Abstract
Cerebrospinal fluid (CSF) rhinorrhea occurs due to communication between the intracranial subarachnoid space and the sinonasal mucosa. It could be due to trauma, raised intracranial pressure (ICP), tumors, erosive diseases, and congenital skull defects. Some leaks could be spontaneous without any specific etiology. The potential leak sites include the cribriform plate, ethmoid, sphenoid, and frontal sinus. Glucose estimation, although non-specific, is the most popular and readily available method of diagnosis. Glucose concentration of > 30 mg/dl without any blood contamination strongly suggests presence and the absence of glucose rules out CSF in the fluid. Beta-2 transferrin test confirms the diagnosis. High-resolution computed tomography and magnetic resonance cisternography are complementary to each other and are the investigation of choice. Surgical intervention is indicated, when conservative management fails to prevent risk of meningitis. Endoscopic closure has revolutionized the management of CSF rhinorrhea due to its less morbidity and better closure rate. It is usually best suited for small defects in cribriform plate, sphenoid, and ethmoid sinus. Large defects can be repaired when sufficient experience is acquired. Most frontal sinus leaks, although difficult, can be successfully closed by modified Lothrop procedure. Factors associated with increased recurrences are middle age, obese female, raised ICP, diabetes mellitus, lateral sphenoid leaks, superior and lateral extension in frontal sinus, multiple leaks, and extensive skull base defects. Appropriate treatment for raised ICP, in addition to proper repair, should be done to prevent recurrence. Long follow-up is required before leveling successful repair as recurrences may occur very late.
Keywords: Cerebrospinal fluid pressure, cerebrospinal fluid rhinorrhea, cerebrospinal fluid, endoscopic surgical procedure, skull base
Introduction
Neuroendoscopy has grown rapidly in the recent years as a therapeutic modality of the treatment in a variety of brain and spinal disorders.[1,2,3,4,5] Cerebrospinal fluid (CSF) rhinorrhea occurs when there is a communication between the intracranial subarachnoid space and the sinonasal mucosa. Majority of the cases are traumatic in etiology, mostly caused by the accidental head trauma or iatrogenic injury. CSF leaks may also be secondary to raised intracranial pressure (ICP), tumors, erosive diseases, and due to the congenital skull base defects. Some leaks could be spontaneous without any specific etiology. Most of the traumatic CSF leaks stop after conservative treatment. Cases with persistent CSF rhinorrhea need definitive intervention. The risk of meningitis in untreated patients is reported to be about 10% annually.
Endoscopic closure has revolutionized the surgical management of CSF rhinorrhea due to reduced morbidity and better closure rate.[3,6,7,8,9,10,11,12,13,14,15,16] Transnasal endoscopic repair has about 87 to 100% success rate.
History
The first successful intracranial repair of the CSF leak was reported by Dandy in 1926. Increased morbidity of intracranial approach resulted in introduction of the extracranial repair by Dohlman in 1948. Hirsch performed transnasal surgery in 1952. Endoscopic treatment of this condition was reported by Wigand in 1981. Since then, this technique has gained increasing attention. The advantages of the endoscopic treatment (excellent visualization, precise graft placement, short operating time, and better results) have popularized it worldwide.
Types of Cerebrospinal Fluid Rhinorrhea
It is very important to know the etiology of CSF rhinorrhea in order to properly plan the repair. CSF leak could be traumatic or non-traumatic.
Traumatic
Trauma (80-90%) is the most common cause of CSF leak. It could be due to head injury or following surgery on skull base.[17]
Head injury
Usually, the fracture involves some portion of the anterior cranial fossa floor. The leaks through the cribriform plate or ethmoid sinus roof are due to the tightly adherent dura in these areas. Another frequently seen anterior cranial fossa fracture site is the posterior wall of the frontal sinus. A less common site is the middle cranial fossa fracture involving sphenoid sinus.
The CSF leak is seen in about 15 to 30% cases if a skull base fracture is present. These leaks may be either immediate (within 48 hours) or delayed. Nearly 95% of the delayed leaks will manifest within first 3 months of injury. Patients with head injuries and the periorbital hematoma are at greater risk of unobserved dural tear and delayed CSF leakage. CSF rhinorrhea persisting for more than 7 days carries a significantly increased risk of developing meningitis.[17] Patients who fail conservative trial need surgery to prevent infective complications.[18]
Iatrogenic
Iatrogenic trauma accounts for about 16% of traumatic cases of CSF rhinorrhea. It can occur following endoscopic sinus surgery (ESS), skull base surgery, trans-sphenoid pituitary surgery, and craniofacial resection. The risk of CSF leak after ESS is reported to be around 0.5%. The most common site of injury during ESS is the lateral cribriform lamella, mainly on the right side. The other common sites of injuries include the posterior fovea ethmoidalis, sphenoid sinus, and the posterior aspect of the frontal recess.[19,20]
Spontaneous leaks
Spontaneous leaks could be associated with or without raised ICP.
Leaks secondary to raised ICP
High pressure leaks could account up to 45% of the non-traumatic CSF rhinorrhea. Sustained increased ICP is thought to lead to remodeling and the thinning of the skull base. The increased hydrostatic pressure of long duration is capable of the bone erosion. Bone erosion and creation of an osteodural defect in pneumatized parts of the skull base lead to CSF leak. The cribriform plate, craniopharyngeal canal, sella, and spheno-occipital synchondrosis are some of the possible sites of the predilection of the leak. Arachnoid granulations in proximity to the ethmoid and sphenoid sinus have been implicated as precursors of osteodural leaks. CSF leaks in these cases have been postulated to represent a manifestation of benign intracranial hypertension or pseudo tumor cerebri.[21,22,23,24]
Leaks with normal intracranial pressure
Normal pressure leaks represent 55% of the non-traumatic cases of the CSF rhinorrhea.[25,26] It is hypothesized that the spontaneous leak is due to the physiologic alterations in CSF pressure that lead to point erosions in the skull base. This theory is based on the fact that elevations in ICP up to 80 mm of water can occur for few seconds in normal person.
Congenital
Congenital causes may be associated with or without increased ICP.[27] These defects may involve the failure of the closure of the anterior neuropore. This can lead to the herniation of the meninges and brain through the defect (encephaloceles). Another congenital defect could be due to the persistent craniopharyngeal canal. This is a vertical midline defect connecting the middle cranial fossa to the sphenoid sinus. CSF rhinorrhea in primary empty sella syndrome is thought to be secondary to a congenital widening of the diaphragma sella.
Miscellaneous causes
Other non-traumatic causes of CSF leak include erosion of the skull base by tumors, infection, mucocele, and following radiation.
Locations of the Leak
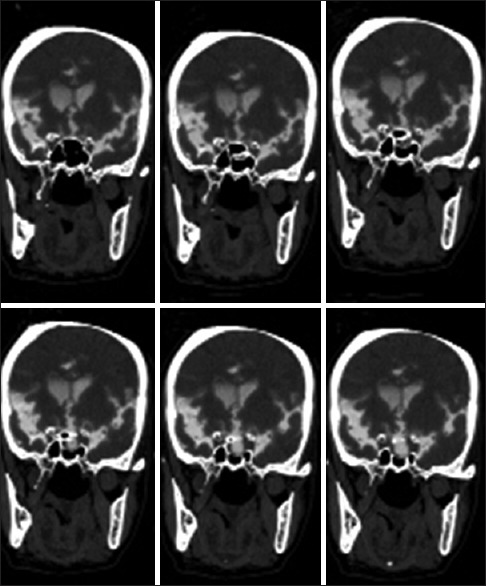
The ethmoid roof, cribriform plate, and sphenoid sinus are the common locations of the defect in CSF rhinorrhea.[26,28] Frontal sinus could also be the site of the leak [Figure 1]. The frequency of the defect location varies in different types of CSF rhinorrhea. The most of the leaks are along the course of anterior ethmoid artery followed by the sphenoid sinus in spontaneous leaks. Defect in the sphenoid sinus could be in the roof, the lateral wall, anterior wall, or the posterior wall [Figures 2 and 3]. The posterior wall defect could be communicating to the posterior fossa. Lateral sphenoid defects are larger than those in ethmoid or mid sphenoid locations while the cribriform plate defects are usually small.[29]
Figure 1.
