Abstract
The objective of this research was to characterize the high level of resistance to stripe that has been observed in the released wheat cultivar, Chuanmai45. A combination of classic genetic analysis, molecular and cytogenetic methods were used to characterize resistance in an F2 population derived from Chuanmai45 and the susceptible Chuanmai42. Inheritance of resistance was shown to be conferred by two genes in Chuanmai45. Fluorescence in situ hybridization (FISH) was used along with segregation studies to show that one gene was located on a 1RS.1BL translocation. Molecular markers were employed to show that the other locus was located on chromosome 4B. The defeated gene, Yr24/26, on chromosome 1BL was present in the susceptible parent and lines that recombined this gene with the 1RS.1BL translocation were identified. The germplasm, loci, and associated markers identified in this study will be useful for application in breeding programs utilizing marker-assisted selection.
Keywords: stripe rust resistance, wheat-rye 1RS.1BL translocations, Chuanmai45
1. Introduction
Wheat stripe rust (yellow rust), caused by Puccinia striiformis (PST) Westend. f. sp. tritici Eriks., is a major disease of wheat (Triticum aestivum L.) worldwide, and can cause yield losses as high as 75% in extremely susceptible cultivars [1]. Growing resistant cultivars is the most economic, effective and environment-friendly approach to control the disease [2]. To date, more than 70 stripe rust resistance genes with official and provisional designations have been reported in wheat [3]. The majority of these genes are race-specific with virulence being detected in various parts of the world, rendering cultivars carrying these genes susceptible [4]. In China, there are very few major genes that are still effective to all pathotypes. It is, therefore, urgent to identify new effective genes for resistance to stripe rust and to pyramid the different genes into wheat cultivars.
Chen et al. [5] analyzed the stripe rust pathogen populations in China and reported that a few races, namely CYR32, CYR33, Su11-4, and Su11-7, have predominated in areas prone to stripe rust. Among the officially-named stripe rust resistance genes, only Yr5, Yr10, Yr15, and Yr24/26 confer resistance to the race CYR32 [5,6,7]. Cultivars with Yr24/26 have been rapidly released in recent years [8]. However, a new virulent stripe rust race, Accession No. 09-6-16-3, also termed v26, is virulent to this locus [9] and this pathotype is increasing in frequency. It is, therefore, essential to identify new stripe rust resistance genes that are effective against the Yr26 virulent race. Molecular markers associated with the new resistance genes are also desirable as they can facilitate the rapid deployment of the new genes through marker-assisted selection (MAS).
Chuanmai45 (CH45), a spring wheat cultivar developed by the Crop Research Institute, Sichuan Academy of Agricultural Sciences, has high yield potential and was released in 2004. It displayed high resistance to stripe rust in experimental plots in both seedlings and adult plants. In the present study, molecular and cytogenetic analyses were used to characterize the stripe rust resistance in CH45, along with further characterization of the defeated Yr24/26 gene, carried by Chuanmai42 (CH42) [10,11]. The concomitant identification of molecular markers should be useful for maker assisted selection (MAS) of stripe rust resistance in common wheat backgrounds.
2. Results
2.1. Assessment of Stripe Rust Resistance
Reactions of different wheat cultivars or lines with known resistance genes to PST No. 09-6-16-3 were listed in Table 1. The resistance reaction of the wheat lines with known genes were consistent with Liu et al. [9]. The lines CN19, containing Yr41, and the Avocet NILs, containing Yr29 and Yr31, all showed high susceptibility. CH45 and its parent, SW1862, showed complete resistance to PST No. 09-6-16-3, while CH42 was highly susceptible (Figure 1, Table 1).
Table 1.
Virulence testing of wheat stripe rust strain pathotype No. 09-6-16-3 on wheat stripe rust differential lines and parental lines at the seedling stage.
| Genotypes/Lines | Yr Gene Location | Infection Types |
|---|---|---|
| Avocet | - | 4 |
| Avocet+Yr1 | 2A | 0 |
| Kalyansona (Yr2, Yr29) | 7B, 1B | 3 |
| Avocet+Yr5 | 2B | 0 |
| Avocet+Yr6 | 7B | 0 |
| Avocet+Yr7 | 2B | 4 |
| Avocet+Yr8 | 2M/2B | 4 |
| Avocet+Yr10 | 1B | 4 |
| Avocet+Yr15 | 1B | 0 |
| Avocet+Yr17, Yr18 | 2A, 7D | 3 |
| Avocet+Yr18 | 7D | 4 |
| Avocet+Yr24 | 1B | 4 |
| Avocet+Yr26 | 1B | 4 |
| Avocet+Yr27 | 2B | 3 |
| Avocet+Yr28 | 4D | 0 |
| Avocet+Yr29 | 1B | 4 |
| Avocet+Yr31 | 2B | 3 |
| Avocet+YrA | unkmown | 4 |
| Avocet+YrCV | 2AL | 0 |
| Avocet+YrSp | 2B | 0 |
| Pavon 76 (Yr6, Yr7, Yr29, Yr30, +) | 1B, 2B, 7B | 1 |
| PBW 343 (Yr9, Yr27, Yr29, +) | 1B, 2B | 4 |
| Seri 82 (Yr2, Yr9, Yr29, Yr30, +) | 1R/1B, 1B, 3B, 7B | 3 |
| Super Kauz (Yr9, Yr27, Yr18, Yr30, +) | 1R/1B, 2B, 3B, 7D | 3 |
| Chuanmai45 | - | 0 |
| SW1862 | - | 0 |
| CN19 (Yr41) | 2B | 4 |
| Chuanmai42 (YrCH42=Yr26) | 1B | 4 |
Figure 1.
