Abstract
A recently proposed paradigm suggests that, like their dietary counterparts, digestion of gastrointestinal endogenous proteins (GEP) may also produce bioactive peptides. With an aim to test this hypothesis, in vitro digests of four GEP namely; trypsin (TRYP), lysozyme (LYS), mucin (MUC), serum albumin (SA) and a dietary protein chicken albumin (CA) were screened for their angiotensin-I converting (ACE-I), renin, platelet-activating factor-acetylhydrolase (PAF-AH) and dipeptidyl peptidase-IV inhibitory (DPP-IV) and antioxidant potential following simulated in vitro gastrointestinal digestion. Further, the resultant small intestinal digests were enriched to obtain peptides between 3–10 kDa in size. All in vitro digests of the four GEP were found to inhibit ACE-I compared to the positive control captopril when assayed at a concentration of 1 mg/mL, while the LYS < 3-kDa permeate fraction inhibited renin by 40% (±1.79%). The LYS < 10-kDa fraction inhibited PAF-AH by 39% (±4.34%), and the SA < 3-kDa fraction inhibited DPP-IV by 45% (±1.24%). The MUC < 3-kDa fraction had an ABTS-inhibition antioxidant activity of 150 (±24.79) µM trolox equivalent and the LYS < 10-kDa fraction inhibited 2,2-Diphenyl-1-picrylhydrazyl (DPPH) by 54% (±1.62%). Moreover, over 190 peptide-sequences were identified from the bioactive GEP fractions. The findings of the present study indicate that GEP are a significant source of bioactive peptides which may influence gut function.
Keywords: gut non-dietary proteins, lysozyme, serum albumin, angiotensin-I converting enzyme (ACE-I) inhibition, renin inhibition, dipeptidyl peptidase IV inhibition, antioxidant peptides
1. Introduction
Gastrointestinal endogenous proteins (GEP) have been proposed [1,2] and recently identified as a potential source of bioactive peptides based on in silico analysis [3]. GEP are made up of gastrointestinal tract (GIT) epithelial turnover and gut microflora proteins [4] as well as soluble secreted proteins. These include the human mucins, digestive enzymes, and serum albumin [4]. Other contributors to GEP include the digestive hormones, immunoglobulins, lysozyme, and other gastric and intestinal peptides [5]. Previous in silico experiments [3] identified 25 GEP as potential sources of bioactive peptides possessing a range of biological activities. The aim of the present study was to investigate if the GEP trypsin (P00761 (TRYP)), human lysozyme (P61626 (LYS)), salivary mucin (P12021 (MUC)), human serum albumin (P02768 (SA)) and the dietary protein chicken albumin (P01012 (CA)) are precursor proteins for bioactive peptides which can be released following GI digestion. The selected proteins were screened for in vitro angiotensin-I converting enzyme (ACE-I; EC 3.4.15.1), renin (EC 3.4.23.15), platelet-activating factor-acetylhydrolase (PAF-AH; EC 3.1.1.47) and dipeptidyl peptidase-IV (DPP-IV; EC 3.4.14.5) inhibitory activities and in vitro antioxidant activities using the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) inhibition assays. The selected proteins were digested using an in vitro gastrointestinal digestion model developed previously as part of the EU COST INFOGEST network [6]. After sequential in vitro gastric and small intestinal digestion, freeze-dried samples were assessed for their in vitro enzyme inhibitory activities.
Inhibition of the enzymes ACE-I, renin, PAF-AH and DPP-IV is known to lower systemic and local blood pressure, and assist in the alleviation of symptoms of several disorders including diabetes mellitus [7], hypercholesterolaemia, inflammatory diseases [8,9], and fibrosis [10]. Inhibition of ACE-I prevents the formation of angiotensin II, a potent vasoconstrictor while renin inhibition prevents the formation of angiotensin I, the precursor of angiotensin II [11]. It is now known that the GIT also contains a local renin angiotensin aldosterone system (RAAS) [12], which plays a role in intestinal fluid and electrolyte balance, and intestinal ischaemia [13]. PAF-AH catalyzes platelet-activating factor (PAF), a pro-inflammatory phospholipid mediator that is involved in various inflammatory diseases of the GIT [14], and elevated levels of PAF-AH are believed to be a risk factor for coronary heart disease [15] and systemic inflammation [16,17]. DPP-IV degrades the incretins including Glucagon-like peptide-2 (GLP-2). GLP-2 is known to help mucosal epithelial cell proliferation in the small intestine, and thus inhibition of DPP-IV in the GIT may enhance epithelial re-growth in the small intestine [18]. DPP-IV inhibition is also known to alleviate the symptoms of hypertension and diabetes mellitus [19] and also plays a role in regulation of satiety [20].
The lumen of the GIT is continually exposed to various pro-oxidants from the diet and the environment, and is thus the site of a significant amount of oxidative reactions [21]. Natural GEP-derived antioxidants could play a protective role against oxidative damage in the lumen. Therefore, the present study not only investigated the in vitro potential of GEP as a source of bioactive peptides with inhibitory activities against ACE-I, renin, DPP-IV and PAF-AH but also the antioxidant potential of these peptides.
2. Results
2.1. Digestion of Proteins and Determination of Protein Content Using Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis SDS-PAGE
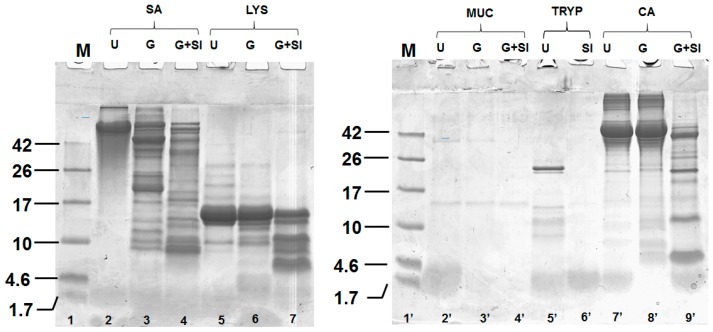
LYS, MUC, SA, CA were subjected to simulated in vitro gastric and small intestinal digestion, and TRYP was subjected to simulated in vitro small intestinal digestion alone. The protein content of the gastric and small intestinal digests are given in Table 1. Tris-tricine sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) analysis was used to confirm the digestion of selected proteins (Figure 1). All of the proteins except LYS were digested extensively by the end of the simulated small intestinal phase of digestion as shown in Figure 1 lanes 2, 3 and 4 for SA, lanes 2′, 3′ and 4′ for MUC and lanes 5′ and 6′ for TRYP. Lanes 7′, 8′ and 9′ show the digestion of CA, which was used as a control (a known dietary source of bioactive peptides). In the case of LYS (lanes 5, 6 and 7), a significant amount of LYS (≈15 kDa band) remained intact even after sequential gastric and small intestinal digestion.
Table 1.
The protein content of the in vitro gastric (G) and small intestinal digests (G + SI) of the gastrointestinal endogenous proteins, porcine trypsin (TRYP), human lysozyme (LYS), porcine salivary mucin (MUC) and human serum albumin (SA), and dietary protein chicken albumin (CA).
| Hydrolysate | Protein Content (%) |
|---|---|
| TRYP G + SI | 16.38 |
| LYS G | 97.89 |
| LYS G + SI | 98.48 |
| MUC G | 45.35 |
| MUC G + SI | 42.89 |
| SA G | 90.12 |
| SA G + SI | 87.71 |
| CA G | 85.56 |
| CA G + SI | 82.26 |
Figure 1.
Tris-tricine SDS-PAGE, of the in vitro gastric and small intestinal digests of the gastrointestinal endogenous proteins, porcine trypsin (TRYP), human lysozyme (LYS), porcine salivary mucin (MUC) and human serum albumin (SA), and dietary protein chicken albumin (CA) conducted under reducing conditions. Each protein was analyzed prior to hydrolysis (U) and after gastric (G) and small intestinal digestion (G + SI).
2.2. Angiotensin-I Converting Enzyme (ACE-I) Inhibition by Digests and Enriched Fractions
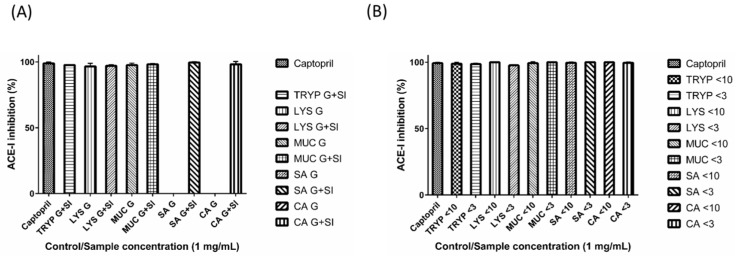
The in vitro small intestinal digest of TRYP, and gastric and small intestinal digests of LYS, MUC, SA and CA were found to inhibit ACE-I significantly (≥ 97% inhibition); while the fractions TRYP < 10-kDa, TRYP < 3-kDa, LYS < 10-kDa, LYS < 3-kDa, MUC < 10-kDa, MUC < 3-kDa, SA < 10-kDa, SA < 3-kDa, CA < 10-kDa, CA < 3-kDa, inhibited ACE-I by 99% (±1.00%), 99% (±0.57%), 100% (±0.01%), 98% (±0.57%), 99% (±1.15%), 100% (±0.06%), 100% (±0.57%), 100% (±0.05%), 100% (±0), and 100% (±0.58%), respectively at a concentration of 1 mg/mL (Figure 2). These values were found to be comparable to the ACE-I inhibition values obtained for the positive control captopril (99% (±0.57%)) which was also assayed at a concentration of 1 mg/mL. To ensure that the observed ACE-I inhibition was due to the peptides present in the digested GEP fractions, rather than to the presence of the salts or other potentially interfering digestion medium constituents, all samples were desalted using dialysis as described previously. Dialyzed samples showed inhibition comparable to captopril when assayed at 1 mg/mL.
Figure 2.
Angiotensin I converting enzyme (ACE-I) inhibition by (A) gastric (G) and small intestinal digests (G + SI) of the gastrointestinal endogenous proteins, porcine trypsin (TRYP), human lysozyme (LYS), porcine salivary mucin (MUC) and human serum albumin (SA), and dietary protein chicken albumin (CA); and (B) <10- and <3-kDa fractions of the small intestinal digests. All of the samples and the positive control captopril were tested at the concentration of 1 mg/mL.
2.3. Renin Inhibition by Digests and Enriched Fractions
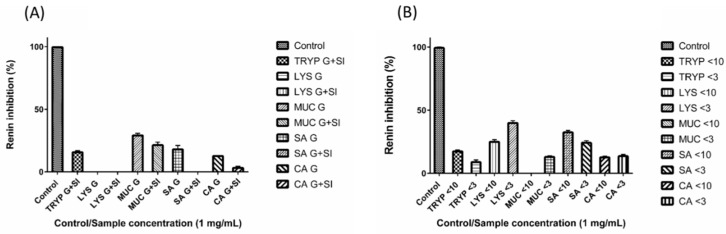
Among all the tested digests and fractions, the LYS < 3-kDa permeate fraction inhibited renin to the greatest extent (40% ±1.79 inhibition) (Figure 3). The small intestinal enriched fractions TRYP < 10-kDa, TRYP < 3-kDa, LYS < 10-kDa, MUC < 3-kDa, SA < 10-kDa, SA < 3-kDa, CA < 10-kDa and CA < 3-kDa inhibited renin by 17% (±1.19%), 9% (±1.70%), 25% (±1.76%), 13% (±0.93%), 32% (±1.51%), 24% (±1.64%), 13% (±0.82%), and 14% (±1.41%) respectively at a concentration of 1 mg/mL. TRYP SI, MUC G, MUC G + SI, SA G, and CA G were found to inhibit renin by 16% (±1.20%), 29% (±.68%), 21% (±2.39%), 18% (±3.17%), and 13% (±0.29%) respectively at a concentration of 1 mg/mL. From the gastric and small intestinal digests tested for renin inhibition LYS G, LYS G + SI, SA G + SI and CA G + SI were found to have negligible renin inhibitory potential. The fraction MUC < 10-kDa also showed no renin inhibition. The positive control (Z-Arg-Arg-Pro-Phe-His-Sta-Ile-His-Lys-(Boc)-Ome), showed 100% (±0.39%) inhibition when assayed at a concentration of 10 µM.