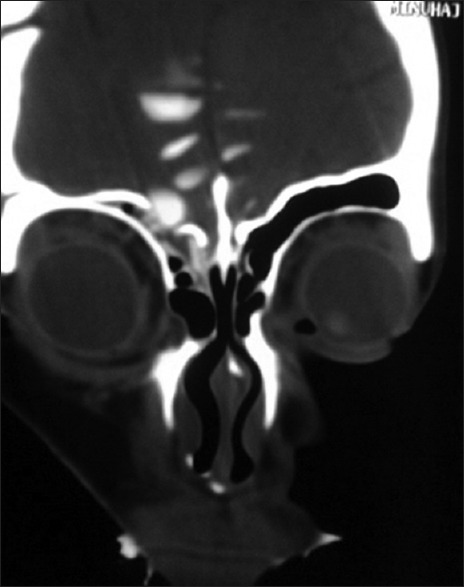
CT cisternography showing defect in the frontal sinus
Figure 2.
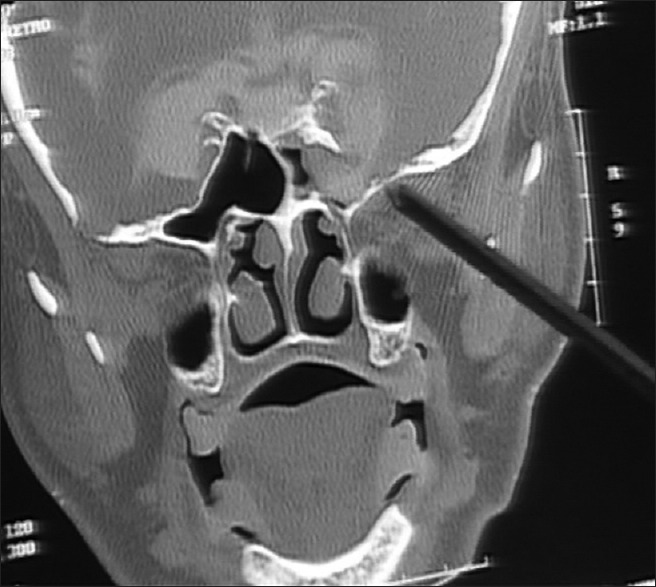
CT cisternography showing defect in the lateral wall of sphenoid sinus
Figure 3.
CT cisternography showing defect in sphenoid sinus
The most common defect location is usually the anterior ethmoid at the attachment of the medial concha, followed by the junction between the ethmoid and sphenoid sinus in iatrogenic cases. The frontal sinus aditus and the medial ethmoid region can also be involved in iatrogenic cases.[20]
Diagnosis
Detecting cerebrospinal fluid in the fluid
It is essential to identify fluid leaking from the nose as CSF. Drops of fluid placed on absorbent filter paper may result in the double-ring sign (a central circle of blood and an outer clear ring of CSF). Absorbent filter test, chloride, and total protein estimation of the fluid are not specific for CSF.
Estimation of glucose using Glucostix test strips has been a traditional method for the detection of CSF in nasal and ear discharge. Glucose detection using Glucostix test strips is not recommended as a confirmatory test due to its lack of specificity and sensitivity. Interpretation of the results is confounded by the contamination from glucose-containing fluid (tears, nasal mucus, and blood). Meningitis or other intracranial infections lower the concentration of glucose in CSF giving a false-negative result.[30] Glucose can be detected in airways secretions in diabetes mellitus, stress hyperglycemia, and inflamed nasal epithelium due to viral illness.[31] Glucose estimation is most popular and readily available Whereas the other tests are either not available or are very costly. Presence of CSF is strongly suspected when the glucose concentration is >30 mg/dl, if there is no blood contamination in the fluid. The specimen usually does not contain CSF if the results show an absence of glucose in the fluid.
Beta-2 transferrin can be detected by immunofixation electrophoresis. With sensitivity of 94% to 100%, and specificity of 98% to 100%, this assay has become the gold standard in detection of CSF leakage. The quantity of the specimen should be preferably 2 ml of serum and 2 ml of nasal fluid (minimum of 0.5 ml serum and 1 ml of nasal fluid). There is usually a delay of 5 to 7 days in order to obtain the report.
Imaging to detect site of the leak
Imaging is an important component in the investigation of unilateral watery discharge suspicious of CSF. It may be difficult to demonstrate the exact site of the leak. Fine detail coronal computed tomography (CT) with sub millimeter thickness through the anterior skull base may show small dehiscence and fractures. Magnetic resonance imaging (MRI) can be used to define soft tissue pathology such as inflammatory tissue, meningoencephalocele, or tumor. CT cisternogram can be helpful in defining the exact site of the leak in presence of active CSF rhinorrhea. Per-operative intrathecal fluorescein may be helpful in some cases when other imaging tests do not prove positive.[32]
All the investigations could provide improved CSF rhinorrhea detection when the rhinorrhea is active. Although Valsalva maneuver or jugular venous compression could improve leak detection rate, Valsalva maneuver remains controversial and is not widely used during imaging by many authors.[33,34] The localization of the leak to the right or left nasal cavity may be difficult because of the tendency of the fluid to cross sides and flow from both the nostrils. Sometime, all the investigations may fail to show site of the leak.
Primary Investigations
Although all the investigations are useful in the diagnosis of the CSF leak, high-resolution (HR) CT and MRI scans are primary investigations of choice. These two primary investigations are helpful in the detection of most of the leaks.[35,36]
HR CT scan and MR cisternography are complementary to each other in CSF rhinorrhea cases. Combined modalities of CT and MRI have a higher sensitivity and specificity however obtaining two investigations are not cost-effective in some situations. CT cisternography in these circumstances offers an acceptable method.
Computed tomography scan
HR thin-section axial and coronal scans of cranial and facial region should include all the paranasal sinuses and petrous temporal bones. Coronal CT image could demonstrate fractures and bone defects well than MRI. This can also show protruding soft-tissue (meningoencephalocele) through the bony defect. CT scan could also demonstrate focal fluid accumulation in the sinuses (ethmoid, frontal, sphenoid, and maxillary sinuses), and pneumocephalus in some cases. Most of the mild pneumocephalus usually resolve spontaneously within one week. Delayed pneumocephalus could be due intracranial hypotension secondary to CSF leak. The sensitivity of HR CT scan is from 88.25 to 93%.[29] CT imaging detects the fluid poorly and may not identify exact site of leak when there are multiple fractures or dehiscence.[33] HR CT is not widely available, especially in a resource-constrained country like India.
Magnetic resonance
Thin-section MR cisternography is performed with heavily T2-weighted, fast spin-echo, fat-saturated sequences in CSF rhinorrhea. A short repetition time can be used to achieve similar result with slightly faster imaging times. Although prone position is uncomfortable, it may improve rhinorrhea detection rate, especially in rapid echo-planar imaging with Valsalva maneuver. The intrathecal injection of 0.5 ml of gadopentetate dimeglumine, diluted in 3-5 ml of CSF, for MR cisternography has been found to have high sensitivity and specificity for detection of active CSF rhinorrhea.
Advances in MR imaging techniques have improved sensitivity of MR cisternography from 89% to 100% even in inactive leaks. Therefore, HR CT, MR cisternography, or combinations of both techniques have replaced the previously used invasive procedures.[29] The magnetic resonance cisternogram with coronal fast spin echo T2-weighted images could demonstrate a defect in the cribriform plate and herniation of meninges and brain tissue with adjacent CSF into the bone defect.
MR cisternography, on the other hand, offers poor spatial and bony resolution. The high T2 signal from CSF rhinorrhea may be difficult to differentiate from sinusitis on axial images. Modification of MRI technique by using both T2 MRI images with fluid-attenuated inversion recovery (FLAIR) imaging is very helpful in differentiating CSF from non-CSF fluid.[23]
Secondary Investigations
Secondary investigations such as CT cisternography, radionuclide cisternography, fluorescence cisternography, and diagnostic nasal endoscopy may be useful if MR cisternography and HR CT scan do not show site of leak.
Computed tomography cisternography
Cisternography with an intrathecal injection of nonionic iodinated myelographic contrast medium usually localizes the CSF leak. The image thickness should be in sub millimeter. Both pre- and post-cisternographic images should be acquired and HU values of pre- and post-cisternographic images should be compared. It is important to find out HU value as the post-cisternographic sinus contents may not visually show an increase in attenuation. Increase in HU values by more than 50% in post-cisternographic image indicates leak. It also helps to differentiate extracranial contrast material accumulation from sclerotic sinus walls, dense insipissated sinus secretions and the blood. This is a useful single investigation in resource-constrained situations, especially in active leak [Figures 1–3].[9] The overall incidence of CSF rhinorrhea detection varies from 22 to 100%. The accuracy of active rhinorrhea detection with CT cisternography is comparatively higher (65-85%) than in inactive leak. Computer-reconstructed coronal images are less accurate as compared to direct coronal images. CT cisternography may have a problem in detecting low-flow rhinorrhea or rhinorrhea with hair line communication. Lack of free distribution of contrast in the CSF space or very small amount of dilute contrast media may not be distinguished from the surrounding bone in low flow rhinorrhea.[33] Another limitation of this procedure is its invasive nature.
