Abstract
Muscle-based biohybrid actuators have generated significant interest as the future of biorobotics but so far they move without having much control over their actuation behavior. Integration of microelectrodes into the backbone of these systems may enable guidance during their motion and allow precise control over these actuators with specific activation patterns. Here, we addressed this challenge by developing aligned CNT forest microelectrode arrays and incorporated them into scaffolds for stimulating the cells. Aligned CNTs were successfully embedded into flexible and biocompatible hydrogel exhibiting excellent anisotropic electrical conductivity. Bioactuators were then engineered by culturing cardiomyocytes on the CNT microelectrode-integrated hydrogel constructs. The resulting cardiac tissue showed homogeneous cell organization with improved cell-to-cell coupling and maturation, which was directly related to the contractile force of muscle tissue. This centimeter-scale bioactuator has excellent mechanical integrity, embedded microelectrodes and is capable of spontaneous actuation behavior. Furthermore, we demonstrated that a biohybrid machine can be controlled by an external electrical field provided by the integrated CNT microelectrode arrays. In addition, due to the anisotropic electrical conductivity of the electrodes provided from aligned CNTs, significantly different excitation thresholds were observed in different configurations such as the ones in parallel vs. perpendicular direction to the CNT alignment.
Keywords: Carbon Nanotubes, Microelectrode arrays, Hybrid hydrogels, Cardiac tissue engineering, Bioactuators
Graphical Abstract

1. Introduction
Nature can be an inspiration to develop innovative methods aimed at solving medical, environmental and engineering challenges[1]. In particular, using muscle tissue as an inspiration, tissue-engineering approaches can be used to build biological machines[2]. Furthermore, since they are built from cells, these biological machines are capable of dynamically sensing and adapting to environmental cues including responding to externally applied stimuli. These devices and actuators could be used to handle numerous challenges specifically for in vitro biological applications, including drug screening and bio-robotics[3]. Fabricating contractile muscular tissues (vasculature, skeletal and cardiac muscles)[4] and integrated them with adaptable soft materials can allow for deformation, locomotion, and control with greater degrees of freedom. These systems can be combined in a simple, low-power, and cost-effective platform when compared to systems made using conventional robotics composed of metallic and electroactive polymeric structures[5]. Recent advances in engineering cardiac tissues in vitro has led to devices with strong spontaneous contractile behavior allowing more precisely controlled actuation from external signal sources (i.e. electrical or optical stimulation)[6, 7]. These advantageous properties of cardiac muscle have inspired the development of biological machines that include cantilevers[8], muscular thin film based bio-hybrid actuators[9], crab-like robots[10], jellyfish[11], a self-propelled swimming robots[12], and walking “biological biomorph” cantilevers at the millimeter-scale [13]. However, despite significant technological advances, biological machines capable of long-term operation with reproducible and precise control over their direction within a biological environment remain a challenge.
To address this and similar challenges, self-propelling biological machines can be integrated with cell stimulating mini- or microelectrodes that provide users with precise control over their actuation behavior [7, 9]. Integration of electrodes onto biological machines for the purpose of external stimulation is challenging. Any direct contact between the electrodes and the biological samples, in general, may cause (a) hydrolysis of the culture medium when an applied electric potential (> ± 1V) exceeds the water window and hence causes bubble formation and a localized pH gradient; (b) joule heating of the medium; (c) contamination of the culture medium due to products of electrode corrosion; (d) surface fouling of electrodes, as a result of electrochemical reactions on the electrode surface that can further lead to damage in bioinspired constructs[14]. To circumvent some of these problems, microelectrodes or microelectrode arrays that can provide stimulation with low electrical potentials should be used. Furthermore, microelectrodes can be fabricated on flexible substrates with precisely defined positions and could therefore provide a highly reproducible and well-quantified electric field over the whole tissue constructs [15, 16].
Electrical and chemical stability is a critical requirement while developing microelectrodes for tissue stimulation. Cannizzaro and Tandon et al have carefully studied different electrode materials, their configuration, magnitude of input voltage and electrode aging (especially in cardiac tissue engineering), and noted that carbon can be used as an ideal electrode material[17, 18]. Carbon has several benefits that include high capacitance, fast charge transfer, and high resistance to corrosion compared to titanium, titanium nitride, and stainless steel[19]. However, a pair of carbon rod electrodes is difficult to integrate onto flexible microporous substrates due to their large size and rigid behavior. As an alternative to carbon, electrodes based on carbon nanotubes (CNTs) are promising candidates and are amenable for easy integration into biological machines. Previously, CNTs have been shown to possess favorable biocompatibility, fibrous morphology, flexibility, high electrical and mechanical properties, strong tissue adhesion and possess optimal long-term stimulation ability in contrast to conventional metallic electrodes[20]. More recently, aligned CNTs were used to fabricate wearable and stretchable devices with high durability, fast response and low creep[21, 22]. Similarly, vertically aligned CNT microelectrode arrays are emerging materials with numerous attractive properties for stimulation of biohybrid actuators. For example, they can be used to generate electric fields using low potentials to induce cell polarization. Despite their advantages, few efforts have been made to integrate them into substrates for creating flexible biological machines.
In our previous work, we have observed that the use of CNT-GelMA conductive gels enhanced the beating behavior of cardiomyocytes [23, 24]. The cells began to beat in a shorter timeframe, maintained their beating activity for a longer time and had more stable beating patterns. The pacing of the cells was done via external carbon rod or Pt wire electrodes placed far away from the cells inside a culture dish. In the current study, we have created a smart substrate with integrated pacing electrodes. To make the electrodes, rather than using dispersed CNTs inside the gels, which cannot be used for stimulation, we used an alternate strategy. The advantage of this system is twofold, first, it allows pacing in close contact to the cells which allowed the use of lower pacing voltages. In addition, since the signals can be applied to specific regions of the biohybrid actuator, the platform enables localized stimulation and hence guidance which will be shown at another study. Generation of embedded, mechanically flexible, and patternable microelectrodes within a tissue construct is an important first step towards future implementation of robotic systems with more complex actuation behaviours. The vertically aligned CNT microelectrode forest arrays were explored for this purpose. The ability to integrate them into cell friendly hydrogel substrates enables novel applications in remotely controlled biohybrid actuators in the future.