The seedlings of CH45 (left) and CH42 (right) inoculated by race No. 09-6-16-3.
2.2. Genetic Analysis of Resistance in Chuanmai45 (CH45)
The resistance to stripe rust in the parents and in the F1 and F2 populations were investigated with infection types (IT) responses of seedlings against v26 (Table 2). The resistant parent (CH45) had an IT of 0, while the two susceptible parents (CH42 and CN19) scored 4. Two F1 and F2 populations from two crosses, CH45/CH42 and CH45/CN19, were tested. Both F1 populations consisted of completely susceptible plants, indicating that the resistance in CH45 was recessive. Furthermore, the F2 generation of both CH45/CH42 (192 plants) and CH45/CN19 (206 plants) segregated in a 7:9 ratio for resistance and susceptibility (Table 2). These results are consistent with the presence of two recessive genes, which are hereafter named throughout the text as yrCH45-1 and yrCH45-2.
Table 2.
Genetic analysis of seedling resistance to stripe rust pathotype No. 09-6-16-3.
| Materials | No. of Plants | Infection Type (R) | Infection Type (S) | Expected Ratio | Chi-Square Test (χ2) | p Value * | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 1 | 2 | 3 | 4 | |||||
| CH45 | 10 | 10 | - | - | - | - | - | - | - | - |
| CH42 | 10 | - | - | - | - | - | 10 | - | - | - |
| CN19 | 10 | - | - | - | - | - | 10 | - | - | - |
| CH45/CH42F1 | 10 | - | - | - | - | - | 10 | - | - | - |
| CH45/CH42F2 | 192 | 13 | 48 | 23 | 29 | 18 | 61 | 7:9 | 0 | 1 |
| CH45/CN19F1 | 10 | - | - | - | - | 8 | 2 | - | - | - |
| CH45/CN19F2 | 206 | 25 | 43 | 28 | 9 | 5 | 96 | 7:9 | 0.7 | 0.4 |
*: p values were calculated from χ2 statistic and show significance at p > 0.05.
2.3. Bulk Segregate Analysis (BSA) of Resistance
Susceptible and resistant bulks of the CH45/CH42 population were developed by selecting 15 resistant lines (IT0-0;) and 15 susceptible lines (IT4) from the F2 population. A total of 168 primer pairs from SSR and expressed sequence tags derived SSR (EST-SSR) primers were screened in the bulk segregate analysis (BSA). There were 40 differentially-amplified fragments between CH42 and the susceptible bulk when compared to CH45 and the resistant bulk (RB). Eight primer pairs (TOP1017, TNAC1009, TNAC1021, TNAC1045, EST123, Xgwm11, Xgwm18, and Xgwm498) that generated strong and repeatable polymorphic bands had been previously assigned to the 1BS chromosome arm [11,12,13].