Intrathecal fluorescein
Non-ophthalmic solution of 0.1 ml of 10% fluorescein is diluted in 10 ml of CSF and injected into the subarachnoid space over a period of 10 minutes. Nasal endoscopy is performed approximately 30 minutes after an intrathecal injection. The surgeon can use this time to perform the initial dissection of the sinuses that would otherwise be required to gain access to the defect. Fluorescein could be directly observed within the sinonasal cavity using standard xenon light sources in most of the cases. A minute amount of fluorescein may be difficult to be observed by standard xenon light sources. A blue-light filter (440-490 nm wave length) can help enhance the visualization of fluorescein.
The intrathecal sodium fluorescein has been found to be useful in localizing the CSF rhinorrhea.[37,38,39] Sensitivity for fluorescein detection varies between 57.7 and 85.6%, while the specificity is 100%. The false-negative rate varies between 15.8 and 43.5%.[40]
Complications such as seizures are reported after its use.[41] These complications from intrathecal application of fluorescein appear to be dose dependent. Diluted concentration of 5%, or lower, helps in minimizing the complications. The side effects, if seen, are transient in dilute concentration. These complications can be further minimized by careful lumbar puncture and slow administration of the dye. The patient should be supervised for 24 hours and a written informed consent from patients for the use of fluorescein is recommended.[42] The US Food and Drug Administration have not approved the use of intrathecal fluorescein for the diagnosis or treatment of CSF rhinorrhea.
Radionuclide cisternography
Radioactive isotopes can be introduced into the CSF by means of lumbar or sub-occipital puncture. The distribution of these agents can be determined by using serial scanning or scintiphotography. Another option is to introduce nasal pledgets in various high-risk areas. These pledgets can be analyzed for the presence of the tracer. Head images are acquired 2, 6, 12, and 24 hours after injection of the isotope. Follow-up 48- or 72-hour scans are possible with indium-111 (111In) and are useful in the detection of intermittent CSF fluid leaks. The sensitivity for CSF leaks is 50 to 100% and the specificity is almost 100% for contemporary radionuclide cisternography.
Despite the relative safety, this technique has several limitations. Radionuclide cisternography is used only when occult CSF leak is suspected and imaging does not show a definite skull base defect. Radionuclide cisternographic examinations do not adequately localize the defect well enough to be the sole diagnostic method. It is, therefore, reserved for complex cases when the diagnosis is in question.[34] The isotope can be absorbed into the circulatory system and can contaminate extracranial tissue. Patient positioning can cause distal pledgets to incorrectly take up the isotope. The readings of the radioactivity should be high to determine a true leak. Borderline readings are not reliable. False-positive results are seen in as high as 33% patients.
Nasal endoscopy
At times, preoperative nasal endoscopy could help in localizing the defect, when all other methods do not help in localizing the CSF rhinorrhea [Figure 4].[43] Valsalva maneuver and jugular compression could improve detection rate.
Figure 4.
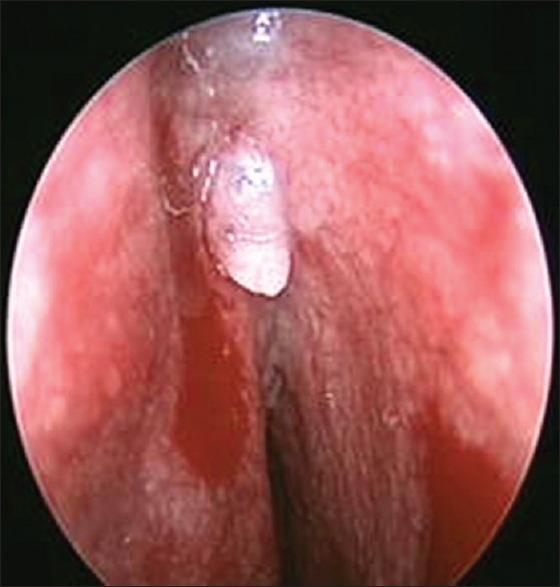
Transnasal endoscopy showing encephalocele defect
Treatment
Conservative management
Most of the traumatic CSF rhinorrhea can be managed with conservative treatment. The routine management involves acetazolamide, laxatives, and the prophylactic antibiotics. Measures such as bed rest with head elevated, avoidance of sneezing, etc., are also effective. A lumbar drain can be useful. The conservative treatment for 2 to 4 weeks can be tried if a CSF leak is caused by trauma or operation.[44]
Operative management
Surgery is indicated to prevent complications if conservative management fails. Success rate of CSF leak by the intracranial approach is in the range of 70 to 90% while it is 87 to 100% in transnasal endoscopic technique. The morbidity of the intracranial approach is significantly higher as compared to the transnasal endoscopic approach. There is a higher incidence of wound infection, severe headache, and anosmia following intracranial approach.
Intracranial approach
Success rate up to 90% is reported after the first intracranial operation and 94% after second-look intracranial approach. This is found to be safe without any postoperative neurological deterioration.[45] Combined intracranial extradural and intradural approach allows the visualization and repair of the entire anterior skull base. It is essentially indicated for patients with extensive bone defects in the cranial base, multiple fractures of the ethmoid bone and the posterior wall of the frontal sinus. It is also indicated when leak is associated with other intracranial lesions, such as intracranial hematomas, and post-traumatic intracranial infection, requiring surgery.[18,45,46] Intracranial approach is also indicated when CSF leak is severe, recurrent, or not amenable to the endoscopic treatment.[47]
Most neurosurgeons prefer the intracranial approach due to the added advantage of allowing the resection of any coexisting intracranial pathology. The success rate, however, is less as compared to transnasal endoscopic technique. A frontal craniotomy often results in a loss of the sense of smell. Though uncommon, it may be complicated by postoperative intracerebral hemorrhage, cerebral edema, epilepsy, frontal lobe dysfunction with memory and concentration deficits, and osteomyelitis. The hospital stay and return to the normal activity are longer. It also results in hair loss along the incision line and it is difficult to approach sphenoid sinus rhinorrhea by this approach.
The extracranial approaches
Extracranial approaches can be open extracranial techniques and endoscopic approaches. These approaches have lower morbidity and higher success rates as compared to intracranial approaches. These approaches provide the good exposure of the sphenoid, parasellar, and posterior ethmoid regions. It offers excellent visualization of rhinorrhea in the posterior wall of the frontal sinus, the cribriform plate, and the fovea ethmoidalis. An extradural approach is also indicated for defects larger than 5 cm, especially in the posterior wall of the frontal sinus which is difficult to manage endoscopically.[18]
Endoscopic technique
Endoscopic closure has revolutionized the surgical management of CSF rhinorrhea and has reduced the morbidity associated with it.[6] The sense of smell is almost always preserved using this technique. The length of stay in hospital is usually restricted to 36 hours, and a craniotomy can be avoided. Easy access, precision, and accuracy of the surgery make the endoscopic technique very valuable method in CSF rhinorrhea. It is now the procedure of choice for the treatment of CSF rhinorrhea.[7,8]
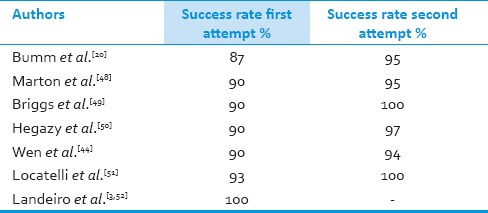
Nasal endoscopic repair has a success rate of 87 to 100% after the first attempt and about 94 to 100% after a second attempt [Table 1]. Transnasal repair using the endoscope is a safe and effective method in CSF leaks.[3,11,12,13,14,15]
Table 1.
