Abstract
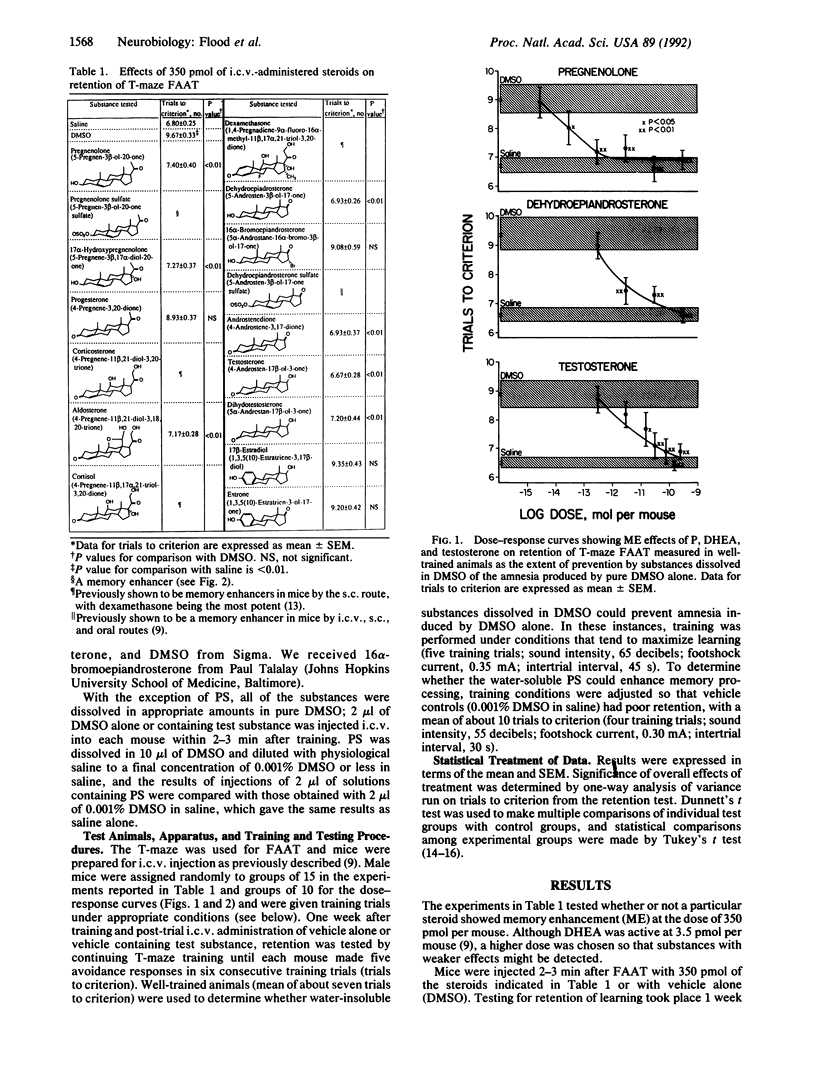
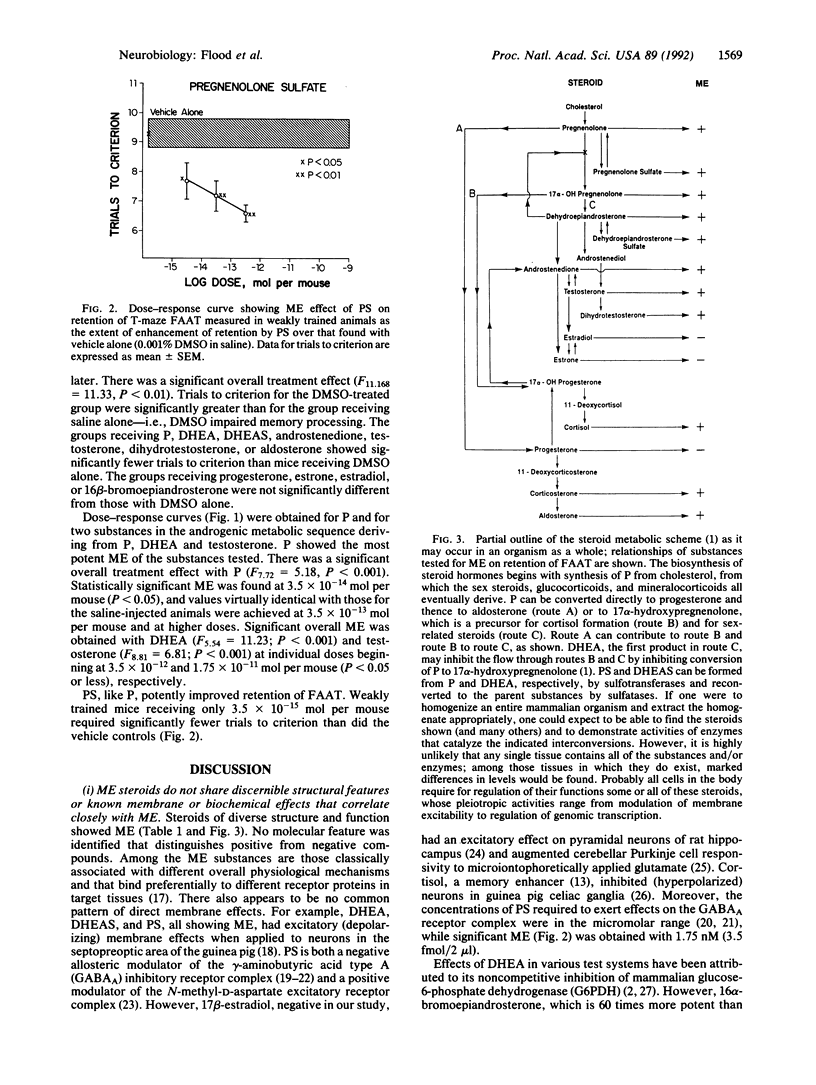
Immediate post-training intracerebroventricular administration to male mice of pregnenolone (P), pregnenolone sulfate (PS), dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEAS), androstenedione, testosterone, dihydrotestosterone, or aldosterone caused improvement of retention for footshock active avoidance training, while estrone, estradiol, progesterone, or 16 beta-bromoepiandrosterone did not. Dose-response curves were obtained for P, PS, DHEA, and testosterone. P and PS were the most potent, PS showing significant effects at 3.5 fmol per mouse. The active steroids did not show discernible structural features or known membrane or biochemical effects that correlated with their memory-enhancing capacity. The above, together with the findings that DHEA acted even when given at 1 hr after training and that P, PS, and DHEA improved retention over a much wider dose range than do excitatory memory enhancers, led to the suggestion that the effects of the active steroids converge at the facilitation of transcription of immediate-early genes. P and PS, for which receptors have not yet been demonstrated, may exert their effects by serving as precursors for the formation of a panoply of different steroids, ensuring near-optimal modulation of transcription of immediate-early genes required for achieving the plastic changes of memory processes. Low serum levels of P in aging and the increases of cancer and behavioral disorders in individuals receiving drugs that block synthesis of cholesterol, the immediate precursor of P, suggest possible clinical utility for P.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin B. A., Soltysik S. S. The effect of cerebral ischaemia, resulting in loss of EEG, on the acquisition of conditioned reflexes in goats. Brain Res. 1966 Jul;2(1):71–84. doi: 10.1016/0006-8993(66)90063-1. [DOI] [PubMed] [Google Scholar]
- Beato M. Transcriptional control by nuclear receptors. FASEB J. 1991 Apr;5(7):2044–2051. doi: 10.1096/fasebj.5.7.2010057. [DOI] [PubMed] [Google Scholar]
- Bologa L., Sharma J., Roberts E. Dehydroepiandrosterone and its sulfated derivative reduce neuronal death and enhance astrocytic differentiation in brain cell cultures. J Neurosci Res. 1987;17(3):225–234. doi: 10.1002/jnr.490170305. [DOI] [PubMed] [Google Scholar]
- Carette B., Poulain P. Excitatory effect of dehydroepiandrosterone, its sulphate ester and pregnenolone sulphate, applied by iontophoresis and pressure, on single neurones in the septo-preoptic area of the guinea pig. Neurosci Lett. 1984 Mar 23;45(2):205–210. doi: 10.1016/0304-3940(84)90100-9. [DOI] [PubMed] [Google Scholar]
- Carson-Jurica M. A., Schrader W. T., O'Malley B. W. Steroid receptor family: structure and functions. Endocr Rev. 1990 May;11(2):201–220. doi: 10.1210/edrv-11-2-201. [DOI] [PubMed] [Google Scholar]
