Abstract
Objective
Investigate relationships between early blood pressure (BP) changes, receipt of anti-hypotensive therapy, and 18 – 22 month corrected age (CA) outcomes for extremely preterm infants.
Design
Prospective observational study of infants 230/7 – 266/7 weeks gestational age (GA). Hourly BP values and anti-hypotensive therapy exposure in the first 24 hours were recorded. Four groups were defined: infants who did or did not receive anti-hypotensive therapy in whom BP did or did not rise at the expected rate (defined as an increase in the mean arterial BP of ≥5 mmHg/day). Random-intercept logistic modeling controlling for center clustering, GA, and illness severity was used to investigate the relationship between BP, anti-hypotensive therapies, and infant outcomes.
Setting
Sixteen academic centers of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network.
Main Outcome Measures
Death or neurodevelopmental impairment / developmental delay (NIDD) at 18 – 22 months CA.
Results
Of 367 infants, 203 (55%) received an anti-hypotensive therapy, 272 (74%) survived to discharge, and 331 (90%) had a known outcome at 18 – 22 months CA. With logistic regression, there was an increased risk of death/NIDD with anti-hypotensive therapy versus no treatment (odds ratio: 1.836, 95% confidence interval: 1.092 – 3.086), but not NIDD alone (odds ratio: 1.53, 95% confidence interval: 0.708 – 3.307).
Conclusion
Independent of early BP changes, anti-hypotensive therapy exposure was associated with an increased risk of death/NIDD at 18 to 22 months CA when controlling for risk factors known to affect survival and neurodevelopment.
Keywords: Extremely preterm infant, blood pressure, neurodevelopment, hypotension
INTRODUCTION
Many investigations in the last 25 years suggest preterm infants considered hypotensive in the immediate postnatal period are at increased risk for adverse outcomes.1-14 This observation has led some clinicians to administer therapies in an effort to raise arterial blood pressure (BP) and, presumably, improve an infant’s chances of survival without major morbidity.12-16 To date, no such improvement in outcomes has been observed.1-16 More concerning is the possibility that commonly prescribed anti-hypotensive therapies may increase risks to preterm infants.3,7, 12-14
The evolving physiology of extremely preterm infants and the dynamic nature of the cardiovascular system are some of the inherent challenges to investigating BP management in the immediate newborn period. The wide range of BP values observed at any given postnatal hour and the spontaneous rise in BP which typically occurs after birth5,8,9 make it difficult to determine whether a BP value for a given infant at a specific postnatal age is too high, too low, rising too quickly, or not increasing quickly enough. There are no placebo-controlled randomized trials to guide therapeutic decisions.3,17-19 As such, BP management for this population varies considerably with a wide range in the frequency of anti-hypotensive therapy administration for perceived low BP.12-14,16
Data on the relationship between early BP management and neurodevelopmental impairment / developmental delay (NIDD) are insufficient and difficult to interpret. This is related in part to the limited number of studies reporting rates of NIDD, patient sample sizes, data on specific anti-hypotensive therapies, and heterogeneous study populations.9-11 Perhaps most importantly, previous investigations did not account for the spontaneous rise in BP that occurs after birth in preterm infants.5,8,9 As a result, it is difficult to determine whether infant outcomes after the administration of anti-hypotensive therapies are related to BP increasing in conjunction with therapy. The objectives of this study were to evaluate toddler age outcomes in a cohort of extremely preterm infants divided into groups defined by early changes in BP and receipt of anti-hypotensive therapy in an effort to clarify: 1) whether adverse outcomes in infants with a concerning BP are related to lack of anti-hypotensive therapy administration and; 2) whether survival and / or intact neurodevelopment in treated infants are related to a rise in BP.
