Abstract
Previously we reported that Apolipoprotein E (ApoE) ε4 negatively affects performance in the novel-image-novel-location (NINL) object recognition test in healthy non-demented elderly human study participants. In this study, the participants were invited to return for testing sessions 6 and 18 months after the baseline session. Using a longitudinal study design, effects of ε4 on NINL test performance were assessed in study “dropouts”, participants that did not return for the second and/or third session(s), and “finishers”, participants that returned for all sessions. There were effects of ε4 on dropout rates and NINL total scores as well as sub-scores in both dropouts and finishers. NINL total score was a predictor of ε4 participant dropout. Compared to non-ε4 dropouts, ε4 dropouts had lower NINL scores. In contrast, ε4 finishers had higher NINL scores than non-ε4 finishers. Thus, the NINL test could be a valuable tool in detecting pre-clinical signs of age-related cognitive impairments, particularly those associated with ε4 risk.
Keywords: ApoE, object recognition, aging, cortisol, testosterone, humans
INTRODUCTION
Apolipoprotein E (apoE) is involved in metabolism and redistribution of cholesterol and lipoproteins [1]. In humans, three different alleles encode apoE, ε2, ε3, and ε4. Compared to ε3, ε4 increases the risk to develop age-related cognitive impairments in the absence of frank dementia [2–6] as well as Alzheimer’s disease (AD) [7–11]. Longitudinal studies confirm the effect of ε4 on cognitive decline in normal aging [12–14] as well as on the progression to AD [15–19]. Early detection of age-related cognitive decline would provide the best chance of delaying Mild Cognitive Impairment (MCI) and AD. Therefore, a non-invasive test sensitive to the effects of ε4 could be useful to identify pre-clinical cognitive decline.
Loss of episodic memory, memories based on experiences in specific space and time, occurs during normal aging [20] but is also one of the first signs of AD [21–31]. Object recognition tasks are used to test episodic memory in animals and humans [32]. Novel location recognition, involving a spatial change of a familiar object to a novel location, is sensitive to effects of age in C57BL6/J wild type female and male mice [33] and effects of castration in male mice expressing apoE4, but not in male mice expressing apoE3 or no apoE at all [34]. Furthermore, female mice expressing apoE4 show impaired novel object recognition, involving replacement of a familiar object by a novel object [34, 35]. Based on the objection recognition test used in mice, a human object recognition test (Novel-Image-Novel-Location (NINL)) [36] was developed and shown to be sensitive to effects of ε4 on object recognition in healthy non-demented elderly [6]. Object recognition tests such as NINL might help to identify early cognitive impairment before clinical signs are present.
Reduced cognitive performance and hippocampal size are associated with alterations in circulating glucocorticoid levels. High cortisol levels are suggested to have a negative effect on cognitive performance in men and women [37–40]. In men and women, elevated cortisol levels correlate with a decrease in hippocampal size and poor performance during recall of spatial learning and memory [41]. The correlation between enhanced glucocorticoid levels and cognitive performance might be more complex and sex-dependent. Salivary cortisol levels correlated with performance on the NINL test in men but not in women [6]. The sex dependency of this correlation might be species-dependent. In female rodents, high levels of glucocorticoids were associated with decreased object recognition and spatial learning and memory [42, 43].
In contrast to cortisol, high testosterone levels are associated with improved performance on cognitive tests in women [44–46] and men [47–50]. In the elderly, ε4 was shown to have a sex-dependent effect on salivary testosterone levels; compared to sex-matched non-ε4 carriers, salivary testosterone levels were higher in ε4-carrying men but lower in ε4-carrying women [6]. Consistent with the idea that increasing androgen levels improves cognitive performance in females, testosterone and dihydrotestosterone [35] and selective androgen receptor modulators (SARMs) [51] antagonized impairment of spatial memory retention in female mice expressing apoE4.
In the current investigation, the participants of our original study [6] were invited to return for testing sessions 6 and 18 months after the baseline session. Using a longitudinal study design, effects of ε4 on NINL test performance were assessed in study “dropouts”, participants that did not return for the second and/or third session(s), and “finishers”, participants that returned for all three sessions. As previous reports suggest that many different factors can predict cognitive decline, sex, age, ε4 status, cognitive test scores, cortisol, and testosterone were analyzed as potential predictors of dropout.
METHODS
Subjects
Study participants, including inclusion/exclusion criteria, recruiting, and description of individuals were previously described in detail [6]. In brief, 114 non-demented elderly (age range 62–92) were recruited from two adjacent local retirement communities in Portland, Oregon, USA. All study participants were given a presentation of the planned research and provided informed consent for participation before the start of the baseline session. All procedures were approved by the institutional review boards at Oregon Health and Science University (OHSU) and the Willamette View and Rose Villa retirement communities. At the start of the baseline and 18 month sessions, cognitive status was assessed using the MMSE (Mini-Mental State Examination) [52].
APOE genotyping
APOE genotyping was performed at the General Clinical Research Center of OHSU as previously described in detail [6]. DNA for the assay was isolated from salivary samples and compared to known controls for each APOE genotype. The distribution of the ε4 allele in the study population was ε2/ε2 (0), ε2/ε3 (13 women, 2 men), ε2/ε4 (1 women, 0 men), ε3/ε3 (50 women, 23 men), ε3/ε4 (18 women, 5 men), and ε4/ε4 (2 women, 0 men).
Study design
All the neuropsychological testing was performed in a designated apartment of the Willamette View retirement community in Portland, Oregon. Three sessions, the initial session (baseline) and the 6 and 18 months follow-up sessions, each lasting 1–2 hours, were conducted in the morning starting at 8:30 am. Each session began with collection of a saliva sample. Subsequently, the MMSE was administered in the baseline and 18 month sessions. Following the MMSE in the baseline session, the following standardized cognitive tests were administered: Reaction Time (http://www.delphiforfun.org/Programs/Reaction_times.htm © 2000–2004, Intellitech Systems Inc., Fairborn, OH, USA), Family Pictures [53], and Faces [53]. In the 6-month follow-up session, the Beck Anxiety Inventory [54], Spatial Span Forward and Spatial Span Backward [53], and Wide Range Achievement Test-Reading [55] were administered first. Each session began with a saliva sample, followed by the standardized cognitive tests and the NINL test [6, 36], using a 5 minute test-retest interval.
Object Recognition
The NINL test for humans has been previously described [36]. In short, there are two sets of 12 panels, three images in each panel. The two sets of three-image panels are similar in complexity, but different in content and arrangement within the four quadrants of the panel with one quadrant is always blank. Before the test, the study participants were given an example of the test. Subsequently, a set of 12 panels was presented, 8 seconds for each panel, and the study participant was asked to memorize the panels (reference set). The second set contained panels either identical or slightly changed compared to the reference set by containing one novel image or one image in a novel location. Next, the second set of 12 panels was presented without delay, and the study participants were asked to identify if the panel was identical (no change), or contained a novel image (novel image) or an identical image in a novel location (novel location). In the second set of 12 panels, four panels were identical to the reference set, four panels had identical images with one image moved to a novel location, and four panels had one image replaced with a novel image. For each correctly identified panel, 1 point could be earned. In order to earn each point, the participant had to correctly identify if there was a change, and, in case there was a change, the type of change (novel image or novel location), and where the change occurred. The score obtained for the 12 panels was calculated as the first of two NINL scores (NINL1; maximum of 12 points). Following a 5 minute delay period, without seeing the reference set again, the study participants were presented with a third set of 12 panels and asked to identify the panels according to the same criteria as in NINL1 based on the reference set (NINL2; maximum of 12 points). The third set was identical to the second set but the order of the panels was rearranged. The two scores (NINL1 and NINL2) were added to calculate the NINL total score.
Also, subscores with a maximum of 8 points for no change (NC), novel image (NI), and novel location (NL), were analyzed for each session (4 points maximum for the immediate and delayed set, both sets were added together for calculation of the total subscore). The NC subscore reflects the ability to correctly identify the whether the panels shown were the identical as the panels from the reference set. The NI and NL subscores reflect the ability of the subject to identify the exact novel image or novel location, respectively. Total NINL, NC, NI, and NL scores were analyzed for each session.