Here, we introduce an innovative approach to fabricate and integrate vertically aligned CNT microelectrode arrays into flexible cardiac muscle tissue constructs. After growing vertically aligned CNT forest microelectrode arrays on silicon substrates, they were transferred to hydrogel substrates. Next, a hybrid conductive hydrogel substrate was created by dispersing individual CNTs inside a thin layer of gelatin methacrylamide (GelMA). Then, this layer was bonded onto the hydrogel sheet containing CNT forest microelectrodes. The CNT forest-based microelectrode arrays were sandwiched between two thin hydrogel layers to prevent undesirable CNT delamination and to improve the mechanical stability of the construct. The CNT-GelMA hybrid hydrogel surface was used as the cell culture substrate to induce maturation of cardiac muscle tissues[23, 24]. The resulting 3D bio-hybrid constructs showed excellent mechanical integrity with embedded microelectrode arrays and advanced electrophysiological functions with strong muscle contraction. Therefore, we successfully created a 3D bio-hybrid actuator with controllable actuation and movement under an electrical field generated by integrated electrodes.
2. Results and Discussion
Design of 3D bioelectronics system
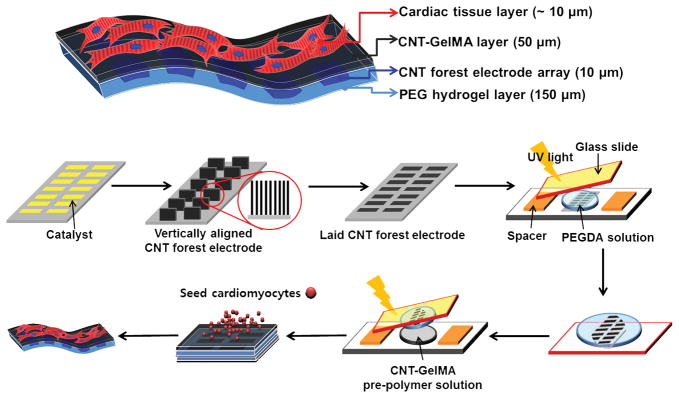
The multi-layer engineered cardiac tissue constructs incorporating vertically aligned CNT forest microelectrode arrays was fabricated according to the process described in Fig. 1. First, a vertically aligned CNT forest microelectrode array was fabricated on a patterned Si wafer by a chemical vapor deposition (CVD) process. The development of biomimetic substrates that are amenable to electrode integration and emulate the properties of the muscle tissue is of great importance for successfully creating muscle-based biological machines. 3D hydrogels are excellent candidates to create biomimetic substrates due to their ability to control physical and chemical properties with their high permeability for the transport of nutrients and metabolites. Before being transferred to hydrogel substrates, vertically aligned CNT forests on Si wafers were first dipped into DI water briefly, pulled out, and air dried. After this process, the vertical CNT forests were flattened onto Si substrates due to elastocapillary induced densification.[25] The transfer of CNT forests onto hydrogels was performed by dispensing a solution of PEG pre-polymer onto the Si wafer, and curing the pre-polymer with UV light, which formed a non-degradable and biocompatible hydrogel. By peeling the PEG layer from the Si substrate, the transfer process was completed and a PEG hydrogel layer with embedded CNT electrode array was successfully obtained with high reproducibility. To provide a biochemical environment suitable for cardiac tissue culture, we dispensed and UV crosslinked the 50 μm thick CNT-GelMA hydrogel layer over the PEG/CNT microelectrode array composite layer. At the end of this process, we created a two layer hydrogel structure where the 50 μm thick CNT-GelMA hydrogel layer was residing on top of the 150 μm thick CNT forest based microelectrode array. Finally, neonatal rat cardiomyocytes were cultured on the CNT-GelMA side of the multilayer structure to yield a 3D hybrid bioactuating construct. The advantage of this stepwise, bottom-up approach is that it allowed for minimally invasive integration of electronic devices with cell and ECM components at the cellular level in 2D and 3D.
Figure 1.
A schematic illustrating the fabrication steps to produce 3D bio-hybrid actuators composed of cardiac tissue on top of a multilayer hydrogel sheet impregnated with aligned CNT microelectrodes.
Vertically-aligned CNT forest microelectrode arrays with anisotropic electrical property
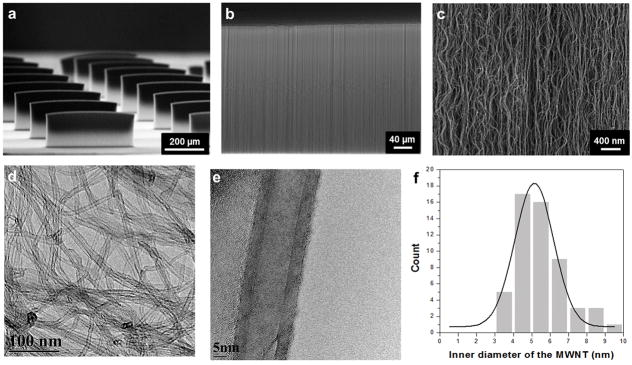
We designed and fabricated thin, microscale CNT forest electrode arrays in order to a) generate an efficient electric field for cultured cells on hydrogels; b) compensate for physical constraints created by electrode presence, which can limit muscle actuation; and c) allow for easy integration into soft biomaterial layers[26]. Vertically aligned CNT arrays were grown on an iron-catalyst patterned Si wafer by CVD using ethylene as the carbon source at 800 °C (Fig. S1). In addition, the length and diameter of CNTs could be easily controlled by changing the reaction time, size of catalyst, and other synthesis conditions. Fig. 2a and b show a tilted scanning electron microscope (SEM) image of well-defined thin film arrays with uniform alignment along the growth direction. The dimensions of each CNT forest, i.e. CNT microelectrode, were measured to be 460 μm (width), 300 μm (height), and 50 μm (thick). The array of microelectrodes were spaced 200 μm apart in order to be able to induce spontaneous beating, changes in repolarization characteristics and conduction path and velocity. This design is similar to those used in commercially available multielectrode array (MEA) designs [27]. Unlike the solid metallic electrodes used in MEAs, the CNT microelectrodes can maintain the flexibility of the final construct after electrode integration[15]. The CNT forests showed a visually discernible morphology with high tortuosity and a 3D interconnected network of nanotubes (Fig. 2c). To characterize the diameter distribution of CNTs inside the CNT forests, we analyzed the samples by high-resolution transmission electron microscopy (HRTEM). Fig. 2d and e show homogeneous shape and size distribution of multiwall nanotubes. The inner diameter and the wall thickness of the nanotubes were around 5 nm and 2 nm (Fig. 2f) with a narrow distribution. In addition, these forest-based conductive materials have been shown to exhibit high electrical conductivity with small amount of nanotube loading and highly reversible electrical property changes under 20% strain change[22, 28]. Therefore, vertically aligned CNT forests represent a unique conducting material made up of nanotube networks with a resilient structural retention that could provide stable electrical conductivity under muscle contraction and deformation.
