Abstract
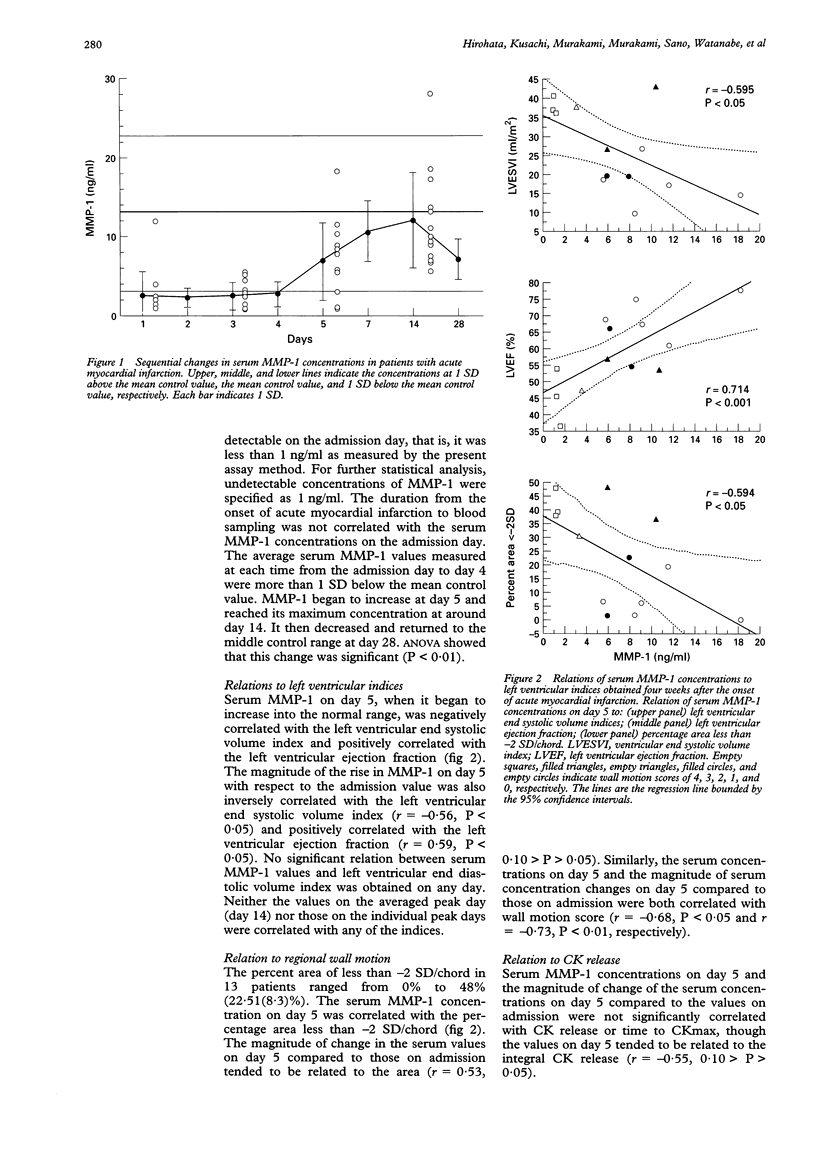
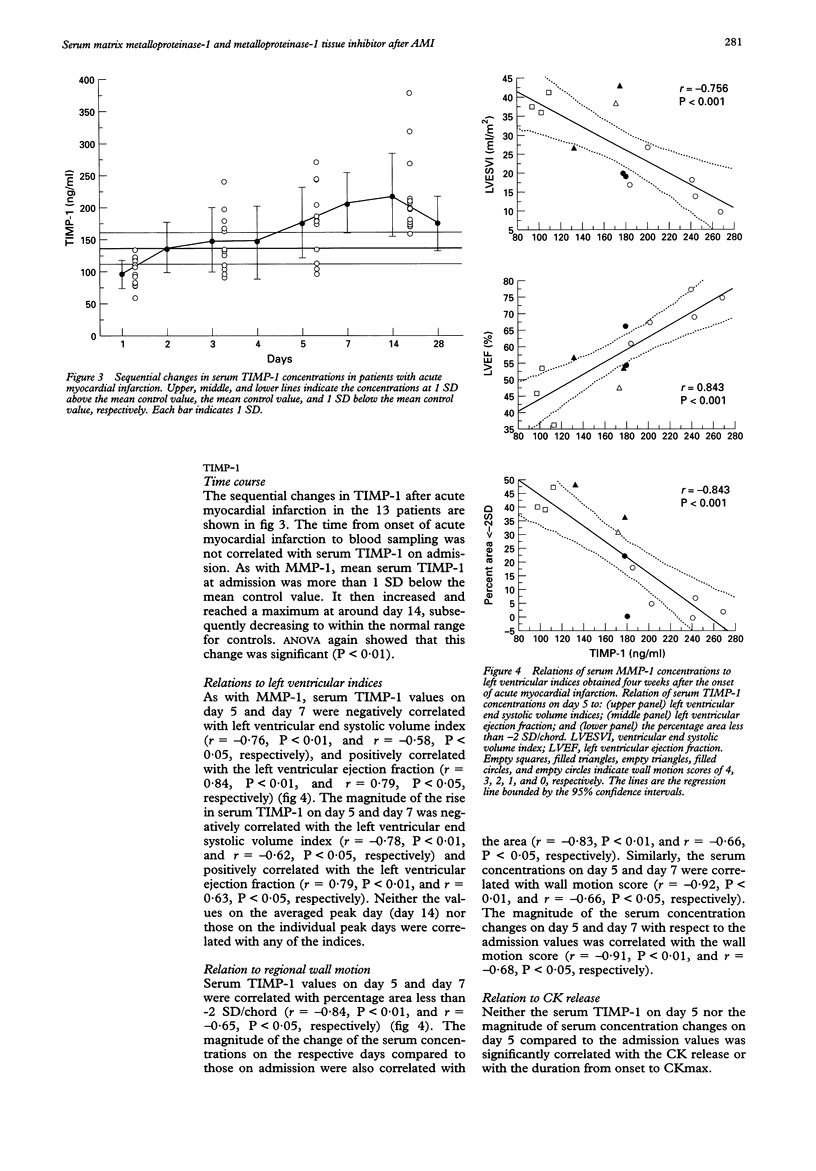
OBJECTIVE: To test the hypothesis that changes in serum matrix metalloproteinase-1 (MMP-1) and tissue inhibitors of metalloproteinase-1 (TIMP-1) after acute myocardial infarction reflect extracellular matrix remodelling and the infarct healing process. PATIENTS: 13 consecutive patients with their first acute myocardial infarction who underwent successful reperfusion. METHODS: Blood was sampled on the day of admission, and on days 2, 3, 4, 5, 7, 14, and 28. Serum MMP-1 and TIMP-1 were measured by one step sandwich enzyme immunoassay. Left ventricular volume indices were determined by left ventriculography performed four weeks after the infarct. RESULTS: Serum concentrations of both MMP-1 and TIMP-1 changed over time. The average serum MMP-1 was more than 1 SD below the mean control values during the initial four days, increased thereafter, reaching a peak concentration around day 14, and then returned to the middle control range. Negative correlations with left ventricular end systolic volume index and positive correlations with left ventricular ejection fraction were obtained for serum MMP-1 on day 5, when it began to rise, and for the magnitude of rise in MMP-1 on day 5 compared to admission. Serum TIMP-1 at admission was more than 1 SD below the mean control value, and increased gradually thereafter, reaching a peak on around day 14. Negative correlations with left ventricular end systolic volume index and positive correlations with left ventricular ejection fraction were obtained for serum TIMP-1 on days 5 and 7, and for the magnitude of rise in TIMP-1 on days 5 and 7 compared to admission. CONCLUSIONS: Both MMP-1 and TIMP-1 showed significant time dependent alteration after acute myocardial infarction. Thus MMP-1 and TIMP-1 may provide useful information in evaluating the healing process as it affects left ventricular remodelling after acute myocardial infarction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold A. Z., Topol E. J. Assessment of reperfusion after thrombolytic therapy for myocardial infarction. Am Heart J. 1992 Aug;124(2):441–447. doi: 10.1016/0002-8703(92)90611-x. [DOI] [PubMed] [Google Scholar]
