Abstract
Addiction is characterized by maladaptive decision-making, a loss of control over drug consumption, and habit-like drug seeking despite adverse consequences. These cognitive changes likely reflect the effects of drugs of abuse on prefrontal cortical neurobiology. Here we review evidence that amphetamine and cocaine fundamentally remodel the structure of excitatory neurons in the prefrontal cortex. We summarize evidence in particular that these psychostimulants have opposing effects in the medial and orbital prefrontal cortices (“mPFC” and “oPFC,” respectively). For example, amphetamine and cocaine increase dendrite length and spine density in the mPFC, while dendrites are impoverished and dendritic spines are eliminated in the oPFC. We will discuss evidence that certain cytoskeletal regulatory proteins expressed in the oPFC and implicated in postnatal (adolescent) neural development also regulate behavioral sensitivity to cocaine. These findings potentially open a window of opportunity for the identification of novel pharmacotherapeutic targets in the treatment of drug abuse disorders in adults, as well as in drug-vulnerable adolescent populations. Finally, we will discuss the behavioral implications of drug-related dendritic spine elimination in the oPFC, with regards to reversal learning tasks and tasks that assess the development of reward-seeking habits, both used to model aspects of addiction in rodents.
Keywords: D-amphetamine, orbitofrontal, prelimbic, cingulate, infralimbic, review, adolescence, dependence, drug abuse, impulsivity
Introduction
Cocaine addiction is characterized by maladaptive decision-making, a loss of control over drug consumption, and habit-like drug seeking despite adverse consequences. These cognitive changes likely reflect the effects of repeated drug exposure on prefrontal cortical neurobiology that then further promote drug use (1–8). Rodents provide an excellent model system by which to characterize the neurobiology of psychostimulant exposure because like humans, rodents will readily self-administer drugs of abuse and engage in complex reward-related decision-making, as well as relapse-like behavior. Also as in humans, individual differences in behavioral response strategies can serve as phenotypic predictors of addiction-like behaviors, such as drug seeking following periods of abstinence and despite adverse consequences (9–10). Experimenter-administered, in addition to self-administered, drugs of abuse can also induce behavioral phenotypes in rodents that are relevant to addiction etiology in humans, e.g., increased propensity to engage in reward-seeking habits following cocaine exposure (11–16).
At least some of the behavioral effects of drug exposure might be due in part to alterations in the underlying structure of neurons. The estimated 100 billion neurons in the brain are organized by synaptic connections between neurons, the majority of which form on specialized protrusions on dendrites termed “dendritic spines.” Dendrites and dendritic spines can be remarkably plastic – for example, changes in spine structure and synaptic efficacy are thought to provide the cellular substrates of learning, memory, mood, and cognition. Further, research using animal models has repeatedly demonstrated that amphetamine and amphetamine-like psychostimulants such as cocaine structurally remodel dendrites and dendritic spines within discrete cortico-limbic circuits. For example, landmark studies reported that experimenter-administered amphetamine increases dendritic branch length and spine density in the nucleus accumbens (NAc), as well as on apical dendrites in layer III of the medial prefrontal cortex (mPFC) (17). Cocaine has the same effects in the NAc, and increases dendritic branching and spine density on apical and basal dendrites in layer V of the mPFC (18). Dendritic spines in the mPFC also proliferate after amphetamine or cocaine self-administration (19–21). These modifications can persist well beyond the period of active drug exposure, suggesting they may be causally associated with long-term craving, maladaptive decision-making, and failures in impulse control associated with addiction.
Here, we will review evidence that drugs of abuse fundamentally remodel prefrontal cortical neurons, and we will highlight relatively recent evidence in particular that cocaine and amphetamine have opposing effects in the orbital, relative to the medial, prefrontal cortex (termed “oPFC” and “mPFC,” throughout).
We will then address potential cytoskeletal regulatory mechanisms within the oPFC of cocaine vulnerability and resilience. When relevant, we will use current knowledge regarding the molecular mechanisms of cortical development during adolescence as a framework. Why is this pertinent? Adolescence is characterized by increased risk-taking, and drug use is often initiated in humans during this period (22). In rodents, cocaine self-administration is more likely to escalate in adolescence than in adulthood (23). Also during this period, the prefrontal cortex is still developing, which may account for vulnerability to the long-term negative consequences of drugs of abuse and exposure to other pathological stimuli in adolescents (24–28). Recent studies indicate that certain cytoskeletal regulatory proteins expressed in the oPFC and implicated in postnatal neural development regulate behavioral sensitivity to cocaine. The isolation of these and other regulatory factors potentially opens a window of opportunity for the identification of novel pharmacotherapeutic strategies and targets in the treatment of drug abuse disorders.
We will conclude by discussing the perspective that drug-induced neural remodeling in the oPFC may be associated with failures in impulse control and maladaptive decision-making in addiction. We will focus on so-called “reversal learning” tasks and tasks aimed at characterizing the development and maintenance of reward-seeking habits, behavioral assays commonly used in rodents to model aspects of addiction. The highlights of this review are outlined in Box 1.
Box 1. Review Highlights.
-
○
Cocaine and amphetamine cause the structural reorganization of excitatory neurons in the medial and orbital prefrontal cortices, two brain regions implicated in addiction etiology. In particular, repeated psychostimulant exposure causes dendritic spine proliferation in the medial prefrontal cortex (mPFC), while spines are eliminated in the orbitofrontal cortex (oPFC).
-
○
A number of cytoskeletal regulatory proteins expressed in the oPFC impact behavioral sensitivity to cocaine. Several of these targets are additionally involved in postnatal (adolescent) prefrontal cortical development. A better understanding of how drugs of abuse impact structural plasticity during adolescent critical periods may elucidate why adolescents are at heightened risk for the development of drug abuse disorders on the one hand, and help to identify novel targets for intervention on the other.
-
○
Modifications in oPFC neuron structure may be associated with drug-related failures in inhibitory control and the development of inflexible, habitual modes of response, aspects of drug abuse disorders that can be modeled in rodents.
Part 1. Psychostimulants remodel dendrites and dendritic spines, with opposite effects in the mPFC and oPFC
This review will focus on the effects of amphetamine and amphetamine-like psychostimulants such as cocaine on neural structure in the frontal cortex, and in this first section, we will highlight the differential effects of cocaine and amphetamine on neurons within the oPFC, relative to the mPFC. We will also briefly discuss the effects of postnatal nicotine exposure in the same regions.
i. Drugs of abuse remodel mPFC neurons, causing dendritic spine proliferation
The mPFC can be separated into the precentral cortex, anterior cingulate cortex, infralimbic cortex, prelimbic cortex, and the medial orbitofrontal cortex (figure 1a). It can also be divided along the dorsoventral axis into a dorsal portion containing the anterior cingulate and prelimbic cortex and a ventral compartment containing the ventral portion of the prelimbic cortex, the infralimbic cortex, and the medial orbitofrontal cortex (for excellent review, see 29). Although each subregion is anatomically and functionally distinct, cortico-striatal projections arising from the dorsomedial structures, as well as the infralimbic cortex, are implicated in drug-seeking behaviors (5), hence sustained interest in the field in characterizing drug-related modifications to the structure and function of excitatory neurons in these brain regions.
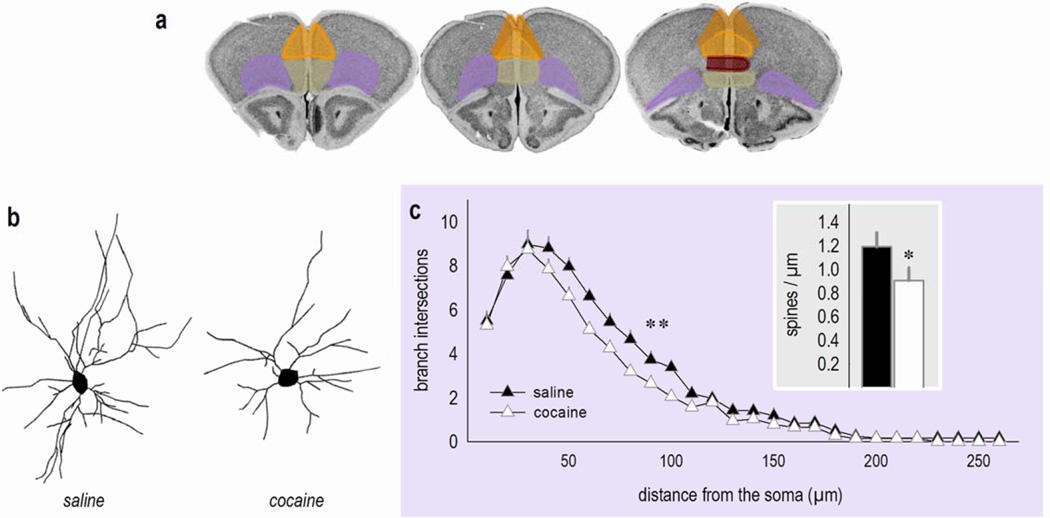
Figure 1. Cocaine: A double threat to neurons in the oPFC?
(a) Sub-regions of the prefrontal cortex transposed onto coronal images from the Mouse Brain Library (202). Pink represents the oPFC and orange, yellow, red and green represent sub-regions of the mPFC: the anterior cingulate, prelimbic, infralimbic, and medial orbital cortices, respectively. These sections correspond roughly to Bregma +1.98, +2.1, and +2.46. (b) Representative deep-layer oPFC neurons from mice treated with saline or cocaine several weeks prior to euthanasia. (c) Sholl analyses indicate that cocaine exposure decreases branch intersections. Inset: Dendritic spines on secondary and tertiary branches are also lost. These findings were originally reported in ref. 81 (for dendrites) and ref. 71 (for dendritic spines), and the reader is referred to these reports for methodological details. Bars and symbols represent means and SEMs, *p<0.05,**p<0.05 40–100 µm from the soma.
In an early investigation in gerbils, a single injection of methamphetamine increased dendritic spine density on excitatory neurons in layers III and V of the dorsal mPFC (30). Using repeated dosing, producing motoric sensitization, Robinson and Kolb (17) subsequently discovered that amphetamine also increases dendritic spine density in the mPFC in rats. Since then, accumulating evidence indicates that amphetamine (18;20;31–33), cocaine (18;19;21;34–39), and (+/−)3, 4-methylenedioxymethamphetamine (40) increase dendritic spine density in the mPFC. Deprenyl, which is metabolized into amphetamine, also increases dendrite branching of mPFC pyramidal neurons in Bonnett monkeys (41), and cocaine exposure increases the thickness and length of prefrontal cortical postsynaptic densities in rats, suggestive of synaptic strengthening (37).
Psychostimulant-elicited dendritic spine proliferation in the mPFC is both rapid and persistent. For example, studies using in vivo dendritic spine imaging indicate that cocaine-induced dendritic spine proliferation is detectable as soon as 2 hours after injection and is most robust after the first exposure (38). Meanwhile, ex vivo studies have documented elevated dendritic spine counts for up to a month (and longer) following exposure (e.g., 19). Interestingly, increased spine counts in drug-exposed animals appear to be attributable to both de novo spine proliferation and enhanced survival of spines that were present prior to the initial cocaine exposure (38). And importantly, amphetamine and amphetamine-like psychostimulant exposure can result in the formation of new synapses. For example, within the prelimbic cortex, amphetamine increases the number of asymmetric, presumed excitatory, axospinous synapses (42), and prenatal exposure to cocaine also increases the number of asymmetric spine synapses in the prelimbic cortex (43). Exposure to methylphenidate increases the density of synaptic contacts in the mPFC and also lengthens mPFC dendrites (44;45). It is important to note, however, that these synaptic modifications may be part of a temporally-dynamic sequence of events, or possibly dependent on developmental factors, since Rasakham and colleagues (39) reported evidence of synapse elimination in the mPFC following cocaine self-administration in rats.
