Abstract
Rationale: Pulmonary arterial hypertension (PAH) is a progressive lung disease of the pulmonary microvasculature. Studies suggest that bone marrow (BM)-derived circulating cells may play an important role in its pathogenesis.
Objectives: We used a genetic model of PAH, the Bmpr2 mutant mouse, to study the role of BM-derived circulating cells in its pathogenesis.
Methods: Recipient mice, either Bmpr2R899X mutant or controls, were lethally irradiated and transplanted with either control or Bmpr2R899X BM cells. Donor cells were traced in female recipient mice by Y chromosome painting. Molecular and function insights were provided by expression and cytokine arrays combined with flow cytometry, colony-forming assays, and competitive transplant assays.
Measurements and Main Results: We found that mutant BM cells caused PAH with remodeling and inflammation when transplanted into control mice, whereas control BM cells had a protective effect against the development of disease, when transplanted into mutant mice. Donor BM-derived cells were present in the lungs of recipient mice. Functional and molecular analysis identified mutant BM cell dysfunction suggestive of a PAH phenotype soon after activation of the transgene and long before the development of lung pathology.
Conclusions: Our data show that BM cells played a key role in PAH pathogenesis and that the transplanted BM cells were able to drive the lung phenotype in a myeloablative transplant model. Furthermore, the specific cell types involved were derived from hematopoietic stem cells and exhibit dysfunction long before the development of lung pathology.
Keywords: pulmonary arterial hypertension, bone marrow cells, hematopoietic stem cells, transplantation, Bmpr2
At a Glance Commentary
Scientific Knowledge on the Subject
Increased numbers of bone marrow–derived cells are found in the lungs of patients with pulmonary arterial hypertension. However, it is unclear whether these cells have a role in disease initiation or are drawn to an ongoing disease process.
What This Study Adds to the Field
This study shows for the first time that bone marrow cells have both initiating and protective roles in pulmonary arterial hypertension and that the bone marrow cells involved are likely derived from hematopoietic stem cells.
Pulmonary arterial hypertension (PAH) is a progressive, fatal disease characterized by elevated pressures in the pulmonary arterial circulation eventually leading to right heart failure. Several lines of investigation suggest that at least some of these cells, those involved in remodeling and the perivascular infiltrate, are bone marrow (BM)-derived cells (1–4).
BM contains many different types of cells and there are data to suggest that some of them are involved in remodeling of small pulmonary arteries (3–7). However, it remains unclear whether BM cells have a pathogenic or protective role in PAH. Studies using monocrotaline- or hypoxia-induced PAH animal models have shown that wild-type BM cells, or BM-derived and ex vivo–expanded mesenchymal stem cells and endothelial progenitor cells, attenuated PAH when injected into animals with PAH (8–16), suggesting that BM cells might have a protective effect. However, other studies show a causative role for wild-type BM cells in disease pathogenesis (17–19).
There is increasing evidence to suggest that BM cells of hematopoietic lineage, particularly myeloid cells, are important in PAH pathogenesis. BM cells of patients with PAH frequently show myeloproliferative abnormalities (20–22), and there is an increased incidence of PAH in myeloproliferative disorders, such as primary myelofibrosis and myeloid leukemia (23, 24). Furthermore, elegant work by Asosingh and colleagues showed that CD133+ progenitor cells from a patient with PAH produced more colonies in a myeloid colony-forming assay, suggesting CD133+ cells of patients with PAH are enriched in myeloid progenitors and, importantly, that these cells, when transplanted in nude mice in short-term studies, could cause elevated pulmonary pressures (25). Regardless of these studies, there remains significant uncertainty about whether BM cells have a direct causative role in long-term PAH pathogenesis and about the nature and origin of the specific cell type involved.
Bone morphogenic protein receptor 2 (BMPR2) gene mutations are the most common cause of heritable PAH (26–31). One naturally occurring mutation in patients with heritable PAH is an arginine-to-termination mutation (R899X) in the cytoplasmic tail domain of BMPR-II, which disrupts signaling functions of the receptor (26, 32–35). The Bmpr2R899X transgenic mouse model expresses the BMPR2 R899X mutation when induced by doxycycline and develops pulmonary pressures and muscularization of pulmonary arteries.
We used this genetic mouse model of PAH to study the role of BM cells in PAH. We used lethal irradiation–based BM transplantation, and functional and molecular analyses in an attempt to clarify the relative contribution of BM cells to PAH pathogenesis.
Methods
Mice
Rosa26-Bmpr2R899X transgenic mice (FVB/N strain) were generated by intercrossing rtTA2-Rosa26 mice and TetO7-BmprR899X mice as previously described. These mice universally expressed Bmpr2R899X transgene on receiving doxycycline chow as previously described (34, 35). Sixteen weeks after doxycycline induction, Rosa26-Bmpr2R899X mice developed elevated pulmonary pressures along with pulmonary artery muscularization whereas the control Rosa26 mice did not. For brevity, Rosa26-Bmpr2R899X mice are referred to as mutant (Mut) or R899X and rtTA2-Rosa26 mice are referred to as control (or wild-type; note that wild type refers to mice with the wild-type Bmpr2 gene) in text and figures (34, 36). All mouse work was approved by the Vanderbilt University Institutional Animal Care and Use Committee.
BM Transplantation and Analysis of Lungs
To investigate the role of BM cells in PAH, we performed BM cross-transplantation experiments by transplanting mutant BM cells into lethally irradiated (900 cGy) control mice and BM cells isolated from control mice into lethally irradiated mutant mice. Recipient mice were fed with doxycycline chow. Sixteen weeks later, recipient mice were subjected to echocardiography and heart catheterization to evaluate heart function and measure right ventricular systolic pressures (RVSPs) (32, 35). Hematoxylin and eosin staining and immunohistochemistry experiments were done as previously described (37).
To determine whether transplanted donor BM cells migrated to the lungs of recipient mice, donor BM cells were isolated from male mice and transplanted into lethally irradiated female recipient mice as described previously. Sixteen to 20 weeks later, female recipient mice were killed, and the distribution of donor-derived BM cells in the fixed lungs was evaluated by fluorescence microscopy and fluorescence in situ hybridization analysis for the Y chromosome as previously described (38).
Evaluation of Hematopoietic Stem Cells and Progenitor Cells and Colony-Forming Unit Assays
Flow cytometric, cfu, and competitive repopulation assays were done as previously described (39, 40). Please see the online supplement for details.
Cytokine, Microarray, and Quantitative PCR Expression Analyses
Cytokine (41) and expression analyses were done as previously described (42). Please see the online supplement for details.
Results
BM Cells from Bmpr2 Mutant Mice Caused PAH in Control Recipient Mice whereas Control BM Cells Were Protective
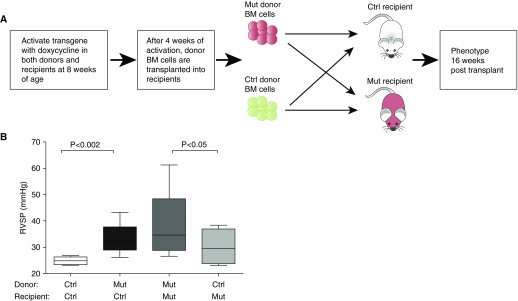
To determine whether BM cells had a direct role in PAH development, we transplanted BM cells isolated from Bmpr2R899X mutant mice into control mice and control BM cells into mutant mice. The control groups consisted of control mice transplanted with control BM cells and mutant mice transplanted with mutant BM cells (Figure 1A; see Table E1 in the online supplement). All the recipients were lethally irradiated to ensure complete myeloablation of the native BM cells. Engraftment of donor Bmpr2R899X BM cells into control mice was confirmed by a previously described real-time PCR assay for engraftment efficiency in recipient mice, at the time of phenotyping and found to be approximately 100% (Figure E1) (43).
