limited to melanoma cells in 3D soft substrates. Increasing 3D matrix rigidity from 90 to 1050-Pa significantly upregulated Nupr1 expression (Figure 1a). We define the melanoma cells that are selected and grown in 90-Pa fibrin gels for 5 days as TRCs.3 Importantly, Nupr1 expression in the cells plated on 2D substrates increased by >7-fold when substrate stiffness was increased from 150-Pa to 8 kPa or rigid plastic (Figure 1b), suggesting that it is the substrate rigidity and not the substrate dimensionality that dictates Nupr1 gene expression. Silencing Nupr1 had no effect on Sox2 expression (Figure 1c); silencing Sox2 had no effect on Nupr1 expression either (Figure 1d). These data suggest that Nupr1 can be regulated by matrix rigidity and that Nupr1 is independent of Sox2.
Figure 1.
Nupr1 is low in soft matrix and independent of Sox2. (a) Nupr1 mRNA expression is lower in 3D fibrin gels than on rigid plastic. 2D: B16-F1 cells were cultured on rigid plastic. 3D 90-Pa, 3D 1050-Pa: cells were cultured in 90 or 1050-Pa fibrin gels for 5 days. After 5 days, the mRNA was extracted for quantitative analysis of Nupr1 mRNA expression by real-time PCR. Cells that are grown in 3D 90 Pa gels are defined as TRCs. (b) Nupr1 mRNA expression increases with substrate stiffness. Cells were cultured on the surface of collagen-1-coated polyacrylamide gels of various stiffnesses or on glass-bottomed dishes for 24 h. All were compared with ‘0.15 kPa'. (c) Silencing Nupr1 does not affect Sox2 expression. Cells were transfected with negative control siRNA, or Nupr1 siRNA #1, #2, for 24 h respectively, and then analyzed for Sox2 mRNA expression by real-time PCR. (d) Silencing Sox2 do not affect Nupr1 expression. TRCs were extracted from 3D fibrin gels and re-plated onto 2D 90-Pa fibrin gel surface for 3 h, and then transfected with Scramble control, or Sox2 shRNA#1, #2, for 12 h (or for 24 h, Supplementary Figure 10c) and then analyzed for Nupr1 mRNA expression by real-time PCR. Mean±s.e.m.; n=3 independent experiments for all subfigures; *P<0.05; **P<0.01; NS, not statistically significant.
Little YAP translocation to TRC nucleus
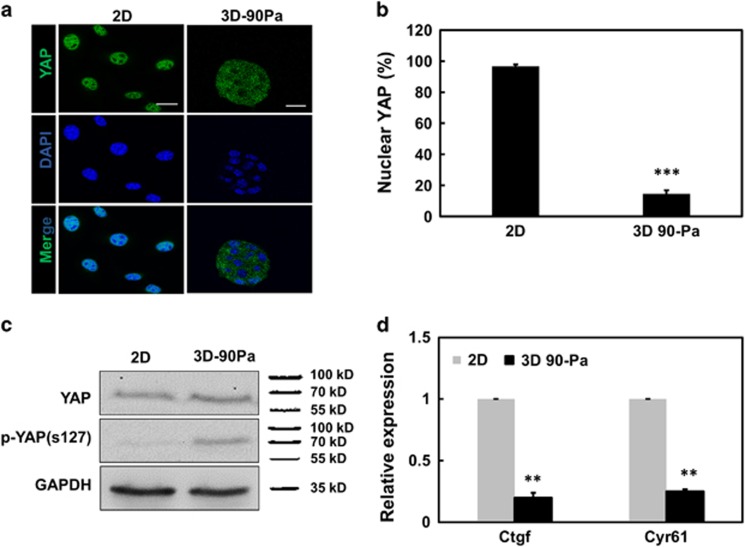
As Nupr1, a nuclear protein, responds to substrate rigidity, we wondered what may be its upstream regulator(s). YAP, and its transcriptional coactivator TAZ, are known as mediators of Hippo signaling and organ growth,11, 12 shuttling between the cytoplasm and the nucleus, where they interact with transcriptional enhancer associate domain transcription factors to regulate transcription. YAP is known to sense cytoskeletal tension and mediate cellular mechanoresponses.13 We assayed endogenous YAP subcellular localization by immunofluorescence for cells plated on 2D rigid plastic and in 3D 90-Pa substrates. Almost all YAP in cells on 2D rigid plastic was nuclear, whereas only ~18% YAP in cells in 3D 90-Pa fibrin matrices was nuclear (Figures 2a and b). Furthermore, immunoblots show that 3D 90-Pa fibrin gels promoted YAP phosphorylation on serine 127 (ser127), whereas the total amounts of YAP in 2D and in 3D 90-Pa were similar (Figure 2c), consistent with published results that phosphorylated YAP do not translocate to the nucleus.14, 15 Ctgf and Cyr61, two known YAP/TAZ endogenous targets, were used as markers of transcriptional activity of YAP. Ctgf and Cyr61 mRNA expression levels in cells in 3D 90-Pa were markedly reduced when compared with those on 2D (Figure 2d), supporting the finding that little YAP from TRCs (the cells in 3D 90-Pa) was translocated from the cytoplasm to the nucleus.
Figure 2.
