Abstract
Labeled problem
Embryo implantation remains the main limiting factor in assisted reproductive medicine (20% success rate).
Methods of study
An endometrial immune profiling was performed among 394 women with the previous history of repeated embryo implantation failures (RIF). The endometrial immune profile documented the ratio of IL‐15/Fn‐14 mRNA as a biomarker of uNK cell activation/maturation (together with the uNK cell count) and the IL‐18/TWEAK mRNA ratio as a biomarker of both angiogenesis and the Th1/Th2 balance. According to their profile, we recommended personalized care to counteract the documented dysregulation and assessed its effects by the live birth rate (LBR) for the next embryo transfer.
Results
Endometrial immune profiles appeared to be dysregulated in 81.7% of the RIF patients compared to control. Overactivation was diagnosed in 56.6% and low activation in 25%. The LBR among these dysregulated/treated patients at the first subsequent embryo transfer was 39.8%.
Conclusion
Endometrial immune profiling may improve our understanding of RIF and subsequent LBR if treated.
Keywords: Birth rates, human endometrium, Immunology, Implantation failure, in vitro fertilization, uterine natural killer cells, uterine receptivity
Introduction
The main limiting factor for live births after in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) remains embryo implantation, despite impressive progress in research, focused mainly on the embryo and its implantation potential. One in every nine couples in Europe and the USA is affected by implantation disorders and pregnancy wastage, and most pregnancy losses occur during embryo implantation.1 The latest figures in France indicate that only 24% of transferred embryos produced by IVF/ICSI result in live births (National report of the French agency of biomedicine, 2012). The live birth rate (LBR) decreases with maternal age and the number of IVF–ICSI treatment cycles.
Endometrial remodeling events leading to endometrial decidualization are required for a successful pregnancy; they begin before implantation at each menstrual cycle, during the mid‐luteal phase, defined as the period of uterine receptivity. This remodeling is a vital process for pregnancy, preparing future maternal immune tolerance, protecting the fetus, and regulating the placentation process. Within the endometrial environment during this stage, known as ‘the implantation window’, a very peculiar influx of immune cells occurs and nearly completely switches local immunity from the adaptive (Th1) to the innate (Th2) type.2 This switch is crucial for implantation. During this period, 65–70% of the immune cells in the endometrium are uterine natural killer (uNK) cells that belong to the innate immunity compartment. Macrophages and dendritic cells are also detected, together with adaptive immune T cells, such as T regulatory cells (Tregs).3, 4
Human implantation may be simply described as a three‐step process that starts with apposition and is followed by the adhesion of a competent blastocyst to the endometrial epithelium (attachment). The third step is the extensive invasion of a receptive endometrium by the trophoblast cells covering the blastocyst. Embryo attachment requires active local endometrial reactivity on the maternal side. The adhesion step is followed immediately by an anti‐inflammatory reaction to enable the induction of the mechanisms of local tolerance, required for effective invasion. Early on, the ideal environment during the implantation window was thought to contain mainly Th2 (compared with Th1) cytokines, which would selectively allow the development of local mechanisms that promote immunotrophism and angiogenesis at the same time that they down‐regulate inflammation and cytotoxic pathways.5 Over time, the concept of pregnancy as a Th2 phenomenon has evolved both the absence and a large excess of Th1 cytokines are thought to be deleterious for implantation and placentation, as is the absence of Th2 cytokines.6 This transient immune switch, together with the adequate uNK cell activation, appears fundamental in enabling the establishment of local maternal tolerance and survival of the fetus. Interleukin (IL)‐15 is directly involved in the post‐ovulatory recruitment and maturation of uNK cells in the uterus7 and, under their control, is essential for adequate Th2 cytokine production.8 Its effects on blood NK cells are different than on uNK cells, for it does not convert them into potent cytolytic cells but instead participates directly in their maturation.9 IL‐18 is a Th2‐promoting cytokine that, through the action of angiopoietin‐2, affects the crucial destabilization of spiral arteries.10, 11 In human endometrium, IL‐18 expression increases during the implantation window.12 Its main role is to remodel the maternal side of vasculature. However, IL‐15 and IL‐18, produced by either epithelial or stromal endometrial cells, are both bivalent: At high levels and when not immunoregulated, they behave as pro‐inflammatory Th1 cytokines (Fig. 1). They reflect the local production of IFN‐γ and TNF‐α, which can activate uNK cells to become cytotoxic.13, 14
Figure 1.
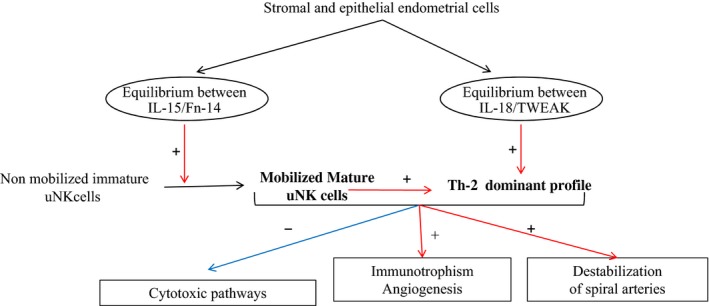
Focus on IL15 and IL18 environment during the implantation window. In the mid‐luteal phase, stromal and endothelial endometrial cells secrete IL‐15, Fn‐14, IL‐18, and TWEAK at specific levels. Increased IL‐15 allows the recruitment and maturation of uNK cells. IL‐18 and mature uNK cells stimulate Th2 cytokine production and lead to a predominantly Th2 balance. This equilibrium promotes immunotrophism and angiogenesis, while inhibiting inflammatory and cytotoxic pathways.
Our group has focused on the maternal–fetal immune dialogue during the implantation by studying distinct immune actors during physiological and pathological implantation processes, first in animals and then in humans. We have observed that the endometrium of women with RIF contains abnormal levels of uNK cells (too few or too many) and dysregulated expression of a group of related cytokines—IL‐12, IL‐15, and IL‐18—involved either in the uNK cell maturation process (IL‐15) or in the essential angiogenic process (IL‐18) compared with fertile women.14, 15, 16 We also demonstrated a crucial role for TWEAK (TNF Weak inducer of Apoptosis) and its receptor, Fn‐14 (fibroblast growth factor‐inducible molecule 14). Comparing the murine abortive model CBA × DBA/2 to a control model CBA x Balc, we showed that TWEAK offers protection against the deleterious effects of a Th1‐dominant (TNF‐rich) environment during implantation and thus increases embryo survival.17 TWEAK and its ligand, Fn‐14, act as immune regulators of the Th1/Th2 cytokine balance in the human endometrium.18 Locally, a high local endometrial expression of TWEAK was able to neutralize the effects of a high IL‐18 expression and impairs the transformation of mature and useful uNK into deleterious cytotoxic killer cells.14 After confirmation that the endometrial immune profile was stable and reproducible for two cycles evaluated 3 months apart, we postulated that combination of mRNA expression of IL‐18/TWEAK and IL‐15/Fn‐14 could help us to define endometrial immune profile and possibly explain the rational of some cases of RIF (PCT/EP2013/065355).
