Abstract
Background:
The human immunodeficiency virus (HIV) pandemic has brought about a resurgence in tuberculosis (TB), especially in developing countries. Previous studies on TB lymphadenitis (TBLN) in South-Eastern Nigeria were done before the advent of the HIV pandemic making a review pertinent.
Aim:
To evaluate the role of TBLN as a cause of superficial lymphadenopathy in the post-HIV/acquired immune deficiency syndrome (AIDS) era of South-Eastern Nigeria.
Materials and Methods:
This is a 15 years (2000-2014) retrospective review of all superficial lymph node biopsies (SLNBs) received at the Department of Morbid Anatomy, University of Nigeria Teaching Hospital, Ituku-Ozalla Enugu, Nigeria.
Results:
One hundred and seventy-two cases of TBLN were identified in this study constituting 14.6% (172/1,180) of SLNBs received at our Hospital's Morbid Anatomy Department during the 15 years period under review. Twenty-eight cases of TBLN were clinically screened for HIV, 23 of which tested positive, representing 82.1% (23/28) of clinically screened cases. Acid fast bacilli demonstration was positive in 15.1% (26/172) of cases using Ziehl-Neelsen stain. 48.8% (84/172) of TBLN cases were males, and 51.2% (88/172) were females with most (22) cases received in 2012 and least (5) cases in 2000. Most TBLN occurred in the 21-25 years age group with a total of 21.5% (37/172) of cases and a male to female ratio of 1:1.5 The most common biopsy site for TBLN was the cervical group followed by the axillary and inguinal groups with 73.8% (127/172), 14% (24/172), and 4.7% (8/172) of cases, respectively.
Conclusions:
There is a remarkable decline in the prevalence of TB lymphadenitis in South-Eastern Nigeria indicating a change in trend from the pre- to the post-HIV/AIDS era with slightly more females now presenting with TBLN and most TB lymphadenitis patients now presenting with associated HIV/AIDS disease. There is an urgent need to provide modern diagnostic facilities in our medical laboratories.
Keywords: Histology, Human immunodeficiency virus/acquired immune deficiency syndrome, Lymphadenitis, Tuberculosis, Ziehl-Neelsen
Introduction
The scourge of tuberculosis (TB) continues to have global dimensions as one of the world's deadliest communicable diseases. In 2013, an estimated 9.0 million people developed TB, and 1.5 million died from the disease, 360,000 of whom were human immunodeficiency virus (HIV)-positive.[1] Though TB is slowly declining each year, it is estimated that 37 million lives were saved between 2000 and 2013 through effective diagnosis and treatment.[1]
TB lymphadenitis (TBLN) remains the most common form of extrapulmonary TB with cervical lymphadenopathy being the most common presentation while inguinal, axillary, mesenteric, mediastinal, and intramammary involvement have also been described.[2,3,4] It is caused by organisms of the Mycobacterium tuberculosis complex[5] and is responsible for 43% of peripheral lymphadenopathy in the developing world.[6]
One of the earliest studies of lymphadenopathy in Nigeria was published by Attah[7] who in 1974 at Ibadan reported TB lymphadenitis as accounting for 30% of superficial lymph node diseases. This was followed by Onuigbo[8,9] who in 1975 at Enugu reported peripheral lymphadenitis as a leading manifestation of TB with the maximal incidence occurring in the 10-29 years age group and males slightly outnumbering females. This was subsequently followed by Ejeckam and Nwabueze[10] who in 1984 at Enugu also reported 31.8% TBLN as the most common cause of superficial lymph node diseases in Enugu from January 1973 to June 1979. Similar studies in Jos,[11] Kano,[12] Ilorin,[13] Benin,[14] and Calabar[15] were also in tandem with 33.05%, 30%, 31.4%, 26.7%, and 45.9%, respectively. Abdurrahman in Zaria, Adedeji et al. in Benin, Oluwole et al. in Ile-Ife, Obafunwa et al. in Jos, and Pindiga et al. in Maiduguri have all reported TB as the most common single histopathological diagnosis causing lymphadenopathy in Nigeria.[16,17,18,19,20] Thus, studies in most Nigerian cities have reported TBLN as the most common lymph node pathology and cervical lymph nodes as the most frequently affected group of lymph nodes.