Figure 2.
SEM images of (a) vertically aligned CNT forests (width: 460 μm and height: 300 μm), (b–c) magnified parts of the CNT forest surface. (d & e) HRTEM images of multiple and individual MWNTs isolated from the CNT forest. (f) Diameter distribution of the MWNTs in the CNT forests.
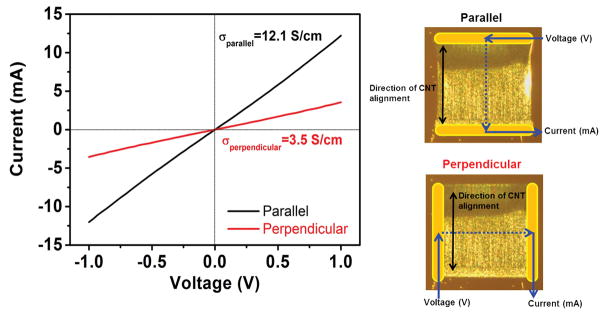
A key electrical conductivity indicator of the embedded microelectrodes is their ability to sustain pacing of cardiac tissue. To measure the conductivity of vertically aligned CNT electrodes, they were first flattened onto the Si wafer where they kept alignment in the lateral direction (Fig. S2). Elastocapillary densification resulted in an increased array density, reducing the layer thickness down to 10 μm. Next, Au contacts were deposited on both sides of flattened CNT forests in order to obtain robust electrical measurements, as shown in Fig. 3 (right panel). Also shown in Fig. 3 (left panel) are the I-V curves measured in configurations parallel and perpendicular to the alignment direction of CNTs. Conductivity parallel to the direction of CNT alignment (12.1 S/cm) was three times higher than that of the one along the perpendicular direction (3.5 S/cm). The parallel direction has enhanced conduction pathways relative to the perpendicular direction, resulting in the electrical anisotropy. The alignment of CNTs (either in the perpendicular direction or the parallel direction to the appied voltage) was the primary cause of the anisotropic conductivity of the fabricated constructs. Therefore, the unique electrical properties of CNT forest microelectrodes, especially when compared to other metal based alternatives, might better mimic the anisotropic conductivity present in the fiber structure of the cardiac muscle tissue.
Figure 3.
Two-point probe I-V curves from the CNT microelectrodes in parallel and perpendicular configurations relative to the nanotube alignment direction of CNT microelectrodes were measured. Au contacts were deposited at the edges of the CNT microelectrodes.
Integration of vertically aligned CNT forest microelectrode arrays into hydrogel layers
Previous studies have relied on PMMA, PEI, or PDMS [29] materials as the primary substrates for electrode fabrication due to their tolerance of large structural deformations and ease of handling. Although these materials may be suitable for the construction of bio-hybrid actuators, they often lack permeability to oxygen and nutrients, which can limit their seamless 3D integration with synthetic tissues [15]. To build bio-inspired synthetic muscle tissue constructs, integration of electronics must be done with 3D microporous biomaterials that resemble the mechanical and biological properties of the native extracellular matrix (ECM). In this work, we tested photocrosslinkable GelMA and PEG hydrogels for the integration of microelectronics. As the first step, CNT microelectrodes fabricated on the Si wafer were directly transferred to the PEG hydrogel substrate without using additional sacrificial layers such as nickel coating[15]. PEG hydrogel, instead of GelMA, was used for electrode transfer because the thin GelMA hydrogel was difficult to peel off the Si wafer due to strong adhesion between GelMA and the Si surface (data not shown). Unlike previously used substrate materials, such as PMMA and PDMS, crosslinked PEG hydrogels are permeable to water, oxygen and nutrients. In addition, they are nontoxic, nonimmunogenic, and non-degradable under physiological conditions with robust mechanical properties [29]. The transfer was achieved by dispensing a 20% PEG pre-polymer solution onto the Si wafer substrate having the CNT forest arrays, followed by UV crosslinking for 50 s. In this step, PEG pre-polymer solution was able to penetrate into the highly porous forest surface,[30] and hence CNTs were embedded in the PEG matrix after UV crosslinking. The flattening process broke the strong bonds between the CNT forests and the silicon substrate. Accordingly, the transfer process occurred quite seamlessly due to the weak adhesion between the flattened CNT forests and the silicon substrate. The thin PEG hydrogel film embedded with CNT forest array was readily peeled off the silicon substrate with intact electrodes (Fig. 4a). The structural integrity, alignment and morphology of transferred CNTs were preserved inside the 150 μm thick hydrogel layer (Fig. 4b). In addition, encapsulation of the CNTs inside the PEG hydrogel prevented possible detachment of fragments or individual CNTs resulting from cracked or destroyed CNT forests during contraction of the tissue constructs. To confirm cytocompatibility, cardiac fibroblasts were seeded on the CNT forest electrodes and cellular viability (i.e. LIVE/DEAD assay) was performed. The CNT forest electrodes did not display any observable cytotoxic effects to the cardiac cells since 95% cellular viability was observed in Fig. 4c.
Figure 4.
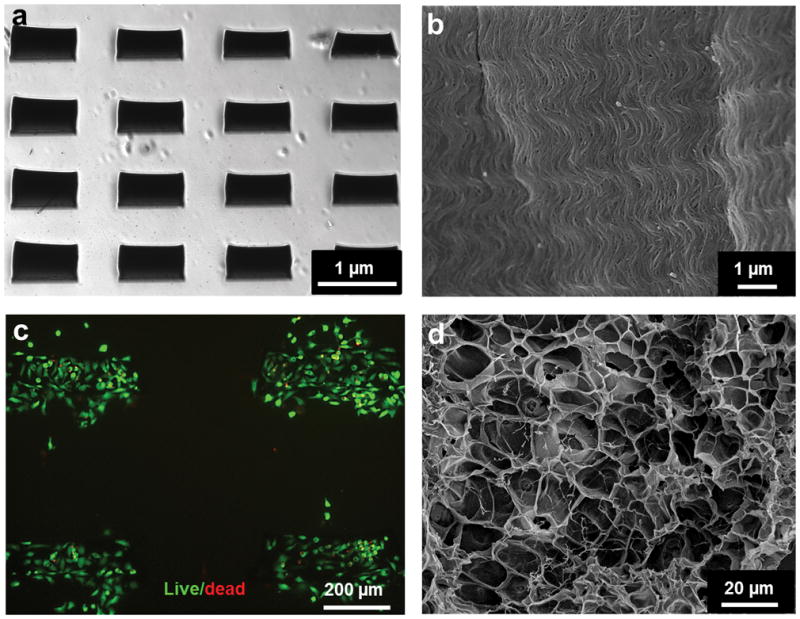
SEM images show that (a) arrays and (b) alignment of CNTs were preserved after they were transferred to the PEG substrate. (c) Cardiac fibroblast cell viability using LIVE/DEAD staining after 24h of seeding on CNT forest microelectrodes. (d) SEM images show porous surfaces of a 1 mg/ml CNT-GelMA layer. This image shows nanofibrous networks of CNTs across and inside a porous gelatin framework.