Here, we report the follow‐up of a cohort of 394 women with RIF who had an endometrial immune profile before a new IVF/ICSI attempt. Those whose profiles showed dysregulation received personalized treatment and recommendations to counteract the specific documented dysregulation and to prepare the endometrium for the embryo transfer (ET). The principal outcomes were the live birth rate (LBR) resulting from this ET. We found that 81.7% of women with RIF had dysregulated endometrial immune profiles. Moreover, the personalized care provided led to a LBR of 39.8% and thus confirmed the interest of this innovative method in the assisted reproduction.
Materials and methods
Protocol Approval and Patient Consent
The Institutional Review Board of St Louis Hospital (2011‐A00994‐37) approved this study, and all women provided written informed consent.
Patient Recruitment
Fertile control group
The control group comprised 26 women who underwent an endometrial sampling 3 months before their ETs and who all successfully gave birth at the first subsequent attempt of fresh or frozen‐thawed ET, without any specific treatment for immune dysregulation. The cause of infertility was a male factor for all 26, and all had a normal ovarian reserve. Their mean age was 32 years.
The cohort of women with RIF
Between 2012 and 2014, we followed 394 women with RIF.
The inclusion criteria for follow‐up in the observational cohort were as follows:
Age younger than 43 years;
Presence of RIF, defined as no ongoing pregnancy lasting longer than 10 weeks (dated from the day of ET), despite multiple ETs over the years, and a cumulative total of at least SIX embryos transferred (on day 3 or day 5) during the intraconjugal IVF/ICSI cycles.
Treatment by IVF/ICSI and effective ET (fresh or frozen) within the 6 months following the endometrial evaluation or spontaneous pregnancy within the two menstrual cycles following the last endometrial immune evaluation.
The mean age of the RIF cohort was 37 years (range: 26–43) with a mean of 6.5 years of infertility. The mean range of previous IVF/ICSI attempts was three with a mean number of 8.9 transferred embryos per patient. In all, 45 of the 394 women (11%) also had one or more miscarriages after previous IVF/ICSI attempts.
Study Design
RIF patients underwent an endometrial biopsy performed in mid‐luteal cycle (confirmed by histological dating) to determine their endometrial immune profile during the implantation window and before a new ET (Fig. 2). If the profile results showed immune dysregulation, treatment was recommended and administered under the physician's supervision before the next fresh or frozen ET. The follow‐up (outcomes of no pregnancy or pregnancy, miscarriage, or live birth) lasted until birth. For 40 women, a second biopsy was performed under therapies, to evaluate their effect on the endometrial immune profile.
Figure 2.
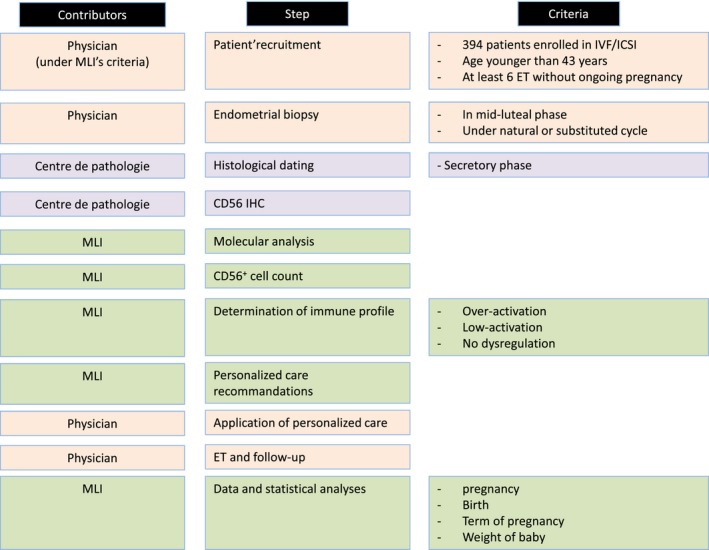
Study design. The study was conducted by three sets of contributors. MatriceLab Innove (MLI) designed the study, performed the endometrial immune profile (with its molecular analyses and CD56+ cell count), suggested personalized care, and remained in contact with physicians to follow‐up each IVF/ICSI attempt. Physicians recruited patients, performed the endometrial biopsies, and provided the personalized care before and after performing the IVF/ICSI. The Centre de pathologie (Passy, France) conducted the histological dating of the biopsies and the CD56 immunohistochemistry (IHC).
Analysis of Endometrial Samples to Determine Immune Uterine Profiles
Biopsy and dating of samples
As the endometrial study must be performed during the mid‐luteal phase, monitoring of ovulation and/or the expected progesterone rise the day before sampling was recommended. Biopsies could be performed during the substituted cycles for women with irregular cycles. The endometrium was gently aspirated by rotating a Pipelle de Cornier within the endometrial cavity. The pipelle content was emptied onto a gauze compress and divided into two parts, one placed in 4% formol (QPath Formol 4% buffered, VWR Chemicals, Fontenay‐sous‐Bois, France) for routine evaluation, endometrial dating, and CD56 immunolabeling by a pathology laboratory (Centre de Pathologie de Passy, France). The second part was placed in RNA later stabilization solution (Qiagen, Courtabeuf, France) for molecular analyses (MatriceLab Innove, France).
RNA extraction and reverse transcription
After confirmation of the histological dating, RNA was extracted from the biopsy sample conserved in RNAlater (Qiagen). Samples were disrupted in a lysis buffer of the RNeasy Plus kit (Qiagen) with TissueLyser LT (Qiagen). RNA was extracted with the RNeasy Plus kit, according to the manufacturer's instructions. The RNA was reverse‐transcribed into cDNA with the first‐strand cDNA synthesis kit for RT‐PCR (Roche, Meylan, France), according to the manufacturer's instructions. The cDNAs were stored at –20°C until use.
Quantitative RT‐PCR
Quantitative RT‐PCR was performed with a LightCycler 480 instrument (Roche Diagnostic) and the Light Cycler 480 SYBR Green I Master mix (Roche Diagnostic). Final concentrations for reaction setup were 0.5 μm of sense and antisense primers and 1/20 of diluted cDNA. Cycling conditions were as follows: denaturation (95°C for 5 min), amplification, and quantitation (95°C for 10 s, 60°C for 10 s, and 72°C for 15 s) repeated 40 times, a melting curve program (65–95°C with a ramp rate of 2.2°C/s), and a cooling step to 4°C. Primer sequences are detailed in Table SI. Each quantitative RT‐PCR assay included a solution without cDNA and inter‐run calibrator (IRC) samples as negative and positive controls. The IRC was obtained from pools of PHA (phytohaemagglutinin A)‐stimulated lymphocytes for TWEAK, Fn‐14, RPL13A, and beta‐2‐microglobulin (β2M) and from the endometrial sample for IL‐18 and IL‐15. The IRC cDNA, after dilution by a factor of 20, underwent the same quantitative RT‐PCR protocol as the unknown samples. PCR efficiency for each quantified target and reference was calculated with known serial dilutions of each specific cDNA. lightcycler®480 Software release 1.5.0 was used to analyze data, and each specific target transcription level was normalized to the geometric mean of the transcription level of the reference gene, with the software's advanced relative quantification workflow. Gene amplification efficiency was specifically determined. For each sample, the results were expressed as the ratio of target/reference cDNA.