Memory Island
As described earlier [6, 36], the Memory Island is a virtual reality task assessing spatial learning and memory in humans. Briefly, participants were placed in front of a 19-inch computer monitor (Dell, USA) with a stereo speaker and subwoofer (Harmon Kardon HK395, Harmon International Industries). Using a joystick (Sidewinder, Microsoft), participants were asked to navigate through the virtual world simulating an island environment of 347 x 287 m2 was composed of four quadrants containing a unique target item. Computer software determined direction, speed, and time spent in each quadrant. Memory Island was only completed during the baseline session.
As previously reported in [6], the Memory Island task included four visible trials followed by four hidden trials. In the visible trials, participants were asked to navigate to each of the four target items marked with a visible flag. The hidden trials contained a target item but no visible flag. Location of the target item required formation of a spatial map. The starting orientation was varied throughout the 8 trials but was kept constant for all participants. When the target item was located within 2 minutes, the trial was determined a success. If the target had not been located within 2 minutes, a directional arrow appeared on the screen to guide the participant to the target. For each trial, a time-stamped coordinate file was generated to calculate total distance moved (feet), velocity (feet/second), time to reach the target (latency, seconds), cumulative distance to the target (feet), and percent time spent in each quadrant.
Salivary Hormones
Saliva samples, collected at the beginning of each session, were analyzed for cortisol and testosterone levels. Using commercial kits for each hormone, the General Clinical Research Center at OHSU determined the level of each hormone in the samples. Performance characteristics for the EIA cortisol (Diagnostic Systems Laboratories, Webster, TX, USA) were as follows: intra-assay precision values of 4.8%, 2.8%, and 1.9% at 0.47, 1.41, and 4.09 µg/dL, respectively, and inter-assay precision values of 15.3% and 9.2% for 0.18 and 1.87 µg/dL, respectively. Performance characteristics for the testosterone EIA (Salimetrics, State College, PA, USA) were as follows: intra-assay precision values 3.3% and 6.7% for 26.3 and 197.3 pg/ml, respectively and inter-assay precision values 5.1% and 9.6% for 13.1 and 200.7 pg/ml, respectively.
Statistical analyses
Data were analyzed using SPSS (version 16.0, SPSS Inc. Chicago IL) and Prism (Graphpad Prism, San Diego, CA) software. Significance was considered at P < 0.05.
Dropout
Following an invitation, some participants did not return for the 6- or 18-month follow-up sessions. These participants were defined as “dropouts” whereas participants that completed all three sessions were defined as “finishers”. Dropout rates were analyzed for effects of ε4 status, sex, and age. Multivariate logistic regression was used to explore the association between the two ε4 groups after accounting for the effects of sex and age.
Baseline data
Previously established cognitive tests were analyzed with a retrospective analysis, separating the dropouts from finishers.
NINL total scores and sub-scores
To determine if NINL scores changed over time in the study, total NINL scores were analyzed using repeated measures ANOVA. The dropouts and finishers of the study were analyzed separately. NINL sub-scores for each session were analyzed using repeated measures ANOVAs. Bonferroni’s correction was used to adjust an experimental-wise error rate. Sub-scores were also analyzed separately for dropouts and finishers.
No difference was observed between cognitive performance of study participants that dropped out of the study after the baseline session and those that dropped out after the 6 month follow up session. To include in the analysis study participants who dropped out of the study after the baseline session, the “Last Observation Carried Forward” (LOCF) imputation method [56] was used for cognitive performance of 6 study participants who dropped out after the baseline session.
Memory Island
Total distance moved, velocity, latency, cumulative distance to the target, ability to reach the target location within the trial time (success or failure), and percent time spent in each quadrant were analyzed using one-way ANOVAs. The data were analyzed separately for dropouts and finishers.
Cortisol and Testosterone Levels
Cortisol and testosterone levels were analyzed separately in male and female groups. Cortisol and testosterone levels were analyzed using repeated measures ANOVA [salivary hormone level X ε4 status X session (repeated measure)]. Hormone levels of dropouts and finishers of the study were analyzed separately. Due to only 1 male ε4 dropout, cortisol and testosterone levels in ε4 and non-ε4 male dropouts were not analyzed. In the female groups, 3 outliers were removed from the finisher group and 2 outliers were removed from the dropout group because the hormone levels were outside the limits of the assay.
RESULTS
Standardized Cognitive Tests
No difference was observed between dropouts and finishers in the MMSE (P = 0.8), Beck Anxiety Inventory (P = 0.7), Wide Range Achievement Test-Reading (P = 0.2), Spatial Span Forward (P = 0.08), Spatial Span Backward (P = 0.7), Reaction Time (P = 0.4), Family Pictures (P = 0.14) and Faces (P = 0.2) tests. As previously reported, there was an effect of sex, but not ε4, on Family Pictures [6]. This effect was not seen when dropouts and finishers were analyzed separately. The MMSE scores were not significantly different between non-ε4 and ε4 finishers over the course of the 18 months testing period (P = 0.09). The scores from the standardized tests and NINL are described in Table 1.
Table 1.
Cognitive test scores of non-ε4 and ε4 dropouts and finishersa
| non-ε4 Dropouts |
ε4 Dropouts | non-ε4 Finishers |
ε4 Finishers | |
|---|---|---|---|---|
| MMSE (Baseline) |
27.67 ± 0.20 | 27.36 ± 0.53 | 27.64 ± 0.17 | 26.60 ± 0.41 |
| MMSE (18 month) |
27.72 ± 0.22 | 28.30 ± 0.50 | ||
| BAIb | 5.00 ± 1.15 | 3.00 ± 0.81 | 4.01 ± 0.44 | 4.67 ± 1.21 |
| WRAT-Rb | 55.27 ± 2.89 | 56.00 ± 3.09 | 58.00 ± 1.00 | 59.14 ± 1.76 |
| SSFb | 6.86 ± 0.35 | 6.72 ± 0.52 | 6.88 ± 0.21 | 7.07 ± 0.34 |
| SSBb | 6.73 ± 0.51 | 7.27 ± 0.42 | 6.58 ± 0.24 | 6.53 ± 0.47 |
| RT | 0.41 ± 0.04 | 0.39 ± 0.02 | 0.38 ± 0.01 | 0.41 ± 0.03 |
| Faces | 68.78 ± 2.13 | 68.27 ± 2.27 | 70.65 ± 0.82 | 70.47 ± 1.81 |
| Family Picturesc | 67.22 ± 6.35 | 50.36 ± 6.82 | 72.81 ± 2.71 | 45.73 ± 5.45 |
| Baseline NINL | 18.28 ± 1.25 | 14.27 ± 1.28* | 18.40 ± 0.52 | 18.93 ± 0.90 |
| 6 month NINL | 16.60 ± 1.20 | 13.38 ± 1.42* | 16.82 ± 0.56 | 19.47 ± 0.98 |
| 18 month NINL | 16.65 ± 0.58 | 19.13 ± 1.17 |
Scores in mean ± SEM from Mini-Mental State Evaluation (MMSE), Beck Anxiety Index (BAI), Wide Range Achievement Test-Reading (WRAT-R), Spatial Span Forward (SSF), Spatial Span Backward (SSB), Reaction Time (RT), Family Pictures, Faces, and Novel-Image-Novel-Location (NINL) were compared between non-ε4 and ε4 dropouts and finishers. NINL was the only cognitive task that demonstrated a difference in performance between non-ε4 and ε4 dropouts and finishers.
indicates 6 missing scores due to participants dropping out before the test was administered. n = 108
indicates there was an effect of sex on the test score which was lost using the current analysis.
indicates score of ε4 dropouts was significantly less than score of non-ε4 dropouts, non-ε4 finishers, and ε4 finishers in the baseline session (P < 0.05).