Repeated psychostimulant exposure causes psychomotor sensitization (reviewed 46), and the mPFC regulates this particular behavioral response to drugs of abuse (47–49). Given that many of the studies examining the effects of psychostimulants on dendritic spine density use dosing regimens capable of producing psychomotor sensitization, it has been hypothesized that drug-induced modifications in dendritic spines could be attributed to progressive drug-induced increases in locomotor activity. At least two investigations using a running wheel to dissociate the effects of locomotor activity from psychostimulant-induced structural modifications, however, indicate that drug-induced dendritic spine proliferation in the mPFC cannot be attributed to locomotor activity (18;38). These and other findings support the perspective that drug-induced remodeling of mPFC neurons contributes to modifications in synaptic plasticity in cortico-cortical and cortico-NAc networks, heightened sensitivity to drug-related cues, and a degradation in the ability of the mPFC to control drug-seeking behaviors in addiction (5).
Another stimulant, nicotine, also remodels excitatory neurons within the mPFC. As with cocaine and amphetamine, repeated nicotine exposure, followed by a drug washout period, causes dendritic spine proliferation and dendrite lengthening in deep-layer mPFC (50). Adolescent nicotine exposure, followed by a subsequent washout period, similarly elongates the basal dendrites of excitatory neurons in the prelimbic cortex, though effects are more modest than following exposure in adulthood; further, nicotine largely spares the infralimbic region (51–52). The structural response of excitatory mPFC neurons to nicotine exposure may be temporally dynamic and/or layer-specific. For example, in another investigation, rats were exposed to nicotine, followed by a washout period. Then, a “challenge” injection was administered, and rats were euthanized the next day (53). In this case, layer III mPFC dendrites were shorter, rather than elongated. Further, dendritic spines were eliminated. In the same report, nicotine lengthened dendrites in the oPFC and increased spine density (53). This pattern is opposite that identified following amphetamine or cocaine, as will be discussed in the next section. Clarification of the long-term vs. acute responses of both mPFC and oPFC neurons to nicotine exposure will be a key addition to a growing body of literature. For further review regarding current knowledge on this topic, the reader is referred to discussions by Gulley and Juraska (54) and Kolb and Muhammad (55).
ii. Psychostimulant exposure leads to impoverished neurons in the oPFC
As summarized in the previous section, the rapid and long-lasting effects of amphetamine and amphetamine-like psychostimulants such as cocaine on neural structure have been intensively studied since the seminal reports of Robinson and Kolb describing drug-induced dendrite elaboration and dendritic spine proliferation in the mPFC and downstream NAc (17;18). Within the prefrontal cortex, the majority of subsequent research regarding neural morphology has remained focused on medial wall structures, sparing the oPFC. This is despite overwhelming evidence implicating oPFC function in addiction etiology (56;59). For example, the oPFC is hypermetabolic in cocaine-addicted individuals with limited drug abstinence, and plasticity in this region is associated with acute sensitivity to drug-associated cues in rodents (56;60). The oPFC is hypo-active following chronic cocaine use and withdrawal (56;57). Further, striatal dopamine D2 receptor binding is reduced in long-term methamphetamine abusers, and receptor occupancy covaries with lower metabolism in the oPFC (57;8). In rodents, acute and repeated cocaine and amphetamine fundamentally impact the neurobiological make-up of the oPFC, altering for example immediate-early gene and neurotransmitter receptor expression patterns (table 1).
Table 1.
Postnatal amphetamine or cocaine exposure alters protein or mRNA expression in the oPFC in rodents.
| AMPH or COC |
Treatment regimen | Target | Effect | Reference |
|---|---|---|---|---|
| AMPH | range 1 – 8 mg/kg s.c., 1 injection per day for 4 days, then 4 injections per day for 9 days (40 injections total) |
Zif268 (mRNA) |
↑ | Shilling et al. (2000) (#203) |
| AMPH | 0.5 mg/kg/infusion i.v. (1 infusion) | Arc (mRNA) |
↑ | Klebaur et al. (2002) (#204) |
| AMPH | 2.5 mg/kg i.p. (5 daily injections), then 1 mg/kg i.p. challenge |
c-fos | ↑ with challenge | Nordquist et al. (2008) (#205) |
| AMPH | 0.5 mg/kg i.p. (1 injection) | Arc (mRNA) |
↑ | Banerjee et al. (2009) (#206) |
| AMPH | 2.5 mg/kg i.p. (1 injection) | AGS1 | ↑ | Schwendt et al. (2010) (#207) |
| AGS3 | NC | |||
| Rhes (mRNA) |
NC | |||
| AMPH | range 1 – 10 mg/kg s.c., 3 injections per day for 4 days (12 injections total) |
5-HT2a (mRNA) |
↓ | Horner et al. (2011) (#208) |
| AMPH | 2 mg/kg i.p. (1 injection) | c-fos (mRNA) |
↑ | Cáceda et al. (2012) (#209) |
| AMPH | 1 mg/kg i.p. (14 daily injections) | Retsat | ↑ | Mychasiuk et al. (2013) (#210) |
| Lpar1 | ↑ | |||
| Csrp1 | ↑ | |||
| Bhlhe40 | ↓ | |||
| Dusp6 | ↓ | |||
| Synpo | ↓ | |||
| Dnajb5 | ↓ | |||
|
Ahdc1 (results of gene array) |
↓ | |||
| AMPH | range 1 – 10 mg/kg i.p., 3 injections per day for 4 days (12 injections total) |
5-HT2a (mRNA) |
NC | Murray et al. (2014) (#211) |
| AMPH; COC |
2 mg/kg i.p. (1 injection) 40 mg/kg i.p. (1 injection) |
Fos | ↑ | Trinh et al. (2003) (#212) |
| ↑ | ||||
| COC | 0.5 mg/kg/infusion i.v. (multiple infusions self-administered per day for 10 days) |
DAT TH |
NC NC |
Grimm et al. (2002) (#213) |
| COC | 0.25 mg/kg/infusion i.v. (multiple infusions self-administered per day for 9–12 days) |
Zif268 (mRNA) |
↑ in control rats receiving yoked COC-cue pairings |
Thomas et al. (2003) (#214) |
| COC | 2 mg/kg/infusion i.v. (1 infusion) | c-fos | ↑ | Samaha et al. (2004) (#215) |
| Arc (mRNA) |
↑ | |||
| COC | 0.75 mg/kg/infusion i.v. (2 infusions) | c-fos (mRNA) |
↑ | Cao et al. (2007) (#216) |
| COC | 0.5 mg/kg/infusion i.v. (multiple infusions self-administered per day for 21 days) OR 15 mg/kg i.p. (21 daily injections) |
ΔFosB | ↑ i.v. and i.p. | Winstanley et al. (2007) (#118) |
| COC | 0.75 mg/kg/infusion i.v. (multiple infusions self-administered per day for 28 days) followed by 22 days extinction or abstinence |
Fos | ↑ after abstinence | Zavala et al. (2007) (#217) |
| GluR1 | NC | |||
| GluR2/3 | NC | |||
| GluR4 | NC | |||
| COC | 0.4 mg/kg/infusion i.v. (multiple infusions self-administered per day for 22 days; 1 or 6 hour sessions) |
DRD2 | ↓ after long access | Briand et al. (2008) (#218) |
| DRD1 (mRNA) |
NC | |||
| COC | 0.6 mg/kg/infusion i.v. (multiple infusions self-administered per day for 10 days) followed by 22 hours abstinence, OR 15 days abstinence, then re-exposure to: 1) a novel chamber; 2) the COC-associated chamber, or 3) the COC-associated chamber and COC-associated cues |
Arc BDNF c-fos Zif268 |
↑ after 22 hours | Hearing et al. (2008) (#219) |
| Arc | ↑ with COC, further ↑ with COC- associated environment |
|||
| c-fos BDNF |
↑, but to same degree as drug naïve rats exposed to a familiar context |
|||
| Zif268 (mRNA) |
↑ with COC- associated environment + cues |
|||
| COC | 0.75 mg/kg/infusion i.v. (multiple infusions self-administered per day for 23 days) |
Arc (mRNA) |
↑ | Zavala et al. (2008) (#220) |
| COC | 0.75 mg/kg/infusion i.v. (multiple infusions self-administered per day for 24 days) followed by 24 days extinction or abstinence |
Fos | ↑ after abstinence | Zavala et al. (2008) (#221) |
| COC | 10 mg/kg i.p. or 40 mg/kg i.p. (1 injection) |
c-fos | ↑ | Caster et al. (2009) (#222) |
| Zif268 (mRNA) |
↑ | |||
| COC | 2 mg/kg/infusion i.v. (1 infusion) | Fos | ↑ | Kufahl et al. (2009) (#223) |
| COC | 25 mg/kg i.p. (5 daily injections), then 25 mg/kg i.p. challenge |
Zif268 | NC | Unal et al. (2009) (#224) |
| Homer 1a (mRNA) |
NC | |||
| COC | 15 mg/kg i.p. (4 daily injections) | Zif268 | NC | Fritz et al. (2011) (#225) |
| COC | 1 mg/kg/infusion, i.v. (multiple infusions self-administered per day for 8 days) followed by cue- or drug- primed reinstatement (10 mg/kg) OR 10 mg/kg i.p. (1 injection) |
Arc | NC i.p.; ↑ prime | Ziólkowska et al. (2011) (#226) |
| Zif268 (mRNA) |
NC | |||
| COC | 15 mg/kg i.p. (4 daily injections) | Zif268 | ↑ | El Rawas et al. (2012) (#227) |
| COC | 250 µg/infusion, i.v. (multiple infusions self-administered per day for 11 or 44 days) in high and low impulsivity rats |
Zif268 | ↓ in high impulsivity rats |
Besson et al. (2013) (#228) |
| 5-HT2c (mRNA) |
NC | |||
| COC | 15 mg/kg i.p. (1 injection) | Zif268 (mRNA) |
↑ | Burton et al. (2013) (#229) |
| COC | 0.25 mg/kg/infusion i.v. (multiple infusions self-administered per day for 25 days) |
Arc | ↑ | Riedy et al. (2013) (#230) |
| Zif268 (mRNA) |
NC | |||
| COC | 2 mg/kg/infusion i.v. (multiple infusions self-administered per day for 7 days), then 2 mg/kg i.v. challenge |
Fos | ↑ | Kufahl et al. (2015) (#231) |
Abbreviations: AMPH, d-amphetamine; COC, cocaine; i.v., intravenous; i.p., intraperitoneal; s.c., subcutaneous; cue, cue-induced reinstatement; prime, drug-primed reinstatement; NC, no change.
Effects: ↑, increase; ↓, decrease; NC, no change. All results reflect drug-exposed compared to drug-naïve controls.
Importantly, the oPFC is thought to be largely structurally and functionally conserved across species (61;62), and the healthy oPFC in both rodents and primates plays a major role in determining reinforcer value and inhibiting inappropriate behaviors, as well as in rapidly learning about predictive associations between stimuli and desired outcomes. These cognitive processes are thought to be impacted in addiction, resulting in increased perceived value of drugs of abuse, in stimulus-elicited drug seeking, and in failures in inhibitory control. In rodents, oPFC thickness atrophies in response to repeated amphetamine exposure (63). With the caveat that this effect is observed in females but not males (63; see also 64), structural atrophy may be linked to decreased oPFC glucose utilization after even short-term cocaine exposure in monkeys (65), as well as diminished oPFC gray matter and oPFC-dependent cognitive flexibility in long-term cocaine addicts (66–69).
How do psychostimulants impact dendritic spine density and structure in the oPFC? To summarize current evidence, amphetamine and cocaine both reduce dendritic spine density on pyramidal neurons within the oPFC (20;32;33;70;71; reviewed 55) (figure 1b–c). In other words, the response of oPFC neurons is opposite that of mPFC neurons. Notably, morphine self-administration and alcohol withdrawal increase dendritic spine density and branching in the oPFC (72;73), which has led to the occasional misperception that psychostimulants also induce dendritic spine proliferation in the oPFC, but this generalization appears to be unfounded (74). Rather, experimenter-administered amphetamine (32;33), self-administered amphetamine (70;20), and experimenter-administered cocaine (71) result in dendritic spine elimination in layers III and V of the oPFC. A great strength reports by Muhammed and Kolb (32;33) and Crombag et al. (20) is that dendritic spines on both mPFC and oPFC principal pyramidal neurons were enumerated within the same subjects, providing further compelling evidence that cocaine and amphetamine have opposing structural effects in these brain regions.
