Summary
Background
In a phase 2 study, mongersen, an oral antisense oligonucleotide targeting Smad7, was effective in inducing clinical remission in approximately 60% of patients with active Crohn's disease (CD).
Aim
In a post hoc analysis to evaluate those patient disease characteristics that may have influenced the efficacy and safety of mongersen therapy.
Methods
Patients with steroid‐dependent/resistant, active CD were randomised to mongersen 10, 40 or 160 mg/day or placebo for 2 weeks; patients were followed for 10 weeks. Clinical remission [Crohn's Disease Activity Index (CDAI) score <150] and clinical response (CDAI score reduction ≥100 points) were assessed at weeks 2, 4 and 12 for these subgroups: disease duration <5/≥5 years, human serum C‐reactive protein (hsCRP) <3/≥3 mg/L, and CDAI at baseline ≤260/>260. Additional patient baseline and disease characteristics were explored.
Results
Clinical remission and response rates were significantly higher in patients receiving mongersen 40 and 160 mg/day but not 10 mg/day vs. placebo and independent of disease duration and hsCRP. Patients with baseline CDAI ≤260 had significantly higher remission rates with 40 and 160 mg/day. In patients with baseline CDAI >260, remission rates were statistically greater with 160 mg/day and numerically better with 40 mg/day vs. placebo. Adverse event rates were similar across treatment groups. Mongersen was safe and well tolerated.
Conclusions
Patients with higher CDAI scores achieved clinical remission most frequently with the highest mongersen dose. Disease duration and baseline human serum C‐reactive protein did not appear to significantly impact efficacy of mongersen in this study (EudraCT Number: 2011‐002640‐27.)
Introduction
Crohn's disease (CD) is a chronic condition characterised by a segmental, transmural inflammation, which can affect any part of the alimentary tract, even though lesions are more common in the terminal ileum and right colon. CD‐related inflammation is responsible for a variety of symptoms/signs and development of local complications and extraintestinal manifestations.1, 2
Smad7, an intracellular protein that binds TGF‐β receptor and prevents TGF‐β1‐associated and SMAD‐associated signalling in CD, has been identified as a novel target for suppression of CD‐associated inflammation.3 In a phase 1 trial, mongersen (GED‐0301), an oral formulation containing the Smad7 antisense oligonucleotide, demonstrated a rapid and durable clinical benefit in patients with active CD, confirming the role of Smad7 in the inflammatory process of CD.4, 5 The results of a subsequent phase 2 study of mongersen in patients with active CD (IGON1 study) were recently reported.6 A marked treatment effect was observed in this study, with clinical remission rates [Crohn's Disease Activity Index (CDAI) <150 points at week 2 and maintained for 2 weeks] in 55% and 65% of patients treated with 40 and 160 mg/day, respectively, for 2 weeks.6
Subgroup analyses from prospective, randomised, controlled trials in patients with active CD indicate that various patient disease characteristics and demographics can affect clinical outcomes.7, 8, 9, 10, 11, 12 In particular, patients with a shorter duration of CD achieve greater clinical benefit compared with patients with longer disease duration, and treatment with biologics and/or immunosuppressive drugs early in duration of CD leads to higher steroid‐free clinical remission rates.13 Similarly, greater CD activity at baseline may negatively influence the efficacy of established therapy.10, 11, 12, 14
Because these and a number of other patient disease characteristics and demographics may impact patient outcomes, we retrospectively explored the impact of duration and disease activity of CD along with sex, body mass index, smoking status, history of CD‐related intestinal resection, steroid status and immunosuppressant use at baseline on the efficacy and safety of mongersen treatment.
Materials and methods
Study design
Data used for this post hoc analysis were from IGON1,6 a previously reported, multicentre, randomised, double‐blind, placebo‐controlled study of mongersen (10, 40, or 160 mg/day) in patients with active CD (CDAI score of 220–400) who were steroid‐dependent and/or steroid‐resistant, as defined by recent guidelines of the European Crohn's and Colitis Organisation,15 and had inflammatory lesions in the terminal ileum and/or right colon.
The study protocol for IGON1 was approved by the Institutional Review Board or Ethics Committee at each of the 17 study centres in Italy and Germany. Written informed consent was obtained from patients before they underwent screening for eligibility; eligible patients underwent randomisation between September 2011 and June 2013 (EudraCT number: 2011‐002640‐27).