Immunohistochemistry (IHC) of uNK Cells
IHC was performed on the biopsy sample tissue conserved in 4% formol on 5‐μm‐thick slides, with an automated streptavidin–biotin method (Benchmark GX, Ventana Medical Systems). The prediluted anti‐CD56 (clone 123C3) murine monoclonal primary antibody (Ventana Medical Systems®; Roche Diagnostics) was applied according to the manufacturer's instructions. Briefly, after deparaffinization of the slides, antigen retrieval was performed for 60 min in a pH 8.4 Cell Conditioning 1 solution. The CD56 primary antibody was then applied for 32 min. Slides for negative controls were prepared by replacing the primary antiserum with non‐immune IgG. Slides were then incubated for 8 min with a biotinylated antimouse secondary antibody. Diaminobenzidine or 3‐amino‐9‐ethylcarbazole was used as the chromogen (iVIEW DAB detection kit, Ventana Medical Systems), and slides were counterstained with hematoxylin for 2 min, incubated in bluing reagent (for 2 min), and mounted. Between each step, slides were rinsed with reaction buffer. The uNK cell count was measured as the mean of CD56+ cells in four representative fields at ×400 magnification.
Treatment Recommended in the Context of Personalized Care after Immune Profiling
The specific rationale and details for each type of personalized care according to the immune profile are detailed in the results section.
Statistical Analysis
Statistical analyses were performed with medcalc Statistical Software version 12.7.2 (MedCalc Software, Ostend, Belgium; http://www.medcalc.org; 2013).
Comparisons between groups (the control group and the different subgroups of women with RIF) used the anova test. The effect of treatment on immune biomarkers was analyzed with the nonparametric paired Wilcoxon test. A P‐value <0.05 was considered statistically significant.
Results
Determination of the Norm of Interpretation
Analyses of our biomarkers in the fertile control group of women who became pregnant were used to determine the norms to interpret each biomarker (Table 1):
Table 1.
Comparison of Endometrial Biomarkers in Control and RIF Subgroups According to Their Immune Profiles
| Immune endometrial profile | Control group | RIF group | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal | Over activation | P value (anova) | Low activation | P value (anova) | Nodysregulation | P value (anova) | ||
| Biomarkers | Number of patient | 26 | 223 (56.6%) | 99 (25%) | 72 (18.3%) | |||
| IL‐18/TWEAK | Range of interpretation | 0.03–0.12 | >0.12 | <0.03 | 0.03–0.12 | |||
| Mean | 0.076 | 0.40* | 0.046 | 0.045** | 0.002 | 0.07 | 0.33 | |
| IL‐15/Fn‐14 | Range of interpretation | 0.3–3 | >3 | <0.3 | 0.3–3 | |||
| Mean | 1.2 | 2.96* | 0.039 | 0.47*** | <0.001 | 1.27 | 0.75 | |
| CD56+ cells count | Range of interpretation | 10–100 | >100 | <10 | 10–100 | |||
| Mean | 45 | 49 | 0.64 | 32** | 0.009 | 42 | 0.46 | |
Norms for each biomarker were determined from a cohort of 26 fertile women. Data for each RIF subgroup were compared to data for the control group and analyzed with anova. A p‐value <0.05 was considered statistically significant.
* = P < 0.05, ** = P < 0.01, *** = P < 0.001.
The mean of the IL‐18/TWEAK mRNA ratio was 0.076, and the standard deviation (SD) 0.037. The distribution was normal. An IL‐18/TWEAK mRNA ratio was considered low when below 0.03 (mean −1 SD) and high when >0.12 (mean + 1 SD). In a previous study, the latter cutoff (>0.12) was also associated with the activation of the uNK cytotoxic receptor NKp46, which attested to the transformation of uNK cells into cytotoxic killer cells.14
The mean of the IL‐15/Fn‐14 mRNA ratio was 1.2, and the standard deviation 0.82. The distribution was normal. An IL‐15/Fn‐14 mRNA ratio was considered low when below 0.3 (mean −1 SD) and high when >3 (mean + 2 SD).
The mean of the CD56+ cell count was 45 cells/field, and the standard deviation 17. The distribution was normal. The CD56+ cell count was therefore considered low when below 10 (mean − 2 SD) and high when >80 (mean + 2 SD).
Figure 3 depicts the dispersion of the raw data observed for the ratios of IL‐18/TWEAK mRNA and IL‐15/Fn‐14 mRNA and for the CD56+ cell count in the fertile controls and the RIF groups.
Figure 3.
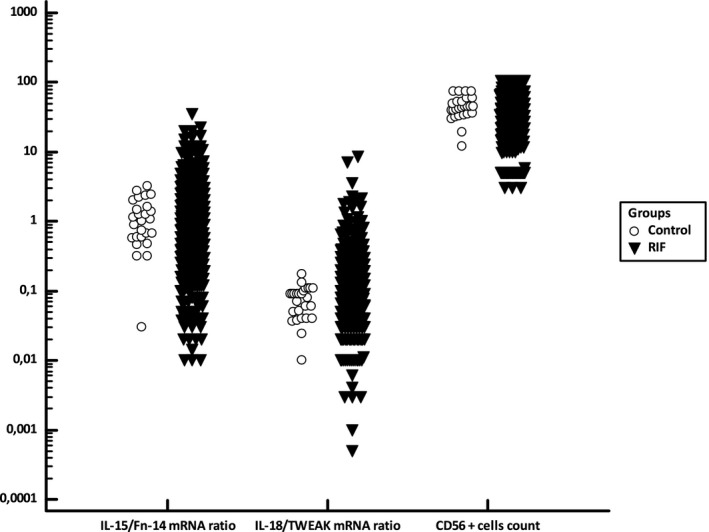
Distribution of the ratios of IL‐15/Fn‐14 mRNA and IL‐18/TWEAK mRNA and of the CD56+ cell count in the control fertile group and in the RIF cohort. Figure 3 presents the data dispersion for the log‐transformed IL‐15/Fn‐14 mRNA ratio, log‐transformed IL‐18/TWEAK mRNA ratio, and log‐transformed CD56+ cell count in the fertile control group and in the RIF cohort.
Endometrial Immune Profiles and Personalized Care in RIF Groups
We used the norms above, defined from the control group, to assess the endometrial immune profiles in the RIF cohort.
Compared with the control group value and as expected, the IL‐15/Fn‐14 mRNA ratio was significantly lower in the RIF low immune activation subgroup (P < 0.001) and significantly higher in the overactivation subgroup (P = 0.039). The same comparisons for the IL‐18/TWEAK mRNA ratio also showed a significantly lower value in the RIF low activation subgroup (P = 0.002) and a significantly higher one in the overactivation group (P = 0.046). Similarly, the CD56+ cell count was significantly lower (P = 0.009) in the RIF low activation group, compared with the controls, but no significant difference was observed between the immune overactivation RIF subgroup and the controls (Table 1).
To establish the endometrial immune profile, a step‐by‐step procedure first considered the IL‐18/TWEAK mRNA ratio (reflecting local angiogenesis and possibly a Th1 deviation), then the CD56+ cell count (reflecting uNK cell mobilization), and finally the IL‐15/Fn‐14 mRNA ratio (indicative of uNK cell maturation and uNK cytotoxic activation).
Immune Overactivation Profile
Applying the definitions above, we found that the RIF cohort included 223 women (56.6%) with endometrial immune overactivation that might induce embryo rejection (Table 2).