[8,9,10,11,12,13,14,15,16,17,18,19,20] In other African cities such as Addis Ababa Ethiopia, Getachew et al.[21] in a study also found that the most common cause of lymphadenopathy (47.8%) was TB. They also determined that, of the lymph nodes biopsied, 57% were cervical, 15.17% axillary, and 8.7% inguinal lymph nodes. Mbise[22] in a study on peripheral lymphadenopathy in children in Dar es Salaam, Tanzania reported that, of the 257 excision peripheral lymph node biopsies of children between 4 days and 15 years of age analyzed, TBLN accounted for 67.3% while nonspecific reactive lymphadenitis, malignant neoplasm, and histiocytosis-X accounted for 20.6%, 11.3%, and 0.8%, respectively. In another study in Jimma, Ethiopia by Bezabih et al.[23] to determine the etiology of superficial enlarged lymph nodes using fine needle aspiration cytology they found a huge burden of benign lymph node enlargements, in general, and TBLN in particular. The latter was also responsible for about two-third of lymph node enlargements in South-Western parts of the country. Tiwari et al.[24] in a study in Nepal, South Asia, found that the most common cause of lymphadenopathy was TB, and the most common group of lymph nodes involved were the cervical lymph nodes. Moreover, in a clinicopathological analysis of lymphadenopathy in adults in Riyadh, Kingdom of Saudi Arabia, Abba et al.[25] found that granulomatous lymphadenopathy due to TB was the most frequent pattern (37.9%) and that TB was also more common in females than males (51% vs. 49%).
The emergence of HIV pandemic has also been associated with an increase in the total incidence of TB[4] and an increased proportion of miliary, disseminated, and extrapulmonary TB cases including lymphadenitis.[26] Previous studies of TBLN in our environment were done before the 80s[8,9,10] and only assessed data of the pre-HIV/acquired immune deficiency syndrome (AIDS) era. Though these studies reported TBLN as the most common cause of superficial lymphadenitis, a more recent study to re-evaluate its prevalence is of great essence in view of the reported associated role of HIV/AIDS.
Materials and Methods
This was a 15 years retrospective study of 1180 superficial lymph nodes biopsies (SLNBs) received at the Department of Morbid Anatomy, University of Nigeria Teaching Hospital, Ituku-Ozalla Enugu, Nigeria from the year 2000 to 2014. This is the core referral center, offering histopathology services to all the five states of the South-East region of Nigeria, as well as the adjoining states of the North-Central and South-South geopolitical regions in Nigeria. The ethical clearance for this study was obtained from the Health Research Ethics Committee of University of Nigeria Teaching Hospital and relevant clinical data of the patients such as histology number, age, sex, site of biopsy, HIV status, and other relevant clinical information of the patients were obtained from the duplicate copies of the request forms and case notes in the Pathology Department, as well as the hospital medical record archive. The tissue blocks of all 1180 SLNBs were retrieved from the departmental archives, and the sections made from them were stained with routine hematoxylin and eosin stains and Ziehl-Neelsen stain, a special histochemical stain for acid-fast bacilli (AFB). The slides were examined using a binocular light microscope (Leica Microsystems - Wetzlar, Germany), and diagnosis of TBLN was made based on the morphological grounds of characteristic histologic picture of chronic granulomatous inflammation with Langhan's giant cells, and a central caseous necrosis [Figures 1 and 2] in addition to the presence of complementary clinical information on the patients. The histological demonstration of AFB by Ziehl-Neelsen stain [Figure 3] was observed in some biopsies but was not used as criteria in diagnosis because of its notoriety of poor sensitivity.[27] The results obtained were analyzed using Predictive Analytics Software Statistics for Windows, Version 18 (Chicago: SPSS Inc.). Tables and photomicrographs are used to clearly present findings.
Figure 1.