Baseline session
When the data from the baseline session were analyzed excluding the dropouts of the study, there was no effect of ε4. There was no difference between non-ε4 and ε4 carriers for NINL total scores (P = 0.3), No Change (NC) sub-scores (P = 0.1), Novel Image (NI) sub-scores (P = 0.8), or Novel Location (NL) sub-scores (P = 0.8). The NINL scores of study participants who dropped out after the baseline session and the 6-month session were not different (P = 0.8).
Predictors of dropout
Multivariate logistic regression analysis revealed that ε4 genotype was a significant predictor of study dropout (χ2 = 30.4, P = 0.02) after accounting for the effects of age and gender. Compared to non-ε4 carriers, ε4 carriers were 2.7 times more likely not to complete the study when accounted for the effects of age and sex (P = 0.03). The 95% confidence interval for the estimated odd ratio was 1.36 to 1. However, neither sex nor age by itself was a predictor of dropout.
Follow-up sessions
Total NINL Scores
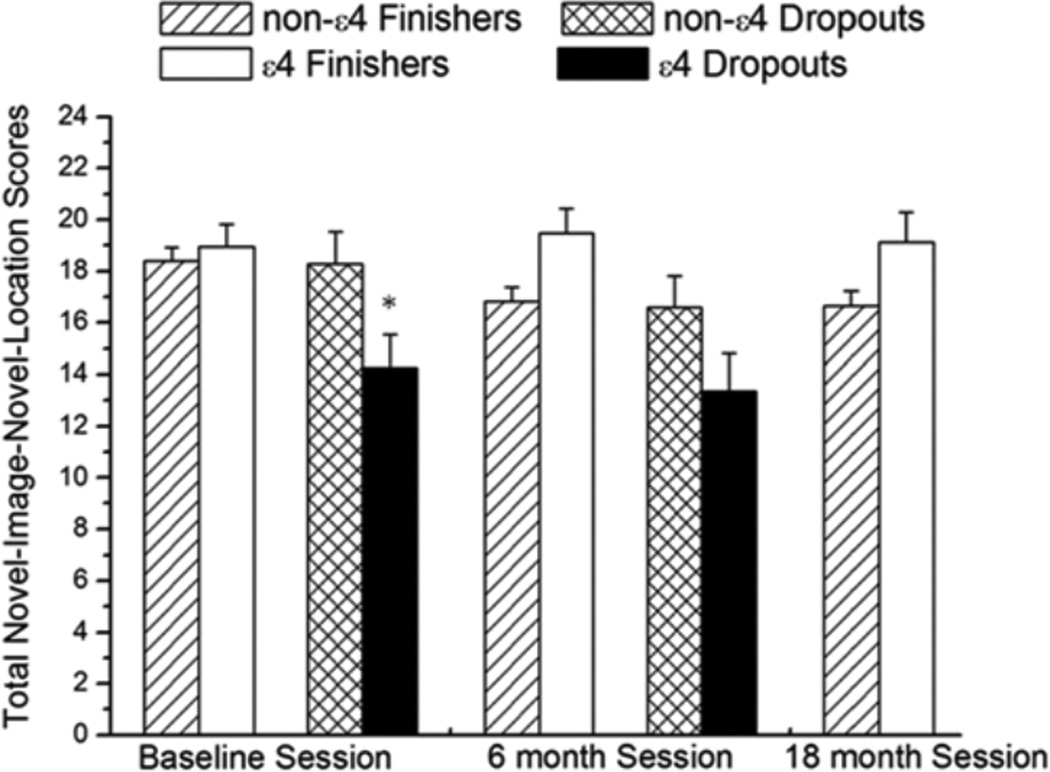
There was an effect of ε4 on the total NINL score for the dropouts and finishers but in opposite directions. In the dropout group, ε4 had a significant effect on NINL score over the two testing sessions [F (1, 21) = 2.5; P = 0.04 (Figure 1)]. The ε4 dropouts scored lower than the non-ε4 dropouts at both the baseline (P = 0.04) and the 6-month (P = 0.03) sessions. In contrast, in the finisher group, ε4 had a significant positive effect on NINL score throughout the testing sessions [F (2, 81) = 3.4; P = 0.03] (Figure 1). Although no difference was observed between the non-ε4 finishers and ε4 finishers in the baseline session (P = 0.7), during each subsequent session, ε4 finishers had higher NINL total scores compared to non-ε4 finishers (6 months P = 0.01; 18 months P < 0.01).
Figure 1.
Total NINL scores for all sessions. In dropouts and finishers, there was a genotype × session interaction. * indicates that a score was significantly lower in the baseline session at P < 0.05. n =114
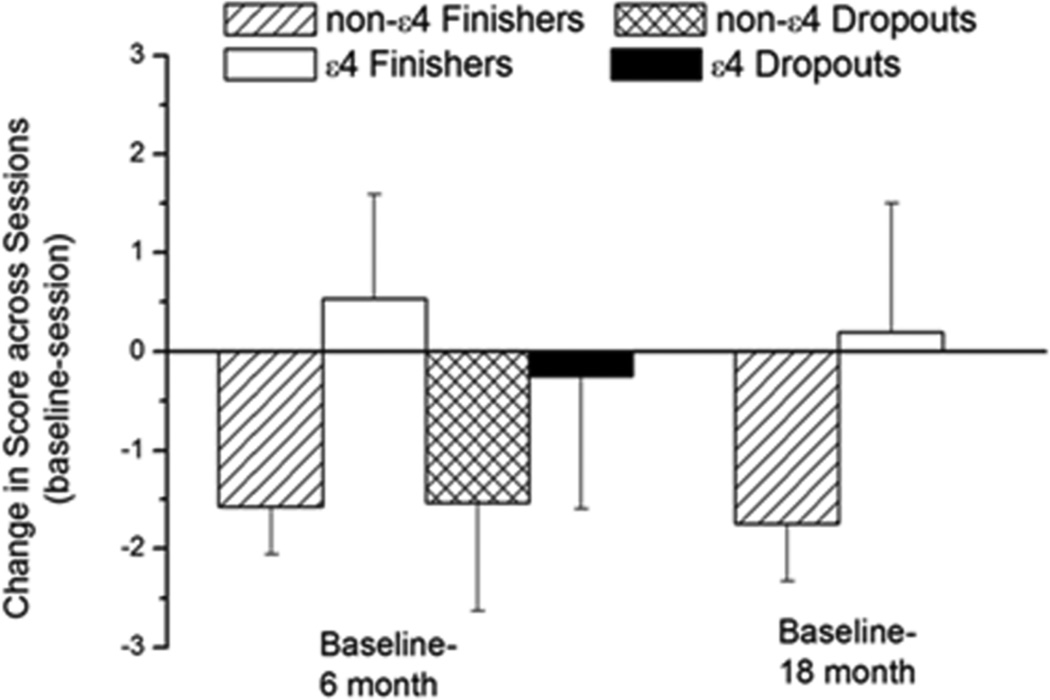
In the dropouts, no difference was observed in the change in total NINL score (ΔNINL total scores) between the two sessions regardless of ε4 status (P = 0.6; Figure 2). The NINL total scores of non-ε4 finishers in both the 6- and 18-month sessions were lower than those at baseline. In contrast, the NINL scores of ε4 finishers in the 6- and 18-month sessions were not different from baseline. In the finisher group, there was a trend of an effect of ε4 on ΔNINL total score between the baseline session and the 6-month session and between the baseline session and the 18-month session but that did not reach significance (P = 0.056).
Figure 2.