Nuclear YAP is low in 3D soft matrix. (a) Confocal immunofluorescence images of YAP and nuclei (4,6-diamidino-2-phenylindole (DAPI)) of cells on 2D rigid plastic (left column) or in 3D 90-Pa (right column) fibrin gel. Cells were culture on 2D rigid dishes or in 90-Pa 3D fibrin gels for 3 days. Scar bars, 20 μm. (b) Percentage of cells with predominantly nuclear YAP. Mean±s.e.m.; n=10 randomly chosen view-fields; ***P<0.001 (c) Western blotting for phosphorylated YAP S127 (p-YAP s127) and YAP in whole-cell lysates of melanoma cells after 3 days of culture on 2D rigid plastic or in 3D 90-Pa fibrin gels. (d) Real-time PCR of Ctgf and Cyr61 as indexes of YAP transcriptional activity on 2D and in 90-Pa 3D fibrin gels. Mean±s.e.m.; n=3; **P<0.01.
Cdc42 regulates Nupr1 via YAP
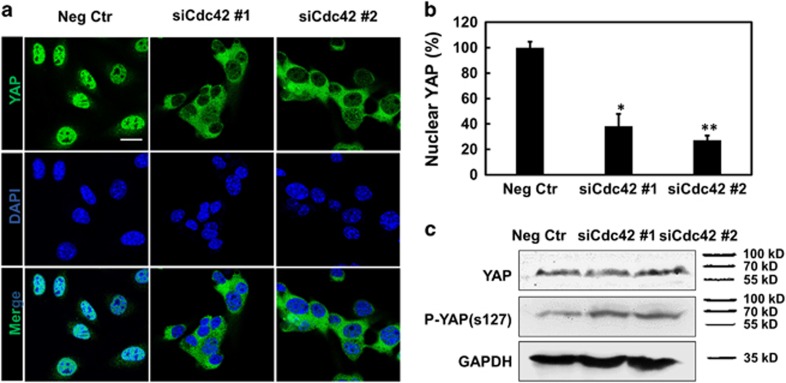
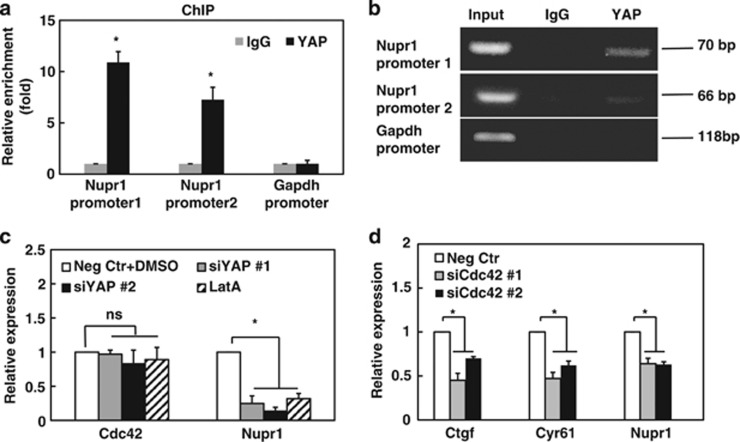
As a key cytoskeleton regulator, Cdc42 is known to regulate the actin cytoskeleton to promote filopodia formation.16, 17 We have reported that Cdc42 is significantly downregulated in TRCs compared with control melanoma cells (the cells on 2D rigid plastic).3 To investigate the relationship between Cdc42 and YAP, we knocked down Cdc42 in the cells on 2D rigid plastic. In comparison with the cells transfected with negative control small interfering RNA (siRNA), the cells transfected with Cdc42 siRNA exhibited dominant YAP cytoplasmic localization (Figures 3a and b) and high levels of phosphorylation of YAP on serine 127 (Figure 3c). To determine the role of nuclear YAP in Nupr1, we performed chromatin immunoprecipitation (ChIP) assay and found that YAP was enriched by 7- to 10-folds at Nupr1 promoter sites for melanoma cells on 2D rigid plastic (Figures 4a and b). For cells cultured in 3D 90-Pa fibrin gels, the YAP enrichment was reduced by ~70% when compared with those on 2D rigid plastic (Supplementary Figure 2a), suggesting the specific role of YAP in Nupr1 gene expression. Silencing YAP downregulated Nupr1 expression but had no effect in Cdc42 expression (Figure 4c). Knocking down transcriptional enhancer associate domains (TEADs) markedly reduced YAP enrichments for cells on 2D rigid plastic (Supplementary Figure 2a) and inhibited Nupr1 expression (Supplementary Figure 2b), suggesting that YAP requires the presence of transcriptional enhancer associate domains to regulate Nupr1 expression at Nupr1 promoter sites. Furthermore, silencing Taz in cells on rigid plastic downregulated Nupr1 (Supplementary Figure 3a), which was significantly lower in TRCs than in cells on rigid plastic (Supplementary Figure 3b). Disrupting F-actin with latrunculin A (Supplementary Figures 4a and c) elevated phosphorylated YAP (Supplementary Figure 5a), increased cytoplasmic YAP (Supplementary Figure 5b) and decreased nuclear YAP (Supplementary Figure 5c). Latrunculin A had no effect on Cdc42 expression but decreased Nupr1 expression (Figure 4c) consistent with published reports that F-actin interacts with Lats1, which in turn regulates phosphorylation of YAP.14 Phosphorylated-YAP was higher in TRCs than in cells on rigid plastic and silencing Cdc42 elevated phosphorylated YAP (Supplementary Figure 6). In addition, silencing Cdc42 downregulated YAP activation markers Ctgf and Cyr61, and also Nupr1 (Figure 4d), likely via lowering F-actin (Supplementary Figures 4b and d) to free up Lats1 so that it can phosphorylate YAP. This conclusion was supported by the results that knocking down Lats1/2 increased nuclear translocation of YAP by twofolds (from ~20 to ~60%) (Supplementary Figures 7a and b) and upregulated Nupr1 gene expression (Supplementary Figure 7c). Together, these data suggest that Cdc42 that promotes assembly of F-actin and Lats1 binding to F-actin is upstream of YAP and regulates Nupr1 via YAP translocation.
