Abstract
Rationale: Interstitial lung diseases (ILDs) are associated with oxidative stress. Plasma biomarkers that are directly linked to oxidative stress responses in this disease have not been identified. Stable oxidation products of tyrosine residues in proteins may reflect the oxidative microenvironment in the lung or a systemic inflammatory state.
Objectives: To determine if levels of protein tyrosine oxidation are elevated in plasma of patients with ILD compared with an age- and sex-matched healthy control cohort.
Methods: Three tyrosine oxidation products (3-chlorotyrosine, 3-nitrotyrosine, and o,o′-dityrosine) were quantified by tandem mass spectrometry in cellular models, a mouse model of injury-induced fibrosis, and in plasma of healthy control subjects and patients with ILD (n = 42 in each group).
Measurements and Main Results: Plasma levels of 3-chlorotyrosine, 3-nitrotyrosine, and o,o′-dityrosine were markedly elevated in patients with ILD compared with control subjects with receiver operating characteristic curves separating these groups of 0.872, 0.893, and 0.997, respectively. In a murine model of lung fibrosis, levels of all three oxidative tyrosine modifications were increased in plasma and lung tissue. Cellular models support a critical role for a heme peroxidase and enzymatic sources of reactive oxygen species in the generation of these oxidized products.
Conclusions: We demonstrate an increase in oxidized tyrosine moieties within proteins in the circulating plasma of patients with ILD. These data support the potential for development of oxidative stress–related biomarkers in early diagnosis, prognostication, and/or in evaluating responsiveness to emerging therapies for ILD.
Keywords: oxidative stress, mass spectrometry, dityrosine, nitrotyrosine, chlorotyrosine
At a Glance Commentary
Scientific Knowledge on the Subject
Interstitial lung diseases (ILDs) are associated with oxidative stress. Plasma biomarkers that are directly linked to oxidative stress responses in this disease have not been identified.
What This Study Adds to the Field
Levels of oxidized tyrosine moieties are increased in the plasma of patients with ILD, supporting the potential use of oxidative stress–related biomarkers in early diagnosis, prognostication, and/or in evaluating responsiveness to emerging therapies for ILD.
Interstitial lung disease (ILD) is a chronic, progressive lung disorder that results in loss of functional alveolar-capillary units, leading to impaired gas-exchange and respiratory failure (1, 2). Mortality and morbidity from ILD remain high, and incidence and prevalence of the disease continues to increase (1, 3). Despite significant advances in the understanding of the pathobiology of lung injury-repair that progresses to fibrosis, identification of curative therapies for ILD have remained elusive (4, 5). Identification of potential plasma biomarkers to further understand the pathobiology and progression of ILD can help identify novel drug targets for patients with ILD.
Oxidative stress has been implicated in ILD pathogenesis (6, 7), although precise mechanisms remain unclear. Studies of lung tissue from patients with ILD demonstrate “signatures” of chronic oxidative damage to the alveolar epithelium (8–10). The cellular/enzymatic sources of reactive oxygen species in lungs of patients with fibrotic lung disease have traditionally been attributed to alveolar inflammatory cells (11–14). However, recent studies indicate that myofibroblasts, key effector cells in fibrogenesis, may contribute to oxidative stress in fibrotic disorders. Our previous studies have demonstrated that the profibrotic cytokine, transforming growth factor (TGF)-β1, is a potent inducer of extracellular hydrogen peroxide (H2O2) production in human lung fibroblasts (15–17), including idiopathic pulmonary fibrosis (IPF)-myofibroblasts (18).
Tyrosine residues in proteins are susceptible to covalent oxidative modifications, including the formation of 3-chlorotyrosine, 3-nitrotyrosine, and o,o′-dityrosine (19). Because these modifications are very stable covalent interactions, protein tyrosine modifications in circulating plasma may serve as a potential biomarker of oxidative injury. In this study, we sought to determine if tyrosine modifications could be observed in various in vitro and in vivo models of fibrosis, and in plasma of patients with ILD. Some of the results of these studies have been previously reported in the form of an abstract (20).
Methods
Cell Culture
Human fetal lung fibroblasts (IMR-90) between passages 10 and 12 were cultured to approximately 90% confluence in gelatin coated plates in Dulbecco’s modified Eagle medium (DMEM) supplemented with 5% fetal bovine serum. Following growth arrest, fresh serum-free DMEM supplemented with TGF-β1 (2 ng/ml; R&D Systems, Minneapolis, MN) was added to stimulate differentiation into myofibroblasts and induce H2O2 production. The cells were washed with serum-free DMEM and incubated with 50 mM myeloperoxidase (MPO) and substrate favoring formation of 3-chlorotyrosine (150 mM sodium chloride), 3-nitrotyrosine (500 μM sodium nitrite), or o,o′-dityrosine (l-tyrosine in phosphate buffer pH 7.4).