Patient sample and clinical assessments
For this analysis, patients were divided into the following subgroups: disease duration since diagnosis at baseline <5 years or ≥5 years, normal (<3 mg/L) or abnormal (≥3 mg/L) human serum C‐reactive protein (hsCRP) value at baseline, and CDAI ≤260 or >260 at baseline. The CDAI categories were selected in order to have a sufficient distribution of patients in each group for a meaningful analysis and to differentiate patients with moderate disease from those with more severe disease. The primary analysis for these subgroups was the intent‐to‐treat population, defined as all randomised patients, regardless of response status, who received at least one dose of study drug. The percentages of patients with CDAI <150 (clinical remission) and those achieving clinical response (CDAI decrease by 100 points compared with baseline) were evaluated for mongersen and placebo patients in each disease duration and severity subgroup at weeks 2, 4 and 12. Additional categories evaluated included male or female, body mass index <25 or ≥25 kg/m2, smoking status, history of CD‐related intestinal resection, steroid status (refractory or dependent) and immunosuppressant use. Safety data were analysed according to the same classes of disease duration and severity for all patients who received at least one dose of study drug.
Statistical analysis
Baseline characteristics among the subgroups were compared by means of descriptive methods. In the analysis of clinical remission/response, patients who dropped out or had missing data were considered nonresponders. Within each disease duration and severity subgroup, the fractions of patients achieving clinical remission or response were compared between each mongersen dose and placebo at each time point using an unconditional exact test. All treatment comparisons used two‐sided tests, and statistical significance was set at P < 0.05. Logistic regression analyses, along with receiver operating characteristic analyses, were performed to evaluate the impact of each of the subgroup variables as a continuous variable on clinical remission and response.
Results
Baseline demographics and clinical characteristics by disease duration and severity
Of the 166 patients assigned to receive one of three mongersen doses or placebo, six had protocol violations and missing data at week 2 and were classified as nonresponders. The remaining 160 patients (96.4%) completed the 2‐week treatment and 4‐week study visit, while 138 patients (83.1%) completed the 12‐week study visit. The reasons for patients withdrawing from the trial after week 4 have been reported previously.6 Table 1 summarises the baseline demographics and disease characteristics of the patients according to disease duration, defined as time since CD diagnosis, baseline hsCRP value and CDAI score. Baseline mean age, sex, mean body mass index and smoking status were similar across the six subgroups. The rate of CD‐related surgery was greater in the subgroups of patients with disease duration ≥5 years [60/96 (62.5%) vs. 10/69 (14.5%); P < 0.0001] and patients with hsCRP <3 mg/L [39/64 (60.9%) vs. 32/102 (31.4%); P = 0.0002].
Table 1.
Demographics and clinical characteristics of the participants to IGON1 study in relation to disease duration, baseline hsCRP value and baseline CDAI score
| Disease duration | hsCRP | CDAI | ||||
|---|---|---|---|---|---|---|
| <5 years | ≥5 years | <3 mg/L | ≥3 mg/L | ≤260 | >260 | |
| Patients, n (%)* | 69/166 (41.6) | 96/166 (57.8) | 64/166 (38.6) | 102/166 (61.4) | 99/166 (59.6) | 67/166 (40.4) |
| Age, mean (years) | 39.5 | 44.6 | 43.0 | 42.3 | 44.3 | 39.9 |
| Female, n (%) | 34/69 (49.3) | 50/96 (52.1) | 34/64 (53.1) | 51/102 (50.0) | 47/99 (47.5) | 38/67 (56.7) |
| BMI, mean (kg/m2) | 23.6 | 22.8 | 22.1 | 23.9 | 23.8 | 22.3 |
| Current smoker, n (%) | 25/69 (36.2) | 36/96 (37.5) | 23/64 (35.9) | 39/102 (38.2) | 38/99 (38.4) | 24/67 (35.8) |
| History of CD‐related intestinal resection, n (%) | 10/69 (14.5) | 60/96 (62.5) | 39/64 (60.9) | 32/102 (31.4) | 37/99 (37.4) | 34/67 (50.7) |
*Disease duration data were missing for one patient.
