Abstract
Rationale: Few studies have examined associations between exposure to air pollution and childhood lung function after implementation of strict air quality regulations in the 1990s.
Objectives: To assess traffic-related pollution exposure and childhood lung function.
Methods: We geocoded addresses for 614 mother–child pairs enrolled during pregnancy in the Boston area 1999–2002 and followed them until a mid-childhood visit (median age, 7.7). We calculated the proximity of the home to the nearest major roadway. We estimated first year of life, lifetime, and prior-year exposure to particulate matter with a diameter smaller than 2.5 μm (PM2.5) by a hybrid model using satellite-derived aerosol optical depth, and to black carbon (BC) by a land-use regression model.
Measurements and Main Results: Residential proximity to roadway and prior-year and lifetime PM2.5 and BC exposure were all associated with lower FVC. Associations with FEV1 were also negative and proportionally similar. Pollution exposures were not associated with the FEV1/FVC ratio or bronchodilator response. Compared with distances greater than or equal to 400 m, living less than 100 m from a major roadway was associated with lower FVC (−98.6 ml; −176.3 to −21.0). Each 2 μg/m3 increment in prior-year PM2.5 was associated with lower FVC (−21.8 ml; −43.9 to 0.2) and higher odds of FEV1 less than 80% predicted (1.41; 1.03–1.93). Each 0.2 μg/m3 increment in prior-year BC was associated with a 38.9 ml (−70.4 to −7.3) lower FVC.
Conclusions: Estimates of long-term exposure to ambient pollution, including proximity to major roadway, PM2.5, and BC (a traffic-related PM2.5 constituent), were associated with lower lung function in this Boston-area cohort of children with relatively low pollution exposures.
Keywords: traffic, outdoor air pollution, spirometry
At a Glance Commentary
Scientific Knowledge on the Subject
Studies have associated long-term exposure to ambient pollution, measured at the community level in the 1990s before dramatic improvements in air quality in the United States and Europe, with reduced lung growth in children. Few studies have examined individual exposure to ambient pollution at levels in compliance with current standards and measures of lung function and reversible airflow obstruction in children.
What This Study Adds to the Field
In this birth cohort study recruited in 1999–2002 in the Boston metropolitan area, we found that long-term exposure to ambient pollution, including proximity to major roadway, fine particulate matter, and black carbon, was associated with lower lung function in a restrictive pattern but not with measures of chronic or reversible airflow obstruction. Our findings suggest that childhood exposure to local traffic and ambient pollution, at relatively low levels within current standards, may reduce lung function and increase risk of clinically relevant lung function deficits in mid-childhood.
Studies dating back to the 1980s have demonstrated that exposure to ambient air pollution is associated with acute respiratory symptoms and reduced lung function in healthy children and those with asthma (1–7). Recent longitudinal studies have found that long-term exposure to ambient pollution is associated with reduced lung growth in children (8–10). These findings are supported by animal models, which have found that chronic exposure to particulate pollution impairs lung development (11, 12).
Air quality has steadily improved over the past several decades across the United States, and evidence is emerging that children’s lung function is improving accordingly. A recent study combining the results of three prospective pediatric cohorts in Southern California found that declining levels in the 1990s and 2000s of particulate matter with a diameter smaller than 2.5 μm (PM2.5), measured at the community-level by central monitors, were associated with improved lung function growth over a 4-year period in children aged 10–15 years (13). PM2.5 levels in the five Southern California communities ranged from approximately 18–28 μg/m3 in 1996 to 15–23 μg/m3 in 2006 (13). Pollution levels in many parts of the United States, including the Boston area, are lower than Southern California. For example, average daily PM2.5 measured by our central monitor in Boston declined from 13.3 μg/m3 in 1996 to 9.0 μg/m3 in 2006, a 32% decline over 10 years. Few studies have examined individual estimates of lifetime exposure to ambient pollutants at these lower pollutant concentrations typical of many U.S. settings in relation to measures of lung function and reversible airflow obstruction in children.
We examined estimates of roadway and ambient air pollution exposures at different time points in childhood and their associations with mid-childhood lung function, including spirometry and bronchodilator response, in a well-characterized birth cohort in the Boston metropolitan area, born after the dramatic improvements in air quality of the 1990s. We hypothesized that long-term exposure to traffic-related air pollution at these lower levels would be associated with reduced lung function and airflow obstruction in children. Some of the results of this study have been previously presented at the American Thoracic Society 2015 International Conference (14).
Methods
Study Population
Study subjects were participants in Project Viva, a prospective prebirth cohort study that recruited women during early pregnancy from Atrius Health, a multispecialty group practice in eastern Massachusetts. Mothers were enrolled between April 1999 and July 2002. Detailed enrollment criteria have been described previously (15). Children were followed from birth. Annual questionnaires were administered to the mothers and the mother–child pairs were invited to a mid-childhood visit (median child age, 7.7 yr) during which spirometry and bronchodilator responsiveness were measured. Of 1,279 mother–child pairs eligible for the mid-childhood visit, a total of 614 mother–child pairs completed questionnaires, provided complete residential address histories, and attended the mid-childhood visit where an acceptable child spirometry measurement was obtained. All mothers provided written informed consent for themselves and their child. This study was approved by the institutional review boards of Brigham and Women’s Hospital and Harvard Pilgrim Health Care.