2.4. In Situ Hybridization of CH45
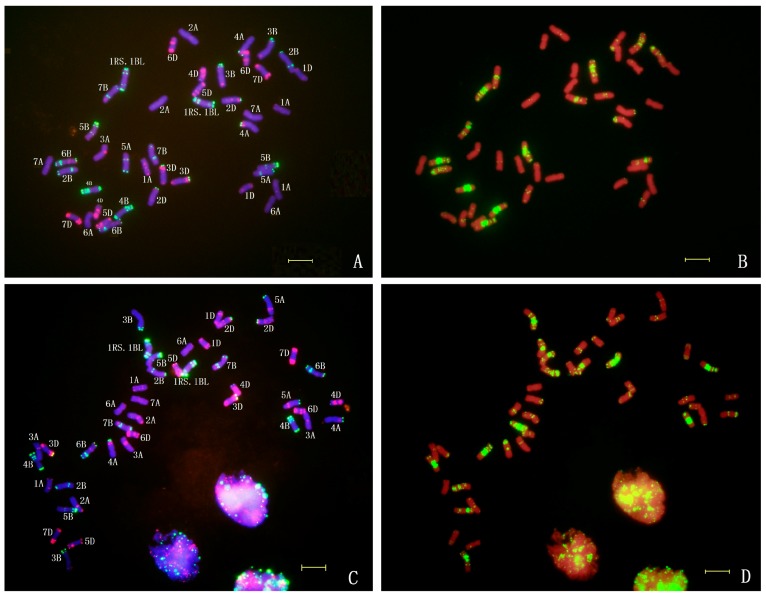
In order to reveal the chromosomal constitution of CH45, we performed sequential, multicolor, fluorescence in situ hybridization (FISH) with the probes Oligo-pSc119.2, Oligo-pTa535, and Oligo-(GAA)7 to mitotic metaphase cells of CH45 and its parents SW1862 (Figure 2). Based on the FISH standard karyotype of wheat and rye chromosomes [14], we showed that both SW1862 (Figure 2A,B) and CH45 (Figure 2C,D) possessed a rye 1R chromosomal short arm substitution on 1BS, indicating that these lines contain 1RS.1BL translocations. Co-incidentally, all 44 lines that were homozygous for 1RS.1BL were also resistant (Table 3). This data, combined with the BSA, give a strong indication that yrCH45-1 was located on the 1RS of this translocation.
Figure 2.
Fluorescence in situ hybridization (FISH) of SW1862 (A,B) and Chuanmai45 (CH45) (C,D) with (A,C) stained by DAPI (blue), Oligo-pTa535 (red), and Oligo-pSc119.2 (green), (B,D) stained by PI (red) and Oligo-(GAA)7 (green).Scale bar indicates 10 μm.
Table 3.
Co-segregation of chromosome 1RS with rust resistance of F2 plants from the cross of CH45/CH42 tested against stripe rust pathotype No. 09-6-16-3.
| No Plant of Homozygous 1RS | No Plant of Non Homozygous 1RS | Expected Ratio | Chi-Square Test (χ2) | p Values * | ||
|---|---|---|---|---|---|---|
| 44 | 148 | 1:3 | 0.4 | 0.50 | ||
| Resistance | Susceptible | Resistance | Susceptible | - | - | - |
| 44 | 0 | - | - | - | - | - |
| - | - | 40 | 108 | 1:3 | 0.32 | 0.57 |
*: p values were calculated from χ2 statistic and show significance at p > 0.05.
The F2 plants that were 1RS.1BL heterozygotes segregated for resistance in a fashion that indicated the presence of another resistance gene independent of the translocation. This subgroup of translocation heterozygotes segregated in a 1:3 ratio for resistance. This, along with the susceptibility of all F1 plants, indicates that this second gene was also recessive in nature. It was temporarily named yrCH45-2, and was independent of yrCH45-1. A BSA analysis was used to identify molecular markers associated with yrCH45-2. A total of 40 markers that differentiated the CH42 and CH45 parental lines were used to screen bulked DNA from lines that segregated for resistance but were heterozygous for the 1RS translocation. Marker Xwmc251 had a loose association with resistance and this was known to reside on chromosome 4B. A total of 16 more SSR primer pairs and nine EST-SSR markers [12] for chromosome 4B were tested and three more were polymorphic within the populations and in linkage disequilibrium with resistance (Figure 3 and Figure 4). Thus, a recessive gene yrCH45-2 on 4BL contributed to the rust resistance ITs in CH45. In conclusion, the yrCH45-1 and yrCH45-2 were located on 1RS.1BL and 4BL of CH45, respectively.
Figure 3.
PCR amplification of Ora12 (A); TOP1017 (B); Xgwm18 (C); Xgwm498 (D); and Xwe173 (E) separated by agarose (A,B,E) and silver-stained polyacrylamide gels (C,D).
Figure 4.
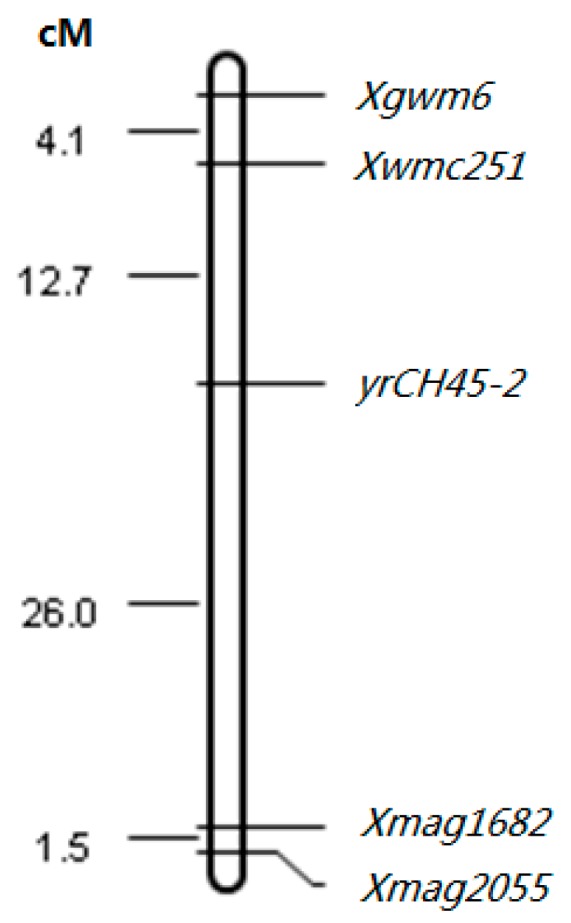
Map position of yrCH45-2 on chromosome 4BL. Heterozygous F2 translocation lines (1RS.1BL) were used to map the resistance locus on 4BL as resistance on the translocation was recessive and would, therefore, not confound phenotypic responses associated with segregation on 4BL.