Figure 1.
Control (Ctrl) recipient mice transplanted with mutant (Mut) bone marrow (BM) cells developed elevated right ventricular systolic pressure (RVSP), whereas Mut mice transplanted with control BM cells had lower RVSP. (A) Experimental design. (B) Lethally irradiated Ctrl mice developed elevated RVSP when transplanted with Mut BM cells (n = 12) compared with the Ctrl group of lethally irradiated Ctrl mice transplanted with Ctrl BM cells (n = 5) (compare the first and second boxplots). Lethally irradiated Mut mice transplanted with Ctrl BM cells showed moderation of RVSP (n = 7) compared with the Ctrl group of lethally irradiated Mut mice transplanted with Mut BM cells (n = 9) (compare the third and fourth boxplots). RVSP was measured 16 weeks after transplantation. Data are shown as box-and-whisker plots, with whiskers indicating Tukey whiskers. An α level of 0.05 was chosen, and P values less than 0.05 were considered statistically significant. All P values were two-tailed. Analyses were performed with Prism 5 for Mac OS X (GraphPad Software Inc., La Jolla, CA).
We found that control recipient mice transplanted with mutant BM cells developed PAH, as evidenced by increased RVSP 16 weeks after transplantation (Figure 1B, compare the first and second boxplots; P = 0.002), whereas mutant recipient mouse cells transplanted with control BM cells had lower RVSP compared with the control group (Figure 1B, compare the third and fourth boxplots; P = 0.05). These data suggested that mutant BM cells alone had the capacity to cause PAH in control recipient mice, whereas control BM cells had a protective effect on the development of PAH in the mutant mice.
Because the lethally irradiated control mice transplanted with control BM cells did not develop PAH, it suggested that lethal irradiation of recipients did not contribute significantly toward the development of PAH. Overall transplantation of BMPR2R899X BM cells into mutant or control recipient mice did not show a significant increase in RV/LV + S (right ventricle/left ventricle plus septum) ratios or evidence of significant ventricular hypertrophy (Figure E2). These data were consistent with our previous findings that mice expressing BMPR2R899X mutant gene were physiologically incapable of RV remodeling—they dilated and failed instead (44). Overall, the BM transplantation data suggested that BM cells might have an important intrinsic role in PAH development.
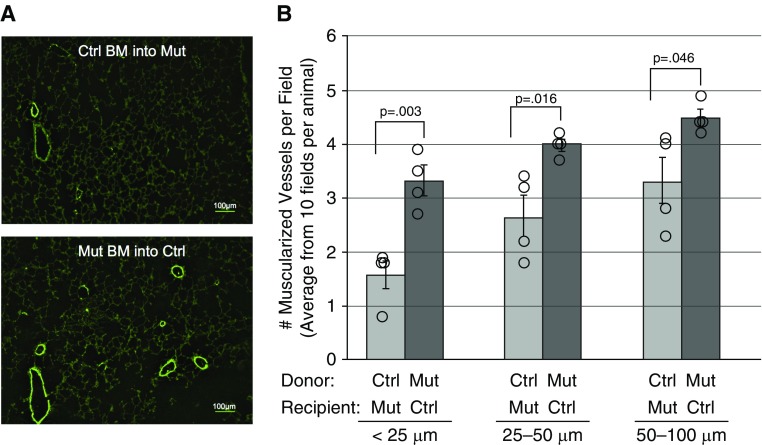
Increased Lung Vascular Muscularization in Mice Transplanted with Bmpr2 Mutant BM Cells
We have shown the BMPR2R899X mutant mice develop muscularization of the pulmonary vessels on activation of the transgene (32, 45, 46). We examined the lungs of recipient mice and found higher numbers of fully muscularized vessels in recipient control mice transplanted with mutant BM cells compared with mutant mice transplanted with control BM cells, at vessel sizes of less than 25 μm in diameter (P = 0.003), less than 25–50 μm (P = 0.016), and 50–100 μm (P = 0.046) per microscope field (Figures 2A and 2B). This suggested that transplanted mutant BM cells could cause vascular remodeling.
Figure 2.
Control (Ctrl) recipient mice transplanted with mutant (Mut) bone marrow (BM) cells had higher muscularization compared with Mut mice transplanted with Ctrl BM cells. (A) Lung sections of recipient mice were stained with anti–smooth muscle actin antibody (fluorescein isothiocyanate conjugated) and visualized by immunofluorescence 16 weeks after transplantation. (B) Three different-sized vessels were counted from 10 microscope fields. Data are presented as means ± SEM and were analyzed by Student's t test. P values less than 0.05 were considered statistically significant. Analyses were performed with Prism 5 for Mac OS X (GraphPad Software Inc., La Jolla, CA).
Increased Number of Inflammatory Cells in and around the Pulmonary Microvasculature in Mice Transplanted with Bmpr2 Mutant BM Cells
Previous studies have shown that inflammatory cells, such as monocytes/macrophages and T cells, tend to accumulate in the lungs of patients with PAH and PAH animal models (47–50). We proceeded to determine whether this was also the case in the recipient mice. Hematoxylin and eosin staining of recipient mouse lung tissue showed that control mice transplanted with mutant BM cells had increased numbers of cells in the lung parenchyma and around the pulmonary vasculature than mutant mice transplanted with control BM cells (Figure E3).
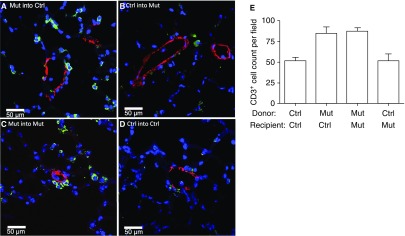
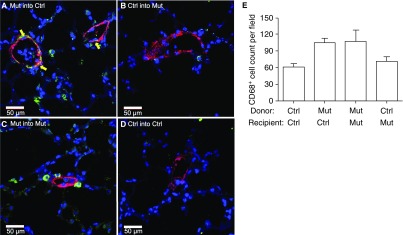
We then determined the nature and relationship of these cells to the vasculature by immunofluorescence. We found that there were more T cells (CD3+) (Figure 3) and macrophages (CD68+) (Figure 4) around pulmonary vessels in the lungs of control or mutant recipient mice transplanted with mutant BM cells. Furthermore, macrophages appeared to be imbedded within the vessel walls (Figure 4A) and were present in higher numbers than T cells in lung sections of mice transplanted with mutant BM cells (Figures 3E and 4E). A quantitative determination of the expression of inflammatory cell markers showed that the lungs of control recipient mice transplanted with mutant BM cells had higher expression of monocyte (CD11b; P = 0.002) and inflammatory cell (TLR4; P = 0.005) markers compared with controls (Figure E4).
Figure 3.
Lungs of recipient mice transplanted with mutant (Mut) bone marrow (BM) cells had increased numbers of T cells (CD3+) compared with lungs of Mut mice transplanted with control (Ctrl) BM cells. Lungs of recipient mice were analyzed 16 weeks after transplantation by costaining with CD3–fluorescein isothiocyanate (green) and α-smooth muscle actin–tetramethylrhodamine (red) antibodies. Nuclei were visualized with 4′,6-diamidino-2-phenylindole (blue). (A) Mut BM transplanted into Ctrl recipient mice. (B) Ctrl BM transplanted into Mut recipient mice. (C) Control group; Mut BM cells transplanted into Mut recipient mice. (D) Control group; Ctrl BM cells transplanted into Ctrl recipient mice. Representative pictures are shown. (E) Average number of CD3+ cells by cell counting from 10 random fields at a magnification of ×10.
