Abstract
Pleomorphic xanthoastrocytoma (PXA) is an uncommon tumor constitutes less than 1% of all astrocytic glial neoplasms was first reported in 1979. PXA commonly occurs in young patients and manifests itself first as seizures followed by focal neurological deficits. The role of radiotherapy or chemotherapy has not yet been established because of the relative infrequency of this disease. PXA is classified as grade II tumor in the WHO classification of tumors of the CNS. In literature 9 to 20 % PXA may undergo malignant change at recurrence or may display at the time of initial presentation. Malignant transformation is mainly associated with high mitotic activity and necrosis. The criteria for PXA with anaplastic features was five or more mitotic activity per 10 high power fields, necrosis, microvascular proliferation, marked cellular anaplasia, and high Ki-67 labeling indices. PXA with anaplastic features management is highly controversial as very sparse literature is available. We are reporting a case of PXA with anaplastic features with atypical radiology and tried to review the up to date literature regarding this rare tumor.
Keywords: PXA with anaplasia, Pleomorphic xanthoastrocytoma, astrocytoma, management of PXA
Introduction
Pleomorphic xanthoastrocytoma (PXA) is an uncommon tumor constitutes less than 1% of all astrocytic glial neoplasms was first reported in 1979.[1,2,3,4] PXA commonly occurs in young patients and manifests itself first as seizures followed by focal neurological deficits.[5] The role of radiotherapy or chemotherapy has not yet been established because of the relative infrequency of this disease.[2,6,7,8] PXA is classified as grade II tumor in the WHO classification of tumors of the CNS.[4,9,10] In literature 9 to 20 % PXA may undergo malignant change at recurrence or may display at the time of initial presentation.[11] Malignant transformation is mainly associated with high mitotic activity and necrosis.[5] The criteria for PXA with anaplastic features was five or more mitotic activity per 10 high power fields, necrosis, microvascular proliferation, marked cellular anaplasia, and high Ki-67 labeling indices.[2,12] PXAs with anaplastic features management is highly controversial as very sparse literature is available.[2,6,13,14] We are reporting a case of PXA with anaplastic features and tried to review the up to date literature regarding this rare tumor.
Case Report
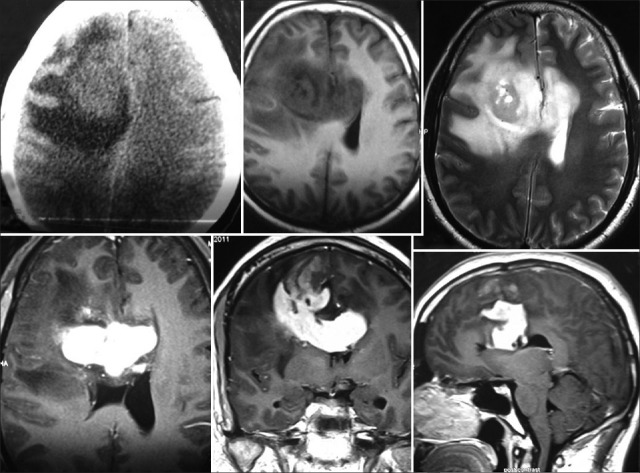
A 35-year-old healthy man complained of early morning headaches associated with vomiting evolving over 10 days. This was not accompanied by any other constitutional or focal neurological symptoms. MRI brain plain and contrast study revealed a heterogenous intensely enhancing right frontal mass lesion which is crossing to opposite hemisphere through corpus callosum with perilesional oedema [Figure 1]. In view of radiological feature we consider differential diagnosis of glioblastoma and Lymphoma. Right frontopareital craniotomy and subtotal resection was performed. Histological examination [Figure 2] showed lesion comprised of large pleomorphic cells with bizarre nuclei and abundant eosinophilic cytoplasm. Eosinophilic granular bodies were seen. There was mitosis with atypical mitotic figures, large foci of necrosis was seen. Lesion also showed focal areas of xanthoma cells with foamy cytoplasm. Reticulin stain showed variably with dense pericellular deposits. A fraction of tumor cells and giant cells showed positivity for Glial fibrillary acidic protein. Staining for the neuronal marker S 100 labeled a small population of tumor cells. CD 68 as was focally positive. CD 34 which is a marker mostly seen in conventional pleomorphic xanthoastrocytoma was negative. Staining for TP53, a marker of astrocytoma, showed no over-expression of the protein. To our surprise the lesion was turned out to be pleomorphic xanthoastrocytoma with anaplastic degeneration, WHO grade III. This patient radiology was atypical for pleomorphic xanthoastrocytoma as they are frequently superficially located tumors. Postoperatively patient underwent stereotactic radiosurgery and patient was under regular follow up.