- Corpéchot C., Synguelakis M., Talha S., Axelson M., Sjövall J., Vihko R., Baulieu E. E., Robel P. Pregnenolone and its sulfate ester in the rat brain. Brain Res. 1983 Jun 27;270(1):119–125. doi: 10.1016/0006-8993(83)90797-7. [DOI] [PubMed] [Google Scholar]
- Demura T., Driscoll W. J., Strott C. A. The nuclear conversion of pregnenolone to progesterone and subsequent binding to the nuclear progesterone-binding protein in the guinea pig adrenal cortex: a possible regulatory role for the pregnenolone-binding protein. Endocrinology. 1990 Sep;127(3):1114–1120. doi: 10.1210/endo-127-3-1114. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood J. F., Roberts E. Dehydroepiandrosterone sulfate improves memory in aging mice. Brain Res. 1988 May 10;448(1):178–181. doi: 10.1016/0006-8993(88)91116-x. [DOI] [PubMed] [Google Scholar]
- Flood J. F., Smith G. E., Bennett E. L., Alberti M. H., Orme A. E., Jarvik M. E. Neurochemical and behavioral effects of catecholamine and protein synthesis inhibitors in mice. Pharmacol Biochem Behav. 1986 Mar;24(3):631–645. doi: 10.1016/0091-3057(86)90569-1. [DOI] [PubMed] [Google Scholar]
- Flood J. F., Smith G. E., Roberts E. Dehydroepiandrosterone and its sulfate enhance memory retention in mice. Brain Res. 1988 May 3;447(2):269–278. doi: 10.1016/0006-8993(88)91129-8. [DOI] [PubMed] [Google Scholar]
- Flood J. F., Vidal D., Bennett E. L., Orme A. E., Vasquez S., Jarvik M. E. Memory facilitating and anti-amnesic effects of corticosteroids. Pharmacol Biochem Behav. 1978 Jan;8(1):81–87. doi: 10.1016/0091-3057(78)90127-2. [DOI] [PubMed] [Google Scholar]
- Gee K. W., Joy D. S., Belelli D. Complex interactions between pregnenolone sulfate and the t-butylbicyclophosphorothionate-labeled chloride ionophore in rat brain. Brain Res. 1989 Mar 13;482(1):169–173. doi: 10.1016/0006-8993(89)90556-8. [DOI] [PubMed] [Google Scholar]
- Gordon G. B., Shantz L. M., Talalay P. Modulation of growth, differentiation and carcinogenesis by dehydroepiandrosterone. Adv Enzyme Regul. 1987;26:355–382. doi: 10.1016/0065-2571(87)90023-9. [DOI] [PubMed] [Google Scholar]
- Hu Z. Y., Bourreau E., Jung-Testas I., Robel P., Baulieu E. E. Neurosteroids: oligodendrocyte mitochondria convert cholesterol to pregnenolone. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8215–8219. doi: 10.1073/pnas.84.23.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S. Y., Chen Y. Z. Membrane receptor-mediated electrophysiological effects of glucocorticoid on mammalian neurons. Endocrinology. 1989 Feb;124(2):687–691. doi: 10.1210/endo-124-2-687. [DOI] [PubMed] [Google Scholar]
- Hunt S. P., Pini A., Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987 Aug 13;328(6131):632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Jensen E. V. Steroid hormone receptors. Curr Top Pathol. 1991;83:365–431. doi: 10.1007/978-3-642-75515-6_11. [DOI] [PubMed] [Google Scholar]