2.5. Recombination of Yr26 in CH45
CH42 contains the Yr26 gene for stripe rust resistance on chromosome 1B [10,11] and several sequence amplified characterized region (SCAR) markers linked to this gene have been produced [15]. The closest flanking marker, Xwe173, is co-dominant and was polymorphic between CH45 and CH42. There were 11 out of the 192 F2 plants from the CH45/CH42 population that were homozygous for both Xwe173 and the 1RS.1BL translocation.
3. Discussion
CH45 continues to show high levels of resistance in the face of changing virulence of stripe rust pathogens in China. Pathotypes CYR32 and CYR33 have caused significant disease epidemics in Southwest China in recent years. The evolution of the v26 pathotype is a major concern as it has virulence for Yr24/26, a gene that has been widely used throughout China. The combination of two loci may help to increase the durability of CH45. The recessive nature of the resistances identified on a rye translocation and on chromosome 4B have helped to differentiate these loci from other known stripe rust resistances. CH45 also has good yield potential and should make a good parent in crossing programs. The markers identified in this study will facilitate the incorporation of both loci into new germplasm.
The locus yrCH45-1 was located on a 1RS.1BL translocation. A previous 1RS.1BL translocation from S. cereale contained the stripe rust gene Yr9. Clearly, the v26 pathogen is virulent on Yr9, however, yrCH45-1 showed an avirulent reaction. Virulence to Yr9 was indicated by high IT scores on PBW343, Seri82, and Super Kauz (Table 1), all of which contain this gene. The original 1RS.1BL translocation had a significant impact in wheat breeding and global wheat production over several decades. It altered yield components [16] and brought in other resistance genes including Sr31, Lr26, and Pm8 [17]. The Sr31 resistance gene provided stable resistance to stem rust for up to 20 years and was probably the main reason this translocation became so common in global germplasm [4]. However, all resistance genes on this original translocation have now broken down and efforts have been underway to search for different sources of rye chromosome 1RS which contain agronomically useful genes [18].
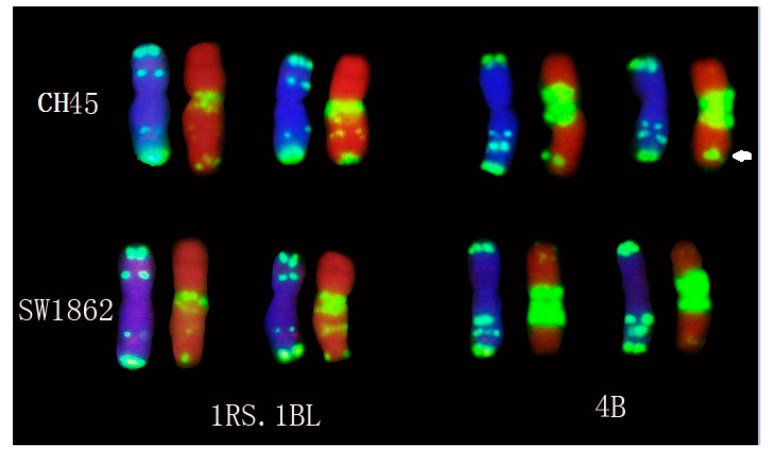
Recently, new 1RS.1BL translocation lines derived from different rye varieties have been developed and will be useful in wheat improvement [19]. Luo et al. [20] also developed new 1RS.1BL and characterized two stripe rust resistance loci termed YrCN17 and YrR212 originating from the 1RS chromosomal segment. These genes were effective in protecting wheat from the races CYR31 and CYR32. It is likely that yrCH45-1 is different to YrCN17 and YrR212, as the former locus was inherited in a recessive fashion whereas the latter two are dominant. Based on the FISH hybridization (Figure 2 and Figure 5), it is also likely that yrCH45-1 is derived from a different source as one of its parents, SW1862,was developed from alternate 1RS.1BL translocations that were distributed by International Maize and Wheat Improvement Centre (CIMMYT) in the 1980s [21]. The germplasm by Luo et al. [20] developed much more recently in China and likely used different Rye sources. Next generation sequencing of core 1RS chromosomes should help to characterize diversity of these translocations and facilitate the search for novel resistances for incorporation into bread wheat [22].
