Abstract
Three gedunin-type limonoids, gedunin (1), 6α-acetoxygedunin (2), and 7-deacetoxy-7-oxogedunin (3), which were isolated from the seed and flower oils of andiroba (Carapa guianensis Aublet, Meliaceae), exhibited hepatoprotective effects at doses of 25 mg/kg, p.o. against d-galactosamine (d-GalN)/lipopolysaccharide (LPS)-induced liver injury in mice. To characterize the mechanisms of action of 1–3 and clarify the structural requirements for their hepatoprotective effects, 17 related limonoids (1–17) isolated from the seed and/or flower oils of C. guianensis were examined in in vitro studies assessing their effects on (i) d-GalN-induced cytotoxicity in primary cultured mouse hepatocytes, (ii) LPS-induced nitric oxide (NO) production in mouse peritoneal macrophages, and (iii) tumor necrosis factor-α (TNF-α)-induced cytotoxicity in L929 cells. The mechanisms of action of 1–3 are likely to involve the inhibition of LPS-induced macrophage activation and reduced sensitivity of hepatocytes to TNF-α; however, these compounds did not decrease the cytotoxicity caused by d-GalN. In addition, the structural requirements of limonoids (1–17) for inhibition of LPS-induced NO production in mouse peritoneal macrophages and TNF-α-induced cytotoxicity in L929 cells were evaluated.
Keywords: hepatoprotective effect, limonoid, andiroba, Carapa guianensis, Meliaceae, structural requirement
1. Introduction
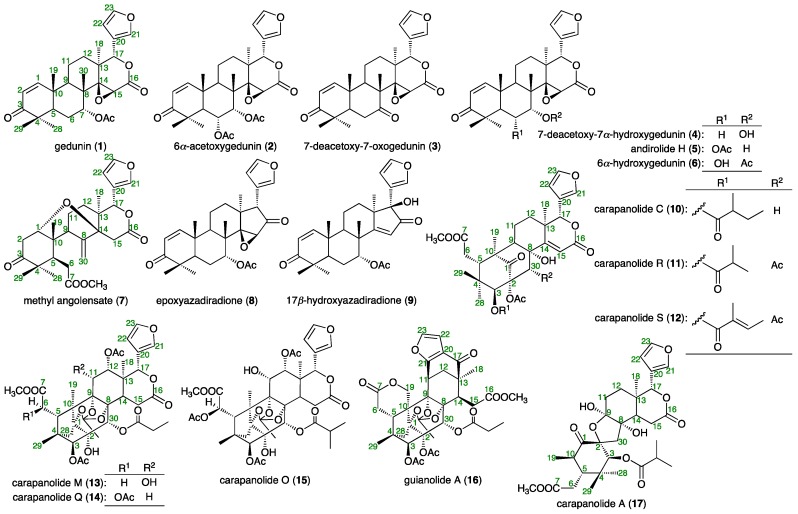
The Meliaceae family is recognized as a rich source of limonoids, which have attracted attention from biogenetic and synthetic perspectives [1,2,3]. Carapa guianensis Aublet (Meliaceae), known locally as andiroba and Brazilian mahogany, is distributed in the tropical rainforests of countries such as Brazil and Colombia, etc. The woody four-cornered andiroba nut has four cells, each of which contains two to three seeds with oil-rich kernels. Extracts of the bark, flowers, and seeds have been used for centuries by the Amazonian people and exhibit various effects: anti-bacterial [4], anti-cancerous [5], anti-tumor [6], anti-fungal [6], insect repellent [7], analgesic [8], anti-malarial [9], anti-inflammatory [10], antiallergic [11], and anti-plasmoidal effects [12], as well as acute and subacute toxicity [13]. In the course of our studies on the chemical constituents from C. guianensis (Carapa guianensis) [14,15,16,17,18,19,20,21,22,23], we have isolated several limonoids, including andirolides A–Y from the flower oil [14,15,16,17], carapanolides A–X [18,19,20,21,22], and guianolides A and B [23] from the seed oil. We have also reported that several limonoids from C. guianensis showed cytotoxic [14,16,18,19,23], antimalarial [15], anti-inflammatory [17,20,22], and triglyceride metabolism-promoting activities [21]. We further evaluated the principal gedunin-type limonoids from the flower oil of C. guianensis, gedunin (1) [15], 6α-acetoxygedunin (2) [14], and 7-deacetoxy-7-oxogedunin (3) [14], which were found to have protective effects against liver injury induced by d-galactosamine (d-GalN)/lipopolysaccharide (LPS) in mice. To characterize the mechanisms of action of limonoids and the structural requirements for their hepatoprotective effects, 17 related limonoids isolated from the flower oil of C. guianensis, such as 7-deacetoxy-7α-hydroxygedunin (4) [21], andirolide H (5) [15], 6α-hydroxygedunin (6) [15], and methyl angolensate (7) [15], as well as limonoids isolated from the seed oil including epoxyazadiradione (8), 17β-hydroxyazadiradione (9) [21], carapanolides C (10) [19], R (11) [21], S (12) [21], M (13) [21], Q (14) [21], and O (15) [21], guianolide A (16) [23], and carapanolide A (17) [18] (Figure 1). This study reports the hepatoprotective effects and possible mechanisms of action of 1–3, as well as the structural requirements for their hepatoprotective effects.
Figure 1.
Limonoids (1–17) from seed and flower oils of C. guianensis.
2. Results and Discussion
2.1. Isolation
In previous studies, compounds 1–7 were isolated from the flower oil of C. guianensis [14,15,16,17], whereas compounds 8–17 were obtained from the seed oil [18,19,20,21,22]. In the present study, principal limonoids (1–3) were identified from the seed oil using normal phase silica gel column chromatography followed by HPLC or recrystallization.
2.2. Protective Effects of Principal Limonoids (1, 2, and 3) on Liver Injury Induced by d-GalN/LPS in Mice
d-GalN/LPS-induced liver injuries are known to develop through immunological responses [24] that progress via two steps. First, expression of inhibitors of apoptosis proteins (IAPs) is inhibited by administration of d-GalN through depletion of uridine triphosphate and increased sensitivity of hepatocytes to tumor necrosis factor-α (TNF-α. Second, release of pro-inflammatory mediators [nitric oxide (NO) and TNF-α from LPS-activated macrophages (Kupffer’s cells) occurs. Apoptosis of hepatocytes induced by TNF-α plays an important role in d-GalN/LPS-induced liver injury [25]. In our previous investigation of compounds from natural medicines possessing hepatoprotective activity, we reported that sesquiterpenes and diarylheptanoids from Curcuma zedoaria [26,27,28,29], saponins from Panax notoginseng [30], coumarins from Angelica furcijuga [31], acid amides from Piper chaba [32,33,34], acylated phenylethanoids from Cistanche tubulosa [35], and triterpenes from Potentilla anserina [36] exhibited significant protective effects against liver injuries induced by d-GalN/LPS in mice.
First, the in vivo hepatoprotective effects of the principal limonoid constituents, gedunin (1), 6α-acetoxygedunin (2), and 7-deacetoxy-7-oxogedunin (3), were evaluated. As shown in Table 1, 1–3 at a dose of 25 mg/kg, p.o. clearly prevented mortalities and significantly inhibited the increase in serum levels of aspartate aminotransaminase (sAST) and alanine transaminase (sALT), which served as markers of acute liver injury [37,38,39]. Notably, 1–3 were more potent than positive compounds curcumin [36,40,41,42] and silybin [43,44], which are well recognized as naturally-occurring hepatoprotective products.
Table 1.