Human neutrophils and monocytes were isolated by buoyant density centrifugation as described previously (21–25). The human monocytes were purified by adhesion, cultured in 35-mm plastic dishes (2 × 106 cells/dish; BD Biosciences, San Jose, CA), and incubated in 2 mM l-glutamine supplemented RPMI-1640 without phenol red (Life Technology, Carlsbad, CA). Monocytes were cultured for 24 hours (23) in Medium 199 (Life Technology) containing 20% autologous serum or in serum-free medium supplemented with recombinant human granulocyte-macrophage colony–stimulating factor (GM-CSF; 50 μg/ml; R&D Systems) to aid differentiation into macrophages. Phorbol 12-myristate 13-acetate (PMA; 20 nM) was used to activate neutrophils/macrophages (106/ml) incubated at 37°C with serum-free oxidant systems favoring formation of 3-chlorotyrosine (150 mM sodium chloride), 3-nitrotyrosine (500 μM sodium nitrite), or o,o′-dityrosine (100 μM tyrosine in phosphate buffer pH 7.4) supplemented with diethylenetetraminopentaacetic acid (100 mM) and plasma or lung proteins (1 mg protein/ml). Following 60-minute incubation with sodium azide (NaN3; 1 mM), catalase (10 μg/ml), or diphenylene iodonium (DPI; 100 μM; Tocris, Bristol, UK), neutrophils/macrophages were removed by centrifugation and the supernatants containing proteins were analyzed.
Mice
Two-month-old female C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) were anesthetized with intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg), and intratracheal bleomycin (1.25 U/kg) was administered to induce lung injury or saline (50 μl total volume) as previously described (26). Then 3 weeks after treatment mice were killed by CO2 inhalation, and lung tissue and plasma were collected. All procedures involving animals were approved by the Institutional Animal Care and Use Committees at the University of Alabama at Birmingham and the University of Michigan.
Human Subjects
Plasma samples were collected from 42 patients with ILD (32 IPF, 10 connective tissue disease–associated ILD [CTD-ILD]) and from 42 healthy control subjects matched for age (healthy = 54.57 ± 6.25; ILD = 55.9 ± 7.14) and sex (male = 23, female = 19 in each group). Pulmonary function tests were performed on patients with ILD, and are reported as percent predicted using the Morris/Polgar equations. This study was approved by the University of Alabama at Birmingham and the University of Michigan Institutional Review Boards.
Mass Spectrometry Analysis
Samples were analyzed as previously outlined (27). In short, protein was precipitated with ice-cold trichloroacetic acid (10% vol/vol) from plasma samples and diluted in 50 mM phosphate buffer pH 7.4. The protein precipitate was delipidated with water/methanol/water-washed diethyl ether (1:3:7; vol/vol/vol). Known concentrations of isotopically labeled internal standards 13C6 tyrosine, 13C6 3-nitrotyrosine, 13C12 o,o′-dityrosine, or 3-chlorotyroine were added, and samples were hydrolyzed for 24 hours in 4 N methane sulfonic acid treated with benzoic acid. Oxidized amino acids were cleaned using Superclean ENVI ChromP columns (3 ml; Supelco Inc., Bellefonte, PA). Oxidized amino acids were quantified by liquid chromatography (LC)-electrospray ionization tandem mass spectrometry (MS/MS) with multiple reaction monitoring MS/MS positive ion acquisition mode using an Agilent 6410 triple quadrupole MS system equipped with an Agilent 1200 LC system (Agilent Technologies, Santa Clara, CA). Labeled precursor amino acid, 13C915N1tyrosine, was added to monitor potential internal artifact formation of 3-chlorotyrosine, 3-nitrotyrosine, and o,o′-dityrosine and was noted to be negligible.
Data Analysis
Analysis of variance (Student’s t test) was used to compare the natural log-transformed data between groups in the murine and human studies. For variables that still failed the equal variance test, Welch analysis of variance was used. Student’s t test was used to compare groups with the respective complete system in the in vitro studies. P values less than or equal to 0.05 were considered significant. Statistical analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC) and JMP Pro 10.0.2 (SAS institute Inc.). Graphs, receiver operating characteristic (ROC) curves, and area under the curve (AUC) calculations were performed using GraphPad Prism version 6.00 (GraphPad Software, La Jolla, CA). Error bars in figures represent group mean ± SD.
Results
Myeloperoxidase Uses H2O2 Derived from Cultured Human Lung Myofibroblasts to Oxidize Tyrosine Residues
We previously demonstrated that myofibroblasts generate extracellular H2O2 (18), which may induce oxidative protein cross-linking reactions involving l-tyrosine (17). However, in the latter study, we used a more promiscuous plant peroxidase (horseradish peroxidase); the potential for a human heme peroxidase to catalyze oxidation of tyrosine residues in proteins secreted by activated myofibroblasts is not known. We hypothesized that MPO can enzymatically oxidize tyrosine moieties using myofibroblast-derived H2O2 as a substrate to generate 3-chlorotyrosine, 3-nitrotyrosine, or o,o′-dityrosine. We used an established cell culture model of myofibroblast differentiation by stimulating human lung fibroblasts (IMR-90) with TGF-β1 (16, 28). The cells were washed with serum-free DMEM and incubated with 50 mM MPO and oxidant systems favoring formation of 3-chlorotyrosine (150 mM sodium chloride), 3-nitrotyrosine (500 μM sodium nitrite), or o,o′-dityrosine (100 μM tyrosine in phosphate buffer pH 7.4). Oxidized amino acids outlined in Figure 1 were quantified by LC-electrospray ionization-MS/MS in protein hydrolysates as described in the Methods section.