Figure 5.
FISH karyotype of chromosomes 1RS.1BL and 4B in SW1862 and CH45. Left chromosomes were stained with DAPI (blue), Oligo-pTa535 (red), and Oligo-pSc119.2 (green), while right chromosomes were stained by PI (red) and Oligo-(GAA)7 (green). Arrow shows the unique hybridization on 4B of CH45.
There are several other loci on chromosome 1B, including Yr10, Yr15, Yr24/26, Yr29, and YrH52. The yrCH45-1 locus is easily differentiated from most of these due to IT data as the pathotype used in this study was virulent on Yr10 and Yr24/26 and Yr29 is an adult plant resistance gene that gives a susceptible IT in seedling tests (Table 1). This CH45 locus can also be differentiated from Yr15 and Yr52H as both of these loci were derived from T. dicoccoides translocations onto chromosome 1BS while yrCH45-1 has its origins in rye.
The yrCH45-2 was located on chromosome 4B and may be a new stripe rust resistance gene. Recently, Yr50 was identified as a dominant gene on chromosome 4BL that was also resistant to the v26 pathotype [23]. Molecular markers place these two loci in similar chromosomal locations; however, the recessive nature of inheritance of yrCH45-2 gives an indication that it is likely different from Yr50. Based on the FISH study of chromosome 4B in CH45 (Figure 2 and Figure 5), we found that the Oligo-(GAA)7 gave rise to a strong signal on 4BL of CH45, while SW1862 did not produce such a signal. Furthermore, Yr50 was introgressed from Thinopyrum intermedium. There is no information in the pedigree of CH45 that suggests it would have Th. intermedium parentage. Allelism tests may not be of use in differentiating these loci as the Th. intermedium DNA does not normally recombine with common wheat, and the similar location would render all progeny of such a test as resistant. Genomic in situ hybridization (GISH) may be useful in identifying Th. intermedium DNA in CH45 and such a result would indicate that yrCH45-2 could be Yr50. The flanking markers of Xwmc251 and Xmag1682 were some distance from the locus yrCH45-2. Research to identify more tightly-linked markers in breeding programs is underway.
The gene resistance Yr24 and Yr26 have been shown to be identical and present in CH42 [10,11]. CH42 was released in 2003 and not only contains Yr24/26 resistance but has been very widely adopted throughout the region due to its significant yield increase over other existing varieties [24]. Original work identified Yr26 as being derived from a T. turgidum landrace [25] that was used in the creation of the wheat-Dasypyrum villosum 6VS/6AL translocation line R64 (92R-149). As the 6VS/6AL translocation also contained the powdery mildew resistance gene, Pm21 [26], R64 was particularly useful in many wheat-growing regions throughout China. Many Chinese breeding programs have used it to develop new cultivars with powdery mildew and stripe rust resistance [8].
The widespread occupation of Yr24/26 containing lines has facilitated the growing presence of v26 [9]. Resistance to CYR32 and CYR33 afforded by Yr24/26 is still useful as these pathotypes remain relatively common in Chinese environments [3]. Closely linked, flanking, co-dominant markers to Lr24/26 have been identified [15,27]. These provide a useful tool in any marker assisted selection strategy. The 1RS.1BL translocation does not recombine with chromosome 1BS from common wheat and its presence can be determined through the use of the TOP1017 marker. This marker is, therefore, diagnostic for the yrCH45-1 locus. There were several lines identified in this study that had recombined the 1RS.1BL segment with Xwe173, a marker for Yr24/26. Given the sound markers available for these loci, it would be relatively easy to maintain this recombination in breeding programs. Such recombinants may be useful in the future should CYR32 or CYR33 develop virulence for the yrCH45-1 locus. The further pyramiding of the yrCH45-2 locus from 4B, again with the use of markers, will help develop lines that should be able to retain resistance for a significant period of time.
4. Materials and Methods
4.1. Plant Materials
CH45 was developed from the cross SW1862/GH430. Cultivars and near-isogenic lines (NIL) with known stripe rust resistance genes in the Avocet background were obtained from Robert A. McIntosh, University of Sydney, Australia. Wheat cultivar CH42 was developed by Wuyun Yang of Sichuan Academy of Agricultural Sciences, and CN19 was developed by Zhenglong Ren of the Sichuan Agricultural University.
4.2. Disease Resistance Screening
Pure isolates of the v26 pathotype were used to inoculate CH45, CH42, CN19, SW1862, and their progenies. The avirulence/virulence formula of the new pathotype is Yr1, 3, 4, H46, 5, 6, 15, 17, 18, 32, Sp, Sd/Yr2, 8, 9, 10, 12, 24 (=26), 31, Su [9].