Figure 4.
Lungs of recipient mice transplanted with mutant (Mut) bone marrow (BM) cells had increased numbers of macrophages (CD68+) compared with lungs of Mut mice transplanted with control (Ctrl) BM cells. Lungs of recipient mice were analyzed 16 weeks after transplantation by costaining with CD68–fluorescein isothiocyanate (green) and α-smooth muscle actin–tetramethylrhodamine (red) antibodies. Nuclei were visualized with 4′,6-diamidino-2- (blue). (A) Mut BM transplanted into Ctrl recipient mice. (B) Ctrl BM transplanted into Mut recipient mice. (C) Control group; Mut BM cells transplanted into Mut recipient mice. (D) Control group; Ctrl BM cells transplanted into Ctrl recipient mice. Note that CD68+ cells appeared to be imbedded within the vessel wall. Representative pictures are shown. Yellow arrows indicate the likely presence of CD68+ cells in the vessel wall. (E) Average number of CD68+ cells by cell counting from 10 random fields at a magnification of ×10.
The inflammatory cells are donor derived
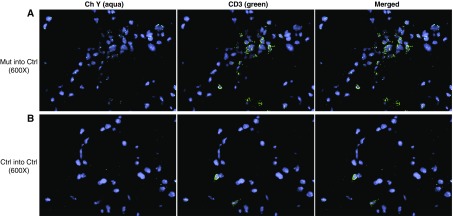
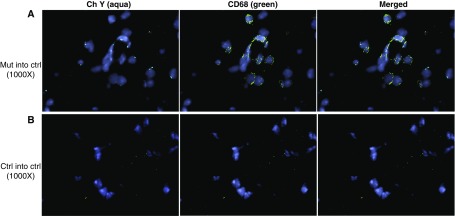
Given that the recipient mice were myeloablated, the inflammatory cells in the recipient mice would be derived from donor BM cells. We sought further evidence of this by tracing donor cells in the lungs of lethally irradiated recipient mice. We used Y chromosome fluorescence in situ hybridization to trace male donor cells in the lungs of female recipient mice. We found that the female recipient control mice had male Bmpr2R899X T cells (CD3+) (Figure 5) and Bmpr2R899X macrophages (CD68+) (Figure 6), as evidenced by the presence of the Y chromosome in the nucleus of CD3+ and CD68+ cells.
Figure 5.
Recipient mice contain donor-derived CD3+ cells. Lungs of female recipient mice transplanted with male bone marrow (BM) cells were analyzed 16 weeks after transplantation. To visualize the Y chromosome (Ch Y) in donor male BM cells, fluorescence in situ hybridization was combined with immunohistochemistry. Lung sections were painted with Y probe (aqua) and then costained with CD3–fluorescein isothiocyanate (T cells, green). Nuclei were visualized with 4′,6-diamidino-2-phenylindole (blue). Representative pictures are shown at an original magnification of ×600. (A) Mutant (Mut) BM transplanted into control (Ctrl) recipient mice. (B) Control group; Ctrl BM transplanted into Ctrl recipient mice.
Figure 6.
Recipient mice contain donor-derived CD68+ cells. Lungs of female recipient mice transplanted with male bone marrow (BM) cells were analyzed 16 weeks after transplantation. To visualize the Y chromosome (Ch Y) in donor male BM cells, fluorescence in situ hybridization was combined with immunohistochemistry. Lung sections were painted with Y probe (aqua) and then costained with CD68–fluorescein isothiocyanate (macrophages, green). Nuclei were visualized with 4′,6-diamidino-2-phenylindole (blue). Representative pictures are shown at an original magnification of ×1,000. (A) Mutant (Mut) BM transplanted into control (Ctrl) recipient mice. (B) Control group; Ctrl BM transplanted into Ctrl recipient mice.
Increased levels of neutrophil chemokine CXCL1 in lungs of mice transplanted with mutant BM cells
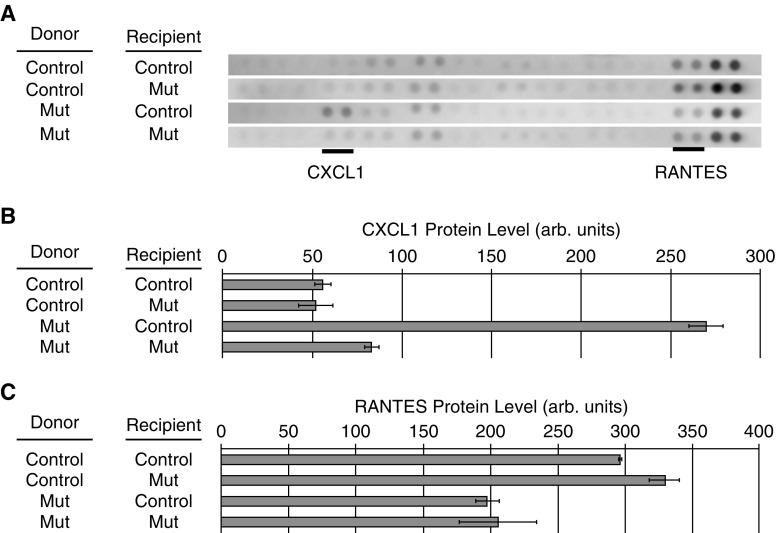
We determined whether there were any differences in the inflammatory milieu in lung tissues between recipient mice transplanted with control and mutant BM cells by mouse cytokine arrays. Of the 40 cytokines tested, 7 were extremely weak or absent in all four groups, 21 had weak but likely present expression, 8 had moderate levels of expression, and 4 had strong expression (Table E2). Of those in the category with at least one sample of moderate expression, CXCL1 had strong expression in the lungs of mice that were transplanted with mutant BM cells compared with mice transplanted with control BM cells and the two control groups (mutant into mutant and control into control). RANTES (regulated upon activation, normal T-cell expressed and secreted) expression was slightly lower in recipient mice transplanted with mutant BM cells (Figures 7A–7C). These data suggest that inflammatory cytokines may play a role in the phenotype produced by the transplanted BM cells.
Figure 7.
Altered chemokine levels in the lungs of recipient mice. Protein extracts from lung tissue of three recipient mice from each transplant group were pooled and applied to R&D Systems mouse cytokine array panels. The four analysis groups were as follows: control (Ctrl) bone marrow (BM) transplanted into Ctrl recipient mice, Ctrl BM transplanted into mutant (Mut) recipient mice, Mut BM cells transplanted into Ctrl recipient mice, and Mut BM cells transplanted in Mut recipient mice. (A) Each pair of dots represents an independent cytokine antibody. Forty cytokines were analyzed per array. The strips containing CXCL1 and RANTES (regulated upon activation, normal T-cell expressed and secreted) are shown, and the spots representing CXCL1 and RANTES are underlined. (B) Normalized densitometry of CXCL1 in each group. (C) Normalized densitometry of RANTES in each group. arb. = arbitrary; CXCL1 = chemokine (C-X-C motif) ligand 1 (melanoma growth–stimulating activity α).