Exposure Assessment
We geocoded each subject’s address at birth and at study visit using ArcGIS (ESRI, Redlands, CA). Distance from the home address to the nearest major roadway (defined as an A1 or A2 U.S. Census Feature Class road) was calculated. A1 roads are primary, multilane highways with limited access and A2 roads are primary highways without limited access, consisting of U.S. highways and certain state and county highways. Based on published findings that traffic-related pollutants decay to background levels exponentially with distance from a freeway (16, 17), we examined associations with lung function using categories of distance (<100 m, 100–<200 m, 200–<400 m, and ≥400 m) and the natural logarithm of proximity to a major roadway.
Daily estimates of PM2.5 at home address were predicted from a hybrid model using moderate resolution imaging spectroradiometer satellite-derived aerosol optical depth (AOD) measurements at a spatial resolution of 10 × 10 km across the Northeast and then resolved to a specific location within a 50 × 50 m grid using land use terms. AOD is a quantitative measure of particle abundance in the atmospheric column. Spatial smoothing was used to estimate exposure on days when AOD measures were not available (e.g., because of cloud coverage or snow) by taking advantage of associations between grid cell AOD values and PM2.5 data from monitors located elsewhere, and associations with available AOD values in neighboring grid cells. The satellite model data are available starting in the year 2000. Details of the PM2.5 prediction model have been described elsewhere (18). We calculated the average of PM2.5 exposure at home address for the first year of life, the 365 days before the date of lung function testing, and the lifetime average of PM2.5 from birth through the date of testing, taking into account the complete residential history of each child.
We estimated first year of life, previous 365-day, and lifetime black carbon (BC) exposure for each child at the home address using daily predictions from a validated spatiotemporal land-use regression model for traffic particles developed for the Boston metropolitan area (R2 = 0.83) (19, 20). This model has been updated and revised from its original version to include data from 148 monitoring stations recording BC between 1995 and 2011 (21, 22).
Data Collected from Study Participants
We obtained questionnaire and interview data, including maternal and household demographics and tobacco exposure, from the mother at study enrollment during the first prenatal visit and at the mid-childhood visit. Trained research assistants measured child height, weight, and lung function (15, 23). Lung function measurements, including the FEV1, the FVC, and the forced expiratory flow at 25–75% of FVC were obtained using the EasyOne Spirometer (NDD Medical Technologies, Andover, MA). Post-bronchodilator spirometric measures were obtained at least 15 minutes after the administration of two puffs (90 μg per puff) of albuterol. Bronchodilator response was calculated as the percent change in absolute FEV1 after albuterol administration: (Post-bronchodilator FEV1 – Prebronchodilator FEV1)/Prebronchodilator FEV1 × 100. Spirometry measurement met American Thoracic Society criteria for acceptability and reproducibility: For inclusion, each child had to produce at least three acceptable spirograms, two of which must have been reproducible (24). Mothers were instructed to hold any child inhaler medications the day of spirometry testing; only 4 out of 614 reported use of a short-acting bronchodilator within 4 hours of testing.
Statistical Analysis
We analyzed the associations of exposures (proximity to major roadway at birth and at visit date; and estimates of PM2.5 and BC for first year of life, lifetime, and prior 365 d) with lung function by linear regression. We selected potential confounders based on expected associations with air pollution exposure and lung function, including child race/ethnicity, household income, household smoking, season (as sine and cosine functions of the visit date to estimate the phase and amplitude of the seasonal cycle), and date of examination (as a continuous linear variable to account for long-term time trends in exposure). We adjusted for spatially varying measures of socioeconomic status at the home census tract level: median household income and the percent of people with at least a college education. To improve the precision of our estimates, we additionally adjusted for the following predictors of lung function: child age, sex, height, and the temperature and humidity the day before the visit.
In secondary analyses, we examined associations between these same exposures and the odds of a clinically relevant impairment in lung function. We built separate logistic regression models examining the odds of an FEV1, FVC, or FEV1/FVC ratio less than 80% predicted based on age, sex, height, and race using the 2012 global lung function equations (25). We adjusted for the same confounders as in the primary analyses.
We performed several sensitivity analyses on our data. To test for residual bias caused by secular trends, we performed stratified analyses of associations between each exposure and FEV1 and FVC by calendar year of lung function testing, then pooled results by meta-analysis and compared these results with primary models. As a sensitivity analysis to test if changes in pollution exposure after the first year of life were associated with differences in mid-childhood lung function, we calculated the difference in annual exposure to PM2.5 and BC in the first year of life compared with the prior 365-day average, and examined associations between the change in exposure during the period of follow-up, and mid-childhood lung function in models that were also adjusted for average exposure in the first year of life.
Because PM2.5 exposure averages in this cohort were generally lower than the current Environmental Protection Agency National Ambient Air Quality Standard annual standard of 12 μg/m3, we performed sensitivity analyses excluding any participants with an estimated 365-day PM2.5 average greater than 12 μg/m3 in the previous year. We also examined previous 365-day PM2.5 exposure as a binary variable (dichotomized as ≤12 vs. >12 μg/m3) in the full cohort.