Clinical remission
Overall proportions of patients with clinical remission (defined as CDAI <150) were significantly higher with mongersen 160 and 40 mg/day compared with placebo, and this was evident for each disease duration subgroup at weeks 2, 4 and 12 (Figure 1a and b). Similar remission rates between mongersen 160 and 40 mg/day or between mongersen 10 mg/day and placebo were observed (Figure 1a and b).
Figure 1.

Clinical remission, defined as a Crohn's Disease Activity Index (CDAI) score <150, at weeks 2, 4 and 12 by disease duration <5 years (a) or ≥5 years (b) for all randomised patients (intent‐to‐treat population) in a post hoc analysis from IGON1,6 a previously reported, multicentre, randomised, double‐blind, placebo‐controlled study of mongersen (10, 40, or 160 mg/day) in patients with active Crohn's disease (baseline CDAI score 220–400). *P ≤ 0.02; † P ≤ 0.0006; ‡ P = 0.002 vs. placebo.
The week 2 remission rate in patients treated with mongersen 160 mg/day was greater than that seen in placebo patients, and this was evident in both subgroups of patients with either normal or abnormal hsCRP value at baseline (Figure 2a and b). At the same time point, the remission rate for the mongersen 40 mg/day patients with normal baseline hsCRP values was significantly different compared with placebo. While the remission rates in patients with abnormal hsCRP values were numerically greater than placebo (50% vs. 24%, respectively) at week 2, the results were not significantly different (Figure 2a and b). Compared with placebo, significantly greater percentages of patients treated with mongersen 160 or 40 mg/day, but not 10 mg/day, achieved clinical remission in the subgroups of patients with either normal or abnormal hsCRP value at baseline, and this was evident at weeks 4 and 12 (Figure 2a and b).
Figure 2.
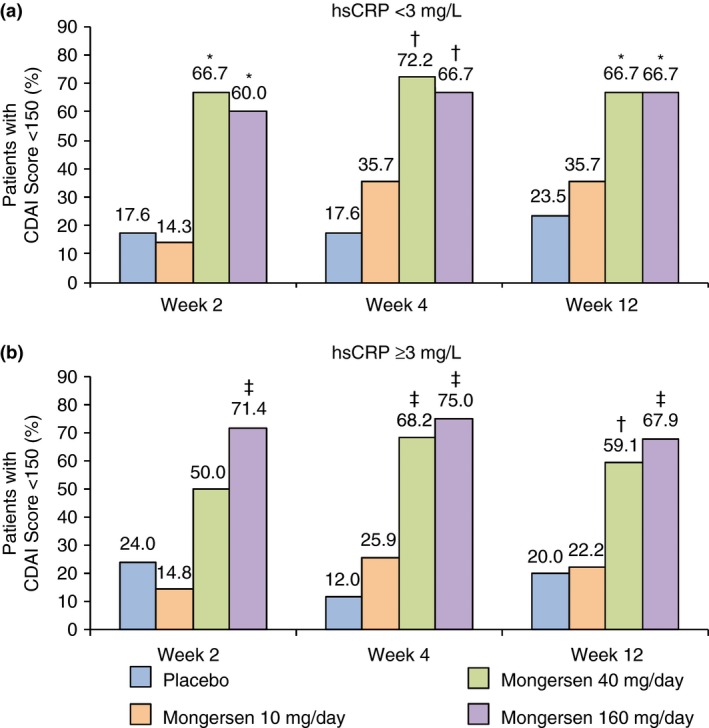
Clinical remission, defined as a Crohn's Disease Activity Index (CDAI) score <150, at weeks 2, 4 and 12 by baseline human serum C‐reactive protein (hsCRP) values <3 mg/L (a) or ≥3 mg/L (b) for all randomised patients (intent‐to‐treat population) in a post hoc analysis from IGON1.6 *P < 0.02; † P < 0.006; ‡ P ≤ 0.0005 vs. placebo.
In each subgroup of patients with CDAI score ≤260 or >260, patients treated with mongersen 160 mg/day had significantly greater remission rates compared with placebo patients, and this was evident at each time point (Figure 3a and b). Moreover, at each time point, in the subgroup of patients with CDAI ≤260, rate of remission was significantly greater in the mongersen 40 mg/day group than in the placebo group (Figure 3a), while in the subgroup of patients with CDAI >260, the difference between the mongersen 40 mg/day and placebo groups was evident only at week 4 (Figure 3b). Among the other patient characteristics and demographics evaluated, differences in clinical remission were seen in the mongersen 160 mg/day group compared with the placebo group at all time periods, regardless of subgroup, except in current smokers and males (nonsignificant at week 2) who had greater remission rates.