Figure 1.
CT scan of brain (plain) showing hypodensity with surrounding edema and MRI of brain (plain) and contrast showing a heterogenous intensely enhancing right frontal mass lesion which is crossing to opposite hemisphere through corpus callosum with perilesional edema which is hypo on T1W and hyper intense on T2 W
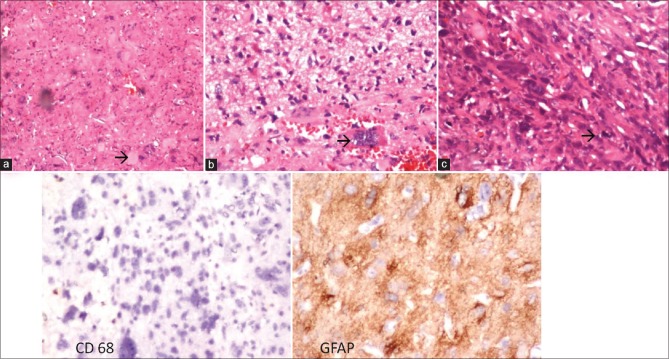
Figure 2.
Histopathology of the pleomorphic xanthoastrocytoma with anaplastic features showing arrow eosinophilic granular bodies (a) ×100, arrow showing pleomorphic giant cells; (b) ×400, arrow showing mitoses; (c) ×400, IHC showing CD 68 negative and GFAP positive
Discussion
It is usually seen in the second decade and more rarely in the first and third decades of life.[13] There is no difference between the sexes in the frequency of PXA.[13] These tumors are ordinarily located in cerebral hemispheres without dural involvement.[13] PXA is one of the desmoplastic glial tumors of the brain. Microscopically, its most conspicuous properties are the pleomorphic, and in part xanthomatous, characteristics of the tumor cells that contain intracytoplasmic lipid. Monstrous giant cells, cell atypia, nuclear irregularity, hyperchro-matism and mitotic activity and necrosis can also be seen.[13] In our case we found all these features. The histological differential diagnosis of PXA against malignant gliomas, which display the same features as PXA and contain necrosis and intracellular lipid, is important. Reticulin network surrounds the individual cells in PXA, where as in glioblastoma the reticulin network surrounds the necrosis.[13] Development of glioblastoma has been reported in some patients with PXA.[2,7] Anaplastic PXA shares several clinical and histologic features with other desmoplastic neuroepithelial neoplasms, such as gliofibroma, gliosarcoma, desmoplastic infantile ganglioglioma and desmoplastic cerebral astrocytoma of infancy.[13]
First and foremost, because of the uncommonness of PXA with anaplastic features,[11,12] the role of standard postoperative therapy has not been established. The cases previously reported were tabulated and analyzed in [Tables 1 and 2]. Seventeen cases of PXA showing anaplastic features at the first presentation have been reported in the literature till now.[15,16,17,18,19]
Table 1.
Previously reported cases
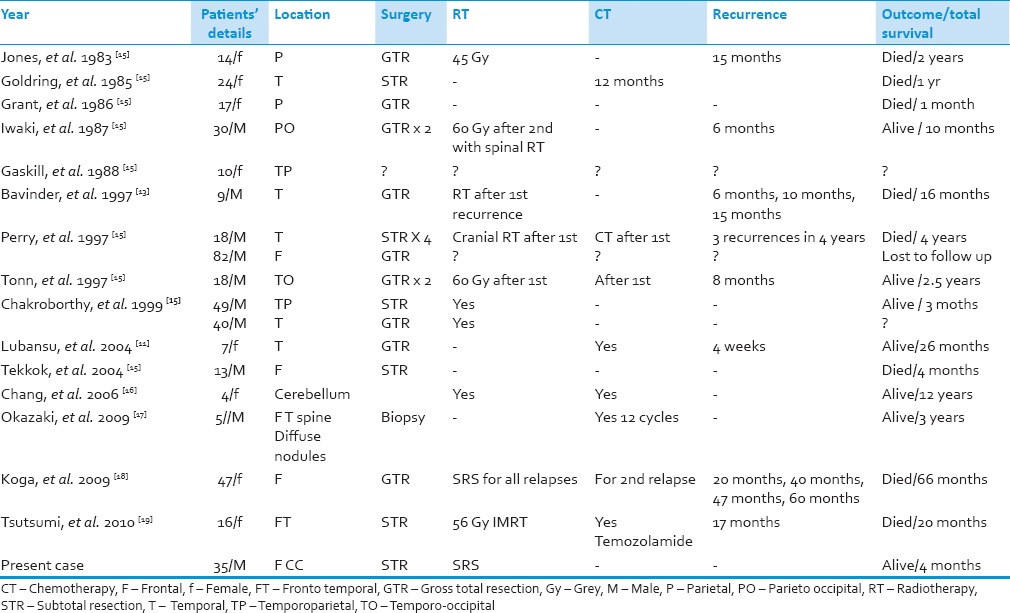
Table 2.