Change in total NINL scores of non-ε4 and ε4 dropouts and finishers (non-ε4 n = 72; ε4 n = 16) between the baseline and the follow-up sessions. In finishers, there was a genotype × session interaction. Decreased scores resulted in a negative change value (−). n = 114
NINL sub-scores
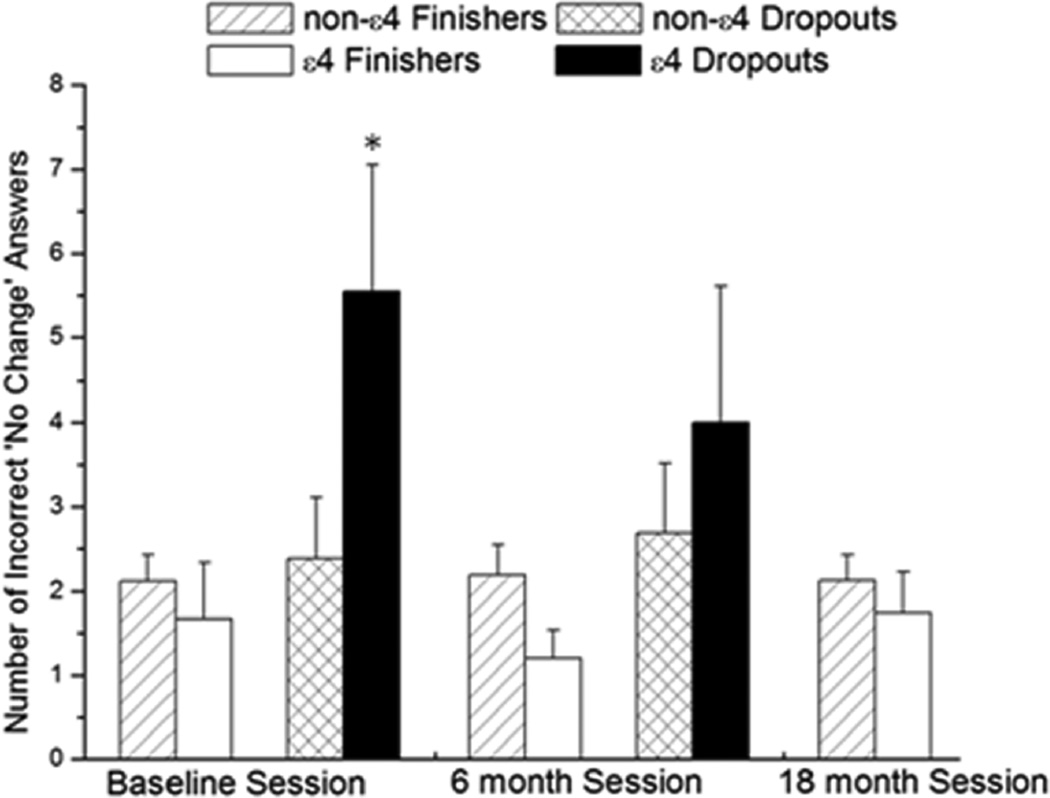
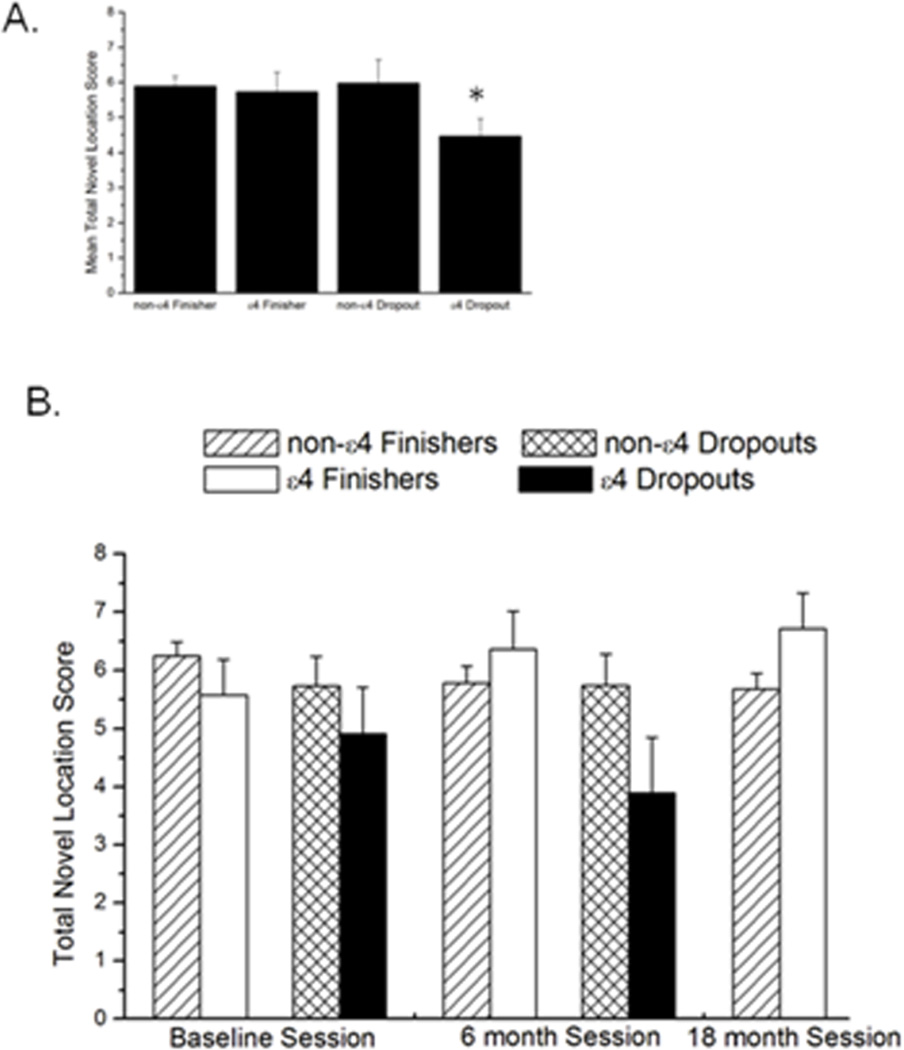
The total No Change (NC) sub-score was not different across the sessions between the non-ε4 and ε4 dropouts (P = 0.2) or non-ε4 and ε4 finishers (P = 0.3; Table 3). In the baseline session, ε4 dropouts had significantly more incorrect ‘no change’ answers than non-ε4 dropouts and non-ε4 and ε4 finishers (P = 0.001; Figure 3). There was no difference in the Novel Image (NI) sub-score between non-ε4 and ε4 carriers over the sessions in either dropouts (P = 0.1) or finishers (P = 0.4; Table 3). For the Novel Location (NL) sub-score, non-ε4 dropouts had significantly higher scores than ε4 dropouts [F (1, 22) = 3.6; P = 0.03]. The ε4 finishers had significantly higher scores compared to the non-ε4 finishers [F (2, 164) = 3.6; P = 0.04 (Figure 4, Table 3)].
Table 3.
NINL subscores
| non-ε4 Dropouts |
ε4 Dropouts | non-ε4 Finishers |
ε4 Finishers | |
|---|---|---|---|---|
| NC Subscore | ||||
| S1 | 6.6 ± 0.3 | 6.3 ± 0.5 | 6.3 ± 0.5 | 6.6 ± 0.5 |
| S2 | 6.3 ± 0.3 | 5.6 ± 0.4 | 5.8 ± 0.2 | 6.4 ± 0.4 |
| S3 | 5.7 ± 0.2 | 6.8 ± 0.3 | ||
| NI Subscore | ||||
| S1 | 5.8 ± 0.7 | 3.5 ± 1.0 | 5.8 ± 0.3 | 6.7 ± 0.3 |
| S2 | 4.5 ± 0.7 | 4.0 ± 0.8 | 5.2 ± 0.3 | 7.0 ± 0.4 |
| S3 | 5.2 ± 0.3 | 6.3 ± 0.7 | ||
| NL Subscore | ||||
| S1 | 5.7 ± 0.3 | 4.9 ± 0.8 | 6.2 ± 0.2 | 5.6 ± 0.6 |
| S2 | 5.7 ± 0.3 | 3.8 ± 0.9 | 5.7 ± 0.3 | 6.4 ± 0.6 |
| S3 | 5.7 ± 0.3 | 6.7 ± 0.6 |
Figure 3.