Figure 3.
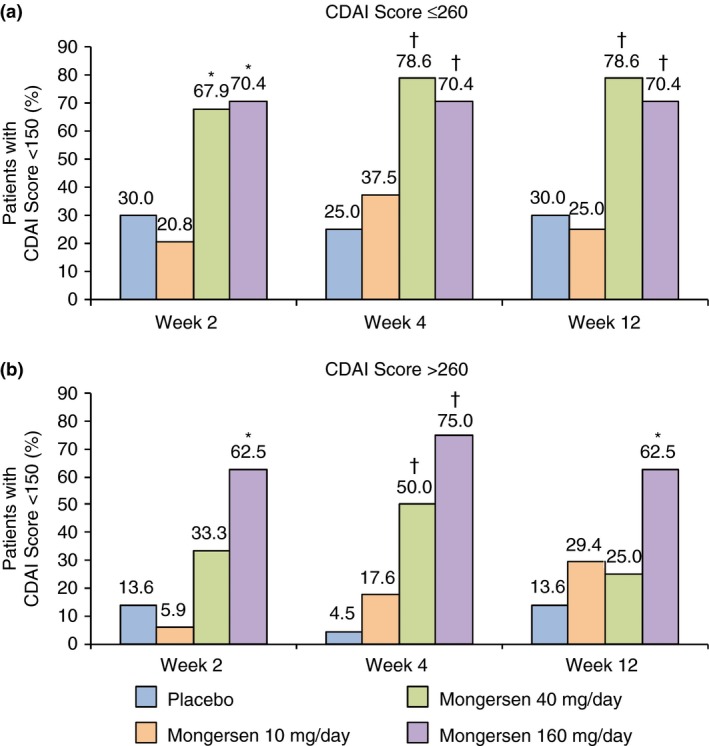
Clinical remission, defined as a Crohn's Disease Activity Index (CDAI) score <150, at weeks 2, 4 and 12 by baseline Crohn's Disease Activity Index (CDAI) scores ≤260 (a) or >260 (b) for all randomised patients (intent‐to‐treat population) in a post hoc analysis from IGON1.6 *P ≤ 0.01; † P ≤ 0.001 vs. placebo.
Clinical response
The overall pattern of results for clinical response‐100 rates by disease duration, baseline hsCRP value, and baseline CDAI score followed the same pattern as the results for clinical remission. However, in contrast to the clinical remission findings, greater response rates were seen for both the mongersen 160 and 40 mg/day dose groups, compared with the placebo group, in each subgroup of patients with CDAI score ≤260 or >260. Clinical response rates at weeks 2, 4 and 12 as a function of disease duration, baseline hsCRP and CDAI score are shown in Table 2.
Table 2.
Proportion of patients achieving a clinical response at weeks 2, 4 and 12 as a function of disease duration, baseline hsCRP value and baseline CDAI score
| Disease duration | hsCRP | CDAI | ||||
|---|---|---|---|---|---|---|
| <5 years | ≥5 years | <3 mg/L | ≥3 mg/L | ≤260 | >260 | |
| Clinical response at 2 weeks | ||||||
| Placebo, n (%) | 6/16 (37.5) | 5/26 (19.2) | 3/17 (17.6) | 8/25 (32.0) | 6/20 (30.0) | 5/22 (22.7) |
| Mongersen 10 mg/day, n (%) | 3/12 (25.0) | 6/29 (20.7) | 3/14 (21.4) | 6/27 (22.2) | 3/24 (12.5) | 6/17 (35.3) |
| Mongersen 40 mg/day, n (%) | 9/22 (40.9) | 8/17 (47.1) | 12/18 (66.7)** | 6/22 (27.3) | 12/28 (42.9) | 6/12 (50.0) |
| Mongersen 160 mg/day, n (%) | 11/19 (57.9) | 17/24 (70.8)** | 11/15 (73.3)** | 17/28 (60.7)* | 14/27 (51.9) | 14/16 (87.5)*** |
| Clinical response at 4 weeks | ||||||
| Placebo, n (%) | 3/16 (18.8) | 4/26 (15.4) | 3/17 (17.6) | 4/25 (16.0) | 4/20 (20.0) | 3/22 (13.6) |
| Mongersen 10 mg/day, n (%) | 3/12 (25.0) | 12/29 (41.4)* | 6/14 (42.9) | 9/27 (33.3) | 8/24 (33.3) | 7/17 (41.2) |
| Mongersen 40 mg/day, n (%) | 14/22 (63.6)* | 8/17 (47.1)* | 10/18 (55.6)* | 13/22 (59.1)** | 16/28 (57.1)* | 7/12 (58.3)* |
| Mongersen 160 mg/day, n (%) | 14/19 (73.7)** | 17/24 (70.8)*** | 12/15 (80.0)** | 19/28 (67.9)*** | 17/27 (63.0)** | 14/16 (87.5)*** |
| Clinical response at 12 weeks | ||||||
| Placebo, n (%) | 4/16 (25.0) | 7/26 (26.9) | 5/17 (29.4) | 6/25 (24.0) | 6/20 (30.0) | 5/22 (22.7) |
| Mongersen 10 mg/day, n (%) | 3/12 (25.0) | 12/29 (41.4) | 6/14 (42.9) | 9/27 (33.3) | 6/24 (25.0) | 9/17 (52.9) |
| Mongersen 40 mg/day, n (%) | 13/22 (59.1)* | 14/17 (82.4)** | 13/18 (72.2)* | 15/22 (68.2)** | 21/28 (75.0)** | 7/12 (58.3)* |
| Mongersen 160 mg/day, n (%) | 12/19 (63.2)* | 19/24 (79.2)** | 13/15 (86.7)** | 18/28 (64.3)** | 17/27 (63.0)* | 14/16 (87.5)*** |
*P < 0.05; **P ≤ 0.005; ***P < 0.0001 vs. placebo.
Logistic regression
Logistic regression models confirmed that the baseline CDAI score, and not the disease duration or baseline hsCRP, influenced the likelihood of clinical remission with mongersen. Throughout the CDAI range, a higher probability of clinical remission at week 4 was demonstrated in patients in the 160 mg/day group compared with the other treatment groups in this model (Figure 4). The findings from the logistic regression models were corroborated with receiver operating characteristic analyses where the baseline CDAI score of 260 corresponded to the optimal cut‐off for prediction of clinical remission, in contrast to the baseline hsCRP and disease duration, which had no impact on clinical remission.