- Austen W. G., Edwards J. E., Frye R. L., Gensini G. G., Gott V. L., Griffith L. S., McGoon D. C., Murphy M. L., Roe B. B. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975 Apr;51(4 Suppl):5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston T. E., Galloway W. A., Mercer E., Murphy G., Reynolds J. J. Purification of rabbit bone inhibitor of collagenase. Biochem J. 1981 Apr 1;195(1):159–165. doi: 10.1042/bj1950159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleutjens J. P., Kandala J. C., Guarda E., Guntaka R. V., Weber K. T. Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol. 1995 Jun;27(6):1281–1292. doi: 10.1016/s0022-2828(05)82390-9. [DOI] [PubMed] [Google Scholar]
- Dollery C. M., McEwan J. R., Henney A. M. Matrix metalloproteinases and cardiovascular disease. Circ Res. 1995 Nov;77(5):863–868. doi: 10.1161/01.res.77.5.863. [DOI] [PubMed] [Google Scholar]
- Enghild J. J., Salvesen G., Brew K., Nagase H. Interaction of human rheumatoid synovial collagenase (matrix metalloproteinase 1) and stromelysin (matrix metalloproteinase 3) with human alpha 2-macroglobulin and chicken ovostatin. Binding kinetics and identification of matrix metalloproteinase cleavage sites. J Biol Chem. 1989 May 25;264(15):8779–8785. [PubMed] [Google Scholar]
- Greene D. G., Carlisle R., Grant C., Bunnell I. L. Estimation of left ventricular volume by one-plane cineangiography. Circulation. 1967 Jan;35(1):61–69. doi: 10.1161/01.cir.35.1.61. [DOI] [PubMed] [Google Scholar]
- Herskowitz A., Choi S., Ansari A. A., Wesselingh S. Cytokine mRNA expression in postischemic/reperfused myocardium. Am J Pathol. 1995 Feb;146(2):419–428. [PMC free article] [PubMed] [Google Scholar]
- Horie M., Yasue H., Omote S., Takizawa A., Nagao M., Nishida S., Kubota J. A new approach for the enzymatic estimation of infarct size: serum peak creatine kinase and time to peak creatine kinase activity. Am J Cardiol. 1986 Jan 1;57(1):76–81. doi: 10.1016/0002-9149(86)90955-0. [DOI] [PubMed] [Google Scholar]
- Kodama S., Iwata K., Iwata H., Yamashita K., Hayakawa T. Rapid one-step sandwich enzyme immunoassay for tissue inhibitor of metalloproteinases. An application for rheumatoid arthritis serum and plasma. J Immunol Methods. 1990 Feb 20;127(1):103–108. doi: 10.1016/0022-1759(90)90345-v. [DOI] [PubMed] [Google Scholar]