METHODS
This study reports the outcomes at 18 to 22 months corrected age (CA) for infants enrolled in a prospective observational study of inborn extremely preterm infants born at 230/7 to 266/7 weeks gestational age (GA) at 16 academic centers of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NRN) enrolled from July 21, 2010 to January 21, 2011. A detailed description of the patient population, explanation of the informed consent process, and in-hospital outcomes have been reported previously.8,14 Briefly, hourly BP measurements (both invasive and non-invasive) and the administration of all anti-hypotensive therapies in the first 24 hours were recorded. Anti-hypotensive therapy included a fluid bolus (≥10 ml/kg of crystalloid), dopamine, dobutamine, hydrocortisone, epinephrine, or any blood product. All treatment decisions were made by the clinical care team. Inborn infants born at 23 through 26 weeks GA admitted to the neonatal intensive care unit were included in this study. Infants were excluded if they died in the delivery room, had a major birth defect, or had intensive care withheld or withdrawn shortly after birth because the clinical care team felt the situation was hopeless. This study was approved by the Institutional Review Board of each participating center. At two centers, infants were enrolled after parents signed a study specific informed consent form. At the remaining 14 centers, this study was incorporated into the ongoing Generic Database (GDB) study of the NRN because all infants in this study qualified for GDB enrollment (on the basis of their GA at birth) and both studies collected de-identified patient information. The Institutional Review Board of some NRN centers allowed for GDB data collection with a waiver of consent.8
The primary outcome for this investigation was the incidence of death or NIDD at 18 to 22 months CA. Neurodevelopmental impairment or developmental delay was defined as a Bayley Scales of Infant Development, Third Edition20 cognitive or motor score < 70, cerebral palsy (Gross Motor Function Classification System level greater than two),21 hearing impairment requiring hearing aids in both ears, or blindness (some or no useful vision in either eye). Secondary outcomes were the rates of any NIDD and individual components of NIDD among infants who survived to 18 – 22 months CA. Outcomes were compared among four infant groups defined by the administration of anti-hypotensive therapy and the rate of rise in BP: 1) infants who did not receive an anti-hypotensive therapy in whom the BP rose as expected; 2) untreated infants in whom BP did not rise at the expected rate; 3) infants who received an anti-hypotensive therapy in the first 24 hours in whom BP rose as expected and; 4) treated infants who did not experience the expected rise in BP. The expected rise in BP was defined a priori as an increase in the mean arterial BP of ≥ 5 mmHg from postnatal hour four to postnatal hour 24. This definition was chosen because this rate of rise was reported previously for extremely preterm infants, this was the average rate of rise in mean arterial BP for this cohort of infants, the average rate of rise was the same for each week gestation across this GA range, and the average rate of rise for this cohort was the same for infants who did versus did not receive an anti-hypotensive therapy.5,8
Data analysis was performed at the NRN Data Coordinating Center (RTI International, Research Triangle Park, NC). Statistical analysis was performed using SAS 9.3 software (SAS Institute Inc., Cary, NC). For univariable analysis, Chi-squared or Fisher’s exact tests were used for comparison of proportions and Analysis of Variance was used for comparison of means. Logistic regression models that included a random intercept to account for potential center clustering were estimated for the outcomes of death / NIDD and NIDD (alone). Infant-level covariates of GA at birth and severity of illness were used as covariates, in addition to anti-hypotensive therapy exposure (treated versus untreated) and early changes in BP (expected rate of rise versus less than the expected rate of rise).14 Severity of illness was defined as the cumulative number of any of the following: a one minute Apgar score less than or equal to three, presence of early anemia (an initial hematocrit ≤ 30%), any pH < 7.10 in the first 24 hours after birth, a positive early blood culture (drawn within 72 hours of birth), or delivery room chest compressions.14
RESULTS
There were 367 infants enrolled in the study (Figure 1). Of these, 158 infants survived the first 24 hours without receiving an anti-hypotensive therapy, including 91 (58%) in whom the mean arterial BP increased by ≥ 5 mmHg and 67 (42%) infants in whom it did not. Blood pressure rose as expected for 128 (65%) of the 198 infants who received an anti-hypotensive therapy. Of the 203 infants who received an anti-hypotensive therapy, 135 (67%) received a fluid bolus, 102 (50%) received a blood product, and 92 (45%), 25 (12%), 18 (9%), and one (<1%) received dopamine, hydrocortisone, dobutamine, and vasopressin, respectively. Five infants who received an anti-hypotensive therapy died in the first 24 hours. In-hospital variables and outcome data for the four infant cohorts are presented in Table 1.
Figure 1.