Table 2.
History, Immune Diagnosis, and Outcome at the Next Embryo Transfer in the RIF Cohort of 394 Patients
| Immune endometrial diagnosis in the RIF cohort | Over immune endometrial activation | Low immune endometrial activation | No immune dysregulation | P value (anova) |
|---|---|---|---|---|
| Number of RIF patients | 223 (56.6%) | 99 (25%) | 72 (18.3%) | |
| Mean age (years) | 36.6 | 37.1 | 37 | 0.24 |
| Number of previous attempt | 3.3 | 3.3 | 3.3 | 0.29 |
| Years of infertility | 6.3 | 6.5 | 6.7 | 0.50 |
| Number of embryos previously transferred | 8.8 | 8.8 | 9.1 | 0.89 |
| Implantation rate (3 weeks) | 29% | 36% | 19% | 0.01 |
| Implantation rate (10 weeks) | 24.7% | 32% | 15% | 0.009 |
| Clinical PR at the first following ET (3 weeks) | 47.1% (105/223) | 55.6% (56/99) | 30.6% (22/72) | 0.005 |
| Ongoing PR at the first following ET (10 weeks) | 37.7% (84/223) | 48.5% (48/99) | 20.8% (15/72) | 0.001 |
| LBR at the first following ET | 36.8% (82/223) | 46.5% (46/99) | 19.4% (14/72) | 0.001 |
| Early miscarriage rates | 9% (21/223) | 8% (8/99) | 9.7% (7/72) | 0.42 |
RIF, Repeated embryo Implantation Failures; PR, Pregnancy Rate; ET, Embryo Transfer.
A P‐value <0.05 was considered statistically significant.
IL‐18/TWEAK mRNA ratios were elevated (>0.12) in 196 women compared to fertile controls. Among these 196 women, 45 also had an overexpressed (>3) IL‐15/Fn‐14 mRNA ratio, 123 a normal (0.3–3) ratio, and 28 a down‐regulated (<0.3) one. For 27 women, IL‐18/TWEAK ratio was normal, but all of them had an elevated IL‐15/Fn‐14 mRNA ratio (>3).
The objective of subsequent recommendations (Fig. 4) was to limit the activation of local immune cells and thereby avoid early embryo rejection or premature destruction of the endometrium, or both.
Figure 4.
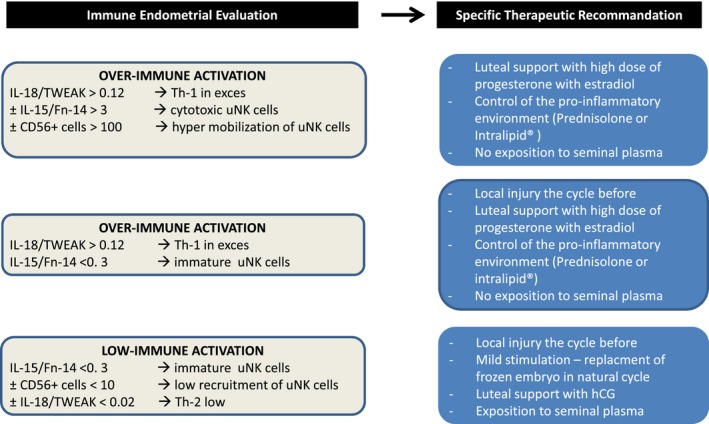
Personalization of treatment according to the endometrial immune profile. This figure summarizes the personalization of care recommended according to the uterine immune profile in the groups with low immune activation, over immune activation, and with or without immature uNK cells.
Recommendations included the following:
-
Modification of the predominantly Th1 endometrial environment
As first‐line treatment, we recommended supplementing treatment from day 3 of ovarian stimulation until the pregnancy test with prednisolone and vitamin E (an antioxidant, I g daily). Prednisolone (20 mg/day) was administered to 184 women diagnosed with immune overactivation. Women who became pregnant continued treatment with prednisolone until 8 weeks after ET and then slowly decreased it, stopping it completely at 10 weeks. Although prednisolone can mobilize NK cells, it is also able:
Corticotherapy is the leading medication worldwide for RIF, but we still lack precise indications for its use based on objective testing.22, 23
For 39 women, we replaced prednisolone with a single slow perfusion of 4% diluted Intralipid® (Fresenius‐Kabi) on days 8–10 of the cycle of embryo transfer. If pregnancy occurred, another slow perfusion was administered at 3 weeks and again at 7 weeks after ET. Intralipid® was proposed when prednisolone treatment was ineffective in normalizing the immune profile (14 patients) and for 25 patients who did not become pregnant with prednisolone. Although no placebo and randomized studies are available, slow perfusion of diluted Intralipid® has been reported to limit the hyperactivation of circulating NK cells and to regulate a Th1‐predominant cytokine balance.24, 25 No side‐effects were recorded during or after the slow perfusion, and all performed in hospital under medical supervision.
-
Adaptation of luteal hormonal support after ET
We recommended high daily vaginal doses of progesterone (1200 mg) for women with this profile. Beside its endocrine role, progesterone can influence the maternal immune system via progesterone‐induced blocking factor (PIBF), which inhibits NK cell activity,26 Th2‐dominant cytokine, and galectin‐1 production by maternal immune cells.27, 28
We also recommended oral estradiol supplementation (4 mg), because it decreases local expression of proinflammatory cytokines, especially for the IL‐18 system in the endometrium.29
Treatment began on the day of oocyte retrieval and continued until 8 weeks after ET in women who were pregnant.
-
Adaptation of mechanical local endometrial stimulation as a function of uNK cell recruitment and maturation
If uNK cell mobilization or activation was normal or high, we recommended the following:- Avoiding any local endometrial injury the cycle before the one during which ET was planned.
- Avoiding sexual intercourse after the ET. Seminal plasma has been shown to induce mobilization and activation by local maternal immune cells.30
If, however, uNK cell mobilization was low (<10 CD56+ cells/field) or if the IL‐15/Fn‐14 mRNA ratio was low, suggesting uNK immaturity (<0.3), we recommended endometrial scratching the cycle preceding ET and sexual intercourse after ET (see infra).
Low Immune Activation Profile
A low immune activation profile was observed for 99 women (25%). It was characterized by low IL‐15/Fn‐14 mRNA expression (reflecting possible the immaturity of uNK cells) or the absence of their mobilization (CD56+ cell count <10) and/or a very low local IL‐18/TWEAK mRNA ratio. We interpreted this profile to indicate a disturbance of the molecular mechanisms involved in effective adhesion and adequate angiogenesis.
A low IL‐18/TWEAK mRNA ratio (<0.02) was observed in 41% (41/99) of women; 84% of this group also had low IL‐15/Fn‐14 mRNA ratios.
The IL‐18/TWEAK mRNA ratio was normal in 58 women, but 42 (72%) had a low IL‐15/Fn‐14 mRNA ratio (<0.3) that suggested uNK cell immaturity. Finally, very low uNK cell recruitment (<10 CD56+ cells/field) was observed despite cell maturity (normal IL‐15/Fn‐14 mRNA ratio) in 28% (16/58).