Success rate of endoscopic transnasal approaches
Indications of Endoscopic Transnasal Approach
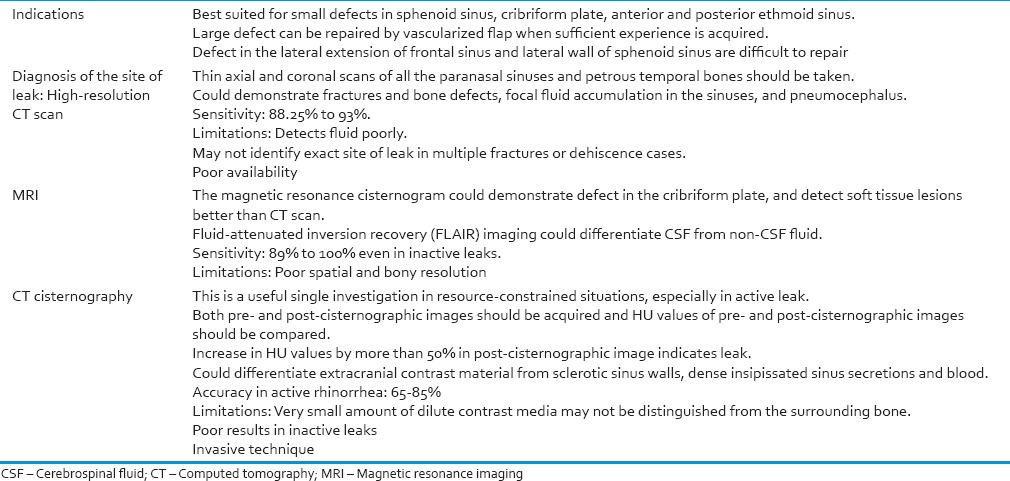
Endoscopic technique is usually best suited for small defects lying in the sphenoid sinus, cribriform plate, anterior and posterior ethmoid sinus. Initially, small leaks should be repaired endoscopically. Large defect can be repaired by vascularized flap when sufficient experience is acquired.[53] Endonasal endoscopic approach is the preferred method for the closure of uncomplicated CSF rhinorrhea, located at the anterior or the posterior ethmoid roof and in the sphenoid sinus, due to its minimal morbidity.[50,53,54,55,56] Endoscopic repair coupled with control of intracranial hypertension could be effective in achieving high success rates.[57] Treatment of frontal CSF rhinorrhea is difficult, especially when defect is in the lateral extension of well-pneumatized frontal sinus. Most of the CSF leaks from the frontal recess and the posterior wall of the frontal sinus can be successfully closed by an endoscopic modified Lothrop procedure.[58,59,60]
Surgical Technique for the Repair of Cerebrospinal Fluid Leaks
Endonasal, endoscopic approaches to the cranial base have undergone significant technique refinement in recent times. These procedures can be done with acceptable morbidity. Repair in the region of ethmoid usually need anterior or anterior and posterior ethmoidectomy. Leaks in the region of sphenoid sinus need sphenoidectomy, while the rhinorrhea from frontal sinus needs exploration of frontal sinus.
Rigid nasal endoscopes (0, 30, and 45 degree) along with the various endoscopic instruments are used in the CSF repair. Usually, the instruments are passed by the side of the telescope but the treatment by a neuroendoscope with a working sheath has been reported. In this technique, instruments are passed through the working channel. It was found to be safe, effective, easy, And it obviates the need for a separate sinoscope.[10]
A 30-cm long telescope has an advantage over 18-cm scope. It keeps camera away from the nasal opening which allows easy manipulations of the instruments. Yellow light filter for the endoscope and a blue filter for the light source may aid in the identification of the defect if intraoperative fluorescein is to be used. General anesthesia is administered and the epinephrine solution soaked in cotton is used to induce vasoconstriction of the whole nasal cavity mucosa. Thorough inspection of the nasal cavity is done to identify the normal structure and any anatomical variation.
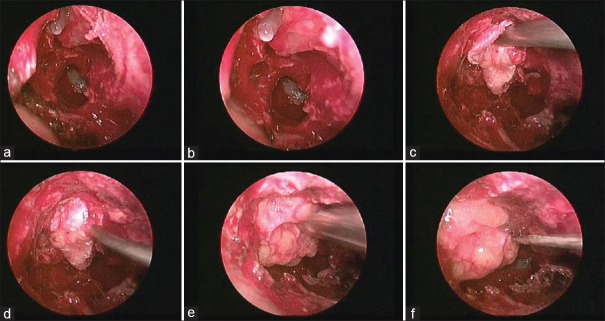
The direct paraseptal approach may be used to reach the defects in the cribriform, or the ethmoid roof. A complete ethmoidectomy is usually needed for adequate exposure when a rhinorrhea is in the cribriform plate and extending in the surrounding bone (ethmoid or in the superior border of the sphenoid sinus). In addition, frontal sinusotomies, sphenoidotomies, and middle-superior turbinectomies may also be required in selected cases. The mucosa is completely stripped away from the defect for at least 5 mm in all the directions once the defect is visualized. Bed is prepared for the graft and any bony projections near the defect are removed for better graft placement and graft take up. Any encephaloceles need to be reduced by using bipolar electrocautery at the stalk. It is important to ablate the encephalocele at the stalk so that it cannot retract into the intracranial compartment and cause hemorrhage. The graft material is then placed to cover the defect [Figures 5 and 6].
Figure 5.
Transnasal endoscopic technique showing meningocele defect (a and b), placement of fascia lata graft (c and d), and fat (e) over the defect. Fibrin glue (f) being used over the graft
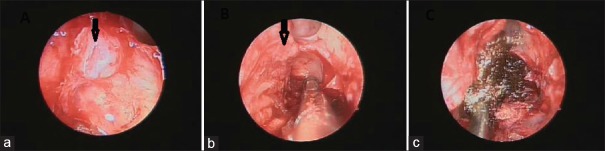
Figure 6.
Transnasal endoscopy showing (a) dural defect (arrow), application of nasoseptal flap (arrow) in image (b) and Surgicel over the flap in image (c)
Graft material could be the cartilage, bone, mucoperichondrium, septal mucosa, turbinate, fascia, abdominal fat, conchal cartilage, free tissue, pedicle tissue, and composite grafts. Wigand et al. were first to use free tissue grafts during an endoscopic approach to treat CSF rhinorrhea. Subsequently, free tissue grafts were used by multiple authors. Free grafts of middle turbinate mucosa were used by Burns et al. and Marks, with success rates of 83% and 94%, respectively. Mucoperichondrium and/or mucoperiosteum free grafts were used in a variety of combinations by Lanza et al.[61] in 1996, with a success rate of 89%. Free tissue grafts have been the preferred material for the repair of CSF leaks by most of the authors and only 9% of the rhinorrhea were repaired using vascularized flaps. The immediate viability is the advantage of a flap over a graft, which in theory increases the ability to heal. However, free-tissue grafts are not as technically demanding as vascularized flap and yielded similar results. Free grafts and flaps can be combined to reinforce the repair. Review and meta-analysis suggest that the choice of the surgical approach and the grafting materials used during the endoscopic endonasal closure of CSF rhinorrhea depends on the availability of the material and on the experience and familiarity of the surgeon. The uses of the various types of graft materials do not seem to alter the outcome.[62]
CSF leaks can be repaired by the bath-plug technique or composite flap from the nasal septa or the turbinates. The bath-plug technique consists of introducing a fat plug with a specifically secured vicryl suture into the intradural space. Traction is placed on the suture to seal the defect much like a bathplug seals a bath. This technique was found to be safe.[63] Conventional techniques may not be sufficient to close CSF leaks due to large defects in the anterior cranial fossa. A composite mucochondrial flap from the nasal septum could be useful for repairing such large defects. The skeletal support, provided by the composite flap to counter the pressure exerted by CSF, is an advantage of the composite flap.[64] The middle turbinate graft can be used as a composite bone/mucosal graft for moderate-sized defects. Separate bone and mucosal grafts from middle turbinate can be used for large defects when intracranial bone placement is desirable. The middle turbinate is an excellent source of the donor material for the repair of almost any endoscopically repairable CSF leak.[65] Lower turbinate graft can be used for the defects larger than 2 cm with good success rate.[66]
Overlay, underlay, combined, and the obliteration techniques can be used for CSF closure. Overlay grafts are placed over the defect and these are outside the bony cranial cavity. The underlay grafts could be of two types (these are inside the bony cranial cavity). The epidural underlay graft is between the bone and the dura matter. The intradural underlay graft is placed in the sub dural space. The combined techniques can be used. In a meta-analysis of the literature, both techniques yielded similar results. In the epidural underlay technique, the intact dura is separated from the edge of the skull base defect to expose an adequate buttress for the stabilization of the graft. The free graft should be designed in such a way that it can be pushed few millimeters between the bone and the dura on all the sides of the defect. Bone or cartilage underlay grafts are advocated for large bony defects associated with herniating brain or meninges. The inlay technique is technically more demanding than the overlay technique. Inlay grafting is also suited to repair defects of the posterior wall of the frontal sinus, the cribriform plate, the ethmoid roof, and the sphenoid sinus in some cases.[67] The onlay (Overlay) technique is recommended if there is a risk of nerves or vessels injury. It is also indicated when an inlay technique is not technically possible. The graft is placed generally over the dural lesion and over the exposed bony margins, which have been denuded of the mucosa. The graft is supported in place with layers of Gel foam/Gel film or Surgicel, followed by a packing of gauze impregnated with an antibiotic ointment or some other method of fixation. Overlay grafts (79%) are more frequently used as compared to inlay grafts (12%). As an alternative, a vascularized tissue flap may be designed transnasally, using middle turbinate mucoperiosteum or septal mucoperichondrium.