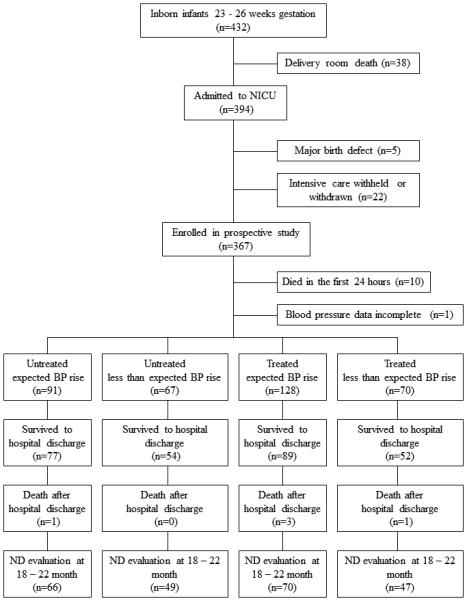
Extremely preterm infant study enrollment classified by study group; NICU = neonatal intensive care unit; BP = blood pressure; ND = neurodevelopmental
Table 1.
In-hospital variables & outcomes for infants based on receipt of anti-hypotensive therapy & rise in blood pressure
| Untreated expected BP rise (n=91) |
Untreated less than expected BP rise (n=67) |
Treated expected BP rise (n=128) |
Treated less than expected BP rise (n=70) |
p-value | |
|---|---|---|---|---|---|
| Birth weight (grams), mean ± SD | 784 ± 165 | 750 ± 144 | 692 ± 150 | 713 ± 168 | <0.001 |
| GA at birth (weeks), mean ± SD | 25.5 ± 0.9 | 25.6 ± 1.0 | 25.1 ± 1.1 | 25.3 ± 1.0 | <0.001 |
| Antenatal corticosteroids, # (%) | 81 (89) | 61 (91) | 118 (92) | 65 (93) | 0.814 |
| Any severity of illness marker, # (%) 1 minute Apgar score ≤ 3 DR chest compressions Positive initial blood culture Any pH <7.10 in the 1st 24 hours First hematocrit <30% |
37 (41) 33 (36) 5 (5) 0 1 (1) 4 (4) |
37 (55) 33 (49) 7 (10) 0 3 (4) 3 (4) |
93 (73) 79 (62) 14 (11) 3 (2) 13 (10) 25 (20) |
56 (80) 49 (70) 8 (11) 5 (7) 11 (16) 12 (17) |
<0.001 <0.001 0.5 0.01¶ 0.003 <0.001 |
| Severe IVH or PVL, # (%) | 11 (12) | 8 (13) | 28 (23) | 21 (31) | 0.008 |
| NEC requiring surgery, # (%) | 4 (5) | 2 (4) | 6 (7) | 2 (4) | 0.881¶ |
| Intervention for ROP, # (%) | 9 (12) | 6 (11) | 18 (20) | 14 (27) | 0.071 |
| BPD, # (%) | 40 (51) | 36 (63) | 58 (62) | 34 (62) | 0.402 |
| Survival to hospital discharge | 77 (85) | 54 (81) | 89 (70) | 52 (74) | 0.055 |
BP = blood pressure; ANOVA = analysis of variance; SD = standard deviation; GA = gestational age; DR = delivery room; IVH = intraventricular hemorrhage; PVL = periventricular leukomalacia; NEC = necrotizing enterocolitis; ROP = retinopathy of prematurity; BPD = bronchopulmonary dysplasia
Fisher's Exact Test
Eighteen to 22 month CA outcomes (death or neurodevelopmental assessment) were known for 331 (90%) infants. This included 99 infants who died and 232 infants who underwent neurodevelopmental testing. Thirty-six infants were lost to follow-up. There were not any significant differences in known in-hospital morbidities or therapies between infants lost to follow-up and hospital survivors with known 18 – 22 month outcomes. On univariable analysis, death or NIDD was significantly higher in treated infants as compared to untreated infants (Table 2) irrespective of whether BP rose as expected. The rates of individual components of NIDD varied across the four groups, but these differences did not reach statistical significance (Table 2; p > 0.05 for each).
Table 2.