Our hypothesis was that RIF resulted from the endometrium's inability to react appropriately to allow effective embryo apposition and adhesion. Because the endometrium is spontaneously anti‐adhesive, embryo adhesion is an active phenomenon that requires the expression of specific chemokines and adhesion molecules to enable embryo attachment. Expression of these adhesion molecules occurs only during the implantation window and depends on the migration and maturation of innate immune cells.31
We therefore recommended the following:
-
IL‐15 stimulation for women with a low IL‐15/Fn‐14 mRNA ratio.
Endometrial scratching or other local injury should be performed in the mid‐luteal phase of the cycle preceding the ET has been described to activate and stimulate subsequent expression of adhesion molecules and, interestingly, of IL‐15, via toll‐like receptor pathways.32
-
Supplementation of the luteal phase with human chorionic gonadotrophin (hCG)
We recommended supplementing the luteal phase with hCG 1500 IU subcutaneously administered 4, 6, and 8 days after oocyte retrieval, that is, during the implantation window. By activation of the mannose receptor, hCG triggers both proliferation and maturation of uNK cells.33 Physiologically produced by the embryo, hCG is known to be directly involved in the local reaction that induces immunological tolerance through adequate angiogenesis and activation of uNK cells at the maternal–fetal interface.34
Sexual intercourse after the ET
Studies of seminal plasma have highlighted its role in preparing for the acceptance of implantation by inducing expression of pro‐inflammatory cytokines and chemokines and the robust recruitment of immune cells.30, 35
Non‐Dysregulated Immune Profile
No endometrial immune dysregulation was observed in 18.3% (72/394) of the RIF cohort. These women underwent no specific personalized care.
Effect of Corticoids or Intralipids on the Endometrial Immune Profile
To assess the most appropriate treatment for the women with endometrial immune overactivation, effect of prednisolone (33 women) and Intralipid® (8) on the immune biomarkers was investigated (Fig 5 and Table 3).
Figure 5.
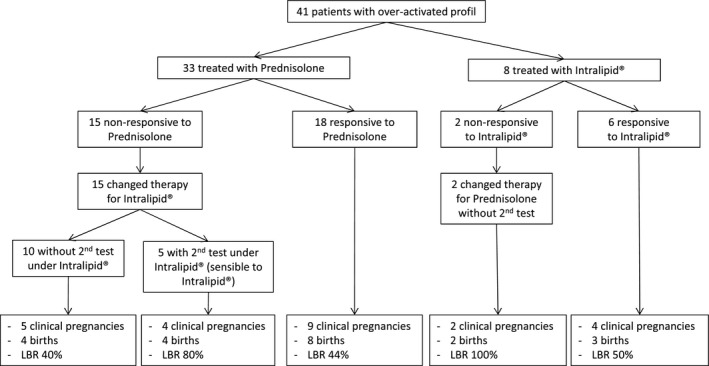
Adaptation of therapy in patients with an overactivated immune profile. Forty‐one women with an overactivated immune profile were first treated with prednisolone or Intralipid® and had another biopsy during the treatment to evaluate its effects on their immune profile. Patients responsive to prednisolone (18 cases) or Intralipid® (six cases) underwent ET with the same treatment in the following cycle. Treatment for women not responsive to prednisolone was changed to Intralipid®, and ET took place under Intralipid®. Inversely, treatment for women unresponsive to Intralipid® was changed to prednisolone, and ET took place under prednisolone.
Table 3.
Comparison of Immune Biomarkers Before and After Treatment in Women with Endometrial Immune Overactivation, According to Response to Treatment
| Number of patients | Initial evaluation | Second evaluation | P value (paired Wilcoxon test) | |
|---|---|---|---|---|
| Immune profiling for responsive patients | ||||
| CD56 cells count (10–100) | 24 | 50 | 43 | 0.82 |
| IL‐18/TWEAK (0.03–0.11) | 24 | 0.21 | 0.075 | 0.0001 |
| IL‐15/Fn‐14 (0.3–3) | 24 | 1.23 | 0.75 | 0.11 |
| Immune profiling for non‐responsive patients | ||||
| CD56 cells count (10–100) | 17 | 51 | 47 | 0.85 |
| IL‐18/TWEAK (0.03–0.11) | 17 | 0.18 | 0.20 | 0.16 |
| IL‐15/Fn‐14 (0.3–3) | 17 | 1.97 | 2.74 | 0.33 |
In 24 responsive patients, the overactivated immune profile was normalized under therapy (prednisolone or Intralipid®), and the IL‐18/TWEAK mRNA ratio decreased significantly (bold value). In 17 non‐responsive women (prednisolone or Intralipid®), the overactivated profile was not normalized, and the biomarkers did not change significantly. Data were analyzed with a nonparametric paired Wilcoxon test. A P‐value <0.05 was considered statistically significant.
Based on the norms defined above in the fertile control group, we studied response to prednisolone or Intralipid® according to whether they induced biomarkers in the normal range. More specifically, we expected the ratios of IL‐18/TWEAK mRNA and IL‐15/Fn‐14 mRNA to fall for women whose ratios had been elevated in the initial evaluation. This reduction would suggest that the drug had a positive effect and was able to restore an immune equilibrium suitable for embryo implantation.
Accordingly, after the first biopsy and before a new ET, 33 women with documented immune overactivation and treated with prednisolone (20 mg daily) and vitamin E underwent a second biopsy. The immune profiles normalized for 18 (54.5%) under prednisolone and did not for 15. Among the 18 patients who responded to prednisolone, the LBR reached 44% (8/18). The 15 patients who did not respond to prednisolone switched to Intralipid® during the following cycle; a normalized profile showed that five responded to Intralipid®. More importantly, the LBR in this subgroup of women who changed treatments reached 53.3% (8/15).
Eight patients were evaluated after a slow perfusion of Intralipid® and vitamin E: The profile was normalized for six. Three of these six patients had live births, and one a miscarriage. The two women who did not respond to Intralipid® became pregnant after prednisolone treatment.
Figure 5 summarizes the choice of therapy as a function of the course of the immune parameters and the subsequent LBR among the 41 patients with a test assessing the efficacy of their treatment (and then changed treatment). Among them, 51.2% (21/41) gave birth to a live baby, compared with 33.5% (61/182) of those without the follow‐up immune profiles.
Table 3 describes the trends in the immune biomarkers evaluated initially and then after treatment in the women who did and did not respond to them.
Outcome: Pregnancy, Miscarriage, and LBR
In the 6 months after the initial endometrial immune evaluation, 325 (82.5%) women with RIF had fresh embryos transferred, 53 received (13.5%) frozen‐thawed embryos, and 16 (4.1%) became pregnant spontaneously. As reported above, 81.7% (322/394) had apparently dysregulated endometrial immune profiles (Table 2), and all received personalized treatment.
Women with endometrial immune overactivation
The profile showed overactivation for 56% of the women with RIF (n = 223). The personalized strategy to reduce it produced a LBR of 36.8% (82/223) at the following ET: For 184 women, prednisolone was given, and for 39, slow perfusion of Intralipid®; all women received vitamin E and high dose of progesterone for luteal support.
The LBR with prednisolone treatment was 35.3% (65/184), with an early miscarriage rate of 10.3% (19/184). The LBR under Intralipid® was 43.6% (17/39), and the early miscarriage rate 5.1% (2/39) (Table 4).
Table 4.