A rhinorrhea within the sphenoid sinus may be repaired with a free graft technique or with an obliterative technique using free abdominal fat. As an alternative, the sphenoid sinus defect may be repaired using self-setting hydroxyapatite bone cement. However, bone cement is not well suited for high-flow leaks, associated with a high intraventricular pressure (e.g., hydrocephalus). The flow of CSF could wash the cement off the defect before it sets. The transmitted pulsations of the brain can also cause microfracturing of the cement. Bone cement has been associated with a higher infection rate also.
Nasal packing is commonly advocated to support the graft in place. Gel foam or Gel film are frequently used to separate the graft from the packing material, to prevent avulsion of the graft or flap during its removal. Whether to use and how long to keep the postoperative packing are based on the surgeon's experience, although most authors recommend removal of the packing in 3 to 5 days after the surgery. Fibrin glue may enhance the adhesion of the graft, and its use may obviate the need for nasal packing. BioGlue could be applied as reinforcement over collagen sponge as the last layer of the repair.[68] Patients are placed on bed rest for 3 to 5 days with head elevation. Perioperative prophylactic antibiotics contribute to the low incidence of meningitis following the repair of CSF leak. Antibiotics should be used after nasal packing to prevent toxic shock syndrome usually caused by the S. aureus and S. epidermidis. This toxic shock syndrome is a potentially fatal illness caused by the bacterial toxin. The patient is asked to avoid nose blowing, sneezing, and Valsalva maneuvers. Stool softeners are advised to avoid straining.
The conventional repair technique with pieces of fat or muscle is usually associated with a relatively high incidence of CSF rhinorrhea, if the dura is widely opened and massive intraoperative CSF leakage is encountered. Watertight dural closure, by direct suturing of the dura, could be a better alternative as it is a simple, safe, and reliable surgical technique for CSF leak repair. Fat or grafts could be avoided in most of the cases after direct suturing.[69] Dural suturing with fascia graft by suture-tying micro instruments could also be performed.[70] The intracranial CSF could compress the double-layer patch graft against the cranial base and could seal the gap in a watertight manner. The double-layer patch graft can be composed of autologous fascial membrane and a commercially available, expanded polytetrafluoroethylene dural substitute. The subdural double-layer patch graft technique is simple and reliable for the prevention of CSF rhinorrhea after transsphenoidal surgery associated with a widely opened dura.[71] Direct suture repair of the dural defect, by a fascial graft of an anteriorly based pericranial flap, is an option when the septal mucosal flap is unavailable for reconstruction in patients with tumor involving nasal septum.[72] This technique is associated with minimal donor site morbidity, and it provides a large flap that can cover the entire ventral skull base. Preoperative radiographic evaluation may guide in surgical planning of the size and site of incisions while harvesting a pericranial flap.[73] Correct localization and repair of the leak could be achieved without any major complications with the help of navigation system. Preliminary report suggests that it is possible to make routine use of the navigation systems in CSF leak.[74]
Microscope can be used for transnasal repair of CSF leak. The decision of whether to use the microscope or the endoscope, or both, is mainly based on the experience and the preference of the surgeon. Both techniques could be highly successful and may be complementary in selected cases.[11] Microscope has the advantage of depth perception. Good success rates using a microscopic approach for the repair of CSF leaks has been reported. Microscopic approach has limitations in visualizing the rhinorrhea in the lateral part of the sphenoid sinus, which can be successfully repaired using a 70° endoscope.
Success Rate
Success rate of endoscopic CSF repair ranged from 87 to 100% after the first attempt and from 94 to 100% after the second attempt [Table 1]. Wen et al. reported 96% and 98% success after third and forth attempts, respectively. The precise locations of leakage prior to surgery, proper patient selection by eliminating cases with large defects, are helpful in ensuring a successful endoscopic CSF repair.[75]
One should be careful in leveling success in CSF rhinorrhea as the recurrences may occur very late. The mean interval until failure was 80 months in Gassner et al.'s series.[76] This stresses the need for long follow-up before we call any procedure as successful.
Adjunctive Techniques
Lumbar drain
A lumbar spinal drain is advocated by many authors to reduce the CSF pressure in large fistula [Table 2]. Most of the authors who use lumbar spinal drains after surgery recommend keeping the drain for 3 to 5 days to reduce the ICP, preserve the position of the graft, and facilitate the process of adhesion. A CSF drain is not necessary in all the patients as some authors reported very good results without a lumbar spinal drain, even in large defects.[77]
Table 2.
Summary of endoscopic transnasal technique of CSF leaks
Lumbar spinal drain is indicated in recurrent or persistent leaks in idiopathic and post-traumatic rhinorrhea associated with hydrocephalus. It is also indicated in large skull base defects with meningocele. A second spinal tap should be performed 24 to 48 hours after removal of the lumbar spinal drain to measure the spinal fluid pressure. Patients with raised ICP and hydrocephalus should undergo ventriculo peritoneal shunt. Hegazy et al.[50] and Lee et al.[78] advocated the use of lumbar drains in the repair of frontal and sphenoid sinus defects with or without meningocele or encephalocele.
Complications of Repair
Meningitis, chronic headache, pneumocephalus, intracranial hematomas, frontal lobe abscess, and the recurrences could be the complications of CSF repair. The incidence of the complications was low in a Meta-analysis. The complications such as meningitis (0.3%), brain abscess (0.9%), subdural hematoma (0.3%), smell disorders (0.6%), and headache (0.3%) were observed.[50]
Recurrence
Patients with spontaneous CSF rhinorrhea, elevated body mass index, lateral sphenoid leaks, and extensive skull base defects are at increased risk for recurrence.[79] Middle-age, obese female patient, and empty sella could also result in recurrence.[80] Higher body-mass index of >30, raised ICP, and diabetes mellitus are associated with high recurrence rate.[66] Patients with multiple CSF leaks could be associated with high failure rate.[81]
High-pressure hydrocephalus is at high risk for recurrence.[50,82,83] The failure up to 50% has been reported in spontaneous CSF leak with raised ICP.[84] The presence or absence of high ICP may be established by means of direct CSF pressure measurement through postoperative lumbar puncture. This allows early intervention and prevention of recurrence.[85]