Figure 3.
Renin inhibition by (A) gastric (G) and small intestinal digests (G + SI) of the gastrointestinal endogenous proteins, porcine trypsin (TRYP), human lysozyme (LYS), porcine salivary mucin (MUC) and human serum albumin (SA), and dietary protein chicken albumin (CA); and (B) <10- and <3- kDa fractions of the small intestinal digests. All of the samples were tested at the concentration of 1 mg/mL. The positive control Z-Arg-Arg-Pro-Phe-His-Sta-Ile-His-Lys-(Boc)-Ome was tested at 10 µM.
2.4. Renin, Platelet-Activating Factor-Acetylhydrolase (PAF-AH) Inhibition by Digests and Enriched Fractions
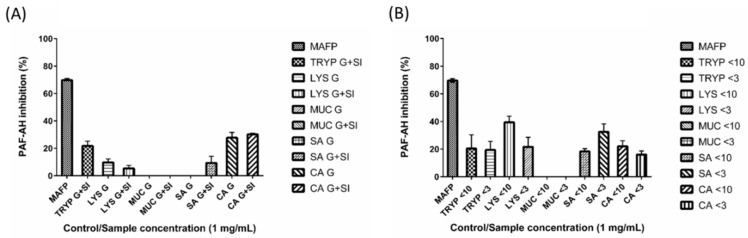
LYS < 10-kDa was found to inhibit PAF-AH by up to 39% (±4.34%), while the fractions TRYP < 10-kDa, TRYP < 3-kDa, LYS < 3-kDa, SA < 10-kDa, SA < 3-kDa, CA < 10-kDa, CA < 3-kDa showed 20% (±9.93%), 19% (±6.25%), 21% (±6.9%), 18% (±1.99%), 33% (±5.69%), and 22% (±4.01%) and 16% (±2.56%)) inhibition respectively at a concentration of 1 mg/mL (Figure 4). The fractions MUC < 10 and MUC < 3 showed no renin inhibition. TRYP SI, LYS G, LYS G + SI, SA G + SI, CA G and CA G + SI inhibited PAF-AH by 22% (±3.50%), 10% (±2.50%), 5% (±2.20%), 9% (±4.79%), 28% (±3.88%), and 30% (±0.81%) respectively at a concentration of 1 mg/mL. The digests MUC G, MUC G + SI and SA G showed no renin inhibition. The positive control MAFP inhibited PAF-AH by 70% (±1.31%).
Figure 4.
Platelet-activating factor-acetylhydrolase (PAF-AH) inhibition by (A) gastric (G) and small intestinal digests (G + SI) of the gastrointestinal endogenous proteins, porcine trypsin (TRYP), human lysozyme (LYS), porcine salivary mucin (MUC) and human serum albumin (SA), and dietary protein chicken albumin (CA); and (B) <10- and <3-kDa fractions of the small intestinal digests. All of the samples were tested at the concentration of 1 mg/mL. The positive control Methyl arachidonyl fluorophosphonate (MAFP) was tested at 260 ng/mL.
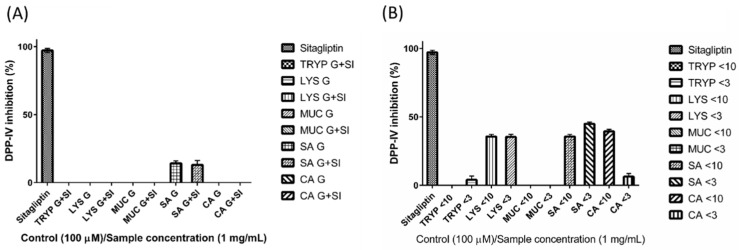
2.5. Dipeptidyl Peptidase-IV Inhibitory (DPP-IV) Inhibition by Digests and Enriched Fractions
The fractions LYS < 10-kDa, LYS < 3-kDa, SA < 10-kDa, SA < 3-kDa, and CA < 10-kDa inhibited DPP-IV by 36% (±1.52%), 35% (±1.93%), 36% (±1.52%), 45% (±1.24%), and 39% (±1.54%)respectively when assayed at a concentration of 1 mg/mL (Figure 5). The positive control Sitagliptin inhibited DPP-IV by 97% when assayed at the same concentration (±1.52%).
Figure 5.
Dipeptidyl peptidase IV (DPP-IV) inhibition by (A) gastric (G) and small intestinal digests (G + SI) of the gastrointestinal endogenous proteins, porcine trypsin (TRYP), human lysozyme (LYS), porcine salivary mucin (MUC) and human serum albumin (SA), and dietary protein chicken albumin (CA); and (B) <10- and <3-kDa fractions of the small intestinal digests. All of the samples were tested at the concentration of 1 mg/mL. The positive control sitagliptin was tested at 100 µM.
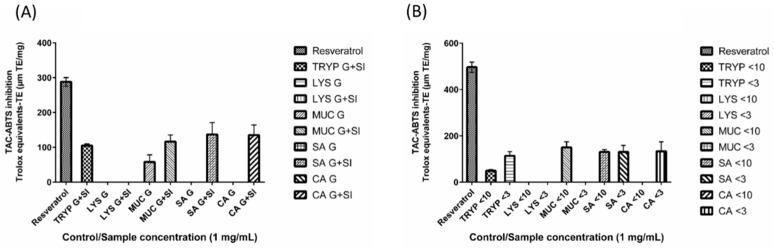
2.6. 2,2′-Azino-bis-3-ethylbenzthiazoline-6-sulphonic Acid Total Antioxidant Capacity (ABTS-TAC) of Digests and Enriched Fractions
Among all of the tested digests and fractions, the fraction MUC < 10-kDa quenched the ABTS radical maximally and had an 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid total antioxidant capacity (ABTS-TAC) value of 150 (± 24.79) µM·TE/mg; while the-fractions TRYP < 10-kDa, TYRP < 3-kDa, SA < 10-kDa, SA < 3-kDa AND CA < 3-kDa had ABTS-TAC values of 50 (± 4.91), 114 (± 17.81), 131 (± 10.54), 131 (± 28.09) and 134 (± 40.84) µM TE (Figure 6). The in vitro digests TRYP SI, MUC G, MUC G + SI, SA G + SI, and CA G + SI were found to have ABTS-TAC of 105 (± 4.62), 58 (± 20.57), 116 (± 19.12), 137 (± 34.27) and 135 (± 29.32) µM·TE/mg. The digests LYS G, LYS G + SI, SA G and CA G were found to have no antioxidant potential using the ABTS-TAC assay. Fractions LYS < 10-kDa, LYS < 3-kDa, MUC < 3-kDa and CA < 10-kDa did not inhibit/quench the ABTS radical. The positive control resveratrol was found to have an ABTS-TAC values ranging from 288 to 497 (±12.38–22.11) TE/mg.
Figure 6.
Total antioxidant capacity by 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) inhibition by (A) gastric (G) and small intestinal digests (G + SI) of the gastrointestinal endogenous proteins, porcine trypsin (TRYP), human lysozyme (LYS), porcine salivary mucin (MUC) and human serum albumin (SA), and dietary protein chicken albumin (CA); and (B) <10- and <3-kDa fractions of the small intestinal digests. All of the samples and the positive control resveratrol were tested at the concentration of 1 mg/mL and the results are expressed in µM trolox equivalents (TE).
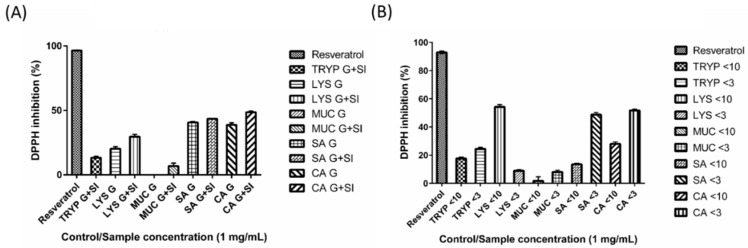
2.7. DPPH Inhibition by Digests and Enriched Fractions
The fractions LYS < 10-kDa, CA < 3-kDa, SA < 3-kDa, were found to scavenge the DPPH radical by 54% (±1.62%), 52% (±0.89%) and 49% (±1.58%), respectively at a concentration of 1 mg/mL (Figure 7). The remaining enriched fractions (TRYP < 10-kDa, TRYP < 3-kDa, LYS < 3-kDa, MUC < 10-kDa, MUC < 3-kDa, SA < 10-kDa, CA < 10-kDa) inhibited the DPPH radical by 18% (±1.12%), 24% (±1.17%), 9% (±0.85%), 2% (±3.03%), 8% (±1.32%), 14% (±0.69%), 28% (±1.35%) respectively at a concentration of 1 mg/mL. The un-fractionated digests TRYP SI, LYS G, LYS G + SI, SA G, SA G + SI, CA G, CA G + SI inhibited the DPPH free radical by 13% (±1.15%), 20% (±1.68%), 30% (±1.84%), 41% (±0.69%), 44% (±0.24%), 39% (±1.59%) and 49% (±1.00%) respectively at a concentration of 1 mg/mL. The MUC G digest showed no DPPH inhibition, while the MUC G + SI digest inhibited DPPH by 7% (±2.29%). The positive control, resveratrol, inhibited DPPH by 93%–97% when assayed at the same concentration (±0.95%–0.36%).
Figure 7.
2,2-diphenyl-1-picrylhydrazyl (DPPH) inhibition by (A) gastric (G) and small intestinal digests (G + SI) of the gastrointestinal endogenous proteins, porcine trypsin (TRYP), human lysozyme (LYS), porcine salivary mucin (MUC) and human serum albumin (SA), and dietary protein chicken albumin (CA); and (B) <10- and <3-kDa fractions of the small intestinal digests. All of the samples and the positive control resveratrol were tested at the concentration of 1 mg/mL.
2.8. Electrospray Ionisation Time of Flight Mass Spectrometry (ESI-TOF-MS) Characterisation of Peptides
A total of 19 and 91 peptides were identified in the <3-kDa fraction of the small intestinal digests of lysozyme and serum albumin respectively and 13 and 70 peptides were identified from the <10-kDa fraction of the small intestinal digests of lysozyme and serum albumin respectively (Table 2). The peptides ranged from 7–36 amino acids in chain length.
Table 2.