Inhibitory effects of gedunin (1), 6α-acetoxygedunin (2), and 7-deacetoxy-7-oxogedunin (3) on d-GalN/LPS-induced liver injury in mice.
| Treatment | Dose (mg/kg, p.o.) | N | sAST | sALT | Mortality | ||
|---|---|---|---|---|---|---|---|
| (Karmen Unit) | Inhibition (%) | (Karmen Unit) | Inhibition (%) | ||||
| Normal (vehicle) | – | 8 | 107 ± 9 ** | – | 20 ± 2 ** | – | 0/8 |
| Control | – | 12 | 5237 ± 1,000 | – | 8533 ± 1795 | – | 4/16 |
| Gedunin (1) | 25 | 7 | 2304 ± 651 * | 56.2 | 2950 ± 710 * | 65.7 | 0/7 |
| 50 | 7 | 1923 ± 576 * | 63.5 | 2824 ± 754 * | 67.2 | 0/7 | |
| 6α-Acetoxygedunin (2) | 25 | 7 | 2384 ± 579 * | 54.7 | 3120 ± 830 * | 63.7 | 0/7 |
| 50 | 7 | 1696 ± 580 ** | 67.9 | 2397 ± 873 ** | 72.2 | 0/7 | |
| 7-Deacetoxy-7-oxo- | 25 | 7 | 2093 ± 742 * | 60.3 | 2899 ± 1024 * | 66.3 | 0/7 |
| gedunin (3) | 50 | 6 | 1759 ± 579 * | 66.7 | 2572 ± 903 ** | 70.2 | 1/7 |
| Control | – | 10 | 6033 ± 1647 | – | 6605 ± 1985 | – | 6/16 |
| Curcumin [36] | 12.5 | 10 | 4770 ± 1218 | 21.1 | 5024 ± 1189 | 24.0 | 0/10 |
| 25 | 10 | 3177 ± 979 | 47.8 | 3253 ± 981 | 50.9 | 0/10 | |
| 50 | 9 | 2220 ± 563 * | 63.8 | 1916 ± 483 * | 71.2 | 1/10 | |
| Control | – | 10 | 4709 ± 461 | – | 7088 ± 917 | – | 4/14 |
| Silybin | 500 | 8 | 1361 ± 191 ** | 71.1 | 1990 ± 439 ** | 71.9 | 0/8 |
Each value represents the mean ± S.E.M.; asterisks denote significant differences from the control group, * p < 0.05, ** p < 0.01.; commercial silybin was purchased from Funakoshi Co., Ltd. (Tokyo, Japan).
2.3. Effects on d-GalN-induced Cytotoxicity in Primary Cultured Mouse Hepatocytes
As a part of our studies to characterize the hepatoprotective compounds from natural medicines, we have investigated several constituents showed inhibitory effect on d-GalN-induced cytotoxicity in primary cultured hepatocytes [26,27,29,31,32,33,34,35,36,43,44,45,46,47,48,49,50,51,52,53,54,55]. Since the principal limonoid constituents from the flower oil of C. guianensis (1–3) showed hepatoprotective effects against d-GalN/LPS-induced liver injury in mice (vide ante), the inhibitory effect of limonoids (1–17) on d-GalN-induced cytotoxicity in primary cultured mouse hepatocytes were examined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. As shown in Table 2, these limonoids (1–17) and curcumin [26,27,29] did not reduce d-GalN-induced cytotoxicity in primary mouse hepatocytes at concentrations of up to 100 μM, whereas silybin (IC50 = 38.8 μM) significantly inhibited cytotoxicity [33,35,36]. Thus, these limonoids (1–17) did not affect cytotoxicity caused by d-GalN.
Table 2.
Inhibitory effects of limonoids (1–17) on d-GalN-induced cytotoxicity in mouse primary hepatocytes.
| Treatment | Inhibition (%) | ||||
|---|---|---|---|---|---|
| 0 µM | 3 µM | 10 µM | 30 µM | 100 µM | |
| Gedunin (1) | 0.0 ± 1.8 | −8.4 ± 1.9 | −3.9 ± 0.4 | −3.2 ± 0.9 | −5.2 ±0.2 |
| 6α-Acetoxygedunin (2) | 0.0 ± 2.4 | −2.6 ± 1.0 | −1.9 ± 0.3 | −2.5 ± 0.7 | −1.8 ± 0.6 |
| 7-Deacetoxy-7-oxogedunin (3) | 0.0 ± 2.2 | −4.6 ± 0.5 | −8.2 ± 0.9 | −8.3 ± 1.1 | −8.9 ± 0.6 |
| 7-Deacetoxy-7α-hydroxygedunin (4) | 0.0 ± 1.6 | −2.0 ± 1.4 | −6.3 ± 0.5 | −8.0 ± 0.4 | −4.1 ± 0.7 |
| Andirolide H (5) | 0.0 ± 2.5 | −6.2 ± 2.6 | −9.0 ± 0.5 | −9.2 ± 0.8 | −0.6 ± 0.8 |
| 6α-Hydroxygedunin (6) | 0.0 ± 2.0 | −7.2 ± 2.4 | −9.4 ± 0.8 | −0.1 ± 0.7 | −2.8 ± 0.5 |
| Methyl angolensate (7) | 0.0 ± 2.2 | −2.1 ± 1.2 | −7.5 ± 0.9 | −6.3 ± 0.7 | −6.6 ± 1.0 |
| Epoxyazadiradione (8) | 0.0 ± 2.1 | −3.1 ± 8.9 | −2.2 ± 2.5 | −10.8 ± 0.6 | −11.9 ± 0.3 |
| 17β-Hydroxyazadiradione (9) | 0.0 ± 2.0 | 15.3 ± 2.3 | −4.1 ± 1.2 | −7.5 ± 1.4 | −7.5 ± 4.0 |
| Carapanolide C (10) | 0.0 ± 1.4 | 8.0 ± 4.3 | 3.4 ± 4.2 | 6.7 ± 2.1 | −7.7 ± 4.4 |
| Carapanolide R (11) | 0.0 ± 2.1 | 21.5 ± 2.8 ** | 27.8 ± 5.0 ** | 46.0 ± 4.7 ** | 36.0 ± 3.2 ** |
| Carapanolide S (12) | 0.0 ± 2.1 | −7.8 ± 3.2 | −3.8 ± 4.1 | −3.7 ± 3.1 | −7.1 ± 3.2 |
| Carapanolide M (13) | 0.0 ± 1.6 | −7.0 ± 0.5 | −7.3 ± 0.7 | 1.0 ± 0.4 | −9.9 ± 1.0 |
| Carapanolide Q (14) | 0.0 ± 1.6 | 2.7 ± 1.9 | −3.5 ± 2.9 | −2.5 ± 2.1 | −6.2 ± 1.7 |
| Carapanolide O (15) | 0.0 ± 1.9 | 7.5 ± 3.9 | −5.3 ± 5.6 | −5.2 ± 3.9 | −2.1 ± 1.7 |
| Guianolide A (16) | 0.0 ± 3.7 | 9.2 ± 4.2 | 11.0 ± 5.3 | 9.8 ± 2.8 | 23.5 ± 3.5 ** |
| Carapanolide A (17) | 0.0 ± 2.0 | −6.8 ± 1.2 | −8.3 ± 0.7 | −4.5 ± 0.6 | −7.0 ± 0.6 |
| Curcumin [26,27,29] | 0.0 ± 3.7 | 0.1 ± 3.8 | 1.1 ± 2.2 | −17.7 ± 1.3 | −44.3 ± 0.3 |
| Silybin [33,35,36] | 0.0 ± 0.3 | 4.8 ± 1.1 | 7.7 ± 0.7 | 45.2 ± 8.8 ** | 77.0 ± 5.5 ** |
Each value represents the mean ± S.E.M. (n = 4); asterisks denote significant differences from the control group, ** p < 0.01.; commercial silybin was purchased from Funakoshi Co., Ltd. (Tokyo, Japan).