Altered functions of cells of hematopoietic lineage in the BM of the mutant mice
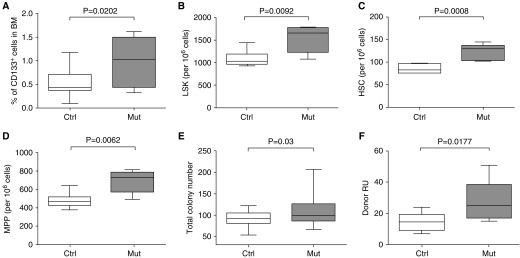
The development of increased RSVP and muscularization in control recipient mice transplanted with mutant BM cells suggested that the phenotype was driven by the transplanted BM cells, especially considering that the recipient mice do not have the mutated Bmpr2 gene. We thus evaluated the BM populations of both mutant and control mice for functional differences. Previous studies in humans have shown that patients with PAH have higher numbers of CD133+ progenitor cells (51). We thus first enumerated various progenitor populations in the BM of mutant and control mice by flow cytometry. Importantly, BM populations were analyzed just 2–4 weeks after induction of the mutant transgene to minimize any potential influence of the developing lung disease on the behavior of the BM cells. We found that, consistent with human PAH, mutant BM had increased numbers of CD133+ cells compared with control BM (Figure 8A) (51). Furthermore, mutant BM cells had elevated numbers of lineage–, Sca-1+, c-Kit+ (LSK) cells (P = 0.009), hematopoietic stem cells (HSCs) (LSK/CD48–CD150+; P = 0.0008), and multipotent progenitors (MPPs) (LSK/CD34+Flt3high; P = 0.006) (Figures 8B–8D). Colony-forming unit assays showed that mutant BM cells produced higher numbers of total colonies than did control BM cells (Figure 8E). No significant difference in specific types of progenitor colonies was found (data not shown).
Figure 8.
Hematopoietic stem cell (HSC) and progenitor cell functions were altered in the mutant (Mut) mice with pulmonary arterial hypertension. Flow cytometric analysis of cell populations was done in the bone marrow (BM) of Mut and control (Ctrl) mice. (A) CD133+ progenitor cells, (B) Lin–Sca-1+c-kit+ (LSK) cells, (C) HSCs (LSK/CD48–CD150+), and (D) multipotent progenitors (MPPs) (LSK/CD34+Flt3high). (E) BM cells from Mut mice produced more colonies in methylcellulose colony-forming assays than BM cells from Ctrl mice. (F) Mut BM cells have higher donor reconstitution units (RU) in long-term competitive transplantation assays than control BM cells. Data are shown as box-and-whisker plots, with whiskers indicating Tukey whiskers. An α level of 0.05 was chosen, and P values less than 0.05 were considered statistically significant. All P values were two-tailed. Analyses were performed with Prism 5 for Mac OS X (GraphPad Software Inc., La Jolla, CA).
To determine whether there were differences in HSC function between the mutant and control mice, we did in vivo competitive repopulation assays. Lethally irradiated 8-week-old control recipient mice were transplanted with 106 BM cells from mutant mice at a 1:1 ratio with 106 competitor green fluorescent protein–expressing control BM cells. We then examined peripheral blood for donor chimerism at 16 weeks post-transplantation. We found that mutant BM cells had a twofold higher reconstituting ability/engraftment potential than did control BM cells (P = 0.018) (Figure 8F). Increased numbers of HSCs and progenitor cells as well as higher reconstituting/engraftment ability of HSCs and higher colony-forming unit potential of the mutant BM suggested the mutant HSCs likely have increased self-renewal, that the progenitor cells have increased differentiation potential, and that differences were cell intrinsic because the BM cell populations were analyzed long before they could be influenced by the developing PAH lung phenotype.
Mutant BM cells develop an early molecular signature of inflammation
To gain insight into the biological and molecular mechanisms involved in the effects of Bmpr2 mutation on BM cells, we compared global gene expression profiles of Bmpr2 mutant and control BM cells. As with the functional studies BM cell expression profiling was done early—2 to 4 weeks after transgene activation—and long before the development of any lung pathology in the mutant mice. We identified 712 genes that were differentially expressed—487 genes were up-regulated and 225 genes were down-regulated (by at least 1.2-fold with a corrected P value of 0.05) in Bmpr2R899X BM cells (Table 1 and Table E3). The top differentially expressed genes in the array data set were confirmed by real-time PCR (Figure E5).
Table 1.
Top 15 Up- and Down-regulated Genes in Mutant Bone Marrow Compared with Control BM Cells
| Gene Symbol | Corrected P Value | Fold Change Mut versus Control | Log2 Fold Change Mut versus Control |
|---|---|---|---|
| Anxa3 | 0.014283723 | 2.7345881 | 1.4513235 |
| Cwc22 | 0.012688569 | 2.5039344 | 1.3241968 |
| Gatm | 4.01 × 10–5 | 2.255848 | 1.1736698 |
| Snora15 | 0.002137598 | 2.1619616 | 1.1123409 |
| Glrx3 | 0.032915503 | 1.8799846 | 0.9107208 |
| Jun | 0.007573118 | 1.8005234 | 0.8484163 |
| Gdpd1 | 8.07 × 10–5 | 1.7881864 | 0.83849716 |
| Ccl3 | 3.60 × 10–4 | 1.7326305 | 0.792964 |
| Olfr10 | 0.039548848 | 1.7275498 | 0.7887273 |
| Zfp931 | 0.002489619 | 1.6726809 | 0.7421622 |
| Fos | 0.001530399 | 1.6628366 | 0.7336464 |
| Sqrdl | 7.19 × 10–5 | 1.5844775 | 0.6640072 |
| Trpv2 | 3.24 × 10–5 | 1.5761803 | 0.6564326 |
| Olfr310 | 0.010107463 | 1.5510877 | 0.6332803 |
| Dpep2 | 0.003690173 | 1.5448326 | 0.62745047 |
| Rhoa | 0.001682325 | −1.6033893 | −0.6811247 |
| Phxr4 | 0.001431052 | −1.6069964 | −0.6843667 |
| Eif4a2 | 0.010703338 | −1.618823 | −0.69494534 |
| Gpr52 | 0.022888012 | −1.6221588 | −0.6979151 |
| Snora73a | 7.03 × 10–4 | −1.6313282 | −0.70604706 |
| Actg1 | 0.001379384 | −1.6758655 | −0.7449064 |
| Tpt1 | 0.004652223 | −1.7219615 | −0.78405285 |
| Cd24a | 0.004322249 | −1.8408889 | −0.88040257 |
| Hspa8 | 6.65 × 10–5 | −1.9101429 | −0.93368053 |
| Malat1 | 0.002931156 | −1.9239621 | −0.94408035 |
| Tuba1b | 0.03283871 | −2.0622864 | −1.0442448 |
| Rpph1 | 3.06 × 10–4 | −2.1314666 | −1.0918465 |
| Snora33 | 0.01737754 | −2.2991877 | −1.2011242 |
| ND6 | 0.00431217 | −3.6850224 | −1.8816733 |
Definition of abbreviations: BM = bone marrow; Mut = mutant.
Expression analysis was done in young (8-wk-old) mice in which the mutant transgene was activated for about 2–4 weeks before the development of any lung phenotype in the mutant mice. Each group consisted of three replicates, with each comprising pooled bone marrow cells from three mice. Thus, in total, nine mutant and nine control mice were used.
Of note, we found that several genes important in the inflammatory response and PAH, such as Fos, Ccl3, cJun, and IL-6, were elevated in the mutant BM cells. Interestingly, Cwc22, a splice regulator and partner of ASF/SF2 splicing factor, previously shown to be important in PAH, was the second highest differentially expressed gene (52).