Incorrect ‘no change’ (NC) responses. In the baseline session, ε4 dropouts had significantly more incorrect ‘NC’ responses than non-ε4 dropouts, non-ε4 and ε4 finishers. * indicates significance in the baseline session at P < 0.05 n = 114
Figure 4.
A. Inset shows average ‘novel location’ (NL) sub-scores over all three sessions. B. Total novel location (NL) sub-scores in the 3 individual sessions. In dropouts and finishers, there was a genotype × session interaction. * indicates a significantly lower collapsed score over time at P < 0.05. n = 114
Memory Island
During the visible trials of the Memory Island task, ε4 dropouts navigated closer to the target location (lower cumulative distance to the target) than ε4 finishers, non-ε4 dropouts and non-ε4 finishers (P = 0.01). While during the last visible trial ε4 dropouts reached the target in less time (lower latency values) than non-ε4 participants (P < 0.001), it was not significantly different from ε4 finishers (P = 0.058). None of the significant differences were predictors of study dropout (P > 0.24).
During the hidden trials, there was no difference in total distance moved, velocity, latency, cumulative distance to the target, success or failure (ability to reach the target location within the trial time), or percentage time spent searching in the target quadrant (containing the platform during the hidden trials) between any of the four groups (non-ε4 dropouts, non-ε4 finishers, ε4 dropouts, and ε4 finishers) in any of the trials (P > 0.35).
Salivary cortisol and testosterone levels
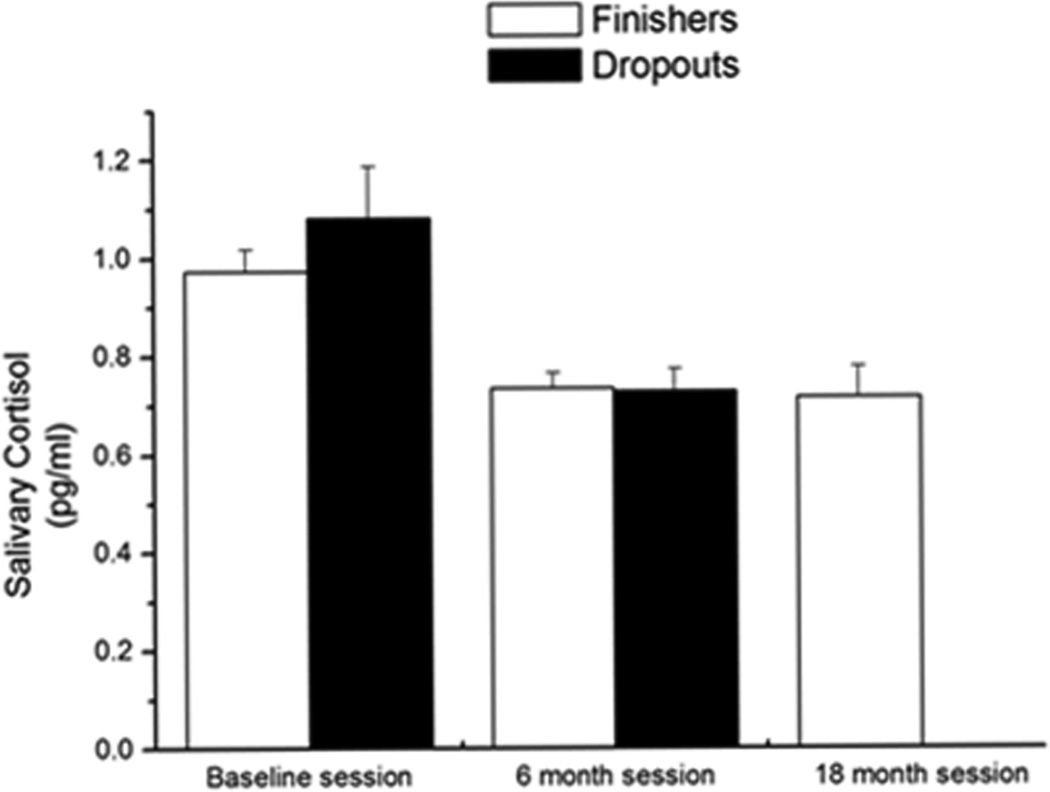
There was no difference in salivary cortisol levels between non-ε4 and ε4 dropouts or non-ε4 and ε4 finishers across the sessions (men: P = 0.6; women: P = 0.7). Furthermore, there was no difference in salivary cortisol levels in men and women (P = 0.5). In the baseline session, there was no difference in salivary cortisol between dropouts and finishers (P = 0.3) and salivary cortisol level was not a predictor of participants dropping out of the study (P = 0.13). However, cortisol levels decreased from the baseline session to the 6-month session in all participants [dropouts: F (1, 22) = 8.4, P = 0.008; finishers: F (2, 68) = 6.15, P = 0.004 (Figure 5)].
Figure 5.
Salivary cortisol levels in dropouts and finishers. In dropouts and finishers, there was a significant decrease in hormone levels over the sessions P < 0.05. n = 107
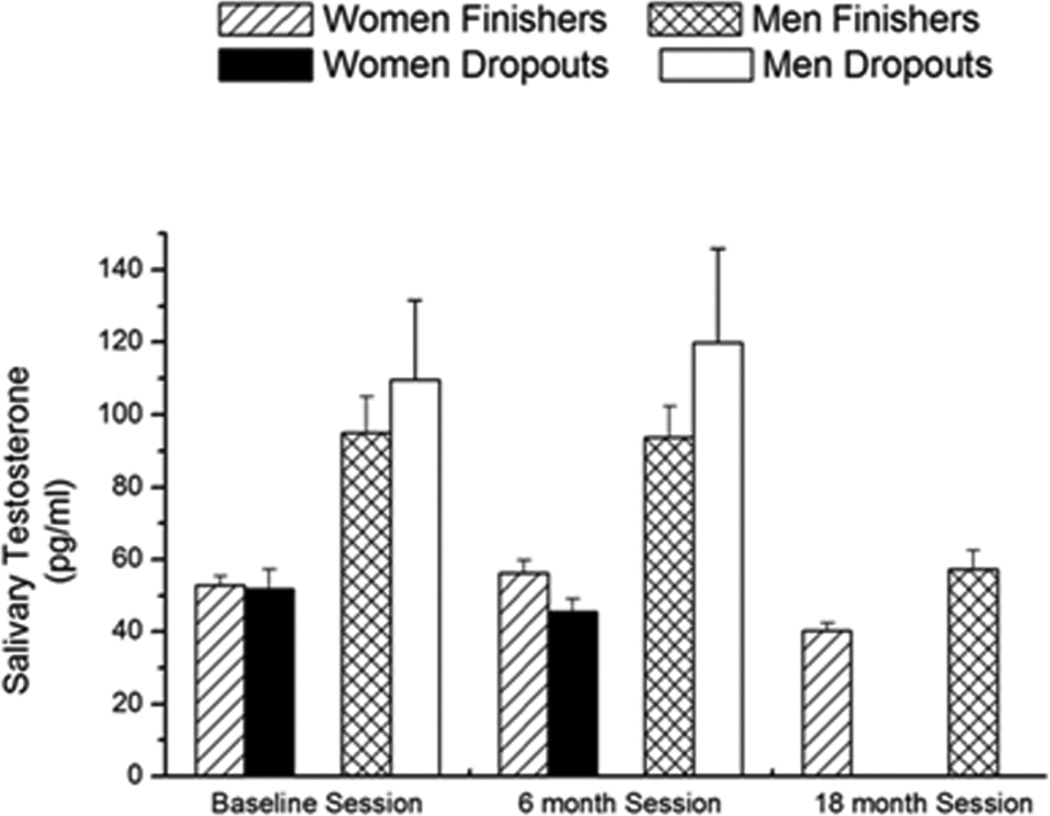
Dropouts and finishers in the study were separated into male and female groups for analyses of salivary testosterone levels. In the baseline session, there was an effect of sex (P < 0.001) on salivary testosterone levels, but there were no differences in testosterone levels between dropouts or finishers (P = 0.3; Figure 6). In the baseline session, ε4 had an effect on testosterone levels (P = 0.04) but testosterone was not a predictor of participants dropping out of the study (P = 0.17). Testosterone levels decreased significantly in the finishers [male finishers: F (2, 70) = 5.342, P = 0.006; female finishers: F (2, 69) = 6.83, P = 0.002] but not in the dropouts [male dropouts: P = 0.38; female dropouts: P = 0.15; Figure 6] and ε4 genotype was not a significant covariate (P > 0.3).