2.4. Effects on LPS-induced NO Production in Mouse Peritoneal Macrophages
The effects of limonoids (1–17) on NO production were examined to provide an estimation of macrophage activation levels in LPS-treated mouse peritoneal macrophages. As shown in Table 3, gedunin-type, gedunin (1, IC50 = 4.6 µM) [17], 6α-acetoxygedunin (2, 7.9 µM) [17], 7-deacetoxy-7-oxogedunin (3, 12.8 µM) [17], 7-deacetoxy-7α-hydroxygedunin (4, 8.7 µM) [17], andirolide H (5, 9.4 µM), 6α-hydroxygedunin (6, 19.1 µM) [17], epoxyazadiradione (8, 8.2 µM), 17β-hydroxyazadiradione (9, 20.3 µM), mexicanolide-type, carapanolides R (11, 68.3 µM) and S (12, 15.5 µM), phragmalin-type limonoids, carapanolides M (13, 41.6 µM), Q (14, 38.0 µM), and O (15, 46.0 µM), and guianolide A (16, 77.9 µM) significantly inhibited NO production without notable cytotoxic effects at the effective concentration. The NO production inhibitory activities of gedunin-type limonoids (1–6, 8, and 9, IC50 = 4.6–20.3 µM) were higher than those of other skeletal-type limonoids such as andirobin-type (7, > 100 µM), mexicanolide or 9,10-seco-mexicanolide-type (10–12 and 17, 15.5 – >100 µM), and phragmalin-type limonoids (13–16, 38.0–77.9 µM). The potencies of 1–6, 8, and 12 were higher than that of the NO synthase inhibitor, NG-monomethyl-l-arginine (l-NMMA, IC50 = 36.0 µM) and equivalent to that of caffeic acid phenethyl ester (CAPE, 11.0 µM), an inhibitor of nuclear factor-κB activation.
Table 3.
Inhibitory effects of limonoids (1–17) on LPS-activated NO production in mouse peritoneal macrophages.
| Treatment | Inhibition (%) | IC50 | ||||
|---|---|---|---|---|---|---|
| 0 µM | 3 µM | 10 µM | 30 µM | 100 µM | (µM) | |
| Gedunin (1) [17] | 0.0 ± 5.6 (100.0 ± 4.1) | 25.1 ± 2.5 ** (102.2 ± 5.3) | 84.5 ± 2.3 ** (119.5 ± 5.3) | 101.8 ± 0.6 ** (94.8 ± 1.4) | 100.9 ± 0.4 ** (3.0 ± 0.4 #) | 4.6 |
| 6α-Acetoxygedunin (2) [17] | 0.0 ± 1.5 (100.0 ± 1.6) | 16.9 ± 1.7 ** (96.8 ± 1.2) | 67.6 ± 4.6 ** (102.3 ± 2.2) | 88.4 ± 3.5 ** (92.5 ± 1.7) | 99.6 ± 0.2 ** (53.6 ± 5.1 #) | 7.9 |
| 7-Deacetoxy-7-oxogedunin (3) [17] | 0.0 ± 6.5 (100.0 ± 5.1) | 7.4 ± 5.2 (100.3 ± 3.9) | 40.9 ± 4.7 ** (98.9 ± 3.2) | 94.0 ± 0.8 ** (98.8 ± 7.4) | 88.1 ± 2.1 ** (83.7 ± 1.2) | 12.8 |
| 7-Deacetoxy-7α-hydroxy-gedunin (4) [17] | 0.0 ± 2.4 (100.0 ± 4.4) | 15.7 ± 4.6 ** (110.3 ± 5.9) | 55.7 ± 4.0 ** (106.6 ± 3.1) | 98.8 ± 0.4 ** (96.3 ± 4.6) | 100.2 ± 0.2 ** (2.6 ± 0.5 #) | 8.7 |
| Andirolide H (5) | 0.0 ± 5.6 (100.0 ± 1.8) | 5.8 ± 6.1 (99.8 ± 4.5) | 63.9 ± 3.0 ** (103.9 ± 6.9) | 97.2 ± 0.9 ** (108.9 ± 2.4) | 99.7 ± 0.5 ** (4.9 ± 0.5 #) | 9.4 |
| 6α-Hydroxygedunin (6) [17] | 0.0 ± 6.2 (100.0 ± 4.5) | 7.7 ± 7.1 (88.4 ± 3.0) | 20.7 ± 4.3 ** (87.6 ± 4.0) | 64.0 ± 3.1 ** (90.4 ± 2.6) | 97.3 ± 0.3 ** (82.2 ± 4.2) | 19.1 |
| Methyl angolensate (7) [17] | 0.0 ± 5.9 (100.0 ± 2.4) | 10.1 ± 4.2 (108.8 ± 11.0) | 20.0 ± 8.1 (108.8 ± 5.5) | 42.2 ± 3.5 ** (111.0 ± 4.5) | 24.0 ± 4.2 * (78.1 ± 5.3 #) | > 100 |
| Epoxyazadiradione (8) | 0.0 ± 0.8 (100.0 ± 4.1) | 10.5 ± 0.9 * (99.6 ± 2.9) | 56.0 ± 4.0 ** (94.8 ± 2.3) | 102.6 ±4.0 ** (81.9 ± 2.7) | 112.3 ± 0.7 ** (10.0 ± 0.5 #) | 8.2 |
| 17β-Hydroxyazadiradione (9) | 0.0 ± 4.9 (100.0 ± 1.8) | −10.4 ± 6.8 (95.4 ± 5.2) | 9.4 ± 7.1 (94.4 ± 1.4) | 65.1 ± 4.5 ** (94.8 ± 4.9) | 97.4 ± 0.7 ** (84.8 ± 3.6) | 20.3 |
| Carapanolide C (10) | 0.0 ± 2.6 (100.0 ± 3.4) | 4.2 ± 8.8 (96.0 ± 4.1) | 20.8 ± 5.0 ** (98.9 ± 3.4) | 20.2 ± 4.9 (91.8 ± 2.8) | 13.2 ± 1.9 (80.0 ± 4.4) | >100 |
| Carapanolide R (11) | 0.0 ± 1.3 (100.0 ± 1.0) | 4.0 ± 2.2 (95.1 ± 1.8) | 8.9 ± 2.3 (98.0 ± 1.7) | 17.4 ± 1.3 (106.0 ± 1.9) | 75.6 ± 1.2 ** (118.4 ± 1.0) | 68.3 |
| Carapanolide S (12) | 0.0 ± 2.8 (100.0 ± 0.7) | 2.5 ± 1.2 (97.9 ± 2.7) | 15.9 ± 1.3 ** (93.8 ± 2.0) | 72.2 ± 3.6 ** (96.8 ± 4.4) | 99.8 ± 0.4 ** (73.7 ± 3.8 #) | 15.5 |
| Carapanolide M (13) | 0.0 ± 2.2 (100.0 ± 2.3) | −1.1 ± 2.7 (99.2 ± 0.6) | 3.2 ± 2.8 (95.1 ± 1.3) | 30.3 ± 3.1 ** (94.2 ± 2.9) | 85.1 ± 1.5 ** (111.9 ± 1.6) | 41.6 |
| Carapanolide Q (14) | 0.0 ± 2.4 (100.0 ± 2.8) | 1.9 ± 0.7 (99.8 ± 1.6) | 14.4 ± 2.2 (96.3 ± 1.6) | 44.3 ± 1.1 ** (95.5 ± 4.1) | 75.3 ± 3.5 ** (115.5 ± 2.2) | 38.0 |
| Carapanolide O (15) | 0.0 ± 2.5 (100.0 ± 4.6) | −2.5 ± 5.4 (104.1 ± 2.5) | 14.7 ± 8.2 (107.9 ± 2.7) | 6.9 ± 5.0 (106.7 ± 2.5) | 102.5 ± 3.2 ** (108.7 ± 5.2) | 46.0 |
| Guianolide A (16) | 0.0 ± 1.8 (100.9 ± 0.9) | 1.9 ± 3.2 (96.9 ± 1.8) | 3.2 ± 1.4 (101.5 ± 1.9) | 12.7 ± 1.6 (98.7 ± 1.8) | 71.3 ± 3.2 ** (106.2 ± 1.6) | 77.9 |
| Carapanolide A (17) | 0.0 ± 1.5 (100.0 ± 1.8) | −0.6 ± 2.1 (103.0 ± 2.6) | 1.2 ± 2.0 (103.9 ± 4.3) | 41.1 ± 1.0 ** (109.5 ± 4.3) | 4.9 ± 1.8 (91.3 ± 1.6) | > 100 |
| l-NMMA [33,36] | 0.0 ± 3.1 (100.0 ± 0.9) | 1.4 ± 2.8 (101.1 ± 5.7) | 19.9 ± 2.8 ** (100.7 ± 6.2) | 43.0 ± 2.1 ** (102.6 ± 4.2) | 70.9 ± 1.6 ** (106.4 ± 4.6) | 36.0 |