We investigated whether associations between exposure to traffic-related pollution and lung function varied according to child sex, household income (≤ vs. >$70,000), child race/ethnicity (white vs. nonwhite), household smoking, and child asthma diagnosis. Asthma diagnosis required maternal report on the mid-childhood questionnaire that her child had ever been diagnosed with asthma by a healthcare professional, and maternal report of child wheezing symptoms or asthma medications in the past 12 months. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Participant characteristics are summarized in Table 1. The children were predominantly of white race (65.5%), and from well-educated families (71% of mothers completed a college degree). Household smoking was rare (12%), and 25.4% of households had an income of less than $70,000 at the time of the visit. Median census tract household income was $65,000, with a fair amount of variability across the cohort. The children were evenly divided by sex and had a median age of 7.7 (range, 6.6–10.9 yr). At the mid-childhood visit, 21.6% of children had an asthma diagnosis.
Table 1.
Participant Characteristics at the Mid-Childhood Visit
| Mean (SD) or n (%) | |
|---|---|
| Child | |
| Race/ethnicity | |
| White | 402 (65.5%) |
| Black | 101 (16.4%) |
| Hispanic | 28 (4.6%) |
| Asian | 15 (2.4%) |
| Other | 68 (11.1%) |
| Female | 328 (53.4%) |
| Age, yr | 7.9 (0.8) |
| Height, cm | 128.2 (7.4) |
| Current asthma | 118 (21.6%) |
| Season of examination | |
| Winter | 141 (23.0%) |
| Spring | 163 (26.5%) |
| Summer | 172 (28.0%) |
| Fall | 138 (22.5%) |
| Mother | |
| College graduate | 434 (70.7%) |
| HH at mid-childhood | |
| Smokers in HH | 71 (11.6%) |
| HH income, $ | 102.7 (43.6) |
| ≤$40,000 | 71 (11.6%) |
| $40,001–$70,000 | 85 (13.8%) |
| >$70,000 | 458 (74.6%) |
| Neighborhood at mid-childhood | |
| Median HH income (census), $ | 64,983 (23,902) |
| ≥College grad (census), % | 42.1 (20.2) |
Definition of abbreviation: HH = household.
Data from 614 mother–child pairs participating in Project Viva.
Neighborhood data are presented at census tract level.
The mean FEV1 was 1.5 L (SD, 0.3) and the mean FVC was 1.8 L (SD, 0.3). The mean percent predicted FEV1 was 95.9% (SD, 13.8) and the mean percent predicted FVC was 103.1% (SD, 13.0).
The median, interquartile range, and range of distance to roadway, PM2.5, and BC measures for different exposure periods are provided in Table 2. For 60% of children, the birth and mid-childhood addresses were different. The median distance from home to a major roadway at birth and mid-childhood was just over 1 km, but there was a wide range of distances with some families living only a few meters from a major roadway and others living several kilometers away. Median lifetime estimates of PM2.5 and BC were slightly higher than the past 365-day estimates and lower than estimates for the first year of life, consistent with the downward trend in particulate pollution exposure for the area. The past 365-day average of PM2.5 estimated by the satellite model of 9.4 μg/m3 was well below the current annual standard of 12 μg/m3. The median 365-day average of BC of 0.46 μg/m3 was substantially lower than the estimated mean annual BC of 0.62 μg/m3 in a study of BC exposure in East Boston that was conducted from 1986 to 1992 (26).
Table 2.
Distributions of Exposure Estimates
| Exposure | n | Median | IQR | Range |
|---|---|---|---|---|
| Distance to major roadway, m | ||||
| At birth | 612 | 1,106 | 1,924 | 3–12,724 |
| At mid-childhood visit | 614 | 1,299 | 2,330 | 8–12,938 |
| PM2.5, μg/m3 | ||||
| First year of life | 580 | 12.09 | 2.63 | 8.26–17.62 |
| Lifetime | 599 | 10.71 | 1.99 | 6.74–16.35 |
| Past 365 d | 611 | 9.40 | 2.57 | 4.08–16.23 |
| BC, μg/m3 | ||||
| First year of life | 586 | 0.68 | 0.25 | 0.20–1.38 |
| Lifetime | 556 | 0.53 | 0.21 | 0.17–1.19 |
| Past 365 d | 571 | 0.46 | 0.20 | 0.13–1.06 |
Definition of abbreviations: BC = black carbon; IQR = interquartile range; PM2.5 = particulate matter with a diameter smaller than 2.5 μm.
Data from 614 children participating in Project Viva.
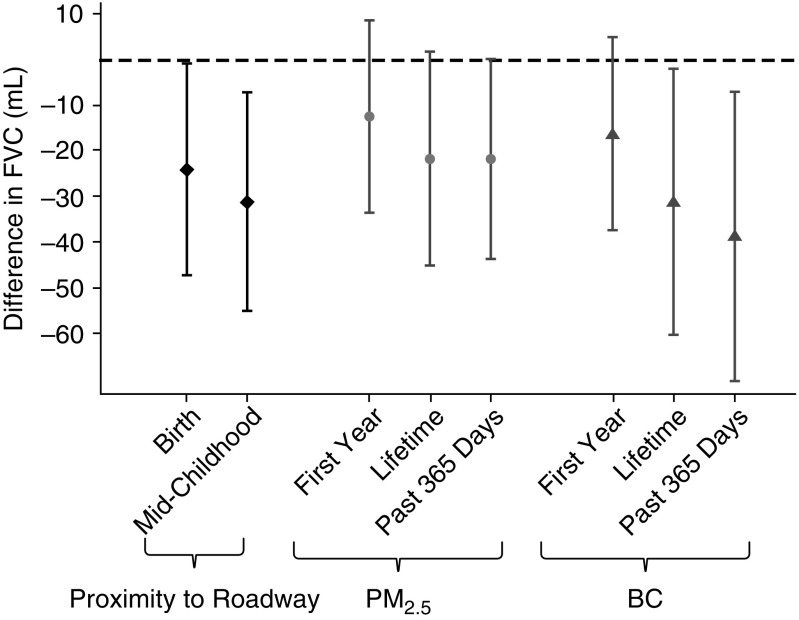
Associations of each of the measures of long-term exposure to pollution with mid-childhood lung function are shown in Figure 1 and Table 3. Residential proximity to nearest major roadway at birth and the time of the mid-childhood visit, and prior 365-day and lifetime estimates of PM2.5 and BC exposure, were all associated with lower FVC. Associations with FEV1 were also negative and proportionally similar, but less precise as reflected by wider confidence intervals. The averages of PM2.5 and BC for the first year of life were weakly associated with lower lung function.