An additional CNT-GelMA hybrid hydrogel layer (thickness: 50μm) was fabricated and placed over the PEG hydrogel layer (thickness: 150μm) containing the CNT forest microelectrode arrays. This new conductive CNT-GelMA layer effectively sandwiched the microelectrodes at the interface between the two hydrogels. The bond between two gel layers was reinforced by the applucation of a UV light. Cardiomyocytes were later seeded on the CNT-GelMA surface. Conductive CNT-GelMA hybrid hydrogel substrates have been shown previously to improve cardiac cell organization, maturation, and cell-to-cell coupling[23]. Fig. 4d shows the porous surface of the CNT-GelMA (1mg/mL concentration of CNTs in 5% GelMA) hydrogel layer. The CNT nanofibrous networks within the GelMA hydrogel layer lead to enhanced electrically conductivity and mechanical properties of the constructs[23]. It is anticipated that the CNT-GelMA would promote the formation of fully matured homogeneous cardiac tissue and offer better mechanical integrity during contraction of the tissue construct. In addition, the acrylic and acrylamide groups present on both GelMA and PEG hydrogels (i.e. PEGDA) generated covalent bonds after UV irradiation which helped minimize the risk of detachment of the two hydrogel layers. Therefore, the CNT forest microelectrode arrays were physically constrained in between two hydrogel layers and can handle cyclic deformations.
Phenotypic and Electrophysiological characteristics of the 3D bio-hybrid system
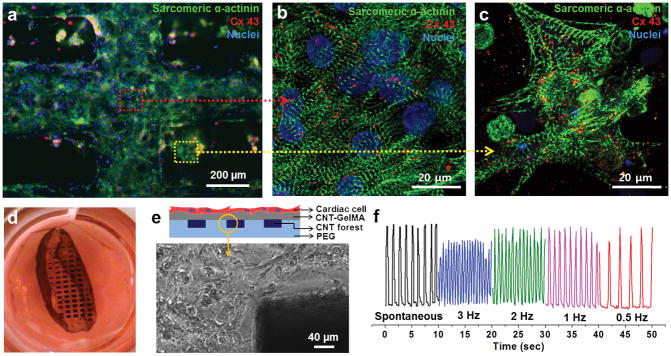
To complete the 3D bio-hybrid fabrication, neonatal rat cardiomyocytes were cultured on the microelectrode-embedded multilayer hydrogel sheets for 5–8 days to form a cardiac tissue layer. The organization and phenotype of cardiomyocytes on the construct was investigated by immunostaining for cardiac biomarkers (i.e. connexin 43 (Cx-43) and sarcomeric α-actinin) after 5 days of culture. Cx-43 and sarcomeric α-actinin are involved in intercellular electrical and metabolic coupling, and muscle contraction, respectively. Fluorescence microscopy of cultured tissue revealed a high density of cardiomyocytes in close contact with the microelectrode arrays (Fig. 5a). Cardiac tissues on these constructs possessed a well-defined, elongated, and interconnected sarcomeric α-actinin structures (Fig. 5b). In addition, we also observed homogeneous distribution of Cx-43 with enhanced synchronous contractile properties of the entire cardiac tissue constructs. These observations are in close agreement with previous reports from our group[23]. Furthermore, cardiac tissues located directly above the CNT forest electrodes (red dot arrow) showed well-interconnected sarcomeric structures with Cx-43 expression that was morphologically different than the ones located above location in between the electrodes (yellow dot arrow) which could be due to the higher mechanical stiffness of hybrid gels embedded with CNT forests compared to the gels having individual CNTs (Fig. 5c).
Figure 5.
Cardiac tissue organization on composite hydrogel layers incorporating CNT forest electrodes. (a) Phenotype of cardiac cells. Immunostaining of sarcomeric α-actinin (green), nuclei (blue), and Cx-43 (red) revealed that cardiac tissues (5-day culture) were created on top of a multilayer hydrogel sheet impregnated with aligned CNT microelectrodes. Magnified image showing the well interconnected sarcomeric structures of cardiac tissue which is located (b) above locations between the electrodes (red dot arrow) and (c) above the electrodes (yellow dot arrow). (d) Photograph of a free-standing 3D bio-hybrid actuator cultured for 8 days. (e) Schematic illustration of a multilayer hydrogel sheet impregnated with aligned CNT microelectrodes (side view). Phase contrast image of the boundary between the CNT forest electrodes and the hydrogel layer shows that the cardiac tissues remained attached to the top hydrogel layer snd stayed intact. (f) The displacement of the CNT forest electrode in multilayer hydrogel sheets (yellow circled tip in b) over time under electrical stimulation (Square wave form, 1.2 V/cm, Frequency: 0.5 Hz – 3 Hz, 50ms pulse width).
Finally, the bio-hybrid constructs made with cardiomyocytes cultured on hydrogel sheets (square shape, 1 cm2), were observed to spontaneously detach from the glass substrate due to constant actuation of the cells and adopted a planar shape with loosely rolled-up edges (Fig. 5d). The CNT forest electrodes within multilayer hydrogel constructs were able to efficiently stimulate the cardiomyocytes and forced them to beat synchronously after only one day of culture. We confirmed that cardiac tissues cultured on the bio-hybrid actuators made on multilayer nanocomposites showed stable spontaneous beating behavior with an average beating rate of ~108 BPM on day 8 (Movie 1). Interestingly, the average beating rate of these 3D bio-hybrid actuators (total thickness of the construct: ~210 μm) showed faster beating rates compared to the rates obtained from cells cultured on CNT-GelMA hydrogels (~50 μm thickness of whole construct and 69.8 ± 19.1 BPM on day 6)[23]. In addition, the beating frequency could be precisely controlled by applying an external electric field (1.2 V/cm) at various frequencies (0.5, 1, 2, and 3 Hz, pulses with 50ms duration) (Fig. 5f, Movie 2) generated by a conventional signal generator. Therefore, we conclude that these 3D bio-hybrid actuators with embedded CNT forest electrodes do not significantly inhibit muscle contractile function, even though they possess higher rigidity and stiffness compared to plain hydrogels. It is expected that the CNT forest electrodes, with a Young’s modulus on the order of 100 MPa to 1 GPa,[31] would have locally stiffer regions, while the overall mechanical strength of the tissue constructs would be dictated by the soft hydrogel substrates. The elastic moduli of 1.0 mg/ml CNT-GelMA and 10% PEGDA hydrogels are 23 kPa[23] and 25 kPa[32] respectively. It is evident that the cardiac tissue layer exhibited forces strong enough to induce contraction of the whole system. Furthermore, microelectrode arrays displayed excellent mechanical integrity within 3D bio-hybrid actuators showing no detachment at the electrode/hydrogel interface. The constructs were able to withstand prolonged muscle tissue contraction, as shown in the phase contrast image taken on day 8 (Fig. 5e). Thus, these 3D biohybrid actuator systems displayed spontaneous synchronous beating and were able to handle electrical stimulation up to 8 days in culture.