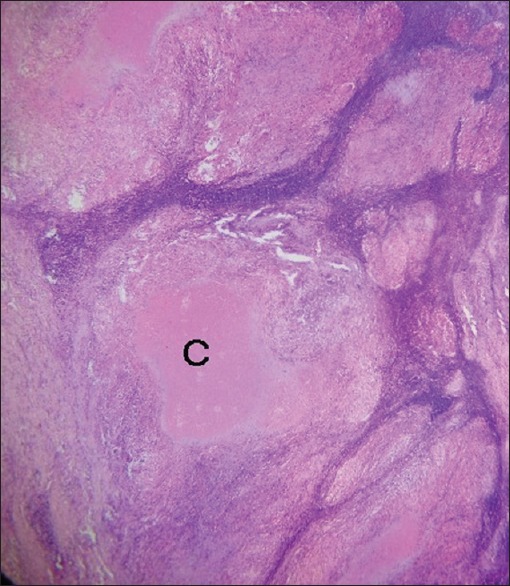
Photomicrograph of tuberculous lymphadenitis showing tuberculoid granulomas with central areas of caseous necrosis [C] (H and E, ×40)
Figure 2.
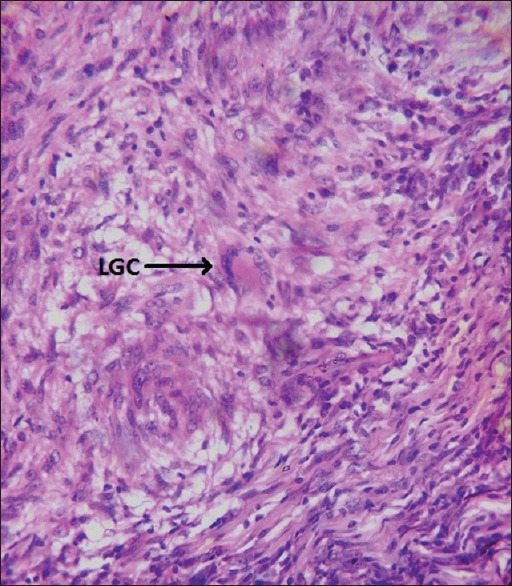
Photomicrograph of tuberculous lymphadenitis showing tuberculoid granulomas with Langhan's type multinucleated giant cell, epithelioid cells, and fibrosis at the periphery (H and E, ×400)
Figure 3.
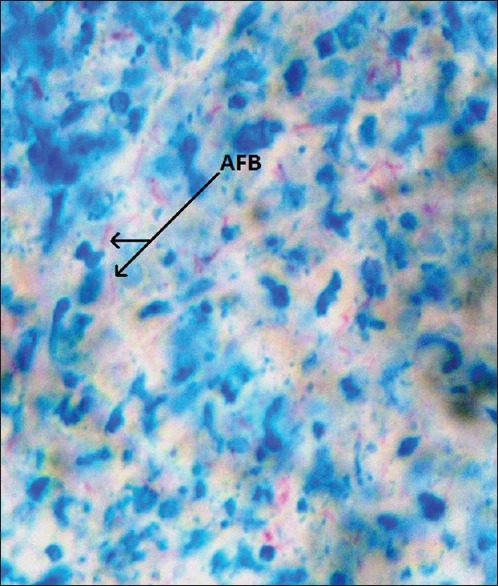
Photomicrograph of tuberculous lymphadenitis showing the acid-fast bacilli of Mycobacterium tuberculosis as the short red rods in view (Ziehl-Neelsen, ×400)
Results
One hundred and seventy-two cases of TBLN were identified in this study constituting 14.6% (172/1,180) of SLNBs received at our Hospital's Morbid Anatomy Department during the 15 years period under review. Only 28 cases of TBLN were clinically screened for HIV with 23 of them testing positive, representing 82.1% (23/28) of clinically screened cases. The AFB demonstration was positive in only 15.1% (26/172) of cases using Ziehl-Neelsen stain [Figure 3]. 48.8% (84/172) of TBLN cases were males, and 51.2% (88/172) were females giving an overall male to female ratio 1:1 with most (22) cases received in 2012 and the least (5) cases in 2000 [Table 1]. The peak number of TBLN occurred in the 21-25 years age group with a total of 21.5% (37/172) of cases and male to female ratio of 1:1.5 [Table 2]. This was followed by the 26-30 years and 31-35 years age groups with 19.8% (34/172) and 12.2% (21/172) of cases, respectively. The least number of TBLN occurred in 61-66 years and 66-70 years age groups with 0.6% (1/172) of cases each. The most common site of biopsy for TBLN was the cervical group followed by the axillary and inguinal groups with 73.8% (127/172), 14% (24/172), and 4.7% (8/172) of cases, respectively [Table 3]. The least biopsied lymph node was the femoral group with only 0.6% (1/172) of TBLN cases.