| CAPE [33,36] | 0.0 ± 2.1 (100.0 ± 1.5) | 5.9 ± 5.2 (95.4 ± 0.7) | 44.4 ± 3.2 ** (70.0 ± 4.0 #) | 86.2 ± 1.1 ** (71.4 ± 6.0 #) | 99.6 ± 0.1 ** (53.0 ± 1.4 #) | 11.0 |
Each value represents the mean ± S.E.M. (n = 4); asterisks denote significant differences from the control group, * p < 0.05, ** p < 0.01; # cytotoxic effects were observed, and values in parentheses indicate cell viability (%) in MTT assay; commercial silybin was purchased from Funakoshi Co., Ltd. (Tokyo, Japan), whereas L-NMMA and CAPE were from Sigma-Aldrich Chemical Co., LLC. (St. Louis, MO, USA).
The structural requirements of gedunin-type limonoids were assessed: (i) 6α-acetoxy and 6α-hydroxy moieties reduced the activity [gedunin (1) > 6α-acetoxygedunin (2), 6α-hydroxygedunin (6)]; (ii) compounds with 7α-acetoxy group exhibited higher activity than those with 7α-hydroxy or 7-keto groups [1 > 7-deacetoxy-7-oxogedunin (3), 7-deacetoxygedunin (4)]; (iii) compounds with an α,β-epoxy-δ-lactone moiety in the D-ring exhibited higher activity than those with an α,β-epoxy or α,β-unsaturated cyclopropane moieties [1 > epoxyanadiradione (8), 17β-hydroxyazadiradione (9)]. For mexicanolide- and phragmalin-type limonoids, the following relationships were suggested: the 30-O-acyl moieties were essential for the activity [carapanolide C (10) ≪ carapanolides R (11), S (12), M (13), Q (14), and O (15)], whereas a 6-acetoxy moiety did not affect the activity [13 ≒ 15], while an 11α-hydroxy moiety reduced the activity [14 > 15].
2.5. Effects on TNF-α-induced Cytotoxicity in L929 Cells
The effects of the limonoids (1–17) on the sensitivity of hepatocytes to TNF-α were assessed by measuring TNF-α-induced decreases in the viability of L929 cells, a TNF-α-sensitive cell line [56], by using the MTT assay. In the absence of a test sample, the cells incubated with 1 ng/mL TNF-α for 44 h were compared with those not incubated with TNF-α. As shown in Table 4, 7-deacetoxy-7-oxogedunin (3, IC50 = 7.3 µM), epoxyazadiradione (8, 10.2 µM), 17β-hydroxyazadiradione (9, 6.9 µM), and carapanolides C (10, 27.0 µM) and A (17, 25.3 µM) inhibited the decrease in cell viability with greater efficacy than silybin (IC50 = 37.2 µM) [36]. The structural requirements of gedunin-type limonoids for the activity were as follows; (i) compounds with a 7-keto group exhibited higher activity than those with 7α-acetoxy or 7α-hydroxy groups [7-deacetoxy-7-oxogedunin (3) > gedunin (1), 7-deacetoxygedunin (4)]; compounds with an α,β-epoxy or α,β-unsaturated cyclopropane moiety in the D-ring exhibited higher activity than those with an α,β-epoxy-δ-lactone moiety [epoxyazadiradione (8), 17β-hydroxyazadiradione (9) > 1]. These structural requirements showed opposite tendencies to those related to NO production inhibitory activity, which is mentioned above.
Table 4.
Inhibitory effects of limonoids (1–17) on TNF-α-induced cytotoxicity in L929 cells.
| Treatment | Inhibition (%) | ||||
|---|---|---|---|---|---|
| 0 µM | 1 µM | 3 µM | 10 µM | 30 µM | |
| Gedunin (1) | 0.0 ± 2.1 | 4.5 ± 1.9 | 21.8 ± 3.7 ** | 38.1 ± 3.8 ** | 36.5 ± 4.1 ** |
| 6α-Acetoxygedunin (2) | 0.0 ± 1.6 | 10.9 ± 1.0 ** | 23.2 ± 1.8 ** | 36.3 ± 2.1 ** | 37.3 ± 1.4 ** |
| 7-Deacetoxy-7-oxogedunin (3) | 0.0 ± 1.1 | 5.8 ± 1.5 | 26.7 ± 4.5 ** | 58.6 ± 7.2 ** | 68.7 ± 4.8 ** |
| 7-Deacetoxy-7α-hydroxygedunin (4) | 0.0 ± 0.3 | −6.5 ± 2.4 | 2.7 ± 2.3 | 36.5 ± 1.8 ** | |
| Andirolide H (5) | 0.0 ± 0.8 | −6.6 ± 3.6 | −0.7 ± 1.2 | 7.6 ± 1.1 | 39.2 ± 1.7 ** |
| 6α-Hydroxygedunin (6) | 0.0 ± 1.3 | 8.1 ± 1.9 | 6.7 ± 1.5 | 12.1 ± 3.0 | 28.3 ± 1.7 ** |
| Methyl angolensate (7) | 0.0 ± 1.4 | −0.5 ± 3.5 | 0.6 ± 2.9 | 13.3 ± 2.6 * | 24.6 ± 2.9 ** |
| Epoxyazadiradione (8) | 0.0 ± 5.3 | 13.7 ± 3.9 | 39.1 ± 6.5 ** | 91.5 ± 11.4 ** | |
| 17β-Hydroxyazadiradione (9) | 0.0 ± 1.5 | 14.1 ± 3.4 | 23.9 ± 3.9 ** | 64.0 ± 3.3 ** | 91.3 ± 8.2 ** |
| Carapanolide C (10) | 0.0 ± 3.7 | 4.9 ± 2.1 | 14.2 ± 3.2 | 27.7 ± 4.3 ** | 54.5 ± 5.5 ** |
| Carapanolide R (11) | 0.0 ± 4.1 | −6.3 ± 4.7 | −1.3 ± 3.8 | 31.7 ± 3.8 ** | |
| Carapanolide S (12) | 0.0 ± 1.5 | −5.5 ± 2.2 | −1.4 ± 1.5 | −2.5 ± 1.2 | |
| Carapanolide M (13) | 0.0 ± 6.5 | −1.5 ± 7.1 | 7.0 ± 4.4 | −5.1 ± 6.2 | |
| Carapanolide Q (14) | 0.0 ± 5.5 | 8.6 ± 4.4 | 1.3 ± 4.2 | 9.2 ± 2.5 | |
| Carapanolide O (15) | 0.0 ± 6.5 | 6.3 ± 4.3 | 1.0 ± 6.4 | 1.5 ± 4.1 | |
| Guianolide A (16) | 0.0 ± 2.9 | −6.2 ± 5.2 | −4.5 ± 1.9 | −7.3 ± 3.0 | |
| Carapanolide A (17) | 0.0 ± 3.7 | 8.8 ± 6.5 | 21.5 ± 5.5 ** | 58.2 ± 4.7 ** | |
| Treatment | Inhibition (%) | ||||
| 0 µM | 3 µM | 10 µM | 30 µM | 100 µM | |
| Silybin [36] | 0.0 ± 2.6 | 5.3 ± 2.8 | 22.0 ± 3.8 ** | 48.0 ± 4.1 ** | 50.8 ± 3.9 ** |
Each value represents the mean ± S.E.M. (n = 4); asterisks denote significant differences from the control group, * p < 0.05, ** p < 0.01.; commercial silybin was purchased from Funakoshi Co., Ltd. (Tokyo, Japan).