Outcomes at 18 – 22 months for infants based on receipt of anti-hypotensive therapy & rise in blood pressure
| Untreated expected BP rise (n=91) |
Untreated less than expected BP rise (n=67) |
Treated expected BP rise (n=128) |
Treated less than expected BP rise (n=70) |
p-value | |
|---|---|---|---|---|---|
| Death or any ND impairment/delay, # (%) | 24 (26) | 19 (28) | 58 (45) | 33 (47) | 0.002 |
| *Any ND impairment/delay, # (%) | 9 (14) | 6 (12) | 16 (23) | 14 (30) | 0.079 |
| Language composite score, mean ± SD | 86 ± 16 | 87 ± 13 | 84 ± 17 | 83 ± 18 | 0.531 |
| Cognitive composite score, mean ± SD | 91 ± 14 | 90 ± 10 | 86 ± 17 | 88 ± 17 | 0.33 |
| Motor composite score, mean ± SD | 89 ± 15 | 89 ± 12 | 86 ± 17 | 86 ± 18 | 0.53 |
| *Language composite score < 70, # (%) | 8 (12) | 2 (4) | 13 (19) | 9 (19) | 0.081 |
| *Cognitive composite score <70, # (%) | 5 (8) | 1 (2) | 11 (16) | 7 (15) | 0.0554 |
| *Motor composite score <70, # (%) | 7 (11) | 4 (8) | 10 (14) | 10 (21) | 0.162 |
| *GMFCS level ≥2, # (%) | 4(6) | 4(8) | 9 (13) | 8 (17) | 0.251 |
| *Blindness, # (%) | 1 (2) | 0 | 0 | 0 | 0.697¶ |
| *Deafness, # (%) | 1 (2) | 1 (2) | 1 (1) | 3 (6) | 0.426¶ |
BP = blood pressure; ANOVA = analysis of variance; ND = neurodevelopment; SD = standard deviation; GMFCS = Gross Motor Function Classification System;
denominator is infants who had a neurodevelopmental assessment at 18 – 22 months corrected age
Fisher's Exact Test
There were significant differences in the incidence of NIDD or the composite outcome of death/NIDD from random-effects logistic regression models (table 3). For each one week increase in GA at birth, both the likelihood of NIDD or death/NIDD decreased. The presence of any marker of severity of illness increased the odds of both NIDD and death/NIDD as did the cumulative number of severity of illness markers. When incorporating these variables and changes in BP into regression models, treatment with any anti-hypotensive therapy was a significant predictor of death/NIDD, but not NIDD alone. In similar regression models incorporating anti-hypotensive treatment, the rise in BP (at the expected rate versus less than the expected rate) was not significantly associated with either outcome.
Table 3.
Estimation results from Logistic regression models with a random intercept
| Covariate | NIDD |
Death / NIDD |
||
|---|---|---|---|---|
| Odds Ratio |
95% Confidence Interval |
Odds Ratio |
95% Confidence Interval |
|
|
| ||||
| Each one week increase in GA at birth | 0.688 | 0.479 – 0.988 | 0.608 | 0.476 – 0.777 |
| Presence of any marker of severity of illness* | 2.733 | 1.123 – 6.652 | 1.872 | 1.074 – 3.261 |
| Higher cumulative number of severity of illness markers | 2.065 | 1.275 – 3.346 | 1.672 | 1.204 – 2.322 |
| Any anti-hyotensive therapy treatment | 1.53 | 0.708 – 3.307 | 1.836 | 1.092 – 3.086 |
| Expected BP rise (versus less than the expected rate) | 0.71 | 0.347 – 1.454 | 0.904 | 0.548 – 1.493 |
NIDD = neurodevelopmental impairment or developmental delay; BP = blood pressure
one minute Apgar score ≤ 3, initial hematocrit ≤ 30%) any pH < 7.10 in the first 24 hours, a positive blood culture drawn within 72 hours of birth, or delivery room chest compressions
DISCUSSION
Previous studies examining the relationship between early BP management and infant outcomes either evaluated hypotensive infants versus normotensive infants or infants with treated versus untreated hypotension.6,9-11 Hypotension was defined as either receipt of an anti-hypotensive therapy (irrespective of BP values) or a BP value below a numeric threshold (e.g. a mean arterial BP less than or equal to the numerical equivalent of the infant’s GA) which was not necessarily used clinically. Difficulties with investigating early BP management include the spontaneous rise in BP that occurs after birth, the positive correlation between BP values and GA at birth, and the wide range in BP values observed at any specific postnatal hour for infants at all GA ranges.5,6,8 The findings of this study underscore the importance of considering multiple factors when examining BP in relation to infant outcomes.