Seeds were planted in small pots with seven plants of the test line along with three plants of the susceptible cultivar Mingxian 169. Seedlings were inoculated with v26 when the first leaf was fully expanded. After inoculation, the seedlings were placed in a dew chamber at 19 °C and 100% relative humidity for 24 h and then transferred to a greenhouse maintained with 14 h light/10 h dark photoperiod at 12–17 °C. Infection types (IT) were scored 14–15 days after inoculation when rust was fully developed on the susceptible check. Infection types were based on a 0–4 scale [28], with 0 for no visible uredia; 0; for small chlorotic flecks without sporulation; 1 for limited uredial development associated with chlorosis and necrosis; 2 for intermediate sporulation with chlorosis and/or necrosis; 3 for abundant sporulation with chlorosis; and 4 for abundant sporulation without chlorosis. Rating of the seedling reactions was simplified to two classes (resistant and susceptible) as there was a clear distinction between these two categories.
4.3. Cytological Analysis
Seedling root tips were collected and then treated with nitrous oxide followed by enzyme digestion, using the procedure of Han et al. [29]. The synthesized oligo-nucleotide probes Oligo-pSc119.2, Oligo-pTa535, and Oligo-(GAA)7 were used for identifying the wheat chromosomes by FISH according to the description of Tang et al. [30]. The protocol of non-denaturing FISH by the synthesized probes was described by Fu et al. [12].
4.4. Molecular Marker Analysis
Genomic DNA from fresh young leaves was extracted by the SDS method [31] and 2 μL of the DNA solution (ca. 100 ng) was used as a template for PCR. Two sets of primers pairs, TOP1017 [32] and Ora12 [33], were used to amplify the 1RS DNA. PCR amplification was carried out in a MyCycleTM Thermal Cycler (Bio-RAD, Hercules, CA, USA). The cycling parameters were 94 °C for 3 min, followed by 40 cycles of 94 °C for 45 s, 55 to 62 °C (depend on the primer) for 45 s, and 72 °C for 2 min, with a final extension at 72 °C for 7 min. An 8 μL aliquot of the product was digested for two hours with 1.0 U each of TaqI (65 °C), HpaII (37 °C), and HaeIII (37 °C). Digested fragments were fractionated by electrophoresis on a 2% agarose gel. The SSR and expressed sequence tags (EST) derived SSR primers and their relevant PCR protocols were described in Xue et al. [13].
5. Conclusions
This study identified two potentially new resistance genes to stripe rust on chromosome 4B and on a 1RS.1BL rye translocation. These are effective against the v26 pathotype in China. The new genes are inherited in a recessive fashion, a feature that helps to distinguish them from other loci. The translocation was shown to recombine with Yr24/26, a 1B locus that has been overcome by v26. Germplasm identified in this study, and markers used here and elsewhere, have the potential to pyramid these loci to provide durable resistance.
Acknowledgments
The study was supported by The Ministry of Science and Technology of China (Grant No. 2011DFG33350, Grant No. 2012DFA32290), Sichuan Youth Science & Technology Foundation (Grant No. 2012JQ0013), and Science & Technology Department of Sichuan Province (Grant No.2015HH0051).
Author Contributions
Ennian Yang and Guangrong Li conceived of and designed the experiments. Guangrong Li, Liping Li, Zhenyu Zhang, Yunliang Peng and Yongqing Zhu performed the experiments. Wuyun Yang, Zujun Yang and Garry M. Rosewarne analyzed the data. Ennian Yang, Zujun Yang and Garry M. Rosewarne wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Roelfs A.P., Singh R.P., Saari E.E. Rust Diseases of Wheat: Concepts and Methods of Disease Management. CIMMYT; Mexico City, Mexico: 1992. [Google Scholar]
- 2.Wellings C.R. Global status of stripe rust: A review of historical and current threats. Euphytica. 2011;179:129–141. doi: 10.1007/s10681-011-0360-y. [DOI] [Google Scholar]
- 3.McIntosh R.A., Dubcovsky J., Rogers J., Morris C., Appels R., Xia X. 2010: Catalogue of Gene Symbols for Wheat: 2010 Supplement. [(accessed on 30 July 2010)]. Available online: http://www.shigen.nig.ac.jp/wheat/komugi/genes/