3. Materials and Methods
3.1. General Experimental Procedures
The following instructions were used to obtain spectroscopic data: melting points, Yanagimoto micromelting point apparatus (Yanaco New Science Inc., Kyoto, Japan); optical rotations, JASCO DIP-1000 digital polarimeter (JASCO Co., Tokyo, Japan); IR spectra, PerkineElmer 1720X FTIR spectrophotometer (PerkineElmer Inc., Waltham, MA, USA); UV spectra, HITACHI U-2000 spectrometer (Hitachi High-Technologies Co., Tokyo, Japan) (acetonitrile as a solvent); 1H and 13C NMR spectra, Agilent VNMRS 600 spectrometer (Agilent Technologies Inc., Santa Clara, CA, USA) (CDCl3 was used as the solvent and TMS as the internal standard); FABMS, JEOL JMS-7000 mass spectrometer (JEOL Ltd., Tokyo, Japan); CD spectra, JASCO J-820 spectrometer (JASCO Co.); and HPLC, JASCO PU-1586 (RI 1531) (JASCO Co.). The following experimental conditions were used for column chromatography: (silica gel, 70–230 mesh; Merck, Darmstadt, Germany); medium-pressure liquid chromatography (MPLC; silica gel, 230–400 mesh; Merck); and TLC (silica gel 60 F254; Merck).
3.2. Material
The flower and seed oils of C. guianensis Aublet (Meliaceae), were collected in Amazon, Brazil in March of 2006, 2011, and 2013. Voucher specimens (CG-01-1, CGS-01-1, and CGS-01-2) were deposited at the Herbarium of the Laboratory of Medicinal Chemistry at Osaka University of Pharmaceutical Sciences as described previously [14,15,16,17,18,19,20,21,22,23].
3.3. Isolation of Compounds 1–3 from the Seed Oil of C. Guianensis
Preliminary silica gel column chromatography was performed to separate the seed oil (2.03 kg, CGS-01-2) of C. guianensis into eight fractions (Fractions A–H) [22]. Fraction C (29.3 g) was rechromatographed on a silica gel (70–230 mesh, 1.0 kg) column using n-hexane–EtOAc (5:1) to yield residues C3 (1.66 g) and C4 (1.02 g). Residue C3 (1.66 g) was rechromatographed on a silica gel (230–400 mesh, 100 g) column using n-hexane–EtOAc (3:1) to give a crystalline solid (590 mg), which was purified by HPLC [ODS, MeOH–H2O (70:30)] to afford compounds 1 (325 mg) and 2 (178 mg). Residue C4 (1.02 g) was rechromatographed on a silica gel (230–400 mesh, 50 g) column using n-hexane–EtOAc (3:1) to give a crystalline solid, which was recrystallized from MeOH to give compound 3 (510 mg). These isolates (1–3) were unambiguously identified by comparison of their physical and spectral data with those of authentic samples [14,15].
3.4. Reagents
LPS from Salmonella enteritidis, minimum essential medium (MEM), and William’s E medium were purchased from Sigma-Aldrich Chemical (St. Louis, MO, USA); fetal bovine serum (FBS) was from Life Technologies (Rockville, MD, USA); and other chemicals were from Wako Pure Chemical Industries, Co., Ltd. (Osaka, Japan). Microplates (96-well) were purchased from Sumitomo Bakelite Co., Ltd. (Tokyo, Japan).
3.5. Animals
Male ddY mice (Kiwa Laboratory Animal Co., Ltd., Wakayama, Japan) were housed at a constant temperature of 23 ± 2°C and were fed a standard laboratory chow (MF, Oriental Yeast Co., Ltd., Tokyo, Japan). The animals were fasted for 24 h prior to the beginning of the experiment but were allowed free access to tap water. All experiments were performed with conscious mice unless otherwise noted. The experimental protocol was approved by the Experimental Animal Research Committee of Kindai University (KAPR-26-001, 1 April 2014).
3.6. Effects on d-GalN/LPS-induced Liver Injury in Mice
The method described by Tiegs et al. [57] was modified and used for this study [33,35,36]. Curcumin [36] and silybin were used as reference compounds.
3.7. Effects on Cytotoxicity Induced by d-GalN in Primary Cultured Mouse Hepatocytes
Assay for the d-GalN-induced cytotoxicity in primary cultured mouse hepatocytes was performed as described previously [33,35,36]. Curcumin [26,27,29] and silybin [33,35,36] were used as reference compounds.
3.8. Effects on Production of NO in LPS-induced Mouse Peritoneal Macrophages
Assay for NO production in TGC-induced mouse peritoneal macrophages was performed as described previously [33,35,36]. NG-Monomethyl-L-arginine (L-NMMA) and caffeic acid phenethyl ester (CAPE) were used as reference compounds [33,36].
3.9. Effects on Cytotoxicity Induced by TNF-α in L929 Cells
Assay for the TNF-α-induced cytotoxicity in L929 cells was performed as described previously [33,35,36]. Silybin was used as a reference compound [36].
3.10. Statistics
All data are expressed as means ± S.E.M. The data analysis was performed with an one-way analysis of variance (ANOVA), followed by Dunnett’s test. Probability (p) values less than 0.05 were considered significant.
4. Conclusions
Three gedunin-type limonoids obtained from the flower oil of C. guianensis, gedunin (1), 6α-acetoxygedunin (2), and 7-deacetoxy-7-oxogedunin (3), showed protective effects against liver injury induced by d-GalN/LPS in mice at a dose of 25 mg/kg, p.o. The mechanisms of action are likely dependent on inhibition of LPS-induced macrophage activation and reduced sensitivity of hepatocytes to TNF-α; however, these compounds did not decrease the cytotoxicity caused by d-GalN. LPS-induced NO production is accompanied with production of several cytokines, such as TNF-α, IL-1, and IL-6, from macrophages through the toll-like receptor 4 (TLR4)-mediated pathways [58,59]. Recently, it was reported that 1 suppressed the activation of macrophages through binding to myeloid differentiation protein 2 (MD-2), and not by affecting TLR4-mediated signaling. Their data supports our results of inhibitory effects of 1 on NO production in LPS-activated macrophages [60]. In addition, the structural requirements of limonoids (1–17) with regard to inhibition of LPS-induced NO production in mouse peritoneal macrophages and TNF-α-induced cytotoxicity in L929 cells were found to show different tendencies as mentioned above. The detailed mechanisms of action for the hepatoprotective effects of limonoids need to be studied further.