- Lanthier A., DiBattista J. A., Patwardhan V. V. Pregnenolone binding sites in the rat olfactory bulb. J Steroid Biochem. 1990 Mar;35(3-4):487–494. doi: 10.1016/0022-4731(90)90258-t. [DOI] [PubMed] [Google Scholar]
- Lanthier A., Patwardhan V. V. Sex steroids and 5-en-3 beta-hydroxysteroids in specific regions of the human brain and cranial nerves. J Steroid Biochem. 1986 Sep;25(3):445–449. doi: 10.1016/0022-4731(86)90259-1. [DOI] [PubMed] [Google Scholar]
- Le Goascogne C., Robel P., Gouézou M., Sananès N., Baulieu E. E., Waterman M. Neurosteroids: cytochrome P-450scc in rat brain. Science. 1987 Sep 4;237(4819):1212–1215. doi: 10.1126/science.3306919. [DOI] [PubMed] [Google Scholar]
- Majewska M. D., Mienville J. M., Vicini S. Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons. Neurosci Lett. 1988 Aug 1;90(3):279–284. doi: 10.1016/0304-3940(88)90202-9. [DOI] [PubMed] [Google Scholar]
- Majewska M. D., Schwartz R. D. Pregnenolone-sulfate: an endogenous antagonist of the gamma-aminobutyric acid receptor complex in brain? Brain Res. 1987 Feb 24;404(1-2):355–360. doi: 10.1016/0006-8993(87)91394-1. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Guthrie P. B., Kater S. B. A role for Na+-dependent Ca2+ extrusion in protection against neuronal excitotoxicity. FASEB J. 1989 Nov;3(13):2519–2526. doi: 10.1096/fasebj.3.13.2572500. [DOI] [PubMed] [Google Scholar]
- Mienville J. M., Vicini S. Pregnenolone sulfate antagonizes GABAA receptor-mediated currents via a reduction of channel opening frequency. Brain Res. 1989 Jun 5;489(1):190–194. doi: 10.1016/0006-8993(89)90024-3. [DOI] [PubMed] [Google Scholar]
- Moore D. D. Promiscuous behaviour in the steroid hormone receptor superfamily. Trends Neurosci. 1989 May;12(5):165–168. doi: 10.1016/0166-2236(89)90061-1. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Cohen D. R., Hempstead J. L., Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987 Jul 10;237(4811):192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Oliver M. F. Might treatment of hypercholesterolaemia increase non-cardiac mortality? Lancet. 1991 Jun 22;337(8756):1529–1531. doi: 10.1016/0140-6736(91)93208-q. [DOI] [PubMed] [Google Scholar]
- Orentreich N., Brind J. L., Rizer R. L., Vogelman J. H. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984 Sep;59(3):551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- Pashko L. L., Schwartz A. G., Abou-Gharbia M., Swern D. Inhibition of DNA synthesis in mouse epidermis and breast epithelium by dehydroepiandrosterone and related steroids. Carcinogenesis. 1981;2(8):717–721. doi: 10.1093/carcin/2.8.717. [DOI] [PubMed] [Google Scholar]