Detailed Outcome of Women with RIF Patient and an Overactivated Immune Profile under Prednisolone and Intralipid® Treatment
| Therapy | Predisolone | Intralipid® |
|---|---|---|
| Number of patients | 184 | 39 |
| Clinical PR at the first following ET (3 weeks) | 46.2% (85/184) | 51.3% (20/39) |
| Ongoing PR at the first following ET (10 weeks) | 35.9% (66/184) | 46.2% (18/39) |
| LBR at the first following ET | 35.3% (65/184) | 43.6% (17/39) |
| Early miscarriage rate | 10.3% (19/184) | 5.1% (2/39) |
RIF, Repeated embryo Implantation Failures; PR, Pregnancy Rate; ET, Embryo Transfer.
Women with low endometrial immune activation profiles
Low immune activation was diagnosed in 25% of the RIF cohort (99/394). The strategy applied (i.e., local injury, luteal support with hCG, as well as sexual intercourse after the ET) produced a LBR of 46.5% (46/99), with an early miscarriage rate of 8% (8/99).
Women with non‐dysregulated profiles
In 18.3% of the cases, no obvious immune endometrial dysregulation was diagnosed. The LBR was significantly lower in the group, at 19.4% (14/72). This finding suggests that other parameters related to the oocyte, the spermatozoon, or the embryo itself affected IVF success. The early miscarriage rate was 9.7% (7/72).
Women with spontaneous pregnancy
Interestingly, in this RIF cohort, 16 women (4%) became pregnant spontaneously, and 14 of them (87.5%) had dysregulated local immune profiles. Seven had low local immune activation and were pregnant following the cycle after the endometrial biopsy. All had either low IL‐15 expression or low uNK cell recruitment, or both. As an endometrial biopsy performed in the mid‐luteal phase has been reported to trigger IL‐15 and uNK cell recruitment, the occurrence of a spontaneous pregnancy following the cycle after the local injury seems understandable.
Seven of the spontaneously pregnant women had endometrial immune overactivation; only one pregnancy ended in miscarriage. In all cases, the pregnancy followed a test cycle during which treatment to reduce the immune overactivation had begun (prednisolone in five cases and Intralipid® in two other cases), thus suggesting positive response to treatment. Finally, two women with normal immune profiles became pregnant spontaneously and gave birth; we have no explanation for these pregnancies.
Impact of maternal age on the outcome
Although 36% (143/394) of the RIF patients gave birth at the ET after their first personalized treatment, age still played, as expected, a clear role. The RIF cohort included 92 women younger than 35 years, 150 aged 35–38 years, 85 aged 39 or 40 years, and 67 older than 40 (41–43). The LBR was 45.6% for those younger than 35 years and 44.6% for those aged 36–38 years, but it decreased significantly to 29.4% for those aged 39 or 40 and reached its lowest rate of 16.4% in RIF patients older than 40 (P < 0.001).
Discussion
Approximately 70–80% of human ETs fail to implant successfully. Many factors may contribute to this failure, but most often it is attributed to poor uterine receptivity combined with poor oocyte quality. Generating good quality embryos is thus an absolute necessity for live births, but so is their transfer into a receptive uterus. The endometrial immune reaction occurring in humans during the implantation window, that is, at the time when the uterus must receive the embryo, is unique and crucial for adequate placentation. Uterine NK cells play an important role in building a healthy placenta by inducing local secretion of angiogenic factors by endometrial cells and modifying the structure of the pre‐existing spiral arteries.4, 36 uNK cells are very different from circulating NK cells—by their phenotype, their repertoire of activating and inhibiting receptors, the cytokines they secrete, and their low cytotoxic potential. In physiological conditions, uNK cells are not spontaneously cytotoxic; rather, their main biological functions are to produce angiogenic and immunotrophic cytokines (IL‐18, TGF beta, IL‐10, etc.), which promote placental growth. However, in a Th1‐dominant environment, uNK become killer cells able to recognize trophoblastic cells as non‐self and reject them. Moreover, the uNK cells are not alone in the endometrium: In a high Th1 environment, dendritic and Treg cells initially helper cells may also become real killers.37, 38
An orchestrated, balanced local immune biological reaction is required during the mid‐luteal phase to enable the active step of embryo attachment but also to regulate the invasion phase. Here, we studied whether the determination of the uterine immune profile with our defined biomarkers and appropriate personalized treatment may improve the success rates after ET in a prospective cohort of 394 women with RIF. Our first objective was to determine whether the uterine environment was favorable to implantation or dysregulated and thus apt to play a role in RIF. We defined a specific combination of biomarkers, including the ratios of endometrial IL‐18/TWEAK mRNA and IL‐15/Fn‐14 mRNA and the CD56+ cell count. The IL‐18/TWEAK mRNA ratio is used to document the local cytotoxic/angiogenic equilibrium, and the IL‐15/Fn‐14 mRNA ratio to assess the state of activation and maturation of uNK cells, while the CD56+ cell count simultaneously measures their mobilization.
In this cohort with its long experience of previous implantation failure, we report immune dysregulation liable to impair the implantation process in more than 80% of the cases. In 56%, the putative mechanism was local immune overactivation, and in 25%, the reverse: Too low a level of immune activation induced a local lack of angiogenic activity among the local innate immune cells. We used these findings to provide the personalized treatment and recommendations cares to modulate local immunity activity, either by reducing it (in case of overactivation) or by activating it (in case of low activation). We then evaluated the effect by measuring the subsequent LBR and the quality of these live births (gestational age and weight). We report a LBR of 39.8% (128/322) at the subsequent ET when dysregulation was diagnosed and treatment personalized. In this cohort of relatively old women (45% were older than 38 years) with a long history of infertility and RIF, the LBR expected at the subsequent ET without any specific intervention was at most 20% per transfer. Thus, our results suggest that this approach can almost double the expected LBR and that these babies of women with RIF had normal birth weights and gestational ages.
Here, we propose a diagnosis based on the assessment of uterine and not circulating NK cells exploration, and we explore both the positive and negative roles of these uNK cells in a large cohort followed up through birth. uNK cells are not the only immune cells present in the endometrium at the time of implantation: Other endometrial immune cells may also be activated to produce negative effects in a dominantly Th1 endometrial environment. Dendritic cells may differentiate into deleterious DC‐1 cells and not into the more helpful DC‐2 cells needed,39 and T cells may differentiate into deleterious Th17 lymphocytes rather than into the Treg cells required for pregnancy.40
We also assume that personalization of treatment when immune dysregulation is diagnosed also seems essential. We showed that prednisolone was able to normalize endometrial immune biomarkers in only 54% of the subgroup tested, while it failed to normalize or even worsened local imbalance in 46%. In other models, prednisolone has been shown to decrease the level of Th1 cytokines while promoting the expression of Th2 cytokines.21 Thus, we may postulate that the local action observed in women responsive to prednisolone is consistent with knowledge accumulated about this drug over the years. Nevertheless, its interaction with uNK cells and locally secreted IL‐15 appears much more complex than expected.41 In cases of obvious lack of response, other treatments seem to be necessary to control the immune overactivation.