Figure 4.

Logistic regression model for clinical remission, defined as a Crohn's Disease Activity Index (CDAI) score <150, at week 4 by baseline CDAI score.
Adverse events by disease duration and severity
The overall rate of adverse events (AEs) did not differ between patients treated with mongersen and those receiving placebo. The numbers of patients who reported an AE and the type of AE have been previously described.6 Placebo patients tended to have greater rates of AEs in the subgroups with baseline hsCRP value >3 mg/L and baseline CDAI score >260 compared to the corresponding subgroups with hsCRP value <3 mg/L and CDAI score ≤260 (Table 3).
Table 3.
Total number of adverse events as a function of disease duration, baseline hsCRP value and baseline CDAI score
| Disease duration | hsCRP | CDAI | ||||
|---|---|---|---|---|---|---|
| <5 years | ≥5 years | <3 mg/L | ≥3 mg/L | ≤260 | >260 | |
| Placebo, n (%) | 10/16 (63) | 18/26 (69) | 7/17 (41) | 21/25 (84) | 10/20 (50) | 18/22 (82) |
| Mongersen 10 mg/day, n (%) | 5/12 (52) | 15/29 (52) | 7/14 (50) | 13/27 (48) | 8/24 (33) | 12/17 (71) |
| Mongersen 40 mg/day, n (%) | 12/22 (55) | 12/17 (71) | 11/18 (61) | 14/22 (64) | 17/28 (61) | 8/12 (67) |
| Mongersen 160 mg/day, n (%) | 12/19 (63) | 9/24 (38) | 4/15 (27) | 17/28 (61) | 15/27 (56) | 6/16 (38) |
Discussion
In this post hoc analysis of all participants in the IGON1 study, disease duration and baseline hsCRP did not appear to significantly impact the efficacy of mongersen. Additionally, significantly higher clinical remission and response rates were observed with mongersen 160 and 40 mg/day compared with the placebo group in most subgroups, while there was no difference between the mongersen 10 mg/day group and the placebo group. Overall, these statistical differences were seen at each time point and were not influenced by the disease duration. These findings differ from previous data generated from trials with infliximab, adalimumab and certolizumab in which shorter disease duration was significantly associated with greater likelihood to induce and maintain remission in CD.10, 11, 14, 16 The reason for such a different response to these treatments is unknown but could reflect the dominant expression of the pharmacologic target in the gut of patients because it is known that TNF production can change during CD course while Smad7 expression is persistently elevated in patients with early and established disease.3 In this context it is, however, noteworthy that the association between the disease duration and therapeutic efficacy of biologics in CD has been inconsistent.7, 9 For example, in infliximab‐resistant patients, no association was found between disease duration and induction of clinical remission with certolizumab at week 26 in the WELCOME study.17 Similarly, in the ENACT‐2 study, the effect of natalizumab on the rates of remission at 6 and 12 months was not affected by disease duration.