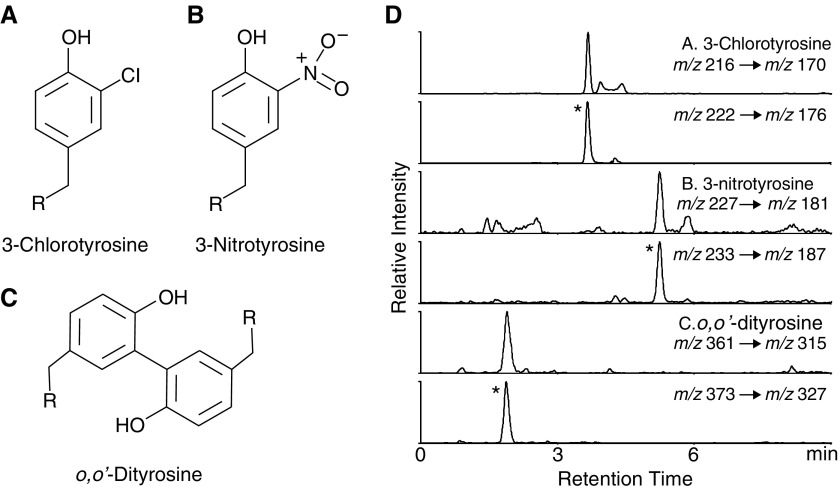
Figure 1.
Chemical structure and extracted ion chromatograms from multiple reaction monitoring mode of the oxidative tyrosine modifications measured. (A–C) The chemical structures of 3-chlorotyrosine, 3-nitrotyrosine, and o,o′-dityrosine. The “R” represents the α-carbon linking the peptide bonds within a protein molecule. (D) Representative chromatogram from plasma of a subject with interstitial lung disease. The protein hydrolysate from plasma samples were separated by reverse phase HPLC and subjected to electrospray ionization/mass spectrometry as described in Methods. Extracted ion chromatograms were derived from the multiple reaction monitoring transitions for 3-chlorotyrosine (A), 3-nitrotyrosine (B), and o,o′-dityrosine (C). The ratio of ion currents for each amino acid compared with the internal standard (asterisk) was used to quantify the levels of oxidized tyrosine; note coelution of authentic oxidized amino acid to the corresponding isotopically labeled internal standard.
Under conditions of endogenous H2O2 production by TGF-β1–differentiated myofibroblasts and MPO, we detected a marked increase in all three oxidized products of tyrosine; the absence of TGF-β1 or MPO resulted in reduction in the formation of these oxidized species (Table 1). The increases in tyrosine oxidation products were also inhibited by the presence of catalase (which competes with MPO to reduce H2O2), NaN3 (a heme peroxidase inhibitor), and DPI (a flavoenzyme inhibitor). These data suggest that, in an ex vivo cell system, the generation of H2O2 by myofibroblasts in the presence of a human heme peroxidase, MPO, can oxidatively modify tyrosine residues to form 3-chlorotyrosine, 3-nitrotyrosine, and o,o′-dityrosine.
Table 1.
Oxidative Tyrosine Modifications in Human Lung Fibroblasts
| Oxidized Tyrosine (Product/Tyrosine [μmol/mol]) |
|||
|---|---|---|---|
| 3-Chlorotyrosine | 3-Nitrotyrosine | o,o′-Dityrosine | |
| Complete system (cells + TGF-β1 + substrate + MPO) | 664 ± 35 | 1,099 ± 122 | 723 ± 100 |
| Complete system | |||
| −TGF-β1 | 16 ± 3.5* | 11 ± 1.2* | 86 ± 8* |
| −MPO | 8 ± 1* | 21 ± 2.1* | 64 ± 7* |
| Complete system | |||
| +Catalase (10 mg/ml) | 23 ± 1.2* | 31 ± 2.1* | 106 ± 18* |
| +NaN3 (1 mM) | 6.1 ± 0.7* | 13 ± 2* | 66 ± 4.5* |
| +DPI (100 μM) | 31 ± 7* | 37 ± 8* | 126 ± 10* |
Definition of abbreviations: DPI = diphenylene iodonium; MPO = myeloperoxidase; TGF = transforming growth factor.
Values are the mean ± SD of triplicate determinations from three independent experiments.
P < 0.05 using Student’s t test comparing values with respective complete system containing TGF-β1, substrate, and MPO.
Ex Vivo Activated Human Neutrophils and Macrophages Generate Oxidized Tyrosine Moieties when Incubated with Human Plasma Proteins and Mouse Lung Tissue Proteins
In addition to activated myofibroblasts, a well-characterized source of H2O2 and MPO are phagocytic cells, including monocytes/macrophages and neutrophils. We sought to determine whether MPO and H2O2 produced by macrophages and neutrophils could oxidize tyrosine residues to generate 3-chlorotyrosine, 3-nitrotyrosine, or o,o′-dityrosine. Human neutrophils and monocytes were isolated from plasma from healthy control subjects by density centrifugation. Monocytes were incubated for 24 hours in medium supplemented with GM-CSF to allow differentiation into macrophages (23). Subsequently, cells were exposed to 1 mg/ml protein (human plasma or lung tissue from control mice) in oxidant systems favoring formation of 3-chlorotyrosine (150 mM sodium chloride), 3-nitrotyrosine (500 μM sodium nitrite), and o,o′-dityrosine (100 μM tyrosine in phosphate buffer pH 7.4). Cells were stimulated with PMA. PMA induces phagocytes to secrete MPO and activates their membrane-bound NADPH oxidase, thus generating superoxide anion, which subsequently dismutates into H2O2, a substrate for MPO. When PMA-stimulated macrophages and neutrophils were introduced to human plasma proteins (1 mg/ml), an increase in the formation of 3-chlorotyrosine, 3-nitrotyrosine, and o,o′-dityrosine was noted (Table 2). Similar results in tyrosine oxidation were obtained when mouse lung protein (1 mg/mL) was incubated instead of plasma (data not shown).