Table 1.
Annual distribution of tuberculous lymphadenitis
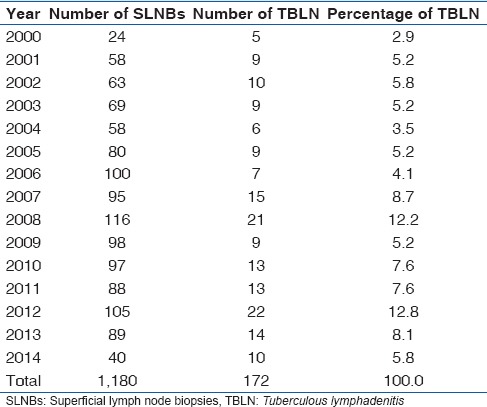
Table 2.
Age and sex distribution of tuberculous lymphadenitis
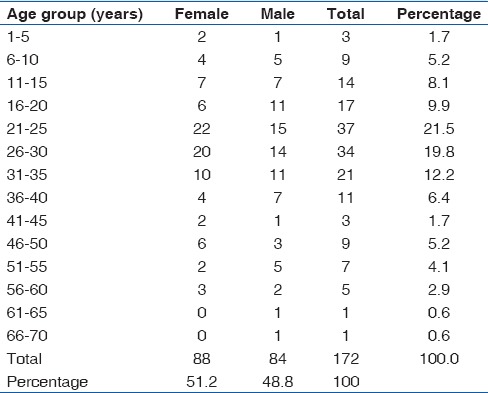
Table 3.
Biopsy sites of tuberculous lymphadenitis
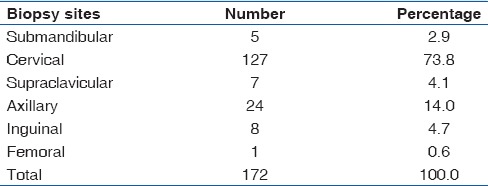
In adults, the highest number of TBLN was received in 2008 with a total of 13% (19/146) of cases while the least was in 2000 with 2.7% (4/146) of cases. In adults, TBLN accounted for 146 cases, 48.6% (71/146) of cases in male, and 51.4% (75/146) of cases in females with a male to female ratio of 1:1.1. The highest number of TBLN occurred in 21-25 years age group with a total of 25.3% (37/146) of cases. The male to female ratio within this age group was 1:1.5. The least number of TBLN occurred in 61-65 years and 66-70 years age groups with only one case of TBLN each. The most common site of lymph node biopsy in adult TBLN was the cervical group of lymph nodes with 73.3% (107/146) of cases followed by the axillary group with 13% (19/146) of cases. The least biopsied lymph node was the femoral group with only 0.7% (1/146) of cases.
In children (≤15 years), TBLN accounted for 15.1% (26/172) of total TBLN cases, 50% (13/26) of cases in male, and 50% (13/26) of cases in females with a male to female ratio of 1:1. The highest number of TBLN was received in 2007 with a total of 19.2% (5/26) of cases while no case of childhood TBLN was recorded in 2006 and 2009. The highest number of TBLN occurred in 11-15 years age group with a total of 53.8% (14/26) of cases. The male to female ratio within this age group was 1:1. The least number of childhood TBLN occurred in 1-5 years age group with 11.5% (3/26) of cases with no case of TBLN in the <1 year age group. In children, the most common biopsy site for TBLN was the cervical group with 76.9% (20/26) of cases followed by axillary and inguinal groups with 19.2% (5/26) and 3.8% (1/26) of cases, respectively.
Discussion
TBLN continues to be a significant form of patient presentation at our Hospital constituting a total of 172 cases and 14.6% of 1180 SLNBs received at our Hospital's Morbid Anatomy Department during the 15 years period under review. This shows a significant drop from being the most common cause of superficial lymphadenopathy with a 31.8% prevalence rate in previous studies in our environment[10] to now 14.6% which is the fourth, the most common becoming metastases (38.6%) followed by nonspecific reactive hyperplasia (21.5%), and lymphomas (20.1%).