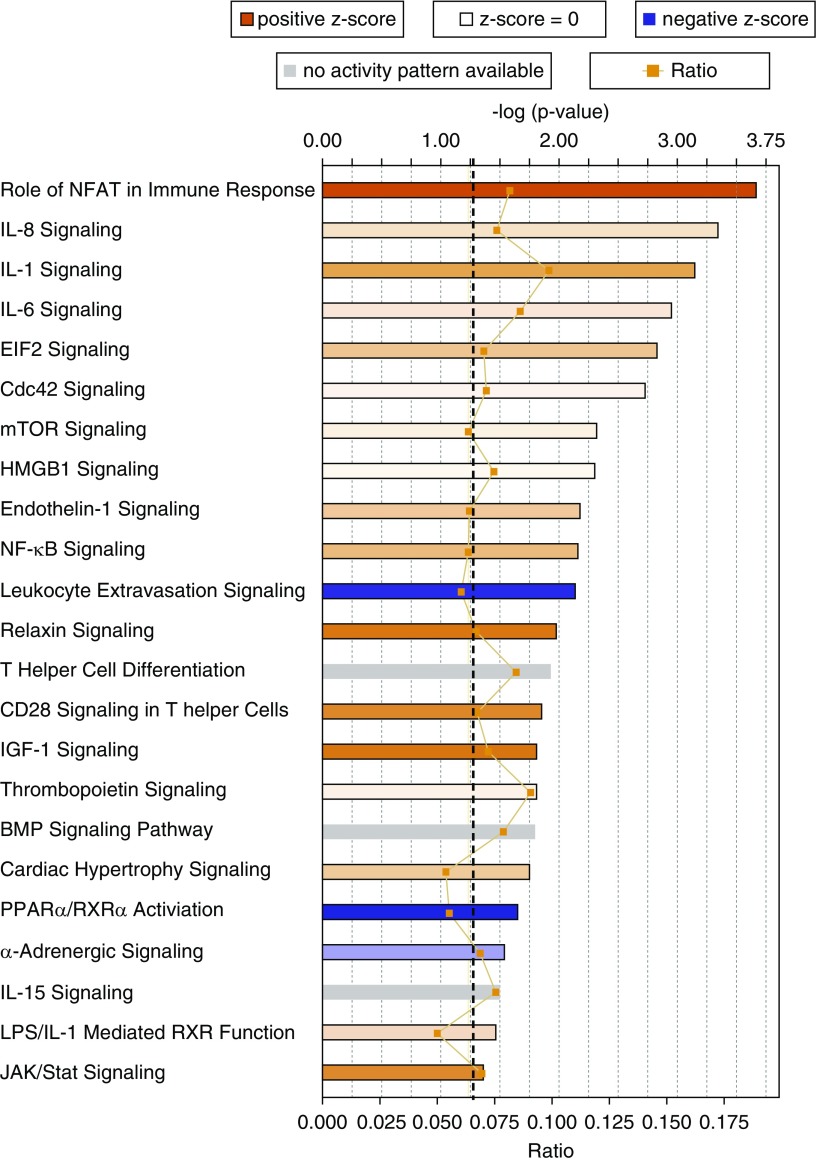
Pathway analysis showed that there was activation of several key inflammatory signaling pathways with known roles in PAH such as NFAT, IL-8, IL-1, IL-6, NF-κB, CD28, JAK/Stat, endothelin, CD28, and T-helper cells differentiation in the mutant BM cells (Figure 9). IL-6 is secreted by monocytes and T cells and data suggest that IL-6 might be one of the key cytokines in the pathogenesis of PAH (53).
Figure 9.
Canonical pathways significantly overrepresented in the gene expression data. The most statistically significant canonical pathways in the differential expression data (comparing mutant bone marrow cells with control bone marrow cells) are shown. Orange–red is indicative of pathway activation; blue is indicative of pathway repression. Color strength shows the degree of activation and repression. The orange line shows the ratio of genes found in each pathway over the total number of genes known to be in that pathway. The dashed black line represents the threshold line and corresponds to a P value of 0.05. The significance of the association between the dataset and the pathway was measured in two ways: (1) a ratio of the number of molecules from the dataset that map to the pathway divided by the total number of molecules that map to the canonical pathways (indicated by the orange line and boxes), and (2) Fisher’s exact test was used to calculate a P value determining the probability that the association between the genes in the observed values and the canonical pathway is explained by chance alone. BMP = bone morphogenetic protein; EIF2 = eukaryotic initiation factor 2; HMGB1 = high-mobility group box 1; IGF = insulin-like growth factor; JAK = Janus kinase; mTOR = mammalian target of rapamycin; NFAT = nuclear factor of activated T cells; NF-κB = nuclear factor-κB; PPARα/RXRα = peroxisome proliferator–activated receptor-α/retinoid X receptor-α.
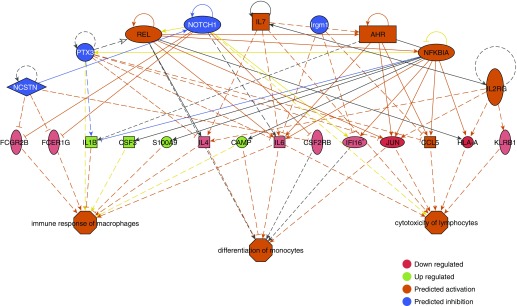
We then analyzed our data to determine the cascade of upstream regulators, that is, mechanistic networks of genes, which may account for the gene expression changes seen in our data set. This analysis showed that Fas and Ccl5 were predicted to be the top mechanistic regulators of the differential gene expression pattern seen in the array analysis (P = 5.2 × 10−14 and 3.6 × 10−13, respectively) (Figure E6). This is notable because both Ccl5 and Fas have a role in PAH with Fas involved in the cross-talk between autophagy and apoptosis in PAH through its interactions with Cav-1 and Ccl5 (54). Interestingly, even at the gene expression level and only 2 weeks postactivation of the mutant transgene, a downstream effects analysis predicted activation of several inflammatory cell functions such as immune response of macrophages, differentiation of monocytes, and cytotoxicity of lymphocytes (Figure 10).
Figure 10.
Ingenuity pathway analysis regulator effects analyses of expression data predict activation of inflammatory cell functions in mutant bone marrow cells shortly after mutant transgene activation. The Regulator Effects algorithm connects upstream regulators to downstream cellular functions to explain how the activation or inhibition of an upstream regulator affects the downstream target molecule expression and the impact of the molecular expression on functions and diseases. The regulators are colored by their predicted activation state: red (down-regulated), green (up-regulated), orange (predicted activation), and blue (predicted inhibition). The intensity of color indicates the degree of activation or repression. Darker colors indicate higher absolute z-scores. Fisher’s exact test with a cutoff P value less than 0.01 was used determine the probability that the association between upstream regulator in expression data and the downstream cell function is explained by chance alone. The lines connecting the nodes are orange when leading to activation of the downstream node, blue or dark gray when leading to its inhibition, and yellow if the findings underlying the relationship are inconsistent with the state of the downstream node. Pointed arrowheads indicate that the downstream node is expected to be activated, whereas blunt arrowheads indicate that the downstream node is expected to be inhibited. Dashed lines indicate virtual relationships composed of the net effect of the paths between the root regulator and the target. *Multiple identifiers in the expression data set for this gene.
Discussion
We present data that show that Bmpr2R899X mutant BM cells can cause PAH with pulmonary vascular muscularization when transplanted into lethally irradiated control mice, whereas control BM cells can moderate increased pulmonary pressures and decrease remodeling when transplanted into lethally irradiated mutant mice. Functional and expression analysis showed that mutant BM cells had altered function and were primed for an inflammatory response early, long before any feedback from developing lung pathology. Overall, our data suggest that BM cells may play a key, initiating role in PAH and, given that normal BM cells offered relative protection for the mutant recipients against the development of PAH, our findings further suggest the possibility that normal BM cells might be useful as therapy in PAH in the form of a BM transplant.