Table 2.
Oxidative Tyrosine Modifications in Human Macrophages and Neutrophils
| Oxidized Tyrosine (Product/Tyrosine [μmol/mol]) |
|||
|---|---|---|---|
| 3-Chlorotyrosine | 3-Nitrotyrosine | o,o′-Dityrosine | |
| Monocytes | |||
| Complete system (cells + PMA + substrate + plasma) | 116 ± 47 | 171 ± 31 | 139 ± 22 |
| Complete system | |||
| −PMA | 12 ± 6* | 31 ± 3* | 15 ± 7* |
| −Cells | 14 ± 7* | 22 ± 3* | 20 ± 10* |
| Complete system | |||
| +Catalase (10 mg/ml) | 36 ± 15* | 21 ± 5* | 26 ± 10* |
| +NaN3 (1 mM) | 18 ± 6* | 16 ± 7* | 17 ± 8* |
| +DPI (100 μM) | 32 ± 9* | 35 ± 14* | 41 ± 15* |
| Neutrophils | |||
| Complete system (cells + PMA + substrate + plasma) | 223 ± 37 | 264 ± 21 | 414 ± 63 |
| Complete system | |||
| −PMA | 14 ± 6* | 16 ± 4* | 22 ± 7* |
| −Cells | 5 ± 1* | 24 ± 5* | 18 ± 3* |
| Complete system | |||
| +Catalase (10 mg/ml) | 10 ± 3* | 31 ± 12* | 16 ± 7* |
| +NaN3 (1 mM) | 3 ± 2* | 14 ± 3* | 12 ± 2* |
| +DPI (100 μM) | 21 ± 6* | 47 ± 12* | 35 ± 9* |
Definition of abbreviations: DPI = diphenylene iodonium; PMA = phorbol 12-myristate 13-acetate.
Values are the mean ± SD of triplicate determinations from three independent experiments.
P < 0.05 using Student’s t test comparing values with respective complete system containing PMA, substrate, and plasma.
Formation of these oxidized moieties required activation of the neutrophils and macrophages with PMA and was inhibited by azide, DPI, and catalase (Table 2), implicating a heme protein and H2O2 in the reaction. Macrophages exposed to GM-CSF for 24 hours produced an approximately threefold higher levels of oxidized tyrosines than untreated macrophages, which is consistent with the known effect of GM-CSF on monocyte-to-macrophage differentiation with increased secretion of MPO. Similar results were obtained with activated human neutrophils (Table 2). These results support the concept that activated phagocytic cells can oxidize tyrosine moieties in mouse lung tissue and human plasma proteins.
Oxidized Tyrosine Moieties Are Elevated in Lung Tissue and Plasma in a Mouse Model of Lung Fibrosis
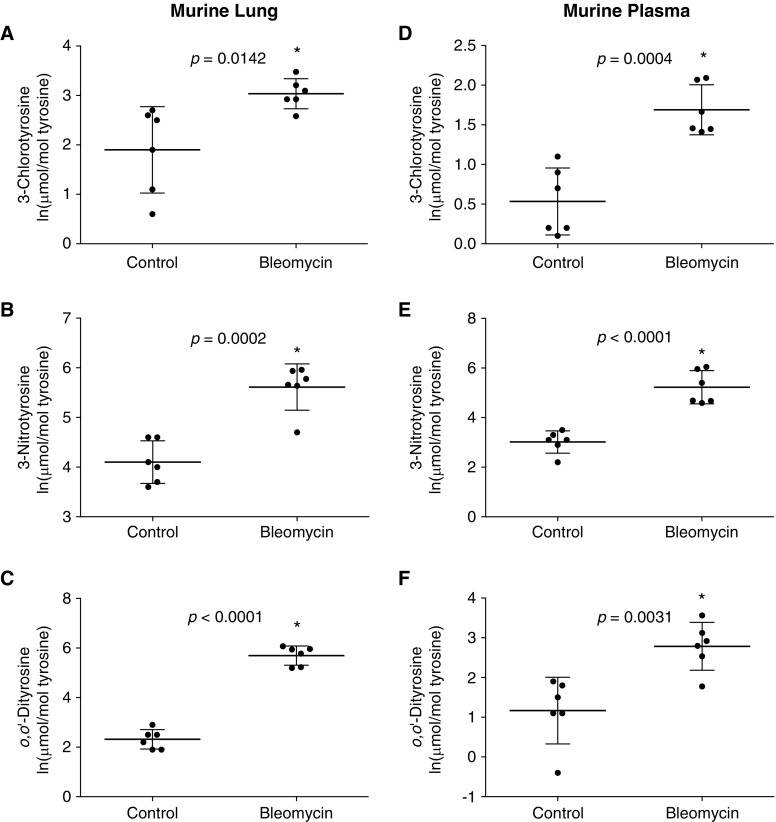
We then determined if tyrosine oxidation seen in vitro also occurs in vivo within the lung tissue of the bleomycin-induced mouse model of lung fibrosis (29, 30). In mouse lung tissue, 3 weeks post-bleomycin injury, an increase in 3-chlorotyrosine, 3-nitrotyrosine, and o,o′-dityrosine was observed when compared with lung tissue from control mice (Figures 2A–2C). The natural log-transformed 3-chlorotyrosine levels were increased in bleomycin-injured lung tissue versus control (uninjured) lung tissue (3.04 ± 0.30 vs. 1.9 ± 0.87, respectively; P = 0.0142), which corresponds to a 3.10-fold increase on a linear scale. The 3-nitrotyrosine levels were increased in bleomycin-injured versus control lung tissue (5.61 ± 0.47 vs. 4.10 ± 0.43, respectively, natural log transformed; P = 0.0002), which corresponds to a 4.52-fold increase on a linear scale. Finally, the o,o′-dityrosine lung tissue levels were increased in bleomycin-injured versus control (5.69 ± 0.39 vs. 2.32 ± 0.39, respectively, natural log transformed; P < 0.0001), which corresponds to a 29.08-fold increase on a linear scale. These data suggest that tyrosine oxidation products are increased within fibrotic lung tissues of bleomycin-injured mice.