Summary from the previously reported cases
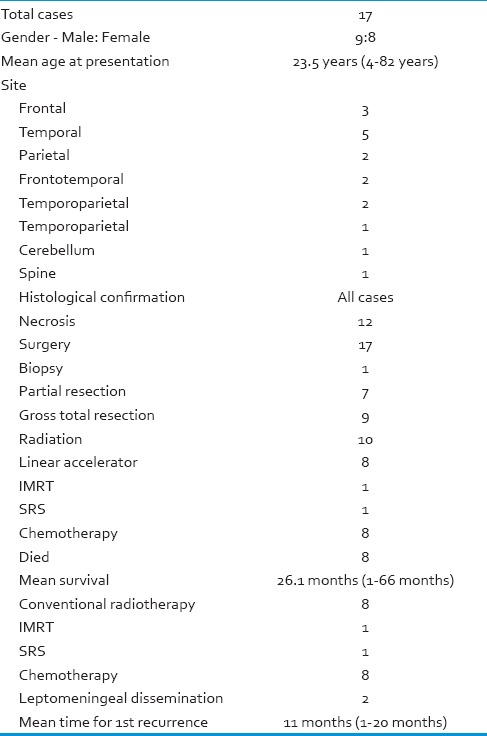
Among 13 patients whose follow-up information was available, eight patients died, and the average overall survival was 26.1 months, ranges from 1 to 66 months. One patient of cerebellar PXA with anaplastic features has progression free survival of 12 years.[16] Eight of 17 patients underwent conventional fractionated radiotherapy and one patient underwent IMRT without apparent benefit. The case reported by Koga et al. showed control up to certain extent with stereotactic radiosurgery for the disseminated nodules of PXA with anaplastic features at each relapse. By the use of repetitive stereotactic radiation, the patient was free from neurological deficit for 50 months despite frequent tumor recurrences within a short duration.[18] Leptomeningeal dissemination is not an uncommon pattern of recurrence for PXA with anaplastic features and has been observed in two cases. Leptomeningeal dissemination of PXA, with or without anaplastic features, commonly presents as multiple nodular lesions, in contrast to the diffuse dissemination seen in high-grade astrocytomas.[5] In some of the cases the presentation itself showed dissemination with nodules.[11,17]
PXA with anaplastic features can show transformation to glioblastoma.[8,18,20] PXA with anaplastic features at diagnosis typically has a poor prognosis.[11,21] In Koga et al. case report with institution of stereotactic radiosurgery survival was prolonged up to 66 months even with multiple nodular dissemination for several times.[18]
Chemotherapy for PXA has been generally considered ineffective.[2,7] Many regimes including carboplatin/VP-16, cyclophosphamide, vincristine and temozolamide were used in the treatment of PXA with anaplastic features but none worked well. Recent study of Marucci et al., assessment of MGMT promoter methylation status in PXA including two cases of PXA with anaplastic features raised doubts about the benefits of treating indistinctly aggressive PXA with Temozolamide.[22]
In Gianni et al. study the significance of the mitotic index, the presence of necrosis, and the extent of resection were analyzed, and found that the mitotic index and the extent of resection were the main predictors for recurrence-free survival and overall survival rates.[2] Korshumov et al., proposed grading system for PXA based on mitoses per 20 high-power fields, those not having mitoses (Grade 1), those with mitoses but without necrosis (Grade 2), and tumors with elevated mitotic index and necrotic foci (Grade 3). No recurrence or death in the Grade 1 group, whereas 36% of Grade 2 and 100% of Grade 3 group showed recurrence.[23] Grade 3 tumors in their classification corresponds to the PXA with anaplastic features.