Figure 1.
Associations of pollution exposure estimates and FVC in mid-childhood. Results scaled from the 75th to the 25th percentile of the log-transformed distance to major roadway, per 2 μg/m3 for particulate matter with a diameter smaller than 2.5 μm (PM2.5), and per 0.2 μg/m3 for black carbon (BC). All models adjusted for child age, sex, race/ethnicity, and height; household income and household smoking; census tract median household income and census tract % of population with at least a college degree; and time (as a continuous variable), season (as sine and cosine terms), and the temperature and humidity of the day before the spirometry examination.
Table 3.
Association between Estimates of Pollution Exposure at Different Periods of Life and Measures of Lung Function
| β (95% CI) |
||
|---|---|---|
| Exposure | FEV1 (ml) | FVC (ml) |
| Proximity to major roadway, m, log transformed | ||
| Birth | −12.1 (−34.0 to 9.8) | −24.1 (−47.4 to −0.8) |
| Mid-childhood visit | −19.4 (−41.8 to 3.1) | −31.2 (−55.1 to −7.3) |
| PM2.5, μg/m3 | ||
| First year of life | −7.1 (−26.8 to 12.6) | −12.5 (−33.6 to 8.5) |
| Lifetime | −14.3 (−36.5 to 7.9) | −21.8 (−45.2 to 1.7) |
| Past 365 d | −24.1 (−44.8 to −3.4) | −21.8 (−43.9 to 0.2) |
| BC, μg/m3 | ||
| First year of life | −11.4 (−31.3 to 8.5) | −16.3 (−37.4 to 4.8) |
| Lifetime | −13.8 (−41.7 to 14.0) | −31.2 (−60.5 to −1.8) |
| Past 365 d | −19.9 (−49.8 to 10.0) | −38.9 (−70.4 to −7.3) |
Definition of abbreviations: BC = black carbon; CI = confidence interval; PM2.5 = particulate matter with a diameter smaller than 2.5 μm.
Data from 614 children participating in Project Viva.
Results scaled from the 75th to the 25th percentile of the log-transformed distance to major roadway, per 2 μg/m3 for PM2.5, and per 0.2 μg/m3 for BC. All models adjusted for child age, sex, race/ethnicity, and height; household income and household smoking; census tract median household income and census tract % of population with at least a college degree; and time (as a continuous variable), season (as sine and cosine terms), and the temperature and humidity of the day before the spirometry examination.
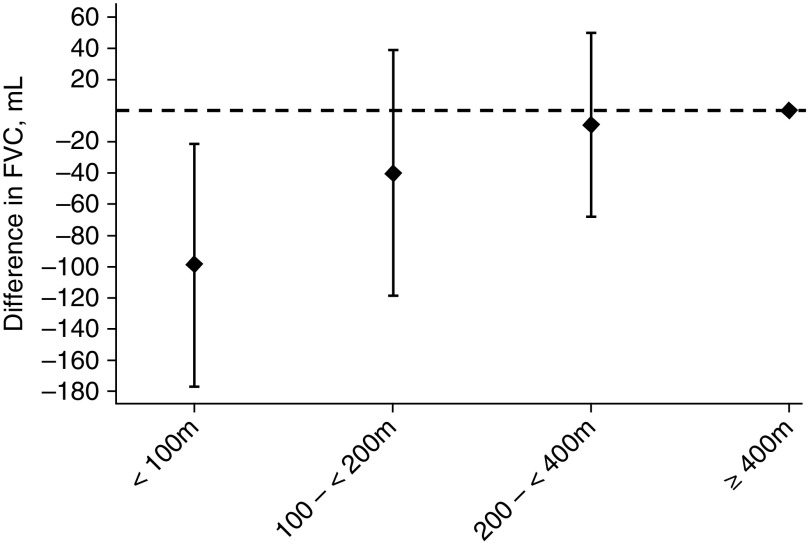
In Figure 2, the difference in FVC in each distance category compared with the reference category of greater than or equal to 400 m is shown. Participants living less than 100 m from a major road had an FEV1 that was 82.9 ml lower (95% confidence interval [CI], −156 to −10.0), and an FVC that was 98.6 ml lower (95% CI, −176.3 to −21.0) than those living greater than or equal to 400 m from a major road. Expressed as a percentage of the mean FEV1 and FVC, children living less than 100 m from a major road had a 5.7% lower FEV1 and a 5.6% lower FVC compared with those living greater than 400 m from a major road. There were no convincing associations between any exposure and measures of airflow obstruction (see Table E1 in the online supplement), nor with the post-bronchodilator change in FVC.