Infants given an anti-hypotensive therapy had a higher rate of death / NIDD irrespective of early BP changes. The significance of this finding remains unclear. It is possible parameters other than a numeric threshold for low BP may better identify infants at risk for a poor outcome who would benefit from therapy. Incorporation of indirect measures of systemic or cerebral blood flow may also partially explain the observed variability in BP management, including why some infants without low BP received an anti-hypotensive therapy while other infants with low BP did not.3,14,22,23
Although not statistically significant (p = 0.055), there was a trend towards higher mortality in treated infants (29%) versus untreated infants (17%) in this study, irrespective of the BP rate of rise. Other reports have also suggested that infants who receive an anti-hypotensive therapy have a higher mortality rate.5-7,24 Definitive conclusions regarding this association cannot be made since to date there are no randomized trials. Potential confounders include higher illness severity in treated infants, a greater likelihood of a condition known to increase mortality (e.g. sepsis), inclusion of infants in extremis who received an anti-hypotensive therapy but were likely to die irrespective of the degree of therapeutic support provided, and omission of other factors which may predispose an infant to both perceived low BP requiring intervention and death (such as a low Apgar score or early acidosis). Attempts were made to control for these concerns in the current study such as excluding infants deemed terminally ill in the first 24 hours and incorporating severity of illness into the regression analyses using factors which both impact BP management and are associated with lower survival.14
In a previous multi-center study, the ELGAN Study investigators evaluated the relationship between indicators of hypotension and rates of NIDD at 24 months CA.9 Similar to this study, those investigators found little evidence of an association between early BP values and subsequent neurodevelopment. Other studies report similar findings.10,11,18 The association between anti-hypotensive therapy and hearing loss reported by Fanaroff et al was not observed in this investigation, possibly due to the low rate of deafness overall (less than three percent).6
Data from this study and others demonstrate the complex relationship between early blood pressure management, mortality, and neurodevelopment. Death and NIDD are distinct outcomes and it is likely that circumstances which lead to death are distinct from but overlap with factors which lead to brain injury such that these therapies – or factors influencing the decision to administer them – may influence mortality risk differently than they influence the risk of NIDD.25,26 Alternatively, factors beyond the immediate postnatal period may have more influence on toddler age outcomes thus masking the impact of early BP management on neurodevelopment.25 Lastly, given the complexity of the immature cardiovascular system, anti-hypotensive therapies may inconsistently influence cardiac function such that they do not uniformly alter early postnatal cerebrovascular blood flow or oxygen delivery.18,22,23
Study strengths include consistent prospective data collected by trained research personnel, inclusion of a large multicenter population of extremely preterm infants, analysis based on GA rather than birth weight, and systematic assessment of neurodevelopment by examiners masked to the infant’s early BP management who were trained in the standardized administration of the Bayley Scales of Infant Development, Third Edition. Importantly, this study may have been underpowered to demonstrate statistically significant, but clinically relevant, differences in some infant outcomes. Additional study limitations reported previously include the observational study design, a lack of data for some variables that may have contributed to BP management decisions, variability in infant enrollment across NRN centers, and inconsistency in how BP values were obtained.14 There was substantial heterogeneity in the treatment of perceived low BP and this also may limit applicability of the study findings.
CONCLUSIONS
Extremely preterm infants who received an anti-hypotensive therapy in the first 24 hours after birth had a significantly higher rate of death or impaired neurodevelopment at 18 – 22 months CA than untreated infants irrespective of early changes in BP. These results cannot be explained by differences in the markers of illness severity collected for this study which include factors known to impact infant survival and morbidity. There is limited evidence to suggest anti-hypotensive therapies improve outcomes for preterm infants and growing concern these therapies may be harmful. It is possible therapeutic interventions for perceived low BP increase the risk of adverse outcomes in extremely preterm infants.