Figure 6.
Salivary testosterone levels in dropouts and finishers. In dropouts and finishers, there was a significant decrease in hormone levels over the sessions P < 0.05. n = 105
DISCUSSION
The main findings of this study are that the NINL task and ε4 are predictors of study dropout in a longitudinal study design of non-demented human “super agers”. To the best of our knowledge no study has identified ε4 as a predictor of study dropout. Participants with ε4 were 2.7 times more likely to drop out of the study than non- ε4 participants and ε4 participants with low NINL scores were 1.3 times more likely to drop out of the study than ε4 participants with a high NINL score. Therefore, the NINL test could be a useful tool in the assessment of early cognitive changes that occur prior to those detected in the clinic using traditional cognitive tests.
Analyzing the effect of ε4 on cognitive tests by dropouts and finishers, some of the effects observed in the original study were lost [6], yet, other interesting effects, were detected. In the baseline session, non-ε4 participants scored higher on the NINL test than ε4 participants [6]. Analyzing the data without the participants that dropped out of the study, there was no difference in performance on the NINL task between non-ε4 and ε4 finishers. However, examination of the data from the baseline session of only the participants that dropped out of the study showed a significant effect of ε4. Therefore, the effect of ε4 observed in the original study was due to the performance of ε4 participants who subsequently would drop out of the study. In the following sessions, ε4 was not an indicator of lower NINL scores. As such, there is a paradoxical effect of ε4 on NINL score, indicating that the NINL task is sensitive to detect cognitive effects of ε4 in healthy non-demented elderly.
Recent studies have demonstrated an effect of ε4 on cognitive performance while others have not. First, a study demonstrated a longitudinal decline in memory, as assessed with the Auditory-Verbal Learning Test (AVLT-LTM), started earlier (before age 60) and showed a greater acceleration in ε4 carriers when compared to non-carriers [57]. These data are consistent with earlier studies of this group showing a more rapid decline in memory in ε4 carriers that was correlated with reduced cerebral metabolism 5–10 years before the onset of cognitive symptoms [58–60]. On the other hand, another study demonstrated that when age and education are added to the analysis, the effect of ε4 disappeared [61]. Another study demonstrated that ε4 did not have an effect on cognitive performance among non-demented 70-year-olds [62]. Although these data seem contradictory, the effect of ε4 might require more specific tests. In our study, instead of using years of formal education, we used WRAT-R as a measure of general intelligence because years of formal education might not be a direct indicator of cognitive abilities due to generational expectations, especially in this age group. Using the WRAT-R as a measure of general cognitive function did not yield significant differences between ε4 and non-ε4 participants. Similar to the results from the Luciano study [62], we found no difference in the performance on a battery of traditional cognitive tests based on ε4 status and dropout/finisher group controlling for age and gender. Taking this information into consideration, we propose that the NINL is a unique test and more sensitive than other traditional cognitive tasks and can be used to detect effects of ε4 on cognition.
There was a significant difference in the change in NINL score between baseline and the 6 or 18 month in non-ε4 and ε4 finishers. In fact, NINL scores of ε4 finishers did not significantly change, suggesting a protective effect of ε4 in the participants that remained in the study. As there was no significant change between the 6- to 18- month sessions, our data suggests that the most important change occurred between the baseline session and the 6-month session and would likely be detectable during a 6 or 12 months follow up visit in a clinical setting. No difference was observed in the NC or NI NINL sub-scores between non-ε4 and ε4 finishers or dropouts across time for, but there was a significant difference in the NL sub-score. Thus, the change of a familiar image to a novel location was difficult for the ε4 dropouts to identify. The addition of the spatial component increases the sensitivity of the NINL task over other object recognition tasks. Consistent with this, in our mouse studies novel location recognition is more sensitive to effects of age in C57BL6/J wild type female and male mice [33] and effects of castration in male mice expressing apoE4 [34]. Perhaps related to the difficulty of ε4 dropouts to identify a novel location change, in the baseline session, ε4 dropouts had significantly more incorrect ‘NC’ answers, thus inflating their NC scores. The ε4 dropouts might have more often used the NC sub-score as a default when they either did not notice a change or did not know the correct answer than the ε4 finishers.
In contrast to the differences between non-ε4 and ε4 dropouts and finishers in the NINL task, very little variation was observed in the Memory Island task. In the baseline study, there was an effect of ε4, which disappeared when the data was analyzed separately for dropouts and finishers. Furthermore, performance on the Memory Island task was not a predictor of dropouts of the study. In addition, no difference was observed between the non-ε4 and ε4 finishers in the MMSE scores at the baseline and 18-month sessions. The spatial navigation and computer use involved in the Memory Island, but not NINL, test might have contributed to this difference in test sensitivity.
Cortisol has been suggested to play a role in hippocampal atrophy [41]. In our initial study [6], cortisol correlated with cognitive performance in only the male study participants. As such, we hypothesized that cortisol would be a predictor of study dropout. However, unlike apoE genotype and NINL score, cortisol was not a predictor of study dropout. Therefore, while cortisol might be a predictor of cognitive performance on some tasks it cannot be assumed to be a predictor for overall cognitive decline.
Another hormone suggested to enhance cognitive function is testosterone [47–50], yet there are contradicting reports about testosterone’s role in cognitive function and the potential role in cognitive impairment [45, 63–66]. In our study, testosterone levels did not correlate with performance on the object recognition test. In addition, there was no difference in testosterone levels of the dropout and finisher groups. The only observed differences in testosterone levels were in the baseline session between non-ε4 and ε4 participants. These data suggest that testosterone is not an indicator of cognitive performance on the NINL task.
Although neither cortisol nor testosterone had an effect on performance in the NINL test, over the course of the longitudinal study both hormones decreased in the finisher population. The decrease in cortisol levels occurred between the baseline and the 6 months sessions, but was not significantly changed between the 6- and 18-month sessions. This observation could be due to a decrease in anxiety levels as the participants were participating in the tests for the second time and were more familiar with the study design. In both male and female finishers, salivary testosterone levels were significantly lower in the 18-month than the baseline session, demonstrating an age-related decline in testosterone levels in the oldest-old population [67, 68]. However, no difference was observed in salivary testosterone levels of the finisher group between the baseline and 6-month session.
We began our study with 114 participants in the baseline session. Of the 114 participants, 26 were ε4 carriers, or 22.8% of the study population, which is a similar distribution of other studies of healthy elderly people [69–72]. We demonstrate that ε4 had a significant effect on dropout rate and that total NINL score was a predictor of whether participants would drop out of the study. There is a small possibility that the participants did not return to the study because they became ill or passed away. Alternatively, although test performance was not communicated to the study participants in any way, it is conceivable that the participants who performed poorly on the cognitive tasks were aware of their performance deficiencies and therefore did not return.
Within the group of ε4 participants, there were two distinct groups; participants who performed well on the NINL test and returned for all three sessions and participants who performed poorly and did not return for all three sessions. It is possible that the finishers were more familiar with the tests and this familiarity contributed to their test performance during follow up test sessions. Remarkable, the dropouts who participated in two study sessions had significantly lower scores in the second session, suggesting that if test familiarity contributed to test performance during follow up visits it did so differently in dropouts and finishers. In any event, these results suggest that the NINL test is sensitive to detect cognitive decline associated with the ε4 allele. Recent evidence supports the concept of distinct ε4subgroups, based on the presence and amount of cognitive decline, when the cognitive decline occurs during AD, where brain atrophy is found, and the amount of brain atrophy [73–75]. Currently, it is not clear what causes the divergence, but there seem to be two likely possibilities; an interaction with a genetic factor besides apoE4 and the individual health and cognitive history.