Figure 2.
Distance to major roadway and FVC in mid-childhood. All models adjusted for child age, sex, race/ethnicity, and height; household income and household smoking; census tract median household income and census tract % of population with at least a college degree; and time (as a continuous variable), season (as sine and cosine terms), and the temperature and humidity of the day before the spirometry examination.
There were 78 children (12.7%) with an FEV1 less than 80% predicted. Only 25 (4.1%) had an FVC less than 80% predicted and 40 (6.5%) had an FEV1/FVC less than 80% predicted. A 2 μg/m3 difference in past 365-day PM2.5 was associated with 1.41 (95% CI, 1.03–1.93) times the odds of an FEV1 less than 80% predicted (Table 4). Associations between other exposures and odds of low spirometry measurements were weak, with wide CIs.
Table 4.
Association between Estimates of Pollution Exposure and Odds of Low Lung Function
| OR (95% CI) |
|||
|---|---|---|---|
| Exposure | FEV1 <80% Pred | FVC <80% Pred | FEV1/FVC <80% Pred |
| Proximity to major roadway, m, log transformed | |||
| Birth | 0.95 (0.68–1.33) | 1.26 (0.71–2.23) | 0.93 (0.61–1.42) |
| Mid-childhood visit | 1.07 (0.77–1.50) | 1.22 (0.69–2.15) | 1.16 (0.76–1.78) |
| PM2.5, μg/m3 | |||
| First year of life | 1.11 (0.84–1.46) | 0.82 (0.49–1.36) | 1.00 (0.69–1.45) |
| Lifetime | 1.26 (0.91–1.73) | 1.02 (0.57–1.81) | 1.10 (0.71–1.69) |
| Past 365 d | 1.41 (1.03–1.93) | 1.24 (0.72–2.14) | 1.09 (0.72–1.65) |
| BC, μg/m3 | |||
| First year of life | 1.21 (0.90–1.64) | 1.03 (0.62–1.72) | 0.95 (0.62–1.46) |
| Lifetime | 1.07 (0.71–1.61) | 1.22 (0.62–2.40) | 0.80 (0.44–1.47) |
| Past 365 d | 0.98 (0.63–1.52) | 1.26 (0.62–2.57) | 0.75 (0.39–1.43) |
Definition of abbreviations: BC = black carbon; CI = confidence interval; OR = odds ratio; PM2.5 = particulate matter with a diameter smaller than 2.5 μm; pred = predicted.
Data from 614 children participating in Project Viva.
Results scaled from the 75th to the 25th percentile of the log transformed distance to major roadway, per 2 μg/m3 for PM2.5, and per 0.2 μg/m3 for BC. All models adjusted for child age, sex, race/ethnicity, and height; household income and household smoking; census tract median household income and census tract % of population with at least a college degree; and time (as a continuous variable), season (as sine and cosine terms), and the temperature and humidity of the day before the spirometry examination.
Results of the sensitivity analyses are discussed in the online supplement.
Discussion
In this birth cohort study in the Boston area, we found that living near a major roadway and past-year and lifetime estimates of exposure to PM2.5 and BC were all associated with reduced lung function in mid-childhood. We found that the associations were similar for FEV1 and FVC (i.e., a restrictive pattern). Although we did not assess lung function over time or measure total lung capacity, the similar associations of air pollution exposure with FEV1 and FVC may suggest that long-term pollution exposure predominantly affects lung growth rather than children’s predisposition for chronic or reversible airflow obstruction. Alternatively, traffic-related pollution exposure could cause obstruction and closure of some of the smallest, most peripheral airways in a nonuniform pattern, resulting in a proportional decline in FEV1 and FVC (27–29). The absence of any association between pollution exposure and bronchodilator response is consistent with either mechanism and suggests that the airway smooth muscle is relatively spared.
Exposures occurring closer to the time of the lung function examination had stronger associations with lung function than exposures that included earlier and more distant time intervals. This suggests that exposure to traffic-related pollution in more recent years may influence childhood lung function more strongly than distant early life exposures. Supporting the conclusion that exposures during childhood may have proximate effects on lung function, a study by Gauderman and colleagues (13) of three cohorts in Southern California found that improvements in PM2.5 and PM10 concentrations in five communities during a 4-year period of follow-up in the 1990s to 2000s were associated with improvements in lung function growth from age 11 to 15 years. Children living in communities whose air quality improved during the period of observation had steeper trajectories of lung function growth than those living in communities whose air quality did not improve. Our study compliments the Gauderman study by examining multiple estimates of traffic-related pollution at each child’s home address (rather than community-level measurements), at pollution levels within current Environmental Protection Agency standards, and we found a similar pattern of associations with lung function.
However, studies by Schultz and colleagues (30, 31) of PM10 exposure in the Stockholm area found that PM10 exposure in the first year of life was more strongly associated with lung function at age 8 and adolescence than later exposure averages. These studies examined larger particles of PM10, which includes PM2.5 and coarse particles, such as road dust. The authors concluded that early life particulate air pollution exposure may be a stronger determinant of mid-childhood lung function than later life exposure (30). However, the Schultz studies may not have been as well-poised as our study to compare effects of different exposure windows on lung function. In the Schultz studies, PM10 concentration calculations using a dispersion model were only made for a single year and then applied to the other years of follow-up, such that differences in annual exposure for any child would only result from an address change. Any local or regional changes in PM10 levels over time were not included. By contrast, we estimated daily PM2.5 exposures for each child’s lifetime using a satellite-based model with daily calibration of the estimates to stationary monitors. The precision of our air pollution exposure estimates should be consistent for every exposure window examined.