Acknowledgments
This work was supported by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2014–2018 (Toshio Morikawa), as well as JSPS KAKENHI Grant Numbers 15K08008 (Toshio Morikawa) and 15K08009 (Kiyofumi Ninomiya). We also thank Akira Yoshino, a representative of NGO Green Heart, Oita, Japan (http://ngo-greenheart.jp) for collecting and identifying the plant materials.
Author Contributions
Kiyofumi Ninomiya, Takashi Kikuchi, Takeshi Yamada, Osamu Muraoka, Reiko Tanaka, and Toshio Morikawa conceived and designed the experiments; Kiyofumi Ninomiya, Seiya Miyazawa, Kaiten Ozeki, Natsuko Matsuo, and Toshio Morikawa performed the experiments; Kiyofumi Ninomiya and Toshio Morikawa analyzed the data; Osamu Muraoka and Reiko Tanaka contributed the materials; Kiyofumi Ninomiya, Reiko Tanaka, and Toshio Morikawa wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Liao S.G., Chen H.D., Yue J.M. Plant orthoesters. Chem. Rev. 2009;109:1092–1140. doi: 10.1021/cr0782832. [DOI] [PubMed] [Google Scholar]
- 2.Fang X., Di Y.T., Hao X.J. The advances in the limonoid chemistry of the Meliaceae family. Curr. Org. Chem. 2011;15:1363–1391. [Google Scholar]
- 3.Tan Q.G., Luo X.D. Meliaceous limonoids: Chemistry and biological activities. Chem. Rev. 2011;111:7437–7522. doi: 10.1021/cr9004023. [DOI] [PubMed] [Google Scholar]
- 4.Pereira da Silva V.P., Oliveira R.R., Figueiredo M.R. Isolation of limonoids from seeds of Carapa guianensis Aublet (Meliaceae) by high-speed countercurrent chromatography. Phytochem. Anal. 2009;20:70–81. doi: 10.1002/pca.1100. [DOI] [PubMed] [Google Scholar]
- 5.Nakanishi K., Sasaki S., Kiang A.K., Goh J., Kakisawa H., Ohashi M., Goto M., Watanabe J., Yokotani H., Matsumura C., et al. Phytochemical survey of Malaysian plants preliminary chemical and pharmacological screening. Chem. Pharm. Bull. 1965;55:457–464. doi: 10.1248/cpb.13.882. [DOI] [PubMed] [Google Scholar]
- 6.Waterman A.M. The effect of water-soluble extracts from the heartwood of tropical American woods on the growth of two wood-decay fungi. Trop. Woods. 1946;88:1–11. [Google Scholar]
- 7.Prophiro J.S., da Silva Mario A.N., Kanis L.A., da Rocha L.C.B.P., Duque-Luna J.E., da Silva O.S. First report on susceptibility of wild Aedes aegypty (Diptera: Culicidae) using Carapa guianensis (Meliaceae) and Copaifera sp. (Leguminosae) Parasitol. Res. 2012;110:699–705. doi: 10.1007/s00436-011-2545-7. [DOI] [PubMed] [Google Scholar]
- 8.Penido C., Costa K.A., Pennaforte R.J., Costa M.F., Pereira J.F., Siani A.C., Henriques M.G. Anti-allergic effects of natural tetranortriterpenoids isolated from Carapa guianensis Aublet on allergen-induced vascular permeability and hyperalgesia. Inflamm. Res. 2005;54:295–303. doi: 10.1007/s00011-005-1357-6. [DOI] [PubMed] [Google Scholar]
- 9.Bickii J., Njifutie N., Foyere J.A., Basco L.K., Ringwald P.J. In vitro antimalarial activity of limonoids from Khaya grandifoliola C.D.C. (Meliaceae) J. Ethnopharmacol. 2000;69:27–33. doi: 10.1016/S0378-8741(99)00117-8. [DOI] [PubMed] [Google Scholar]
- 10.Penido C., Conte F.P., Chagas M.S.S., Rodrigue C.A.B., Pereira J.F.G., Henriques M.G.M.O. Antiinflammatory effects of natural tetranortriterpenoids isolated from Carapa guianensis Aublet on zymosan-induced arthritis in mice. Inflamm. Res. 2006;55:457–464. doi: 10.1007/s00011-006-5161-8. [DOI] [PubMed] [Google Scholar]
- 11.Ferraris F.K., Rodrigues R., da Silva V.P., Figueiredo R., Penido C., Henriques M.G.M.O. Modulation of T lymphocyte and eosinophil functions in vitro by natural tetranortriterpenoids isolated from Carapa guianensis Aublet. Int. Immunopharmacol. 2011;11:1–11. doi: 10.1016/j.intimp.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Miranda R.N.C., Jr., Dolabela M.F., da Silva M.N., Povoa M.M., Maia J.G.S. Antiplasmoidal activity of the andiroba (Carapa guianensis Aublet., Meliaceae) oil and its limonoid-rich fraction. J. Ethnopharmacol. 2012;142:679–683. doi: 10.1016/j.jep.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 13.Costa-Silva H., Lima C.R., Silva E.J., Araújo A.V., Fraga M.C., Ribeiro E Ribeiro A., Arruda A.C., Lafayette S.S., Wanderley A.G. Acute and subacute toxicity of the Carapa guianensis Aublet (Meliaceae) seed oil. J. Ethnopharmacol. 2008;116:495–500. doi: 10.1016/j.jep.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka Y., Yamada T., In Y., Muraoka O., Kajimoto T., Tanaka R. Absolute stereostructure of andirolides A–G from the flower of Carapa guianensis. Tetrahedron. 2011;67:782–792. doi: 10.1016/j.tet.2010.11.028. [DOI] [Google Scholar]
- 15.Tanaka Y., Sakamoto A., Inoue T., Yamada T., Kikuchi T., Kajimoto T., Muraoka O., Sato A., Wataya Y., Kim H.-S., et al. Andirolides H–P from the flower of andiroba (Carapa guianensis, Meliaceae) Tetrahedron. 2012;68:3669–3677. doi: 10.1016/j.tet.2011.12.076. [DOI] [Google Scholar]
- 16.Sakamoto A., Tanaka Y., Inoue T., Kikuchi T., Kajimoto T., Muraoka O., Yamada T., Tanaka R. Andirolides Q–V from the flower of andiroba. Fitoterapia. 2013;90:20–29. doi: 10.1016/j.fitote.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto A., Tanaka Y., Yamada T., Kikuchi T., Muraoka O., Ninomiya K., Morikawa T., Tanaka R. Andirolides W–Y from the flower oil of andiroba. Fitoterapia. 2015;100:81–87. doi: 10.1016/j.fitote.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Inoue T., Nagai Y., Mitooka A., Ujike R., Muraoka O., Yamada T., Tanaka R. Carapanolides A and B: Unusal 9,10-seco-mexicanolides having a 2R,9S-oxygen bridge from the seeds of Carapa guianensis. Tetrahedron Lett. 2012;53:6685–6688. doi: 10.1016/j.tetlet.2012.09.108. [DOI] [Google Scholar]
- 19.Inoue T., Matsui Y., Kikuchi T., In Y., Muraoka O., Yamada T., Tanaka R. Carapanolides C–I from the seeds of andiroba (Carapa guianensis, Miliaceae) Fitoterapia. 2014;96:56–64. doi: 10.1016/j.fitote.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Matsui Y., Kikuchi T., Inoue T., Muraoka O., Yamada T., Tanaka R. Carapanolides J–L from seeds of Carapa guianensis (andiroba) and their effects on LPS-activated NO production. Molecules. 2014;19:17130–17140. doi: 10.3390/molecules191117130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue T., Matsui Y., Kikuchi T., Yamada T., In Y., Muraoka O., Sakai C., Ninomiya K., Morikawa T., Tanaka R. Carapanolides M–S from seeds of andiroba (Carapa guianensis, Meliaceae) and triglyceride metabolism-promoting activity in high glucose-pretreated HepG2 cells. Tetrahedron. 2015;71:2753–2760. doi: 10.1016/j.tet.2015.03.017. [DOI] [Google Scholar]