TB has severally been reported as the most frequent cause of lymphadenopathy[8,9,10,11,12,13,14,15,16,17,18,19,20] and the most common form of extrapulmonary TB,[2,4,28] but the trend appears to be changing as Anunobi et al.[29] in a similar study in Lagos reported chronic nonspecific lymphadenitis (34%) as the most common cause of lymph node enlargement followed by metastatic lymph node lesions (33.6%), TB (17.4%), and lymphomas (14.2%). In our study, TBLN is now slightly more common in females than males (51.2% vs. 48.8%) and also peaks in the 21-25 years age group with a total of 37 (21.5%) cases. This; however, remains consistent with a similar study in Ethiopia[21] which also a reported mean age of 24.2 years and also with previous studies done three decades ago in our environment[9,10] which also reported a maximal incidence in the 10-29 years age group but then with males slightly outnumbering females. However, a study of lymphadenopathy in adults in Riyadh, Kingdom of Saudi Arabia, by Abba et al.[25] reported that TB was more common in females than males (51% vs. 49%). This is consistent with our finding that TBLN is now slightly more common in females than males (51.2% vs. 48.8%) [Table 2]. The most common biopsy site for TBLN in our study was the cervical group of superficial lymph nodes with 127 (73.8%) cases followed by the axillary group with 24 (14%). This is also in agreement with previous studies in Enugu,[9,10] other Nigerian cities,[11,12,13,14,15,16,17,18,19,20] and the world.[21,22,23,24,25]
In adults, TBLN accounted for 146 cases of lymphadenopathy and was more common in the younger age group, peaking at the 21-25 years age group with an overall male to female ratio of 1:1.1. This is largely in agreement with previous reports in our environment[9,10] and other Nigerian cities,[14,30] affecting the youths in their prime age, though now slightly affecting more females. The least cases of TBLN occurred in late middle age and the elderly with 61-65 years and 66-70 years age groups having only one case of TBLN each.
In children (≤15 years), TBLN accounted for 26 (15.1%) cases with 13 (50%) cases in male and 13 (50%) cases in females with a male to female ratio of 1:1. The highest number of TBLN occurred in 11-15 years age group with a total of 14 (53.8%) cases. The male to female ratio within this age group was 1:1. The least number of childhood TBLN occurred in 1-5 years age group with three (11.5%) cases with no case of TBLN in the <1 year age group. The absence of TBLN in the <1 year age group may be due to protection offered by transferred maternal immunity from maternal breast milk while the increase in the number of TBLN cases with increase in age from >1 year groups could be due to decrease in the acquired maternal immunity, as well as increased exposure to other TB predisposing factors such as co-infection with HIV, malnutrition, and young age.[31] The biopsy sites for TBLN in adults and children were consistent with those of the general population.
However, in a study to determine the diseases that commonly presented with lymphadenopathy in Port of Spain, Trinidad, and Raju[32] analyzed 651 diagnostic lymph node biopsies and found that nonspecific reactive hyperplasia was common, and TBLN was rare. This result is discordant with our findings, those of other Nigerian cities[9,10,11,12,13,14,15,16,17,18,19,20] and other developing countries.[21,22,23,24,25] The predominance of TB in Nigeria, predilection of cervical lymph node and female sex in superficial lymphadenopathy in most Nigerian cities, is in agreement with our studies and those from the rest of the African continent. TB remains a serious though preventable cause of superficial lymphadenopathy in many parts of Africa.[33]
There is nevertheless a resurgence of TB, especially in developing countries[34] and much of it is thought to be related to HIV infection[3,35] with reports of increasing TB lymphadenopathy in association with HIV positivity.[36,37] The higher rate of TBLN in some African countries like Zambia and Malawi is reported to be due to higher HIV infection rates.[36,38] The effect of HIV infection in increasing the incidence of TBLN was observed in our study as 23 of the 28 cases of TBLN screened for HIV were positive giving a HIV positivity of 82.1% within the screened TBLN population. Thus, though the incidence of TBLN appears to be declining in our environment, HIV/AIDS endemicity remains a significant factor. It is pertinent that since TBLN is the most common presentation of extrapulmonary TB, and TB is an HIV/AIDS-defining illness, all suspected TBLN patients should be evaluated for HIV infection.