Our study adds a unique contribution to the emerging and somewhat conflicting literature on long-term pollution at relatively low exposure levels and the presence of airflow obstruction in children. Although some studies have also found proportional associations of air pollution with FEV1 and FVC in a restrictive pattern (8, 13), other studies have found a larger-magnitude association with FEV1 than FVC, suggesting an obstructive effect involving the larger airways (9, 30, 31). Two recent studies that examined long-term PM2.5 and BC exposure in the Boston area using the same models as the present study found restrictive patterns of effects on adult lung function and lung function decline (21, 32). One possible explanation for the restrictive pattern of associations we found is that air pollution may slow lung growth in children (and accelerate lung function decline in adults). Another possible explanation is that exposure to traffic-related particles may contribute to small airway inflammation and closure in both children and adults. Nonuniform small airway closure accompanied by a restrictive pattern on spirometry has been observed in subjects with asthma during bronchoprovocation and in adults exposed to World Trade Center dust (27, 29). This idea could be tested in future studies.
To put our estimates in context, the average growth velocity in FVC among white boys and girls between the ages of 7 and 10 years is 200 ml per year (33). We found that children living within 100 m of a major roadway had an FVC in mid-childhood that was, on average, 99 ml lower than those living more than 400 m from a major road. This difference is equivalent to 6 months of lung growth. We found evidence that every 2 μg/m3 difference in prior-year exposure to PM2.5 was associated with 1.41 times the odds of an FEV1 less than 80% predicted, suggesting that for some children, PM2.5 exposure may result in clinically significant lung function impairment in childhood. Secondary analyses also suggested that children who experienced greater improvements in air quality after the first year of life had better lung function, on average, compared with those whose air quality did not improve as much (see Table E4). Our finding that recent exposures have the strongest associations with lung function, in conjunction with the Gauderman and colleagues (13) finding that reductions in pollution levels over a 4-year period may accelerate lung function growth, suggests that interventions to lower ambient pollution may have immediate benefits.
There are several limitations to our study. This investigation included a population of predominantly white, well-educated women and their children residing in the Boston area. Generalizability to other groups may be limited. We measured lung function only once, and were therefore unable to examine the effects of air pollution exposure on the growth of lung function over time. We used distance to roadway as a correlate of long-term traffic-related ambient air pollution exposure, which may not reflect actual pollution exposures and could be a measure of other exposures, such as noise or social stress that was not captured by the socioeconomic measures of household income, race/ethnicity, or household smoking, and census tract income and education. Similarly, it is conceivable that other spatially varying exposures may be correlated with PM2.5 or BC exposure and affect the relationships we identified with lung function. However, given the consistent associations we identified in models that were adjusted for potential confounders and used very different approaches for measuring pollution exposure, we deem this to be unlikely.
Our study included very few, if any, families living in poverty. All study participants were enrolled in a health insurance plan at the time of enrollment. It is therefore difficult to draw any major conclusions from our finding that associations of proximity to roadway and PM2.5 with lung function were strongest among children with a household income above $70,000. It is possible that children from lower income families had other exposures, not captured by adjustment for measures of socioeconomic status, that overwhelmed the long-term effects of air pollution on lung function. The respective contributions from nonwhite racial/ethnic groups were relatively small and therefore it is difficult to interpret the meaning of the differential associations with proximity to roadway in these very small subgroups. Our study includes a large number of comparisons. For this reason, we focus our conclusions on the overall patterns and consistency of associations, and not on tests of statistical significance. Because this was an observational study, we cannot infer causality from any of the associations observed.
This study also has several strengths. Although most studies have assessed air pollution exposure during mid-childhood using central site monitors, we were able to examine each child’s lifetime average exposure at home address using validated spatially and temporally resolved models of PM2.5 and BC exposure, and to compare associations of early childhood, lifetime, and later-childhood exposure averages with lung function. This allowed us to consider the relative contribution of different exposure windows during lung development on lung function by mid-childhood. Our study protocol included prebronchodilator and post-bronchodilator spirometry measurement, allowing us to examine associations between long-term air pollution exposure and both chronic and reversible airflow obstruction. In addition, our results were adjusted for a robust list of potential individual, family, and community-level confounders and predictors of lung function.
In conclusion, we found that proximity to a major roadway, and prior-year and lifetime exposure to PM2.5 and BC, were associated with reduced lung function but not airflow obstruction in mid-childhood. We found that more recent exposures had stronger associations with lung function than early life exposures, and that associations with FEV1 and FVC were proportionally similar in magnitude. Our findings are consistent with a growing body of evidence that chronic fine particulate exposure, even at low levels within current standards, impairs lung function in childhood and increases risk of clinically significant decrements in lung function. These findings have potential policy implications regarding the need for continued pollution control and urban planning to protect the respiratory health of children.
Acknowledgments
Acknowledgment
The authors thank the mothers and children of Project Viva. In addition, they thank Drs. Robert Brown (Massachusetts General Hospital) and Kenneth Berger (New York University) for their guidance in respiratory physiology.