- 22.Miyake T., Ishimoto S., Higuchi K., Minoura K., Kikuchi T., Yamada T., Muraoka O., Tanaka R. Carapanolides T–X from Carapa guianensis (andiroba) seeds. Molecules. 2015;20:20955–20966. doi: 10.3390/molecules201119737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue T., Matsui Y., Kikuchi T., In Y., Yamada T., Muraoka O., Matsunaga S., Tanaka R. Guianolides A and B, new carbon skeletal limonoids from the seeds of Carapa guianensis. Org. Lett. 2013;15:3018–3021. doi: 10.1021/ol400924u. [DOI] [PubMed] [Google Scholar]
- 24.Freudenberg M.A., Galanos C. Tumor necrosis factor-α mediates lethal activity of killed gram-negative and gram-positive bacteria in d-galactosamine-treated mice. Infect. Immun. 1991;59:2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Josephs M.D., Bahjat F.R., Fukuzuka K., Ksontini R., Solorzano C.C., Edwards C.K., 3rd, Tannahill C.L., MacKay S.L., Copeland E.M., 3rd, Moldawer L.L. Lipopolysaccharide and d-galactosamine-induced hepatic injury is mediated by TNF-α and not by Fas ligand. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;278:R1196–R1201. doi: 10.1152/ajpregu.2000.278.5.R1196. [DOI] [PubMed] [Google Scholar]
- 26.Matsuda H., Ninomiya K., Morikawa T., Yoshikawa M. Inhibitory effect and action mechanism of sesquiterpenes from Zedoariae Rhizoma on d-galactosamine/lipopolysaccharide-induced liver injury. Bioorg. Med. Chem. Lett. 1998;8:339–344. doi: 10.1016/S0960-894X(98)00021-3. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda H., Morikawa T., Ninomiya K., Yoshikawa M. Hepatoprotective constituents from Zedoariae Rhizoma: Absolute stereostructures of three new carabrane-type sesquiterpenes, curcumenolactonea A, B, and C. Bioorg. Med. Chem. 2001;9:909–916. doi: 10.1016/S0968-0896(00)00306-0. [DOI] [PubMed] [Google Scholar]
- 28.Morikawa T., Matsuda H., Ninomiya K., Yoshikawa M. Medicinal foodstuffs. XXIX. potent protective effects of sesquiterpenes and curcumin from Zedoariae Rhizoma on liver injury induced by d-galactosamine/lipopolysaccharide or tumor necrosis factor-α. Biol. Pharm. Bull. 2002;25:627–631. doi: 10.1248/bpb.25.627. [DOI] [PubMed] [Google Scholar]
- 29.Morikawa T. Search for bioactive constituents from several medicinal food: Hepatoprotective, antidiabetic, and antiallergic activities. J. Nat. Med. 2007;61:112–126. doi: 10.1007/s11418-006-0105-8. [DOI] [Google Scholar]
- 30.Yoshikawa M., Morikawa T., Kashima Y., Ninomiya K., Matsuda H. Structures of new dammarane-type triterpene saponins from the flower buds of Panax notoginseng and hepatoprotective effects of principal ginseng saponins. J. Nat. Prod. 2003;66:922–927. doi: 10.1021/np030015l. [DOI] [PubMed] [Google Scholar]
- 31.Yoshikawa M., Nishida N., Ninomiya K., Ohgushi T., Kubo M., Morikawa T., Matsuda H. Inhibitory effects of coumarin and acetylene constituents from the roots of Angellica furcijuga on d-galactosamine/lipopolysaccharide-induced liver injury in mice and on nitric oxide production in lipopolysaccharide-activated mouse peritoneal macrophages. Bioorg. Med. Chem. 2006;14:456–463. doi: 10.1016/j.bmc.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 32.Matsuda H., Ninomiya K., Morikawa T., Yasuda D., Yamaguchi I., Yoshikawa M. Protective effects of amide constituents from the fruit of Piper chaba on d-galactosamine/TNF-α-induced cell death in mouse hepatocytes. Bioorg. Med. Chem. Lett. 2008;18:2038–2042. doi: 10.1016/j.bmcl.2008.01.101. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda H., Ninomiya K., Morikawa T., Yasuda D., Yamaguchi I., Yoshikawa M. Hepatoprotective amide constituents from the fruit of Piper chaba: Structural requirements, mode of action, and new amides. Bioorg. Med. Chem. 2009;17:7313–7323. doi: 10.1016/j.bmc.2009.08.050. [DOI] [PubMed] [Google Scholar]
- 34.Morikawa T. Search for TNF-α sensitivety degradation principles from medicinal foods—Hepatoprotective amide constituents from Thai natural medicine Piper chaba. Yakugaku Zasshi. 2010;130:785–791. doi: 10.1248/yakushi.130.785. [DOI] [PubMed] [Google Scholar]
- 35.Morikawa T., Pan Y., Ninomiya K., Imura K., Matsuda H., Yoshikawa M., Yuan D., Muraoka O. Acylated phenylethanoid oligoglycosides with hepatoprotective activity from the desert plant Cistanche tubulosa. Bioorg. Med. Chem. 2010;18:1882–1890. doi: 10.1016/j.bmc.2010.01.047. [DOI] [PubMed] [Google Scholar]
- 36.Morikawa T., Ninomiya K., Imura K., Yamaguchi T., Aakgi Y., Yoshikawa M., Hayakawa T., Muraoka O. Hepatoprotective triterpene from traditional Tibetan medicine Potentilla anserina. Phytochemistry. 2014;102:169–181. doi: 10.1016/j.phytochem.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Osumi W., Jin D., Imai Y., Tashiro K., Li Z.-L., Otsuki Y., Maemura K., Komeda K., Hirokawa F., Hayashi M., et al. Recombinant human soluble thrombomodulin improved lipopolysaccharide/d-galactosamine-induced acute liver failure in mice. J. Pharmacol. Sci. 2015;129:233–239. doi: 10.1016/j.jphs.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Cho H.I., Hong J.M., Choi J.W., Choi H.S., Kwak J.H., Lee D.U., Lee S.K., Lee S.M. β-Caryophyllene alleviates d-galactosamine and lipopolysaccharide-induced hepatic injury through suppression of the TLR4 and RAGE signaling pathways. Eur. J. Pharmacol. 2015;764:613–621. doi: 10.1016/j.ejphar.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Sass G., Heinlein S., Agli A., Bang R., Schümann J., Tiegs G. Cytokine expression in three mouse models of experimental hepatitis. Cytokine. 2002;19:115–120. doi: 10.1006/cyto.2002.1948. [DOI] [PubMed] [Google Scholar]