Despite multiple studies indicating that dendritic spines on oPFC neurons are eliminated following psychostimulant exposure, it is important to note that Ferrario et al. (21) found no evidence of oPFC spine elimination (or proliferation) following short- or extended-access cocaine self-administration. In this case, spine densities in layer III were simply unchanged. Nonetheless, evidence that cocaine eliminates deep-layer dendritic spines (71) is provocative, given that: 1) cocaine causes dendritic spine proliferation in the adjacent mPFC, as already discussed above. 2) Further, amygdalo-cortical interactions are implicated in addiction-related behaviors (3;7), and amygdala projections innervate deep-layer prefrontal cortex (e.g., 75). Thus, cocaine may fundamentally imbalance synaptic plasticity between amygdalo-oPFC and amygdalo-mPFC neurocircuits, contributing to heightened sensitivity to drug-related conditioned stimuli and promoting drug seeking at the expense of engaging inhibitory response strategies. Supporting this perspective, inactivation of amygdalo-mPFC projections in cocaine self-administering rats prevents cue-induced reinstatement of cocaine seeking, an animal model of relapse (76).
One report has indicated that early-life (adolescent) cocaine exposure, followed by acute re-exposure in adulthood, eliminates dendritic spines on oPFC neurons and also increases the head diameter of remaining dendritic spines (71). This is notable because measures such as dendritic spine head diameter directly relate to spine function, as spines with larger heads, mushroom-type spines, are more likely to be synapse-containing (77;78). And indeed, expression of the post-synaptic marker PSD95 in the oPFC, but not mPFC, is elevated in nonhuman primates euthanized immediately following extended-access cocaine self-administration (79), suggestive of synaptic strengthening.
To reiterate, early-life cocaine exposure, followed by acute re-exposure in adulthood, eliminates oPFC dendritic spines, but increases the head diameter of remaining spines (71). Cocaine-induced spine head enlargement may reflect a neural response that preserves or even under some circumstances enhances neuroplasticity in the face of drug-induced spine elimination. Whether this phenomenon is behaviorally ‘protective’ or deleterious is unclear. For example, blocking F-actin polymerization in the oPFC, needed for dendritic spine enlargement, exaggerates locomotor activity in response to cocaine (71), suggesting that spine head growth is in some ways protective. On the other hand, acute amphetamine causes cognitive impairments in a food-reinforced instrumental conditioning task, and these deficiencies are associated with acute drug-induced hyper-activity of oPFC neurons (80). In humans, cocaine exposure acutely hyper-activates the oPFC, and this response is associated with drug craving (56). Future research should clearly dissociate the rapid effects of cocaine on neural structure in the oPFC from the more prolonged, durable consequences since these modifications may have separable influences on behavioral outcomes. For example, acute structural plasticity could contribute to drug craving and disorganized decision-making, while the long-term degradation of neural structure following repeated cocaine exposure could contribute to impaired oPFC-dependent impulse control and habit-like drug seeking.
As discussed, repeated cocaine exposure can eliminate dendritic spines on excitatory neurons in deep-layer oPFC. Repeated cocaine exposure also simplifies the dendritic arbors of deep-layer excitatory neurons in the oPFC, reducing overall dendrite length and decreasing branch intersections in Sholl analyses (81; figure 1b–c). Chronic ethanol exposure does not appear to remodel dendrite arbors of excitatory neurons in the oPFC (82;83), thus these effects may be selective to amphetamine-like psychostimulants, or potentially cocaine specifically. Interestingly, oPFC dendrite arbors and dendritic spines also remodel following exposure to elevated levels of stress hormones, but in this case, oPFC spines are eliminated while arbors become more complex (84–86). Thus, even relative to prolonged stressor exposure, cocaine appears to pose a “double threat” to the dendritic structures of oPFC neurons, simplifying arbors on principal pyramidal neurons and eliminating dendritic spines (figure 1b–c). In this sense, cocaine is more comparable to prenatal stress, which causes oPFC dendrite regression and spine elimination, detectable multiple weeks following the stressor exposure period (87). Identification of whether common behavioral phenotypes are linked to changes in neural structure may be a fruitful topic of future research.
Part 2. Cytoskeletal regulatory factors in the cerebral cortex regulate behavioral sensitivity to cocaine
i. Rho regulatory factors
Dendritic spine morphology is regulated by an underlying actin cytoskeleton, which determines the spine’s shape and its signaling properties. Cytoskeletal remodeling of dendrites and dendritic spines is orchestrated in part by Rho family GTPases including RhoA (Rho), Rac1, and Cdc42, which coordinate the actin cytoskeletal rearrangements required for dendrite elaboration or simplification. Rho activation decreases branch extensions in multiple neural systems, while interference with Rho promotes dendrite growth (e.g., 88–93). Interference with Rho also promotes activity-dependent remodeling of dendritic spines (94). Regulators of Rho activity are thus well-poised to coordinate structural and behavioral responses to cocaine and other drugs of abuse.
One endogenous Rho inhibitor in the brain is p190RhoGAP, which is activated by integrin receptor binding to extracellular matrix proteins and activation of regulatory partners such as Arg kinase (also called Abl2; 95–99). p190rhogap−/− mice are not viable, and while p190rhogap+/− mice appear superficially normal, they exhibit significant vulnerabilities to genetic and chemical perturbations. For example, simultaneous heterozygosity for mutations in both p190RhoGAP and Arg kinase results in increased Rho activity and hippocampal dendritic arbor destabilization, accompanied by deficits in a novel object recognition task (92). Further, mice deficient in p190RhoGAP or Arg kinase are hyper-vulnerable to cocaine, generating a sensitization-like locomotor response following a single cocaine injection (100;71). Thus, disinhibition of Rho is associated with behavioral vulnerability to cocaine.
Arg-p190RhoGAP interactions are stimulated by integrin receptor binding (98). Integrins are heterodimeric α/β subunit-containing transmembrane receptors that mediate cell adhesion to the extracellular matrix and control signaling pathways that coordinate changes in cytoskeletal rearrangements (101). In neurons, integrins are localized to the synaptic cleft where they initiate biochemical signaling cascades that contribute to synapse maturation, synaptic transmission and plasticity, and dendrite formation and stability. Mice lacking β1-integrin, Arg kinase, or both Arg and β1-integrin, are deficient in an oPFC-dependent test of behavioral flexibility (reversal learning) (71;100;102;103). This is significant because poor performance in this task in drug-naïve rodents is associated with increased cocaine self-administration (104). Further, as with Arg and p190RhoGAP deficiency, β1-integrin knockdown elicits a sensitization-like locomotor response following a single cocaine injection (103). These studies utilized mice with forebrain-specific knockout, suggesting that cortical β1-integrin expression and signaling in particular may be a resiliency factor against pathological stimuli such as cocaine exposure.
While the loss of β1-integrin in the forebrain augments cocaine-induced locomotor hyperactivity (103), independent investigations have indicated that self-administered cocaine does not impact the expression of β1-integrin in the NAc (105). Rather, in the NAc, cocaine self-administration regulates β3-integrin expression (105). Given that drugs of abuse have opposite effects on dendritic spines in the oPFC relative to NAc, future studies might capitalize on brain region-specific fluctuations in spine regulatory factors, e.g., to develop pharmacological strategies that promote spinogenesis in the oPFC – reversing the effects of repeated psychostimulant exposure – but spare dendritic spine density in the NAc. Such strategies could possibly be combined with those that reverse drug-induced spinogenesis in the NAc, but spare oPFC spines. Together, these effects could normalize widespread, divergent structural consequences of psychostimulant exposure, potentially to therapeutic-like ends.
This model presumes, however, that dendritic spine proliferation in the NAc is associated with cocaine seeking behaviors, drug craving, etc., but this may not be the case. For example, knock out of the Rho-GEF Kalirin 7 blocks cocaine-induced dendritic spine proliferation in the NAc and blunts cocaine conditioned place preference, a protective-like response. On the other hand, Kalirin 7 knockout mice also develop exaggerated cocaine-induced locomotor sensitization and self-administer more cocaine than wild type mice (106;107). For further discourse regarding the complex roles that drug-related dendritic spine proliferation in the NAc may play in cocaine vulnerability vs. resilience, see discussions by Chandler and Kalivas (108), Smith et al. (109), and others.
Even with these caveats, accumulating evidence indicates that integrin-mediated signaling events are impacted by cocaine exposure in rodents (105;110) and possibly involved in cocaine addiction in humans (111;112), motivating the authors of the present review to better understand the role of the downstream Arg kinase in models of addiction vulnerability. To this end, we have investigated the effects of Arg deficiency on the expression of prefrontal cortical dopamine D1- and D2-type receptors. In both cases, Arg kinase deficiency results in progressive receptor loss during postnatal development (100). Additionally, infusion of the Abl-family kinase inhibitor STI-571 selectively in the oPFC mimics behavioral vulnerability to cocaine observed in arg−/− mice, augmenting drug-induced locomotor sensitization and interfering with oPFC-dependent learning and memory (100;71).
At a basic level, dendritic spine morphology is regulated by actin cycling between monomeric globular (G)-actin and filamentous (F)-actin (113;114). Chronic cocaine exposure decreases prefrontal cortical levels of F-actin (115), consistent with spine elimination in the oPFC following cocaine exposure (71). Notably, however, modestly elevated F-actin and PSD95 levels have also been reported (37). This apparently contradictory finding may simply reflect differences in tissue dissection strategies that resulted in the enrichment of mPFC tissues in the more recent investigation (37) since dendritic spines proliferate in the mPFC following psychostimulant exposure, as discussed in Part 1 of this review.
Other spine-regulatory factors include transcription factors implicated in cocaine action such as deltaFosB (116). deltaFosB overexpression in the NAc causes dendritic spine proliferation (117), and under certain circumstances, deltaFosB induction in the oPFC has protective benefits with regards to the cognitive deficiencies induced by cocaine (118;119). These benefits could conceivably be attributable to a proliferative influence on oPFC dendritic spines.
We have noted several instances in which manipulations of neurobiological factors related to dendritic spine stability and density in the oPFC regulate locomotor sensitivity to drugs of abuse. One remaining question thus pertains to how the oPFC itself might regulate psychomotor sensitization. The rodent NAc receives projections from the oPFC, but they are quite sparse (120). Further, Winstanley et al. (119) recently provided indirect but compelling evidence that hyper-sensitization caused by over-expression of deltaFosB in the oPFC was unlikely to be due to effects on the NAc. The rodent oPFC strongly innervates the dorsal striatum, however (120). This connectivity may be associated with its ability to regulate drug-induced locomotor sensitization.
ii. Adolescent critical periods and metaplasticity in neural structure
Adolescence is characterized by increased risk-taking, vulnerability to the development of neuropsychiatric disorders such as drug addiction, and activity-dependent neocortical refinement that culminates in synaptic reorganization and dendritic spine pruning by early adulthood (121–126;22;78). Synapse and spine stabilization processes may impose a biological “set-point” for prefrontal cortical-dependent neuropsychiatric vulnerabilities and cognitive capacity in adulthood (127), and structural stabilization may confer resilience to impulsive decision-making, addiction, and addictive-like behaviors in the transition from adolescence to adulthood (125;22). Within the prefrontal cortex, certain aspects of oPFC development have a prolonged maturation timecourse (128). This protracted developmental trajectory may result in a substantial window of opportunity for aberrant dendritic spine reorganization and remodeling due to pathological events such as cocaine exposure. On the other hand, ‘beneficial’ experiences may also impact developmental trajectories. For example, in rodents, early-life housing with same-age conspecifics – increasing the likelihood of social play – appears to facilitate typical dendritic spine pruning in the oPFC relative to housing with adult cagemates (129).