Footnotes
Support by the National Institutes of Health (NIEHS F32ES023352, P30ES000002, P01-ES009825; NICHD K24 HD069408, R37 HD034568; NIAID R01AI102960) and the U.S. Environmental Protection Agency (EPA; R832416, RD834798). This publication’s contents are solely the responsibility of the grantee and do not necessarily represent the official views of the EPA. Furthermore, EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Author Contributions: M.B.R. developed the data analysis plan under the supervision of D.R.G. and M.A.M. and wrote the first version of the manuscript. S.L.R.-S. conducted all data analyses. A.A.L., E.O., and M.W.G. supervised the collection and quality control of data in the Viva cohort study and reviewed the analysis plan. E.O., M.W.G., and D.R.G. obtained funding. I.K. and J.S. developed the PM2.5 model, and J.S. and B.A.C. developed the black carbon model. H.L.-G., A.Z., B.A.C., J.S., D.R.G., and M.A.M. advised on the data analysis. D.R.G., M.A.M., J.S., and P.K. planned the overall study design as part of the Harvard Clean Air Research Center. All authors contributed to the interpretation of the data, revised the manuscript, and approved the final manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201506-1058OC on November 17, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.O’Connor GT, Neas L, Vaughn B, Kattan M, Mitchell H, Crain EF, Evans R, III, Gruchalla R, Morgan W, Stout J, et al. Acute respiratory health effects of air pollution on children with asthma in US inner cities. J Allergy Clin Immunol. 2008;121:1133–1139.e1. doi: 10.1016/j.jaci.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Neas LM, Dockery DW, Koutrakis P, Tollerud DJ, Speizer FE. The association of ambient air pollution with twice daily peak expiratory flow rate measurements in children. Am J Epidemiol. 1995;141:111–122. doi: 10.1093/oxfordjournals.aje.a117399. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz J, Dockery DW, Neas LM, Wypij D, Ware JH, Spengler JD, Koutrakis P, Speizer FE, Ferris BG., Jr Acute effects of summer air pollution on respiratory symptom reporting in children. Am J Respir Crit Care Med. 1994;150:1234–1242. doi: 10.1164/ajrccm.150.5.7952546. [DOI] [PubMed] [Google Scholar]
- 4.Hoek G, Dockery DW, Pope A, Neas L, Roemer W, Brunekreef B. Association between PM10 and decrements in peak expiratory flow rates in children: reanalysis of data from five panel studies. Eur Respir J. 1998;11:1307–1311. doi: 10.1183/09031936.98.11061307. [DOI] [PubMed] [Google Scholar]
- 5.Thurston GD, Lippmann M, Scott MB, Fine JM. Summertime haze air pollution and children with asthma. Am J Respir Crit Care Med. 1997;155:654–660. doi: 10.1164/ajrccm.155.2.9032209. [DOI] [PubMed] [Google Scholar]
- 6.Romieu I, Meneses F, Ruiz S, Sienra JJ, Huerta J, White MC, Etzel RA. Effects of air pollution on the respiratory health of asthmatic children living in Mexico City. Am J Respir Crit Care Med. 1996;154:300–307. doi: 10.1164/ajrccm.154.2.8756798. [DOI] [PubMed] [Google Scholar]
- 7.Peters JM, Avol E, Gauderman WJ, Linn WS, Navidi W, London SJ, Margolis H, Rappaport E, Vora H, Gong H, Jr, et al. A study of twelve Southern California communities with differing levels and types of air pollution. II. Effects on pulmonary function. Am J Respir Crit Care Med. 1999;159:768–775. doi: 10.1164/ajrccm.159.3.9804144. [DOI] [PubMed] [Google Scholar]
- 8.Rojas-Martinez R, Perez-Padilla R, Olaiz-Fernandez G, Mendoza-Alvarado L, Moreno-Macias H, Fortoul T, McDonnell W, Loomis D, Romieu I. Lung function growth in children with long-term exposure to air pollutants in Mexico City. Am J Respir Crit Care Med. 2007;176:377–384. doi: 10.1164/rccm.200510-1678OC. [DOI] [PubMed] [Google Scholar]
- 9.Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, McConnell R, Kuenzli N, Lurmann F, Rappaport E, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351:1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 10.Gauderman WJ, McConnell R, Gilliland F, London S, Thomas D, Avol E, Vora H, Berhane K, Rappaport EB, Lurmann F, et al. Association between air pollution and lung function growth in southern California children. Am J Respir Crit Care Med. 2000;162:1383–1390. doi: 10.1164/ajrccm.162.4.9909096. [DOI] [PubMed] [Google Scholar]
- 11.Mauad T, Rivero DHRF, de Oliveira RC, Lichtenfels AJ de FC, Guimarães ET, de Andre PA, Kasahara DI, Bueno HM de S, Saldiva PHN. Chronic exposure to ambient levels of urban particles affects mouse lung development. Am J Respir Crit Care Med. 2008;178:721–728. doi: 10.1164/rccm.200803-436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camarinho R, Garcia PV, Rodrigues AS. Chronic exposure to volcanogenic air pollution as cause of lung injury. Environ Pollut. 2013;181:24–30. doi: 10.1016/j.envpol.2013.05.052. [DOI] [PubMed] [Google Scholar]