Recurrence rate of the CSF rhinorrhea in the lateral part of the sphenoid sinus is high.[25] Extended endoscopic approaches, including the pterygomaxillary fossa approach, may be useful in selected instances to properly repair such defect.[86] Likewise, high recurrence rate up to about 44% is reported in frontal sinus with superior and lateral extension.[87,88] Frontal sinus CSF leaks have traditionally been repaired using an external approach with osteoplastic flaps and obliteration of the sinus. Recurrent frontal CSF leaks could also be successfully repaired by an open-endoscopic approach. The endoscopic modified Lothrop technique can be used as an effective alternative approach to repair CSF leaks in poorly accessible areas of the frontal sinus.[89]
Transnasal endoscopic surgery is an effective treatment for recurrent CSF rhinorrhea.[36] All patients with increased ICP should get appropriate treatment for raised ICP in addition to the endoscopic repair.[81,90]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Yadav YR, Shenoy R, Mukerji G, Parihar V. Water jet dissection technique for endoscopic third ventriculostomy minimises the risk of bleeding and neurological complications in obstructive hydrocephalus with a thick and opaque third ventricle floor. Minim Invasive Neurosurg. 2010;53:155–8. doi: 10.1055/s-0030-1263107. [DOI] [PubMed] [Google Scholar]
- 2.Yadav YR, Parihar V, Sinha M, Jain N. Endoscopic treatment of supra sellar arachnoid cyst. Neurol India. 2010;58:280–83. doi: 10.4103/0028-3886.63772. [DOI] [PubMed] [Google Scholar]
- 3.Landeiro JA, Lázaro B, Melo MH. Endonasal endoscopicrepair of cerebrospinal fluid rhinorrhea. Minim Invasive Neurosurg. 2004;47:173–7. doi: 10.1055/s-2004-818451. [DOI] [PubMed] [Google Scholar]
- 4.Yadav YR, Parihar V, Agarwal M, Sherekar S, Bhatele PR. Endoscopic vascular decompression of the trigeminal nerve. Minim Invasive Neurosurg. 2011;54:110–4. doi: 10.1055/s-0031-1283129. [DOI] [PubMed] [Google Scholar]
- 5.Yadav YR, Yadav S, Sherekar S, Parihar V. A new minimally invasive tubular brain retractor system for surgery of deep intracerebral hematoma. Neurol India. 2011;59:74–7. doi: 10.4103/0028-3886.76870. [DOI] [PubMed] [Google Scholar]
- 6.Sanderson JD, Kountakis SE, McMains KC. Endoscopic management of cerebrospinal fluidleaks. Facial Plast Surg. 2009;25:29–37. doi: 10.1055/s-0028-1112229. [DOI] [PubMed] [Google Scholar]
- 7.Golusinski W, Waśniewska E, Kulczyński B. Endoscopic reconstruction of the anterior skull base in cerebrospinal rhinorrhea. Otolaryngol Pol. 2003;57:75–9. [PubMed] [Google Scholar]
- 8.Kirtane MV, Gautham K, Upadhyaya SR. Endoscopic CSF rhinorrheaclosure: Our experience in 267 cases. Otolaryngol Head Neck Surg. 2005;132:208–12. doi: 10.1016/j.otohns.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Tahir MZ, Khan MB, Bashir MU, Akhtar S, Bari E. Cerebrospinal fluid rhinorrhea: An institutional perspective from Pakistan. Surg Neurol Int. 2011;2:174. doi: 10.4103/2152-7806.90689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Husain M, Jha D, Vatsal DK, Husain N, Gupta RK. Neuroendoscopic transnasal repair of cerebrospinal fluid rhinorrhea. Skull Base. 2003;13:73–8. doi: 10.1055/s-2003-820561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Banhawy OA, Halaka AN, El-Hafiz Shehab El-Dien A, Ayad H. Subcranial transnasal repair of cerebrospinal fluid rhinorrhea with free autologous grafts by the combined overlay and underlay techniques. Minim Invasive Neurosurg. 2004;47:197–202. doi: 10.1055/s-2004-818513. [DOI] [PubMed] [Google Scholar]
- 12.Schick B, Ibing R, Brors D, Draf W. Long-term study of endonasal duraplasty and review of the literature. Ann Otol Rhinol Laryngol. 2001;110:142–7. doi: 10.1177/000348940111000209. [DOI] [PubMed] [Google Scholar]
- 13.Bachert C, Verhaeghe B, van Cauwenberge P, Daele J. Endoscopic endonasal surgery (EES) in skull base repairs and CSF leakage. Acta Otorhinolaryngol Belg. 2000;54:179–89. [PubMed] [Google Scholar]
- 14.Kansu L, Akkuzu B, Avci S. Endoscopic treatment of idiopathic spontaneous although cerebrospinal fluid rhinorrhea: A case report. Kulak Burun Bogaz Ihtis Derg. 2009;19:36–40. [PubMed] [Google Scholar]
- 15.Nyquist GG, Anand VK, Mehra S, Kacker A, Schwartz TH. Endoscopic endonasal repair of anterior skull base non-traumatic cerebrospinal fluid leaks, meningoceles, and encephaloceles. J Neurosurg. 2010;113:961–6. doi: 10.3171/2009.10.JNS08986. [DOI] [PubMed] [Google Scholar]
- 16.Sughrue ME, Aghi MK. Reconstruction of dural defects of the endonasal skull base. Neurosurg Clin N Am. 2010;21:637–41. doi: 10.1016/j.nec.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Abuabara A. Cerebrospinal fluid rhinorrhoea: Diagnosis and management. Med Oral Patol Oral Cir Bucal. 2007;12:E397–400. [PubMed] [Google Scholar]
- 18.Bell RB, Dierks EJ, Homer L, Potter BE. Management of cerebrospinal fluid leak associated with craniomaxillofacial trauma. J Oral Maxillofac Surg. 2004;62:676–84. doi: 10.1016/j.joms.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 19.Platt MP, Parnes SM. Management of unexpected cerebrospinal fluid leak during endoscopic sinus surgery. Curr Opin Otolaryngol Head Neck Surg. 2009;17:28–32. doi: 10.1097/MOO.0b013e32831fb593. [DOI] [PubMed] [Google Scholar]
- 20.Bumm K, Heupel J, Bozzato A, Iro H, Hornung J. Localization and infliction pattern of iatrogenic skull base defects following endoscopic sinus surgery at a teaching hospital. Auris Nasus Larynx. 2009;36:671–6. doi: 10.1016/j.anl.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Schlosser RJ, Woodworth BA, Wilensky EM, Grady MS, Bolger WE. Spontaneous cerebrospinal fluid leaks: A variant of benign intracranial hypertension. Ann Otol Rhinol Laryngol. 2006;115:495–500. doi: 10.1177/000348940611500703. [DOI] [PubMed] [Google Scholar]
- 22.Owler BK, Allan R, Parker G, Besser M. Pseudotumour cerebri, CSF rhinorrhoea and the role of venous sinus stenting in treatment. Br J Neurosurg. 2003;17:79–83. doi: 10.3109/02688690309177979. [DOI] [PubMed] [Google Scholar]
- 23.Al-Sebeih K, Karagiozov K, Elbeltagi A, Al-Qattan F. Non-traumaticcerebrospinal fluid rhinorrhea: Diagnosis and management. Ann Saudi Med. 2004;24:453–8. doi: 10.5144/0256-4947.2004.453. Comment in Ann Saudi Med 2005;25:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlosser RJ, Bolger WE. Spontaneous nasal cerebrospinal fluid leaks and empty sella syndrome: A clinical association. Am J Rhinol. 2003;17:91–6. [PubMed] [Google Scholar]
- 25.Lopatin AS, Kapitanov DN, Potapov AA. Endonasal endoscopic repair of spontaneous cerebrospinal fluid leaks. Arch Otolaryngol Head Neck Surg. 2003;129:859–63. doi: 10.1001/archotol.129.8.859. [DOI] [PubMed] [Google Scholar]
- 26.Banks CA, Palmer JN, Chiu AG, O’Malley BW, Jr, Woodworth BA, Kennedy DW. Endoscopic closure of CSF rhinorrhea: 193 cases over 21 years. Otolaryngol Head Neck Surg. 2009;140:826–33. doi: 10.1016/j.otohns.2008.12.060. [DOI] [PubMed] [Google Scholar]
- 27.Kim S, Park CH, Park K. Cerebrospinal fluid rhinorrhea caused by a congenital defect of stapesmimickingotorrhea: Radionuclide cisternographic findings. Clin Nucl Med. 2000;25:634–5. doi: 10.1097/00003072-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 28.McMains KC, Gross CW, Kountakis SE. Endoscopic management of cerebrospinal fluid rhinorrhea. Laryngoscope. 2004;114:1833–7. doi: 10.1097/00005537-200410000-00029. [DOI] [PubMed] [Google Scholar]