- 4.Ellis J.G., Lagudah E.S., Spielmeyer W., Dodds P.N. The past, present and future of breeding rust resistant wheat. Front Plant Sci. 2014;5 doi: 10.3389/fpls.2014.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W.Q., Wu L.R., Liu T.G., Xu S.C., Jin S.L., Peng Y.L., Wang B.T. Race dynamics, diversity, and virulence evolution in Puccinia striiformis f. sp. tritici, the causal agent of wheat stripe rust in China from 2003 to 2007. Plant Dis. 2009;93:1093–1101. doi: 10.1094/PDIS-93-11-1093. [DOI] [PubMed] [Google Scholar]
- 6.Wan A.M., Zhao Z.H., Chen X.M., He Z.H., Jin S.L., Jia Q.Z., Yao G., Yang J.X., Wang B.T., Li G.B., et al. Wheat stripe rust epidemic and virulence of Puccinia striiformis f. sp. tritici in China in 2002. Plant Dis. 2004;88:896–904. doi: 10.1094/PDIS.2004.88.8.896. [DOI] [PubMed] [Google Scholar]
- 7.Kang Z., Zhao J., Han D., Zhang H., Wang X., Wang C., Han Q., Guo J., Huang L. Status of Wheat Rust Research and Control in China. BGRI 2010 Technical Workshop; St. Petersburg, Russia: 2010. pp. 50–69. [Google Scholar]
- 8.He Z.H., Xia X.C., Chen X.M., Zhuang Q.S. Progress of wheat breeding in China and the future perspective. Acta Agron. Sin. 2011;37:202–215. doi: 10.3724/SP.J.1006.2011.00202. [DOI] [Google Scholar]
- 9.Liu G.T., Peng Y.L., Chen W.Q., Zhang Z.Y. First detection of virulence in Puccinia striiformis f. sp. tritici in China to resistance genes Yr24 (=Yr26) present in wheat cultivar Chuanmai 42. Plant Dis. 2010;94 doi: 10.1094/PDIS-94-9-1163C. [DOI] [PubMed] [Google Scholar]
- 10.Li G.Q., Li Z.F., Yang W.Y., Zhang Y., He Z.H., Xu S.C., Singh R.P., Qu Y.Y., Xia X.C. Molecular mapping of stripe rust resistance gene YrCH42 in Chinese wheat cultivar Chuanmai 42 and its allelism with Yr24 and Yr26. Theor. Appl. Genet. 2006;112:1434–1440. doi: 10.1007/s00122-006-0245-y. [DOI] [PubMed] [Google Scholar]
- 11.Wen W.E., Li G.Q., He Z.H., Yang W.Y., Xu M.L., Xia X.C. Development of an STS marker tightly linked to Yr26 against wheat stripe rust using the resistance gene-analog polymorphism (RGAP) technique. Mol. Breed. 2008;22:507–515. doi: 10.1007/s11032-008-9194-2. [DOI] [Google Scholar]
- 12.Xue S., Zhang Z., Lin F., Kong Z., Cao Y., Li C., Yi H., Mei M., Zhu H., Wu J., et al. A high-density intervarietal map of the wheat genome enriched with markers derived from expressed sequence tags. Theor. Appl. Genet. 2008;117:181–189. doi: 10.1007/s00122-008-0764-9. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa G., Nakamura T., Ashida T., Saito M., Nasuda S., Endo T., Wu J., Matsumoto T. Localization of anchor loci representing five hundred annotated rice genes to wheat chromosomes using PLUG markers. Theor. Appl. Genet. 2009;118:499–514. doi: 10.1007/s00122-008-0916-y. [DOI] [PubMed] [Google Scholar]
- 14.Fu S., Chen L., Wang Y., Li M., Yang Z., Qiu L., Yan B., Ren Z., Tang Z. Oligonucleotide probes for ND-FISH analysis to identify rye and wheat chromosomes. Sci. Rep. 2015;5 doi: 10.1038/srep10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C.M., Zhang Y.P., Han D.J., Kang Z.S., Li G.P., Cao A.Z., Chen P.D. SSR and STS markers for wheat stripe rust resistance gene Yr26. Euphytica. 2008;159:359–366. doi: 10.1007/s10681-007-9524-1. [DOI] [Google Scholar]
- 16.Singh R.P., Huerta-Espino J., Rajaram S., Crossa J. Agronomic effects from chromosome translocations 7DL.7Ag and 1RS.1BL in spring wheat. Crop Sci. 1998;38:27–33. doi: 10.2135/cropsci1998.0011183X003800010005x. [DOI] [Google Scholar]
- 17.Villareal R.L., Rajaram S., Mujeeb-Kazi A., Del Toro E. The effect of chromosome 1RS.1BL translocation on the yield potential of certain spring wheats (Triticum aestivum L.) Plant Breed. 1991;106:77–81. doi: 10.1111/j.1439-0523.1991.tb00482.x. [DOI] [Google Scholar]
- 18.Lelley T., Eder C., Grausgruber H. Influence of 1RS.1BL wheat-rye chromosome translocation on genotype by environment interaction. J. Cereal. Sci. 2004;39:313–320. doi: 10.1016/j.jcs.2003.11.003. [DOI] [Google Scholar]