Various molecular genetic changes are associated with PXA,[2,24,25] which were entirely different from the other diffusely infiltrating astrocytomas. The CDK4, CDKN2A, TP53 tumor suppressor genes, EGFR, and MDM2 genes are well known to occur in diffuse infiltrating astrocytic glioma including anaplastic astrocytoma and glioblastoma, but are not involved in the pathogenesis of PXA.[24] These genetic differences seem to be responsible for the distinct biological behavior of PXA. CD34 expression was more common in PXAs of WHO grade II (37 of 44 tumors, 84%) than in PXAs with anaplastic features (7 of 16 tumors, 44%) our tumor also not showing positivity of the CD 34.[26]
Conclusions
PXA with anaplastic features is a rare entity with sparse literature of review. Presence of necrosis, mitotic activity and subtotal resection are supposed to be poor prognostic factors. Conventional radiotherapy and chemotherapy including new drugs like temozolamide are not useful in the treatment of the PXA with anaplastic features. Stereotactic radiosurgery may be useful in the treatment of halting progression but needs several other studies. Even though this tumor comes under astrocytomas, genetically this tumor is entirely different from the conventional low and high grade astrocytomas.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Fouladi M, Jenkins J, Burger P, Langston J, Merchant T, Heideman R, et al. Pleomorphic xanthoastrocytoma: favorable outcome after complete surgical resection. Neurooncology. 2001;3:184–92. doi: 10.1093/neuonc/3.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giannini C, Scheithauer BW, Burger PC, Brat DJ, Wollan PC, Lach B, et al. Pleomorphic xanthoastrocytoma: What do we really know about it? Cancer. 1999;85:2033–45. [PubMed] [Google Scholar]
- 3.Gomez JG, Garcia JH, Colon LE. A variant of cerebral glioma called pleomorphic xanthoastrocytoma: Case report. Neurosurgery. 1985;16:703–6. doi: 10.1227/00006123-198505000-00025. [DOI] [PubMed] [Google Scholar]
- 4.Kepes JJ, Rubinstein LJ, Eng LF. Pleomorphic xanthoastrocytoma: a distinctive meningocerebral glioma of young subjects with relatively favorable prognosis. A study of 12 cases. Cancer. 1979;44:1839–52. doi: 10.1002/1097-0142(197911)44:5<1839::aid-cncr2820440543>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima T, Kumabe T, Shamoto H, Watanabe M, Suzuki H, Tominaga T. Malignant transformation of pleomorphic xanthoastrocytoma. Acta Neurochir (Wien) 2006;148:67–71. doi: 10.1007/s00701-005-0549-8. [DOI] [PubMed] [Google Scholar]
- 6.Cervoni L, Salvati M, Santoro A, Celli P. Pleomorphic xanthoastrocytoma: some observations. Neurosurg Rev. 1996;19:13–6. doi: 10.1007/BF00346603. [DOI] [PubMed] [Google Scholar]
- 7.Thomas C, Golden B. Pleomorphic xanthoastrocytoma: Report of two cases and brief review of the literature. Clin Neuropathol. 1993;12:97–101. [PubMed] [Google Scholar]
- 8.Whittle IR, Gordon A, Misra BK, Shaw JF, Steers AJ. Pleomorphic xanthoastrocytoma. Report of four cases. J Neurosurg. 1989;70:463–8. doi: 10.3171/jns.1989.70.3.0463. [DOI] [PubMed] [Google Scholar]
- 9.Stuart G, Appleton DB, Cooke R. Pleomorphic xanthoastrocytoma: Report of two cases. Neurosurgery. 1988;22:422–7. doi: 10.1227/00006123-198802000-00028. [DOI] [PubMed] [Google Scholar]
- 10.Giannini CPW, Louis DN, Liberski P. Pleomorphic xanthoastrocytoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. World Health Organization Classification of Tumours of the Central Nervous System. 4th ed. Lyon, France: International Agency for Research on Cancer; 2007. pp. 22–4. [Google Scholar]
- 11.Lubansu A, Rorive S, David P, Sariban E, Seligmann R, Brotchi J, et al. Cerebral anaplastic pleomorphic xanthoastrocytoma with meningeal dissemination at first presentation. Childs Nerv Syst. 2004;20:119–22. doi: 10.1007/s00381-003-0854-6. [DOI] [PubMed] [Google Scholar]