The constellation of cytoskeletal regulatory proteins discussed in the prior section – β1-integrin, Arg, and p190RhoGAP – is involved in hippocampal and cortical development during adolescence (92;100;71;103;101). And as discussed, the absence of Arg kinase increases behavioral vulnerability to cocaine (100;71). These patterns suggest that perturbations in normative structural maturation during adolescence increase vulnerability to cocaine. Further support for this perspective comes from additional experiments using Arg-deficient mice, in which oPFC dendritic spines are lost beginning at roughly postnatal day 31 (71). Psychomotor sensitivity to cocaine is normal at time points preceding dendritic spine loss, and heightened motoric sensitivity emerges only with the onset of dendritic spine elimination associated with Arg kinase deficiency. This temporal convergence provides empirical support for the hypothesis that structural instability in adolescence contributes to vulnerability to the drug (71).
Cocaine-induced locomotor sensitization can be mitigated in adult rodents by treatment with broad-spectrum NMDA receptor antagonists, as well as ifenprodil, an NR2B-selective NMDA receptor antagonist (130;131). Additionally, cocaine exposure can increase NR2B expression in the prefrontal cortex (132). These findings are provocative from a developmental perspective because NR2A signaling stabilizes cortical synapses during early-life critical periods (133) and in response to environmental stimuli (134;135), suggesting that enhancing NR2A–mediated signaling during adolescent critical periods by blocking NR2B may have long-term behavioral benefits. In support of this perspective, ifenprodil treatment in adolescent mice exposed to cocaine blocks the sensitized response to a challenge injection administered in adulthood, 4 weeks later, even in the absence of further ifenprodil treatment (71).
NR2B blockade can also enhance Arg kinase signaling by preventing a substrate, cortactin, from translocating from the dendritic spine (136); this mechanism could further contribute to the protective effects of ifenprodil in adolescent mice (71). The authors do not mean to suggest that ifenprodil is only effective during adolescence, however; ifenprodil also has protective benefits in the context of alcohol-, nicotine-, heroin-, and morphine-seeking behaviors in mature rodents, suggestive of potentially widespread applications (137–141).
Of the studies reporting that psychostimulant exposure eliminates dendritic spines on excitatory neurons in the oPFC, the authors of one of these – ref. 71 – administered cocaine during the equivalent of early adolescence in rodents, then administered a “challenge” injection in adulthood prior to euthanasia. Dendritic spines were eliminated, and remaining spine heads were enlarged. This experimental design raises the issue of metaplastic sensitivity of oPFC neurons to cocaine exposure. The term “metaplasticity” was originally used to refer to a type of synaptic plasticity in which the history of plasticity at that synapse influenced its response to subsequent stimuli. It has increasingly been applied, however, to biochemical, physiological, and morphological phenomena (142). For example, Shen and colleagues reported that a rat’s cocaine exposure history determines the structural response of dendritic spines in the NAc to subsequent cocaine injection (143). With regards to dendritic spine head enlargement in the oPFC following adolescent cocaine exposure and a subsequent challenge injection in adulthood (71), whether head enlargement can be attributed to a history of cocaine exposure in adolescence, or to a metaplastic response to further exposure in adulthood remains unclear. Dissociating these possibilities may be important since the likelihood of occasional cocaine use transitioning to cocaine dependence in humans peaks in late adolescence/young adulthood, later than for alcohol and marijuana (144), and developmentally-regulated metaplastic effects of repeated drug exposure on oPFC structure and function may play a role.
Part 3. Psychostimulant exposure impacts complex behavior
i. Cocaine-related impulsivity and behavioral inflexibility can be modeled in rodents
In humans, individuals who initiate cocaine use in adolescence are at increased risk of developing dependence (145) and have decreased likelihood of seeking treatment (146). Interventions aimed at correcting perturbations in the cellular structure of oPFC neurons, particularly during critical adolescent developmental periods, may have beneficial effects, as discussed in Part 2 of this review. Experiments in this vein may capitalize on tests of impulse control and behavioral flexibility, aspects of oPFC-dependent day-to-day function that are impacted by cocaine exposure in both humans and rodent models. For example, adolescent cocaine exposure impairs response reversal in instrumental and water maze tasks for up to 5 weeks following exposure (147;148;81), evidence of long-term detriments to oPFC-dependent cognitive and behavioral flexibility. We will conclude our review with a discussion of these and other tasks commonly used to assess the functional consequences of cocaine exposure in rodents.
The term “reversal learning” most commonly refers to tasks in which animals must associate specific stimuli — such as visual cues — with desired outcomes — such as food reinforcers — and then update these stimulus-outcome associations as they are modified by the experimenter. These discrimination-based reversal tasks, like other reversal tasks, require simultaneous inhibition of a previously acquired response and deployment of a previously withheld response. Across multiple species, oPFC lesions impair performance in discrimination-based reversals, and impairments can be attributed to increased perseverative responding, as opposed to, for example, response extinction (149–156; see for further discussion, 157;158). In these tasks, the oPFC is not critical to the initial stimulus-outcome associative conditioning, since lesions do not impair initial discrimination of reward-associated stimuli and associated outcomes. Instead, the oPFC is necessary for altering behavior as contingencies shift, for using specific information about desired outcomes in the expression of decision-making strategies, for “on the fly” decision-making (159;160).
oPFC-dependent response reversals can be tested in multiple model systems, and reversal tasks have been used to probe oPFC function following cocaine exposure with a high degree of concordance across species. For example, repeated cocaine exposure in both nonhuman primates and humans impairs reversal performance (161;68). Furthermore, the severity (amount and duration) of cocaine use correlates with deficits in reversal tasks in humans (69). Repeated cocaine exposure also impairs reversal performance in rodents (162;163). Of particular note, reversal deficits in cocaine self-administering rats are detectable up to three months following exposure (164), further evidence that chronic cocaine exposure persistently degrades oPFC function. Given that cocaine-related dendritic spine elimination in the oPFC also persists multiple weeks beyond the exposure period (71), structural remodeling in this region, coupled with drug-induced irregularities in the electrophysiological and neurochemical properties of oPFC systems (see 158;59), may be causally associated with cocaine-induced failures in impulse control.
Another reversal task utilized in rodents has been termed “instrumental reversal” conditioning. In this case, mice or rats are trained to nose poke for food reinforcers in a chamber with multiple response operandi. Once animals have acquired the reinforced response, the location of the reinforced aperture is “reversed” to another previously non-reinforced aperture. The initial acquisition of the instrumental response is largely unrelated to reversal performance (165), and the reversal phase is oPFC-dependent. Specifically, lesions of the lateral oPFC delay the acquisition of the new response, while lesions of the medial oPFC increase perseverative errors; this can be attributed to energized response rates directed toward the previously-reinforced response because response rates on the newly-reinforced aperture are unaffected (166). Repeated cocaine exposure in mice similarly impairs the acquisition of a new response, characteristic of damage to the lateral oPFC (167;81). DePoy et al. in the same report (81) provided evidence that the same cocaine exposure protocol simplifies dendrite arbors in the lateral and ventrolateral oPFC (recapitulated in figure 1b–c here), suggesting that cognitive deficiencies may be associated with these neural rearrangements.
Instrumental reversal conditioning in drug-naïve mice is predictive of cocaine sensitivity, in that poor reversal performance is associated with higher rates of cocaine-reinforced responding (104). This finding suggests that instrumental reversal tasks could be used as a tool to isolate neurobiological factors associated with cocaine vulnerability and resilience in drug-naïve organisms.
What factors regulate instrumental reversal learning? oPFC-selective (but not mPFC-selective) knockdown of Brain-derived neurotrophic factor (Bdnf) impairs response acquisition in an instrumental reversal task in adult male mice (168;169), recapitulating the effects of repeated cocaine exposure. In female mice, oPFC-selective Bdnf knockdown results in instrumental responding for food reinforcers that greatly outpaces reinforcer delivery, a possible indicator of impulsive-like behavior (170). BDNF is essential to dendrite pruning and dendritic spine stability, including in the mature cortex (171–173), and accordingly, Bdnf knockdown-induced behavioral abnormalities can be corrected by application of a Rho-kinase inhibitor (170). Rho-kinase is a substrate of the RhoA GTPase, a primary regulator of cell shape throughout the central nervous system (as discussed in Part 2). Thus, oPFC BDNF may facilitate goal-directed decision-making via its actions on local structural plasticity.
In a large study, response patterns in an instrumental reversal task were characterized in 51 strains of mice (165). Among these genetically diverse strains, optimal reversal performance was associated with elevated brain Synapsin-3 (Syn3) mRNA levels. Syn3 codes for synapsin 3, a member of the synapsin family, which anchors synaptic vesicles to the cytoskeletal network of presynaptic terminals. Laughlin and colleagues (165) propose a model in which Syn3 regulates dopamine D2 receptor function. This could impact impulse control since reduced D2 activity or expression, due to genetic or pharmacological interventions or individual differences, interferes with reversal performance (100;165;174;175). D2 receptors are expressed both pre- and post-synaptically in the prefrontal cortex, and D2 receptor knockout causes dendritic spine elimination in deep-layer prefrontal cortex (176). Thus, low D2 levels – e.g., due to genetic perturbations or individual differences in receptor expression levels – may impact addiction-related behaviors by sensitizing oPFC neurons to cocaine-induced dendritic spine elimination.
ii. Goal-directed decision-making and reward-seeking habits
The oPFC has long been associated with the expression of goal-directed behaviors. For example, oPFC lesions in monkeys and rats resulted in a resistance to the extinction of a food-reinforced response in classical studies (177;178). Butter and colleagues (177) attributed this finding to the inability of monkeys with lesions to suppress “strong, habitual modes of response.”
A bias towards the development of cue-elicited habits at the expense of engaging goal-oriented behavioral response strategies is considered an etiological factor in addiction (5;179;180). The most common way to test whether reward-related decision-making occurs according to outcome-based (goal-directed) vs. stimulus-response (habitual) associative contingencies in rodents is by assessing reward-related responding following devaluation of a reinforcer. This can be accomplished by pairing the reinforcer with, for example, temporary malaise induced by post-ingestion injection of lithium chloride. If responding associated with the outcome persists despite devaluation, responding is interpreted as being under the control of a stimulus-response habit, while response inhibition by contrast reflects goal-directed modes of response (181). In rodents, responding for cocaine can rapidly assume habitual qualities (182;183). Moreover, a history of repeated cocaine or amphetamine exposure also results in insensitivity to changes in reinforcer value in food-reinforced tasks, evidence of persistent biases towards stimulus-elicited habits (11;14–16;184;185).
Response-outcome contingency degradation can also be used to classify response strategies. Here, mice or rats are typically trained to generate two distinct reinforced responses, then the likelihood of reinforcement associated with one response is reduced – or “degraded.” Rodents and humans that are sensitive to the predictive relationship between actions and their outcomes – that are goal-directed – will inhibit responding associated with non-contingent reinforcer delivery, providing evidence of knowledge of the response-outcome relationship (181;186). By contrast, equivalent engagement of both responses reflects reflexive habits. Using this task, Gourley and colleagues found that acute cocaine exposure can interrupt response-outcome learning and memory and thereby bias response strategies towards habit-based, as opposed to goal-directed, responding (12). Further, this task has been used to provide evidence that cocaine-induced habit-like response strategies are detectable 7 weeks following repeated cocaine exposure in mice (13). The same cocaine dosing protocol simplifies the dendritic arbors of deep-layer excitatory oPFC neurons (81). Durable neural remodeling could contribute to a drug-induced bias towards reward-seeking habits.
oPFC-selective knockdown of Bdnf and surgical disconnection of the oPFC from the downstream dorsal striatum also cause habit behavior, as assessed using response-outcome contingency degradation (168). This report focused on ventrolateral and dorsolateral oPFC projections targeting the ventrolateral compartment of the dorsal striatum (120;187). Instrumental conditioning — that is, learning that a response produces a reinforcer — increases immediate-early gene expression in this region of the dorsal striatum, supporting the perspective that it is involved in associating a particular behavioral response with a particular reinforcer (188).