In this context, we began proposing a slow perfusion of Intralipid® as an alternative for women who either failed to become pregnant with prednisolone or whose immune profile remained dysregulated with it. The safety of Intralipid® during the initial period of implantation and during pregnancy has been indirectly evaluated, insofar as Intralipid® is the diluent of the principal anesthetic drugs, the propofol used during general anesthesia for oocyte retrieval and during surgery for pregnancy.42 Almost nothing is known about its exact action on the endometrium during the implantation window. Surprisingly, while some media advertising suggests that it is currently used mainly in the UK and USA, almost no studies are available. Slow perfusion of Intralipid® has been proposed as a low‐cost alternative to immunotherapy in women with previous implantation failure and documented high levels of circulating cytotoxic NK cells.24, 25 No randomized study has yet tested placebos vs Intralipid®. The reported utility of this old and unexplored drug led Shreeve and Sadek to recommended controlled, large‐scale confirmatory studies to test its efficacy.43 Although randomized placebo‐controlled clinical trials are needed to confirm the results presented here, the follow‐up of pregnancies and births in our RIF cohort certainly indicates that the personalized treatments that we proposed have no deleterious effects on newborns. Moreover, the LBR in women whose endometrial immune profiles were normalized by treatment with prednisolone or Intralipid® strongly suggests that these treatments have a specific effect on the endometrial immune profile.
In conclusion, for the first time we used endometrial immune biomarkers to determine the endometrial immune profile at the time of uterine receptivity to help physicians in women with unexplained RIF. In a large cohort of these women, the LBR reached 40% among the 81.5% of women diagnosed with a form of immune dysregulation, so the result was perceived as encouraging. Based on the observational cohort study presented here, two controlled studies as well as a prospective randomized study (NCT‐02262117) are ongoing.
Supporting information
Table SI. Primers sequences used in quantitative RT‐PCR.
Acknowledgments
We first thank the patients who agreed to the retrospective collection of their pregnancy outcomes. We also want to thank the physicians who agreed to participate in this procedure: Alexandre F, Alvarez S, Aubriot FX, Ayel JP, Bailly M, Benifla JL, Bidet M, Bstandig B, Bouret D, Bulwa S, Cedrin I, Cornelie C, Chelly C, Chevalier N, Cornet D, Creux H, Delvigne A, Delaroche L, Descat E, Douard S, Elbaze R, Farges F, Fari M, Godefroy A, Grossetti A, Gout C, Frydman R, Herve F, Jacquesson L, Kerbrat G, Larue L, Lechat X, Lenoble C, Levy G, Massari A,Mathieu‐D'Argent E, Nathan C, Neuraz A, Oger P, Olivennes F, Parneix I, Patillot A, Schumacker A, Roubach AC, Rodrigue M, Teboul J, Divry V, Zitoun P. We particularly thank physicians of the reproductive unit of the hospital Les Bluets (Paris) for their enthusiasm and support. Finally, we also want to thank Henri Hamon and the Medifirst team who adapted the secure online medical file for infertility to include the new data provided by the immune profile and the information support needed to extract the relevant data and generate the reports.
Lédée N, Petitbarat M, Chevrier L, Vitoux D, Vezmar K, Rahmati M, Dubanchet S, Gahéry H, Bensussan A, Chaouat G. The uterine immune profile may help women with repeated unexplained embryo implantation failure after in vitro fertilization. Am J Reprod Immunol 2016; 75: 388–401
References
- 1. Teklenburg G, Salker M, Heijnen C, Macklon NS, Brosens JJ: The molecular basis of recurrent pregnancy loss: impaired natural embryo selection. Mol Hum Reprod 2010; 16:886–895. [DOI] [PubMed] [Google Scholar]
- 2. Gellersen B, Brosens IA, Brosens JJ: Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 2007; 25:445–453. [DOI] [PubMed] [Google Scholar]
- 3. Loke YW, King A, Burrows TD: Decidua in human implantation. Hum Reprod 1995; 10(Suppl. 2):14–21. [DOI] [PubMed] [Google Scholar]
- 4. Hanna J, Goldman‐Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson‐Yaron S, Prus D, Cohen‐Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O: Decidual NK cells regulate key developmental processes at the human fetal‐maternal interface. Nat Med 2006; 12:1065–1074. [DOI] [PubMed] [Google Scholar]
- 5. Wegmann TG, Lin H, Guilbert L, Mosmann TR: Bidirectional cytokine interactions in the maternal‐fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 1993; 14:353–356. [DOI] [PubMed] [Google Scholar]
- 6. Chaouat G: The Th1/Th2 paradigm: still important in pregnancy? Semin Immunopathol 2007; 29:95–113. [DOI] [PubMed] [Google Scholar]
- 7. Kitaya K, Yamaguchi T, Honjo H: Central role of interleukin‐15 in postovulatory recruitment of peripheral blood CD16(‐) natural killer cells into human endometrium. J Clin Endocrinol Metab 2005; 90:2932–2940. [DOI] [PubMed] [Google Scholar]
- 8. Eriksson M, Meadows SK, Wira CR, Sentman CL: Unique phenotype of human uterine NK cells and their regulation by endogenous TGF‐beta. J Leukoc Biol 2004; 76:667–675. [DOI] [PubMed] [Google Scholar]
- 9. Verma S, Hiby SE, Loke YW, King A: Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol Reprod 2000; 62:959–968. [DOI] [PubMed] [Google Scholar]
- 10. Croy BA, Esadeg S, Chantakru S, van den Heuvel M, Paffaro VA, He H, Black GP, Ashkar AA, Kiso Y, Zhang J: Update on pathways regulating the activation of uterine Natural Killer cells, their interactions with decidual spiral arteries and homing of their precursors to the uterus. J Reprod Immunol 2003; 59:175–191. [DOI] [PubMed] [Google Scholar]
- 11. Goldman‐Wohl DS, Ariel I, Greenfield C, Lavy Y, Yagel S: Tie‐2 and angiopoietin‐2 expression at the fetal‐maternal interface: a receptor ligand model for vascular remodelling. Mol Hum Reprod 2000; 6:81–87. [DOI] [PubMed] [Google Scholar]
- 12. Huang HY, Chan SH, Yu HT, Wang HS, Lai CH, Soong YK: Interleukin‐18 system messenger RNA and protein expression in human endometrium during the menstrual cycle. Fertil Steril 2006; 86:905–913. [DOI] [PubMed] [Google Scholar]
- 13. Hayakawa S, Nagai N, Kanaeda T, Karasaki‐Suzuki M, Ishii M, Chishima F, Satoh K: Interleukin‐12 augments cytolytic activity of peripheral and decidual lymphocytes against choriocarcinoma cell lines and primary culture human placental trophoblasts. Am J Reprod Immunol 1999; 41:320–329. [DOI] [PubMed] [Google Scholar]