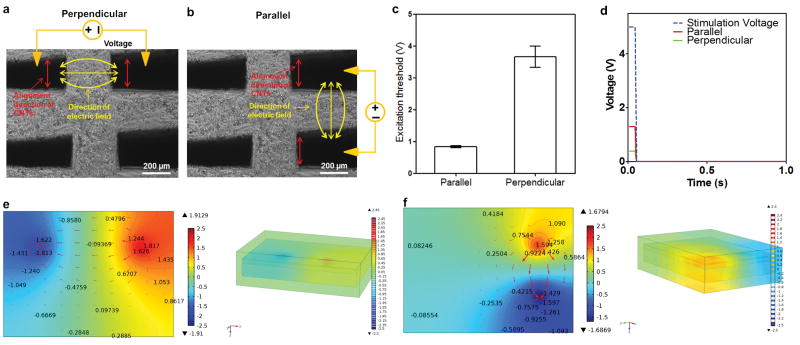
The effect of CNT forest based microelectrode arrays with anisotropic conductivity was further investigated using a 3D biohybrid actuator (Fig. 6). To evaluate the electrical stimulation ability of the embedded CNT forest electrodes, we have applied the electrical signals to the cells by using two CNT forest electrodes instead of the whole microelecrode array. The micromanipulator allows us to lower the micromanipulator probes until they make contact to the CNT forest electrodes as shown in Figure S3. Compared to GelMA hydrogels these CNT forest electrodes have higher mechanical integrity and can be readily probed. During the probing step, the whole construct still maintained its mechanical integrity while the probes were lowered through a small hole on the construct until they reached the middle CNT forest electrode layer. Electrical pulse signals with different frequencies were applied to the CNT forest electrodes using a pulse generator via the micromanipulator probes. A video camera attached to a microscope with a 10x magnification was used to capture the movement of the cardiac tissue created on the multilayer construct. The beating frequency of the cardiac tissue was precisely controllable by applying an electrical signal to the CNT microelectrodes in either along a perpendicular (Fig. 6a) or parallel (Fig. 6b) alignment direction, which generated an electric field perpendicular or parallel to the alignment direction of the CNTs, at different frequencies as 0.5, 1, 2, and 3 Hz. The excitation threshold which is the minimum voltage needed to induce synchronous contraction of the whole construct at each frequency was characterized for each both electrode configuration. We observed that the application of the electrical signal in the direction parallel to the alignment direction (0.8 ± 0.03 V) resulted in drastically reduced excitation thresholds compared to those stimulated with signals applied to the electrodes aligned in the perpendicular direction (3.7 ± 0.3 V) (Fig. 6c) (Movie 3 and 4). We modeled the electrical signal propagation generated by a pair of anisotropic CNT microelectrodes and examined the potential distribution in the parallel and perpendicular configuration (see supporting information). Furthermore, we took into account the roughness of the CNT electrode surfaces (SEM images, Fig. 4b) (Fig. S5). The wavy nature of the CNT fibers made them act as scattering elements when the electrical field was in the direction perpendicular to the nanotube alignment. This in effect can decrease the ratio between the two (perpendicular and parallel) components of the electric field. The distribution of electrical potentials in perpendicular and parallel electrode configurations are shown in Fig. 6e and f respectively. Modeling results indicated that the drop in electrical potential was close to the microelectrodes, and also did not produce uniform electric field. Our model showed that the average potential acting on the cardiac cells significantly differed between the parallel and the perpendicular configurations (Fig. 6d). When a square wave of 1 Hz at 5V (50 ms pulse width) was applied across a pair of CNT electrodes, the potential drop across the cardiac layer was about 1.8 V in the configuration parallel to the alignment direction, which was 2.67 times (0.6 V) than the one observed in the direction perpendicular to the alignment configuration. The main reason that could explain this difference is that the spreading of the field profile occurs in parallel to the electric field even in the perpendicular configuration. In the parallel configuration, the electric field is more confined in between the pair of electrodes providing the cardiac cells with sufficient electrical field for stimulation at lower thresholds to induce beating.
Figure 6.
(a) and (b) Phase contrast images that show the direction of the applied field which is along the parallel and the perpendicular direction to the alginment of the CNT forests. (c) The excitation threshold of cardiac tissue in the parallel configuration was three times lower than that in the perpendicular configuration. (d) Numerically calculated maximum voltage applied on the cardiac cell layer using the two different electrode configurations (Frequency range: 0.5 Hz – 3 Hz, Wave: Pulse signal, Pulse width: 50ms). Top views of the numerically calculated electric field applied to the muscle cells in perpendicular (e) and parallel (f) electrode configurations with top and cross sectional views.