Abundant evidence indicates that the medial compartment of the dorsal striatum — which receives projections from the medial and ventrolateral oPFC (120;187) — is also essential to goal-directed action selection, while the dorsolateral striatum is instead intimately linked with stimulus-response habits (189;190). Consistent with this model, site-selective inhibition of the cytoskeletal regulator Arg kinase decreases synaptic marker expression and causes habits when targeted to the dorsomedial striatum, but breaks habits when targeted to the dorsolateral striatum (12). Further, methamphetamine increases dendritic spine density in the dorsolateral striatum, associated with stimulus-elicited habits, but eliminates dendritic spines (particularly synapse-associated mushroom-shaped spines) in the dorsomedial striatum, tightly coupled with goal-directed response strategies (191). These structural modifications – in concert with impoverished neural structure within the oPFC – could be causally associated with drug-induced biases towards stimulus-elicited habits.
In non-human primates, oPFC neurons encode both stimulus-outcome and response-outcome associative contingencies (192), further implicating the oPFC in goal-directed action selection and suggesting that damage to the oPFC – e.g., through repeated cocaine exposure – could contribute to a reliance instead on stimulus-response habits elicited by reward-associated cues. These cues can include the context in which an appetitive reinforcer, such as cocaine, is acquired. Studies using cocaine self-administration approaches indicate that prolonged oPFC inactivation enhances context-elicited reinstatement of drug seeking, sparing drug-seeking behaviors induced by other conditioned stimuli (193;194). Independent investigations indicate that oPFC-selective ablation of GABAAα1 receptor subunit expression causes a decrease in synaptic marker expression in the oPFC, and in concert, mice develop reward-seeking habits, but only in the context in which the instrumental response was originally trained (195).
Together, these findings provide evidence that, in the development of adaptive response strategies, the healthy oPFC gates the influence of contextual cues associated with appetitive outcomes. Repeated cocaine exposure could degrade this function to promote the development and maintenance of context-elicited habitual drug seeking through repeated stimulation of the dopamine D1 receptor for example (see 196), dendrite and dendritic spine elimination (as discussed above), and/or imbalance between dopamine D1- and D2-type receptors, given that D2 is highly expressed on basal arbors that are eliminated by cocaine exposure (197;81).
Conclusions
Dendrites and dendritic spines can be remarkably plastic, and their structure is sculpted by multiple external stimuli including exposure to drugs of abuse and stressors. Studies aimed at identifying molecular mechanisms of cocaine-induced dendritic spine remodeling – particularly in the NAc – strongly suggest that cellular structural modifications can directly regulate behavioral outcomes. These include potentially adaptive consequences; for example, cocaine-induced dendritic spine proliferation in the NAc has been associated with certain behavioral resiliencies (e.g., 109), and blockade of cocaine-induced dendritic spine modifications in both the oPFC and NAc can increase – though in other cases, occlude – behavioral vulnerability to cocaine (115;71;198;199). Meanwhile, the correction of long-term or metaplastic modifications following prolonged cocaine exposure may have behavioral benefits (200;143).
Animal models, and particularly rodent models, provide a strong venue to disentangle these issues and resolve knowledge gaps: First, rodent models are amenable to determining neurobiological vulnerability and resiliency factors associated with drugs of abuse using both prospective and retrospective approaches, and using experimental designs and manipulations that can establish causal relationships between structural plasticity and behavioral outcomes. Second, we and others argue that the identification of signaling cascades – in addition to specific proteins – that could serve as promising therapeutic targets will be critical (201). Third, developmental critical periods and neurobiological correlates and mechanisms of sex differences in drug vulnerability and resilience may be more rapidly isolated using rodent, rather than primate, systems. A final challenge that could be tackled using rodent models may be the identification of approaches that can normalize drug-induced structural modifications associated with maladaptive behaviors across multiple brain regions. Such studies may benefit from leveraging differential expression of relevant protein targets across structures, and/or attempting to augment new learning regarding the negative consequences of drug self-administration and habitual reward seeking.
Synopsis.
Exposure to cocaine and amphetamine structurally reorganizes excitatory neurons in the medial and orbital prefrontal cortices (mPFC and oPFC), inducing dendritic spine proliferation in the mPFC, and eliminating spines in the oPFC. Modifications may be causally associated with addiction etiology. Certain cytoskeletal regulatory proteins expressed in the oPFC and implicated in postnatal neural development also regulate behavioral sensitivity to cocaine, potentially opening a window of opportunity for the identification of novel pharmacotherapeutic targets in the treatment of drug abuse disorders.
Acknowledgments
This work was supported by the Emory University Research Council, DA 034808, and DA 036737. The Yerkes National Primate Research Center is supported by the Office of Research Infrastructure Programs/OD P51OD11132. The authors thank Dr. Anthony Koleske, Ms. Kelsey Zimmermann, Mr. Andrew Swanson, and Ms. Elizabeth Pitts for valuable comments on this manuscript and Dr. Marina Wheeler for contributions to an early version of this document.
Footnotes
The authors have no conflicts to disclose.
References
- 1.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 2.Robbins TW, Everitt BJ. Drug addiction: Bad habits add up. Nature. 1999;398:567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- 3.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 4.Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- 5.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 6.Torregrossa MM, Corlett PR, Taylor JR. Aberrant learning and memory in addiction. Neurobiol Learn Mem. 2011;96:609–623. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torregrossa MM, Taylor JR. Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology. 2013;226:659–672. doi: 10.1007/s00213-012-2750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci USA. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 10.Vanderschuren LJML, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 11.Schoenbaum G, Setlow B. Cocaine makes actions insensitive to outcomes but not extinction: Implications for altered orbitofrontal-amygdalar function. Cereb Cortex. 2005;15:1162–1169. doi: 10.1093/cercor/bhh216. [DOI] [PubMed] [Google Scholar]
- 12.Gourley SL, Olevska, Gordon J, Taylor JR. Cytoskeletal determinants of stimulus-response habits. J Neurosci. 2013;33:11811–11816. doi: 10.1523/JNEUROSCI.1034-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinton EA, Wheeler MG, Gourley SL. Early-life cocaine interferes with BDNF-mediated behavioral plasticity. Learn Mem. 2014;21:253–257. doi: 10.1101/lm.033290.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeBlanc KH, Maidment NT, Ostlund SB. Repeated cocaine exposure facilitates the expression of incentive motivation and induces habitual control in rats. PLoS One. 2013;8:e61355. doi: 10.1371/journal.pone.0061355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbit LH, Chieng BC, Balleine BW. Effects of repeated cocaine exposure on habit learning and reversal by N-Acetylcysteine. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.37. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitzer-Torbert N, Apostolidis S, Amoa R, O’Rear C, Kaster M, Stowers J, Ritz R. Post-training cocaine administration facilitates habit learning and requires the infralimbic cortex and dorsolateral striatum. Neurobiol Learn Mem. 2015;118:105–112. doi: 10.1016/j.nlm.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and the prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 19.Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Crombag HS, Gorny G, Li Y, Kolb B, Robinson PV. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cerebral Cortex. 2005;15:341–348. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- 21.Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 22.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong WC, Ford KA, Pagels NE, McCutcheon JE, Marinelli M. Adolescents are more vulnerable to cocaine addiction: behavioral and electrophysiological evidence. J Neurosci. 2013;33:4913–4922. doi: 10.1523/JNEUROSCI.1371-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neuro. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 25.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;4:78–96. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 26.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 27.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry. 2013;3:e238. doi: 10.1038/tp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Dawirs RR, Teuchert-Noodt G, Busse M. Single doses of methamphetamine cause changes in the density of dendritic spines in the prefrontal cortex of gerbils (Meriones unguiculatus) Neuropharmacology. 1991;30:275–282. doi: 10.1016/0028-3908(91)90155-5. [DOI] [PubMed] [Google Scholar]
- 31.Heijtz RD, Kolb B, Forssberg H. Can a therapeutic dose of amphetamine during pre-adolescence modify the pattern of synaptic organization in the brain? Eur J Neurosci. 2003;18:3394–3399. doi: 10.1046/j.0953-816x.2003.03067.x. [DOI] [PubMed] [Google Scholar]
- 32.Muhammad A, Kolb B. Maternal separation altered behavior and neuronal spine density without influencing amphetamine sensitization. Behav Brain Res. 2011;223:7–16. doi: 10.1016/j.bbr.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Muhammad A, Kolb B. Mild prenatal stress-modulated behavior and neuronal spine density without affecting amphetamine sensitization. Dev Neurosci. 2011;33:85–98. doi: 10.1159/000324744. [DOI] [PubMed] [Google Scholar]
- 34.Frankfurt M, Wang HY, Marmolejo N, Bakshi K, Friedman E. Prenatal cocaine increases dendritic spine density in cortical and subcortical brain regions of the rat. Dev Neurosci. 2009;31:71–75. doi: 10.1159/000207495. [DOI] [PubMed] [Google Scholar]
- 35.Frankfurt M, Salas-Ramirez K, Friedman E, Luine V. Cocaine alters dendritic spine density in cortical and subcortical brain regions of the postpartum and virgin female rat. Synapse. 2011;65:955–961. doi: 10.1002/syn.20918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salas-Ramirez KY, Frankfurt M, Alexander A, Luine VN, Friedman E. Prenatal cocaine exposure increases anxiety, impairs cognitive function and increases dendritic spine density in adult rats: influence of sex. Neuroscience. 2010;169:1287–1295. doi: 10.1016/j.neuroscience.2010.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esparza MA, Bollati F, Garcia-Keller C, Virgolini MB, Lopez LM, Brusco A, Shen H-W, Kalivas PW, Cancela LM. Stress-induced sensitization to cocaine: actin cytoskeleton remodeling within mesocorticolimbic nuclei. Eur J Neurosci. 2012;36:3103–3117. doi: 10.1111/j.1460-9568.2012.08239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munoz-Cuevas FJ, Athilingam J, Piscopo D, Wilbrecht L. Cocaine-induced structural plasticity in frontal cortex correlates with conditioned place preference. Nat Neurosci. 2013;16:1367–1369. doi: 10.1038/nn.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rasakham K, Schmidt HD, Kay K, Huizenga MN, Calcagno N, Pierce RC, Spires-Jones TL, Sadri-Vakili G. Synapse density and dendritic complexity are reduced in the prefrontal cortex following seven days of forced abstinence from cocaine self-administration. PLoS One. 2014;9:e102524. doi: 10.1371/journal.pone.0102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ball KT, Wellman CL, Fortenberry E, Rebec GV. Sensitizing regimens of (+/−)3, 4-methylenedioxymethamphetamine (ecstasy) elicit enduring and differential structural alterations in the brain motive circuit of the rat. Neuroscience. 2009;160:264–274. doi: 10.1016/j.neuroscience.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shankaranarayana Rao BS, Lakshmana MK, Meti BL, Raju TR. Chronic (−) deprenyl administration alters dendritic morphology of layer III pyramidal neurons in the prefrontal cortex of adult Bonnett monkeys. Brain Res. 1999;821:218–223. doi: 10.1016/s0006-8993(98)01362-6. [DOI] [PubMed] [Google Scholar]