Figure 3.
Silencing Cdc42 downregulates nuclear YAP. (a) Confocal immunofluorescence images of YAP and nuclei (4,6-diamidino-2-phenylindole (DAPI)). Cells were treated with negative control siRNA, or Cdc42 siRNA #1, #2 for 24 h, respectively. Scar bar, 20 μm. (b) Percentage of cells with predominantly nuclear YAP. Mean±s.e.m; n=10 randomly chosen view-fields. *P<0.05; **P<0.01. (c) Western blotting for phosphorylated YAP S127 (p-YAP s127) and YAP in whole-cell lysates of melanoma cells. Control cells were transfected with negative control siRNA, or Cdc42 siRNA #1, #2 for 24 h, respectively.
Figure 4.
Silencing Cdc42 downregulates nuclear YAP enriched at Nupr1 promoter sites. (a) YAP is enriched at Nupr1 promoter sites, assayed using ChIP assay. ChIP was performed using normal rabbit IgG (negative control) or YAP antibody on control cells lysates. Two sets of primers were used for Nupr1 promoter region. To exclude nonspecific effect, the relative enrichment of Gapdh promoter was also shown. Relative enrichment was determined by real-time PCR. Mean±s.e.m.; n=3; *P<0.05. (b) Representative blots of the reverse transcriptase–PCR (RT–PCR). Similar results are observed in two other blots. (c) YAP impacts Nupr1 expression. Control cells were treated with negative control siRNA, DMSO, YAP siRNA#1 or #2 for 24 h, or 1 μM Latrunculin A (LatA), and then analyzed for Nupr1 mRNA expression by real-time PCR. Silencing YAP with siRNA#1 or #2 markedly reduced YAP protein levels (Supplementary Figure 11a). (d) Silencing Cdc42 decreases expression of Ctgf, Cyr61 and Nupr1. Cells were transfected with negative control siRNA, or Cdc42 siRNA#1, #2 for 24 h, respectively, and then analyzed for Ctgf, Cyr61 and Nupr1 mRNA expression by real-time PCR. Mean±s.e.m.; n=3 independent experiments for all subfigures; *P<0.05; NS, not statistically significant.
Nupr1 is a negative regulator of melanoma cell growth in vitro and in vivo
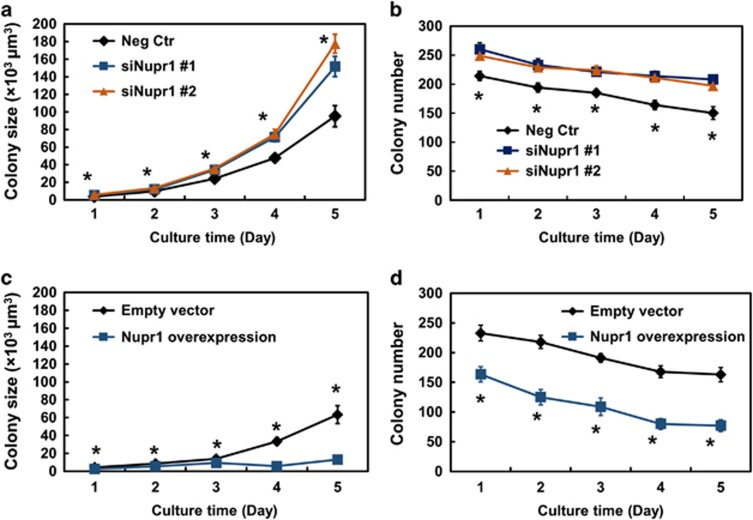
To determine the functional role of Nupr1 in melanoma cells, we altered Nupr1 levels and assayed melanoma cell proliferation. Compared with the cells grown in 3D 90-Pa gels transfected with negative controls, silencing Nupr1 significantly increased colony size and colony number (Figures 5a and b; Supplementary Figures 8 and 9). In contrast, overexpressing Nupr1 markedly reduced colony size and number (Figures 5c and d; Supplementary Figures 8 and 9). These results suggest that Nupr1 negatively regulates TRC growth.
Figure 5.
Nupr1 negatively regulates colony growth in culture. Silencing Nupr1 using two different siRNAs significantly increases colony size (a) and number (b). Cells were transfected with Nupr1 siRNA #1 or #2 for 24 h, and then cultured in 90-Pa fibrin gels. Colony number and colony size were quantified until day 5. Mean±s.e.m.; n=2 independent experiments; *P<0.05, indicating significant differences between Neg Ctr and siNupr1 #1 or #2. (c, d) Overexpressing Nupr1 for 24 h before plating significantly inhibits colony size and number. Significant differences between empty vector and Nupr1 overexpression from day 1 through day 5. Mean±s.e.m.; n=2 independent experiments, *P<0.05.