Gastrointestinal endogenous proteins (GEP)-derived peptide sequences found in the <3- and <10-kDa fractions of the in vitro small intestinal digests of lysozyme and serum albumin. The samples were analyzed using electrospray ionization time of flight mass spectrometry (ESI-TOF MS).
| Parent Protein and Hydrolysate Fraction | Peptide Amino acid Sequence | Protein Fragment | Observed Mass (Da) | Theoretical Mass (Da) | Observed m/z | Theoretical m/z | Theoretical Z |
|---|---|---|---|---|---|---|---|
| Lysozyme < 3-kDa fraction | ALLQDNIADAV | f(101–111) | 1141.60 | 1141.60 | 571.81 | 571.81 | 2 |
| ALLQDNIADAVA | f(101–112) | 1212.64 | 1212.64 | 607.33 | 607.32 | 2 | |
| ARTLKRLGMDGYRGISL | f(27–43) | 1906.06 | 1906.06 | 477.52 | 477.52 | 4 | |
| DPQGIRAWV | f(120–128) | 1040.54 | 1040.54 | 521.28 | 521.28 | 2 | |
| GIFQINSRYW a | f(73–82) | 1298.66 | 1298.64 | 650.34 | 650.33 | 2 | |
| GMDGYRGISLANWM | f(34–47) | 1569.71 | 1569.71 | 785.86 | 785.86 | 2 | |
| LGMDGYRGISL | f(33–43) | 1180.59 | 1180.59 | 591.30 | 591.30 | 2 | |
| LGMDGYRGISLA | f(33–44) | 1251.63 | 1251.63 | 626.82 | 626.82 | 2 | |
| LLQDNIADAV b | f(102–111) | 1071.54 | 1071.54 | 536.78 | 536.78 | 2 | |
| NAGDRSTDYG | f(64–73) | 1054.43 | 1054.43 | 528.22 | 528.22 | 2 | |
| NAGDRSTDYGIFQ | f(64–76) | 1442.64 | 1442.64 | 722.33 | 722.33 | 2 | |
| NAGDRSTDYGIFQI | f(64–77) | 1555.73 | 1555.73 | 778.87 | 778.87 | 2 | |
| NAGDRSTDYGIFQIN | f(64–78) | 1669.77 | 1669.77 | 835.89 | 835.89 | 2 | |
| NYNAGDRSTD | f(62–71) | 1111.45 | 1111.45 | 556.73 | 556.73 | 2 | |
| NYNAGDRSTDYGIF | f(62–75) | 1591.69 | 1591.69 | 796.85 | 796.85 | 2 | |
| NYNAGDRSTDYGIFQ | f(62–76) | 1719.76 | 1719.75 | 860.89 | 860.88 | 2 | |
| NYNAGDRSTDYGIFQI | f(62–77) | 1832.84 | 1832.83 | 917.43 | 917.42 | 2 | |
| PQGIRAWVAW | f(121–130) | 1182.63 | 1182.63 | 592.32 | 592.32 | 2 | |
| YNAGDRSTDYGIF | f(63–75) | 1477.65 | 1477.65 | 739.83 | 739.83 | 2 | |
| Lysozyme < 10-kDa fraction | CNDGKTPGAV | f(83–92) | 960.43 | 960.43 | 481.22 | 481.22 | 2 |
| CNDGKTPGAVNACHLSCS c,d | f(83–100) | 1773.73 | 1773.74 | 592.25 | 592.25 | 3 | |
| GIFQINSRYW | f(73–82) | 1282.65 | 1282.65 | 642.33 | 642.33 | 2 | |
| GMDGYRGISLANWM | f(34–47) | 1569.72 | 1569.71 | 785.87 | 785.86 | 2 | |
| NAGDRSTDYG | f(64–73) | 1054.43 | 1054.43 | 528.22 | 528.22 | 2 | |
| NAGDRSTDYGIFQI | f(64–77) | 1555.74 | 1555.73 | 778.88 | 778.87 | 2 | |
| NYNAGDRSTD | f(62–71) | 1111.45 | 1111.45 | 556.73 | 556.73 | 2 | |
| NYNAGDRSTDY | f(62–72) | 1274.52 | 1274.52 | 638.27 | 638.27 | 2 | |
| NYNAGDRSTDYG | f(62–73) | 1331.53 | 1331.54 | 666.77 | 666.78 | 2 | |
| NYNAGDRSTDYGIFQI | f(62–77) | 1832.85 | 1832.83 | 917.43 | 917.42 | 2 | |
| SALLQDNIADAV | f(100–111) | 1228.63 | 1228.63 | 615.32 | 615.32 | 2 | |
| YNAGDRSTDYG | f(63–73) | 1217.49 | 1217.49 | 609.75 | 609.75 | 2 | |
| ALLQDNIADAVACA e | f(101–114) | 1434.66 | 1434.67 | 718.34 | 718.34 | 2 | |
| Serum albumin < 3-kDa fraction | AEAKDVFLGMFL | f(344–355) | 1339.69 | 1339.68 | 1339.69 | 670.85 | 2 |
| AEFAEVSKLVTDL | f(250–262) | 1420.75 | 1420.74 | 1420.75 | 711.38 | 2 | |
| AEFAEVSKLVTDLT f | f(250–263) | 1549.79 | 1549.79 | 1549.79 | 775.90 | 2 | |
| AEFAEVSKLVTDLTK | f(250–264) | 1649.89 | 1649.89 | 1649.89 | 825.95 | 2 | |
| AEFAEVSKLVTDLTKVHT | f(250–267) | 1987.10 | 1987.06 | 1987.10 | 994.54 | 2 | |
| AEVENDEMPADLPSLA | f(315–330) | 1699.76 | 1699.76 | 1699.76 | 850.89 | 2 | |
| AEVSKLVTDLT | f(253–63) | 1174.64 | 1174.64 | 1174.64 | 588.33 | 2 | |
| AKVFDEFKPL | f(395–404) | 1192.65 | 1192.65 | 1192.65 | 597.33 | 2 | |
| AKVFDEFKPLVEEPQ | f(395–409) | 1774.91 | 1774.91 | 1774.91 | 888.46 | 2 | |
| ALEVDETYVPK | f(514–524) | 1262.64 | 1262.64 | 1262.64 | 632.33 | 2 | |
| ALEVDETYVPKE | f(514–525) | 1391.68 | 1391.68 | 1391.68 | 696.85 | 2 | |
| ALEVDETYVPKEF | f(514–526) | 1538.75 | 1538.75 | 1538.75 | 770.38 | 2 | |
| ALVLIAFA | f(45–52) | 816.51 | 816.51 | 816.51 | 409.26 | 2 | |
| ALVLIAFAQ | f(45–53) | 944.57 | 944.57 | 944.57 | 473.29 | 2 | |
| ALVLIAFAQY | f(45–54) | 1107.62 | 1107.63 | 1107.62 | 554.82 | 2 | |
| AVMDDFAAFVEK | f(570–581) | 1341.63 | 1341.63 | 1341.63 | 671.82 | 2 | |
| DDNPNLPR | f(131–138) | 939.44 | 939.44 | 939.44 | 470.73 | 2 | |
| DEFKPLVEEPQNLI | f(399–412) | 1669.86 | 1669.86 | 1669.86 | 835.94 | 2 | |
| DEFKPLVEEPQNLIK | f(399–413) | 1797.95 | 1797.95 | 1797.95 | 899.98 | 2 | |
| DETYVPKE | f(518–525) | 979.45 | 979.45 | 979.45 | 490.73 | 2 | |
| DLGEENFKALV | f(37–47) | 1233.63 | 1233.62 | 1233.63 | 617.82 | 2 | |
| DLGEENFKALVL | f(37–48) | 1346.71 | 1346.71 | 1346.71 | 674.36 | 2 | |
| DLPSLAADFVES | f(325–336) | 1262.60 | 1262.60 | 1262.60 | 632.31 | 2 | |
| DLPSLAADFVESK | f(325–337) | 1390.70 | 1390.70 | 1390.70 | 696.36 | 2 | |
| DVFLGMFLYE g | f(348–357) | 1248.77 | 1248.57 | 1248.77 | 625.29 | 2 | |
| EDHVKLVNEVTEFA | f(61–74) | 1628.81 | 1628.80 | 1628.81 | 815.41 | 2 | |
| ENDEMPADLPSL | f(318–329) | 1329.58 | 1329.58 | 1329.58 | 665.80 | 2 | |
| ENDEMPADLPSLAADFVES | f(318–336) | 2048.90 | 2048.89 | 2048.90 | 1025.45 | 2 | |
| ENDEMPADLPSLAADFVESK | f(318–337) | 2177.01 | 2176.98 | 2177.01 | 1089.50 | 2 | |
| EQLKAVMDD | f(566–574) | 1047.49 | 1047.49 | 1047.49 | 524.75 | 2 | |
| EQLKAVMDDF h | f(566–575) | 1176.55 | 1176.55 | 1176.55 | 589.28 | 2 | |
| EQLKAVMDDFA h | f(566–576) | 1247.59 | 1247.59 | 1247.59 | 624.80 | 2 | |
| EQLKAVMDDFAA h | f(566–577) | 1318.62 | 1318.62 | 1318.62 | 660.32 | 2 | |
| EVDETYVPK | f(516–524) | 1078.52 | 1078.52 | 1078.52 | 540.27 | 2 | |
| EVDETYVPKE | f(516–525) | 1207.57 | 1207.56 | 1207.57 | 604.79 | 2 | |
| EVDETYVPKEF | f(516–526) | 1354.63 | 1354.63 | 1354.63 | 678.32 | 2 | |
| EVDETYVPKEFN | f(516–527) | 1468.67 | 1468.67 | 1468.67 | 735.34 | 2 | |
| EVDETYVPKEFNAET i | f(516–530) | 1770.79 | 1770.78 | 1770.79 | 886.40 | 2 | |
| FDEFKPLVEEPQNLIK | f(398–413) | 1945.02 | 1945.02 | 1945.02 | 973.52 | 2 | |
| FKDLGEEN | f(35–42) | 950.44 | 950.43 | 950.44 | 476.22 | 2 | |
| FKDLGEENFKALV | f(35–47) | 1508.79 | 1508.79 | 1508.79 | 755.40 | 2 | |
| FKDLGEENFKALVL | f(35–48) | 1621.87 | 1621.87 | 1621.87 | 811.94 | 2 | |
| FPKAEFAEVSKLVTDLT | f(247–263) | 1894.01 | 1894.01 | 1894.01 | 632.34 | 3 | |
| FYAPELLFFAK | f(173–183) | 1344.71 | 1344.71 | 1344.71 | 673.36 | 2 | |
| KPLVEEPQN | f(402–410) | 1052.55 | 1052.55 | 1052.55 | 527.28 | 2 | |
| KPLVEEPQNLI | f(402–412) | 1278.72 | 1278.72 | 1278.72 | 640.37 | 2 | |
| KVPQVSTPT | f(438–446) | 955.53 | 955.53 | 955.53 | 478.77 | 2 | |
| KVPQVSTPTLV | f(438–448) | 1167.69 | 1167.69 | 1167.69 | 584.85 | 2 | |
| KVPQVSTPTLVEV | f(438–450) | 1395.79 | 1395.80 | 1395.79 | 698.91 | 2 | |
| KVPQVSTPTLVEVS | f(438–451) | 1482.83 | 1482.83 | 1482.83 | 742.42 | 2 | |
| LDELRDEG | f(206–213) | 945.44 | 945.44 | 945.44 | 473.73 | 2 | |
| LEVDETYVPK | f(515–524) | 1191.60 | 1191.60 | 1191.60 | 596.81 | 2 | |
| LEVDETYVPKE | f(515–525) | 1320.65 | 1320.64 | 1320.65 | 661.33 | 2 | |
| LQHKDDNPNLPR | f(127–138) | 1445.73 | 1445.74 | 1445.73 | 482.92 | 3 | |
| LVAASQAALG | f(599–608) | 899.51 | 899.51 | 899.51 | 450.76 | 2 | |
| LVNEVTEFA | f(66–74) | 1020.52 | 1020.51 | 1020.52 | 511.26 | 2 | |
| LVNEVTEFAK | f(66–75) | 1148.61 | 1148.61 | 1148.61 | 575.31 | 2 | |
| LVNEVTEFAKTCVADESAENCDK c,d | f(66–88) | 2512.11 | 2512.13 | 2512.11 | 629.04 | 4 | |
| LVRPEVDVM g | f(139–147) | 1072.56 | 1072.56 | 1072.56 | 537.29 | 2 | |
| NDEMPADLPSLA i | f(318–330) | 1272.56 | 1272.55 | 1272.56 | 637.28 | 2 | |
| NDEMPADLPSLAADF | f(318–333) | 1604.71 | 1604.70 | 1604.71 | 803.36 | 2 | |
| NDEMPADLPSLAADFVES | f(318–336) | 1919.86 | 1919.85 | 1919.86 | 960.93 | 2 | |
| NDEMPADLPSLAADFVESK | f(318–337) | 2047.95 | 2047.94 | 2047.95 | 1024.98 | 2 | |