- Kostuk W. J., Kazamias T. M., Gander M. P., Simon A. L., Ross J., Jr Left ventricular size after acute myocardial infarction. Serial changes and their prognostic significance. Circulation. 1973 Jun;47(6):1174–1179. doi: 10.1161/01.cir.47.6.1174. [DOI] [PubMed] [Google Scholar]
- Lefebvre V., Peeters-Joris C., Vaes G. Modulation by interleukin 1 and tumor necrosis factor alpha of production of collagenase, tissue inhibitor of metalloproteinases and collagen types in differentiated and dedifferentiated articular chondrocytes. Biochim Biophys Acta. 1990 May 22;1052(3):366–378. doi: 10.1016/0167-4889(90)90145-4. [DOI] [PubMed] [Google Scholar]
- Manicourt D. H., Fujimoto N., Obata K., Thonar E. J. Levels of circulating collagenase, stromelysin-1, and tissue inhibitor of matrix metalloproteinases 1 in patients with rheumatoid arthritis. Relationship to serum levels of antigenic keratan sulfate and systemic parameters of inflammation. Arthritis Rheum. 1995 Aug;38(8):1031–1039. doi: 10.1002/art.1780380803. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Murawaki Y., Kawasaki H., Burkhardt H. Serum collagenase activity in chronic liver diseases. Pathol Res Pract. 1994 Oct;190(9-10):929–933. doi: 10.1016/S0344-0338(11)80998-2. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cawston T. E., Reynolds J. J. An inhibitor of collagenase from human amniotic fluid. Purification, characterization and action on metalloproteinases. Biochem J. 1981 Apr 1;195(1):167–170. doi: 10.1042/bj1950167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris R. M., Whitlock R. M., Barratt-Boyes C., Small C. W. Clinical measurement of myocardial infarct size. Modification of a method for the estimation of total creatine phosphokinase release after myocardial infarction. Circulation. 1975 Apr;51(4):614–620. doi: 10.1161/01.cir.51.4.614. [DOI] [PubMed] [Google Scholar]
- Pfeffer M. A., Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990 Apr;81(4):1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- Rosalki S. B. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967 Apr;69(4):696–705. [PubMed] [Google Scholar]