- 29.Schuknecht B, Simmen D, Briner HR, Holzmann D. Nontraumatic skull based efects with spontaneous CSF rhinorrhea and arachnoid herniation: Imaging findings and correlation with endoscopic sinus surgery in 27 patients. AJNR Am J Neuroradiol. 2008;29:542–9. doi: 10.3174/ajnr.A0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan DT, Poon WS, IP CP, Chiu PW, Goh KY. How useful is glucose detection in diagnosing cerebrospinal fluid leak?. The rational use of CT and Beta-2 transferrin assay in detection of cerebrospinal fluid fistula. Asian J Surg. 2004;27:39–42. doi: 10.1016/S1015-9584(09)60242-6. [DOI] [PubMed] [Google Scholar]
- 31.Philips BJ, Meguer JX, Redman J, Baker EH. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 2003;29:2204–10. doi: 10.1007/s00134-003-1961-2. [DOI] [PubMed] [Google Scholar]
- 32.Lund VJ, Savy L, Lloyd G, Howard D. Optimum imaging and diagnosis of cerebrospinal fluid rhinorrhoea. J Laryngol Otol. 2000;114:988–92. doi: 10.1258/0022215001904572. [DOI] [PubMed] [Google Scholar]
- 33.Selcuk H, Albayram S, Ozer H, Ulus S, Sanus GZ, Kaynar MY, et al. Intrathecal gadolinium-enhanced MR cisternography in the evaluation of CSF leakage. AJNR Am J Neuroradiol. 2010;31:71–5. doi: 10.3174/ajnr.A1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd KM, Del Gaudio JM, Hudgins PA. Imagingof skull base cerebrospinal fluid leaks in adults. Radiology. 2008;248:725–36. doi: 10.1148/radiol.2483070362. [DOI] [PubMed] [Google Scholar]
- 35.Mostafa BE, Khafagi A. Combined HRCT and MRI in the detection of CSF rhinorrhea. Skull Base. 2004;14:157–62. doi: 10.1055/s-2004-832259. discussion 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui S, Han D, Zhou B, Zhang L, Li Y, Ge W, et al. Endoscopic endonasal surgery for recurrent cerebrospinal fluid rhinorrhea. Acta Otolaryngol. 2010;130:1169–74. doi: 10.3109/00016481003602090. [DOI] [PubMed] [Google Scholar]
- 37.Gendeh BS, Wormald PJ, Forer M, Goh BS, Misiran K. Endoscopic repair of spontaneous cerebro-spinal fluid rhinorrhoea: A report of 3 cases. Med J Malaysia. 2002;57:503–8. [PubMed] [Google Scholar]
- 38.Bateman N, Mason J, Jones NS. Use of fluorescein for detecting cerebrospinal fluid rhinorrhoea: A safe technique for intrathecal injection. ORL J Otorhinolaryngol Relat Spec. 1999;61:131–2. doi: 10.1159/000027657. [DOI] [PubMed] [Google Scholar]
- 39.Lund VJ. Endoscopic management of cerebrospinal fluid leaks. Am J Rhinol. 2002;16:17–23. [PubMed] [Google Scholar]
- 40.Seth R, Rajasekaran K, Benninger MS, Batra PS. The utility of intrathecal fluorescein in cerebrospinal fluid leak repair. Otolaryngol Head Neck Surg. 2010;143:626–32. doi: 10.1016/j.otohns.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Anari S, Waldron M, Carrie S. Delayed absence seizure: A complication of intrathecal fluorescein injection. A case report and literature review. Auris Nasus Larynx. 2007;34:515–8. doi: 10.1016/j.anl.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Keerl R, Weber RK, Draf W, Wienke A, Schaefer SD. Use of sodium fluorescein solution for detection of cerebrospinal fluid fistulas: An analysis of 420 administrations and reported complications in Europe and the United States. Laryngoscope. 2004;114:266–72. doi: 10.1097/00005537-200402000-00016. [DOI] [PubMed] [Google Scholar]
- 43.Presutti L, Mattioli F, Villari D, Marchioni D, Alicandri-Ciufelli M. Transnasal endoscopic treatment of cerebrospinal fluid leak: 17 years’ experience. Acta Otorhinolaryngol Ital. 2009;29:191–6. [PMC free article] [PubMed] [Google Scholar]
- 44.Wen W, Xu G, Zhang X, Shi J, Xie M, Li Y, et al. Surgical management of cerebrospinal fluid rhinorrhea. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2002;37:366–9. [PubMed] [Google Scholar]
- 45.Scholsem M, Scholtes F, Collignon F, Robe P, Dubuisson A, Kaschten B, et al. Surgical management of anterior cranial base fractures with cerebrospinal fluid fistulae: A single-institution experience. Neurosurgery. 2008;62:463. doi: 10.1227/01.neu.0000316014.97926.82. [DOI] [PubMed] [Google Scholar]
- 46.Tosun F, Gonul E, Yetiser S, Gerek M. Analysis of different surgical approaches for the treatment of cerebrospinal fluid rhinorrhea. Minim Invasive Neurosurg. 2005;48:355–60. doi: 10.1055/s-2005-915636. [DOI] [PubMed] [Google Scholar]
- 47.Darakchiev BJ, Pensak ML. Cerebrospinal fluid dynamics in skull base surgery. Curr Opin Otolaryngol Head Neck Surg. 2004;12:404–7. doi: 10.1097/01.moo.0000134442.22229.2c. [DOI] [PubMed] [Google Scholar]
- 48.Marton E, Billeci D, Schiesari E, Longatti P. Transnasal endoscopic repair of cerebrospinal fluid fistulas and encephaloceles: Surgical indications and complications. Minim Invasive Neurosurg. 2005;48:175–81. doi: 10.1055/s-2005-870904. [DOI] [PubMed] [Google Scholar]
- 49.Briggs RJ, Wormald PJ. Endoscopic transnasal intradural repair of anterior skull base cerebrospinal fluid fistulae. J Clin Neurosci. 2004;11:597–9. doi: 10.1016/j.jocn.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Hegazy HM, Carrau RL, Snyderman CH, Kassam A, Zweig J. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea: A meta-analysis. Laryngoscope. 2000;110:1166–72. doi: 10.1097/00005537-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 51.Locatelli D, Rampa F, Acchiardi I, Bignami M, De Bernardi F, Castelnuovo P. Endoscopic endonasal approaches for repair of cerebrospinal fluid leaks: Nine-year experience. Neurosurgery. 2006;58(4 Suppl 2) doi: 10.1227/01.NEU.0000193924.65297.3F. ONS-246-56; discussion ONS-256-7. [DOI] [PubMed] [Google Scholar]
- 52.Landeiro JA, Flores MS, Lázaro BC, Melo MH. Surgical management of cerebrospinal fluid rhinorrhea under endoscopic control. Arq Neuropsiquiatr. 2004;62:827–31. doi: 10.1590/s0004-282x2004000500016. [DOI] [PubMed] [Google Scholar]
- 53.Kerr JT, Chu FW, Bayles SW. Cerebrospinal fluid rhinorrhea: Diagnosis and management. Otolaryngol Clin North Am. 2005;38:597–611. doi: 10.1016/j.otc.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 54.Martin TJ, Loehrl TA. Endoscopic CSF leak repair. Curr Opin Otolaryngol Head Neck Surg. 2007;15:35–9. doi: 10.1097/MOO.0b013e3280123fce. [DOI] [PubMed] [Google Scholar]
- 55.Alameda YA, Busquets JM, Portela JC. Anterior skull base cerebrospinal fluid fistulas in Puerto Rico: Treatment and outcome. Bol Asoc MedPR. 2009;101:29–33. [PubMed] [Google Scholar]
- 56.Bibas AG, Skia B, Hickey SA. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhoea. Br J Neurosurg. 2000;14:49–52. doi: 10.1080/02688690042924. [DOI] [PubMed] [Google Scholar]
- 57.Woodworth BA, Palmer JN. Spontaneous cerebrospinal fluid leaks. Curr Opin Otolaryngol Head Neck Surg. 2009;17:59–65. doi: 10.1097/MOO.0b013e3283200017. [DOI] [PubMed] [Google Scholar]
- 58.Shi JB, Chen FH, Fu QL, Xu R, Wen WP, Hou WJ, et al. Frontal sinus cerebrospinalfluid leaks: Repairin 15 patients using an endoscopic surgical approach. ORL J Otorhinolaryngol Relat Spec. 2010;72:56–62. doi: 10.1159/000275675. [DOI] [PubMed] [Google Scholar]
- 59.Becker SS, Duncavage JA, Russell PT. Endoscopic endonasal repair of difficult-to-access cerebrospinal fluid leaks of the frontal sinus. Am J Rhinol Allergy. 2009;23:181–4. doi: 10.2500/ajra.2009.23.3291. [DOI] [PubMed] [Google Scholar]