We show that transplanted mutant BM cells could drive the disease phenotype by themselves because the lungs of control recipients expressed wild-type Bmpr2 (Figure 1B). We found early functional abnormalities in the mutant BM cells including increased numbers of CD133+ progenitor cells, increased numbers of MPPs, increased numbers of myeloid colony-forming units, and increased self-renewal capacity of the HSCs. Increased numbers of progenitor cells and increased colony-forming unit potential could be one explanation for the increased numbers of myeloid and T cells seen in the lungs of mice transplanted with the mutant BM (Figures 3 and 4). These findings are consistent with previous studies showing increased numbers of CD133+ cells in patients with PAH (25, 51), and other studies showing that myeloid and inflammatory cells may be relevant in PAH (51, 55). Previous studies, however, have not been able to determine the source of the increased numbers of progenitor cells seen in both mouse models of PAH and humans with PAH. Our data suggest that the increased numbers of progenitor cells might in part be due to more HSCs in the mutant BM compared with the control BM (Figures 8C and 8F).
Cytokine array analysis showed elevation of CXCL1 in the lungs of recipient mice transplanted with mutant BM cells, suggesting that CXCL1 might be important in disease pathogenesis. The increase in CXCL1 was present only in this one group and not in the control group of mutant BM cells in mutant recipient mice. This was not surprising as we have previously published that mice with a Bmpr2 mutation affecting SMAD function had strong induction of a number of cytokines in both vascular smooth muscle and endothelium (37, 56, 57), but Bmpr2 mutations that affected the tail domain, such as R899X, did not show induction of cytokines (32, 57), including when expressed in every cell type. Thus when both donor cells and recipients carry the tail domain R899X mutation the mice would be unable to induce a significant cytokine response, as was the case in the control group. However, when mutant BM cells were transplanted into control mice, increased CXCL1 was likely to be from the recipient lung tissues, which had normal/functional Bmpr2.
RNA expression data were consistent with these data and provided additional clues as to the molecular mechanisms at play. The upstream regulators identify a cascading network of genes that are differentially expressed and point to cell types and functions known to be important in PAH pathogenesis (Figure 10), all within 2 weeks of transgene activation—nearly 12 to 14 weeks before detection of any lung pathology. That these molecular and previously described functional changes would occur so soon after mutant gene activation is a key finding of our study and further suggests that the functional and inflammatory responses seen here were likely BM cell intrinsic. Canonical pathway analysis of the expression data showed increased IL-8, IL-1, and IL-6 signaling (Figure 9), suggesting that in this model of PAH mutant Bmpr2 transgene expression resulted in an overall increased inflammatory response. IL-6 is secreted by monocytes and T cells, and data suggest that IL-6 might be one of the key cytokines in the pathogenesis of PAH (53). Interestingly, IL-6 can up-regulate CXCL1 expression and thus these expression data may provide one explanation for the elevated CXCL1 expression in the lung tissue in mice (Figure 7). IL-8 is produced by macrophages, is a growth factor for endothelial cells, and can induce chemotaxis in granulocytes. IL-8 has also been shown to play a role in early phases of vascular remodeling in PAH (58). These data are also consistent with findings that induction of PAH in Bmpr2+/− mice resulted in significantly increased levels of IL-6 and IL-8 in both the lungs and circulation (59). Because it is known that IL-6 is important in hypoxia-induced PAH (60, 61) and that there might be a feedback loop between IL-6 and BMP signaling (56), our data and the study by Soon and colleagues (59) would suggest that IL-6 signaling may be important in both BMPR2- and non–BMPR2-related PAH.
Our experimental approach used a gene-specific PAH mouse model, which allowed us to more definitively dissect and focus on the functions of BM cells while minimizing some key confounders. For example, we were able to use a myeloablative BM transplantation model, which would minimize any potential effects of recipient BM cells. Furthermore, and importantly, we used BM cells that were not manipulated or expanded ex vivo, which can significantly alter the behavior and innate functioning of BM cells as has been done in several previous studies (8, 10, 12, 15, 62). However, our model also had several relative limitations, which should be carefully considered when relating our data to PAH in general. We used a Bmpr2-specific mouse model that ubiquitously overexpressed a mutant Bmpr2 in all tissues. Overexpression of a mutant Bmpr2 gene may result in endoplasmic reticulum stress and an unfolded protein response, which could confound proper interpretation. In addition, our findings may be specific only for BMPR2-related PAH and not significantly relevant to non-BMPR2 PAH. Studies with other gene-specific models of PAH are thus needed to determine whether the role of BM cells in PAH is generalizable to other types of PAH. However, regardless of these limitations the data presented here still improve our understanding of the role of BM cells in PAH pathogenesis.
Footnotes
Supported by an NHLBI HL102020-01 award (R.H.).
Author Contributions: L.Y. conducted experiments, analyzed data, and wrote the manuscript. X.C. and M.T. conducted experiments and analyzed the data. B.W.N., T.B., S.G., and F.W. conducted experiments. J.C., J.L., and E.A. contributed to the concept and design of the study, interpreted the data, and revised the manuscript for intellectual content. J.W. and R.H. conceived the study; designed the experiments; acquired, analyzed, and interpreted the data; and wrote and edited the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201502-0407OC on December 9, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hayashida K, Fujita J, Miyake Y, Kawada H, Ando K, Ogawa S, Fukuda K. Bone marrow–derived cells contribute to pulmonary vascular remodeling in hypoxia-induced pulmonary hypertension. Chest. 2005;127:1793–1798. doi: 10.1378/chest.127.5.1793. [DOI] [PubMed] [Google Scholar]
- 2.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 3.Angelini DJ, Su Q, Kolosova IA, Fan C, Skinner JT, Yamaji-Kegan K, Collector M, Sharkis SJ, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMα) recruits bone marrow–derived cells to the murine pulmonary vasculature. PLoS One. 2010;5:e11251. doi: 10.1371/journal.pone.0011251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spees JL, Whitney MJ, Sullivan DE, Lasky JA, Laboy M, Ylostalo J, Prockop DJ. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J. 2008;22:1226–1236. doi: 10.1096/fj.07-8076com. [DOI] [PubMed] [Google Scholar]
- 5.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol. 2006;168:659–669. doi: 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montani D, Perros F, Gambaryan N, Girerd B, Dorfmuller P, Price LC, Huertas A, Hammad H, Lambrecht B, Simonneau G, et al. c-kit–positive cells accumulate in remodeled vessels of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2011;184:116–123. doi: 10.1164/rccm.201006-0905OC. [DOI] [PubMed] [Google Scholar]