Figure 2.
Oxidative tyrosine modifications are increased in lung tissue and plasma from mice subjected to bleomycin-induced lung fibrosis. C57BL/6 mice were subjected to lung injury by intratracheal instillation of bleomycin (1.25 U/kg) or saline control. Lung tissue (A–C) and plasma (D–F) were analyzed by mass spectrometry for 3-chlorotyrosine, 3-nitrotyrosine, and o,o′-dityrosine. Values represent mean ± SD of naturally log-transformed data; n = 6, *P ≤ 0.05 compared with (uninjured) control subjects.
Because oxidized tyrosine moieties could be detected at increased quantities within the bleomycin-induced fibrotic mouse lung, we wanted to further analyze if increased tyrosine oxidation could also be detected in circulating plasma. Increases in 3-chlorotyrosine, 3-nitrotyrosine, and o,o′-dityrosine were observed in mice 3 weeks post-bleomycin injury compared with control mice (Figures 2D–2F). The natural log-transformed 3-chlorotyrosine levels increased in bleomycin versus control plasma (1.69 ± 0.32 vs. 0.53 ± 0.42, respectively; P = 0.0004), which corresponds to a 3.19-fold increase on a linear scale. The 3-nitrotyrosine plasma levels increased in bleomycin versus control (5.23 ± 0.67 vs. 3.02 ± 0.45, respectively, natural log transformed; P < 0.0001); this corresponds to a 9.12-fold increase on a linear scale. The o,o′-dityrosine levels were increased in bleomycin versus control plasma (2.79 ± 0.60 vs. 1.17 ± 0.84, respectively, natural log transformed; P = 0.0031), which corresponds to a 5.05-fold increase on a linear scale. These data suggest that tyrosine oxidation is increased in plasma during the fibrotic phase of bleomycin injury-repair in a mouse model.
Oxidative Tyrosine Modifications of Proteins Are Increased in Plasma of Human Patients with ILD
ILD has been characterized as a disease of oxidative stress (6); however, biomarkers of oxidative stress in this disease have not been identified. To determine if oxidized tyrosine moieties can be detected in the peripheral plasma of human patients with ILD, we measured the oxidized tyrosine levels of 42 ILD samples (32 IPF, 10 CTD-ILD) and 42 healthy control subjects. Groups were well matched for age (healthy = 54.57 ± 6.25; ILD = 55.9 ± 7.14) and sex (male = 23, female = 19 in both groups). The patients with ILD in the cohort had average percent predicted FVC of 58.6 ± 21.9% and diffusing capacity of carbon monoxide (DlCO) of 44.3 ± 17.1% (n = 41 for FVC and n = 39 for DlCO; pulmonary function testing at the time of blood draw was not performed on one patient and DlCO measurements were not available on two patients with ILD); 20 of the 42 patients were on oxygen therapy at the time of blood draw.
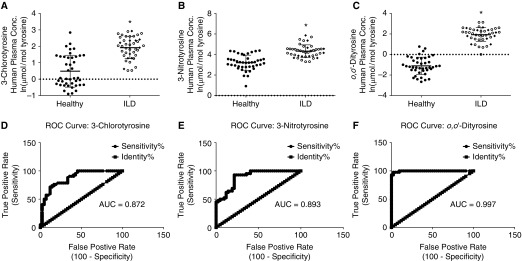
We determined tyrosine oxidation products (3-chlorotyrosine, 3-nitrotyrosine, and o,o′-dityrosine) within proteins in circulating plasma as outlined in Figures 3A–3C. The naturally log-transformed data showed an increase in 3-chlorotyrosine plasma levels in patients with ILD versus healthy control subjects (1.92 ± 0.67 vs. 0.49 ± 0.97, respectively; P < 0.0001); this corresponds to a 4.2-fold increase on a linear scale. An increase in 3-nitrotyrosine levels was observed in patients with ILD versus healthy control subjects (4.33 ± 0.62 vs. 3.20 ± 0.74, respectively, natural log transformed; P < 0.0001); this corresponds to a 3.08-fold increase on a linear scale. The o,o′-dityrosine levels within the plasma showed an almost complete separation between the two groups with an increase in patients with ILD versus healthy control subjects (1.93 ± 0.68 vs. −1.11 ± 0.84, respectively, natural log transformed; P < 0.0001); this corresponds to a 20.76-fold increase on a linear scale. The AUC for the ROC curves for 3-chlorotyrosine, 3-nitrotyrosine, and o,o′-dityrosine were 0.872, 0.893, and 0.997, respectively. These data suggest that tyrosine oxidation can be detected in circulating plasma and levels of these oxidized tyrosine moieties are increased in the plasma of patients with ILD when compared with healthy age- and sex-matched control subjects. Additionally, these data support the potential for oxidized protein tyrosyl residues as biomarkers for fibrotic lung disorders, such as ILD.