- 60.Gendeh BS, Mazita A, Selladurai BM, Jegan T, Jeevanan J, Misiran K. Endonasal endoscopic repair of anterior skull-base fistulas: The Kuala Lumpur experience. J Laryngol Otol. 2005;119:866–74. doi: 10.1258/002221505774783421. [DOI] [PubMed] [Google Scholar]
- 61.Lanza DC, O’Brien DA, Kennedy DW. Endoscopic repair of cerebrospinal fluid fistulae and encephaloceles. Laryngoscope. 1996;106:1119–25. doi: 10.1097/00005537-199609000-00015. [DOI] [PubMed] [Google Scholar]
- 62.Castelnuovo P, Mauri S, Locatelli D, Emanuelli E, Delù G, Giulio GD. Endoscopicrepair of cerebrospinal fluid rhinorrhea: Learning from our failures. Am J Rhinol. 2001;15:333–42. [PubMed] [Google Scholar]
- 63.Wormald PJ, McDonogh M. ‘Bath-plug’ technique for the endoscopic management of cerebrospinal fluid leaks. J Laryngol Otol. 1997;111:1042–6. doi: 10.1017/s0022215100139295. [DOI] [PubMed] [Google Scholar]
- 64.Friedman M, Venkatesan TK, Caldarelli DD. Composite mucochondral flap for repair of cerebrospinal fluid leaks. Head Neck. 1995;17:414–8. doi: 10.1002/hed.2880170510. [DOI] [PubMed] [Google Scholar]
- 65.Marks SC. Middle turbinate graft for repair of cerebral spinal fluid leaks. Am J Rhinol. 1998;12:417–9. doi: 10.2500/105065898780707900. [DOI] [PubMed] [Google Scholar]
- 66.Cassano M, Felippu A. Endoscopic treatment of cerebrospinal fluid leaks with the use of lower turbinate grafts: A retrospective review of 125 cases. Rhinology. 2009;47:362–8. doi: 10.4193/Rhin08.175. [DOI] [PubMed] [Google Scholar]
- 67.Strek P, Zagólski O, Skladzie J, Kurzyflski M, Oleg K, Konior M, et al. Endoscopic treatment of cerebral rhinorrhea. Otolaryngol Pol. 2007;61:69–73. doi: 10.1016/s0030-6657(07)70386-4. [DOI] [PubMed] [Google Scholar]
- 68.Dusick JR, Mattozo CA, Esposito F, Kelly DF. BioGlue for prevention of postoperative cerebrospinal fluid leaks in transsphenoidal surgery: A case series. Surg Neurol. 2006;66:371–6. doi: 10.1016/j.surneu.2006.06.043. discussion 376. [DOI] [PubMed] [Google Scholar]
- 69.Nishioka H, Izawa H, Ikeda Y, Namatame H, Fukami S, Haraoka J. Dural suturing for repair of cerebrospinal fluid leak in transnasal transsphenoidal surgery. Acta Neurochir (Wien) 2009;151:1427–30. doi: 10.1007/s00701-009-0406-2. [DOI] [PubMed] [Google Scholar]
- 70.Ahn JY, Kim SH. A new technique for dural suturing with fascia graft for cerebrospinal fluid leakage in transsphenoidal surgery. Neurosurgery. 2009;65(6 Suppl):65–71. doi: 10.1227/01.NEU.0000327695.32775.BB. discussion 71-2. [DOI] [PubMed] [Google Scholar]
- 71.Kitano M, Taneda M. Subdural patch graft technique for watertight closure of large dural defects in extended transsphenoidal surgery. Neurosurgery. 2004;54:653–60. doi: 10.1227/01.neu.0000108780.72365.dc. discussion 660-1. [DOI] [PubMed] [Google Scholar]
- 72.Zanation AM, Snyderman CH, Carrau RL, Kassam AB, Gardner PA, Prevedello DM. Minimally invasive endoscopic pericranial flap: A new method for endonasal skull base reconstruction. Laryngoscope. 2009;119:13–8. doi: 10.1002/lary.20022. [DOI] [PubMed] [Google Scholar]
- 73.Patel MR, Shah RN, Snyderman CH, Carrau RL, Germanwala AV, Kassam AB, et al. Pericranial flap for endoscopic anterior skull-base reconstruction: Clinical outcomes and radioanatomic analysis of preoperative planning. Neurosurgery. 2010;66:506–12. doi: 10.1227/01.NEU.0000365620.59677.FF. discussion 512. [DOI] [PubMed] [Google Scholar]
- 74.Paludetti G, Sergi B, Rigante M, Campioni P, Galli J. New techniques and technology to repair cerebrospinal fluid rhinorrhea. Acta Otorhinolaryngol Ital. 2004;24:130–6. [PubMed] [Google Scholar]
- 75.Ye H, Zuo J, Zhao H, Liu S, An H, Liu Y. Endonasal endoscopic repair of cerebrospinal fluid rhinorrhea in a series of 69 patients. Br J Neurosurg. 2010;24:244–8. doi: 10.3109/02688690903572087. [DOI] [PubMed] [Google Scholar]
- 76.Gassner HG, Ponikau JU, Sherris DA, Kern EB. CSF rhinorrhea: 95 consecutive surgical cases with long term follow-up at the Mayo Clinic. Am J Rhinol. 1999;13:439–47. doi: 10.2500/105065899781329610. [DOI] [PubMed] [Google Scholar]
- 77.Casiano RR, Jassir D. Endoscopic cerebrospinal fluid rhinorrhea repair: Is a lumbardra in necessary? Otolaryngol Head Neck Surg. 1999;121:745–50. doi: 10.1053/hn.1999.v121.a98754. [DOI] [PubMed] [Google Scholar]
- 78.Lee TJ, Huang CC, Chuang CC, Huang SF. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea and skull base defect: Ten-year experience. Laryngoscope. 2004;114:1475–81. doi: 10.1097/00005537-200408000-00029. [DOI] [PubMed] [Google Scholar]
- 79.Lindstrom DR, Toohill RJ, Loehrl TA, Smith TL. Management of cerebrospinal fluid rhinorrhea: The Medical College of Wisconsin experience. Laryngoscope. 2004;114:969–74. doi: 10.1097/00005537-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 80.Schlosser RJ, Wilensky EM, Grady MS, Bolger WE. Elevated intracranial pressures in spontaneous cerebrospinal fluid leaks. Am J Rhinol. 2003;17:191–5. [PubMed] [Google Scholar]
- 81.Woodworth BA, Prince A, Chiu AG, Cohen NA, Schlosser RJ, Bolger WE, et al. Spontaneous CSF leaks: A paradigm for definitive repair and management of intracranial hypertension. Otolaryngol Head Neck Surg. 2008;138:715–20. doi: 10.1016/j.otohns.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 82.Tosun F, Carrau RL, Snyderman CH, Kassam A, Celin S, Schaitkin B. Endonasal endoscopic repair of cerebrospinal fluid leaks of the sphenoid sinus. Arch Otolaryngol Head Neck Surg. 2003;129:576–80. doi: 10.1001/archotol.129.5.576. [DOI] [PubMed] [Google Scholar]
- 83.Wise SK, Schlosser RJ. Evaluation of spontaneous nasal cerebrospinal fluid leaks. Curr Opin Otolaryngol Head Neck Surg. 2007;15:28–34. doi: 10.1097/MOO.0b013e328011bc76. [DOI] [PubMed] [Google Scholar]
- 84.Mirza S, Thaper A, McClelland L, Jones NS. Sinonasal cerebrospinal fluid leaks: Management of 97 patients over 10 years. Laryngoscope. 2005;115:1774–7. doi: 10.1097/01.mlg.0000175679.68452.75. [DOI] [PubMed] [Google Scholar]
- 85.Carrau RL, Snyderman CH, Kassam AB. The management of cerebrospinal fluid leaks in patients at risk for high-pressure hydrocephalus. Laryngoscope. 2005;115:205–12. doi: 10.1097/01.mlg.0000154719.62668.70. [DOI] [PubMed] [Google Scholar]
- 86.Sautter NB, Batra PS, Citardi MJ. Endoscopic management of sphenoid sinus cerebrospinal fluid leaks. Ann Otol Rhinol Laryngol. 2008;117:32–9. doi: 10.1177/000348940811700108. [DOI] [PubMed] [Google Scholar]
- 87.Woodworth BA, Schlosser RJ, Palmer JN. Endoscopic repair of frontal sinus cerebrospinal fluid leaks. J Laryngol Otol. 2005;119:709–13. doi: 10.1258/0022215054797961. [DOI] [PubMed] [Google Scholar]
- 88.Lee DH, Lim SC, Joo YE. Treatment outcomes of endoscopic repairs of sinonasal cerebrospinal fluid leaks. J Craniofac Surg. 2011;22:1266–70. doi: 10.1097/SCS.0b013e31821c6ad3. [DOI] [PubMed] [Google Scholar]
- 89.Anverali JK, Hassaan AA, Saleh HA. Endoscopic modified Lothrop procedure for repair of lateral frontal sinus cerebrospinal fluid leak. J Laryngol Otol. 2009;123:145–7. doi: 10.1017/S0022215108002326. [DOI] [PubMed] [Google Scholar]
- 90.Zweig JL, Carrau RL, Celin SE, Schaitkin BM, Pollice PA, Snyderman CH, et al. Endoscopic repair of cerebrospinal fluid leaks to the sinonasal tract: Predictors of success. Otolaryngol Head Neck Surg. 2000;123:195–201. doi: 10.1067/mhn.2000.107452. [DOI] [PubMed] [Google Scholar]