- 42.Morshedi MM, Rademacher DJ, Meredith GE. Increased synapses in the medial prefrontal cortex are associated with repeated amphetamine administration. Synapse. 2009;63:126–135. doi: 10.1002/syn.20591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrow BA, Hajszan T, Leranth C, Elsworth JD, Roth RH. Prenatal exposure to cocaine is associated with increased number of spine synapses in rat prelimbic cortex. Synapse. 2007;61:862–865. doi: 10.1002/syn.20430. [DOI] [PubMed] [Google Scholar]
- 44.Zehle S, Bock J, Jezierski G, Gruss M, Braun K. Methylphenidate treatment recovers stress-induced elevated dendritic spine densities in the rodent dorsal anterior cingulate cortex. Dev Neurobiol. 2007;67:1891–1900. doi: 10.1002/dneu.20543. [DOI] [PubMed] [Google Scholar]
- 45.Cavaliere C, Cirillo G, Bianco MR, Adriani W, De Simone A, Leo D, Perrone-Capano C, Papa M. Methylphenidate administration determines enduring changes in neuroglial networks in rats. Eur Neuropsychopharmacol. 2012;22:53–63. doi: 10.1016/j.euroneuro.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Vanderschuren LJMJ, Pierce RC. Sensitization processes in drug addiction. Curr Topics Behav Neurosci. 2009;3:179–195. doi: 10.1007/7854_2009_21. [DOI] [PubMed] [Google Scholar]
- 47.Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 48.Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res Rev. 2003;41:203–228. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- 49.Beyer CE, Steketee JD. Cocaine sensitization: Modulation by dopamine D2 receptors. Cereb Cortex. 2002;12:526–535. doi: 10.1093/cercor/12.5.526. [DOI] [PubMed] [Google Scholar]
- 50.Brown RW, Kolb B. Nicotine sensitization increases dendritic length and spine density in the nucleus accumbens and cingulate cortex. Brain Res. 2001;899:94–100. doi: 10.1016/s0006-8993(01)02201-6. [DOI] [PubMed] [Google Scholar]
- 51.Bergstrom HC, McDonald CG, French HT, Smith RF. Continuous nicotine administration produces selective, age-dependent structural alteration of pyramidal neurons from prelimbic cortex. Synapse. 2008;62:31–39. doi: 10.1002/syn.20467. [DOI] [PubMed] [Google Scholar]
- 52.Bergstrom HC, Smith RF, Mollinedo NS, McDonald CG. Chronic nicotine exposure produces lateralized, age-dependent dendritic remodeling in the rodent basolateral amygdala. Synapse. 2010;64:754–764. doi: 10.1002/syn.20783. [DOI] [PubMed] [Google Scholar]
- 53.Mychasiuk R, Muhammad A, Carroll C, Kolb B. Does prenatal nicotine exposure alter the brain’s response to nicotine in adolescence? A neuroanatomical analysis. Eur J of Neurosci. 2013;38:2491–2503. doi: 10.1111/ejn.12245. [DOI] [PubMed] [Google Scholar]
- 54.Gulley JM, Juraska JM. The effects of abused drugs on adolescent development of corticolimbic circuitry and behavior. Neuroscience. 2013;249:3–20. doi: 10.1016/j.neuroscience.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kolb B, Muhammad A. Harnessing the power of neuroplasticity for intervention. Front Hum Neurosci. 2014 doi: 10.3389/fnhum.2014.00377. pepub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 57.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological bases: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torregrossa MM, Quinn JJ, Taylor JR. Impulsivity, compulsivity, and habit: the role of orbitofrontal cortex revisited. Biol Psychiatry. 2008;63:253–255. doi: 10.1016/j.biopsych.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lucantonio F, Stalnaker TA, Shahm Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci. 2012;15:358–366. doi: 10.1038/nn.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guillem K, Kravitz AV, Moorman DE, Peoples LL. Orbitofrontal and insular cortex: Neural response to cocaine-associated cues and cocaine self-administration. Synapse. 2010;64:1–13. doi: 10.1002/syn.20698. [DOI] [PubMed] [Google Scholar]
- 61.Preuss T. Do rats have prefrontal cortex? The Rose-Woosley-Akert program reconsidered. J Cog Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 62.Wallis JD. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nat Neurosci. 2012;15:13–19. doi: 10.1038/nn.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muhammad A, Kolb B. Prenatal tactile stimulation attenuates drug-induced behavioral sensitization, modifies behavior, and alters brain architecture. Brain Res. 2011;1400:53–65. doi: 10.1016/j.brainres.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 64.Wheeler AL, Lerch JP, Chakravarty MM, Friedel M, Sled JG, Fletcher PJ, Josselyn SA, Frankland PW. Adolescent cocaine exposure causes enduring macroscale changes in mouse brain structure. J Neurosci. 2013;33:1797–1803. doi: 10.1523/JNEUROSCI.3830-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lyons D, Friedman DP, Nader MA, Porrino LJ. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J Neurosci. 1996;16:1230–1238. doi: 10.1523/JNEUROSCI.16-03-01230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal., cingulate, and temporal cortices of cocaine patients. Biological Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 67.Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- 68.Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology. 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moreno-Lopez L, Perales JUC, van Son D, Albein-Urios N, Soriano-Mas C, Martinez-Gonzalaz JM, Wiers RW, Verdejo-Garcia A. Cocaine use severity and cerebellar gray matter are associated with reversal learning deficits in cocaine-dependent individuals. Addict Biol. 2014 doi: 10.1111/adb.12143. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 70.Kolb B, Pellis S, Robinson TE. Plasticity and functions of the orbital frontal cortex. Brain Cogn. 2004;55:104–115. doi: 10.1016/S0278-2626(03)00278-1. [DOI] [PubMed] [Google Scholar]
- 71.Gourley SL, Olevska A, Warren MS, Taylor JR, Koleske AJ. Arg kinase regulates prefrontal dendritic spine refinement and cocaine-induced plasticity. J Neurosci. 2012;32:2314–2323. doi: 10.1523/JNEUROSCI.2730-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002;46:271–279. doi: 10.1002/syn.10146. [DOI] [PubMed] [Google Scholar]
- 73.McGuier NS, Padula AE, Lopez MF, Woodward JJ, Mulholland PJ. Withdrawal from chronic intermittent alcohol exposure increases dendritic spine density in the lateral orbitofrontal cortex of mice. Alcohol. 2015;49:21–27. doi: 10.1016/j.alcohol.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 75.Gabbott PL, Warner TA, Busby SJ. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience. 2006;139:1039–1048. doi: 10.1016/j.neuroscience.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 76.Stefanik MT, Kalivas PW. Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front Behav Neurosci. 2013;7:213. doi: 10.3389/fnbeh.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peters A, Kaiserman-Abramof IR. The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am J Anat. 1970;127:321–355. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- 78.Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Ann Rev Physiol. 2009;77:261–282. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- 79.McIntosh S, Howell L, Hemby S. Dopaminergic dysregulation in prefrontal cortex of rhesus monkeys following cocaine self-administration. Front Psychiatry. 2013;4:88. doi: 10.3389/fpsyt.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Homayoun H, Moghaddam B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. J Neurosci. 2006;26:8025–8039. doi: 10.1523/JNEUROSCI.0842-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DePoy LM, Perszyk RE, Zimmermann KS, Koleske AJ, Gourley SL. Adolescent cocaine exposure simplifies orbitofrontal cortical dendritic arbors. Front Pharmacol. 2014;5:228. doi: 10.3389/fphar.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holmes A, Fitzgerald P, Macpherson K, DeBrouse L, Colcaccio G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DePoy LM, Daut R, Brigman JL, MacPherson KP, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, Lovinger DM, Bussey TJ, Camp MC, Holmes A. Chronic alcohol produces neuroadaptations to prime striatal learning. Proc Natl Acad Sci. 2013;110:14783–14788. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:2870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 86.Gourley SL, Swanson AM, Koleske AJ. Corticosteroid-induced neural remodeling predicts behavioral vulnerability and resilience. J Neurosci. 2013;33:3107–3112. doi: 10.1523/JNEUROSCI.2138-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- 88.Ruchhoeft ML, Ohnuma S, McNiell L, Holt CE, Harris WA. The neuronal architecture of Xenopus retinal ganglion cells is sculpted by rho-family GTPases in vivo. J Neurosci. 1999;19:8454–8463. doi: 10.1523/JNEUROSCI.19-19-08454.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Z, van Aelst L, Cline HT. Rho GTPases regulate distinct aspects of dendritic arbor growth in Xenopus central neurons in vivo. Nat Neurosci. 2000;3:217–225. doi: 10.1038/72920. [DOI] [PubMed] [Google Scholar]
- 90.Wong WT, Faulkner-Jones BE, Sanes JR, Wong RO. Rapid dendritic remodeling in the developing retina: dependence on neurotransmission and reciprocal regulation by Rac and Rho. J Neurosci. 2000;20:5024–5036. doi: 10.1523/JNEUROSCI.20-13-05024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sin WC, Haas K, Ruthazer ES, Cline HT. Dendrite growth increased by visual activity required NMDA receptor and Rho GTPase. Nature. 2002;419:475–480. doi: 10.1038/nature00987. [DOI] [PubMed] [Google Scholar]
- 92.Sfakianos MK, Eisman A, Gourley SL, Bradley WD, Scheetz AJ, Settleman J, Taylor JR, Greer CA, Williamson A, Koleske AJ. Inhibition of Rho via Arg and p190RhoGAP in the postnatal mouse hippocampus regulates dendritic spine maturation, synapse and dendrite stability, and behavior. J Neurosci. 2007;27:10982–10992. doi: 10.1523/JNEUROSCI.0793-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Couch BA, DeMarco GJ, Gourley SL, Koleske AJ. Increased dendrite branching in AβPP/PS1 mice and elongation of dendrite arbors by fasudil administration. J Alzheimers Dis. 2010;20:1003–1008. doi: 10.3233/JAD-2010-091114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arthur WT, Petch LA, Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr Biol. 2000;10:719–722. doi: 10.1016/s0960-9822(00)00537-6. [DOI] [PubMed] [Google Scholar]
- 96.Hernandez SE, Settleman J, Koleske AJ. Adhesion-dependent regulation of p190RhoGAP in the developing brain by the Abl-related gene tyrosine kinase. Curr Biol. 2004;14:691–696. doi: 10.1016/j.cub.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 97.Moresco EM, Donaldson S, Williamson A, Koleske AJ. Integrin-mediated dendrite branch maintenance requires Abelson (Abl) family kinases. J Neurosci. 2005;25:6105–6118. doi: 10.1523/JNEUROSCI.1432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bradley WD, Hernandez SE, Settleman J, Koleske AJ. Integrin signaling through Arg activates p190RhoGAP by promoting its binding to p120RasGAp and recruitment to the membrane. Mol Biol Cell. 2006;17:4827–4836. doi: 10.1091/mbc.E06-02-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bradley WD, Koleske AJ. Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. J Cell Sci. 2009;122:3441–3454. doi: 10.1242/jcs.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gourley SL, Koleske AJ, Taylor JR. Loss of dendrite stabilization by the Abl-related gene (Arg) kinase regulates behavioral flexibility and sensitivity to cocaine. Proc Natl Acad Sci. 2009;106:16859–16864. doi: 10.1073/pnas.0902286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koleske AJ. Molecular mechanisms of dendrite stability. Nat Rev Neurosci. 2013;14:536–550. doi: 10.1038/nrn3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gourley SL, Taylor JR, Koleske AJ. Cell adhesion signaling pathways: First responders to cocaine exposure? Commun Integr Biol. 2011;4:30–33. doi: 10.4161/cib.4.1.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Warren MS, Bradley WD, Gourley SL, Lin YC, Simpson MA, Reichardt LF, Greer CA, Taylor JR, Koleske AJ. Integrin β1 signals through Arg to regulate postnatal dendritic arborization, synapse density and behavior. J Neurosci. 2012;32:2824–2834. doi: 10.1523/JNEUROSCI.3942-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cervantes MC, Laughlin RE, Jentsch JD. Cocaine self-administration behavior in inbred mouse lines segregating different capacities for inhibitory control. Psychopharmacology. 2013;229:515–525. doi: 10.1007/s00213-013-3135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wiggins AT, Smith RJ, Shen H, Kalivas PW. Integrins modulate relapse to cocaine-seeking. J Neurosci. 2011;31:16177–16184. doi: 10.1523/JNEUROSCI.3816-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kiraly DD, Ma XM, Mazzone CM, Xin X, Mains RE, Eipper BA. Behavioral and morphological responses to cocaine require kalirin7. Biol Psychiatry. 2010;68:249–255. doi: 10.1016/j.biopsych.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kiraly DD, Nemirovsky NE, LaRese TP, Tomek SE, Yahn SL, Olive MF, Eipper BA, Mains RE. Constitutive knockout of Kalirin-7 leads to increased rates of cocaine self-administration. Mol Pharmacol. 2013;84:582–590. doi: 10.1124/mol.113.087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chandler LJ, Kalivas PW. Neuroscience: Brain’s defence against cocaine. Nature. 2008;455:743–744. doi: 10.1038/455743a. [DOI] [PubMed] [Google Scholar]