Figure 3.
Oxidative tyrosine modifications are increased in plasma from patients with interstitial lung disease (ILD). Age- and sex-matched plasma from ILD (open circles = idiopathic pulmonary fibrosis; solid diamonds = connective tissue disease–associated ILD) and healthy control subjects (solid circles) were analyzed by mass spectrometry for 3-chlorotyrosine (A), 3-nitrotyrosine (B), and o,o′-dityrosine (C). Receiver operating characteristic (ROC) curves for plasma levels of 3-chlorotyrosine (D), 3-nitrotyrosine (E), and o,o′-dityrosine (F); values represent mean ± SD of naturally log-transformed data; n = 42, *P < 0.0001 compared with healthy control subjects. AUC = area under the curve; conc. = concentration.
Discussion
ILD is a disease associated with oxidative stress (6, 7). However, there are currently no studies that have demonstrated increases in plasma or serum biomarkers that are directly attributable to oxidative stress. Most of the candidate biomarkers for ILD are secreted proteins or immune cells (31–35). In this study, we demonstrate the use of plasma measurements of oxidized covalent modifications of protein tyrosine residues as a potential biomarker of ILD. All three oxidative modifications of tyrosine (3-chlorotyrosine, 3-nitrotyrosine, and o,o′-dityrosine) were markedly elevated in plasma of patients with ILD. These results were replicated in an animal model of fibrotic lung injury, both in lung tissue and in circulating plasma. Mechanisms for the formation of these oxidative modifications were interrogated with the incubation of plasma with activated macrophages and neutrophils; evidence for the requirement for specific substrates, H2O2, and a heme peroxidase (likely MPO, in the case of phagocytes) to generate these oxidized tyrosine moieties is provided. An alternative model for tyrosine oxidation was demonstrated by the stimulation of lung fibroblasts by the profibrotic mediator, TGF-β1; as with the phagocytic system, a requirement for both a heme peroxidase (MPO) and flavoenzyme activity was demonstrated, with the relative products being dependent on the availability of specific substrates.
There are several important implications and limitations to the current study. First, the identity and source of the protein-tyrosine moieties that are oxidized are unknown. Our data suggest that circulating plasma proteins may be directly targeted by inflammatory cells in an activated state. Indeed, the presence of activated, reactive oxygen species generating neutrophils have been demonstrated in the bronchoalveolar lavage fluid of subjects with ILD (36). Alternatively, the elevated levels in plasma may reflect the lung-specific oxidative microenvironment and release of oxidized proteins into the circulation. Second, the current study does not provide insights into whether levels of oxidized tyrosine species are indicative of the disease severity, acute exacerbations, or progression of disease. This requires larger numbers of patients with a well-defined phenotype and longitudinal sampling. It would be of interest to determine if these biomarkers are predictive of subjects at-risk for ILD before symptomatology and clinical diagnosis, such as in family members of affected individuals or workers in environments that pose risk for ILD. Third, we do not know if the marked increases in oxidized tyrosine that we detect in plasma occur in other inflammatory and/or fibrotic lung disorders, such as cystic fibrosis or chronic obstructive pulmonary disease. Levels of o,o′-dityrosine and 3-nitrotyrosine have been shown to be elevated in sputum samples from adult patients with cystic fibrosis (37, 38). Increased levels of 3-nitrotyrosine have been observed in exhaled breath condensate of patients with chronic obstructive pulmonary disease, and in bronchoalveolar lavage fluid from patients with acute respiratory distress syndrome (39, 40). The age of subjects in the healthy and ILD cohorts were not significantly different, yet there was an almost complete separation between the two groups with regard to plasma o,o′-dityrosine (ROC AUC = 0.997). This is an important finding because o,o′-dityrosine has been shown to increase with age (41, 42). Finally, the lack of a validation cohort is a limitation of the current study, and future studies need to address the identity of the specific peroxidases and oxidases that mediate tyrosine oxidation in relevant animal models of lung fibrosis.
Future studies will address the prognostic implications of subjects with ILD with high circulating levels of oxidized protein tyrosines. Identification of the specific protein targets of oxidation will likely provide new insights into disease pathogenesis. Specific therapies against oxidative stress responses in ILD are being developed (26), thus defining biomarkers that are predictive of disease progression or responsive to specific therapies will be a significant advance in the field. Two drugs, pirfenidone and nintedanib, were recently approved drugs for IPF, the most prevalent and aggressive form of ILD; interestingly, pirfenidone seems to mediate its beneficial effects, in part, by modulating inflammatory responses and oxidative stress (43–46). This will afford opportunities for the development of precision/personalized approaches to treatment, and more targeted therapeutics for this disease.
Footnotes
Funded by National Institutes of Health grants R01 HL094230, P30 DK89503, U24 DK097153, R01 AG046210, and P01 HL114470.
Author Contributions: S.P. and V.J.T. conceived of the project, designed the studies, interpreted the data, and wrote the manuscript. A.V.-G., J.B., and L.Z. contributed to mass-spectrometry analyses of tyrosine modifications. M.L.L. contributed to data analysis and manuscript writing. T.K. and J.A.d.A. contributed to sample collection and data interpretation. D.Z. contributed to statistical analyses.