| NEVTEFAKTCVADESAENCDK c,d | f(68–88) | 2299.96 | 2299.98 | 2299.96 | 1150.99 | 2 | |
| NRRPCFSALEVDETYVPKE d,j | f(507–525) | 2199.96 | 2200.09 | 2199.96 | 734.37 | 3 | |
| NYAEAKDVFLG | f(342–352) | 1225.60 | 1225.60 | 1225.60 | 613.81 | 2 | |
| NYAEAKDVFLGM | f(342–353) | 1356.64 | 1356.64 | 1356.64 | 679.33 | 2 | |
| NYAEAKDVFLGMFL | f(342–355) | 1616.83 | 1616.79 | 1616.83 | 809.40 | 2 | |
| QHKDDNPNLPR h | f(128–138) | 1315.62 | 1315.63 | 1315.62 | 439.55 | 3 | |
| QYLQQCPFEDHV d | f(53–64) | 1471.68 | 1471.67 | 1471.68 | 736.84 | 2 | |
| RETYGEM | f(105–111) | 884.37 | 884.37 | 884.37 | 443.19 | 2 | |
| RHPDYSVV | f(361–368) | 971.48 | 971.48 | 971.48 | 486.75 | 2 | |
| RHPDYSVVL | f(361–369) | 1084.57 | 1084.57 | 1084.57 | 543.29 | 2 | |
| SALEVDETYVPK | f(513–524) | 1349.67 | 1349.67 | 1349.67 | 675.84 | 2 | |
| SALEVDETYVPKE | f(513–525) | 1478.72 | 1478.71 | 1478.72 | 740.36 | 2 | |
| SALEVDETYVPKEF | f(513–526) | 1625.79 | 1625.78 | 1625.79 | 813.90 | 2 | |
| TPVSDRVT | f(491–498) | 873.45 | 873.46 | 873.45 | 437.74 | 2 | |
| VFDEFKP | f(397–403) | 880.43 | 880.43 | 880.43 | 441.22 | 2 | |
| VFDEFKPL | f(397–404) | 993.52 | 993.52 | 993.52 | 497.77 | 2 | |
| VFDEFKPLVEEPQ | f(397–409) | 1575.78 | 1575.78 | 1575.78 | 788.90 | 2 | |
| VFDEFKPLVEEPQN | f(397–410) | 1689.83 | 1689.82 | 1689.83 | 845.92 | 2 | |
| VFDEFKPLVEEPQNLI | f(397–412) | 1916.00 | 1915.99 | 1916.00 | 959.00 | 2 | |
| VKLVNEVTEFA | f(64–74) | 1247.68 | 1247.68 | 1247.68 | 624.85 | 2 | |
| VLIAFAQYL | f(47–55) | 1036.60 | 1036.60 | 1036.60 | 519.31 | 2 | |
| VPQVSTPTLVEVS | f(439–451) | 1354.74 | 1354.73 | 1354.74 | 678.37 | 2 | |
| VSTPTLVEVS | f(442–451) | 1030.56 | 1030.55 | 1030.56 | 516.28 | 2 | |
| DVFLGMFL g | f(348–355) | 956.47 | 956.47 | 956.47 | 479.24 | 2 | |
| TLRETYGEMADCCAKQEPERNECFLQHKDDNPNLPR d,i | f(103–138) | 4216.84 | 4216.90 | 4216.84 | 844.39 | 5 | |
| LVNEVTEFAKTC d | f(66–77) | 1318.71 | 1318.68 | 1318.71 | 660.35 | 2 | |
| ENDEMPADLPSLA h | f(318–330) | 1382.60 | 1382.60 | 1382.60 | 692.31 | 2 | |
| LSVVLNQLCVL c | f(477–87) | 1231.69 | 1231.68 | 1231.69 | 616.85 | 2 | |
| Serum albumin < 10-kDa fraction | AEAKDVFLGMF | f(344–354) | 1226.60 | 1226.60 | 614.31 | 614.31 | 2 |
| AEAKDVFLGMFL | f(344–355) | 1339.70 | 1339.68 | 670.86 | 670.85 | 2 | |
| AEFAEVSKLVTDL | f(250–262) | 1420.76 | 1420.74 | 711.39 | 711.38 | 2 | |
| AEFAEVSKLVTDLT | f(250–263) | 1521.91 | 1521.79 | 761.96 | 761.90 | 2 | |
| AEFAEVSKLVTDLTK | f(250–264) | 1649.88 | 1649.89 | 550.97 | 550.97 | 3 | |
| AEVSKLVTDLT | f(253–263) | 1174.65 | 1174.64 | 588.33 | 588.33 | 2 | |
| AKVFDEFKPLVEEPQNLIK | f(395–413) | 2243.25 | 2243.22 | 748.76 | 748.75 | 3 | |
| ALVLIAFAQ | f(45–53) | 944.56 | 944.57 | 473.29 | 473.29 | 2 | |
| ALVLIAFAQY | f(45–54) | 1107.63 | 1107.63 | 554.82 | 554.82 | 2 | |
| APELLFFAK | f(175–183) | 1034.57 | 1034.58 | 518.29 | 518.30 | 2 | |
| AVMDDFAAFVE | f(570–580) | 1213.54 | 1213.53 | 607.78 | 607.77 | 2 | |
| AVMDDFAAFVEK | f(570–581) | 1341.64 | 1341.63 | 671.83 | 671.82 | 2 | |
| DEFKPLVEEPQNL | f(399–411) | 1556.80 | 1556.77 | 779.41 | 779.39 | 2 | |
| DEFKPLVEEPQNLI | f(399–412) | 1669.89 | 1669.86 | 835.95 | 835.94 | 2 | |
| DEFKPLVEEPQNLIK | f(399–413) | 1798.00 | 1797.95 | 900.01 | 899.98 | 2 | |
| DLGEENFKALVLI | f(37–49) | 1459.82 | 1459.79 | 730.92 | 730.90 | 2 | |
| DLGEENFKALVLIA | f(37–50) | 1530.85 | 1530.83 | 766.43 | 766.42 | 2 | |
| DLGEENFKALVLIAFAQ | f(37–53) | 1877.06 | 1876.99 | 939.54 | 939.50 | 2 | |
| DLPSLAADF | f(325–333) | 947.45 | 947.46 | 474.73 | 474.74 | 2 | |
| DLPSLAADFVES | f(325–334) | 1262.61 | 1262.60 | 632.31 | 632.31 | 2 | |
| DLPSLAADFVESK | f(325–335) | 1390.72 | 1390.70 | 696.37 | 696.36 | 2 | |
| DVFLGMFLY g | f(348–366) | 1119.53 | 1119.53 | 560.77 | 560.77 | 2 | |
| DVFLGMFLYE g | f(348–367) | 1248.57 | 1248.57 | 625.29 | 625.29 | 2 | |
| DVFLGMFLYEYA g | f(348–369) | 1482.70 | 1482.67 | 742.36 | 742.34 | 2 | |
| ENDEMPADLPSLA | f(318–330) | 1400.63 | 1400.61 | 701.32 | 701.31 | 2 | |
| EQLKAVMDDF | f(566–575) | 1176.55 | 1176.55 | 589.28 | 589.28 | 2 | |
| EQLKAVMDDFAA | f(566–577) | 1318.63 | 1318.62 | 660.32 | 660.32 | 2 | |
| EQLKAVMDDFAAF | f(566–578) | 1483.73 | 1483.70 | 742.87 | 742.86 | 2 | |
| EVDETYVPKE | f(516–525) | 1207.56 | 1207.56 | 604.79 | 604.79 | 2 | |
| FDEFKPLVEEPQNLIK | f(398–413) | 1945.04 | 1945.02 | 649.36 | 649.35 | 3 | |
| FKDLGEENFKALV | f(35–47) | 1508.81 | 1508.79 | 755.41 | 755.40 | 2 | |
| FKDLGEENFKALVL | f(35–48) | 1621.91 | 1621.87 | 811.96 | 811.94 | 2 | |
| FKDLGEENFKALVLI | f(35–49) | 1735.00 | 1734.96 | 868.51 | 868.49 | 2 | |
| FKDLGEENFKALVLIA | f(35–50) | 1806.04 | 1805.99 | 904.03 | 904.00 | 2 | |
| FKDLGEENFKALVLIAFAQ | f(35–53) | 2152.24 | 2152.16 | 1077.13 | 1077.09 | 2 | |
| FPKAEFAEVSKLVTDLT | f(247–263) | 1894.01 | 1894.01 | 632.35 | 632.34 | 3 | |
| FYAPELLFFA | f(173–182) | 1216.62 | 1216.62 | 609.32 | 609.32 | 2 | |
| FYAPELLFFAK | f(173–183) | 1344.73 | 1344.71 | 673.37 | 673.36 | 2 | |
| FYAPELLFFAKR | f(173–184) | 1500.79 | 1500.81 | 501.27 | 501.28 | 3 | |
| KDLGEENFKALVLIAFAQ | f(36–53) | 2005.11 | 2005.09 | 669.38 | 669.37 | 3 | |
| KVPQVSTPT | f(438–446) | 955.52 | 955.53 | 478.77 | 478.77 | 2 | |
| KVPQVSTPTLVEV | f(438–450) | 1395.81 | 1395.80 | 698.91 | 698.91 | 2 | |
| KVPQVSTPTLVEVS | f(438–451) | 1482.85 | 1482.83 | 742.43 | 742.42 | 2 | |
| KVPQVSTPTLVEVSRN k | f(438–453) | 1710.00 | 1709.92 | 856.01 | 855.97 | 2 | |
| LQHKDDNPNLPR | f(127–138) | 1445.71 | 1445.74 | 482.91 | 482.92 | 3 | |
| LVNEVTEFAKTCVADESAENCDKSLHTLF c,k | f(66–94) | 3210.60 | 3210.50 | 1071.21 | 1071.17 | 3 | |
| NDEMPADLPSLA | f(319–330) | 1271.58 | 1271.57 | 636.80 | 636.79 | 2 | |
| NDEMPADLPSLAADFVESK | f(319–337) | 2048.01 | 2047.94 | 1025.01 | 1024.98 | 2 | |
| NYAEAKDVFLGMF | f(342–354) | 1503.73 | 1503.71 | 752.87 | 752.86 | 2 | |
| NYAEAKDVFLGMFL g | f(342–355) | 1632.81 | 1632.79 | 817.41 | 817.40 | 2 | |
| RHPDYSVVLLL | f(361–371) | 1310.74 | 1310.73 | 656.38 | 656.37 | 2 | |
| SALEVDETYVPK | f(513–524) | 1349.68 | 1349.67 | 675.85 | 675.84 | 2 | |
| TKKVPQVSTPTLVEVSf | f(436–451) | 1739.99 | 1739.97 | 871.00 | 870.99 | 2 | |
| TYETTLEK | f(376–383) | 983.47 | 983.48 | 492.74 | 492.75 | 2 | |
| VFDEFKPL | f(397–404) | 993.50 | 993.52 | 497.76 | 497.77 | 2 | |
| VFDEFKPLVEEPQ | f(397–409) | 1575.81 | 1575.78 | 788.91 | 788.90 | 2 | |
| VFDEFKPLVEEPQN | f(397–410) | 1689.86 | 1689.82 | 845.94 | 845.92 | 2 | |
| VFDEFKPLVEEPQNL | f(397–411) | 1802.96 | 1802.91 | 902.49 | 902.46 | 2 | |
| VFDEFKPLVEEPQNLI | f(397–412) | 1916.05 | 1915.99 | 959.03 | 959.00 | 2 | |
| VFDEFKPLVEEPQNLIK | f(397–413) | 2044.11 | 2044.09 | 682.38 | 682.37 | 3 | |
| VFDEFKPLVEEPQNLIKQ | f(397–414) | 2172.21 | 2172.15 | 725.08 | 725.06 | 3 | |
| VLIAFAQYL | f(47–55) | 1036.58 | 1036.60 | 519.30 | 519.31 | 2 | |
| VPQVSTPTLVEV | f(439–450) | 1267.71 | 1267.70 | 634.86 | 634.86 | 2 | |
| VPQVSTPTLVEVS | f(439–451) | 1354.75 | 1354.73 | 678.38 | 678.37 | 2 | |
| EQLKAVMDDFA | f(566–576) | 1265.60 | 1265.60 | 633.81 | 633.81 | 2 | |
| YAPELLFFAK | f(174–183) | 1197.65 | 1197.64 | 599.83 | 599.83 | 2 | |
| LVRPEVDV | f(139–146) | 925.51 | 925.52 | 463.76 | 463.77 | 2 | |
| EVDETYVPK | f(516–524) | 1078.51 | 1078.52 | 540.26 | 540.27 | 2 | |
| LSQRFPKAEFAEVSKLVTDLT | f(243–263) | 2379.28 | 2378.28 | 595.83 | 595.58 | 4 | |
| VLIAFAQY | f(47–54) | 923.49 | 923.51 | 462.75 | 462.76 | 2 |
a Deamidated W; b Deamidated Q; c Dioxidation(C); d Cys– > Dha(C); e Trioxidation(C); f Formyl(K); g Oxidation(M); h Glu– > pyro-Glu@N-term; i Deamidated(N); j Dehydrated(S); k Arg– > GluSA(R).