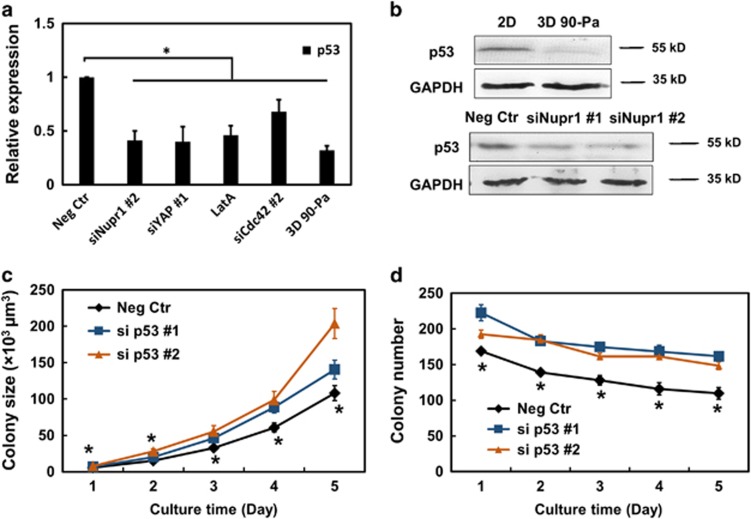
It is reported8 that Nupr1 interacts with tumor-suppressor p53. p53 mRNA levels (Figure 6a) and protein levels (Figure 6b) were much lower in cells plated in 3D soft fibrin gels (TRCs) than in melanoma cells plated on 2D rigid plastic. Silencing Nupr1 decreased both p53 mRNA and protein levels considerably (Figures 6a and b); silencing YAP and Cdc42 in cells on rigid plastic that are upstream of Nupr1 or treating cells on rigid plastic with Latrunculin A to disrupt F-actin significantly decreased p53 expression (Figure 6a). Together, these results suggest that Nupr1 is upstream of p53. Silencing p53 in cells on rigid plastic significantly increased colony size and colony number, consistently with the known role of p53 as a tumor suppressor (Figures 6c and d).
Figure 6.
Nupr1 is upstream of tumor-suppressor p53. (a) p53 is lower in TRCs and silencing Nupr1 decreases p53 expression. Cells were treated with negative control siRNA, Nupr1 siRNA#2, YAP siRNA#1, Cdc42 siRNA#2 for 24 h, and 1 μM Latrunculin A for 12 h, or plated in 90-Pa fibrin gels, and then analyzed for p53 mRNA expression by real-time PCR. Mean±s.e.m.; n=3 independent experiments. *P<0.05. (b) Western blotting assays for p53. 2D: cells were cultured on rigid plastic; 3D 90 Pa: cells cultured in 90-Pa fibrin gels for 5 days. Neg Ctr, siNupr1#1, siNupr1#2: cells were transfected with negative control siRNA, Nupr1 siRNA#1, or #2 for 24 h. (c, d) Silencing p53 using two different siRNAs significantly increases colony size and number. Control cells were transfected with p53 siRNA #1 or #2 for 24 h, and then cultured in 90-pa fibrin gels. Colony number and size were quantified until day 5. Mean±s.e.m.; n=2 independent experiments; *P<0.05, between Neg Ctr and si p53 #1 or #2.
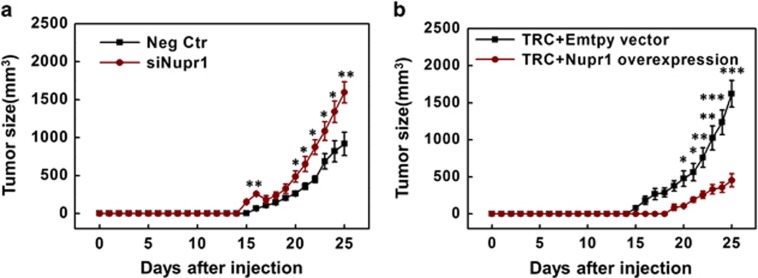
To further explore the role of Nupr1 in vivo, we either silenced Nupr1 in melanoma cells grown on 2D rigid plastic (whose Nupr1 levels were high; Figure 1a; Supplementary Figure 1a) or overexpressed Nupr1 in TRCs (whose Nupr1 levels were low; Figure 1a; Supplementary Figure 1a) and injected those treated cells into mice at 2000 cells per mouse subcutaneously. Silencing Nupr1 in 2D melanoma cells elevated the number of mice with tumors (from 9 out of 16 mice to 12 out of 16 mice); overexpressing Nupr1 in TRCs significantly suppressed the number of mice with tumors (from 15 out of 16 mice to 9 out of 16 mice) (Table 1). Importantly, sizes of the tumors were significantly increased when Nupr1 was silenced and were significantly suppressed when Nupr1 was overexpressed (Figures 7a and b). These findings are consistent with the in vitro colony size results and support the notion that Nupr1 behaves like a tumor-suppressor in vivo.