This analysis shows also that the efficacy of mongersen 160 and 40 mg/day treatment on clinical remission and clinical response did not appear to be significantly influenced by baseline hsCRP value as patients with either normal or abnormal hsCRP value responded equally to the drug. HsCRP is a biomarker of mucosal inflammation in CD, and patients with higher hsCRP values are more likely to benefit from therapy with biologics. The inclusion criteria limited the enrolment of patients to those with known disease only in the terminal ileum and/or right colon, and there is evidence that active inflammation in the terminal ileum is not necessarily accompanied by elevated hsCRP values, thus providing an explanation of why the baseline hsCRP value may not influence the therapeutic effect of mongersen. These findings suggest that baseline hsCRP may not be determinative in identifying patients who are responsive to mongersen, even though this point must be further addressed in larger clinical trials.
In the group of patients receiving mongersen 160 mg/day, there was no association between induction of clinical remission/response and baseline CDAI score, in contrast to the 40 mg/day dose, which demonstrated a higher remission rate in the subgroup of patients with baseline CDAI ≤260. These associations were confirmed by logistic regression analysis, which suggests that patients with greater CDAI may need the highest dose of the drug to achieve remission.
There are some limitations of the data presented. This was a post‐hoc analysis and patients enrolled in the IGON1 study were not randomised taking into account the disease duration and severity. The cut‐off selected to classify disease duration from the time of diagnosis in two major subgroups is similar to that proposed in other studies, but it is arbitrary and does not take into consideration the fact that some patients may be asymptomatic for long periods before being diagnosed with CD. The same criticism can be applied to the CDAI threshold adopted to differentiate patients with moderate (220‒260) vs. more severe disease (>260).
In summary, this study suggests that the clinical benefit seen in patients treated with mongersen 40 or 160 mg/day might not be influenced by disease duration and baseline hsCRP, while the mongersen 160 mg/day dose is necessary to control clinical activity in patients with more severe disease. These results are extremely useful for designing the future phase 3 trials with mongersen.
Authorship
Guarantor of the article: Giovanni Monteleone.
Author contributions: Giovanni Monteleone: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content and study supervision. Antonio Di Sabatino, Sandro Ardizzone, Francesco Pallone and Markus F. Neurath: enrolment of patients and critical revision of the manuscript for important intellectual content. Keith Usiskin, Xiaojiang Zhan and Guillermo Rossiter: analysis of data and critical revision of the manuscript for important intellectual content. All authors approved the final version of the article, including the authorship list.
Acknowledgments
Declaration of personal interests: Giovanni Monteleone is the holder of a patent for the use of Smad7 antisense oligonucleotides in Crohn's disease. Antonio Di Sabatino, Sandro Ardizzone and Francesco Pallone have no conflicts to declare. Keith Usiskin, Xiaojiang Zhan and Guillermo Rossiter are employees of Celgene Corporation. Markus F. Neurath provided expert advice to Giuliani SpA and received grant support for developing 6‐thio‐GTP analogues.
Declaration of funding interests: This work was supported by Giuliani SpA, Milan, Italy, acting under contract to Nogra Pharma. The authors received editorial support from Peloton Advantage, LLC, Parsippany, NJ, USA, funded by Celgene Corporation, Summit, NJ, USA.