- 12.Hirose T, Ishizawa K, Sugiyama K, Kageji T, Ueki K, Kannuki S. Pleomorphic xanthoastrocytoma: a comparative pathological study between conventional and anaplastic types. Histopathology. 2008;52:183–93. doi: 10.1111/j.1365-2559.2007.02926.x. [DOI] [PubMed] [Google Scholar]
- 13.Bayindir C, Balak N, Karasu A, Kasaroğlu D. Anaplastic pleomorphic xanthoastrocytoma. Childs Nerv Syst. 1997;13:50–6. doi: 10.1007/s003810050040. [DOI] [PubMed] [Google Scholar]
- 14.Bucciero A, De Caro MI, Tedeschi E, De Stefano V, Bianco M, Soricelli A, et al. Atypical pleomorphic xanthoastrocytoma. J Neurosurg Sci. 1998;42:153–7. [PubMed] [Google Scholar]
- 15.Tekkök IH, Sav A. Anaplastic pleomorphic xanthoastrocytomas: Review of literature with reference to malignancy. Pediatr Neurosurg. 2004;40:171–81. doi: 10.1159/000081935. [DOI] [PubMed] [Google Scholar]
- 16.Chang HT, Latorre JG, Hahn S, Dubowy R, Schelper RL. Pediatric cerebellar pleomorphic xanthoastrocytoma with anaplastic features: A case of long-term survival after multimodality therapy. Childs Nerv Syst. 2006;22:609–13. doi: 10.1007/s00381-005-0005-3. [DOI] [PubMed] [Google Scholar]
- 17.Okazaki T, Kageji T, Matsuzaki K, Horiguchi H, Hirose T, Watanabe H, et al. Primary anaplastic pleomorphic xanthoastrocytoma with widespread neuroaxis dissemination at diagnosis––a pediatric case report and review of the literature. J Neurooncol. 2009;94:431–7. doi: 10.1007/s11060-009-9876-6. [DOI] [PubMed] [Google Scholar]
- 18.Koga H, Zhang S, Kumanishi T, Washiyama K, Ichikawa T, Tanaka R, et al. Analysis of p53 gene mutations in low- and high-grade astrocytomas by polymerase chain reaction-assisted single-strand conformation polymorphism and immunohistochemistry. Acta Neuropathol. 1994;87:225–32. doi: 10.1007/BF00296737. [DOI] [PubMed] [Google Scholar]
- 19.Tsutsumi S, Abe Y, yasumoto Y, Ito M. Anaplatic pleomorphic xanthoastrocytoma with a component of anaplastic astrocytoma presenting as skull base tumor followed by downward extracranial extension. Neurol Med Chir (Tokyo) 2010;50:1108–12. doi: 10.2176/nmc.50.1108. [DOI] [PubMed] [Google Scholar]
- 20.Kepes JJ, Rubinstein LJ, Ansbacher L, Schreiber DJ. Histopathological features of recurrent pleomorphic xanthoastrocytomas: Further corroboration of the glial nature of this neoplasm. A study of 3 cases. Acta Neuropathol. 1989;78:585–93. doi: 10.1007/BF00691285. [DOI] [PubMed] [Google Scholar]
- 21.Hirose T, Giannini C, Scheithauer BW. Ultrastructural features of pleomorphic xanthoastrocytoma: A comparative study with glioblastoma multiforme. Ultrastruct Pathol. 2001;25:469–78. doi: 10.1080/019131201753343502. [DOI] [PubMed] [Google Scholar]
- 22.Marucci G, Morandi L. Assessment of MGMT promoter methylation status in Pleomorphic Xantho Astrocytoma. J Neurooncol. 2011;105:397–400. doi: 10.1007/s11060-011-0605-6. [DOI] [PubMed] [Google Scholar]
- 23.Korshunov A, Golanov A. Pleomorphic xanthoastrocytomas: Immunohistochemistry, grading and clinico-pathologic correlations. An analysis of 34 cases from a single institute. J Neurooncol. 2001;52:63–72. doi: 10.1023/a:1010648006319. [DOI] [PubMed] [Google Scholar]
- 24.Kaulich K, Blaschke B, Numann A, von Deimling A, Wiestler OD, Weber RG, et al. Genetic alterations commonly found in diffusely infiltrating cerebral gliomas are rare or absent in pleomorphic xanthoastrocytomas. J Neuropathol Exp Neurol. 2002;61:1092–9. doi: 10.1093/jnen/61.12.1092. [DOI] [PubMed] [Google Scholar]
- 25.Paulus W, Lisle DK, Tonn JC, Wolf HK, Roggendorf W, Reeves SA, et al. Molecular genetic alterations in pleomorphic Xanthoastrocytoma. Acta Neuropathol. 1996;91:293–7. doi: 10.1007/s004010050428. [DOI] [PubMed] [Google Scholar]
- 26.Reifenberger G, Kaulich K, Weistler OD, Blümcke I. Expression of CD 34 antigen in pleomorphic xanthoastrocytoma. Acta Neuropathol. 2003;105:358–64. doi: 10.1007/s00401-002-0652-3. [DOI] [PubMed] [Google Scholar]