- 14. Petitbarat M, Rahmati M, Serazin V, Dubanchet S, Morvan C, Wainer R, de Mazancourt P, Chaouat G, Foidart JM, Munaut C, Ledee N: TWEAK appears as a modulator of endometrial IL‐18 related cytotoxic activity of uterine natural killers. PLoS ONE 2011; 6:e14497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ledee‐Bataille N, Dubanchet S, Coulomb‐L'hermine A, Durand‐Gasselin I, Frydman R, Chaouat G: A new role for natural killer cells, interleukin (IL)‐12, and IL‐18 in repeated implantation failure after in vitro fertilization. Fertil Steril 2004; 81:59–65. [DOI] [PubMed] [Google Scholar]
- 16. Ledee‐Bataille N, Bonnet‐Chea K, Hosny G, Dubanchet S, Frydman R, Chaouat G: Role of the endometrial tripod interleukin‐18, ‐15, and ‐12 in inadequate uterine receptivity in patients with a history of repeated in vitro fertilization‐embryo transfer failure. Fertil Steril 2005; 83:598–605. [DOI] [PubMed] [Google Scholar]
- 17. Mas AE, Petitbarat M, Dubanchet S, Fay S, Ledee N, Chaouat G: Immune regulation at the interface during early steps of murine implantation: involvement of two new cytokines of the IL‐12 family (IL‐23 and IL‐27) and of TWEAK. Am J Reprod Immunol 2008; 59:323–338. [DOI] [PubMed] [Google Scholar]
- 18. Petitbarat M, Serazin V, Dubanchet S, Wayner R, de Mazancourt P, Chaouat G, Ledee N: Tumor necrosis factor‐like weak inducer of apoptosis (TWEAK)/fibroblast growth factor inducible‐14 might regulate the effects of interleukin 18 and 15 in the human endometrium. Fertil Steril 2009; 94:1141–1143. [DOI] [PubMed] [Google Scholar]
- 19. Eddy JL, Krukowski K, Janusek L, Mathews HL: Glucocorticoids regulate natural killer cell function epigenetically. Cell Immunol 2014; 290:120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moustaki A, Argyropoulos KV, Baxevanis CN, Papamichail M, Perez SA: Effect of the simultaneous administration of glucocorticoids and IL‐15 on human NK cell phenotype, proliferation and function. Cancer Immunol Immunother 2011; 60:1683–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elenkov IJ: Glucocorticoids and the Th1/Th2 balance. Ann N Y Acad Sci 2004; 1024:138–146. [DOI] [PubMed] [Google Scholar]
- 22. Lunghi L, Pavan B, Biondi C, Paolillo R, Valerio A, Vesce F, Patella A: Use of glucocorticoids in pregnancy. Curr Pharm Des 2010; 16:3616–3637. [DOI] [PubMed] [Google Scholar]
- 23. Boomsma CM, Keay SD, Macklon NS: Peri‐implantation glucocorticoid administration for assisted reproductive technology cycles. Cochrane Database Syst Rev 2012; 6:CD005996. [DOI] [PubMed] [Google Scholar]
- 24. Roussev RG, Acacio B, Ng SC, Coulam CB: Duration of intralipid's suppressive effect on NK cell's functional activity. Am J Reprod Immunol 2008; 60:258–263. [DOI] [PubMed] [Google Scholar]
- 25. Coulam CB, Acacio B: Does immunotherapy for treatment of reproductive failure enhance live births? Am J Reprod Immunol 2012; 67:296–304. [DOI] [PubMed] [Google Scholar]
- 26. Szekeres‐Bartho J, Par G, Dombay G, Smart YC, Volgyi Z: The antiabortive effect of progesterone‐induced blocking factor in mice is manifested by modulating NK activity. Cell Immunol 1997; 177:194–199. [DOI] [PubMed] [Google Scholar]
- 27. Szekeres‐Bartho J, Halasz M, Palkovics T: Progesterone in pregnancy; receptor‐ligand interaction and signaling pathways. J Reprod Immunol 2009; 83:60–64. [DOI] [PubMed] [Google Scholar]
- 28. Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo‐Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, Tirado I, Markert UR, Klapp BF, Poirier F, Szekeres‐Bartho J, Rabinovich GA, Arck PC: A pivotal role for galectin‐1 in fetomaternal tolerance. Nat Med 2007; 13:1450–1457. [DOI] [PubMed] [Google Scholar]
- 29. Ledee N, Dubanchet S, Lombroso R, Ville Y, Chaouat G: Downregulation of human endometrial IL‐18 by exogenous ovarian steroids. Am J Reprod Immunol 2006; 56:119–123. [DOI] [PubMed] [Google Scholar]
- 30. Robertson SA: Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res 2005; 322:43–52. [DOI] [PubMed] [Google Scholar]
- 31. Singh H, Aplin JD: Adhesion molecules in endometrial epithelium: tissue integrity and embryo implantation. J Anat 2009; 215:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gnainsky Y, Granot I, Aldo PB, Barash A, Or Y, Schechtman E, Mor G, Dekel N: Local injury of the endometrium induces an inflammatory response that promotes successful implantation. Fertil Steril 2010; 94:2030–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kane N, Kelly R, Saunders PT, Critchley HO: Proliferation of uterine natural killer cells is induced by human chorionic gonadotropin and mediated via the mannose receptor. Endocrinology 2009; 150:2882–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perrier dS, Berndt S, Tsampalas M, Charlet‐Renard C, Dubois M, Bourgain C, Hazout A, Foidart JM, Geenen V: Dialogue between blastocyst hCG and endometrial LH/hCG receptor: which role in implantation? Gynecol Obstet Invest 2007; 64:156–160. [DOI] [PubMed] [Google Scholar]
- 35. Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA: Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod 2007; 13:491–501. [DOI] [PubMed] [Google Scholar]
- 36. Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, Croy BA: Assessment of requirements for IL‐15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol 2003; 171:2937–2944. [DOI] [PubMed] [Google Scholar]
- 37. Hanna J, Mandelboim O: When killers become helpers. Trends Immunol 2007; 28:201–206. [DOI] [PubMed] [Google Scholar]
- 38. Blois SM, Klapp BF, Barrientos G: Decidualization and angiogenesis in early pregnancy: unravelling the functions of DC and NK cells. J Reprod Immunol 2011; 88:86–92. [DOI] [PubMed] [Google Scholar]
- 39. Tirado‐Gonzalez I, Barrientos G, Freitag N, Otto T, Thijssen VL, Moschansky P, von Kwiatkowski P, Klapp BF, Winterhager E, Bauersachs S, Blois SM: Uterine NK cells are critical in shaping DC immunogenic functions compatible with pregnancy progression. PLoS ONE 2012; 7:e46755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sharma S: Natural killer cells and regulatory T cells in early pregnancy loss. Int J Dev Biol 2014; 58:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perez SA, Mahaira LG, Demirtzoglou FJ, Sotiropoulou PA, Ioannidis P, Iliopoulou EG, Gritzapis AD, Sotiriadou NN, Baxevanis CN, Papamichail M: A potential role for hydrocortisone in the positive regulation of IL‐15‐activated NK‐cell proliferation and survival. Blood 2005; 106:158–166. [DOI] [PubMed] [Google Scholar]
- 42. Jarahzadeh MH, Jouya R, Mousavi FS, Dehghan‐Tezerjani M, Behdad S, Soltani HR: Propofol or Thiopental sodium in patients undergoing reproductive assisted technologies: differences in hemodynamic recovery and outcome of oocyte retrieval: a randomized clinical trial. Iran J Reprod Med 2014; 12:77–82. [PMC free article] [PubMed] [Google Scholar]
- 43. Shreeve N, Sadek K: Intralipid therapy for recurrent implantation failure: new hope or false dawn? J Reprod Immunol 2012; 93:38–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Primers sequences used in quantitative RT‐PCR.