Table 1. Nupr1 suppresses tumor growth in vivo.
| Mouse model cell line | C57BL/6 mice (subcutaneous injection) |
|---|---|
| B16-F1 (2000 cells) | |
| Neg Ctr (2D) | 9/16 |
| siNupr1 (2D) | 12/16 |
| TRC+empty vector | 15/16 |
| TRC+Nupr1 overexpression | 9/16* |
Abbreviations: 2D, two dimensional; Nupr1, nuclear protein 1; TRC, tumor-repopulating cell.
*P<0.05, Fisher's exact test.
Figure 7.
Nupr1 negatively regulates tumor growth in vivo. From the published results on limiting dilution assays, it is known that 100 subcutaneously injected TRCs but not 100 melanoma cells grown on 2D rigid plastic can generate tumors; when 100 000 cells grown on rigid plastic are injected, however, 100% mice have tumors,2 presumably a small population of the cells grown on rigid plastic are TRC-like cells.2 Hence, we chose 2000 tumor cells to inject subcutaneously in order to determine the potential differences in tumorigenic efficiency (see Table 1) and tumor growth rates when Nupr1 was perturbed. (a) In all, 2000 cells cultured on 2D rigid plastic were transfected with negative control siRNA (Neg Ctr) or Nupr1 siRNAs (siNupr1) for 24 h and then subcutaneously injected to mice. The tumor volume was quantified using the formula of length times the square of width times 0.52. (b) Overexpressing Nupr1 suppresses tumor growth. 2000 TRCs transfected with empty vector (TRC+empty vector) or Nupr1 complementary DNA plasmids (TRC+Nupr1 overexpression) for 12 h were subcutaneously injected to mice. The volume of skin melanoma was measured for 25 days. Mean±s.e.m.; n=9 (Neg Ctr) and 12 (siNupr1) in a; n=15 (empty vector) and 9 (Nupr1 overexpression) in b; data were pooled from two independent experiments as results from the two experiments were similar, in each experiment eight mice were used per condition; *P<0.05; **P<0.01; ***P<0.001. Welch's unpaired t-test.
Nupr1 negatively regulates nestin and Tert
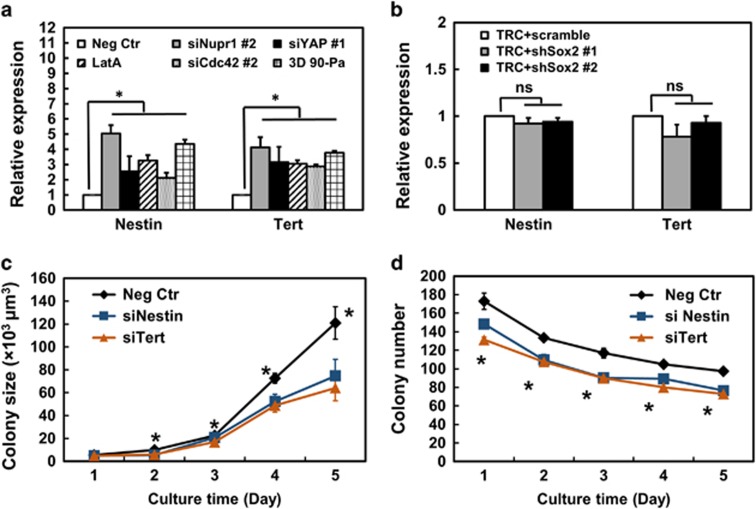
To further explore what else may be regulated by Nupr1, we examined Nestin and Tert, which are upregulated in TRCs2 (Figure 8a). Silencing Sox2 had no effects on either Nestin or Tert, suggesting these two molecules are independent of Sox2 (Figure 8b). Knocking down Nupr1, YAP or Cdc42, or disrupting F-actin with Latrunculin A, led to upregulation of Nestin and Tert (Supplementary Figure 8a), suggesting that Nupr1 and molecules that are upstream of Nupr1 negatively regulate Nestin and Tert. Importantly, silencing either Nestin or Tert decreased both colony size and colony number (Figures 8c and d), suggesting that Nestin and Tert facilitate cell growth. Together, these results suggest that Nestin and Tert are independent of Sox2 and dependent on Nupr1 and promote cell proliferation.
Figure 8.
Nestin and Tert are independent of Sox2 and promote cell proliferation. (a) Nestin and Tert are regulated via the Cdc42-YAP-Nupr1 pathway. Cells were treated with negative control siRNA, Nupr1 siRNA#2, YAP siRNA#1, Cdc42 siRNA #2 for 24 h, and 1 μM Latrunculin A for 12 h, or plated in 90-Pa fibrin gels, and then analyzed for Nestin and Tert mRNA expression by real-time PCR. Mean±s.e.m.; n=3 independent experiments. *P<0.05. (b) Silencing Sox2 does not affect expression of Nestin and Tert. TRCs were transfected with scrambled control shRNA or Sox2 shRNAs for 12 h and their mRNAs were used to quantify the expression levels of Nestin and Tert by real-time PCR. Mean±s.e.m.; n=3 independent experiments; NS, no statistical significance. Extending the shRNA Sox2 transfection duration to 24 h did not have any effect on Nestin and Tert expression either (Supplementary Figure 10c). (c, d) Silencing Nestin or Tert significantly inhibits colony size and number. Cells were transfected with negative control, Nestin or Tert siRNA, and then cultured in 90-Pa fibrin gels. Colony number and colony size were quantified till day 5. Mean±s.e.m.; n=3 independent experiments; all P<0.05 between Neg Ctr and siNestin or siTert except at day 1 in c.