3. Discussion
To date several studies have investigated the quantity of GEP found in the digestive contents of animals [4,22,23] and humans [24,25,26], and the effect of dietary constituents such as protein (and its structure), fiber and anti-nutritional factors on the GEP [27,28,29,30]. It is now widely accepted that GEP contribute significantly to the amino acid pool in the GIT, and consequently are now recognized as important in addition to estimation of the true amino acid requirements of humans [5,31]. However, in spite of the knowledge that up to 80% of the GEP secreted into the gut lumen are digested and reabsorbed by the end of the terminal ileum, the possibility that they may also give rise to bioactive peptides has only recently been considered [1]. Quantitatively, in mammals, GEP are secreted in an equal or greater amount as dietary proteins ingested per day [26]. However, while an extensive range of dietary proteins have been investigated for their ability to generate bioactive peptides during food processing, fermentation and gastrointestinal digestion in vivo, GEP have not been widely investigated. Therefore, analogous to enzymatically-derived dietary bioactive peptides, peptides derived from GEP in the gut lumen may also possess gut-specific bioactivities [2].
The present study used an in vitro digestion model to examine GEP as a source of peptides with physiological effects including heart-health regulatory, GIT health beneficial, anti-inflammatory and weight gain prevention. Four GEP and one dietary protein (chicken albumin) were selected to examine their potential to generation bioactive peptides following simulated GIT digestion. This work demonstrates that in vitro gastric and small intestinal digests of GEP possess biological activity relevant to the GIT and overall human health. These biological activities include potential renin, ACE-I, DPP-IV inhibitory and antioxidant activities.
GEP used in this work included TRYP, LYS, MUC, and SA. These were subjected to simulated, sequential gastric and small intestinal digestion using pepsin and pancreatin (containing proteases from the exocrine cells of the porcine pancreas), while TRYP was only subjected to small intestinal digestion. Pepsin and proteolytic enzymes present in pancreatin digested all of the four GEP as shown using SDS-PAGE (Figure 1), and the extent of digestion generally compared favorably to the protein CA (Figure 1). LYS was not completely digested and this finding is in agreement with a previous study that demonstrated that hen egg lysozyme, which is 57.5% homologous (BLAST identity match) to human lysozyme [32], was only partially digested in the GIT [33].
All of the gastric and small intestinal digests, and the enriched fractions containing peptides <10- and <3-kDa in size inhibited ACE-I when assayed at a concentration of 1 mg/mL and results obtained were comparable to ACE-I inhibition values obtained for captopril and for food derived ACE-I inhibitory peptides identified previously. To ensure that the observed ACE-I inhibition was from the peptides in the tested GEP digests rather than the salts and chemical constituents of the in vitro digestion medium, the samples were desalted by subjecting them to dialysis, and the dialyzed samples also showed inhibition comparable to captopril (data not shown). In comparison, dietary bioactive peptides, from various sources including cereals, legumes, meat, fish and poultry, have been documented to show a wide range of ACE-I inhibition ranging from 1% to ≥90% inhibition [34,35,36]. The ACE-I inhibition activity observed suggests that the enzyme may be inhibited by a broad-spectrum of peptidic inhibitors. From a gut perspective, inhibition of ACE-I is important since it is now known that an extensive RAAS exists within the GIT. While the GIT-RAAS is known to balance the fluid and electrolyte dynamics in the gut, it is not entirely clear as to what the exact role of ACE-I inhibitors may be in the context of the gut. It is important to note that, postprandial or functional hyperemia (an increased blood flow to the gut after a meal (postprandial state)) is a known phenomenon that helps in the digestion of food [37,38], and it may be that the dietary protein- and GEP-derived ACE-I inhibitory peptides play a protective role against a severe rise in local hypertension in the GIT.
Only a few GEP digests including TRYP SI, MUC G, MUC G + SI, SA G and CA G inhibited renin. While the LYS G + SI digest did not inhibit renin the fractions LYS <10-kDa and <3-kDa were found to inhibit renin by 25% and 40% each, indicating that low molecular weight peptides derived from LYS may be responsible for the observed inhibition. In comparison to previously reported values for renin and PAF-AH inhibition by peptides from dietary proteins, inhibition values obtained in the present work were comparable. For example, a previous study investigated the renin inhibition of a papain digest of the red seaweed Palmaria palmata and reported that a reverse phase high pressure liquid chromatography (RP-HPLC) fraction of the latter hydrolysate inhibited renin by 58.97% (±1.26%) when assayed at a concentration of 1 mg/mL [39].
Although the small intestinal digest of LYS and SA inhibited PAF-AH by 5% and 9%, their enriched fractions (LYS < 10-kDa and SA < 3-kDa) inhibited the PAF-AH by 39% and 33% respectively. This suggests that smaller peptides derived from LYS and SA may be more effective inhibitors of PAF-AH. The PAF-AH inhibitory values obtained in this work were comparable to those obtained previously for a papain hydrolysate of Palmaria palmata which inhibited PAF-AH by 30.37% [40].
A similar trend was observed in the case of DPP-IV inhibition by GEP subjected to GI digestion. While the gastric and small intestinal digests of all of the proteins showed either no inhibition or low inhibition values (SA G and SA G + SI), following enrichment of the small intestinal digests a substantial increase in the DPP-IV inhibitory activity was observed. For example, the SA < 3-kDa, SA < 10-kDa, LYS < 10-kDa and LYS < 3-kDa were found to inhibit DPP-IV by greater than 35% at a concentration of 1 mg/mL. In particular, the DPP-IV inhibition exerted by SA < 3-kDa (45% (±1.24%)), is comparable to the DPP-IV inhibition reported for the gastrointestinal digests of oat flour, alcalase digest of oat glutelin and a tryptic digest of highland barley glutelin that were found to have IC50 values of 0.99, 0.13 and 1.83 mg/mL respectively [41]. In relation to gut health, the optimal inhibition of DPP-IV may aid in remediation of non-steroidal anti-inflammatory drug-induced intestinal ulcers [42], by possibly enhancing the beneficial amino acid- and bile acid-induced bicarbonate secretion in the small intestine [43], besides promoting epithelial regrowth [18] and stimulating intestinal growth [44].
The extent of inhibition of the ACE-I, renin, DPP-IV and PAF-AH enzymes observed in the current work is comparable to that of various food protein digests [40,45,46,47]. The inhibition of all of the above mentioned enzymes by peptides is associated with a positive impact on cardiovascular health and reduction of high blood pressure [19,48,49]. However, ACE-I, renin, DPP-IV and PAF-AH are enzymes that are also present in the gut, and recent evidence suggests that inhibition of these enzymes can impact positively on gut health and may help to prevent necrosis, ulcerative colitis and other inflammatory disorders of the human gut [8,9,13,17].
Furthermore, the digested GEP and enriched fractions were also found to have considerable antioxidant activity in vitro. Among the digests that were able to inhibit ABTS ion (TRYP SI, MUC G, MUC G + SI, SA G + SI, and CA G + SI), SA G + SI had the highest total antioxidant capacity value (137 µM·TE/mg), which is much higher than the previously reported values (0.47 µM·TE/mg protein) for the 3-kDa fraction of in vitro digests of human milk [50]. However, the highest TAC-ABTS values obtained for GEP in the present study are significantly lower than the values for human plasma (430–569 µM·TE) which was analyzed for TAC-ABTS using ABTS and hydrogen peroxide [51] previously. In addition, a small increase in the ABTS scavenging was observed after fraction enrichment, particularly for MUC protein. However, in the antioxidant potential measurement using the DPPH radical assay, we found a greater difference between the un-fractionated GEP digest fractions and the enriched fraction of the same GEP. For example, LYS G + SI inhibited DPPH by only 30% (±1.84%) but the <10-kDa fraction of the same sample inhibited DPPH by 54% (±1.62), followed by the SA <3-kDa that also inhibited DPPH by 49% (±1.58%). The antioxidant activity of GEP-derived peptides may be of great importance to gut health as an exclusive dependence on diet-derived antioxidants may not be entirely possible given that our diet varies considerably [1]. The gut undergoes a significant turnover of the epithelial lining and is constantly exposed to the external environment and various oxidants on a daily basis, thus GEP may form an intrinsic source of potential antioxidant peptides that can offset this regular damage.
In this work, the GEP serum albumin and lysozyme were found to inhibit enzymes in all of the in vitro bioassays carried out and the results were comparable to enzyme inhibition values obtained for the dietary protein chicken albumin (CA). In particular, the <10- and <3-kDa fractions of SA and LYS were found to be the most active. Consequently, the latter two fractions were examined further using ESI-TOF MS to characterize the peptides present. Thirty-two peptides were identified in the enriched fractions (10- and 3-kDa) of LYS, and 161 different peptides in the SA fractions. Most of the identified peptides were within the general range of the chain length reported for bioactive peptides (3–30 amino acids) [52,53], but tending towards the longer chain length (7–36 amino acids). Overall, among all of the 22 amino acids, LYS has the highest amounts of the amino acids A (10.8%), R (10.8%) and N (7.7%). In terms of the most commonly found amino acids in bioactive peptide sequences, LYS sequences contained significant amounts of A (10.8%), R (10.8%), G (8.5%), L (6.2%), V (6.9%), Q (4.6%) and Y (4.6%). In the <3 and <10 kDa fractions of LYS, the major amino acids observed were A, V, N, Q and Y. Most of the peptide fractions identified were from the f(62–130) region of the protein, and a few from the f(27–47) region of the protein. In particular, from the LYS fractions that showed the highest DPPH inhibition, we have identified 10 peptide sequences that have an N residue at the N-terminal of the peptide. These results are in agreement with the findings of Rajapakse, et al. [54], who identified the two peptides NADFGLNGLEGLA and NGLEGLK, containing an N-terminal N residue. These peptides, generated from squid, showed the greatest antioxidant effect in an in vitro analysis based on lipid peroxidation studies previously. Overall, the SA protein derived peptides contained the highest amounts (up to 10%) of the amino acids A, E, L, T and K. In terms of the most commonly found amino acids in bioactive peptide sequences, SA was found to be rich in L (10.4%), V (7%), F (5.3%), T (4.8%), P (4.1%) and R (4.1%). We observed the highest frequency of occurrence of peptides with D, E, K, F, L, V, A, R, T and S in the peptides identified in the <3- and <10-kDa fraction of SA. Within the <3- and <10-kDa fraction of SA, over 80 peptide fragments with a hydrophobic or aromatic amino acid residues of A, V, L and F were identified, and the presence of these peptides is known to have a positive impact on the renin inhibition [55] and antioxidant potential of peptides [56,57,58]. Thus, while both the LYS and SA fractions were found to contain peptides with previously identified bioactive amino acids such as A, L, V, R, T and F [54,59,60], the observed range of bioactivity in this study suggests that apart from the constituting amino acids, the peptide structure and certain other amino acids may also play a role in the bioactivity of various hydrolysates.
A majority of the peptides identified in the present study are novel sequences that have not been reported from these GEP previously. However, we found two peptides in the LYS < 3-kDa fractions (DPQGIRAWV (f(120–128)), and PQGIRAWVAW (f(121–130))) that are subsequences [61] of a previously identified antimicrobial sequence reported from human lysozyme DNIADAVACAKRVVRDPQGIRAWVAWRNR (f(87–115)) [35,62]. Future work will specifically aim to chemically synthesize the identified peptides and examine their individual biological activities, which will allow us to determine which particular GEP-derived peptide sequences result in ACE-I, renin, DPP-IV, PAF-AH and antioxidant activity.