- 109.Smith LN, Jedynak JP, Fontenot MR, Hale CF, Dietz KC, Taniguchi M, Thomas FS, Zirlin BC, Birnbaum SG, Huber KM, Thomas MJ, Cowan CW. Fragile X mental retardation protein regulates synaptic and behavioral plasticity to repeated cocaine exposure. Neuron. 2014;82:645–658. doi: 10.1016/j.neuron.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wiggins AT, Pacchioni AM, Kalivas PW. Integrin expression is altered after acute and chronic cocaine. Neurosci Lett. 2009;450:321–323. doi: 10.1016/j.neulet.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mash DC, ffrench-Mullen J, Adi N, Qin Y, Buck A, Pablo J. Gene expression in human hippocampus from cocaine abusers identifies genes which regulate extracellular matrix remodeling. PLoS One. 2007;12:e1187. doi: 10.1371/journal.pone.0001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Drgon T, Zhang PW, Johnson C, Walther D, Hess J, Nino M, Uhl GR. Genome wide association for addiction: replicated results and comparisons of two analytical approaches. PLoS One. 2010;5:e8832. doi: 10.1371/journal.pone.0008832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- 114.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Bio. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Toda S, Shen H, Peters J, Cagle S, Kalivas P. Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J Neurosci. 2006;26:1579–1587. doi: 10.1523/JNEUROSCI.4132-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nestler EJ. Cellular basis of memory for addiction. Dialogues Clin Neurosci. 2013;15:431–434. doi: 10.31887/DCNS.2013.15.4/enestler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Robison AJ, Vialou V, Mazei-Robison M, Feng J, Kourrich S, Collins M, Wee S, Koob G, Turecki G, Neve R, Thomas M, Nestler EJ. Behavioral and structural responses to chronic cocaine require a feedforward loop involving deltaFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J Neurosci. 2013;33:4295–4307. doi: 10.1523/JNEUROSCI.5192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Winstanley CA, LaPlant Q, Theobald DE, Green TA, Bachtell RK, Perrotti LI, DiLeone RJ, Russo SJ, Garth WJ, Self DW, Nestler EJ. DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Winstanley CA, Green TA, Theobald DE, Renthal W, LaPlant Q, DiLeone RJ, Chakravarty S, Nestler EJ. DeltaFosB induction in orbitofrontal cortex potentiates locomotor sensitization despite attenuating the cognitive dysfunction caused by cocaine. Pharmacol Biochem Behav. 2009;93:278–284. doi: 10.1016/j.pbb.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schilman EA, Uylings HB, Galis-de Graaf Y, Joel D, Groenewegen HJ. The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci Lett. 2008;432:40–45. doi: 10.1016/j.neulet.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 121.Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cerebral Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 122.Rakic P, Bourgeois J-P, Goldman-Rakic PS. Synaptic development of the cerebral cortex: Implications for learning, memory, and mental illness. Prog Brain Res. 1994;201:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- 123.Anderson SA, Classey JD, Condé F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67:7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- 124.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 125.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 126.Spear LP. Assessment of adolescent neurotoxicity: Rationale and methodological considerations. Neurotox Teratol. 2007;29:1–9. doi: 10.1016/j.ntt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van Eden CG, Uylings HBM. Postnatal volumetric development of the prefrontal cortex in the rat. J Comp Neurol. 1985;241:268–274. doi: 10.1002/cne.902410303. [DOI] [PubMed] [Google Scholar]
- 129.Bell HC, Pellis SM, Kolb B. Juvenile peer play experiences and the development of the orbitofrontal and medial prefrontal cortices. Behav Brain Res. 2010;207:7–13. doi: 10.1016/j.bbr.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 130.Kalivas PW, Alesdatter JE. Involvement of N-methyl-D-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1993;267:486–495. [PubMed] [Google Scholar]
- 131.Schumann J, Yaka R. Prolonged withdrawal from repeated noncontingent cocaine exposure increases NMDA receptor expression and ERK activity in the nucleus accumbens. J Neurosci. 2009;29:6955–6963. doi: 10.1523/JNEUROSCI.1329-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Loftis JM, Janowsky A. Regulation of NMDA receptor subunits and nitric oxide synthase expression during cocaine withdrawal. J Neurochem. 2000;75:2040–2050. doi: 10.1046/j.1471-4159.2000.0752040.x. [DOI] [PubMed] [Google Scholar]
- 133.Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci. 1999;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci. 1999;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- 135.Yashiro K, Philpot BD. Regulation of NMDA subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lin YC, Yeckel MF, Koleske AJ. Abl2/Arg controls dendritic spine and dendrite arbor stability via distinct cytoskeletal control pathways. J Neurosci. 2013;33:1846–1857. doi: 10.1523/JNEUROSCI.4284-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vengeliene V, Bachteler D, Danysz W, Spanagel R. The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacology. 2005;48:822–829. doi: 10.1016/j.neuropharm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 138.Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci. 2011;108:19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci USA. 2013;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ma YY, Chu NN, Guo CY, Han JS, Cui CL. NR2B-containing NMDA receptor is required for morphine- but not stress-induced reinstatement. Exp Neuro. 2007;203:309–319. doi: 10.1016/j.expneurol.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 141.Ma YY, Yu P, Guo CY, Cui CL. Effects of ifenprodil on morphine-induced conditioned place preference and spatial learning and memory in rats. Neurochem Res. 2011;36:383–391. doi: 10.1007/s11064-010-0342-9. [DOI] [PubMed] [Google Scholar]
- 142.Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387–399. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- 143.Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wagner FA, Anthony JC. From first drug use to drug dependence: developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- 145.O’Brien MS, Anthony JC. Risk of becoming cocaine dependent: Epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology. 2005;30:1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- 146.Kessler R, Aguilar-Gaxiola S, Berglund P, Caraveo-Anduaga J, DeWit D, Greenfield S, Kolody B, Olfson M, Wega W. Patterns and predictors of treatment seeking after onset of a substance use disorder. Arch Gen Psychiatry. 2001;58:1065–1071. doi: 10.1001/archpsyc.58.11.1065. [DOI] [PubMed] [Google Scholar]
- 147.Santucci AC, Capodilupo S, Bernstein J, Gomez-Ramirez M, Milefsky R, Mitchell H. Cocaine in adolescent rats produces residual memory impairments that are reversible with time. Neurotoxicol Teratol. 2004;26:651–661. doi: 10.1016/j.ntt.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 148.Santucci AC. Adolescent cocaine exposure impairs working memory and enhances fear memory in rats. Exp Clin Psychopharmacol. 2008;16:77–85. doi: 10.1037/1064-1297.16.1.77. [DOI] [PubMed] [Google Scholar]
- 149.Butter CM. Perseveration in extinction and in discrimination reversal tasks following selective frontal ablations in Macaca mulatta. Physiol Behav. 1969;4:163–171. [Google Scholar]
- 150.Jones B, Mishkin M. Limbic lesions and the problem of stimulus-reinforcement associations. Exp Neurol. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- 151.Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- 152.Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- 153.Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Izquierdo A, Suda R, Murray E. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hornak J, O’Doherty J, Bramham J, Rolls E, Morris R, Bullock P, Polkey C. Reward-related reversal learning after surgical excisions in the orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- 156.Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28:1124–1130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- 158.Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology. 2012;219:607–620. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stalnaker TA, Cooch NK, Schoenbaum G. What the orbitofrontal cortex does not do. Nat Neurosci. 2015;18:620–627. doi: 10.1038/nn.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McDannald MA, Jones JL, Takahashi YK, Schoenbaum G. Learning theory: a driving force in understanding orbitofrontal function. Neurobiol Learn Mem. 2014;108:22–27. doi: 10.1016/j.nlm.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jentsch JD, Olausson P, de la Garza R, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- 162.Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experiences rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- 163.McCracken CB, Grace AA. Persistent cocaine-induced reversal learning deficits are associated with altered limbic cortico-striatal local field potential synchronization. J Neurosci. 2013;33:17469–17482. doi: 10.1523/JNEUROSCI.1440-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn Mem. 2007;14:325–328. doi: 10.1101/lm.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Laughlin RE, Grant TL, Williams RW, Jentsch JD. Genetic dissection of behavioral flexibility: reversal learning in mice. Bio Psychiatry. 2011;69:1109–1116. doi: 10.1016/j.biopsych.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR. Dissociable regulation of instrumental action within mouse prefrontal cortex. Eur J Neurosci. 2010;32:1726–1734. doi: 10.1111/j.1460-9568.2010.07438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Krueger DD, Howell JL, Oo H, Olausson P, Taylor JR, Nairn AC. Prior chronic cocaine exposure in mice induces persistent alterations in cognitive function. Behav Pharmacol. 2009;20:695–704. doi: 10.1097/FBP.0b013e328333a2bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gourley SL, Olevska A, Zimmermann KS, Ressler KJ, Dileone RJ, Taylor JR. The orbitofrontal cortex regulates outcome-based decision-making via the lateral striatum. Eur J Neurosci. 2013;38:2382–2388. doi: 10.1111/ejn.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gourley SL, Howell JL, Rios M, DiLeone RJ, Taylor JR. Prelimbic cortex bdnf knock-down reduces instrumental responding in extinction. Learn Mem. 2009;16:756–760. doi: 10.1101/lm.1547909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.DePoy LM, Noble B, Allen AG, Gourley SL. Developmentally divergent effects of Rho-kinase inhibition on cocaine- and BDNF-induced behavioral plasticity. Behav Brain Res. 2013;243:171–175. doi: 10.1016/j.bbr.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Woronowicz A, Cawley NX, Chang S-Y, Koshimizu H, Phillips AW, Xiong Z-G, Loh YP. Carboxypeptidase E knockout mice exhibit abnormal dendritic arborization and spine morphology in central nervous system neurons. J Neurosci Res. 2010;88:64–72. doi: 10.1002/jnr.22174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vigers AJ, Amin DS, Talley-Farnham T, Gorski JA, Xu B, Jones KR. Sustained expression of brain-derived neurotrophic factor is required for maintenance of dendritic spines and normal behavior. Neuroscience. 2012;212:1–18. doi: 10.1016/j.neuroscience.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Reichardt LF. Neurotrophin-regulated signaling pathways. Phil Trans R Soc B. 2006;31:1545–1564. doi: 10.1098/rstb.2006.1894. (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee B, Groman S, London ED, Jentsch JD. Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology. 2007;32:2125–2134. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- 175.De Steno DA, Schmauss C. A role for dopamine D2 receptors in reversal learning. Neuroscience. 2009;162:118–127. doi: 10.1016/j.neuroscience.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang H-D, Stanwood GD, Grandy DK, Deutch AY. Dystrophic dendrites in prefrontal cortical pyramidal cells of dopamine D1 and D2 but not D4 receptor knockout mice. Brain Res. 2009;1300:58–64. doi: 10.1016/j.brainres.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Butter CM, Mishkin M, Rosvold HE. Conditioning and extinction of a food-rewarded response after selective ablations of frontal cortex in rhesus monkeys. Exp Neurol. 1963;7:65–75. doi: 10.1016/0014-4886(63)90094-3. [DOI] [PubMed] [Google Scholar]