Originally Published in Press as DOI: 10.1164/rccm.201505-0992OC on November 17, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 2.Thannickal VJ, Toews GB, White ES, Lynch JP, III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 4.Selman M, Thannickal VJ, Pardo A, Zisman DA, Martinez FJ, Lynch JP., III Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches. Drugs. 2004;64:405–430. doi: 10.2165/00003495-200464040-00005. [DOI] [PubMed] [Google Scholar]
- 5.Thannickal VJ, Flaherty KR, Hyzy RC, Lynch JP., III Emerging drugs for idiopathic pulmonary fibrosis. Expert Opin Emerg Drugs. 2005;10:707–727. doi: 10.1517/14728214.10.4.707. [DOI] [PubMed] [Google Scholar]
- 6.Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med. 2005;172:417–422. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacNee W, Rahman I. Oxidants/antioxidants in idiopathic pulmonary fibrosis. Thorax. 1995;50:S53–S58. doi: 10.1136/thx.50.suppl_1.s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuwano K, Nakashima N, Inoshima I, Hagimoto N, Fujita M, Yoshimi M, Maeyama T, Hamada N, Watanabe K, Hara N. Oxidative stress in lung epithelial cells from patients with idiopathic interstitial pneumonias. Eur Respir J. 2003;21:232–240. doi: 10.1183/09031936.03.00063203. [DOI] [PubMed] [Google Scholar]
- 9.Kuwano K, Kunitake R, Kawasaki M, Nomoto Y, Hagimoto N, Nakanishi Y, Hara N. P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1996;154:477–483. doi: 10.1164/ajrccm.154.2.8756825. [DOI] [PubMed] [Google Scholar]
- 10.Kuwano K, Hagimoto N, Maeyama T, Fujita M, Yoshimi M, Inoshima I, Nakashima N, Hamada N, Watanabe K, Hara N. Mitochondria-mediated apoptosis of lung epithelial cells in idiopathic interstitial pneumonias. Lab Invest. 2002;82:1695–1706. doi: 10.1097/01.lab.0000045084.81853.76. [DOI] [PubMed] [Google Scholar]
- 11.Cantin AM, North SL, Fells GA, Hubbard RC, Crystal RG. Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis. J Clin Invest. 1987;79:1665–1673. doi: 10.1172/JCI113005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crystal RG. Oxidants and respiratory tract epithelial injury: pathogenesis and strategies for therapeutic intervention. Am J Med. 1991;91:39S–44S. doi: 10.1016/0002-9343(91)90282-3. [DOI] [PubMed] [Google Scholar]
- 13.Strausz J, Müller-Quernheim J, Steppling H, Ferlinz R. Oxygen radical production by alveolar inflammatory cells in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1990;141:124–128. doi: 10.1164/ajrccm/141.1.124. [DOI] [PubMed] [Google Scholar]
- 14.Behr J, Maier K, Krombach F, Adelmann-Grill BC. Pathogenetic significance of reactive oxygen species in diffuse fibrosing alveolitis. Am Rev Respir Dis. 1991;144:146–150. doi: 10.1164/ajrccm/144.1.146. [DOI] [PubMed] [Google Scholar]
- 15.Thannickal VJ, Aldweib KD, Fanburg BL. Tyrosine phosphorylation regulates H2O2 production in lung fibroblasts stimulated by transforming growth factor beta1. J Biol Chem. 1998;273:23611–23615. doi: 10.1074/jbc.273.36.23611. [DOI] [PubMed] [Google Scholar]
- 16.Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem. 1995;270:30334–30338. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 17.Larios JM, Budhiraja R, Fanburg BL, Thannickal VJ. Oxidative protein cross-linking reactions involving L-tyrosine in transforming growth factor-beta1-stimulated fibroblasts. J Biol Chem. 2001;276:17437–17441. doi: 10.1074/jbc.M100426200. [DOI] [PubMed] [Google Scholar]
- 18.Waghray M, Cui Z, Horowitz JC, Subramanian IM, Martinez FJ, Toews GB, Thannickal VJ. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J. 2005;19:854–856. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- 19.Giulivi C, Traaseth NJ, Davies KJ. Tyrosine oxidation products: analysis and biological relevance. Amino Acids. 2003;25:227–232. doi: 10.1007/s00726-003-0013-0. [DOI] [PubMed] [Google Scholar]
- 20.Locy ML, Vivekanandan-Giri A, Byun J, Pennathur S, Thannickal VJ. Plasma tyrosine oxidative modifications as a biomarker in human idiopathic pulmonary fibrosis [abstract] Am J Respir Crit Care Med. 2015;191:A2524. [Google Scholar]
- 21.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ståhlman M, Davidsson P, Kanmert I, Rosengren B, Borén J, Fagerberg B, Camejo G. Proteomics and lipids of lipoproteins isolated at low salt concentrations in D2O/sucrose or in KBr. J Lipid Res. 2008;49:481–490. doi: 10.1194/jlr.D700025-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Takeshita J, Byun J, Nhan TQ, Pritchard DK, Pennathur S, Schwartz SM, Chait A, Heinecke JW. Myeloperoxidase generates 5-chlorouracil in human atherosclerotic tissue: a potential pathway for somatic mutagenesis by macrophages. J Biol Chem. 2006;281:3096–3104. doi: 10.1074/jbc.M509236200. [DOI] [PubMed] [Google Scholar]