3. Conclusions
In summary, we synthesized vertically aligned CNT microelectrode arrays using well established CVD growth patterns and mechanisms. The microelectrode arrays had a 200 μm separation between each CNT forest and were next flattened on the silicon wafer breaking the bonds between the CNTs and the silicon substrate. Later, the forest arrays were successfully transferred onto mechanically stable PEG hydrogels where they were found to retain their dense array structure and structural integrity. Following transfer, we carried out I-V measurements in two different configurations to confirm anisotropic conductivity of the CNT electrodes. Furthermore, the CNT electrodes on a PEG substrate did not exhibit any toxicity to cells and preserved high cell viability when seeded with cardiac fibroblasts. However, neither CNT arrays nor PEG hydrogels could support cardiomyocyte adhesion. Hence an additional CNT-GelMA hybrid hydrogel layer was fabricated and was deposited on top of the CNT forest embedded gel substrate as a cell adhesive layer. The microelectrodes were, therefore, sandwiched at the interface between two hydrogel layers, which formed covalent bonds under UV irradiation thereby crosslinking the structure, stabilizing it and preventing delamination. Finally, the constructs were seeded with cardiomyocytes and cultured for 5–8 days and showed homogenous Cx-43 distribution and partial uniaxial alignment of sarcomeric structures. After detaching from the glass slide, the constructs spontaneously adopted a planar shape and continued their synchronous beating behavior. The constructs exhibited excellent mechanical integrity and were able to sustain spontaneous cardiomyocyte beating for long periods (8 days of culture). The beating frequency of the 3D bio-hybrid actuators could be precisely controlled by applying an electrical signal along the parallel and perpendicular directions relative to the alignment direction of the CNT forest at 0.5, 1, 2, and 3 Hz (at 5V). In addition, we observed that the application of the electrical signal in the direction parallel to the CNT alignment resulted in drastically reduced excitation thresholds compared to those stimulated with a signal applied in the direction perpendicular to the CNT alignment direction. Modeling results indicated that the average voltage acting on the cardiac cells was significantly higher when the stimulating electric fields were in parallel to the alignment of CNT forest electrodes. Therefore, the high integration fidelity and unique electrical property of these CNT forest microelectrodes could be utilized to develop a growing number of diagnostic, therapeutic, and treatment tools for various cardiac, neurological, and other biomedical applications.
4. Experimental
Materials
High purity gases, ethylene, hydrogen, and argon (purity > 99.99%) for CNT synthesis was purchased from Plaxair (Ontario, Canada). Sigma-Aldrich (Wisconsin, USA) was used as a supplier for Gelatin (Type A, 300 bloom from porcine skin), polyethylene glycol diacrylate (PEGDA), 3-(trimethoxysily) propyl methacrylate (TMSPMA), and methacrylic anhydride (MA). CAD Art (Washington, USA) provided the photomasks and EXPO Photonic Solutions Inc. (Ontario, Canada).
Preparation and characterization of vertically aligned CNT forest microelectrodes
The fabrication process is illustrated in Fig. 1. First, the vertically aligned CNT forest microelectrode arrays were fabricated on a Si wafer by a CVD process as described in previous reports [25]. E-beam evaporated Fe film (4 nm thick) deposited on Si/SiO2 wafers was used as the catalyst. CVD growth was done using a gas mixture ethylene/hydrogen/argon (70:70:70 sccm) at 800 °C in a 1 in. tube furnace (Lindberg). The growth time was between 30 and 60 min with no preannealing step. Patterning of the iron-catalyst films were carried out by standard photolithography using AZ3330 photoresist and LOR15A as the lift-off resist (Fig S1). HRTEM images were obtained using a JEOL JEM-2010. SEM (Hitachi Model S4700, Japan) was used to assess the structure of the CNT-GelMA hybrid hydrogels. In order to determine the electrical conductivity of the CNT forest, vertically aligned CNT forests were first flattened onto the Si wafer, and then Au electrodes were coated on each side to form electrical contacts. The current-voltage (I–V) curves were obtained in the parallel direction and in the perpendicular direction by a two-point probe method.
Preparation and characterization of CNT electrode incorporated cardiac tissue constructs
Vertically aligned CNT forests were grown on Si wafers and then were dipped into DI water briefly, pulled out, and air dried. During this process, the vertically aligned CNT forests were flattened onto the Si wafer. In order to successfully create the CNT forest microelectrodes inside hydrogels, they were encapsulated between two hydrogel sheets; CNT-GelMA and PEG hydrogel layer. GelMA and CNT-GelMA hybrid hydrogels (1.0 mg/ml CNT in 5% GelMA) and PEG hydrogels were prepared based on our previously published reports [23, 33]. 20% PEG prepolymer solution was dispensed onto a Si water with the CNT forest microeectrode array and crosslinked using UV light (6.9 mW /cm2 (360–480 nm)) and had a final thickness of 150 μm determined by a spacer. To generate a cardiac tissue actuator the CNT forest/PEG hydrogel layer was released from the Si wafer manually and placed over a CNT-GelMA pre-polymer solution. The final hybrid cell culture scaffold, with a 50 μm thickness was made after exposure to UV light for 50 sec which not only polymerized the gel prepolymer solution but also enhanced the attachmewnt of CNT-GelMA to the underlying substrate. SEM (Hitachi Model S4700, Japan) was used to assess the structure of CNT forest on the PEG hydrogel and the CNT-GelMA hybrid hydrogel. After freezing with liquid nitrogen the swollen hydrogels were lyophilized. These lyophilized samples were coated with Pt/Pd using a sputter coater for SEM imaging.
Cell isolation and culture
We followed well-established protocols approved by the Institute’s Committee on Animal Care for isolation of neonatal rat (2-day-old Sprague-Dawley) ventricular cardiomyocytes. Post extraction and enrichment via 1h preplating, the cardiomyocytes were ready to be seeded. Culturing of seeded cells was performed in Dulbecco’s modified eagle medium (DMEM, Gibco, USA) with added 10 % fetal bovine serum (FBS), 1% L-Glutamine and 100 units/ml penicillin-streptomycin (All from Gibco, USA) without electric field stimulation up to 8 days.
Cell characterization
To test the cytocompatiblity of the CNT forest electrodes, the they were first transferred onto a PEG hydrogel layer. Then fibronectin (50 ug/ml) was coated on this sample. We used a Live/Dead assay based on the manufacturer’s instructions (Invitrogen). An inverted fluorescence microscope (Nikon, Eclipse TE 2000U, Japan) was used to collect Live/Dead images. Immunostaining of cardiac tissue constructs was performed on samples fixed for 20 min in 4% paraformaldehyde followed by DPBS a wash at room temperature. After fixation and washing, the cells were treated with 0.15% Triton X-100 in DPBS for 10 min. Finally, cell staining with a cardiac biomarker (sarcomeric α-actinin, Cx-43) was performed in the presence of a blocking buffer for 45 min at the manufacturer‘s suggested dilution. Similarly, a DAPI counterstain was performed at 1:20,000 dilution in DPBS for 45 min. Inverted laser scanning confocal imaging was then carried out using a confocal microscope (Leica SP5X MP, Germany).
Electrophysiological assessment of the biohybrid actuators
Cultured cardiomyocytes were imaged using a microscope equipped with a CCD camera inside a temperature controlled chamber. Using a microscope with a 10x magnification (Nikon, Eclipse TE 2000U, Japan), a video camera (Sony XCD-X710) was used to capture the movement of the cells. Video capture software was used to obtain digitized beating videos with twenty frames per second (20 fps). A custom developed MATLAB code (MathWorks Inc., Natick, MA) was used for the image processing software as described in previous reports[23, 34]. After 6 to 8 days of culture the samples were detached from the substrates by themselves. Biohybrid actuators were stimulated at RT (~ 20 °C) and their actuation behavior was monitored in culture medium. Panasonic HDC-HS9 digital camera (1920 × 1080 pixels, 24 fps, and recording quality) was used to capture movies of the biohybrid actuators.