The diagnosis of TBLN in this study was made based on a histologic picture of chronic granulomatous inflammation with Langhan's giant cells and central caseous necrosis, and the presence of complementary clinical information on the patients. This diagnostic modality is usually sufficient but in some cases, granulomas lack caseation or are poorly formed posing a diagnostic challenge. Some cases of granuloma lacking caseation or other specific features were seen but were excluded in this review. It is possible that some of these were early TB lesions yet to develop caseous necrosis though they were negative for AFB. Histological demonstration of AFB by Ziehl-Neelsen stain in TB is the goal standard for a diagnosis of TB, but it is notorious for its poor sensitivity,[27,37,38,39,40] especially in extrapulmonary TB owing to the paucibacillary nature of the specimen,[27] as well as the effect of formalin and xylene on the stainability of mycobacteria by Ziehl-Neelsen method.[39,40] In our study, AFB demonstration using Ziehl-Neelsen stain yielded only 15% positivity. This underlies the need for more sensitive techniques such as fluorescence auramine staining, immunofluorescence, and nucleic acid hybridization as necessary diagnostic tools for a more accurate diagnosis of TB lymphadenitis.[12,27,41] An integrated approach of combining low-cost polymerase chain reaction together with classic microbiological assays and histopathology can significantly improve diagnostic accuracy; enabling early treatment, improved quality of life HIV patients, reduction of hospital stays, relapses, and drug resistance.[42] These facilities are presently not available in most health centers in the developing world, where the scourge of TB is prevalent. So much is globally spent annually in the control of TB, but little is utilized in providing diagnostic facilities. This highlights the need to provide modern diagnostic facilities in our medical laboratories.
Conclusion
There is a remarkable decline in the prevalence of TB lymphadenitis in South-Eastern Nigeria indicating a change in trend from the pre- to the post-HIV/AIDS era with slightly more females now presenting with TBLN and most TB lymphadenitis patients now presenting with associated HIV/AIDS disease. There is an urgent need to provide modern diagnostic facilities in our medical laboratories.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Acknowledgment
The authors acknowledge the technical support provided by staff of the Department of Morbid Anatomy, University of Nigeria Teaching Hospital, Ituku-Ozalla Enugu, Nigeria.
References
- 1.World Health Organization. Global Tuberculosis Report 2014. Geneva: WHO Press; 2014. [Last accessed 2015 Apr 12]. Available from: http://www.who.int/tb/publications/global_report/gtbr14_main_text.pdf . [Google Scholar]
- 2.Golden MP, Vikram HR. Extrapulmonary tuberculosis: An overview. Am Fam Physician. 2005;72:1761–8. [PubMed] [Google Scholar]
- 3.Sharma S, Sarin R, Khalid UK, Singla N, Sharma PP, Behera D. Clinical profile and treatment outcome of tuberculous lymphadenitis in children using DOTS strategy. Indian J Tuberc. 2010;57:4–11. [PubMed] [Google Scholar]
- 4.Chand P, Dogra R, Chauhan N, Gupta R, Khare P. Cytopathological pattern of tubercular lymphadenopathy on FNAC: Analysis of 550 consecutive cases. J Clin Diagn Res. 2014;8:FC16–9. doi: 10.7860/JCDR/2014/9956.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontanilla JM, Barnes A, von Reyn CF. Current diagnosis and management of peripheral tuberculous lymphadenitis. Clin Infect Dis. 2011;53:555–62. doi: 10.1093/cid/cir454. [DOI] [PubMed] [Google Scholar]