In summary, in this study, participants with ε4 were more likely to drop out of the study than non-ε4 participants. Furthermore, ε4 participants with a low NINL score were more likely to drop out of the study than ε4 participants with high NINL score. Therefore, NINL could be a valuable tool to assess pre-clinical cognitive decline.
Table 2.
Distribution of participants remaining in the study compared to participants starting the study by ε4 genotype.
| Baseline | 6 month | 18 month | ||||
|---|---|---|---|---|---|---|
| non-ε4 | 87 | 100 %a | 84/87 | 97 % | 72/87 | 81 % |
| ε4 | 27 | 100 % | 24/27 | 88 % | 16/27 | 58 % |
| Total | 114 | 100 % | 108/114 | 95 % | 88/114 | 76 % |
indicates percentage of participants remaining in the study.
Acknowledgments
This work was funded by EMF AG-NS-0201, the Medical Research Foundation of Oregon, a pilot project of the Layton Center for Aging and Alzheimer’s disease, PHS grant 5 M01 RR000334 and NIA T32-AG023477.
REFERENCES
- 1.Mahley RW. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 2.De Blasi S, et al. APOE polymorphism affects episodic memory among non demented elderly subjects. Exp Gerontol. 2008 doi: 10.1016/j.exger.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Adamson MM, et al. Apolipoprotein E varepsilon4 influences on episodic recall and brain structures in aging pilots. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds CA, et al. Longitudinal memory performance during normal aging: twin association models of APOE and other Alzheimer candidate genes. Behav Genet. 2006;36(2):185–194. doi: 10.1007/s10519-005-9027-6. [DOI] [PubMed] [Google Scholar]
- 5.de Frias CM, et al. Cholesterol and triglycerides moderate the effect of apolipoprotein E on memory functioning in older adults. J Gerontol B Psychol Sci Soc Sci. 2007;62(2):P112–P118. doi: 10.1093/geronb/62.2.p112. [DOI] [PubMed] [Google Scholar]
- 6.Berteau-Pavy F, Park B, Raber J. Effects of sex and APOE ε4 on object recognition and spatial navigation in the elderly. Neuroscience. 2007;147:6–17. doi: 10.1016/j.neuroscience.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Farrer LA, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 8.Corder EH, et al. The apolipoprotein E ε4 allele and sex-specific risk of Alzheimer's disease. JAMA. 1995;273:373–374. [PubMed] [Google Scholar]
- 9.Corder EH, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 10.Roses AD. Apolipoprotein E and Alzheimer's disease: A rapidly expanding field with medical and epidemiological consequences. Annals of New York Academy of Sciences. 1996;802:50–57. doi: 10.1111/j.1749-6632.1996.tb32598.x. [DOI] [PubMed] [Google Scholar]
- 11.Selkoe DK, Schenk D. Alzheimer's disease: molecular understanding predicts amyloid-based therapeutics. Annual Reviews of Pharmacological Toxicology. 2003;43:545–584. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- 12.Adak S, et al. Predicting the rate of cognitive decline in aging and early Alzheimer disease. Neurology. 2004;63(1):108–114. doi: 10.1212/01.wnl.0000132520.69612.ab. [DOI] [PubMed] [Google Scholar]
- 13.Howieson DB, et al. Natural history of cognitive decline in the old old. Neurology. 2003;60(9):1489–1494. doi: 10.1212/01.wnl.0000063317.44167.5c. [DOI] [PubMed] [Google Scholar]
- 14.Brayne C, et al. Apolipoprotein E genotype in the prediction of cognitive decline and dementia in a prospectively studied elderly population. Dementia. 1996;7(3):169–174. doi: 10.1159/000106873. [DOI] [PubMed] [Google Scholar]
- 15.Bennett DA, et al. Amyloid mediates the association of apolipoprotein E e4 allele to cognitive function in older people. J Neurol Neurosurg Psychiatry. 2005;76(9):1194–1199. doi: 10.1136/jnnp.2004.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett DA, et al. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer's disease. Neurology. 2003;60(2):246–252. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- 17.Wilson RS, et al. The apolipoprotein E epsilon 4 allele and decline in different cognitive systems during a 6-year period. Arch Neurol. 2002;59(7):1154–1160. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal NT, et al. The apolipoprotein E epsilon4 allele and incident Alzheimer's disease in persons with mild cognitive impairment. Neurocase. 2005;11(1):3–7. doi: 10.1080/13554790490903038. [DOI] [PubMed] [Google Scholar]
- 19.Lahiri DK, Sambamurti K, Bennett DA. Apolipoprotein gene and its interaction with the environmentally driven risk factors: molecular, genetic and epidemiological studies of Alzheimer's disease. Neurobiol Aging. 2004;25(5):651–660. doi: 10.1016/j.neurobiolaging.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Bondi M, et al. Episodic memory changes are associated with the APOE-ε4 allele in nondemented older adults. Neurology. 1995;45:2203–2206. doi: 10.1212/wnl.45.12.2203. [DOI] [PubMed] [Google Scholar]
- 21.Storandt M, et al. Psychometric differentiation of mild senile demintia of the Azheimer type. Archives in Neurology. 1984;41:497–499. doi: 10.1001/archneur.1984.04050170043013. [DOI] [PubMed] [Google Scholar]
- 22.Eslinger P, et al. Neuropsychological detection of abnormal mental decline in older persons. JAMA. 1985;253:670–674. [PubMed] [Google Scholar]
- 23.Knopman D, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Archives in Neurology. 1989;46:141–145. doi: 10.1001/archneur.1989.00520380041011. [DOI] [PubMed] [Google Scholar]
- 24.Fuld P, et al. Object-memory evaluation for prospective detection of dementia in normal functioning elderly: predictive and normative data. Journal of Clinical Experimental Neuropsychology. 1990;12:520–528. doi: 10.1080/01688639008400998. [DOI] [PubMed] [Google Scholar]
- 25.Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly. Predictors of dementia. Neurology. 1991;41:1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 26.Welsh K, et al. Detection of abnormal memory decline in mild cases of Alzheimer's disease using CERAD neuropsychological measures. Archives in Neurology. 1991;48:278–281. doi: 10.1001/archneur.1991.00530150046016. [DOI] [PubMed] [Google Scholar]
- 27.Petersen R, et al. Memory function in very early Alzheimer's disease. Neurology. 1994;44:867–872. doi: 10.1212/wnl.44.5.867. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs D, et al. Neuropsychological detection and characterization of preclinical Alzheimer's disease. Neurology. 1995;45:957–962. doi: 10.1212/wnl.45.5.957. [DOI] [PubMed] [Google Scholar]
- 29.Caselli R, et al. Preclinical memory decline in cognitive normal apolipoprotein E-epsilon 4 homozygotes. Neurology. 1999;53:201–207. doi: 10.1212/wnl.53.1.201. [DOI] [PubMed] [Google Scholar]
- 30.Fox N, et al. Presymptomatic cognitive deficits in individuals at risk of familial Alzheimer's disease. A longitudinal prospective study. Brain. 1998;121:1631–1639. doi: 10.1093/brain/121.9.1631. [DOI] [PubMed] [Google Scholar]
- 31.Tierney M, et al. Prediction of probable Alzheimer's disease in memory impaired patients: A prospective longitudinal study. Neurology. 1996;46:661–665. doi: 10.1212/wnl.46.3.661. [DOI] [PubMed] [Google Scholar]