- 19.Ren T.H., Chen F., Yan B.J., Zhang H.Q., Ren Z.L. Genetic diversity of wheat-rye 1RS.1BL translocation lines derived from different wheat and rye sources. Euphytica. 2012;183:133–146. doi: 10.1007/s10681-011-0412-3. [DOI] [Google Scholar]
- 20.Luo P.G., Zhan H.Y., Shu K., Zhang H.Q., Luo H.Y., Ren Z.L. Stripe rust (Puccinia striiformis f. sp. tritici) resistance in wheat with wheat-rye 1BL/1RS chromosomal translocation. Can. J. Plant Pathol. 2008;30:254–259. doi: 10.1080/07060661.2008.10540540. [DOI] [Google Scholar]
- 21.Rabinovich S.V. Importance of wheat-rye translocations for breeding modern cultivars of Triticum aestivum L. Euphytica. 1998;100:323–340. doi: 10.1023/A:1018361819215. [DOI] [Google Scholar]
- 22.Fluch S., Kopecky D., Burg K., Šimková H., Taudien S., Petzold A., Kubaláková M., Platzer M., Berenyi M., Krainer S., et al. Sequence composition and gene content of the short arm of rye (Secale cereale) chromosome 1. PLoS ONE. 2012;7:601. doi: 10.1371/journal.pone.0030784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J., Chang Z., Zhang X., Yang Z., Li X., Jia J., Zhan H., Guo H., Wang J. Putative Thinopyrum intermedium-derived stripe rust resistance gene Yr50 maps on wheat chromosome arm 4BL. Theor. Appl. Genet. 2013;126:265–274. doi: 10.1007/s00122-012-1979-3. [DOI] [PubMed] [Google Scholar]
- 24.Tang Y.L., Rosewarne G.M., Li C.S., Wu X.L., Yang W.Y., Wu C. Physiological factors underpinning grain yield improvements of synthetic derived wheat in south western China. Crop Sci. 2014;55:98–122. doi: 10.2135/cropsci2014.02.0124. [DOI] [Google Scholar]
- 25.Ma J.X., Zhou R.H., Dong Y.S., Wang L.F., Wang X.M., Jia J.Z. Molecular mapping and detection of the yellow rust resistance gene Yr26 in wheat transferred from Triticum turgidum L. using microsatellite markers. Euphytica. 2001;120:219–226. doi: 10.1023/A:1017510331721. [DOI] [Google Scholar]
- 26.Qi L.L., Chen P.D., Liu D.J., Zhou B., Zhang S.Z. The gene Pm21: A new source for resistance to wheat powdery mildew. Acta Agron. Sin. 1995;21:257–262. [Google Scholar]
- 27.Zhang X., Han D., Zeng Q., Duan Y., Yuan F., Shi J., Wang Q., Wu J., Huang L., Kang Z. Fine mapping of wheat stripe rust resistance gene Yr26 based on collinearity of wheat with Brachypodium distachyon and rice. PLoS ONE. 2013;8:601. doi: 10.1371/journal.pone.0057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bariana H.S., McIntosh R.A. Cytogenetic studies in wheat. XV. Location of rust resistance genes in VPM1 and their genetic linkage with other disease resistance genes in chromosome 2A. Genome. 1993;36:476–482. doi: 10.1139/g93-065. [DOI] [PubMed] [Google Scholar]
- 29.Han F.P., Lamb J.C., Birchler J.A. High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. Proc. Natl. Acad. Sci. USA. 2006;103:3238–3243. doi: 10.1073/pnas.0509650103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang Z.X., Yang Z.J., Fu S.L. Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and pAWRC.1 for FISH analysis. J. Appl. Genet. 2014;55:313–318. doi: 10.1007/s13353-014-0215-z. [DOI] [PubMed] [Google Scholar]
- 31.Yang Z.J., Li G.R., Jia J.Q., Zeng X., Lei M.P., Zeng Z.X., Zhang T., Ren Z.L. Molecular cytogenetic characterization of wheat—Secale africanum amphiploids and derived introgression lines with stripe rust resistance. Euphytica. 2009;167:197–202. doi: 10.1007/s10681-008-9861-8. [DOI] [Google Scholar]
- 32.Bartos J., Paux E., Kofler R., Havrankova M., Kopecky D., Suchankova P., Safar J., Simkova H., Town C.D., Lelley T., et al. A first survey of the rye (Secale cereale) genome composition through BAC end sequencing of the short arm of chromosome 1R. BMC Plant Biol. 2008;8 doi: 10.1186/1471-2229-8-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuchida M., Fukushima T., Nasuda S., Masoudi-Nejad A., Ishikawa G., Nakamura T., Endo T.R. Dissection of rye chromosome 1R in common wheat. Genes Genet. Syst. 2008;83:43–53. doi: 10.1266/ggs.83.43. [DOI] [PubMed] [Google Scholar]