The present study, although limited in its scope, due to its in vitro nature, is the first to illustrate that GEP are a source bioactive peptides with in vitro ACE-I, renin, PAF-AH and DPP-IV inhibitory and antioxidant activities. The known amino acid composition of the peptides identified in the present work may have contributed to the observed biological activities in vitro. However, the peptide structure and certain lesser studied amino acids that were also found to be present in abundance may also have positively contributed to the observed in vitro bioactivities. Indeed, some of the food derived bioactive peptides with IC50 values in the range of 100 to 500 µM can be of nutritive/physiological importance in that they can be active after oral administration [63]. It is known that peptides greater than 4 amino acids in size are usually not absorbed into the blood. However, the bioavailability of the peptides in this work should be further studied using in vitro transcytosis assays using cell models with epithelial cell lines including the human epithelial cororectal adenocarcinoma cells (Caco-2) or human brain microvascular endothelial cell lines (hCMEC/D3) as previously reported [64]. Further in vivo work is essential to determine if GEP can indeed give rise to bioactive peptides during their digestion in the gastrointestinal tract. However, the present study points towards the possibility that the GEP may have the potential to constitute a feedback-like peptidergic system within the gut lumen, particularly considering that the GEP are constantly present in the gut and can be modulated by dietary constituents.
4. Experimental Section
4.1. Materials and Reagents
Human recombinant LYS (P61626), human recombinant SA (P02768), porcine TRYP (P00761), porcine MUC (Apomucin; (P12021)), CA (P01012), dimethyl sulfoxide, porcine salivary amylase, porcine pepsin and porcine pancreatin, the antioxidant resveratrol and the ACE-I inhibitor captopril, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and all of the tris-tricine SDS-PAGE reagents were supplied by Sigma Aldrich (Dublin, Ireland). The ACE-I inhibition assay kit was supplied by Dojindo EU, GmbH (Kumamoto, Japan), the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) inhibition based total antioxidant capacity assay kit was supplied by BioVision Inc. (Milpitas, CA, USA), the ABTS total antioxidant capacity assay kit was supplied by Zen-Bio, Inc. (Research Triangle Park, NC, USA) and the renin, PAF-AH and DPP-IV inhibition assay kits were all supplied by the Cayman Chemical Company (Cambridge BioSciences, Cambridge, UK). The molecular weight cut-off filters were obtained from Millipore (Tullagreen, Carrigtwohill, Cork city, Ireland). The 3.5 kDa dialysis membrane was supplied by the medical supply company (Medical supply company (MSC), Dublin, Ireland). All other chemicals used were of analytical grade (≥99% purity). Unless otherwise stated, all reagents were made using Milli-Q deionized water.
4.2. Simulated Gastrointestinal Digestion of Selected Gastrointestinal Endogenous Proteins (GEP) and a Food Protein Comparator
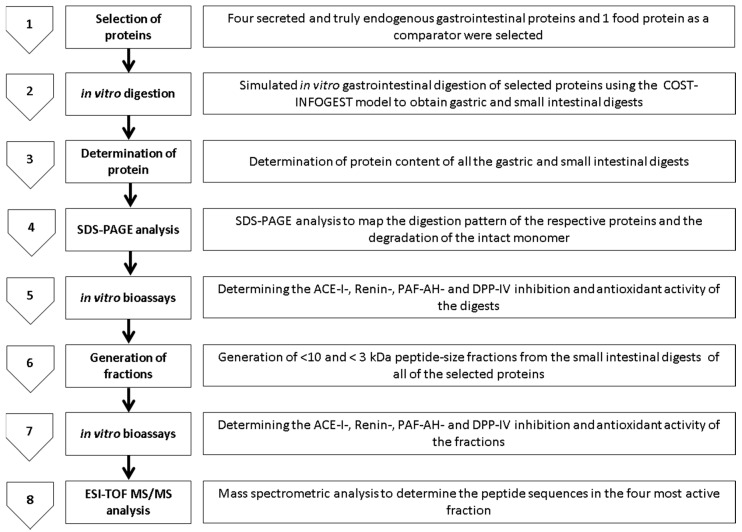
Four secreted endogenous gut proteins were selected for the present study, namely, porcine trypsin (TRYP), human recombinant lysozyme (LYS), porcine salivary mucin (MUC) and serum albumin (SA). Chicken albumin (CA) was included in the study for the purpose of comparison, as it is a known source of food-derived bioactive peptides [65,66,67,68]. The molecular weights and the chain lengths of the proteins examined in the present study are presented in Table 3.
Table 3.
Gastrointestinal endogenous proteins (GEP) and dietary protein examined in the in vitro study.
| Protein | Chain Length | Molecular Weight (Mw) (KDa) | Uniprot Accession No. |
|---|---|---|---|
| Gut cryptome protein | – | – | – |
| Trypsin (TRYP) | 223 | 24 | P00761 |
| Lysozyme (LYS) | 148 | 17 | P61626 |
| Porcine salivary mucin (Apomucin) (MUC) | 1150 | 110 | P12021 |
| Serum albumin (SA) | 609 | 69 | P02768 |
| Dietary protein | – | – | – |
| Chicken albumin (CA) | 385 | 43 | P01012 |
All of the studied proteins were subjected to simulated static in vitro gastrointestinal digestion using the INFOGEST method described previously [6]. Selected proteins were subjected to sequential gastric and small intestinal digestions with the exception of TRYP. TRYP was subjected only to small intestinal digestion as it enters the GIT lumen at the duodenum [69]. The simulated in vitro sequential digestion was performed in triplicate. Samples (0.5 mL × 3) of the hydrolysates of the chosen GEP at a concentration of 0.5–0.0125 g/mL were collected after the gastric phase (G) and small intestinal phase (G + SI) of digestion, freeze-dried and stored at −30 °C until further analysis. The overall methodology and major steps followed in the present study are shown in Figure 8. Furthermore, MWCO-based ultra-filtration was applied to all of the small intestinal in vitro digests, to obtain <10- and <3-kDa sized peptide-enriched fractions (labelled as TRYP/LYS/MUC/SA/CA <3- and <-10 kDa respectively). All of the samples were desalted using a 3.5 kDa dialysis membrane.
Figure 8.
The in vitro experimental design and methodologies used to study the angiotensin I converting enzyme (ACE-I), renin, Platelet-activating factor-acetylhydrolase (PAF-AH) and dipeptidyl peptidase IV (DPP-IV) inhibition, and Total antioxidant capacity by 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) inhibition based antioxidant activity of the gastric and small intestinal hydrolysates and the small intestinal <10- and <3-kDA fractions of gastrointestinal endogenous proteins porcine trypsin, porcine salivary mucin, human recombinant lysozyme and serum albumin, and food protein chicken albumin. SDS-PAGE: Tris tricine sodium dodecyl sulphate polyacrylamide gel electrophoresis; ESI-TOF MS/MS: Electrospray ionization time of flight mass spectrometry.
4.3. Total Protein Analysis
The total protein content was determined using the Dumas method in accordance with the AOAC method 992.15 (1990) and using a LECO FP628 Protein analyzer (LECO Corp., St. Joseph, MI, USA). A conversion factor of 6.25 was used to estimate the protein content from the nitrogen content.
4.4. Tris Tricine Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
Tris tricine SDS-PAGE peptide analysis was carried out using previously described methods [70,71]. GEP protein samples were diluted 1:10 with reducing PAGE sample buffer (2% or 4% SDS and 100 mM dithiothreitol (DTT)) to obtain a concentration of 1 mg/mL. 5–10 µL of each diluted sample was loaded onto a SDS-PAGE resolving gel (49.5% T, 3% C). The components of the resolving and stacking gels are given in supplementary Table S1.
The loaded gel was run in a Bio-Rad Laboratories Miniprotean gel apparatus at a voltage of 100 V for 2 h or until the dye front approached the bottom of the gel. Gels were stained using Coomassie brilliant blue R250 (0.3%) for 1 h at 20 °C. Destaining was carried out using 10% v/v acetic acid and 10% v/v 2-propanol. Gels were destained for 48 h at 20 °C, with gentle shaking. Gels were scanned on a molecular Gel Doc XR system (Bio-Rad Laboratories, Berkeley, CA, USA) and the gel images were analyzed using QuantityOne and ImageLab software programs (Bio-Rad Laboratories, Berkeley, CA, USA).
4.5. ACE-I Inhibition Assay
This assay was carried out using an ACE-I inhibitor assay kit in accordance with the manufacturer’s instructions. All fractions were assayed at a concentration of 1 mg/mL sample dissolved in HPLC grade water in triplicate. The known ACE-I inhibitor Captopril was used as a positive control at a concentration of 1 mg/mL. ACE-I inhibition percentage values for each peptide were calculated using the absorbance values obtained at 450 nm and the equation:
| % ACE-I inhibition = (Absorbance of Blank 1 − Absorbance value of the Inhibitor)/(Absorbance of Blank 1 − Blank 2) × 100 |
where, blank 1 is control without the addition of any inhibitor, blank 2 is the reagent blank, and the inhibitor is the positive control or test sample (protein digest/peptide fraction).
4.6. Renin Inhibition Assay
The renin inhibition assay was carried out using a renin inhibitor screening kit in accordance with the manufacturer’s instructions. Z-Arg-Arg-Pro-Phe-His-Sta-Ile-His-Lys-(Boc)-Ome (Sigma Aldrich, Dublin, Ireland), a known renin inhibitor, was used as a positive control at a concentration of 10 µM. The % inhibition of renin was calculated using the fluorescence values obtained and the following formula:
| % renin inhibition = (100% Initial activity values (AF) − fluorescence value obtained for the test sample)/100% initial activity (AF) × 100 |
where, test sample is protein digest/peptide fraction.
4.7. PAF-AH Inhibition Assay
This assay was performed using a PAF-AH inhibitor kit supplied by Cambridge BioSciences, Cambridge, UK in accordance with the manufacturer’s instructions. All of the digests and enriched fractions were assessed in triplicate. Methyl arachidonyl fluorophosphonate (MAFP), a known PAF-AH inhibitor was used as a positive control at a concentration of 260 ng/mL.
4.8. DPP-IV Inhibition
The DPP-IV inhibitory activities of the digested protein samples were determined using a DPP-IV inhibitor assay kit (Cambridge BioSciences, Cambridge, UK) in accordance with the manufacturer’s instructions. Sitagliptin, a known DPP-IV inhibitor, was used as a positive control at a concentration of 100 µM. The percentage inhibition of DPP-IV was calculated as follows:
| % Inhibition of DPP-IV = (RFU DPP-IV activity − RFU Inhibitor)/(RFU DPP−IV activity) × 100 |
where, RFU DPP-IV activity is the fluorescence (measured in relative fluorescence units (RFU)) of the measured without addition of any inhibitor, and RFU Inhibitor is the RFU measured in the presence of the Sitagliptin or the test digests or enriched fractions.
4.9. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS)-Based Total Antioxidant Capacity (ABTS-TAC) Assay
The assay was carried out using an ABTS antioxidant assay kit and performed as described by the manufacturer. Trolox was used to obtain a standard curve and the assay buffer was used as a blank. Resveratrol was used as a positive control. Antioxidant activity was calculated using the trolox standard curve and expressed as µM trolox equivalents (TE).
4.10. DPPH Inhibition
The DPPH inhibition assay was performed in accordance with previously described methods [72,73]. Briefly, triplicates of the digest or enriched fraction samples (1 mg/mL) and the positive control (resveratrol) were prepared in the assay buffer. The samples and positive control solutions (285 µL) were each combined with a freshly prepared methanolic DPPH solution in a Nunc™ 96-well microWell™ plate to obtain a final DPPH concentration of 100 µM. The plate was incubated in dark at room temperature for 30 min. The absorbance was then read at 515 nm. The control reaction mixture contained DPPH and methanol without sample. The percentage DPPH inhibition was calculated using the following equation:
| % Inhibition DPPH = (Abs Control − Abs Inhibitor)/Abs Control × 100 |
where, Abs Control is the absorbance of the DPPH solution without any resveratrol or test peptides and Abs Inhibitor is the absorbance of the test samples or positive control.
4.11. Electrospray Ionization Time of Flight Mass Spectrometry (ESI-TOF MS) Characterisation of Peptides Present in the Most Active Fractions
The four fractions which showed the greatest extent of renin, DPP-IV, PAF-AH inhibition and antioxidant activities were selected for peptide characterization. Briefly, the nano LC-MS/MS analysis was performed using an Eksigent Nano-LC Ultra 1D Plus system (Eksigent of AB Sciex, Framingham, CA, USA) coupled to the quadrupole-time-of-flight (Q-ToF) TripleTOF® 5600 system from AB Sciex Instruments (Framingham, MA, USA) that was equipped with a nano-electrospray ionization source.