- 178.Kolb B, Nonneman AJ, Singh RK. Double dissociation of spatial impairments and perseveration following selective prefrontal lesions in rats. J Comp Physiol Psych. 1974;87:772–780. doi: 10.1037/h0036970. [DOI] [PubMed] [Google Scholar]
- 179.Pierce RC, Vanderschuren LJMJ. Kicking the habit: The neural basis of ingrained behaviors in cocaine addiction. Neurosci Biobehav Rev. 2010;35:212–219. doi: 10.1016/j.neubiorev.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schwabe L, Dickinson A, Wolf OT. Stress, habits, and drug addiction: a psychoneuroendocrinological perspective. Exp Clin Psychopharmacol. 2011;19:53–63. doi: 10.1037/a0022212. [DOI] [PubMed] [Google Scholar]
- 181.Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Miles FJ, Everitt BJ, Dickinson A. Oral cocaine seeking by rats: Action or habit? Behav Neurosci. 2013;117:927–938. doi: 10.1037/0735-7044.117.5.927. [DOI] [PubMed] [Google Scholar]
- 183.Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci. 2010;30:15457–15463. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nelson A, Killcross S. Amphetamine exposure enhances habit formation. J Neurosci. 2006;26:3805–3812. doi: 10.1523/JNEUROSCI.4305-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nordquist RE, Voom R, de Mooij-van Malsen GJ, Joosten RN, Pennartz CM, Vanderschuren LJ. Augmented reinforcer value and accelerated habit formation after repeated amphetamine treatment. Eur Neuropsychopharmacology. 2007;17:532–540. doi: 10.1016/j.euroneuro.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 186.Dickinson A. Contemporary Animal Learning Theory. Cambridge: Cambridge University Press; 1980. [Google Scholar]
- 187.Mailly P, Aliane V, Groenewegen HJ, Haber SN, Deniau J-M. The rat prefrontostriatal system analyzed in 3D: Evidence for multiple interacting functional units. J Neurosci. 2013;33:5718–5727. doi: 10.1523/JNEUROSCI.5248-12.2013. (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Maroteaux M, Valjent E, Longueville S, Topilko P, Girault JA, Hervé D. Role of the plasticity-associated transcription factor zif268 in the early phase of instrumental learning. PLos One. 2014;9:e81868. doi: 10.1371/journal.pone.0081868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. 2008;28:1437–1448. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yin HH, Mulcare SP, Hilário MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jedynak JP, Uslaner JM, Esteban JA, Robinson TE. Methamphetamine-induced structural plasticity in the dorsal striatum. Eur J Neurosci. 2007;25:847–853. doi: 10.1111/j.1460-9568.2007.05316.x. [DOI] [PubMed] [Google Scholar]
- 192.Luk C-H, Wallis JD. Choice coding in frontal cortex during stimulus-guided or action-guided decision-making. J Neurosci. 2013;33:1864–1871. doi: 10.1523/JNEUROSCI.4920-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of the orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lasseter HC, Ramirez DR, Xie X, Fuchs RA. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. Eur J Neurosci. 2009;30:1370–1381. doi: 10.1111/j.1460-9568.2009.06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Swanson AM, Allen AG, Shapiro LP, Gourley SL. GABAAα1-mediated plasticity in the orbitofrontal cortex regulates context-dependent action selection. Neuropsychopharmacology. 2015;40:1027–1036. doi: 10.1038/npp.2014.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lasseter HC, Xie X, Arguello AA, Wells AM, Hodges MA, Fuchs RA. Contribution of a mesocorticolimbic subcircuit to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2014;39:660–669. doi: 10.1038/npp.2013.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Brock JW, Farooqui S, Ross K, Prasad C. Localization of dopamine D2 receptor protein in rat brain using polyclonal antibody. Brain Res. 1992;578:244–250. doi: 10.1016/0006-8993(92)90253-6. [DOI] [PubMed] [Google Scholar]
- 198.Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, Greengard P, Nairn AC, Nestler EJ, Cowan CW. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Besnard A, Bouveyron N, Kappes V, Pascoli V, Pagès C, Heck N, Vanhoutte P, Caboche J. Alterations of molecular and behavioral responses to cocaine by selective inhibition of Elk-1 phosphorylation. J Neurosci. 2011;31:14296–14307. doi: 10.1523/JNEUROSCI.2890-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Giza JI, Jung Y, Jeffrey RA, Neugebauer NM, Picciotto MR, Biederer T. The synaptic adhesion molecular SynCAM 1 contributes to cocaine effects on synapse structure and psychostimulant behavior. Neuropsychopharmacology. 2013;38:628–638. doi: 10.1038/npp.2012.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Penzes P, Cahill ME, Jones KA, Van Leeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disease. Nat Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rosen G, et al. The mouse brain library @ www.Mbl.Org. International Mouse Genome Conference. 2000;14:166. [Google Scholar]
- 203.Shilling PD, Kelsoe JR, Kuczenski R, Segal DS. Differential regional zif268 messenger RNA expression in an escalating dose/binge model of amphetamine-induced psychosis. Neuroscience. 2000;96:83–90. doi: 10.1016/s0306-4522(99)00510-2. [DOI] [PubMed] [Google Scholar]
- 204.Klebaur JE, Ostrander MM, Norton CS, Watson SJ, Akil H, Robinson TE. The ability of amphetamine to evoke arc (Arg 3.1) mRNA expression in the caudate, nucleus accumbens and neocortex is modulated by environmental context. Brain Res. 2002;930:30–36. doi: 10.1016/s0006-8993(01)03400-x. [DOI] [PubMed] [Google Scholar]
- 205.Nordquist RE, Vanderschuren LJ, Jonker AJ, Bergsma M, de Vries TJ, Pennartz CM, Voom P. Expression of amphetamine sensitization is associated with recruitment of a reactive neuronal population in the nucleus accumbens core. Psychopharmacology. 2008;198:113–126. doi: 10.1007/s00213-008-1100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Banerjee PS, Aston J, Khundakar AA, Zetterström TS. Differential regulation of psychostimulant-induced gene expression of brain derived neurotrophic factor and the immediate-early gene Arc in the juvenile and adult brain. Eur J Neurosci. 2009;29:465–476. doi: 10.1111/j.1460-9568.2008.06601.x. [DOI] [PubMed] [Google Scholar]
- 207.Schwendt M, McGinty JF. Amphetamine up-regulates activator of G-protein signaling 1 mRNA and protein levels in rat frontal cortex: the role of dopamine and glucocorticoid receptors. Neuroscience. 2010;168:96–107. doi: 10.1016/j.neuroscience.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Horner KA, Gilbert YE, Noble ES. Differential regulation of 5-HT2A receptor mRNA expression following withdrawal from a chronic escalating dose regiment of D-amphetamine. Brain Res. 2011;1390:10–20. doi: 10.1016/j.brainres.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 209.Cáceda R, Binder EB, Kinkead B, Nemeroff CB. The role of endogenous neurotensin in psychostimulant-induced disruption of prepulse inhibition and locomotion. Schizophr Res. 2012;136:88–95. doi: 10.1016/j.schres.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mychasiuk R, Muhammad A, Ilnytskyy S, Kolb B. Persistent gene expression changes in NAc, mPFC, and OFC associated with previous nicotine or amphetamine exposure. Behav Brain Res. 2013;256:655–661. doi: 10.1016/j.bbr.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 211.Murray RC, Hebbard JC, Logan AS, Vanchipurakel GA, Gilbert YE, Horner KA. Stress and withdrawal from d-amphetamine alter 5-HT2A receptor mRNA expression in the prefrontal cortex. Neurosci Lett. 2014;559:44–49. doi: 10.1016/j.neulet.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 212.Trinh JV, Nehrenberg DL, Jacobsen JP, Caron MG, Wetsel WC. Differential psychostimulant-induced activation of neural circuits in dopamine transporter knockout and wild type mice. Neuroscience. 2003;118:297–310. doi: 10.1016/s0306-4522(03)00165-9. [DOI] [PubMed] [Google Scholar]
- 213.Grimm JW, Shaham Y, Hope BT. Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas. Behav Pharmacol. 2002;13:379–388. doi: 10.1097/00008877-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Thomas KL, Arroyo M, Everitt BJ. Induction of the learning and plasticity-associated gene Zif268 following exposure to a discrete cocaine-associated stimulus. Eur J Neurosci. 2003;17:1964–1972. doi: 10.1046/j.1460-9568.2003.02617.x. [DOI] [PubMed] [Google Scholar]
- 215.Samaha AN, Mallet N, Ferguson SM, Gonon F, Robinson TE. The rate of cocaine administration alters gene regulation and behavioral plasticity: Implications for addiction. J Neurosci. 2004;24:6362–6370. doi: 10.1523/JNEUROSCI.1205-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cao J, Lotfipour S, Loughlin SE, Leslie FM. Adolescent maturation of cocaine-sensitive neural mechanisms. Neuropsychopharmacology. 2007;32:2279–2289. doi: 10.1038/sj.npp.1301349. [DOI] [PubMed] [Google Scholar]
- 217.Zavala AR, Biswas S, Harlan RE, Neisewander JL. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145:438–452. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology. 2008;33:2969–2980. doi: 10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hearing MC, Miller SW, See RE, McGinty JF. Relapse to cocaine seeking increases activity-regulated gene expression differentially in the prefrontal cortex of abstinent rats. Psychopharmacology. 2008;198:77–91. doi: 10.1007/s00213-008-1090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zavala AR, Osredkar T, Joyce JN, Neisewander JL. Upregulation of Arc mRNA expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking behavior. Synapse. 2008;62:421–431. doi: 10.1002/syn.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zavala AR, Browning JR, Dickey ED, Biswas S, Neisewander JL. Region-specific involvement of AMPA/Kainate receptors in Fos protein expression induced by cocaine-conditioned cues. Eur Neuropsychopharmacology. 2008;18:600–611. doi: 10.1016/j.euroneuro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Caster JM, Kuhn CM. Maturation of coordinated immediate early gene expression by cocaine during adolescence. Neuroscience. 2009;160:13–31. doi: 10.1016/j.neuroscience.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kufahl PR, Pentkowski NS, Heintzelman K, Neisewander JL. Cocaine-induced Fos expression is detectable in the frontal cortex and striatum of rats under isoflurane but not α-chloralose anesthesia: implications for FMRI. J Neurosci Methods. 2009;181:241–248. doi: 10.1016/j.jneumeth.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Unal CT, Beverley JA, Willuhn I, Steiner H. Long-lasting dysregulation of gene expression in corticostriatal circuits after repeated cocaine treatment in adult rats: effects on zif 268 and homer 1a. Eur J Neurosci. 2009;29:1615–1626. doi: 10.1111/j.1460-9568.2009.06691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Fritz M, El Rawas R, Salti A, Klement S, Bardo MT, Kemmler G, Dechant G, Saria A, Zernig G. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict Biol. 2011;16:273–284. doi: 10.1111/j.1369-1600.2010.00285.x. [DOI] [PubMed] [Google Scholar]
- 226.Ziólkowska B, Kielbinski M, Gieryk A, Soria G, Maldonado R, Przewlocki R. Regulation of the immediate-early genes arc and zif268 in a mouse operant model of cocaine seeking reinstatement. J Neural Transm. 2011;118:877–887. doi: 10.1007/s00702-011-0583-z. [DOI] [PubMed] [Google Scholar]
- 227.El Rawas R, Klement S, Kummer KK, Fritz M, Dechant G, Saria A, Zernig G. Brain regions associated with the acquisition of conditioned place preference for cocaine vs. social interaction. Front Behav Neurosci. 2012;6:63. doi: 10.3389/fnbeh.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Besson M, Pelloux Y, Dilleen Y, Theobald DE, Lyon A, Belin-Rauscent A, Robbins TW, Dalley JW, Everitt BJ, Belin D. Cocaine modulation of frontostriatal expression of Zif268, D2, and 5-HT2c receptors in high and low impulsive rats. Neuropsychopharmacology. 2013;38:1963–1973. doi: 10.1038/npp.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Burton CL, Rizos Z, Diwan M, Nobrega JN, Fletcher PJ. Antagonizing 5-HT2A receptors with M100907 and stimulating 5-HT2C receptors with Ro60-0175 blocks cocaine-induced locomotion and zif268 mRNA expression in Sprague-Dawley rats. Behav Brain Res. 2013;240:171–181. doi: 10.1016/j.bbr.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 230.Riedy MD, Keefe KA. Lack of increased immediate early gene expression in rats reinstating cocaine-seeking behavior to discrete sensory cues. PLoS One. 2013;8:e72883. doi: 10.1371/journal.pone.0072883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kufahl PR, Peartree NA, Heintzelman KL, Chung M, Neisewander JL. Region-specific effects of isoflurane anesthesia on Fos immunoreactivity in response to intravenous cocaine challenge in rats with a history of repeated cocaine administration. Brain Res. 2015;1594:256–266. doi: 10.1016/j.brainres.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]