- 6.Dandapat MC, Mishra BM, Dash SP, Kar PK. Peripheral lymph node tuberculosis: A review of 80 cases. Br J Surg. 1990;77:911–2. doi: 10.1002/bjs.1800770823. [DOI] [PubMed] [Google Scholar]
- 7.Attah EB. Peripheral lymphadenopathy in Nigeria. Trop Geogr Med. 1974;26:257–60. [PubMed] [Google Scholar]
- 8.Onuigbo WI. Cervical lymph node biopsy in the Igbos of Nigeria. Int Surg. 1975;60:410. [PubMed] [Google Scholar]
- 9.Onuigbo WI. Tuberculous peripheral lymphadenitis in the Igbos of Nigeria. Br J Surg. 1975;62:323–5. doi: 10.1002/bjs.1800620419. [DOI] [PubMed] [Google Scholar]
- 10.Ejeckam GC, Nwabueze ED. Pathology of superficial lymph node in children and young adults in Enugu, Nigeria. J Trop Pediatr. 1984;30:307–9. doi: 10.1093/tropej/30.6.307. [DOI] [PubMed] [Google Scholar]
- 11.Okolo SN, Nwana EJ, Mohammed AZ. Histopathologic diagnoses of lymphadenopathy in children in Jos, Nigeria. Niger Postgrad Med J. 2003;10:165–7. [PubMed] [Google Scholar]
- 12.Ochicha O, Edino ST, Mohammed AZ, Umar AB, Atanda AT. Pathology of peripheral lymph node biopsies in Kane, Northern Nigeria. Ann Afr Med. 2007;6:104–8. doi: 10.4103/1596-3519.55725. [DOI] [PubMed] [Google Scholar]
- 13.Adeniji KA, Anjorin AS. Peripheral lymphadenopathy in Nigeria. Afr J Med Med Sci. 2000;29:233–7. [PubMed] [Google Scholar]
- 14.Olu-Eddo AN, Ohanaka CE. Peripheral lymphadenopathy in Nigerian adults. J Pak Med Assoc. 2006;56:405–8. [PubMed] [Google Scholar]
- 15.Asindi AA, Ekanem IA, Khalil MI. Painless peripheral lymphadenopathy in Nigerian children. Trop Geogr Med. 1992;44:270–4. [PubMed] [Google Scholar]
- 16.Abdurrahman MB. Aetiology of persistent peripheral lymphadenopathy in Nigerian children. Trop Geogr Med. 1980;32:126–31. [PubMed] [Google Scholar]
- 17.Adedeji MO, Aghahowa J. The diagnostic value of lymphnode biopsies in Benin-City, Nigeria. East Afr Med J. 1989;66:127–33. [PubMed] [Google Scholar]
- 18.Oluwole SF, Odesanmi WO, Kalidasa AM. Peripheral lymphadenopathy in Nigeria. Acta Trop. 1985;42:87–96. [PubMed] [Google Scholar]
- 19.Obafunwa JO, Olomu IN, Onyia NJ. Primary peripheral lymphadenopathy in Jos, Nigeria. West Afr J Med. 1992;11:25–8. [PubMed] [Google Scholar]
- 20.Pindiga UH, Dogo D, Yawe T. Histopathology of primary peripheral lymphadenopathy in North Eastern Nigeria. Niger J Surg Res. 1999;1:69–70. [Google Scholar]
- 21.Getachew A, Demissie M, Gemechu T. Pattern of histopathologic diagnosis of lymph node biopsies in a teaching hospital in Addis Ababa, 1981-1990 G.C. Ethiop Med J. 1999;37:121–7. [PubMed] [Google Scholar]
- 22.Mbise RL. Peripheral lymphadenopathy in children in Dar es Salaam, Tanzania. A study from biopsy material. Ann Trop Paediatr. 1984;4:83–5. doi: 10.1080/02724936.1984.11748314. [DOI] [PubMed] [Google Scholar]
- 23.Bezabih M, Mariam DW. Determination of aetiology of superficial enlarged lymph nodes using fine needle aspiration cytology. East Afr Med J. 2003;80:559–63. doi: 10.4314/eamj.v80i11.8763. [DOI] [PubMed] [Google Scholar]
- 24.Tiwari M, Aryal G, Shrestha R, Rauniyar SK, Shrestha HG. Histopathologic diagnosis of lymph node biopsies. Nepal Med Coll J. 2007;9:259–61. [PubMed] [Google Scholar]
- 25.Abba AA, Bamgboye AE, Afzal M, Rahmatullah RA. Lymphadenopathy in adults. A clinicopathological analysis. Saudi Med J. 2002;23:282–6. [PubMed] [Google Scholar]
- 26.Mert A, Tabak F, Ozaras R, Tahan V, Oztürk R, Aktuglu Y. Tuberculous lymphadenopathy in adults: A review of 35 cases. Acta Chir Belg. 2002;102:118–21. doi: 10.1080/00015458.2002.11679277. [DOI] [PubMed] [Google Scholar]