- Regelson W., Loria R., Kalimi M. Hormonal intervention: "buffer hormones" or "state dependency". The role of dehydroepiandrosterone (DHEA), thyroid hormone, estrogen and hypophysectomy in aging. Ann N Y Acad Sci. 1988;521:260–273. doi: 10.1111/j.1749-6632.1988.tb35284.x. [DOI] [PubMed] [Google Scholar]
- Robel P., Bourreau E., Corpéchot C., Dang D. C., Halberg F., Clarke C., Haug M., Schlegel M. L., Synguelakis M., Vourch C. Neuro-steroids: 3 beta-hydroxy-delta 5-derivatives in rat and monkey brain. J Steroid Biochem. 1987;27(4-6):649–655. doi: 10.1016/0022-4731(87)90133-6. [DOI] [PubMed] [Google Scholar]
- Roberts E. A systems approach to aging, Alzheimer's disease, and spinal cord regeneration. Prog Brain Res. 1990;86:339–355. doi: 10.1016/s0079-6123(08)63190-8. [DOI] [PubMed] [Google Scholar]
- Roberts E., Bologa L., Flood J. F., Smith G. E. Effects of dehydroepiandrosterone and its sulfate on brain tissue in culture and on memory in mice. Brain Res. 1987 Mar 17;406(1-2):357–362. doi: 10.1016/0006-8993(87)90807-9. [DOI] [PubMed] [Google Scholar]
- Roberts E., Matthysse S. Neurochemistry: at the crossroads of neurobiology. Annu Rev Biochem. 1970;39:777–820. doi: 10.1146/annurev.bi.39.070170.004021. [DOI] [PubMed] [Google Scholar]
- Rusak B., Robertson H. A., Wisden W., Hunt S. P. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science. 1990 Jun 8;248(4960):1237–1240. doi: 10.1126/science.2112267. [DOI] [PubMed] [Google Scholar]
- Saffen D. W., Cole A. J., Worley P. F., Christy B. A., Ryder K., Baraban J. M. Convulsant-induced increase in transcription factor messenger RNAs in rat brain. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7795–7799. doi: 10.1073/pnas.85.20.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar S. M., Sharp F. R., Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988 Jun 3;240(4857):1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Smith S. S., Waterhouse B. D., Woodward D. J. Sex steroid effects on extrahypothalamic CNS. II. Progesterone, alone and in combination with estrogen, modulates cerebellar responses to amino acid neurotransmitters. Brain Res. 1987 Sep 29;422(1):52–62. doi: 10.1016/0006-8993(87)90539-7. [DOI] [PubMed] [Google Scholar]
- Sonka J. Dehydroepiandrosterone. Metabolic effects. Acta Univ Carol Med Monogr. 1976;71:1-137, 146-71. [PubMed] [Google Scholar]
- Wahli W., Martinez E. Superfamily of steroid nuclear receptors: positive and negative regulators of gene expression. FASEB J. 1991 Jun;5(9):2243–2249. doi: 10.1096/fasebj.5.9.1860615. [DOI] [PubMed] [Google Scholar]
- Wong M., Moss R. L. Electrophysiological evidence for a rapid membrane action of the gonadal steroid, 17 beta-estradiol, on CA1 pyramidal neurons of the rat hippocampus. Brain Res. 1991 Mar 8;543(1):148–152. doi: 10.1016/0006-8993(91)91057-8. [DOI] [PubMed] [Google Scholar]
- Wu F. S., Gibbs T. T., Farb D. H. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol Pharmacol. 1991 Sep;40(3):333–336. [PubMed] [Google Scholar]