- 7.Davie NJ, Crossno JT, Jr, Frid MG, Hofmeister SE, Reeves JT, Hyde DM, Carpenter TC, Brunetti JA, McNiece IK, Stenmark KR. Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L668–L678. doi: 10.1152/ajplung.00108.2003. [DOI] [PubMed] [Google Scholar]
- 8.Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, Hyman AL, Kadowitz PJ. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1120–H1128. doi: 10.1152/ajpheart.00173.2006. [DOI] [PubMed] [Google Scholar]
- 9.Umar S, de Visser YP, Steendijk P, Schutte CI, Laghmani H, Wagenaar GT, Bax WH, Mantikou E, Pijnappels DA, Atsma DE, et al. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009;297:H1606–H1616. doi: 10.1152/ajpheart.00590.2009. [DOI] [PubMed] [Google Scholar]
- 10.Patel KM, Crisostomo P, Lahm T, Markel T, Herring C, Wang M, Meldrum KK, Lillemoe KD, Meldrum DR. Mesenchymal stem cells attenuate hypoxic pulmonary vasoconstriction by a paracrine mechanism. J Surg Res. 2007;143:281–285. doi: 10.1016/j.jss.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Liang OD, Mitsialis SA, Chang MS, Vergadi E, Lee C, Aslam M, Fernandez-Gonzalez A, Liu X, Baveja R, Kourembanas S. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells. 2011;29:99–107. doi: 10.1002/stem.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow–derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96:442–450. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi M, Nakamura T, Toba T, Kajiwara N, Kato H, Shimizu Y. Transplantation of endothelial progenitor cells into the lung to alleviate pulmonary hypertension in dogs. Tissue Eng. 2004;10:771–779. doi: 10.1089/1076327041348563. [DOI] [PubMed] [Google Scholar]
- 14.Raoul W, Wagner-Ballon O, Saber G, Hulin A, Marcos E, Giraudier S, Vainchenker W, Adnot S, Eddahibi S, Maitre B. Effects of bone marrow–derived cells on monocrotaline- and hypoxia-induced pulmonary hypertension in mice. Respir Res. 2007;8:8. doi: 10.1186/1465-9921-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yip HK, Chang LT, Sun CK, Sheu JJ, Chiang CH, Youssef AA, Lee FY, Wu CJ, Fu M. Autologous transplantation of bone marrow–derived endothelial progenitor cells attenuates monocrotaline-induced pulmonary arterial hypertension in rats. Crit Care Med. 2008;36:873–880. doi: 10.1097/CCM.0B013E318165B7EA. [DOI] [PubMed] [Google Scholar]
- 16.Luan Y, Zhang ZH, Wei DE, Lu Y, Wang YB. Effects of autologous bone marrow mononuclear cells implantation in canine model of pulmonary hypertension. Circ J. 2012;76:977–985. doi: 10.1253/circj.cj-11-1175. [DOI] [PubMed] [Google Scholar]
- 17.Launay JM, Hervé P, Callebert J, Mallat Z, Collet C, Doly S, Belmer A, Diaz SL, Hatia S, Côté F, et al. Serotonin 5-HT2B receptors are required for bone-marrow contribution to pulmonary arterial hypertension. Blood. 2012;119:1772–1780. doi: 10.1182/blood-2011-06-358374. [DOI] [PubMed] [Google Scholar]
- 18.Amsellem V, Lipskaia L, Abid S, Poupel L, Houssaini A, Quarck R, Marcos E, Mouraret N, Parpaleix A, Bobe R, et al. CCR5 as a treatment target in pulmonary arterial hypertension. Circulation. 2014;130:880–891. doi: 10.1161/CIRCULATIONAHA.114.010757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satoh K, Satoh T, Kikuchi N, Omura J, Kurosawa R, Suzuki K, Sugimura K, Aoki T, Nochioka K, Tatebe S, et al. Basigin mediates pulmonary hypertension by promoting inflammation and vascular smooth muscle cell proliferation. Circ Res. 2014;115:738–750. doi: 10.1161/CIRCRESAHA.115.304563. [DOI] [PubMed] [Google Scholar]
- 20.Zetterberg E, Popat U, Hasselbalch H, Prchal J, Palmblad J. Angiogenesis in pulmonary hypertension with myelofibrosis. Haematologica. 2008;93:945–946. doi: 10.3324/haematol.12426. [DOI] [PubMed] [Google Scholar]
- 21.Popat U, Frost A, Liu E, May R, Bag R, Reddy V, Prchal JT. New onset of myelofibrosis in association with pulmonary arterial hypertension. Ann Intern Med. 2005;143:466–467. doi: 10.7326/0003-4819-143-6-200509200-00017. [DOI] [PubMed] [Google Scholar]
- 22.Popat U, Frost A, Liu E, Guan Y, Durette A, Reddy V, Prchal JT. High levels of circulating CD34 cells, dacrocytes, clonal hematopoiesis, and JAK2 mutation differentiate myelofibrosis with myeloid metaplasia from secondary myelofibrosis associated with pulmonary hypertension. Blood. 2006;107:3486–3488. doi: 10.1182/blood-2005-08-3319. [DOI] [PubMed] [Google Scholar]
- 23.Adir Y, Humbert M. Pulmonary hypertension in patients with chronic myeloproliferative disorders. Eur Respir J. 2010;35:1396–1406. doi: 10.1183/09031936.00175909. [DOI] [PubMed] [Google Scholar]
- 24.Guilpain P, Montani D, Damaj G, Achouh L, Lefrere F, Le Pavec J, Marfaing-Koka A, Dartevelle P, Simonneau G, Humbert M, et al. Pulmonary hypertension associated with myeloproliferative disorders: a retrospective study of ten cases. Respiration. 2008;76:295–302. doi: 10.1159/000112822. [DOI] [PubMed] [Google Scholar]
- 25.Asosingh K, Farha S, Lichtin A, Graham B, George D, Aldred M, Hazen SL, Loyd J, Tuder R, Erzurum SC. Pulmonary vascular disease in mice xenografted with human BM progenitors from patients with pulmonary arterial hypertension. Blood. 2012;120:1218–1227. doi: 10.1182/blood-2012-03-419275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, III, Loyd JE, Nichols WC, Trembath RC International PPH Consortium. Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 27.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 28.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machado RD, Aldred MA, James V, Harrison RE, Patel B, Schwalbe EC, Gruenig E, Janssen B, Koehler R, Seeger W, et al. Mutations of the TGF-β type II receptor BMPR2 in pulmonary arterial hypertension. Hum Mutat. 2006;27:121–132. doi: 10.1002/humu.20285. [DOI] [PubMed] [Google Scholar]
- 30.Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, Ward K, Yacoub M, Mikhail G, Rogers P, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-β family. J Med Genet. 2000;37:741–745. doi: 10.1136/jmg.37.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aldred MA, Vijayakrishnan J, James V, Soubrier F, Gomez-Sanchez MA, Martensson G, Galie N, Manes A, Corris P, Simonneau G, et al. BMPR2 gene rearrangements account for a significant proportion of mutations in familial and idiopathic pulmonary arterial hypertension. Hum Mutat. 2006;27:212–213. doi: 10.1002/humu.9398. [DOI] [PubMed] [Google Scholar]
- 32.West J, Harral J, Lane K, Deng Y, Ickes B, Crona D, Albu S, Stewart D, Fagan K. Mice expressing BMPR2R899X transgene in smooth muscle develop pulmonary vascular lesions. Am J Physiol Lung Cell Mol Physiol. 2008;295:L744–L755. doi: 10.1152/ajplung.90255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West J. Cross talk between Smad, MAPK, and actin in the etiology of pulmonary arterial hypertension. Adv Exp Med Biol. 2010;661:265–278. doi: 10.1007/978-1-60761-500-2_17. [DOI] [PubMed] [Google Scholar]