To assess the response of cardiomyocytes to external electrical fields, we utilized established protocols based on a modified carbon electrode system[18]. A biphasic square waveform (50 ms pulses of 0–7 V/cm at 0.5, 1, 2 and 3 Hz) was applied from an electric pulse generator to samples located between two Pt wire electrodes inside an experimentation chamber. In this setup, the samples were placed between two Pt wire electrodes (spaced ~3cm apart in cell culture media). To assess the stimulation ability of embedded CNT forest electrodes, the electrical signal was applied by a pair of the micromanipulation probes attached to the CNT forest electrodes which were aligned in the parallel and perpendicular directions to the applied field (Figure S3). The minimum voltage needed to induce synchronous contraction (excitation threshold) at each frequency is characterized for both electrode systems.
Modelling of the electrical field
An analysis of the electric field profile acting on the cardiac tissue generated by an applied voltage using CNT forest electrodes is presented. We modeled the electrical field generated by the vertically aligned CNT forest microelectrodes, using commercially available software (Multiphysics, Comsol, electrostatics module) (see supporting information).
Statistical analysis
Statistical significance was performed by measuring one-way or two-way ANOVA tests (GraphPad Prism 5.02, GraphPad Software). To analyze and assess significant differences between selected treatments, we utilized Tukey’s multiple comparison tests. Differences were characterized as significant for p<0.05.
Supplementary Material
Acknowledgments
This work was supported by the Institute for Soldier Nanotechnology, National Institutes of Health (HL092836, EB02597, AR057837, HL099073), the National Science Foundation (DMR0847287), the Office of Naval Research Young Investigator award, ONR PECASE Award, a Discovery grant from National Sciences and Engineering Research Council of Canada.
Footnotes
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Dr. Su Ryon Shin, Biomaterials Innovation Research Center, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02139, USA. Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, MA 02115, USA
Courtney Shin, Biomaterials Innovation Research Center, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02139, USA. Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Dr. Adnan Memic, Center of Nanotechnology, King Abdulaziz University, Jeddah, 21589, Saudi Arabia
Samaneh Shadmehr, Department of Chemistry & Waterloo Institute for Nanotechnology, University of Waterloo, 200 University Ave. West, Waterloo, Ontario, N2L 3G1, Canada.
Mario Miscuglio, Biomaterials Innovation Research Center, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02139, USA. Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Dr. Hyun Young Jung, Department of Mechanical and Industrial Engineering, Northeastern University, Boston, Massachusetts 02115, USA
Dr. Sung Mi Jung, Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA
Dr. Hojae Bae, College of Animal Bioscience and Technology, Department of Bioindustrial Technologies, Konkuk University, Hwayang-dong, Kwangjin-gu, Seoul 143-701, Korea
Prof. Dr. Ali Khademhosseini, Biomaterials Innovation Research Center, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02139, USA. Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. Department of Physics, King Abdulaziz University, Jeddah 21569, Saudi Arabia. Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, MA 02115, USA. Department of Maxillofacial Biomedical Engineering and Institute of Oral Biology, School of Dentistry, Kyung Hee University, Seoul 130-701, Republic of Korea
Prof. Xiaowu (Shirley) Tang, Email: tangxw@uwaterloo.ca, Department of Chemistry & Waterloo Institute for Nanotechnology, University of Waterloo, 200 University Ave. West, Waterloo, Ontario, N2L 3G1, Canada.
Dr. Mehmet R. Dokmeci, Email: mdokmeci@rics.bwh.harvard.edu, Biomaterials Innovation Research Center, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02139, USA. Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, MA 02115, USA.
References
- 1.Dvir T, Timko BP, Kohane DS, Langer R. Nature Nanotechnology. 2011;6:13. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricotti L, Menciassi A. Biomed Microdevices. 2012;14:987. doi: 10.1007/s10544-012-9697-9. [DOI] [PubMed] [Google Scholar]; Sakar MS, Neal D, Boudou T, Borochin MA, Li Y, Weiss R, Kamm RD, Chen CS, Asada HH. Lab Chip. 2012;12:4976. doi: 10.1039/c2lc40338b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yurke B, Turberfield AJ, Mills AP, Jr, Simmel FC, Neumann JL. Nature. 2000;406:605. doi: 10.1038/35020524. [DOI] [PubMed] [Google Scholar]; Grosberg A, Alford PW, McCain ML, Parker KK. Lab Chip. 2011;11:4165. doi: 10.1039/c1lc20557a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herr H, Dennis RG. J Neuroeng Rehabil. 2004;1:6. doi: 10.1186/1743-0003-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Akiyama Y, Sakuma T, Funakoshi K, Hoshino T, Iwabuchi K, Morishima K. Lab Chip. 2013;13:4870. doi: 10.1039/c3lc50490e. [DOI] [PubMed] [Google Scholar]; Akiyama Y, Hoshino T, Iwabuchi K, Morishima K. PLoS One. 2012;7:e38274. doi: 10.1371/journal.pone.0038274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cvetkovic C, Raman R, Chan V, Williams BJ, Tolish M, Bajaj P, Sakar MS, Asada HH, Saif MT, Bashir R. Proc Natl Acad Sci U S A. 2014;111:10125. doi: 10.1073/pnas.1401577111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vunjak-Novakovic G, Tandon N, Godier A, Maidhof R, Marsano A, Martens TP, Radisic M. Tissue Eng Part B Rev. 2010;16:169. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia Z, Valiunas V, Lu Z, Bien H, Liu H, Wang HZ, Rosati B, Brink PR, Cohen IS, Entcheva E. Circ Arrhythm Electrophysiol. 2011;4:753. doi: 10.1161/CIRCEP.111.964247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xi J, Schmidt JJ, Montemagno CD. Nat Mater. 2005;4:180. doi: 10.1038/nmat1308. [DOI] [PubMed] [Google Scholar]