- 32.Dere E, et al. The case for episodic memory in animals. Neurosci Biobehav Rev. 2006;30(8):1206–1224. doi: 10.1016/j.neubiorev.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Benice TS, et al. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137(2):413–423. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 34.Pfankuch T, et al. Role of circulating androgen levels in effects of apoE4 on cognitive function. Brain Research. 2005;1053:88–96. doi: 10.1016/j.brainres.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 35.Raber J, et al. Androgens protect against apolipoprotein E4-induced cognitive deficits. Journal of Neuroscience. 2002;22:5204–5209. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizk-Jackson A, et al. Effects of sex on object recognition and spatial navigation in humans. Behavioral Brain Research. 2006;173:181–190. doi: 10.1016/j.bbr.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 37.Magri F, et al. Stress and dementia: the role of the hypothalamicpituitary-adrenal axis. Aging Clin Exp Res. 2006;18(2):167–170. doi: 10.1007/BF03327435. [DOI] [PubMed] [Google Scholar]
- 38.MacLullich AM, et al. Plasma cortisol levels, brain volumes and cognition in healthy elderly men. Psychoneuroendocrinology. 2005;30(5):505–515. doi: 10.1016/j.psyneuen.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Li G, et al. Salivary cortisol and memory function in human aging. Neurobiol Aging. 2006;27(11):1705–1714. doi: 10.1016/j.neurobiolaging.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 40.Lupien SJ, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30(3):225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Lupien SJ, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1(1):69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 42.Aisa B, et al. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32(3):256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 43.Sapolsky R. Stress, the aging brain, and the mechanisms of neuron death. Cambridge: MIT Press; 1992. [Google Scholar]
- 44.Konrad C, et al. The functional anatomy of semantic retrieval is influenced by gender, menstrual cycle, and sex hormones. J Neural Transm. 2008;115(9):1327–1337. doi: 10.1007/s00702-008-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yaffe K, et al. Sex hormones and cognitive function in older men. J Am Geriatr Soc. 2002;50(4):707–712. doi: 10.1046/j.1532-5415.2002.50166.x. [DOI] [PubMed] [Google Scholar]
- 46.Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab. 1999;84(10):3681–3685. doi: 10.1210/jcem.84.10.6086. [DOI] [PubMed] [Google Scholar]
- 47.Janowsky JS, Chavez B, Orwoll E. Sex steroids modify working memory. J Cogn Neurosci. 2000;12(3):407–414. doi: 10.1162/089892900562228. [DOI] [PubMed] [Google Scholar]
- 48.Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behav Neurosci. 1994;108(2):325–332. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- 49.Cherrier MM, et al. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology. 2001;57(1):80–88. doi: 10.1212/wnl.57.1.80. [DOI] [PubMed] [Google Scholar]
- 50.Cherrier MM, et al. The role of aromatization in testosterone supplementation: effects on cognition in older men. Neurology. 2005;64(2):290–296. doi: 10.1212/01.WNL.0000149639.25136.CA. [DOI] [PubMed] [Google Scholar]
- 51.Acevedo SF, Tittle S, Raber J. Transgenic expression of androgen receptors improves spatial memory retention in both sham-irradiated and 137Cs gamma-irradiated female mice. Radiat Res. 2008;170(5):572–578. doi: 10.1667/RR1435.1. [DOI] [PubMed] [Google Scholar]
- 52.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 53.Wechsler D. WMS-III administration and scoring manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 54.Beck AT, et al. An inventroy for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology. 1988;65:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 55.Jastak S, Wilkinson G. The wide range achievement test, revised. Wilmington, DE: Jastak Associates; 1994. [Google Scholar]
- 56.Wood AM, et al. Comparison of imputation and modelling methods in the analysis of a physical activity trial with missing outcomes. Int J Epidemiol. 2005;34(1):89–99. doi: 10.1093/ije/dyh297. [DOI] [PubMed] [Google Scholar]
- 57.Caselli RJ, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361(3):255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baxter LC, et al. Apolipoprotein E epsilon 4 affects new learning in cognitively normal individuals at risk for Alzheimer's disease. Neurobiol Aging. 2003;24(7):947–952. doi: 10.1016/s0197-4580(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 59.Caselli RJ, et al. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 2004;62(11):1990–1995. doi: 10.1212/01.wnl.0000129533.26544.bf. [DOI] [PubMed] [Google Scholar]
- 60.Caselli RJ, et al. Correlating cerebral hypometabolism with future memory decline in subsequent converters to amnestic pre-mild cognitive impairment. Arch Neurol. 2008;65(9):1231–1236. doi: 10.1001/archneurol.2008.1. [DOI] [PubMed] [Google Scholar]
- 61.Welsh-Bohmer KA, et al. Neuropsychological performance in advanced age: influences of demographic factors and Apolipoprotein E: findings from the Cache County Memory Study. Clin Neuropsychol. 2009;23(1):77–99. doi: 10.1080/13854040801894730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luciano M, et al. Apolipoprotein E is not related to memory abilities at 70 years of age. Behav Genet. 2009;39(1):6–14. doi: 10.1007/s10519-008-9236-x. [DOI] [PubMed] [Google Scholar]
- 63.Muller M, et al. Sex hormone binding globulin and incident Alzheimer's disease in elderly men and women. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hogervorst E, et al. Low free testosterone is an independent risk factor for Alzheimer's disease. Exp Gerontol. 2004;39(11–12):1633–1639. doi: 10.1016/j.exger.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 65.Hogervorst E, Combrinck M, Smith AD. Testosterone and gonadotropin levels in men with dementia. Neuro Endocrinol Lett. 2003;24(3–4):203–208. [PubMed] [Google Scholar]
- 66.Viega S, et al. Sex hormones and brain aging. Experimental Gerontology. 2004;39:1623–1631. doi: 10.1016/j.exger.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 67.Yeap BB. Testosterone and ill-health in aging men. Nat Clin Pract Endocrinol Metab. 2009;5(2):113–121. doi: 10.1038/ncpendmet1050. [DOI] [PubMed] [Google Scholar]
- 68.Brown M. Skeletal muscle and bone: effect of sex steroids and aging. Adv Physiol Educ. 2008;32(2):120–126. doi: 10.1152/advan.90111.2008. [DOI] [PubMed] [Google Scholar]
- 69.Scuteri A, et al. apoE4 allele and the natural history of cardiovascular risk factors. Am J Physiol Endocrinol Metab. 2005;289(2):E322–E327. doi: 10.1152/ajpendo.00408.2004. [DOI] [PubMed] [Google Scholar]
- 70.Dick IM, et al. Apolipoprotein E4 is associated with reduced calcaneal quantitative ultrasound measurements and bone mineral density in elderly women. Bone. 2002;31(4):497–502. doi: 10.1016/s8756-3282(02)00851-7. [DOI] [PubMed] [Google Scholar]
- 71.Eggertsen G, et al. Influence of variation at the apolipoprotein E locus on lipid and lipoprotein levels in CAPD patients. Nephrol Dial Transplant. 1997;12(1):141–144. doi: 10.1093/ndt/12.1.141. [DOI] [PubMed] [Google Scholar]
- 72.Eggertsen G, et al. Apolipoprotein E polymorphism in a healthy Swedish population: variation of allele frequency with age and relation to serum lipid concentrations. Clin Chem. 1993;39(10):2125–2129. [PubMed] [Google Scholar]
- 73.van der Vlies AE, et al. Most rapid cognitive decline in APOE epsilon4 negative Alzheimer's disease with early onset. Psychol Med. 2009:1–5. doi: 10.1017/S0033291709005492. [DOI] [PubMed] [Google Scholar]
- 74.Sluimer JD, et al. Whole-brain atrophy rate and cognitive decline: longitudinal MR study of memory clinic patients. Radiology. 2008;248(2):590–598. doi: 10.1148/radiol.2482070938. [DOI] [PubMed] [Google Scholar]
- 75.Sluimer JD, et al. Whole-brain atrophy rate in Alzheimer disease: identifying fast progressors. Neurology. 2008;70(19 Pt 2):1836–1841. doi: 10.1212/01.wnl.0000311446.61861.e3. [DOI] [PubMed] [Google Scholar]