- 34.Johnson JA, Hemnes AR, Perrien DS, Schuster M, Robinson LJ, Gladson S, Loibner H, Bai S, Blackwell TR, Tada Y, et al. Cytoskeletal defects in Bmpr2-associated pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2012;302:L474–L484. doi: 10.1152/ajplung.00202.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West J, Niswender KD, Johnson JA, Pugh ME, Gleaves L, Fessel JP, Hemnes AR. A potential role for insulin resistance in experimental pulmonary hypertension. Eur Respir J. 2013;41:861–871. doi: 10.1183/09031936.00030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yasuda T, Tada Y, Tanabe N, Tatsumi K, West J. Rho-kinase inhibition alleviates pulmonary hypertension in transgenic mice expressing a dominant-negative type II bone morphogenetic protein receptor gene. Am J Physiol Lung Cell Mol Physiol. 2011;301:L667–L674. doi: 10.1152/ajplung.00423.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tada Y, Majka S, Carr M, Harral J, Crona D, Kuriyama T, West J. Molecular effects of loss of BMPR2 signaling in smooth muscle in a transgenic mouse model of PAH. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1556–L1563. doi: 10.1152/ajplung.00305.2006. [DOI] [PubMed] [Google Scholar]
- 38.Trotman W, Beckett T, Goncz KK, Beatty BG, Weiss DJ. Dual Y chromosome painting and in situ cell-specific immunofluorescence staining in lung tissue: an improved method of identifying donor marrow cells in lung following bone marrow transplantation. Histochem Cell Biol. 2004;121:73–79. doi: 10.1007/s00418-003-0598-0. [DOI] [PubMed] [Google Scholar]
- 39.Harrison DE, Jordan CT, Zhong RK, Astle CM. Primitive hemopoietic stem cells: direct assay of most productive populations by competitive repopulation with simple binomial, correlation and covariance calculations. Exp Hematol. 1993;21:206–219. [PubMed] [Google Scholar]
- 40.Yan L, Womack B, Wotton D, Guo Y, Shyr Y, Davé U, Li C, Hiebert S, Brandt S, Hamid R. Tgif1 regulates quiescence and self-renewal of hematopoietic stem cells. Mol Cell Biol. 2013;33:4824–4833. doi: 10.1128/MCB.01076-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talati M, West J, Zaynagetdinov R, Hong CC, Han W, Blackwell T, Robinson L, Blackwell TS, Lane K. BMP pathway regulation of and by macrophages. PLoS One. 2014;9:e94119. doi: 10.1371/journal.pone.0094119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang HC, Zheng S, VanBuren V, Zhao Z. Discovering disease-specific biomarker genes for cancer diagnosis and prognosis. Technol Cancer Res Treat. 2010;9:219–230. doi: 10.1177/153303461000900301. [DOI] [PubMed] [Google Scholar]
- 43.An N, Kang Y. Using quantitative real-time PCR to determine donor cell engraftment in a competitive murine bone marrow transplantation model. J Vis Exp. 2013;73:e50193. doi: 10.3791/50193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hemnes AR, Brittain EL, Trammell AW, Fessel JP, Austin ED, Penner N, Maynard KB, Gleaves L, Talati M, Absi T, et al. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;189:325–334. doi: 10.1164/rccm.201306-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talati M, West J, Blackwell TR, Loyd JE, Meyrick B. BMPR2 mutation alters the lung macrophage endothelin-1 cascade in a mouse model and patients with heritable pulmonary artery hypertension. Am J Physiol Lung Cell Mol Physiol. 2010;299:L363–L373. doi: 10.1152/ajplung.00295.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, Hoedt-Miller M, Tada Y, Ozimek J, Tuder R, et al. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res. 2004;94:1109–1114. doi: 10.1161/01.RES.0000126047.82846.20. [DOI] [PubMed] [Google Scholar]
- 47.Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, Scheed A, Ritter C, Dahal BK, Vater A, et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:897–908. doi: 10.1164/rccm.201202-0335OC. [DOI] [PubMed] [Google Scholar]
- 48.Burke DL, Frid MG, Kunrath CL, Karoor V, Anwar A, Wagner BD, Strassheim D, Stenmark KR. Sustained hypoxia promotes the development of a pulmonary artery–specific chronic inflammatory microenvironment. Am J Physiol Lung Cell Mol Physiol. 2009;297:L238–L250. doi: 10.1152/ajplung.90591.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall S, Brogan P, Haworth SG, Klein N. Contribution of inflammation to the pathology of idiopathic pulmonary arterial hypertension in children. Thorax. 2009;64:778–783. doi: 10.1136/thx.2008.106435. [DOI] [PubMed] [Google Scholar]
- 50.Pinto RF, Higuchi M de L, Aiello VD. Decreased numbers of T-lymphocytes and predominance of recently recruited macrophages in the walls of peripheral pulmonary arteries from 26 patients with pulmonary hypertension secondary to congenital cardiac shunts. Cardiovasc Pathol. 2004;13:268–275. doi: 10.1016/j.carpath.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Farha S, Asosingh K, Xu W, Sharp J, George D, Comhair S, Park M, Tang WH, Loyd JE, Theil K, et al. Hypoxia-inducible factors in human pulmonary arterial hypertension: a link to the intrinsic myeloid abnormalities. Blood. 2011;117:3485–3493. doi: 10.1182/blood-2010-09-306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cogan J, Austin E, Hedges L, Womack B, West J, Loyd J, Hamid R. Role of BMPR2 alternative splicing in heritable pulmonary arterial hypertension penetrance. Circulation. 2012;126:1907–1916. doi: 10.1161/CIRCULATIONAHA.112.106245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roy M, Richard JF, Dumas A, Vallières L. CXCL1 can be regulated by IL-6 and promotes granulocyte adhesion to brain capillaries during bacterial toxin exposure and encephalomyelitis. J Neuroinflammation. 2012;9:18. doi: 10.1186/1742-2094-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SJ, Smith A, Guo L, Alastalo TP, Li M, Sawada H, Liu X, Chen ZH, Ifedigbo E, Jin Y, et al. Autophagic protein LC3B confers resistance against hypoxia-induced pulmonary hypertension. Am J Respir Crit Care Med. 2011;183:649–658. doi: 10.1164/rccm.201005-0746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yeager ME, Nguyen CM, Belchenko DD, Colvin KL, Takatsuki S, Ivy DD, Stenmark KR. Circulating myeloid-derived suppressor cells are increased and activated in pulmonary hypertension. Chest. 2012;141:944–952. doi: 10.1378/chest.11-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hagen M, Fagan K, Steudel W, Carr M, Lane K, Rodman DM, West J. Interaction of interleukin-6 and the BMP pathway in pulmonary smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1473–L1479. doi: 10.1152/ajplung.00197.2006. [DOI] [PubMed] [Google Scholar]
- 57.Majka S, Hagen M, Blackwell T, Harral J, Johnson JA, Gendron R, Paradis H, Crona D, Loyd JE, Nozik-Grayck E, et al. Physiologic and molecular consequences of endothelial Bmpr2 mutation. Respir Res. 2011;12:84. doi: 10.1186/1465-9921-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li A, Varney ML, Valasek J, Godfrey M, Dave BJ, Singh RK. Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis. 2005;8:63–71. doi: 10.1007/s10456-005-5208-4. [DOI] [PubMed] [Google Scholar]
- 59.Soon E, Crosby A, Southwood M, Yang P, Tajsic T, Toshner M, Appleby S, Shanahan CM, Bloch KD, Pepke-Zaba J, et al. Bone morphogenetic protein receptor type II deficiency and increased inflammatory cytokine production: a gateway to pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:859–872. doi: 10.1164/rccm.201408-1509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Savale L, Tu L, Rideau D, Izziki M, Maitre B, Adnot S, Eddahibi S. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res. 2009;10:6. doi: 10.1186/1465-9921-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104:236–244. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanki-Horimoto S, Horimoto H, Mieno S, Kishida K, Watanabe F, Furuya E, Katsumata T. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006;114(Suppl 1):I181–I185. doi: 10.1161/CIRCULATIONAHA.105.001487. [DOI] [PubMed] [Google Scholar]