- 24.Vivekanandan-Giri A, Slocum JL, Byun J, Tang C, Sands RL, Gillespie BW, Heinecke JW, Saran R, Kaplan MJ, Pennathur S. High density lipoprotein is targeted for oxidation by myeloperoxidase in rheumatoid arthritis. Ann Rheum Dis. 2013;72:1725–1731. doi: 10.1136/annrheumdis-2012-202033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith CK, Vivekanandan-Giri A, Tang C, Knight JS, Mathew A, Padilla RL, Gillespie BW, Carmona-Rivera C, Liu X, Subramanian V, et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 2014;66:2532–2544. doi: 10.1002/art.38703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vivekanandan-Giri A, Byun J, Pennathur S. Quantitative analysis of amino acid oxidation markers by tandem mass spectrometry. Methods Enzymol. 2011;491:73–89. doi: 10.1016/B978-0-12-385928-0.00005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 29.Vittal R, Horowitz JC, Moore BB, Zhang H, Martinez FJ, Toews GB, Standiford TJ, Thannickal VJ. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol. 2005;166:367–375. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders YY, Hagood JS, Liu H, Zhang W, Ambalavanan N, Thannickal VJ. Histone deacetylase inhibition promotes fibroblast apoptosis and ameliorates pulmonary fibrosis in mice. Eur Respir J. 2014;43:1448–1458. doi: 10.1183/09031936.00095113. [DOI] [PubMed] [Google Scholar]
- 31.Yokoyama A, Kondo K, Nakajima M, Matsushima T, Takahashi T, Nishimura M, Bando M, Sugiyama Y, Totani Y, Ishizaki T, et al. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology. 2006;11:164–168. doi: 10.1111/j.1440-1843.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 32.Prasse A, Probst C, Bargagli E, Zissel G, Toews GB, Flaherty KR, Olschewski M, Rottoli P, Müller-Quernheim J. Serum CC-chemokine ligand 18 concentration predicts outcome in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:717–723. doi: 10.1164/rccm.200808-1201OC. [DOI] [PubMed] [Google Scholar]
- 33.Kinder BW, Brown KK, McCormack FX, Ix JH, Kervitsky A, Schwarz MI, King TE., Jr Serum surfactant protein-A is a strong predictor of early mortality in idiopathic pulmonary fibrosis. Chest. 2009;135:1557–1563. doi: 10.1378/chest.08-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, Li K, Choi J, Vuga LJ, Lindell KO, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185:67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilani SR, Vuga LJ, Lindell KO, Gibson KF, Xue J, Kaminski N, Valentine VG, Lindsay EK, George MP, Steele C, et al. CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLoS One. 2010;5:e8959. doi: 10.1371/journal.pone.0008959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bitterman PB, Rennard SI, Keogh BA, Wewers MD, Adelberg S, Crystal RG. Familial idiopathic pulmonary fibrosis. Evidence of lung inflammation in unaffected family members. N Engl J Med. 1986;314:1343–1347. doi: 10.1056/NEJM198605223142103. [DOI] [PubMed] [Google Scholar]
- 37.Van Der Vliet A, Nguyen MN, Shigenaga MK, Eiserich JP, Marelich GP, Cross CE. Myeloperoxidase and protein oxidation in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2000;279:L537–L546. doi: 10.1152/ajplung.2000.279.3.L537. [DOI] [PubMed] [Google Scholar]
- 38.Yuan S, Hollinger M, Lachowicz-Scroggins ME, Kerr SC, Dunican EM, Daniel BM, Ghosh S, Erzurum SC, Willard B, Hazen SL, et al. Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Sci Transl Med. 2015;7:276ra27. doi: 10.1126/scitranslmed.3010525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osoata GO, Hanazawa T, Brindicci C, Ito M, Barnes PJ, Kharitonov S, Ito K. Peroxynitrite elevation in exhaled breath condensate of COPD and its inhibition by fudosteine. Chest. 2009;135:1513–1520. doi: 10.1378/chest.08-2105. [DOI] [PubMed] [Google Scholar]
- 40.Sittipunt C, Steinberg KP, Ruzinski JT, Myles C, Zhu S, Goodman RB, Hudson LD, Matalon S, Martin TR. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:503–510. doi: 10.1164/ajrccm.163.2.2004187. [DOI] [PubMed] [Google Scholar]
- 41.Huggins TG, Staton MW, Dyer DG, Detorie NJ, Walla MD, Baynes JW, Thorpe SR. o-Tyrosine and dityrosine concentrations in oxidized proteins and lens proteins with age. Ann N Y Acad Sci. 1992;663:436–437. doi: 10.1111/j.1749-6632.1992.tb38692.x. [DOI] [PubMed] [Google Scholar]
- 42.Feeney MB, Schöneich C. Tyrosine modifications in aging. Antioxid Redox Signal. 2012;17:1571–1579. doi: 10.1089/ars.2012.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirano A, Kanehiro A, Ono K, Ito W, Yoshida A, Okada C, Nakashima H, Tanimoto Y, Kataoka M, Gelfand EW, et al. Pirfenidone modulates airway responsiveness, inflammation, and remodeling after repeated challenge. Am J Respir Cell Mol Biol. 2006;35:366–377. doi: 10.1165/rcmb.2005-0452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu H, Drew P, Cheng Y, Visner GA. Pirfenidone inhibits inflammatory responses and ameliorates allograft injury in a rat lung transplant model. J Thorac Cardiovasc Surg. 2005;130:852–858. doi: 10.1016/j.jtcvs.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Misra HP, Rabideau C. Pirfenidone inhibits NADPH-dependent microsomal lipid peroxidation and scavenges hydroxyl radicals. Mol Cell Biochem. 2000;204:119–126. doi: 10.1023/a:1007023532508. [DOI] [PubMed] [Google Scholar]
- 46.Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther. 2014;349:209–220. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]