- 27.Chakravorty S, Sen MK, Tyagi JS. Diagnosis of extrapulmonary tuberculosis by smear, culture, and PCR using universal sample processing technology. J Clin Microbiol. 2005;43:4357–62. doi: 10.1128/JCM.43.9.4357-4362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohapatra PR, Janmeja AK. Tuberculous lymphadenitis. J Assoc Physicians India. 2009;57:585–90. [PubMed] [Google Scholar]
- 29.Anunobi CC, Banjo AA, Abdulkareem FB, Daramola AO, Abudu EK. Review of the histopathologic patterns of superficial lymph node diseases, in Lagos (1991-2004) Niger Postgrad Med J. 2008;15:243–6. [PubMed] [Google Scholar]
- 30.Olu-eddo AN, Omoti CE. Diagnostic evaluation of primary cervical adenopathies in a developing country. Pan Afr Med J. 2011;10:52. [PMC free article] [PubMed] [Google Scholar]
- 31.Narasimhan P, Wood J, MacIntyre CR, Mathai D. Risk factors for tuberculosis. Pulm Med. 2013;2013:11. doi: 10.1155/2013/828939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raju GC. Lymphoreticular disease in Trinidad. Trop Geogr Med. 1987;39:376–9. [PubMed] [Google Scholar]
- 33.Kasili EG, Shah TS. Lymphoreticular disease in Kenya. Pathological pattern of the superficial lymphadenopathies. Trop Geogr Med. 1974;26:242–56. [PubMed] [Google Scholar]
- 34.Abdulkareem FB, Elesha SO, Banjo AA. Tuberculosis revisited - A review of surgical biopsies and autopsy specimens (1992-1996). Nig. Qt J Hosp Med. 2000;10(2):126–8. [Google Scholar]
- 35.FitzGerald JM, Grzybowski S, Allen EA. The impact of human immunodeficiency virus infection on tuberculosis and its control. Chest. 1991;100:191–200. doi: 10.1378/chest.100.1.191. [DOI] [PubMed] [Google Scholar]
- 36.Bem C, Patil PS, Bharucha H, Namaambo K, Luo N. Importance of human immunodeficiency virus-associated lymphadenopathy and tuberculous lymphadenitis in patients undergoing lymph node biopsy in Zambia. Br J Surg. 1996;83:75–8. doi: 10.1002/bjs.1800830124. [DOI] [PubMed] [Google Scholar]
- 37.Steinbrook R. HIV in India - A complex epidemic. N Engl J Med. 2007;356:1089–93. doi: 10.1056/NEJMp078009. [DOI] [PubMed] [Google Scholar]
- 38.Boeree MJ, Kamenya A, Liomba G, Ngwira B, Subramanyam VR, Harries AD. Tuberculosis lymphadenitis, a diagnostic problem in areas of high prevalence of HIV and tuberculosis. Malawi Med J. 1998;11:56–9. doi: 10.1016/s0035-9203(97)90081-x. [DOI] [PubMed] [Google Scholar]
- 39.Mudduwa LK, Nagahawatte Ade S. Diagnosis of tuberculous lymphadenitis: Combining cytomorphology, microbiology and molecular techniques - A study from Sri Lanka. Indian J Pathol Microbiol. 2008;51:195–7. doi: 10.4103/0377-4929.41680. [DOI] [PubMed] [Google Scholar]
- 40.Patel JM, Patel KR, Shah K, Patel NU, Baria H, Patel PD. Comparison of fine-needle aspiration technique with Ziehl-Neelsen stains in diagnosis of tuberculous lymphadenitis. Int J Med Sci Public Health. 2015;4:400–3. [Google Scholar]
- 41.Kidane D, Olobo JO, Habte A, Negesse Y, Aseffa A, Abate G, et al. Identification of the causative organism of tuberculous lymphadenitis in ethiopia by PCR. J Clin Microbiol. 2002;40:4230–4. doi: 10.1128/JCM.40.11.4230-4234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortez MV, Oliveira CM, Monte RL, Araújo JR, Braga BB, Reis DZ, et al. HIV-associated tuberculous lymphadenitis: The importance of polymerase chain reaction (PCR) as a complementary tool for the diagnosis of tuberculosis - A study of 104 patients. An Bras Dermatol. 2011;86:925–31. doi: 10.1590/s0365-05962011000500010. [DOI] [PubMed] [Google Scholar]