This article was accepted for publication after full peer‐review.
References
- 1. Randall CW, Vizuete JA, Martinez N, et al From historical perspectives to modern therapy: a review of current and future biological treatments for Crohn's disease. Therap Adv Gastroenterol 2015; 8: 143–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boyapati R, Satsangi J, Ho GT. Pathogenesis of Crohn's disease. F1000Prime Rep 2015; 7: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF‐beta1 signaling in chronic inflammatory bowel disease. J Clin Invest 2001; 108: 601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Monteleone G, Fantini MC, Onali S, et al Phase I clinical trial of Smad7 knockdown using antisense oligonucleotide in patients with active Crohn's disease. Mol Ther 2012; 20: 870–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zorzi F, Calabrese E, Monteleone I, et al A phase 1 open‐label trial shows that smad7 antisense oligonucleotide (GED0301) does not increase the risk of small bowel strictures in Crohn's disease. Aliment Pharmacol Ther 2012; 36: 850–7. [DOI] [PubMed] [Google Scholar]
- 6. Monteleone G, Neurath MF, Ardizzone S, et al Mongersen, an oral Smad7 antisense oligonucleotide, and Crohn's disease. N Engl J Med 2015; 372: 1104–13. [DOI] [PubMed] [Google Scholar]
- 7. Parsi MA, Achkar JP, Richardson S, et al Predictors of response to infliximab in patients with Crohn's disease. Gastroenterology 2002; 123: 707–13. [DOI] [PubMed] [Google Scholar]
- 8. Reinisch W, Wang Y, Oddens BJ, Link R. C‐reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn's disease: a post‐hoc analysis from ACCENT I. Aliment Pharmacol Ther 2012; 35: 568–76. [DOI] [PubMed] [Google Scholar]
- 9. Arnott ID, McNeill G, Satsangi J. An analysis of factors influencing short‐term and sustained response to infliximab treatment for Crohn's disease. Aliment Pharmacol Ther 2003; 17: 1451–7. [DOI] [PubMed] [Google Scholar]
- 10. Vermeire S, Louis E, Carbonez A, et al Demographic and clinical parameters influencing the short‐term outcome of anti‐tumor necrosis factor (infliximab) treatment in Crohn's disease. Am J Gastroenterol 2002; 97: 2357–63. [DOI] [PubMed] [Google Scholar]
- 11. Schreiber S, Reinisch W, Colombel JF, et al Subgroup analysis of the placebo‐controlled CHARM trial: increased remission rates through 3 years for adalimumab‐treated patients with early Crohn's disease. J Crohns Colitis 2013; 7: 213–21. [DOI] [PubMed] [Google Scholar]
- 12. Sandborn WJ, Colombel JF, Panes J, et al Exploring the use of adalimumab for patients with moderate Crohn's disease: subanalyses from induction and maintenance trials. J Crohns Colitis 2013; 7: 958–67. [DOI] [PubMed] [Google Scholar]
- 13. Sandborn WJ, Colombel JF, D'Haens G, et al One‐year maintenance outcomes among patients with moderately‐to‐severely active ulcerative colitis who responded to induction therapy with adalimumab: subgroup analyses from ULTRA 2. Aliment Pharmacol Ther 2013; 37: 204–13. [DOI] [PubMed] [Google Scholar]
- 14. Schreiber S, Colombel JF, Bloomfield R, et al Increased response and remission rates in short‐duration Crohn's disease with subcutaneous certolizumab pegol: an analysis of PRECiSE 2 randomized maintenance trial data. Am J Gastroenterol 2010; 105: 1574–82. [DOI] [PubMed] [Google Scholar]
- 15. Van Assche G, Dignass A, Panes J, et al The second European evidence‐based consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. J Crohns Colitis 2010; 4: 7–27. [DOI] [PubMed] [Google Scholar]
- 16. D'Haens G, Baert F, Van Assche G, et al Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet 2008; 371: 660–7. [DOI] [PubMed] [Google Scholar]
- 17. Feagan BG, Sandborn WJ, Wolf DC, et al Randomised clinical trial: improvement in health outcomes with certolizumab pegol in patients with active Crohn's disease with prior loss of response to infliximab. Aliment Pharmacol Ther 2011; 33: 541–50. [DOI] [PubMed] [Google Scholar]