- 13.Gauderman WJ, Urman R, Avol E, Berhane K, McConnell R, Rappaport E, Chang R, Lurmann F, Gilliland F. Association of improved air quality with lung development in children. N Engl J Med. 2015;372:905–913. doi: 10.1056/NEJMoa1414123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice MB, Rifas-Shiman SL, Litonjua AA, Oken E, Gillman MW, Kloog I, Gibson H, Zanobetti A, Coull B, Schwartz J, Koutrakis P, et al. Long-term exposure to traffic-related air pollution and lung function in children [abstract] Am J Respir Crit Care Med. 2015:191–A3201. doi: 10.1164/rccm.201506-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, Rich-Edwards JW, Rifas-Shiman SL, Sagiv S, Taveras EM, et al. Cohort profile: Project Viva. Int J Epidemiol. 2015;44:37–48. doi: 10.1093/ije/dyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc. 2002;52:1032–1042. doi: 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Levy JI. Factors influencing the spatial extent of mobile source air pollution impacts: a meta-analysis. BMC Public Health. 2007;7:89. doi: 10.1186/1471-2458-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kloog I, Melly SJ, Ridgway WL, Coull BA, Schwartz J. Using new satellite based exposure methods to study the association between pregnancy PM₂.₅ exposure, premature birth and birth weight in Massachusetts. Environ Health. 2012;11:40. doi: 10.1186/1476-069X-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gryparis A, Coull B, Schwartz J, Suh H. Semi-parametric latent variable regression models for spatio-temporal modeling of mobile source particles in the greater Boston area. J R Stat Soc Ser C Appl Stat. 2007;56:183–209. [Google Scholar]
- 20.Zanobetti A, Coull BA, Gryparis A, Kloog I, Sparrow D, Vokonas PS, Wright RO, Gold DR, Schwartz J. Associations between arrhythmia episodes and temporally and spatially resolved black carbon and particulate matter in elderly patients. Occup Environ Med. 2014;71:201–207. doi: 10.1136/oemed-2013-101526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lepeule J, Litonjua AA, Coull B, Koutrakis P, Sparrow D, Vokonas PS, Schwartz J. Long-term effects of traffic particles on lung function decline in the elderly. Am J Respir Crit Care Med. 2014;190:542–548. doi: 10.1164/rccm.201402-0350OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleisch AF, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, Melly S, Coull BA, Zanobetti A, Gillman MW, Gold DR, et al. Prenatal exposure to traffic pollution: associations with reduced fetal growth and rapid infant weight gain. Epidemiology. 2015;26:43–50. doi: 10.1097/EDE.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tse SM, Gold DR, Sordillo JE, Hoffman EB, Gillman MW, Rifas-Shiman SL, Fuhlbrigge AL, Tantisira KG, Weiss ST, Litonjua AA. Diagnostic accuracy of the bronchodilator response in children. J Allergy Clin Immunol. 2013;132:554–559.e5. doi: 10.1016/j.jaci.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 25.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MSM, Zheng J, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franco Suglia S, Gryparis A, Schwartz J, Wright RJ. Association between traffic-related black carbon exposure and lung function among urban women. Environ Health Perspect. 2008;116:1333–1337. doi: 10.1289/ehp.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olive JT, Jr, Hyatt RE. Maximal expiratory flow and total respiratory resistance during induced bronchoconstriction in asthmatic subjects. Am Rev Respir Dis. 1972;106:366–376. doi: 10.1164/arrd.1972.106.3.366. [DOI] [PubMed] [Google Scholar]
- 28.Pride NB, Permutt S, Riley RL, Bromberger-Barnea B. Determinants of maximal expiratory flow from the lungs. J Appl Physiol. 1967;23:646–662. doi: 10.1152/jappl.1967.23.5.646. [DOI] [PubMed] [Google Scholar]
- 29.Berger KI, Reibman J, Oppenheimer BW, Vlahos I, Harrison D, Goldring RM. Lessons from the World Trade Center disaster: airway disease presenting as restrictive dysfunction. Chest. 2013;144:249–257. doi: 10.1378/chest.12-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultz ES, Gruzieva O, Bellander T, Bottai M, Hallberg J, Kull I, Svartengren M, Melén E, Pershagen G. Traffic-related air pollution and lung function in children at 8 years of age: a birth cohort study. Am J Respir Crit Care Med. 2012;186:1286–1291. doi: 10.1164/rccm.201206-1045OC. [DOI] [PubMed] [Google Scholar]
- 31.Schultz ES, Hallberg J, Bellander T, Bergström A, Bottai M, Chiesa F, Gustafsson PM, Gruzieva O, Thunqvist P, Pershagen G, et al. Early life exposure to traffic-related air pollution and lung function in adolescence. Am J Respir Crit Care Med. 2016;193:171–177. doi: 10.1164/rccm.201505-0928OC. [DOI] [PubMed] [Google Scholar]
- 32.Rice MB, Ljungman PL, Wilker EH, Dorans KS, Gold DR, Schwartz J, Koutrakis P, Washko GR, O’Connor GT, Mittleman MA. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham Heart Study. Am J Respir Crit Care Med. 2015;191:656–664. doi: 10.1164/rccm.201410-1875OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Dockery DW, Wypij D, Gold DR, Speizer FE, Ware JH, Ferris BG., Jr Pulmonary function growth velocity in children 6 to 18 years of age. Am Rev Respir Dis. 1993;148:1502–1508. doi: 10.1164/ajrccm/148.6_Pt_1.1502. [DOI] [PubMed] [Google Scholar]