Discussion
Our current reveals an important role of Nupr1 in inhibiting growth of tumorigenic cancer cells in vitro and in vivo and the pathway that is critical for Nupr1 expression. Published reports show that Sox2 is critical in pluripotency maintenance18 of embryonic stem cells and fate determination19, 20 of adult stem cells. Sox2 upregulation has been found in tumor-initiating cells or precursors of osteosarcomas,21 glioblastoma,22 lung and esophageal squamous cell carcinomas,23 and breast cancer.24 In our previous studies, we have shown that Sox2 is markedly upregulated in melanoma TRCs.2, 3 More recently, we have discovered an essential role of Sox2 in mediating efficient extravasation of melanoma TRCs via downregulating Cdc42 and thus F-actin in a zebrafish model.25 However, Sox2 may not be the only critical factor in various carcinomas as in many carcinomas there is no evidence that Sox2 is upregulated. Our current study reveals that Nupr1, independent of Sox2, is low in three types of carcinoma TRCs (melanoma, ovarian cancer and breast cancer) and is a negative regulator of TRC growth. We find that regulation of growth of TRCs by the soft mechanical microenvironment is complex. As the matrix stiffness becomes very low (~100 Pa), Cdc42 is lowered, which leads to decreases in F-actin, which in turn frees up Lats1 so that it binds to and phosphorylate YAP, preventing translocation of YAP into the nucleus. As a result, Nupr1 is downregulated; downregulation of Nupr1, in turn, leads to downregulation of p53 and upregulation of Nestin and Tert. All these help to promote growth and self-renewal of TRCs (Supplementary Figure 10). This pathway appears to be independent of the Sox2-mediated pathway but is regulated by the soft matrices of the microenvironment. At the present, we do not know how important Nupr1 is in TRCs in many other carcinomas, but the abrogation of the inhibitory role of Nupr1 in soft matrices and the upregulation of Nupr1 in stiff matrices support the postulate of physical microenvironment barrier in cancer progression26 and are consistent with the role of 3D stiff matrices in inhibiting carcinoma progression.3 In the future, we need to determine how Nupr1, together with other tumor suppressors, inhibits carcinoma progression in human subjects and to find ways to perturb this pathway to stop cancer progression.
Materials and methods
Animals
Four-week old C57BL/6 female mice were obtained from Center of Medical Experimental Animals of Hubei Province (Wuhan, China). The mice were randomly assigned to be in the control group or the treated group. As a minimum of six mice per group was required for having a statistical power, each group had eight mice, The experimentalists were blinded from the expected outcome of the treatment. All animals received humane care in compliance with the Principles of Laboratory Animal Care Formulated by the National Society of Medical Research and the guide for the US National Institutes of Health. The protocol was approved by the Animal Care and Use Committee of Huazhong University of Science and Technology.
Cell lines and cell culture
Human ovarian cancer cell line A2780, human MCF-7 breast cancer cell line and murine melanoma cell line B16-F1 were purchased from China Center for Type Culture Collection (CCTCC, Wuhan, China). Cells were cultured on rigid dishes with RPMI-1640, Dulbecco's modified Eagle's medium or minimum essential medium cell culture medium supplemented with 10% fetal bovine serum (Life Technologies, Carlsbad, CA, USA), and 1% penicillin and streptomycin at 37 °C with 5% CO2. Cells were passaged every 3–4 days using TrypLE (Life Technologies). Cell samples were randomly allocated to each well of the culture dishes and randomly chosen for intervention. For all cell culture experiments, at least three independent experiments were performed per condition.
3D fibrin gel preparation
Salmon fibrinogen and thrombin were purchased from Reagent Proteins (San Diego, CA, USA). 3D fibrin gels were prepared as described previously.2, 3 In brief, fibrinogen was diluted into 20 mg/ml with T7 buffer (pH 7.4, 50 mM Tris, 150 mM NaCl). Cells were detached from 2D rigid dishes. Fibrinogen and single-cell solution mixture was made by mixing the same volume of fibrinogen solution and cell solution, resulting in 1 or 8 mg/ml fibrin gels (the stiffness of 1 and 8 mg/ml fibrin gels is 90 and 1050 Pa, respectively). In all, 250 μl cell/fibrinogen mixture was seeded into each well of 24-well plate and mixed well with pre-added 5 μl thrombin (100 U/ml). The cell culture plate was then incubated in 37 °C cell culture incubator for 25 min. Finally, 1 ml of minimum essential medium containing 10% fetal bovine serum and antibiotics was added.
2D polyacrylamide gel preparation
Polyacrylamide gels were prepared using the protocol reported previously.27 Gel stiffness was varied by altering the concentrations of bis-acrylamide crosslinker (0.04%, 0.0.5% and 0.3%) and acrylamide (3%, 5% and 5%) and the corresponding gel stiffness is 0.15, 2 and 8.0 kPa, respectively. The gel surface was activated with Sulpho-SANPAH under ultraviolet light for 5 min and coated with 100 μg/ml collagen-1 overnight.