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Extremely preterm infants who receive anti-hypotensive therapies have worse in-hospital outcomes.
The relationship between toddler age outcomes, early blood pressure values, and anti-hypotensive therapies is unclear.
Investigating these relationships is challenging in part because of changes in blood pressure values occurring shortly after birth.
WHAT THIS STUDY ADDS.
Infants who received an anti-hypotensive therapy were more likely to have an adverse outcome at 18 to 22 months corrected age than untreated infants irrespective of whether or not blood pressure increased.
These results cannot be explained by differences in the markers of severity of illness investigated.
ACKNOWLEDGEMENTS
The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) provided grant support, including funding from the Best Pharmaceuticals for Children Act, for the Neonatal Research Network’s Early Blood Pressure Observational Study.
Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, Drs. Abhik Das (DCC Principal Investigator) and Lei Li (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to the authors, participated in this study:
NRN Steering Committee Chair: Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006-2011).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904) – Abbot R. Laptook, MD; William Oh, MD; Angelita M. Hensman, RNC-NIC BSN; Kristin Basso, RN MaT.
Case Western Reserve University, Rainbow Babies & Children's Hospital (U10 HD21364) – Avroy A. Fanaroff, MD; Bonnie S. Siner, RN; Deanne E. Wilson-Costello, MD.
Cincinnati Children's Hospital Medical Center, University Hospital, and Good Samaritan Hospital (U10 HD27853) – Kurt Schibler, MD; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Lenora Jackson, CRC; Kristin Kirker, CRC; Estelle E. Fischer, MHSA MBA.
Duke University School of Medicine, University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (U10 HD40492) – Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Kimberley A. Fisher, PhD FNP-BC IBCLC; Sandy Grimes, RN BSN.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, UL1 RR25008) – David P. Carlton, MD; Ellen C. Hale, RN BS CCRC.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856) – Brenda B. Poindexter, MD MS; Leslie D. Wilson, BSN CCRC; Dianne E. Herron, RN; Cassandra Stahlke, BS CCRC.
RTI International (U10 HD36790) – Dennis Wallace, PhD; Jeanette O’Donnell Auman, BS; Margaret Cunningham, BS; Carolyn M. Petrie Huitema, MS; James W. Pickett II, BS; Kristin M. Zaterka-Baxter, RN BSN.
Stanford University, Lucile Packard Children's Hospital (U10 HD27880) – Krisa P. Van Meurs, MD; David K. Stevenson, MD; M. Bethany Ball, BS CCRC; Melinda S. Proud, RCP.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119) – Ivan D. Frantz III, MD; John M. Fiascone, MD; Anne Furey, MPH; Brenda L. MacKinnon, RNC; Ellen Nylen, RN BSN.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216) – Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN.
University of Iowa Children's Hospital and Mercy Medical Center (U10 HD53109, UL1 RR24979) – Edward F. Bell, MD; Dan L. Ellsbury, MD; Karen J. Johnson, RN BSN; Donia D. Campbell, RNC-NIC; Rachael M. Hyland, BA.
University of New Mexico Health Sciences Center (U10 HD53089) – Robin K. Ohls, MD; Conra Backstrom Lacy, RN; Sandra Brown, BSN.
The University of North Carolina at Chapel Hill (UL1 RR25747) – Carl L. Bose, MD; Gennie Bose, RN; Janice Bernhardt, MS RN; Cindy Clark, RN.
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children's Medical Center Dallas (U10 HD40689, M01 RR633) – Pablo J. Sánchez, MD; Luc P. Brion, MD; Lizette E. Torres, RN; Diana M. Vasil, RNC-NIC; Lijun Chen, RN PhD; Alicia Guzman.
University of Texas Health Science Center at Houston Medical School, Children's Memorial Hermann Hospital – Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Georgia E. McDavid, RN; Patti L. Pierce Tate, RCP; Sharon L. Wright, MT (ASCP).
University of Utah, University Hospital, Intermountain Medical Center, and Primary Children's Medical Center (U10 HD53124, UL1 RR25764) – Karen A. Osborne, RN BSN CCRC; Jill Burnett, RNC; Cynthia Spencer, RNC; Kimberlee Weaver-Lewis, RN BSN; Karie Bird, RN; Karen Zanetti, RN; Laura Cole, RN.