- Sheehan F. H., Bolson E. L., Dodge H. T., Mathey D. G., Schofer J., Woo H. W. Advantages and applications of the centerline method for characterizing regional ventricular function. Circulation. 1986 Aug;74(2):293–305. doi: 10.1161/01.cir.74.2.293. [DOI] [PubMed] [Google Scholar]
- Shell W. E., Kjekshus J. K., Sobel B. E. Quantitative assessment of the extent of myocardial infarction in the conscious dog by means of analysis of serial changes in serum creatine phosphokinase activity. J Clin Invest. 1971 Dec;50(12):2614–2625. doi: 10.1172/JCI106762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricklin G. P., Li L., Jancic V., Wenczak B. A., Nanney L. B. Localization of mRNAs representing collagenase and TIMP in sections of healing human burn wounds. Am J Pathol. 1993 Dec;143(6):1657–1666. [PMC free article] [PubMed] [Google Scholar]
- Tyagi S. C., Kumar S. G., Banks J., Fortson W. Co-expression of tissue inhibitor and matrix metalloproteinase in myocardium. J Mol Cell Cardiol. 1995 Oct;27(10):2177–2189. doi: 10.1016/s0022-2828(95)91443-9. [DOI] [PubMed] [Google Scholar]
- Vaziri N. D., Kennedy S. C., Kennedy D., Gonzales E. Coagulation, fibrinolytic, and inhibitory proteins in acute myocardial infarction and angina pectoris. Am J Med. 1992 Dec;93(6):651–657. doi: 10.1016/0002-9343(92)90198-k. [DOI] [PubMed] [Google Scholar]
- Weisman H. F., Healy B. Myocardial infarct expansion, infarct extension, and reinfarction: pathophysiologic concepts. Prog Cardiovasc Dis. 1987 Sep-Oct;30(2):73–110. doi: 10.1016/0033-0620(87)90004-1. [DOI] [PubMed] [Google Scholar]
- White H. D., Norris R. M., Brown M. A., Brandt P. W., Whitlock R. M., Wild C. J. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987 Jul;76(1):44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y., Obata K., Fujimoto N., Yamashita K., Hayakawa T., Shimmei M. Increased levels of stromelysin-1 and tissue inhibitor of metalloproteinases-1 in sera from patients with rheumatoid arthritis. Arthritis Rheum. 1995 Jul;38(7):969–975. doi: 10.1002/art.1780380713. [DOI] [PubMed] [Google Scholar]
- Zhang J., Fujimoto N., Iwata K., Sakai T., Okada Y., Hayakawa T. A one-step sandwich enzyme immunoassay for human matrix metalloproteinase 1 (interstitial collagenase) using monoclonal antibodies. Clin Chim Acta. 1993 Oct 15;219(1-2):1–14. doi: 10.1016/0009-8981(93)90192-7. [DOI] [PubMed] [Google Scholar]