Lyophilized samples were re-suspended in 500 µL of H2O with 0.1% of TFA, and 20 µL of each sample at different times of processing were cleaned and concentrated using Zip-Tip C18 with standard bed format (Millipore Corporation, Bedford, MA, USA) according to manufacturer’s guidelines, and kept at −20 °C until analysis. Then, 5 µL of the supernatant was injected into the LC-MS/MS system.
After 5 min of pre-concentration, the trap column was automatically switched in-line onto a nano-HPLC capillary column (3 µm, 75 µm × 12.3 cm, C18) (Nikkyo Technos Co, Ltd., Tokyo, Japan). Mobile phase A contained 0.1% v/v formic acid in water, and mobile phase B, contained 0.1% v/v FA in 100% acetonitrile. A linear gradient from 5%–35% of solvent B over 90 min, and 10 min from 35%–65% of solvent B, at a flow rate of 0.3 μL/min and a column temperature of 30 °C was used. The column outlet was directly coupled to a nano-electrospray ion (nESI) source. Operating conditions for the Q/ToF mass spectrometer were positive polarity and information-dependent acquisition (IDA) mode was used. A 0.25-s ToF MS scan from m/z of 100 to 1200 was performed, followed by 0.05-s product ion scans from m/z of 100 to 1500 on the 50 most intense 1+ to 5+ charged ions. Automated spectral processing, peak list generation, database search, and de novo sequencing for the identification of peptides were performed using Mascot Distiller v2.4.2.0 software (Matrix Science, Inc., Boston, MA, USA) (hppt://www.matrixscience.com). The UniProt protein database was used to identify the peptides with a significance threshold p < 0.1 and a False Discovery Rate of 1.5%. The taxonomy used for identification in the database was homo sapiens. The tolerance on the mass measurement was 0.3 Da in MS mode and MS/MS ions.
Acknowledgments
The authors acknowledge the financial support provided by the Centre of Research Excellence (CoRE) fund from the Tertiary Education Commission and the Ministry of Education, New Zealand. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| ACE-I | angiotensin-I Converting Enzyme |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DPP-IV | dipeptidyl peptidase-IV |
| GEP | Gastrointestinal endogenous proteins |
| GLP-2 | Glucagon-like peptide-2 |
| MAFP | Methyl arachidonyl fluorophosphonate |
| MWCO | Molecular weight cut-off |
| PAF-AH | platelet-activating factor-acetylhydrolase |
| RAAS | renin angiotensin aldosterone system |
| SDS-PAGE | sodium dodecyl sulphate polyacrylamide gel electrophoresis |
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/17/4/482/s1.
Author Contributions
Maria Hayes and Lakshmi A. Dave conceived and designed the experiments; Lakshmi A. Dave performed the experiments; Leticia Mora performed the ESI-TOF MS analysis; Lakshmi A. Dave and Maria Hayes analyzed the data; Maria Hayes contributed reagents, materials and analysis tools; Lakshmi A. Dave wrote the first draft of the manuscript. Shane M. Rutherfurd, Paul J. Moughan, Maria Hayes and Carlos A. Montoya provided critical contributions to the draft.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Moughan P.J., Rutherfurd S.M., Montoya C.A., Dave L.A. Food-derived bioactive peptides—A new paradigm. Nutr. Res. Rev. 2014;27:16–20. doi: 10.1017/S0954422413000206. [DOI] [PubMed] [Google Scholar]
- 2.Dave L.A., Hayes M., Montoya C.A., Rutherfurd S.M., Moughan P.J. Human gut endogenous proteins as a potential source of angiotensin-I-converting enzyme (ACE-I)-, renin inhibitory and antioxidant peptides. Peptides. 2016;76:30–44. doi: 10.1016/j.peptides.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Dave L.A., Montoya C.A., Rutherfurd S.M., Moughan P.J. Gastrointestinal endogenous proteins as a source of bioactive peptides—An in silico study. PLoS ONE. 2014;9:482. doi: 10.1371/journal.pone.0098922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miner-Williams W., Moughan P.J., Fuller M.F. Endogenous components of digesta protein from the terminal ileum of pigs fed a casein-based diet. J. Agric. Food Chem. 2009;57:2072–2078. doi: 10.1021/jf8023886. [DOI] [PubMed] [Google Scholar]
- 5.Moughan P.J., Rutherfurd S.M. Gut luminal endogenous protein: Implications for the determination of ileal amino acid digestibility in humans. Br. J. Nutr. 2012;108(Suppl. S2):S258–S263. doi: 10.1017/S0007114512002474. [DOI] [PubMed] [Google Scholar]
- 6.Minekus M., Alminger M., Alvito P., Ballance S., Bohn T., Bourlieu C., Carriere F., Boutrou R., Corredig M., Dupont D., et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014;5:1113–1124. doi: 10.1039/c3fo60702j. [DOI] [PubMed] [Google Scholar]
- 7.D’Alessio D.A. What if gut hormones aren’t really hormones: DPP-4 inhibition and local action of GLP-1 in the gastrointestinal tract. Endocrinology. 2011;152:2925–2926. doi: 10.1210/en.2011-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pucar L.B., Detel D., Varljen J. Dipeptidyl peptidase IV in inflammatory bowel diseases (DPP IV/CD26) Arhiv. Ind. Hyg. Toxicol. 2012;63:75–100. doi: 10.2478/10004-1254-63-2012-2185. [DOI] [PubMed] [Google Scholar]
- 9.Carl-McGrath S., Grantzdorffer I., Lendeckel U., Ebert M.P., Rocken C. Angiotensin II-generating enzymes, angiotensin-converting enzyme (ACE) and mast cell chymase (CMA1), in gastric inflammation may be regulated by H. Pylori and associated cytokines. Pathology. 2009;41:419–427. doi: 10.1080/00313020902885037. [DOI] [PubMed] [Google Scholar]
- 10.Kanasaki K., Shi S., Kanasaki M., He J., Nagai T., Nakamura Y., Ishigaki Y., Kitada M., Srivastava S.P., Koya D. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes. 2014;63:2120–2131. doi: 10.2337/db13-1029. [DOI] [PubMed] [Google Scholar]
- 11.Coates D. The angiotensin converting enzyme (ACE) Int. J. Biochem. Cell Biol. 2003;35:769–773. doi: 10.1016/S1357-2725(02)00309-6. [DOI] [PubMed] [Google Scholar]
- 12.Fandriks L. The renin-angiotensin system and the gastrointestinal mucosa. Acta Physiol. (Oxf.) 2011;201:157–167. doi: 10.1111/j.1748-1716.2010.02165.x. [DOI] [PubMed] [Google Scholar]
- 13.Garg M., Angus P.W., Burrell L.M., Herath C., Gibson P.R., Lubel J.S. Review article: The pathophysiological roles of the renin-angiotensin system in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2012;35:414–428. doi: 10.1111/j.1365-2036.2011.04971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H., Tan X.-D., Qu X.-W., Chang H., Remick D.G., Gonzalez-Crussi F., Hsueh W. Platelet-activating factor (PAF) up-regulates plasma and tissue PAF-acetylhydrolase activity in the rat: Effect of cycloheximide. Pediatr. Res. 1997;42:597–603. doi: 10.1203/00006450-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Packard C.J., O’Reilly D.S.J., Caslake M.J., McMahon A.D., Ford I., Cooney J., Macphee C.H., Suckling K.E., Krishna M., Wilkinson F.E., et al. Lipoprotein-associated phospholipase a2 as an independent predictor of coronary heart disease. N. Engl. J. Med. 2000;343:1148–1155. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 16.Sudhir K. Lipoprotein-associated phospholipase A2, a novel inflammatory biomarker and independent risk predictor for cardiovascular disease. J. Clin. Endocrinol. Metab. 2005;90:3100–3105. doi: 10.1210/jc.2004-2027. [DOI] [PubMed] [Google Scholar]
- 17.Shi Y., Zhang P., Zhang L., Osman H., Mohler Iii E.R., Macphee C., Zalewski A., Postle A., Wilensky R.L. Role of lipoprotein-associated phospholipase A2 in leukocyte activation and inflammatory responses. Atherosclerosis. 2007;191:54–62. doi: 10.1016/j.atherosclerosis.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Okawada M., Holst J.J., Teitelbaum D.H. Administration of a DPP-IV inhibitor enhances the intestinal adaptation in a mouse model of short bowel syndrome. Surgery. 2011;150:217–223. doi: 10.1016/j.surg.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salles T.A., dos Santos L., Barauna V.G., Girardi A.C. Potential role of dipeptidyl peptidase IV in the pathophysiology of heart failure. Int. J. Mol. Sci. 2015;16:4226–4249. doi: 10.3390/ijms16024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinehr T., Roth C.L., Enriori P.J., Masur K. Changes of dipeptidyl peptidase IV (DPP-IV) in obese children with weight loss: Relationships to peptide YY, pancreatic peptide, and insulin sensitivity. J. Pediatr. Endocrinol. Metab. 2010;23:101–108. doi: 10.1515/JPEM.2010.23.1-2.101. [DOI] [PubMed] [Google Scholar]
- 21.Halliwell B., Zhao K., Whiteman M. The gastrointestinal tract: A major site of antioxidant action? Free Radic. Res. 2000;33:819–830. doi: 10.1080/10715760000301341. [DOI] [PubMed] [Google Scholar]
- 22.Hodgkinson S.M., Moughan P.J., Reynolds G.W., James K.A. The effect of dietary peptide concentration on endogenous ileal amino acid loss in the growing pig. Br. J. Nutr. 2000;83:421–430. [PubMed] [Google Scholar]
- 23.Montagne L., Toullec R., Lalles J.P. Intestinal digestion of dietary and endogenous proteins along the small intestine of calves fed soybean or potato. J. Anim. Sci. 2001;79:2719–2730. doi: 10.2527/2001.79102719x. [DOI] [PubMed] [Google Scholar]
- 24.Baglieri A., Mahe S., Benamouzig R., Savoie L., Tome D. Digestion patterns of endogenous and different exogenous proteins affect the composition of intestinal effluents in humans. J. Nutr. 1995;125:1894–1903. doi: 10.1093/jn/125.7.1894. [DOI] [PubMed] [Google Scholar]
- 25.Miner-Williams W., Deglaire A., Benamouzig R., Fuller M.F., Tome D., Moughan P.J. Endogenous proteins in terminal ileal digesta of adult subjects fed a casein-based diet. Am. J. Clin. Nutr. 2012;96:508–515. doi: 10.3945/ajcn.111.033472. [DOI] [PubMed] [Google Scholar]
- 26.Miner-Williams W., Deglaire A., Benamouzig R., Fuller M.F., Tomé D., Moughan P.J. Endogenous proteins in the ileal digesta of adult humans given casein-, enzyme-hydrolyzed casein-or crystalline amino-acid-based diets in an acute feeding study. Eur. J. Nutr. 2014;68:363–369. doi: 10.1038/ejcn.2013.270. [DOI] [PubMed] [Google Scholar]
- 27.Deglaire A., Moughan P.J., Rutherfurd S.M., Bos C., Tome D. Feeding dietary peptides to growing rats enhances gut endogenous protein flows compared with feeding protein-free or free amino acid-based diets. J. Nutr. 2007;137:2431–2436. doi: 10.1093/jn/137.11.2431. [DOI] [PubMed] [Google Scholar]
- 28.Gilani G.S., Cockell K.A., Sepehr E. Effects of antinutritional factors on protein digestibility and amino acid availability in foods. J. AOAC Int. 2005;88:967–987. [PubMed] [Google Scholar]
- 29.Rutherfurd S.M., Cui J., Goroncy A.K., Moughan P.J. Dietary protein structure affects endogenous ileal amino acids but not true ileal amino acid digestibility in growing male rats. J. Nutr. 2015;145:193–198. doi: 10.3945/jn.114.198283. [DOI] [PubMed] [Google Scholar]
- 30.Ouellet D.R., Demers M., Zuur G., Lobley G.E., Seoane J.R., Nolan J.V., Lapierre H. Effect of dietary fiber on endogenous nitrogen flows in lactating dairy cows. J. Dairy Sci. 2002;85:3013–3025. doi: 10.3168/jds.S0022-0302(02)74387-7. [DOI] [PubMed] [Google Scholar]
- 31.Rutherfurd S.M., Fanning A.C., Miller B.J., Moughan P.J. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J. Nutr. 2015;145:372–379. doi: 10.3945/jn.114.195438. [DOI] [PubMed] [Google Scholar]
- 32.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 33.Jiménez-Saiz R., Benedé S., Miralles B., López-Expósito I., Molina E., López-Fandiño R. Immunological behavior of in vitro digested egg-white lysozyme. Mol. Nutr. Food Res. 2014;58:614–624. doi: 10.1002/mnfr.201300442. [DOI] [PubMed] [Google Scholar]
- 34.Iwaniak A., Minkiewicz P., Darewicz M. Food-originating ace inhibitors, including antihypertensive peptides, as preventive food components in blood pressure reduction. Compr. Rev. Food Sci. Food Saf. 2014;13:114–134. doi: 10.1111/1541-4337.12051. [DOI] [PubMed] [Google Scholar]
- 35.BIOPEP. [(accessed on 25 August 2015)]. Available online: http://www.uwm.edu.pl/biochemia/index.php/en/biopep.