- 40.Lee H.I., McGregor R.A., Choi M.S., Seo K.I., Jung U.J., Yeo J., Kim M.J., Lee M.K. Low doses of curcumin protect alcohol-induced liver damage by modulation of the alcohol metabolic pathway, CYE2E1 and AMPK. Life Sci. 2013;93:693–699. doi: 10.1016/j.lfs.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 41.Nabavi S.F., Daglia M., Moghaddam A.H., Habtemariam S., Nabavi S.M. Curcumin and liver disease: From chemistry to meidicne. Comp. Rev. Food Sci. Food Saf. 2013;13:62–77. doi: 10.1111/1541-4337.12047. [DOI] [PubMed] [Google Scholar]
- 42.Palipoch S., Punsawad C., Koomhin P., Suwannalert P. Hepatoprotective effect of curcumin and α-tocopherol against cisplatin-induced oxidative stress. BMC Comp. Alt. Med. 2014;14:111. doi: 10.1186/1472-6882-14-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fehér J., Deák G., Muzes G., Láng I., Niederland V., Nékám K., Kárteszi M. Liver-protective action of silymarin therapy in chronic alcoholic liver diseases. Orv. Hetil. 1989;130:2723–2727. [PubMed] [Google Scholar]
- 44.Skottová N., Krecman V. Silymarin as a potential hypocholesterolaemic drug. Physiol. Res. 1998;47:1–7. [PubMed] [Google Scholar]
- 45.Yoshikawa M., Xu F., Morikawa T., Ninomiya K., Matsuda H. Anastatins A and B, new skeletal flavonoids with hepatoprotective activities from the desert plant Anastatica hierochuntica. Bioorg. Med. Chem. Lett. 2003;13:1045–1049. doi: 10.1016/S0960-894X(03)00088-X. [DOI] [PubMed] [Google Scholar]
- 46.Xu F., Morikawa T., Matsuda H., Ninomiya K., Yoshikawa M. Structures of sesquiterpenes and hepatoprotective constituents from the Egyptian herbal medicine Cyperus longus. J. Nat. Prod. 2004;67:569–576. doi: 10.1021/np030368k. [DOI] [PubMed] [Google Scholar]
- 47.Matsuda H., Morikawa T., Xu F., Ninomiya K., Yoshikawa M. New isoflavones and pterocarpanes with hepatoprotective activity from the stems of Erycibe expansa. Planta Med. 2004;70:1201–1209. doi: 10.1055/s-2004-835852. [DOI] [PubMed] [Google Scholar]
- 48.Li N., Morikawa T., Matsuda H., Ninomiya K., Li X., Yoshikawa M. New flavanone oligoglycosides, theaflavanosides I, II, III, and IV, with hepatoprotective activity from the seeds of tea plant (Camellia sinensis) Heterocycles. 2007;71:1193–1201. [Google Scholar]
- 49.Ninomiya K., Morikawa T., Zhang Y., Nakamura S., Matsuda H., Muraoka O., Yoshikawa M. Bioactive constituents from Chinese natural medicines. XXIII. Absolute structures of new megastigmane glycosides, sedumosides A4, A5, A6, H, and I, and hepatoprotective megastigmanes from Sedum sarmentosum. Chem. Pharm. Bull. 2007;55:1185–1191. doi: 10.1248/cpb.55.1185. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y., Morikawa T., Nakamura S., Ninomiya K., Matsuda H., Muraoka O., Yoshikawa M. Bioactive constituents from Chinese natural medicines. XXV. New flavonol bisdesmosides, sarmenosides I, II, III, and IV, with hepatoprotective activity from Sedum sarmentosum. Heterocycles. 2007;71:1565–1576. [Google Scholar]
- 51.Ninomiya K., Morikawam T., Xie H., Matsuda H., Yoshikawa M. Bioactive constituents from Chinese natural medicines. XXXI. Hepatoprotective principles from Sinocrassula indica: Structres of sinocrassosides A8, A9, A10, A11, and A12. Heterocycles. 2008;75:1983–1995. [Google Scholar]
- 52.Nakamura S., Li X., Matsuda H., Ninomiya K., Morikawa T., Yamaguti K., Yoshikawa M. Bioactive constituents from Chinese natural medicines. XXVI. chemical structures and hepatoprotective effects of constituents from roots of Rhodiola sachalinensis. Chem. Pharm. Bull. 2007;55:1505–1511. doi: 10.1248/cpb.55.1505. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura S., Okazaki Y., Ninomiya K., Morikawa T., Matsuda H., Yoshikawa M. Medicinal flowers. XXIV. Chemical structures and hepatoprotective effects of constituents from flowers of Hedychium coronarium. Chem. Pharm. Bull. 2008;56:1704–1709. doi: 10.1248/cpb.56.1704. [DOI] [PubMed] [Google Scholar]
- 54.Chaipech S., Morikawa T., Ninomiya K., Yoshikawa M., Pongpiriyadacha Y., Hayakawa T., Muraoka O. Structures of two new phenolic glycosides, kaempferiaosides A and B, and hepatoprotective constituents from the rhizomes of Kaempferia parviflora. Chem. Pharm. Bull. 2012;60:62–69. doi: 10.1248/cpb.60.62. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura S., Xu F., Ninomiya K., Nakashima S., Oda Y., Morikawa T., Muraoka O., Yoshikawa M., Matsuda H. Chemical structures and hepatoprotective effects of constituents from Cassia auriculata leaves. Chem. Pharm. Bull. 2014;62:1026–1031. doi: 10.1248/cpb.c14-00420. [DOI] [PubMed] [Google Scholar]
- 56.Kouroku Y., Fujita E., Jimbo A., Mukasa T., Tsuru T., Momoi M.Y., Momoi T. Locallization of active from of caspase-8 in mouse L929 cells induced by TNF treatment and polyglutamine aggregates. Biochem. Biophys. Res. Commun. 2000;270:972–977. doi: 10.1006/bbrc.2000.2463. [DOI] [PubMed] [Google Scholar]
- 57.Tiegs G., Wolter M., Wendel A. Tumor necrosis factor is a terminal mediator in galactosamine/endotoxin-induced hepatitis in mice. Biochem. Pharmacol. 1989;38:627–631. doi: 10.1016/0006-2952(89)90208-6. [DOI] [PubMed] [Google Scholar]
- 58.Peng X.X., Zhang S.H., Wang X.L., Ye T.J., Li H., Yan X.F., Wei L., Wu Z.P., Hu J., Zou C.P., et al. Panax Notoginseng flower saponins (PNFS) inhibit LPS-stimulated NO overproduction and iNOS gene overexpression via the suppression of TLR4-mediated MAPK/NF-kappa B signaling pathways in RAW264.7 macrophages. Chin. Med. 2015;10 doi: 10.1186/s13020-015-0045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao Y., Chen X., Fang L., Liu F., Cai R., Peng C., Qi Y. Rhein exerts pro- and anti-inflammatory actions by targeting IKKβ inhibition in LPS-activated macrophages. Free Radic. Biol. Med. 2014;72:104–112. doi: 10.1016/j.freeradbiomed.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Borges P.V., Moret K.H., Maya-Monteiro C.M., Souza-Silva F., Alves C.R., Batista P.R., Caffarena E.R., Pacheco P., das Graças Henriques M., Penido C. Gedunin binds to myeloid differentiation protein 2 and impairs lipopolysaccharide-induced toll-like receptor 4 signaling in macrophages. Mol. Pharmacol. 2015;88:949–961. doi: 10.1124/mol.115.098970. [DOI] [PubMed] [Google Scholar]