Real-time qPCR analysis
Total mRNA was isolated from the cells using the Trizol reagent according to the supplier's instruction (Life Technologies). Reverse transcription (RT) was performed using the TransScript First-strand cDNA Synthesis Super Mix (TransGen, Beijing, China), according to the manufacturer's protocol. Real-time quantitative PCR (qPCR) was performed using GoTaq qPCR Master Mix (Promega, Madison, MI, USA). The data were normalized against mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The sequences of all the primers for real-time qPCR are listed in Supplementary Table 1.
Immunofluorescence
B16 in plastic dish or 3D fibrin gel culture were fixed with the fix buffer consisting of 4% formaldehyde (BioLegend, Shanghai, China) for 10 min at room temperature. Cells were then permeabilized with 0.5% Triton X-100 (BioLegend) for 2 min and treated with blocking serum (Solarbio, Beijing, China, SL1) for >5 h. Primary antibody, anti-YAP (CST, Danvers, MA, USA, 1:100, #14074) for immunofluorescence was incubated overnight. After being washed to remove unbound primary antibodies, cells were incubated with the secondary antibody Alexa Fluor 488 (Abcam, Cambridge, MA, USA, 1:2000, ab150069) and 4,6-diamidino-2-phenylindole (Biosharp, Wuhan, China, BS130A) for 2 h under dark conditions. Images were acquired with a Leica SP2 confocal microscope (Mennheim, Germany). For quantifications of YAP subcellular localizations, YAP immunofluorescence signal was scored as predominantly nuclear versus evenly distributed/predominantly cytoplasmic in 80–100 cells for each experimental condition.
Western blotting assay
To quantify the expression levels of YAP, phospho-YAP, Nupr1, cells were lysed with RIPA Lysis buffer (Beyotime, Jiangsu, China). Each sample were separated by 8–15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, blocked with 5% bovine serum albumin overnight at 4 °C and incubated with primary antibodies to YAP (rabbit, 1:1000, CST, #14074), phospho-YAP (Ser127) (rabbit, 1:1000, CST, #13008), p8 (T-14) (goat, 1:1000, Santa Cruz, Dallas, TX, USA, sc-23283) and GAPDH (mouse, 1:1000, Abcam, ab8245) for 2 h at room temperature. Primary antibodies were detected with goat anti-rabbit IgG-horseradish peroxidase (1:2000, Santa Cruz, sc-2004), donkey anti-goat IgG-horseradish peroxidase (1:2000, Santa Cruz, sc-2020) or anti-mouse IgG-horseradish peroxidase (1:2000, Santa Cruz, sc-2005). The blots were developed using Immobilon Western Chemiluminescent HRP Substrate (Millipore, Billerica, MA, USA).
F-actin staining
Cells were fixed with 4% paraformaldehyde for 10 min at room temperature. The F-actin was stained using 0.76 mM rhodamine-phalloidin (Sigma, St Louis, MO, USA, 94072 Atto 565 phalloidin) and the nuclei was stained with 10 mg/ml 4,6-diamidino-2-phenylindole (Biosharp, BS130A) for 30 min at 37 °C. The samples were rinsed three times with 1X phosphate-buffered saline before imaging. F-actin content was quantified along the lines shown using Image J (NIH, Bethesda, MA, USA).
ChIP assay
We performed ChIP assay following the manufacturer's instructions (EZ-ChIP kit, Millipore). Briefly, cells were subjected to cross-linking with 1% formaldehyde in medium for 10 min at 37 °C and then lysed on ice. Chromatin was sonicated to shear DNA to an average length of 0.2–1.0 kb. ChIP was performed using control rabbit IgG or antibody against YAP (CST, #14074). The immunoprecipitation was heated to reverse the formaldehyde cross-linking and the DNA fragments in the precipitates was purified for real-time qPCR and PCR analysis. Primers were sets corresponding to Nupr1 and GAPDH (negative control) promoter regions. The sequences of these promoter regions can be found in Transcriptional Regulatory Element Database (Cold Spring Harbor Laboratory, Long Island, NY, USA). Primers used were:
Mus Gapdh promoter: forward, 5'-TCTTCTTGTGCAGTGCCAGGT-3'; reverse, 5'-CACACTTCGCACCAGCATCC-3'. Mus Nupr1 promoter 1: forward, 5'-ACAAGGAGACACAGGCAAGACT-3'; reverse, 5'-TGGCTGTTGGTGGCAAGGT-3'.
Mus Nupr1 promoter 2: forward, 5'-TGCTTGGGTGAGTCCTGTGAG-3' reverse, 5'-GACAGCAGTCCTGAGCAGAGA-3'.
Transfection
Cells were transfected with siRNA, short hairpin RNA (shRNA) or complementary DNA using Lipofectamine 2000 (Invitrogen) following the manufacturer's protocol. Silencer negative control no. 1 siRNA (Invitrogen, AM4611) was used a negative control in RNA interference experiment. The sequences of siRNA or shRNA are listed in Supplementary Table 2. The Nupr1 complementary was obtained from OriGene (Rockville, MD, USA, MG200140). pEGFP-N1 vector (Clotech, Mountain View, CA, USA) was used as control. Knocking down or overexpressing efficiency for various genes were shown in Supplementary Figure 11.