Wayne State University, University of Michigan, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385) – Seetha Shankaran, MD; Beena G. Sood, MD MS; Rebecca Bara, RN BSN; Mary Johnson, RN BSN.
Yale University, Yale-New Haven Children’s Hospital (U10 HD27871, UL1 RR24139) – Richard A. Ehrenkranz, MD; Monica Konstantino, RN BSN; JoAnn Poulsen, RN.
FUNDING
This study was funded by the Best Pharmaceuticals for Children Act.
Footnotes
All Authors reside and work in the United States of America
AUTHOR CONTRIBUTORSHIP STATEMENT
Beau Batton was the principal study investigator, wrote the first draft of the manuscript, and oversaw subsequent revisions. Lei Li was responsible for all statistical analyses, co-wrote portions of the manuscript relevant to data analysis and interpretation, and actively participated in the review and revision of all drafts of the manuscript. Nancy Newman assisted with patient enrollment, data collection, and participated in the review and revision of all drafts of the manuscript. Abhik Das was responsible for statistical analysis and interpretation and actively participated in the review and revision of all drafts of the manuscript. Kristi Watterberg, Bradley Yoder, Roger Faix, Matthew Laughon, and Barbara Stoll assisted with patient enrollment, data collection, and actively participated in the review and revision of all drafts of the manuscript. Rosemary Higgins was a senior study investigator, assisted with data collection, and actively participated in the review and revision of all drafts of the manuscript. Michele Walsh was a senior study investigator, assisted with patient enrollment, data collection, and actively participated in the review and revision of all drafts of the manuscript. All authors participated in the study design and implementation.
COMPETING INTERESTS
None.
LICENSE FOR PUBLICATION STATEMENT
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non-exclusive for government employees) on a worldwide basis to the BMJ and co-owners or contracting owning societies (where published by the BMJ on their behalf), and its Licensees to permit this article (if accepted) to be published in Archives of Disease in Childhood and any other BMJ products and to exploit all subsidiary rights, as set out in our license.
REFERENCES
- 1.Watkins A, West C, Cooke R. Blood pressure and cerebral haemorrhage and ischaemia in very low birthweight infants. Early Hum Dev. 1989;19:103–110. doi: 10.1016/0378-3782(89)90120-5. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham S, Symon A, Elton R, et al. Intra-arterial blood pressure reference ranges, death and morbidity in very low birthweight infants during the first seven days of life. Early Hum Dev. 1999;56:151–165. doi: 10.1016/s0378-3782(99)00038-9. [DOI] [PubMed] [Google Scholar]
- 3.Short B, Van Meurs K, Evans J. Summary proceedings from the cardiology group on cardiovascular instability in preterm infants. Pediatrics. 2006;117:S34–S39. doi: 10.1542/peds.2005-0620F. [DOI] [PubMed] [Google Scholar]
- 4.Lundstrom K, Pryds O, Greisen G. The haemodynamic effects of dopamine and volume expansion in sick preterm infants. Early Hum Dev. 2000;57:157–163. doi: 10.1016/s0378-3782(00)00048-7. [DOI] [PubMed] [Google Scholar]
- 5.Batton B, Batton D, Riggs T. Blood pressure in the first 7 days in premature infants born at postmenstrual age 23 to 25 weeks. Am J Perinatol. 2007;24:107–115. doi: 10.1055/s-2007-970178. [DOI] [PubMed] [Google Scholar]
- 6.Fanaroff J, Wilson-Costello D, Newman N, et al. Symptomatic hypotension is associated with neonatal morbidity and hearing loss in extremely low birth weight infants. Pediatrics. 2006;117:1131–1135. doi: 10.1542/peds.2005-1230. [DOI] [PubMed] [Google Scholar]
- 7.Dempsey E, Al Hazzani F, Barrington K. Permissive hypotension in the extremely low birthweight infant with signs of good perfusion. Arch Dis Child Fetal Neonatal Ed. 2009;94:F241–F244. doi: 10.1136/adc.2007.124263. [DOI] [PubMed] [Google Scholar]