- 36.Minkiewicz P., Dziuba J., Iwaniak A., Dziuba M., Darewicz M. Biopep database and other programs for processing bioactive peptide sequences. J. AOAC Int. 2008;91:965–980. [PubMed] [Google Scholar]
- 37.Kvietys P.R. The Gastrointestinal Circulation. Morgan & Claypool Life Sciences; San Rafael, CA, USA: 2010. Chapter 5: Postprandial hyperemia. [PubMed] [Google Scholar]
- 38.Kvietys P.R., McLendon J.M., Granger D.N. Postprandial intestinal hyperemia: Role of bile salts in the ileum. Am. J. Physiol. Gastrointest. Liver Physiol. 1981;241:G469–G477. doi: 10.1152/ajpgi.1981.241.6.G469. [DOI] [PubMed] [Google Scholar]
- 39.Fitzgerald C., Mora-Soler L., Gallagher E., O’Connor P., Prieto J., Soler-Vila A., Hayes M. Isolation and characterization of bioactive pro-peptides with in vitro renin inhibitory activities from the macroalga palmaria palmata. J. Agric. Food Chem. 2012;60:7421–7427. doi: 10.1021/jf301361c. [DOI] [PubMed] [Google Scholar]
- 40.Fitzgerald C., Gallagher E., O’Connor P., Prieto J., Mora-Soler L., Grealy M., Hayes M. Development of a seaweed derived platelet activating factor acetylhydrolase (PAF-AH) inhibitory hydrolysate, synthesis of inhibitory peptides and assessment of their toxicity using the zebrafish larvae assay. Peptides. 2013;50:119–124. doi: 10.1016/j.peptides.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Wang F., Yu G., Zhang Y., Zhang B., Fan J. Dipeptidyl peptidase IV inhibitory peptides derived from oat (avena sativa L.), buckwheat (fagopyrum esculentum), and highland barley (hordeum vulgare trifurcatum (L.) trofim) proteins. J. Agric. Food Chem. 2015;63:9543–9549. doi: 10.1021/acs.jafc.5b04016. [DOI] [PubMed] [Google Scholar]
- 42.Inoue T., Higashiyama M., Kaji I., Rudenkyy S., Higuchi K., Guth P.H., Engel E., Kaunitz J.D., Akiba Y. Dipeptidyl peptidase IV inhibition prevents the formation and promotes the healing of indomethacin-induced intestinal ulcers in rats. Dig. Dis. Sci. 2014;59:1286–1295. doi: 10.1007/s10620-013-3001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inoue T., Wang J.-H., Higashiyama M., Rudenkyy S., Higuchi K., Guth P.H., Engel E., Kaunitz J.D., Akiba Y. Dipeptidyl peptidase IV inhibition potentiates amino acid- and bile acid-induced bicarbonate secretion in rat duodenum. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;303:G810–G816. doi: 10.1152/ajpgi.00195.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sueyoshi R., Woods Ignatoski K.M., Okawada M., Hartmann B., Holst J., Teitelbaum D.H. Stimulation of intestinal growth and function with DPP4 inhibition in a mouse short bowel syndrome model. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;307:G410–G419. doi: 10.1152/ajpgi.00363.2013. [DOI] [PubMed] [Google Scholar]
- 45.Lafarga T., Rai D.K., O’Connor P., Hayes M. A bovine fibrinogen-enriched fraction as a source of peptides with in vitro renin and angiotensin-i-converting enzyme inhibitory activities. J. Agric. Food Chem. 2015;63:8676–8684. doi: 10.1021/acs.jafc.5b03167. [DOI] [PubMed] [Google Scholar]
- 46.Darewicz M., Borawska J., Vegarud G.E., Minkiewicz P., Iwaniak A. Angiotensin I-converting enzyme (ACE) inhibitory activity and ACE inhibitory peptides of salmon (salmo salar) protein hydrolysates obtained by human and porcine gastrointestinal enzymes. Int. J. Mol. Sci. 2014;15:14077–14101. doi: 10.3390/ijms150814077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin Y., Yan J., Yu Y., Qi Y. Screening and identification of DPP-IV inhibitory peptides from deer skin hydrolysates by an integrated approach of lC–MS/MS and in silico analysis. J. Funct. Foods Part A. 2015;18:344–357. doi: 10.1016/j.jff.2015.07.015. [DOI] [Google Scholar]
- 48.Atlas S.A. The renin-angiotensin aldosterone system: Pathophysiological role and pharmacologic inhibition. J. Manag. Care Pharm. 2007;13:9–20. doi: 10.18553/jmcp.2007.13.s8-b.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samsamshariat S., Basati G., Movahedian A., Pourfarzam M., Sarrafzadegan N. Elevated plasma platelet-activating factor acetylhydrolase activity and its relationship to the presence of coronary artery disease. J. Res. Med. Sci. 2011;16:674–679. [PMC free article] [PubMed] [Google Scholar]
- 50.Hernández-Ledesma B., Quirós A., Amigo L., Recio I. Identification of bioactive peptides after digestion of human milk and infant formula with pepsin and pancreatin. Int. Dairy J. 2007;17:42–49. doi: 10.1016/j.idairyj.2005.12.012. [DOI] [Google Scholar]
- 51.Kambayashi Y., Binh N.T., Asakura H.W., Hibino Y., Hitomi Y., Nakamura H., Ogino K. Efficient assay for total antioxidant capacity in human plasma using a 96-well microplate. J. Clin. Biochem. Nutr. 2009;44:46–51. doi: 10.3164/jcbn.08-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moller N.P., Scholz-Ahrens K.E., Roos N., Schrezenmeir J. Bioactive peptides and proteins from foods: Indication for health effects. Eur. J. Nutr. 2008;47:171–182. doi: 10.1007/s00394-008-0710-2. [DOI] [PubMed] [Google Scholar]
- 53.Shahidi F., Zhong Y. Bioactive peptides. J. AOAC Int. 2008;91:914–931. [PubMed] [Google Scholar]
- 54.Rajapakse N., Mendis E., Byun H.G., Kim S.K. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J. Nutr. Biochem. 2005;16:562–569. doi: 10.1016/j.jnutbio.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Girgih A.T., Alashi A.M., He R., Malomo S.A., Raj P., Netticadan T., Aluko R.E. A novel hemp seed meal protein hydrolysate reduces oxidative stress factors in spontaneously hypertensive rats. Nutrition. 2014;6:5652–5666. doi: 10.3390/nu6125652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chi C.-F., Hu F.-Y., Wang B., Li Z.-R., Luo H.-Y. Influence of amino acid compositions and peptide profiles on antioxidant capacities of two protein hydrolysates from skipjack tuna (katsuwonus pelamis) dark muscle. Mar. Drugs. 2015;13:2580–2601. doi: 10.3390/md13052580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitts D.D., Weiler K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharm. Des. 2003;9:1309–1323. doi: 10.2174/1381612033454883. [DOI] [PubMed] [Google Scholar]
- 58.Chen H.M., Muramoto K., Yamauchi F. Structural analysis of antioxidative peptides from soybean β-conglycinin. J. Agric. Food Chem. 1995;43:574–578. doi: 10.1021/jf00051a004. [DOI] [Google Scholar]
- 59.Udenigwe C.C., Aluko R.E. Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int. J. Mol. Sci. 2011;12:3148–3161. doi: 10.3390/ijms12053148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu J., Aluko R.E., Nakai S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: Quantitative structure-activity relationship study of di- and tripeptides. J. Agric. Food Chem. 2006;54:732–738. doi: 10.1021/jf051263l. [DOI] [PubMed] [Google Scholar]
- 61.Minkiewicz P., Darewicz M., Iwaniak A., Sokołowska J., Starowicz P., Bucholska J., Hrynkiewicz M. Common amino acid subsequences in a universal proteome—Relevance for food science. Int. J. Mol. Sci. 2015;16:20748. doi: 10.3390/ijms160920748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ibrahim H.R., Thomas U., Pellegrini A. A helix-loop-helix peptide at the upper lip of the active site cleft of lysozyme confers potent antimicrobial activity with membrane permeabilization action. J. Biol. Chem. 2001;276:43767–43774. doi: 10.1074/jbc.M106317200. [DOI] [PubMed] [Google Scholar]
- 63.FitzGerald R.J., Meisel H. Milk protein-derived peptide inhibitors of angiotensin-I-converting enzyme. Br. J. Nutr. 2000;84:33–37. doi: 10.1017/S0007114500002221. [DOI] [PubMed] [Google Scholar]
- 64.Hayes M., Moens L.F., Auty M.A.E., Lea T.E. Transport of a prolyl endopeptidase inhibitory peptide across the blood brain barrier demonstrated using the hCMEC/D3 cell line transcytosis assay. J. Agric. Food Chem. 2016;64:146–150. doi: 10.1021/acs.jafc.5b04696. [DOI] [PubMed] [Google Scholar]
- 65.Fujita H., Usui H., Kurahashi K., Yoshikawa M. Isolation and characterization of ovokinin, a bradykinin B1 agonist peptide derived from ovalbumin. Peptides. 1995;16:785–790. doi: 10.1016/0196-9781(95)00054-N. [DOI] [PubMed] [Google Scholar]
- 66.Miguel M., Aleixandre A. Antihypertensive peptides derived from egg proteins. J. Nutr. 2006;136:1457–1460. doi: 10.1093/jn/136.6.1457. [DOI] [PubMed] [Google Scholar]
- 67.Nimalaratne C., Bandara N., Wu J. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed chicken egg white. Food Chem. 2015;188:467–472. doi: 10.1016/j.foodchem.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 68.Oda A., Kaneko K., Mizushige T., Lazarus M., Urade Y., Ohinata K. Characterization of ovolin, an orally active tryptic peptide released from ovalbumin with anxiolytic-like activity. J. Neurochem. 2012;122:356–362. doi: 10.1111/j.1471-4159.2012.07777.x. [DOI] [PubMed] [Google Scholar]
- 69.Voet D., Voet J.G., Pratt C.W. Fundamentals of Biochemistry: Life at the Molecular Level. 4th ed. Wiley; Hoboken, NJ, USA: 2013. [Google Scholar]
- 70.Schagger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 71.Stryer L. Biochemistry. 4th ed. W. H. Freeman and Company; New York, NY, USA: 1995. [Google Scholar]
- 72.Nicklisch S.C.T., Waite J.H. Optimized DPPH assay in a detergent-based buffer system for measuring antioxidant activity of proteins. MethodsX. 2014;1:233–238. doi: 10.1016/j.mex.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goupy P., Dufour C., Loonis M., Dangles O. Quantitative kinetic analysis of hydrogen transfer reactions from dietary polyphenols to the DPPH radical. J. Agric. Food Chem. 2003;51:615–622. doi: 10.1021/jf025938l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.