Colony number assay
By changing the focal planes along the z axis (the direction of gel depth), the colony number was counted view by view. At least three wells of colonies were counted per condition per day.
Statistical analysis
All statistics were performed using a two-tailed Student's t-test with unequal variance except the Fisher's exact test and Welch's unpaired t-test for analyzing data from mice experiments.
Acknowledgments
This work was supported by funds from Huazhong University of Science and Technology, by US NIH grant GM072744, and by University of Illinois at Urbana-Champaign.
Author contributions
NW conceived the project; NW, QJ and HJ designed the experiments. QJ, WZ, WY, FY, SZ, RS, JWC, JJC, YZ, FW and YZ carried out the experiments and analyzed the data. NW and QJ wrote the manuscript with inputs from all other authors.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Oncogenesis website (http://www.nature.com/oncsis)
Supplementary Material
References
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell 2011; 147: 275–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Tan Y, Zhang H, Zhang Y, Xu P, Chen J et al. Soft fibrin gels promote selection and growth of tumorigenic cells. Nat Mater 2012; 11: 734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Tajik A, Chen J, Jia Q, Chowdhury F, Wang L et al. Matrix softness regulates plasticity of tumour-repopulating cells via H3K9 demethylation and Sox2 expression. Nat Commun 2014; 5: 4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury UR, Samant RS, Fodstad O, Shevde LA. Emerging role of nuclear protein 1 (Nupr1) in cancer biology. Cancer Metastasis Rev 2009; 28: 225–232. [DOI] [PubMed] [Google Scholar]
- Ree AH, Tvermyr M, Engebraaten O, Rooman M, Røsok O, Hovig E et al. Expression of a novel factor in human breast cancer cells with metastatic potential. Cancer Res 1999; 59: 4675–4680. [PubMed] [Google Scholar]
- Vasseur S, Iovanna JL. p8 gene is necessary for tumor establishment. Med Sci 2003; 19: 1259–1264. [DOI] [PubMed] [Google Scholar]
- Brannon KM, Million Passe CM, White CR, Bade NA, King MW, Quirk CC. Expression of the high mobility group A family member p8 is essential to maintaining tumorigenic potential by promoting cell cycle dysregulation in LβT2 cells. Cancer Lett 2007; 254: 146–155. [DOI] [PubMed] [Google Scholar]
- Clark DW, Mitra A, Fillmore RA, Jiang WG, Samant RS, Fodstad O et al. Nupr1 interacts with p53, transcriptionally regulates p21 and rescues breast epithelial cells from doxorubicin-induced genotoxic stress. Curr Cancer Drug Targets 2008; 8: 421–430. [DOI] [PubMed] [Google Scholar]
- Hamidi T, Cano CE, Grasso D, Garcia MN, Sandi MJ, Calvo EL et al. Nupr1 works against the metabolic stress-induced autophagy-associated cell death in pancreatic cancer cells. Autophagy 2013; 9: 95–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang WG, Davies G, Martin TA, Kynaston H, Mason MD, Fodstad O. Com-1/p8 acts as a putative tumour suppressor in prostate cancer. Int J Mol Med 2006; 18: 981–986. [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Dev Cell 2010; 19: 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 2008; 13: 188–192. [DOI] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M et al. Role of YAP/TAZ in mechanotransduction. Nature 2011; 474: 179–183. [DOI] [PubMed] [Google Scholar]
- Sun Y, Yong KM, Villa-Diaz LG, Zhang X, Chen W, Philson R et al. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat Mater 2014; 13: 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 2007; 21: 2747–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 1995; 81: 53–62. [DOI] [PubMed] [Google Scholar]
- Myers JP, Robles E. Ducharme-Smith A, Gomez TM. Focal adhesion kinase modulates Cdc42 activity downstream of positive and negative axon guidance cues. J Cell Sci 2012; 125: 2918–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong H, Hohenstein KA, Donovan PJ. Regulation of self-renewal and pluripotency by Sox2 in human embryonic stem cells. Stem Cells 2008; 26: 1931–1938. [DOI] [PubMed] [Google Scholar]
- Park SB, Seo KW, So AY, Seo MS, Yu KR, Kang SK et al. SOX2 has a crucial role in the lineage determination and proliferation of mesenchymal stem cells through Dickkopf-1 and c-MYC. Cell Death Differ 2012; 19: 534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Hochedlinger K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell 2013; 12: 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu-Roy U, Seo E, Ramanathapuram L, Rapp TB, Perry JA, Orkin SH et al. Sox2 maintains self renewal of tumor-initiating cells in osteosarcomas. Oncogene 2012; 31: 2270–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangemi RM, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P et al. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells 2009; 27: 40–48. [DOI] [PubMed] [Google Scholar]
- Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet 2009; 41: 1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson DA, Bhakta NR, Kessenbrock K, Prummel KD, Yu Y, Takai K et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 2015; 526: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhou W, Jia Q, Chen J, Zhang S, Yao W et al. Efficient extravasation of tumor-repopulating cells depends on cell deformability. Sci Rep 2016; 6: 19304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 2011; 17: 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YL, Pelham RJ Jr.. Preparation of a flexible, porous polyacrylamide substrate for mechanical studies of cultured cells. Methods Enzymol 1998; 298: 489–496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.