- 8.Batton B, Li L, Newman N, Das A, et al. Evolving blood pressure dynamics for extremely preterm infants. J Perinatol. 2014;34:301–305. doi: 10.1038/jp.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logan J, O’Shea T, Allred E, et al. Early postnatal hypotension and developmental delay at 24 months of age among extremely low gestational age newborns. Arch Dis Child Fetal Neonatal Ed. 2011;96:F321–F328. doi: 10.1136/adc.2010.183335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batton B, Zhu X, Fanaroff J, et al. Blood pressure, anti-hypotensive therapy, and neurodevelopment in extremely preterm infants. J Pediatr. 2009;154:351–357. doi: 10.1016/j.jpeds.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Alderliesten T, Lemmers P, van Haastert I, et al. Hypotension in preterm neonates: low blood pressure alone does not affect neurodevelopmental outcome. J Pediatr. 2014;164:986–991. doi: 10.1016/j.jpeds.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 12.Laughon M, Bose C, Allred E, et al. Factors associated with treatment for hypotension in extremely low gestational age newborns during the first postnatal week. Pediatrics. 2007;119:273–280. doi: 10.1542/peds.2006-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dempsey E, Barrington K. Evaluation and treatment of hypotension in the preterm infant. Clin Perinatol. 2009;36:75–86. doi: 10.1016/j.clp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Batton B, Li L, Newman N, Das A, et al. Use of antihypotensive therapies in extremely preterm infants. Pediatrics. 2013;131:e1865–e1873. doi: 10.1542/peds.2012-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Penny D, Kim N, et al. Mechanisms of blood pressure increase induced by dopamine in hypotensive preterm neonates. Arch Dis Child Fetal Neonat Ed. 1999;81:F99–F104. doi: 10.1136/fn.81.2.f99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stranak Z, Semberova J, Barrington K, et al. International survey on diagnosis and management of hypotension in extremely preterm babies. Eur J Pediatr. 2014;173:793–798. doi: 10.1007/s00431-013-2251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batton B, Li L, Newman N, Das A, et al. Feasibility study of early blood pressure management in extremely preterm infants. J Pediatr. 2012;161:65–69. doi: 10.1016/j.jpeds.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dempsey E, Barrington K, Marlow N, et al. Management of hypotension in preterm infants (the HIP Trial): A randomized controlled trial of hypotension management in extremely low gestational age newborns. Neonatology. 2014;105:275–281. doi: 10.1159/000357553. [DOI] [PubMed] [Google Scholar]
- 19.Vain N, Barrington K. Feasibility of evaluating treatment of early hypotension in extremely low birthweight infants. J Pediatr. 2012;161:4–7. doi: 10.1016/j.jpeds.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Bayley N. Bayley Scales of Infant Development. 3rd Pyschological Corp; San Antonio, TX: 2006. [Google Scholar]
- 21.Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 22.Kooi E, van der Laan M, Verhagen E, et al. Volume expansion does not alter cerebral tissue oxygen extraction in preterm infants with clinical signs of poor perfusion. Neonatology. 2013;103:308–14. doi: 10.1159/000346383. [DOI] [PubMed] [Google Scholar]
- 23.Bonestroo H, Lemmers P, Baerts W, et al. Effect of anti-hypotensive treatment on cerebral oxygenation of preterm infants without PDA. Pediatrics. 2011;128:e1502–1510. doi: 10.1542/peds.2010-3791. [DOI] [PubMed] [Google Scholar]
- 24.Barrington K. Hypotension and shock in the preterm infant. Semin Fetal Neonat Med. 2008;13:16–23. doi: 10.1016/j.siny.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Marlow N. Is survival and neurodevelopmental impairment at 2 years of age the gold standard outcome for neonatal studies? Arch Dis Child Fetal Neonatal Ed. 2015;100:F82–F84. doi: 10.1136/archdischild-2014-306191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lilienfeld A, Parkhurst E. A study of the association of factors of pregnancy and parturition with the development of cerebral palsy: a preliminary report. Am J Hygiene. 1951;53:262–282. doi: 10.1093/oxfordjournals.aje.a119453. [DOI] [PubMed] [Google Scholar]