- 9.Feinberg AW, Feigel A, Shevkoplyas SS, Sheehy S, Whitesides GM, Parker KK. Science. 2007;317:1366. doi: 10.1126/science.1146885. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Park J, Yang S, Baek J, Kim B, Lee SH, Yoon ES, Chun K, Park S. Lab Chip. 2007;7:1504. doi: 10.1039/b705367c. [DOI] [PubMed] [Google Scholar]
- 11.Nawroth JC, Lee H, Feinberg AW, Ripplinger CM, McCain ML, Grosberg A, Dabiri JO, Parker KK. Nat Biotechnol. 2012;30:792. doi: 10.1038/nbt.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams BJ, Anand SV, Rajagopalan J, Saif MT. Nat Commun. 2014;5:3081. doi: 10.1038/ncomms4081. [DOI] [PubMed] [Google Scholar]
- 13.Chan V, Park K, Collens MB, Kong H, Saif TA, Bashir R. Sci Rep. 2012;2:857. doi: 10.1038/srep00857. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chan V, Jeong JH, Bajaj P, Collens M, Saif T, Kong H, Bashir R. Lab Chip. 2012;12:88. doi: 10.1039/c1lc20688e. [DOI] [PubMed] [Google Scholar]
- 14.Ahadian S, Ramon-Azcon J, Ostrovidov S, Camci-Unal G, Kaji H, Ino K, Shiku H, Khademhosseini A, Matsue T. Biomed Microdevices. 2013;15:109. doi: 10.1007/s10544-012-9692-1. [DOI] [PubMed] [Google Scholar]
- 15.Tian B, Liu J, Dvir T, Jin L, Tsui JH, Qing Q, Suo Z, Langer R, Kohane DS, Lieber CM. Nature materials. 2012;11:986. doi: 10.1038/nmat3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim DH, Ghaffari R, Lu N, Rogers JA. Annu Rev Biomed Eng. 2012;14:113. doi: 10.1146/annurev-bioeng-071811-150018. [DOI] [PubMed] [Google Scholar]; Kim DH, Lu N, Ma R, Kim YS, Kim RH, Wang S, Wu J, Won SM, Tao H, Islam A, Yu KJ, Kim TI, Chowdhury R, Ying M, Xu L, Li M, Chung HJ, Keum H, McCormick M, Liu P, Zhang YW, Omenetto FG, Huang Y, Coleman T, Rogers JA. Science. 2011;333:838. doi: 10.1126/science.1206157. [DOI] [PubMed] [Google Scholar]
- 17.Cannizzaro C, Tandon N, Figallo E, Park H, Gerecht S, Radisic M, Elvassore N, Vunjak-Novakovic G. Methods Mol Med. 2007;140:291. doi: 10.1007/978-1-59745-443-8_16. [DOI] [PubMed] [Google Scholar]
- 18.Tandon N, Cannizzaro C, Chao PH, Maidhof R, Marsano A, Au HT, Radisic M, Vunjak-Novakovic G. Nature protocols. 2009;4:155. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tandon N, Marsano A, Maidhof R, Wan L, Park H, Vunjak-Novakovic G. J Tissue Eng Regen Med. 2011;5:e115. doi: 10.1002/term.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cellot G, Cilia E, Cipollone S, Rancic V, Sucapane A, Giordani S, Gambazzi L, Markram H, Grandolfo M, Scaini D, Gelain F, Casalis L, Prato M, Giugliano M, Ballerini L. Nature Nanotechnology. 2008;4:126. doi: 10.1038/nnano.2008.374. [DOI] [PubMed] [Google Scholar]; Bareket-Keren L, Hanein Y. Front Neural Circuits. 2012;6:122. doi: 10.3389/fncir.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foroughi J, Spinks GM, Wallace GG, Oh J, Kozlov ME, Fang S, Mirfakhrai T, Madden JD, Shin MK, Kim SJ, Baughman RH. Science. 2011;334:494. doi: 10.1126/science.1211220. [DOI] [PubMed] [Google Scholar]
- 22.Yamada T, Hayamizu Y, Yamamoto Y, Yomogida Y, Izadi-Najafabadi A, Futaba DN, Hata K. Nat Nanotechnol. 2011;6:296. doi: 10.1038/nnano.2011.36. [DOI] [PubMed] [Google Scholar]
- 23.Shin SR, Jung SM, Zalabany M, Kim K, Zorlutuna P, Kim SB, Nikkhah M, Khabiry M, Azize M, Kong J, Wan KT, Palacios T, Dokmeci MR, Bae H, Tang XS, Khademhosseini A. ACS Nano. 2013;7:2369. doi: 10.1021/nn305559j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dvir T, Timko BP, Brigham MD, Naik SR, Karajanagi SS, Levy O, Jin H, Parker KK, Langer R, Kohane DS. Nat Nanotechnol. 2011;6:720. doi: 10.1038/nnano.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shin SR, Aghaei-Ghareh-Bolagh B, Gao X, Nikkhah M, Jung SM, Dolatshahi-Pirouz A, Kim SB, Kim SM, Dokmeci MR, Tang XS, Khademhosseini A. Adv Funct Mater. 2014;24:6136. doi: 10.1002/adfm.201401300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazloumi M, Shadmehr S, Rangom Y, Nazar LF, Tang XS. ACS Nano. 2013;7:4281. doi: 10.1021/nn400768p. [DOI] [PubMed] [Google Scholar]
- 26.Ahadian S, Ramon-Azcon J, Estili M, Liang X, Ostrovidov S, Shiku H, Ramalingam M, Nakajima K, Sakka Y, Bae H, Matsue T, Khademhosseini A. Sci Rep. 2014;4:4271. doi: 10.1038/srep04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liau B, Zhang D, Bursac N. Regen Med. 2012;7:187. doi: 10.2217/rme.11.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abadi PP, Hutchens SB, Greer JR, Cola BA, Graham S. Nanoscale. 2012;4:3373. doi: 10.1039/c2nr30474k. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Yang R, Shi Z, Zhang L, Shi D, Wang E, Zhang G. ACS Nano. 2011;5:3645. doi: 10.1021/nn103523t. [DOI] [PubMed] [Google Scholar]
- 30.Shin MK, Oh J, Lima M, Kozlov ME, Kim SJ, Baughman RH. Advanced Materials. 2010;22:2663. doi: 10.1002/adma.200904270. [DOI] [PubMed] [Google Scholar]
- 31.Volder MFLD, Park SJ, Tawfick SH, Vidaud DO, John HA. journal of micromechanics and microengineering. 2011;21:12. [Google Scholar]
- 32.Hutson CB, Nichol JW, Aubin H, Bae H, Yamanlar S, Al-Haque S, Koshy ST, Khademhosseini A. Tissue Eng Part A. 2011;17:1713. doi: 10.1089/ten.tea.2010.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Biomaterials. 2010;31:5536. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SB, Koo KI, Bae H, Dokmeci MR, Hamilton GA, Bahinski A, Kim SM, Ingber DE, Khademhosseini A. Lab Chip. 2012;12:3976. doi: 10.1039/c2lc40345e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.